Abstract
Sympathetic outflow to brown adipose tissue (BAT) contributes to both thermoregulation and energy expenditure in rats through regulation of BAT thermogenesis. Acute cold exposure in mature animals augments BAT thermogenesis; however, the enhanced BAT thermogenic response returns to normal shortly after cessation of the cold exposure. In this study, we sought to determine whether cold exposure in early neonatal life could induce enhanced responses in the sympathetic outflow to BAT and whether this altered sympathetic regulation would be sustained after the cold stimulus was removed. BAT sympathetic nerve activity (SNA) was recorded in urethane–chloralose-anesthetized, artificially ventilated rats that were raised from birth in either 18 or 30°C environments and then, at 8 weeks of age, were maintained in 23°C for at least 4 weeks. An acute hypothermic stimulus, disinhibition of a brainstem thermogenic network in the raphe pallidus, or electrical stimulation in this raphe site produced increases in BAT SNA that were twice as great in rats reared at 18°C as in those reared at 30°C. The norepinephrine content of the interscapular BAT (IBAT) and the number of sympathetic ganglion cells projecting to interscapular BAT were 70% greater in the 18°C-reared rats. We conclude that neonatal exposure to a cold environment induces a permanent developmental alteration in the capacity for sympathetic stimulation of BAT thermogenesis that may be mediated, in part, by a greater number of sympathetic ganglion cells innervating BAT in cold-reared animals.
Keywords: raphe pallidus, sympathetic ganglion, sympathetic development, brown adipose tissue, thermogenesis, cold acclimation, bicuculline, fast blue
Development within the mammalian nervous system continues well after birth. Consequently, postnatal sensory experiences exert a formative influence on the maturation of numerous components of the nervous system, including visual and auditory pathways (Hubel and Wiesel, 1970; Moore, 1985; Thoenen, 1995), the somatosensory and olfactory systems (Woolsey et al., 1981; Brunjes and Frazier, 1986), and the networks controlling respiration (Ling et al., 1997; Erickson et al., 1998). The finding that alterations in early postnatal environment can result in changes in nervous system function that are maintained throughout life has lead to the concept of developmental plasticity in which altered levels of growth factors or other signaling molecules resulting from exposure to environmental factors can affect cell number (perhaps through influences on the apoptotic process) or function in the nervous system to produce permanent alterations in behavior or organ function. The present study was designed to determine whether a similar developmental plasticity could influence thermoregulatory responses in adult animals. Specifically, we tested the hypothesis that a reduction in neonatal rearing temperature would result in a sustained amplification of the thermogenic responses in brown adipose tissue (BAT) that are known to play a critical role in heat production during acute cold exposure in normal (Foster, 1984) and in cold-acclimated (Foster and Frydman, 1979) rats.
Cold acclimation, produced by repeated cold stress in adult animals, leads to a greater cold tolerance and an augmented metabolic heat production in subsequent cold challenges (LeBlanc et al., 1967;Talan and Engel, 1988). Both a centrally mediated enhancement of the level of sympathetic outflow to BAT (Kawate et al., 1994) and an upregulation of BAT metabolic processes contribute to these enhanced thermogenic responses. The amplified BAT thermogenesis in cold acclimation is lost, however, if the interval between cold exposures is increased from 2 to 4 weeks (Talan and Engel, 1988), indicating a return to normal conditions in the absence of the conditioning cold stimulus. It has not been determined whether neonatal cold exposure can induce alterations in the control of BAT sympathetic nerve activity (SNA) that are similar to those seen in cold acclimation, but are sustained on the return to a normothermic environment.
Although the hypothalamus plays a critical role in the initiation and control of various processes that increase BAT metabolic activity, including the thermogenic response to environmental cold (Boulant, 1980), the pathway(s) by which changes in hypothalamic neuronal discharge influence the sympathetic preganglionic neurons controlling BAT remain largely unknown. Recently, we have discovered a potential role for neurons in the rostral medullary raphe nuclei, including raphe pallidus (RPa), in the central thermogenic network regulating BAT SNA (Morrison et al., 1999). This information has provided the unique opportunity to determine the effect of altering neonatal environmental temperature on the responses resulting from activation of neurons in the brainstem circuits driving BAT thermogenesis.
A preliminary report of these results has been published previously (Morrison and Young, 1998).
MATERIALS AND METHODS
Animals. One-day-old male and female Sprague Dawley rats with multiparous foster mothers were obtained from Charles River Breeding Laboratories (Wilmington, MA). On arrival, litters were culled to 10 pups, and each litter with foster mother was placed in a standard plastic cage (length, 18.5 in; width, 10 in; height, 8 in) lined with wood shavings. Cages were then placed in one of two temperature-controlled chambers set at 18 or 30°C (±0.2°C). Because the cages were covered only by wire grids, the temperatures in the cages were similar to the internal temperatures of the chambers. The chambers (Model ST50 GC/M; Sure-Temp, Apex, NC; internal volume, 50 ft3) were equipped with glass doors, and illumination was provided by room lighting as well as by a timer-controlled internal light set to coincide with the 14/10 hr light/dark cycle of the room. Litters and mothers were left undisturbed, except for weekly cage changes. Pups were weaned at 21–22 d, removed from the chambers at 60 d of age, and housed in a room at 21 ± 0.2°C for at least 30 d until participation in the acute experiments described below. Rats were provided ad libitum access to water and standard laboratory chow (Prolab R-M-H 3000; Agway, Syracuse, NY). The animals used in this study were maintained in accordance with the guidelines and approval of the Animal Care and Use Committee of Northwestern University.
Neural recordings and stimulations. Rats were anesthetized intravenously with urethane (0.8 gm/kg) and chloralose (80 mg/kg) after induction with 3% isoflurane in 100% O2. A femoral artery, a femoral vein, and the trachea were cannulated for measurement of arterial pressure, drug injection, and artificial ventilation, respectively. Heart rate was determined from the arterial pressure signal. Animals were positioned prone in a stereotaxic frame (incisor bar, −11.0 mm) with a spinal clamp on the T10 vertebra, paralyzed with d-tubocurarine (0.3 mg initial dose, 0.1 mg/hr supplements), and artificially ventilated with 100% O2 (50 cycles per minute; tidal volume, 3 ml). Small adjustments in minute ventilation were made as necessary to maintain end-tidal CO2 between 4 and 5%. Throughout most of the experiment, colonic temperature was maintained at 37.5°C with a heating plate beneath the animal and a heat lamp. To provide a natural stimulus for the activation of the sympathetic nerve discharge to BAT, body temperature was lowered once in each animal by turning off the heat sources and placing dry ice in contact with the metal heating plate beneath the animal for ∼5 min. This caused body temperature to fall from 37.5 to 34–35°C within 10 min, at which time the heat sources were turned on and body temperature returned to 37.5°C.
Postganglionic sympathetic nerve activity to BAT was recorded from the central cut end of a small nerve bundle dissected from the ventral surface of the right interscapular BAT (IBAT) after the fat pad was divided along the midline and reflected laterally. Nerve activity was recorded with bipolar hook electrodes in a monopolar configuration, filtered (1–300 Hz) and amplified (50,000 times) with a Cyberamp 380 (Axon Instruments, Foster City, CA), and digitized and recorded (Neurodata, Woods Hole, MA) on videocassette-recorder tape along with the arterial pressure and stimulus trigger pulses.
A stimulating electrode and, subsequently, a microinjection pipette (tip outside diameter, 20 μm) were positioned stereotaxically in the RPa after a partial occipital craniotomy and reflection of the atlanto-occipital membrane. Relative to the calamus scriptorius, the coordinates for the RPa were as follows: anteroposterior, 3.0 mm; mediolateral, 0.0 mm; and dorsoventral, −2.7 mm. At the end of each experiment, the microinjection pipette was retracted vertically from the RPa, refilled with a 1% solution of fast green dye, and lowered to the site of microinjection (and stimulation). Dye was electrophoretically deposited (15 μA anodal direct current for 15 min). After perfusion and histological processing, the locations of the microinjection sites in the RPa were plotted on camera lucida drawings of sections through the rostral medulla (Paxinos and Watson, 1986). Stimuli, applied through a monopolar tungsten microelectrode (30 μm exposed tip), consisted of twin pulses of 1 msec duration, 6 msec interpulse interval, 5–150 μA, delivered at 0.4 Hz.
During the experimental protocol, arterial pressure, heart rate, and BAT SNA were recorded (1) during an initial control period with body temperature maintained at ∼37.5°C, (2) during a reduction in body temperature, including responses to electrical stimuli applied to RPa, (3) during a second control period 30 min after the return to normal body temperature, and (4) during a microinjection of vehicle (saline) and of the GABAA antagonist, bicuculline methiodide (60 nl, 500 μm), into RPa. No differences were observed between the parameters recorded during the first and second control periods, and no responses were ever noted to microinjection of saline into RPa.
After digitization at 1 kHz, arterial pressure and BAT SNA signals were analyzed with software written in the ASYST (Keithley Instruments, Cleveland, OH) programming environment. The amplitude of the BAT SNA and the mean frequency of the bursts in nerve activity were derived from autospectral analysis. For each experimental condition, an average autospectrum of a 20.5 sec period of sympathetic nerve activity to BAT was obtained by dividing the data record into nine 4.1 sec segments with a 50% overlap. The power value of the average autospectrum at each frequency point was computed as the mean value of the powers at that frequency in the individual autospectra of these nine segments. The amplitude of the sympathetic nerve activity to BAT was taken as the root mean square value of the total power in the 1–10 Hz band of the averaged autospectrum and is expressed in the text simply as “units.” The mean burst frequency of BAT SNA was obtained as the weighted mean frequency within the 2 Hz interval containing the greatest power. Parameters defining the inverse relationship between body temperature and BAT SNA were obtained by measuring BAT SNA amplitude at several levels of body temperature as the temperature was lowered during the hypothermic portion of the protocol described above. These paired data points were fit to a “logistic” equation (Kent et al., 1972) to determine the gain of the relationship (slope of the linear portion of the curve) and the operating (set) point of the relationship (midpoint of the linear portion of the curve).
Ganglion cell counting. To compare the relative number and size of ganglion cells contributing axons to the sympathetic postganglionic nerves innervating the interscapular BAT in animals raised at 18 and 30°C, we used the approach of Vera et al. (1997), injecting the retrograde tracer, fast blue, into the right interscapular fat pad. In each animal, eight fast blue injections (1 μl of a 2% solution) were made in a two-by-four grid: two rows (1 and 3 mm lateral to the vertebral processes) of four injections each (beginning at the rostral edge of the fat pad and separated by 2.0 mm). After survival times of 10–12 d, animals were anesthetized with 75 mg/kg sodium pentobarbital and perfused transcardially with 50 ml of saline, followed by 500 ml of 4% paraformaldehyde. The right sympathetic chain ganglia were removed from the superior cervical to the T5 level and placed in fixative for 1 hr. Individual ganglia were then sectioned at 50 μm on a freezing microtome, mounted in gelatin-coated slides, air-dried, and examined under a fluorescent microscope. The number of fluorescent cell profiles in each section was counted, and the total number of retrogradely labeled cells in each ganglion in each animal was determined. Correction factors were not applied to these counts because (1) the diameters of the ganglion cells were less than half of the section thickness, (2) the sizes of the ganglion cells did not differ between the two groups of animals, and (3) identical procedures were followed for preparing the tissue in both animal groups. To obtain an estimate of the diameters of the ganglion cells in animals reared at 18 and 30°C, computerized images of six fluorescent cell profiles were traced from sections of the middle cervical ganglia from each of five animals at each rearing temperature. The area of each cell profile was computed, as was a corresponding cell diameter, assuming that the cell was spherical. A mean cell diameter was determined for each animal, and these values were used to assess differences in ganglion cell size attributable to rearing temperature.
Analysis of BAT catecholamines. For norepinephrine (NE) analysis, interscapular BAT was weighed and homogenized in iced 0.2N perchloric acid. After addition of the internal standard, dihydroxybenzylamine (Sigma, St. Louis, MO), catecholamines were isolated from the perchloric acid extract by adsorption onto alumina (Woelm neutral, ICN Nutritional Biochemicals) in the presence of 2m Tris (hydroxymethyl)-aminomethane buffer, pH 8.7 (Sigma), containing 2% EDTA. Catecholamines were eluted from the alumina with 0.2N perchloric acid. Analysis of BAT catecholamines in the alumina eluates was performed using the method of Eriksson and Persson (1982).
Data analysis. Data are displayed as means ± SE, unless otherwise noted. Statistical ANOVAs and covariance were performed using Data Desk 5.0 statistical software (Data Description, Ithaca, NY). In comparisons of NE content between groups before weaning, the ANOVA model included litter as an additional variable in a nested analysis. Post hoc, pair-wise comparisons after ANOVA used Sheffé's test. Statistical differences were also assessed with Student's paired t test, with p < 0.05.
RESULTS
The initial resting mean arterial pressures and heart rates in the animals raised at 18°C were not different from those of the animals raised at 30°C (Table 1). The mean body weight of the males raised at 18°C (583 ± 25 gm,n = 11) was significantly greater (p < 0.05) than that of the males raised at 30°C (485 ± 29 gm, n = 6), although the weights of the females did not differ between the two rearing temperatures (18°C: 345 ± 15 gm, n = 19; 30°C: 348 ± 24 gm, n = 8).
Table 1.
Cardiovascular variables in rats reared at 18°C and 30°C and changes evoked by acute hypothermia and disinhibition of raphe pallidus neurons
| Rearing temperature | Control | Acute hypothermia | BIC in RPa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MAP (mmHg) | PP (mmHg) | HR (bpm) | ΔMAP (mmHg) | ΔPP (mmHg) | ΔHR (bpm) | ΔMAP (mmHg) | ΔPP (mmHg) | ΔHR (bpm) | |
| 18°C | 108 ± 4 | 59 ± 2 | 345 ± 8 | +11 ± 3 | +9 ± 2 | +41 ± 7 | +18 ± 3 | +12 ± 2* | +70 ± 7 |
| 30°C | 106 ± 4 | 57 ± 4 | 357 ± 7 | +11 ± 4 | +14 ± 3 | +55 ± 9 | +22 ± 4 | +23 ± 5 | +84 ± 14 |
MAP, Mean arterial pressure; PP, pulse pressure; HR, heart rate; Δ, evoked change;BIC, bicuculline; RPa, raphe pallidus.
Significant difference (p < 0.05) between 18°C- and 30°C-reared rats.
Effect of rearing temperature on BAT SNA responses to acute hypothermia
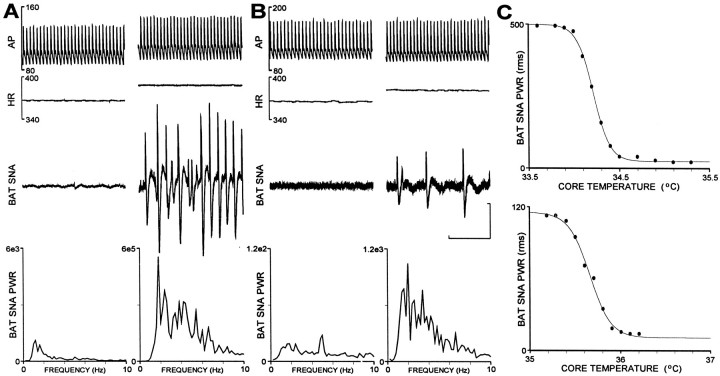
At normal body temperature (37–38°C), there was little or no activity on the sympathetic nerve to BAT in rats raised at either 18 or 30°C (Fig.1A,B, left panels, BAT SNA traces). The mean levels of normothermic control BAT SNA were not different between animals raised at 18°C (25 ± 2 units) and those raised at 30°C (30 ± 4 units). As body temperature was lowered by ventral contact with a chilled surface and removal of external heating sources, BAT SNA increased in the form of isolated bursts (Fig.1A,B, right panels, BAT SNA traces) indicating the synchronous discharge of sympathetic ganglion cell axons within the nerve bundle. The amplitude and frequency of the bursts in BAT SNA increased as body temperature fell, reaching a level of maximum total power that was sustained for a short time and then declined if body temperature was reduced further. Neither the threshold temperature for an increase in BAT SNA nor the body temperature at which the maximum increase in BAT SNA occurred was different between animals raised at 18°C (threshold, 35.6 ± 0.2°C; maximum BAT SNA, 34.7 ± 0.2°C) and those raised at 30°C (threshold, 35.5 ± 0.2°C; maximum BAT SNA, 34.6 ± 0.3°C). However, the maximum level of BAT SNA and the gain of the relationship between body temperature and BAT SNA were significantly greater in animals raised at 18°C than in those raised at 30°C.
Fig. 1.
Comparison of the effects of acute hypothermia on sympathetic nerve activity to brown adipose tissue (BAT SNA) in animals raised at 18 and 30°C. A, Arterial pressure (AP), heart rate (HR), BAT SNA, and the averaged power spectrum of BAT SNA (BAT SNA PWR) in a rat raised at 18°C under normothermic, control conditions (left panel) and after core temperature had been lowered to 33.8°C (right panel). Note differences in scale factorfor BAT SNA PWR. B, Increase in BAT SNA when body temperature was lowered to 35.2°C in an animal raised at 30°C. Note differences in scale factor for BAT SNA PWR between panels A and B. C, Reciprocal relationship between falling core temperature and reflex rise in BAT SNA during the hypothermia. Top trace: logistic curve fit for data in A from an animal raised at 18°C indicated a reflex gain (slope of the linear portion of the curve) of 1321 BAT SNA PWR units/°C and an operating point (core temperature at the center of the linear portion of the curve) of 34.2°C. Bottom trace: curve fitting for data inB from an animal raised at 30°C indicated a reflex gain of 229 BAT SNA PWR units/°C and an operating point of 35.7°C. Horizontal calibration represents 1 sec for the top three traces in A and B, and the vertical calibration represents 50μV for the BAT SNAtraces in panels A andB.
A representative example of the responses to acute hypothermia in a rat raised at 18°C is shown in Figure 1. The BAT SNA amplitude increased from 20 units at body temperatures >34.9°C (Fig.1A, left panel, BAT SNA PWR spectrum) to 471 units at a body temperature of 33.9°C (Fig. 1A,right panel). Fitting the body temperature measurements and the corresponding BAT SNA amplitudes to a logistic function (Kent et al., 1972) yielded a reflex gain of 1321 units/°C of BAT SNA and an operating point temperature of 34.2°C (Fig.1C, top trace). In contrast, the amplitude of the BAT SNA in a rat raised at 30°C increased from 15 units at body temperatures >36.0°C (Fig. 1B, left panel, BAT SNA PWR spectrum) to a maximum of 108 units at a body temperature of 35.4°C (Fig. 1B, right panel). This response had a reflex gain of 229 units of BAT SNA/°C and an operating point temperature of 35.7°C (Fig.1C, bottom trace).
In the rats raised at 18°C, the mean maximum level of BAT SNA occurring during the acute hypothermic stimulus was 365 ± 54 units, representing an increase of 353 ± 54 units (1331 ± 180% of control), which was significantly (p < 0.01) greater than the mean maximum level of BAT SNA (197 ± 32 units) achieved in animals raised at 30°C, representing an increase of 165 ± 28 units (520 ± 62% of control). The mean gain of the relationship between falling body temperature and increased BAT SNA was 568 ± 95 units/°C in the rats raised at 18°C, which was significantly (p < 0.005) greater than that in the rats raised at 30°C (196 ± 30 units/°C). The mean operating point temperatures were not different between rats reared at 18°C (35.1 ± 0.2°C) and those reared at 30°C (35.1 ± 0.3°C). Mean arterial pressure and heart rate were also increased in both groups of animals during the hypothermic response (Fig.1A,B, top traces), although the peak increases in mean arterial pressure and heart rate were not different between the rats raised at 18°C and those raised at 30°C (Table 1).
Effect of rearing temperature on BAT SNA responses to disinhibition of RPa neurons
A second stimulus that increases BAT SNA and thermogenesis is disinhibition of neurons in the rostral RPa (Morrison et al., 1999). This site may contain sympathetic premotor neurons providing an excitatory input to the spinal sympathetic preganglionic neurons for BAT, and reduced inhibition of RPa neurons may underlie the increase in BAT thermogenesis in response to a fall in body temperature (Morrison, 1999; Morrison et al., 1999). To determine whether exposure to a lowered environmental temperature in the early postnatal period would alter the BAT SNA response to activation of RPa neurons, we recorded the BAT SNA response to the blockade of local GABAA receptors in RPa with microinjections (60 nl) of bicuculline (500 μm).
Disinhibition of raphe pallidus neurons produced rapid and large increases in the sympathetic outflow to BAT in both groups of animals. In the example in Figure2A from an animal raised at 18°C, the control level of BAT SNA (18 units) was increased to a maximum of 755 units (4194% of control) at 6 min after microinjection of bicuculline into RPa. BAT SNA was increased in a similar manner by disinhibition of RPa neurons in the animal raised at 30°C (Fig. 2B); however, the peak amplitude of BAT SNA (201 units; 773% of control) that was reached at 7 min after the bicuculline microinjection was markedly less than that in the animal raised at 18°C.
Fig. 2.
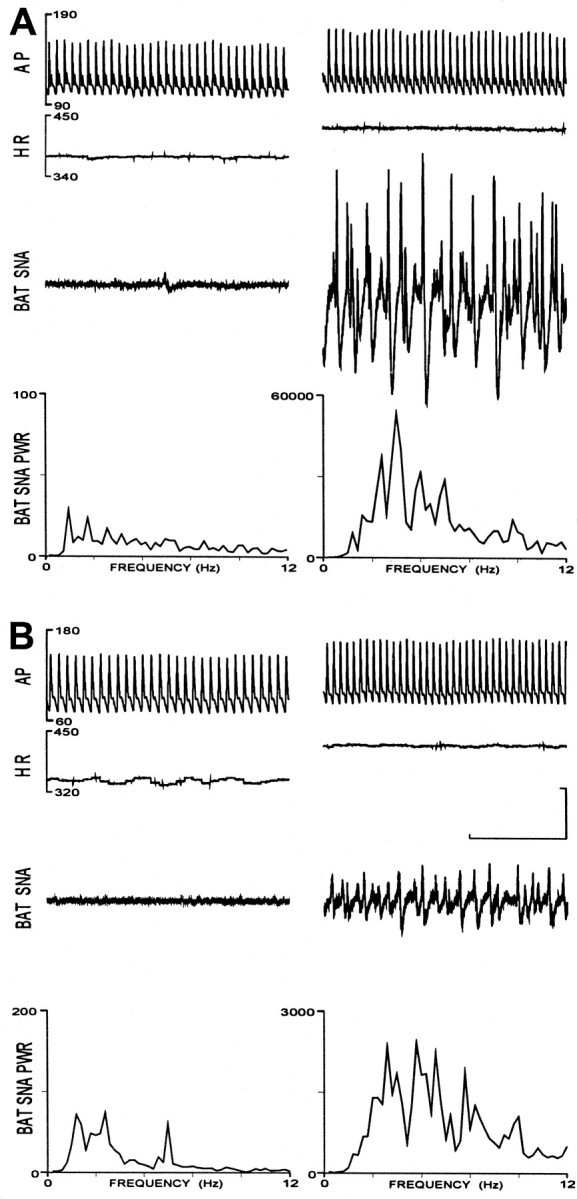
Comparison of the effects of disinhibition of raphe pallidus (RPa) neurons on the sympathetic nerve activity to brown adipose tissue (BAT SNA) in animals raised at 18 and 30°C. A, Arterial pressure (AP), heart rate (HR), BAT SNA, and the averaged power spectrum of BAT SNA (BAT SNA PWR) in a rat raised at 18°C, under normothermic, control conditions (left) and 6 min after bicuculline was microinjected (60 nl, 500 μm) into the RPa (right). Note differences in the scale for BAT SNA PWR. B, Same traces as in A in an animal raised at 30°C under normothermic, control conditions (right) and 7 min after a bicuculline microinjection into RPa (left). Note differences in scale factor for BAT SNA PWR between Aand B. Horizontal calibration represents 1 sec for thetop three traces in A andB, and vertical calibration represents 50 μV for the BAT SNA traces in A and B.
Microinjection of bicuculline into RPa evoked mean maximum levels of BAT SNA that were significantly (p < 0.005) higher in animals raised at 18°C (706 ± 103 units) than those in the rats raised at 30°C (352 ± 84 units). From control levels that were not different between groups, disinhibition of raphe pallidus neurons evoked increases in BAT SNA of 682 ± 101 units (2820 ± 294% of control) in animals raised at 18°C, which were significantly greater (p < 0.05) than the 317 ± 78 units (1746 ± 545% of control) increase in animals raised at 30°C. Bicuculline microinjection into RPa also increased the mean arterial pressure and heart rate (Fig.2A,B), although the peak increases were not different between the two groups of rats (Table 1).
Effect of rearing temperature on BAT SNA responses to stimulation in RPa
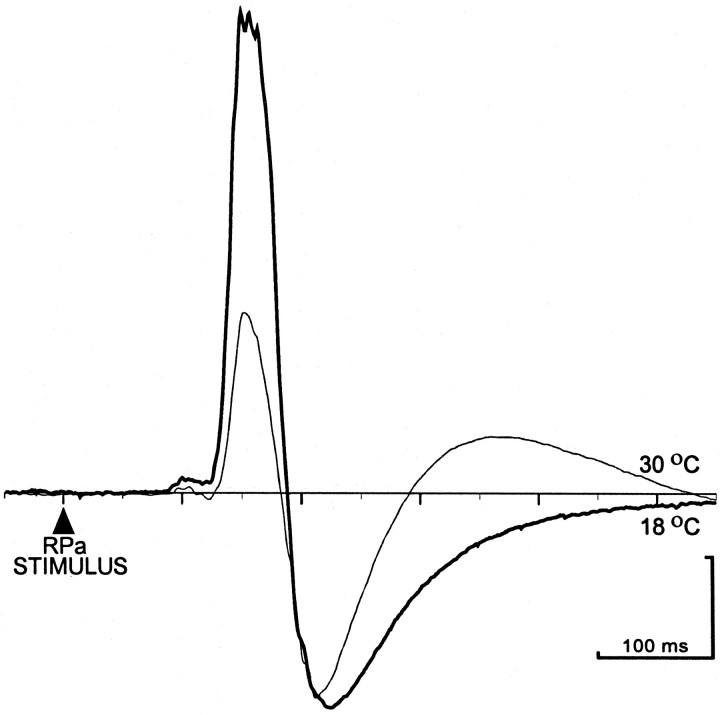
Electrical stimulation (twin pulses, 5–150 μA, 0.5 Hz) in RPa evoked excitatory potentials in BAT SNA (Fig.3) with significantly larger peak amplitudes and areas in the rats raised at 18°C than in those raised at 30°C. In the example in Figure 3, the peak amplitude (232 μV) and area (7489 μV·msec) of the RPa stimulus-evoked potential in BAT SNA were 3.0 and 3.2 times greater, respectively, in the animal raised at 18°C than those in the rat raised at 30°C (77 μV and 2324 μV·msec). On average, the mean peak amplitude of the RPa stimulus-evoked potentials in BAT SNA in the rats raised at 18°C (204 ± 31 μV) was 2.1 times greater (p< 0.05) than that (98 ± 16 μV) in the rats raised at 30°C. Similarly, the mean area of the potentials was 2.2 times greater (p < 0.05) in the rats raised at 18°C (6269 ± 987 μV·msec) than that (2833 ± 412 μV·msec) in the rats raised at 30°C. Differences in rearing temperature did not induce any differences in the mean onset latency (124 ± 1.7 msec), mean peak latency (157 ± 2.3 msec), or duration (61 ± 2.9 msec) of the RPa stimulus-evoked potentials. Neither the threshold stimulus intensity (9 ± 1.3 μA) nor the body temperature at which the stimulations were performed (34.3 ± 0.3°C) was different between the two groups of animals.
Fig. 3.
Comparison of averaged excitatory potentials evoked on a sympathetic nerve to BAT SNA by electrical stimulation in raphe pallidus (RPa) in an animal raised at 18°C (heavy trace) and an animal raised at 30°C (light trace). Traces are peristimulus averages of the responses in BAT SNA to 10 stimuli consisting of paired pulses, 6 msec interval, 100 μA, 0.4 Hz. Calibration: 50 μV; 100 msec.
Representative bicuculline microinjection and electrical stimulation sites in RPa are shown in Figure 4. They were located in the rostral raphe pallidus and the overlying raphe magnus at the level of the caudal portion of the facial nucleus. There were no differences in the locations of the bicuculline microinjection sites or the electrical stimulation sites between animals raised at 18°C and those raised at 30°C. The locations of these microinjections correspond to the raphe region within which bicuculline evokes a maximal increase in BAT SNA and in which neuronal c-fos expression is induced during acute cold exposure (Morrison et al., 1999).
Fig. 4.
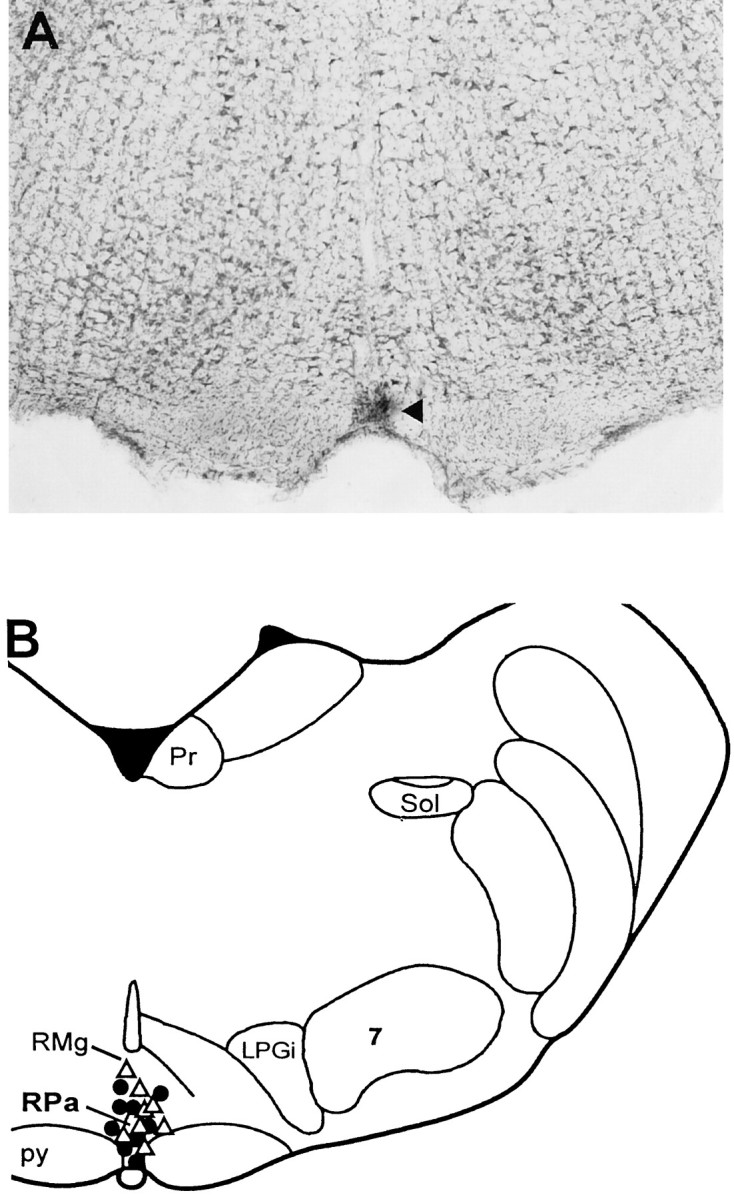
Locations of electrical stimulation and bicuculline microinjection sites in the rostral raphe pallidus (RPa). A, Histological section through the rostral RPa containing fast green dye (arrow) deposited from the tip of a bicuculline microinjection pipette.B, Representative bicuculline microinjection sites from nine animals raised at 18°C (●) and seven animals raised at 30°C (▵), plotted on an atlas (Paxinos and Watson, 1986) drawing at interaural −2.30 mm. Microinjection sites in remaining animals were omitted for clarity. Pr, prepositus hypoglossal nulceus;RMg, nucleus raphe magnus; Sol, nucleus of the solitary tract; LPGi, lateral paragigantocellular nucleus;7, facial nucleus; py, pyramidal tract.
Effect of rearing temperature on NE levels in IBAT
The impact of exposure to 18 and 30°C for the first 2 months of life on body weight, IBAT weight, and IBAT NE levels in 4-month-old male and female rats is presented in Table2. In both male and female animals, body weights and IBAT weights were greater in the rats housed at 18°C than in those reared at 30°C; however, the increase in IBAT weight was proportional to the gain in body weight in the 18°C-reared animals. The NE levels in IBAT were elevated (both as nanograms per tissue and as nanograms per gram of tissue) in the rats raised in the colder environment. In contrast, cardiac NE levels (nanograms per gram of tissue) were either the same (males) or lower (females) in animals reared at 18°C versus those reared at 30°C. Thus, exposure to a cold environment (18 vs 30°C) for the first 60 d of life results in greater NE levels in BAT but not in heart, a difference that persisted for at least 2 months after relocation of both 18°C-reared and 30°C-reared animals to housing at a common temperature.
Table 2.
Effect of rearing temperature on body, IBAT, and cardiac weights and on IBAT and cardiac norepinephrine content
| Body weight (gm) | IBAT weight | IBAT NE | Cardiac weight | Cardiac NE | |||||
|---|---|---|---|---|---|---|---|---|---|
| mg | % BW | ng | ng/gm | mg | %BW | ng | ng/gm | ||
| Male | |||||||||
| 18°C (n = 24) | 546 ± 8* | 573 ± 272-160 | 0.105 ± 0.004 | 721 ± 30* | 1286 ± 52* | 1328 ± 17* | 0.244 ± .003 | 601 ± 272-160 | 450 ± 19 |
| 30°C (n = 24) | 466 ± 7 | 490 ± 20 | 0.105 ± 0.004 | 393 ± 16 | 842 ± 54 | 1103 ± 22 | 0.237 ± .003 | 478 ± 29 | 433 ± 24 |
| Female | |||||||||
| 18°C (n = 16) | 336 ± 52-160 | 373 ± 222-160 | 0.111 ± 0.006 | 862 ± 35* | 2436 ± 173* | 1016 ± 17* | 0.303 ± .0062-160 | 849 ± 56 | 832 ± 492-160 |
| 30°C (n = 23) | 311 ± 5 | 319 ± 14 | 0.103 ± 0.004 | 524 ± 16 | 1695 ± 72 | 886 ± 13 | 0.286 ± .004 | 890 ± 31 | 1008 ± 36 |
IBAT, Interscapular brown adipose tissue;NE, norepinephrine; BW, body weight.
,
F2-160: Significant difference (p < 0.001)* and (p < 0.05)** between 18°C- and 30°C-reared rats.
Effect of rearing temperature on number of sympathetic ganglion cells innervating IBAT
To determine whether the enhanced BAT SNA responses and the elevated IBAT NE levels in rats raised at 18°C might reflect a neuroanatomic alteration resulting from rearing temperature, we compared the number and size of sympathetic ganglion cells retrogradely labeled after fast blue injections into the IBAT in 18°C-reared and 30°C-reared rats. Figure 5 illustrates the greater number of ganglion cells innervating IBAT found in animals raised at 18°C in comparison to animals raised at 30°C. The results derived from counting ganglion cells containing fast blue fluorescence are presented in Table 3. Injection of fast blue into IBAT labeled the greatest number of ganglion cells in the middle cervical–stellate ganglia, with decreasing numbers in the first four thoracic ganglia ipsilateral to the IBAT that was injected (Table 3). The mean diameter of middle cervical ganglion cells innervating BAT in animals (n = 5) reared at 18°C was 25 ± 1.8 μm, which was not different from that in animals (n = 5) reared at 30°C (24 ± 1.5 μm). The total number of ganglion cells labeled by fast blue injection into IBAT, as well as the number in the middle cervical–stellate ganglia and in the first thoracic ganglion, was significantly greater in the rats reared at 18°C than in those reared at 30°C (Table 3). The total number of ganglion cells retrogradely labeled from IBAT was 72% greater in animals reared at 18°C than in those reared at 30°C, primarily because of populations of retrogradely labeled cells in the middle cervical–stellate ganglia that were 73% greater and in the first thoracic ganglion that were 63% greater. These data are consistent with the size of the differences noted above in BAT SNA response amplitudes and in IBAT NE levels and suggest that sympathetic postganglionic nerve bundles to BAT in 18°C-reared animals contained a greater number of axons than those in 30°C-reared rats.
Fig. 5.
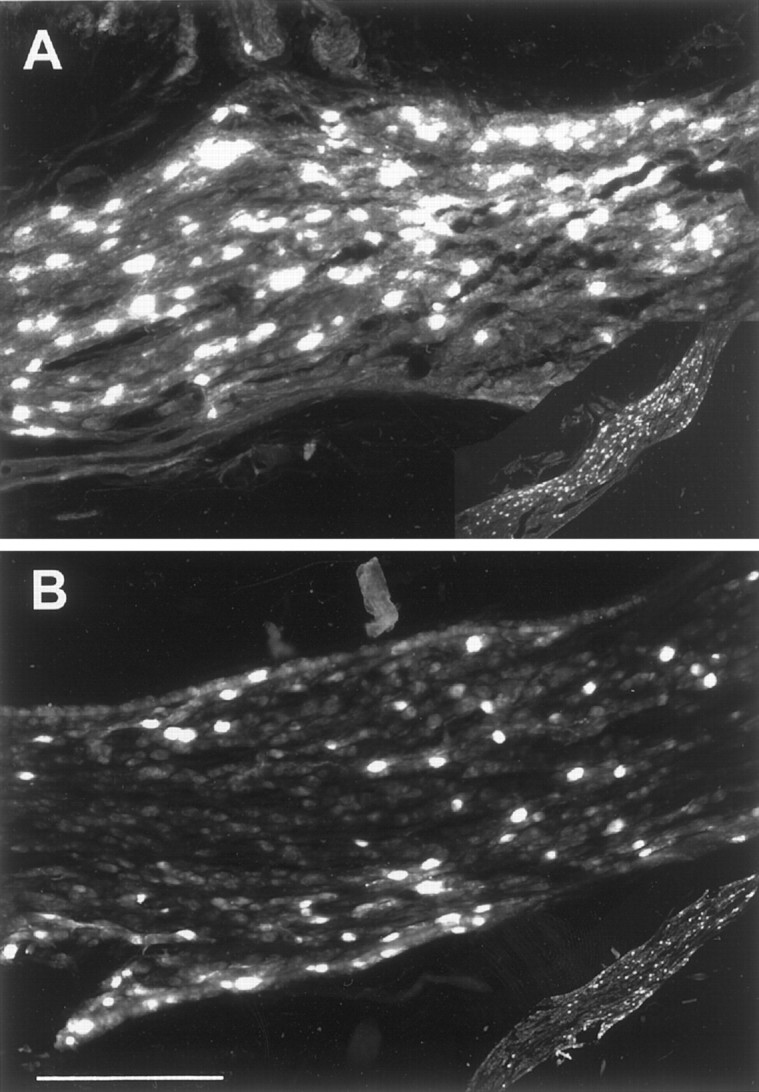
Comparison of the retrograde labeling of middle cervical ganglion cells in an animal raised at 18°C (A) and an animal raised at 30°C (B) after fast blue dye deposits in the ipsilateral IBAT. Histological sections have been illuminated to reveal fast blue fluorescence in ganglion cells innervating IBAT.Insets at bottom right show low-power images of these sections. The section from the middle cervical ganglion of the animal raised at 18°C contained 303 retrogradely labeled neurons (A), whereas that from the same site in an animal raised at 30°C contained 117 neurons with fast blue fluorescence (B). Scale bar: high-power images, 300 μm; low-power images, 2400 μm.
Table 3.
Effect of rearing temperature on numbers of sympathetic ganglion cells innervating interscapular brown adipose tissue
| Rearing temperature | Total per rat | Middle cervical/stellate | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| 18°C (n = 9) | 2659 ± 3183-150 | 2000 ± 3033-150 | 354 ± 383-160 | 213 ± 45 | 88 ± 24 | 56 ± 17 |
| 30°C (n = 11) | 1545 ± 111 | 1154 ± 74 | 217 ± 42 | 151 ± 30 | 60 ± 19 | 33 ± 10 |
Numbers are means ± SEM of cells per ganglion retrogradely labeled from unilateral fast blue injections into right interscapular brown adipose tissue.
F3-150: ,
F3-160: Significant difference (p < 0.01)* and (p < 0.05)** between 18°C- and 30°C-reared rats.
DISCUSSION
The principal findings of this study are that early postnatal exposure to a cold (18°C) environment induces a permanent enhancement of the responses to thermogenic stimuli in the sympathetic outflow to BAT and that a cold-induced hyperplasia within the sympathetic pathway to BAT may contribute to the exaggerated sympathetic responses in cold-reared animals. These data provide evidence that exposure during early postnatal life to an environment that activates BAT thermogenesis can produce a sustained change in the neural regulation of this thermoregulatory system.
The increased number of sympathetic ganglion cells innervating BAT in animals reared at 18°C is likely to be a consequence of an activity-induced reduction in the apoptotic processes that occur during this period (Aguayo et al., 1976; Wright et al., 1983; Smet et al., 1986; Messina et al., 1996; Tafreshi et al., 1998), a proposal supported by the finding that neonatal rats housed under similar conditions show heightened indices of sympathetic nerve activity to BAT by the second week of neonatal life (Bertin et al., 1990). Moreover, although norepinephrine levels in BAT begin to diverge in 18- and 30°C-reared rats after the first week of life (Bertin et al., 1990) (J. B. Young, unpublished observations), gene expression for nerve growth factor and neurotrophin-3 in BAT does not differ as a function of rearing temperature from birth through weaning (J. B. Young, N. Boufath, and J. Weiss, unpublished observations). Additionally, in the present study, we found that ganglion cell sizes were not different between rats raised at 18 and 30°C. Also arguing against a potential role for BAT-derived NGF in the augmented number of sympathetic ganglion cells innervating BAT in 18°C-reared rats is the demonstration that BAT NGF production declined when adult rats were exposed to cold temperatures and that addition of norepinephrine to cultures of BAT cells reduced their NGF production (Nisoli et al., 1996). Together these observations implicate presynaptic signaling rather than target-derived factors in the augmented ganglion cell numbers in rats raised at 18°C. We cannot rule out, however, a role for possible cold-induced alterations in ganglion cell function that may have influenced our observations, such as an increased ability to transport fast blue or a greater incidence of coupling between neurons.
Other CNS pathways exhibit a comparable developmental plasticity in which a modified perinatal environment produced by controlling a particular sensory stimulus results in a sustained alteration in an associated neuronal system performance. Kuno and colleagues (Kuno, 1956) determined that the sweating response to body heating was influenced by the environment in which an individual had lived for the first 2 years of life. Because the density of sweat glands in adult skin did not vary by race or by location of rearing, Kuno concluded that the observed differences in rate of perspiration derived from developmental alterations in the neural regulation of this response. Ventilatory responses to hypoxemia are greatly attenuated in adult rats in which the peripheral chemoreceptor reflex was suppressed during the first month of life by living in a hyperoxic environment (Ling et al., 1996, 1997). Litter size and neonatal handling are additional factors in perinatal life that can alter development of the neural system involved in homeostasis (Plagemann et al., 1999; Young, 2000). The sympathetic nervous system is composed of multiple, functionally specific subunits (Janig and McLachlan, 1992), each of which may be susceptible to the developmental influences of exposure to a different set of environmental conditions. Extending this model of neuronal plasticity for the sympathetic nervous system, sympathetic function in the adult would reflect the net result of the host of environmental factors to which the individual was exposed during early life.
Neurons in the preoptic region of the hypothalamus play a central role in the integration of information on body and environmental temperature and in the elaboration of autonomic and behavioral responses required to maintain a constant body temperature (Boulant and Dean, 1986; Gordon and Heath, 1986). Although activation of BAT thermogenesis in the rat is a major component of the response to a cold environment, the central pathways mediating increases in BAT SNA have not been determined. Recently, the RPa has been identified as a potential site of the sympathetic premotor neurons providing the principal excitatory input to spinal sympathetic preganglionic neurons controlling BAT thermogenesis (Morrison et al., 1999). The finding that the level of BAT SNA is very low under control, normothermic conditions, but is dramatically increased by disinhibition of RPa neurons (Morrison et al., 1999) (Fig. 2), is consistent with the existence of a potent, tonic, GABA-mediated inhibition of the RPa neurons controlling BAT thermogenesis that is relieved during periods when BAT thermogenesis is stimulated. These results lead to the hypothesis that the reduction in environmental temperature experienced by rats reared at 18°C stimulated an elevated level of activity in their BAT thermogenic pathway, including cold-sensitive neurons in the hypothalamus (Boulant and Dean, 1986) and BAT sympathetic premotor neurons in the RPa, which, in turn, induced an increase in the number of ganglion cells innervating BAT and resulted in the enhanced responses recorded in their BAT SNA. Although our data indicate that RPa neurons can influence those components of the BAT thermogenic pathway that are enhanced in rats reared at 18°C, we did not determine whether an increase in the responsiveness or the number of RPa neurons contributed to the increased amplitude of the responses recorded in rats reared at 18°C. The finding that disinhibition of RPa neurons produced large increases in BAT SNA, but only small changes in the visceral vasoconstrictor outflow in the splanchnic nerve (Morrison, 1999), suggests that the sympathetic efferents regulated by RPa neuronal activity are those specifically involved in thermoregulation or metabolism. Thus, we would not expect responses evoked in cardiovascular sympathetic efferents to differ between rats reared at 18°C and those reared at 30°C, although this was not tested in our study.
The close parallel between the relative amplitudes of the evoked increases in BAT SNA determined in the rats raised at 18°C and those raised at 30°C and the relative difference in the number of ganglion cells innervating IBAT between the 18°C-reared animals and their 30°C-reared counterparts suggests that the enhanced sympathetic responses in the cold-reared animals were mediated to a significant degree by the greater number of postganglionic axons in the nerves innervating BAT. Specifically, the maximal increase in BAT SNA activated by hypothermia, disinhibition of RPa neurons, and electrical stimulation of RPa was 85, 100, and 110% greater, respectively, in the 18°C-reared rats than in the 30°C-reared animals. The finding that these values are of the same order as both the 72% difference in the number of sympathetic ganglion cells retrogradely labeled from IBAT and the 83% difference in IBAT norepinephrine content in 18°C- versus 30°C-reared animals suggests that the simplest explanation for the enhanced BAT sympathetic responses in the rats reared at 18°C is the greater number of postganglionic axons in the nerves innervating their BAT. A similar relationship between postganglionic sympathetic burst amplitude and the number of active preganglionic and postganglionic axons has been proposed, based on the sequential reduction in the heights of the spontaneous bursts in inferior cardiac postganglionic sympathetic discharge produced by sequential section of preganglionic rami to the stellate ganglion (Ninomiya et al., 1993). Although based on indirect evidence, this hypothesis is plausible if individual postganglionic axons do not discharge, to a significant degree, more than once during an individual sympathetic burst. Recordings of individual postganglionic muscle vasoconstrictor axons in awake human subjects indicate that, although such units can exhibit multiple discharges during a single sympathetic burst, these events are rare (Macefield and Wallin, 1999; Macefield et al., 1999). It was not determined in this study whether cold-rearing also induces a greater number of BAT sympathetic preganglionic neurons or their antecedent premotor neurons or whether the synaptic gain within the pathway leading to the excitation of ganglion cells innervating BAT is augmented.
In this study, we characterized two aspects of the inverse sigmoid relationship between core temperature and the amplitude of BAT SNA: (1) the threshold temperature at which BAT SNA began to increase and (2) the operating point temperature, determined as the midpoint on the linear portion of the curve. Our finding that both the mean threshold temperature (35.5°C) and the mean operating point temperature (35.1°C) were markedly less than the normal core temperature of the awake rat (37.5°C) would suggest that the temperature regulatory mechanisms in these animals are suppressed by anesthesia. Chloralose, one component of the anesthesia used in these experiments, has been suggested to lower the threshold temperature and reduce the response dynamics of thermoregulation (Grewe et al., 1995). Additionally, our stimulus for producing an acute hypothermia involved ventral contact with a chilled surface, which would likely have activated cutaneous cold receptors before reducing core temperature. Thus, coupled with the effects of anesthesia, the potential for the networks controlling BAT SNA to be differentially sensitive to activation of cutaneous receptors versus stimulation of cold-sensitive neurons in the hypothalamus may have masked a developmentally induced difference in thermal sensitivity between rats reared at 18 and 30°C.
Because animals raised at either 18 or 30°C had a similar range of temperatures from threshold to maximal BAT SNA responses, the greater gain of the relationship between core temperature and BAT SNA amplitude in the 18°C-reared rats was strongly influenced by the enhanced maximal levels of BAT SNA that could be generated by 18°C-reared rats in comparison to those reared at 30°C. This developmental adaptation would allow cold-reared rats to achieve a greater maximal metabolic thermogenic response to cold stress more rapidly than their counterparts raised in a warmer environment.
Footnotes
This research was supported by National Institutes of Health Grant DK-20378.
Correspondence should be addressed to Dr. Shaun F. Morrison, Department of Physiology (M211), Northwestern University Medical School, 303 E. Chicago Avenue, Chicago, IL 60611. E-mail:s-morrison2@northwestern.edu.
REFERENCES
- 1.Aguayo AJ, Peyronnard JM, Terry LC, Romine JS, Bray GM. Neonatal neuronal loss in rat superior cervical ganglia: retrograde effects on developing preganglionic axons and Schwann cells. J Neurocytol. 1976;5:137–155. doi: 10.1007/BF01181653. [DOI] [PubMed] [Google Scholar]
- 2.Bertin R, Mouroux I, De Marco F, Portet R. Norepinephrine turnover in brown adipose tissue of young rats: effects of rearing temperature. Am J Physiol. 1990;259:R90–R96. doi: 10.1152/ajpregu.1990.259.1.R90. [DOI] [PubMed] [Google Scholar]
- 3.Boulant JA. Hypothalamic control of thermoregulation. In: Morgane PJ, Panksepp J, editors. The handbook of the hypothalamus. Dekker; New York: 1980. pp. 1–82. [Google Scholar]
- 4.Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol. 1986;48:639–654. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- 5.Brunjes PC, Frazier LL. Maturation and plasticity in the olfactory system of vertebrates. Brain Res Rev. 1986;11:1–45. doi: 10.1016/s0006-8993(86)80188-3. [DOI] [PubMed] [Google Scholar]
- 6.Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, Vidruk EH, Jr, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. J Physiol (Lond) 1998;509:519–526. doi: 10.1111/j.1469-7793.1998.519bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson BM, Persson BA. Determination of catecholamines in rat heart tissue and plasma samples by liquid chromatography and electrochemical detection. J Chromatogr. 1982;228:143–154. doi: 10.1016/s0378-4347(00)80427-2. [DOI] [PubMed] [Google Scholar]
- 8.Foster DO. Quantitative contribution of brown adipose tissue thermogenesis to overall metabolism. Can J Biochem Cell Biol. 1984;62:618–622. doi: 10.1139/o84-082. [DOI] [PubMed] [Google Scholar]
- 9.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 10.Gordon CJ, Heath JE. Integration and central processing in temperature regulation. Annu Rev Physiol. 1986;48:595–612. doi: 10.1146/annurev.ph.48.030186.003115. [DOI] [PubMed] [Google Scholar]
- 11.Grewe W, Janig W, Kummel H. Effects of hypothalamic thermal stimuli on sympathetic neurones innervating skin and skeletal muscle of the cat hindlimb. J Physiol (Lond) 1995;488:139–152. doi: 10.1113/jphysiol.1995.sp020952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol (Lond) 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janig W, McLachlan EM. Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci. 1992;15:475–481. doi: 10.1016/0166-2236(92)90092-m. [DOI] [PubMed] [Google Scholar]
- 14.Kawate R, Talan MI, Engel BT. Sympathetic nervous activity to brown adipose tissue increases in cold-tolerant mice. Physiol Behav. 1994;55:921–925. doi: 10.1016/0031-9384(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 15.Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- 16.Kuno Y. Human perspiration. Thomas; Springfield, IL: 1956. [Google Scholar]
- 17.LeBlanc J, Robinson D, Sharman DF, Tousignant P. Catecholamines and short-term adaptation to cold in mice. Am J Physiol. 1967;213:1419–1422. doi: 10.1152/ajplegacy.1967.213.6.1419. [DOI] [PubMed] [Google Scholar]
- 18.Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol (Lond) 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response. Respir Physiol. 1997;110:261–268. doi: 10.1016/s0034-5687(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 20.Macefield VG, Wallin BG. Firing properties of single vasoconstrictor neurones in human subjects with high levels of muscle sympathetic activity. J Physiol (Lond) 1999;516:293–301. doi: 10.1111/j.1469-7793.1999.293aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macefield VG, Rundqvist B, Sverrisdottir YB, Wallin BG, Elam M. Firing properties of single muscle vasoconstrictor neurons in the sympathoexcitation associated with congestive heart failure. Circulation. 1999;100:1708–1713. doi: 10.1161/01.cir.100.16.1708. [DOI] [PubMed] [Google Scholar]
- 22.Messina A, Jaworowski A, Bell C. Detection of jun but not fos protein during developmental cell death in sympathetic neurons. J Comp Neurol. 1996;372:544–550. doi: 10.1002/(SICI)1096-9861(19960902)372:4<544::AID-CNE4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Moore DR. Postnatal development of the mammalian central auditory system and the neural consequences of auditory deprivation. Acta Otolaryngol [Suppl] 1985;421:19–30. doi: 10.3109/00016488509121753. [DOI] [PubMed] [Google Scholar]
- 24.Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol. 1999;276:R962–973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- 25.Morrison SF, Young JB. Developmental plasticity in sympathetic outflow: lowered neonatal temperature enhances sympathetic regulation of brown adipose tissue. Soc Neurosci Abstr. 1998;24:367. [Google Scholar]
- 26.Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol. 1999;276:R290–297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- 27.Ninomiya I, Malpas SC, Matsukawa K, Shindo T, Akiyama T. The amplitude of synchronized cardiac sympathetic nerve activity reflects the number of activated pre- and postganglionic fibers in anesthetized cats. J Auton Nerv Syst. 1993;45:139–147. doi: 10.1016/0165-1838(93)90125-e. [DOI] [PubMed] [Google Scholar]
- 28.Nisoli E, Tonello C, Benarese M, Liberini P, Carruba M. Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology. 1996;137:495–503. doi: 10.1210/endo.137.2.8593794. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 2. Academic; Sydney, Australia: 1986. [Google Scholar]
- 30.Plagemann A, Harder T, Rake A, Melchior K, Rohde W, Dorner G. Increased number of galanin neurons in the paraventricular hypothalamic nucleus of neonatally overfed weanling rats. Brain Res. 1999;818:160–163. doi: 10.1016/s0006-8993(98)01264-5. [DOI] [PubMed] [Google Scholar]
- 31.Smet P, Rush RA, Straznicky C. The thoracic sympathetic neurons of the chick: normal development and the effects of nerve growth factor. Histol Histopathol. 1986;1:315–322. [PubMed] [Google Scholar]
- 32.Tafreshi AP, Zhou XF, Rush RA. Endogenous nerve growth factor and neurotrophin-3 act simultaneously to ensure the survival of postnatal sympathetic neurons in vivo. Neuroscience. 1998;83:373–380. doi: 10.1016/s0306-4522(97)00385-0. [DOI] [PubMed] [Google Scholar]
- 33.Talan MI, Engel BT. Habituation and dishabituation to repeated mild cold exposures in C57BL/6J mice. Physiol Behav. 1988;44:753–757. doi: 10.1016/0031-9384(88)90057-1. [DOI] [PubMed] [Google Scholar]
- 34.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 35.Vera PL, Haase EB, Schramm LP. Origins of the sympathetic innervation of the cervical end of the uterus in the rat. Brain Res. 1997;747:140–143. doi: 10.1016/s0006-8993(96)01107-9. [DOI] [PubMed] [Google Scholar]
- 36.Woolsey TA, Durham D, Harris RM, Simons DJ, Valentino KL. Somatosensory development. In: Aslin RN, Alberts JR, Peterson MR, editors. Development of perception. Psychobiological perspectives, Vol 1. Audition, somatic perception and the chemical senses. Academic; New York: 1981. pp. 259–292. [Google Scholar]
- 37.Wright LL, Cunningham TJ, Smolen AJ. Developmental neuron death in the rat superior cervical sympathetic ganglion: cell counts and ultrastructure. J Neurocytol. 1983;12:727–738. doi: 10.1007/BF01258147. [DOI] [PubMed] [Google Scholar]
- 38.Young JB. Effects of neonatal handling on sympathoadrenal activity and body composition in adult male rats. Am J Physiol. 2000;279:1745–1752. doi: 10.1152/ajpregu.2000.279.5.R1745. [DOI] [PubMed] [Google Scholar]