Abstract
Recently, a 22 kDa protein termed p75NTR-associated death executor (NADE) was discovered to be a necessary factor for p75NTR-mediated apoptosis in certain cells. However, the possible role for p75NTR/NADE in pathological neuronal death has yet been undetermined. In the present study, we have examined this possibility in vivo and in vitro. Exposure of cortical cultures to zinc induced both p75NTR and NADE in neurons, whereas exposure to NMDA, ionomycin, iron, or H2O2 induced neither. In addition, zinc exposure increased neuronal NGF expression and its release into the medium. A function-blocking antibody of p75NTR (REX) inhibited association between p75NTR and NADE as well as neuronal death induced by zinc. Conversely, NGF augmented zinc-induced neuronal death. Caspase inhibitors reduced zinc-induced neuronal death, indicating that caspases were involved. Because reduction of NADE expression with cycloheximide or NADE antisense oligonucleotides attenuated zinc-induced neuronal death, NADE appears to contribute to p75NTR-induced cortical neuronal death as shown in other cells. Because zinc neurotoxicity may be a key mechanism of neuronal death after transient forebrain ischemia, we next examined this model. After ischemia, p75NTR and NADE were induced in degenerating rat hippocampal CA1 neurons. There was a close correlation between zinc accumulation and p75NTR/NADE induction. Suggesting the role of zinc here, injection of a metal chelator, CaEDTA, into the lateral ventricle completely blocked the induction of p75NTR and NADE. Our results suggest that co-induction of p75NTR and NADE plays a role in zinc-triggered neuronal death in vitro and in vivo.
Keywords: neurotrophin, nerve growth factor, apoptosis, caspase, TFL-Zn, calcium, oxidative injury
Neurotrophins can act at two different kinds of receptors: the agonist-selective high-affinity receptors called Trks (Trk-A, -B, and -C) and the agonist-nonselective low-affinity receptor called p75NTR (Barbacid, 1995). Whereas the signaling cascades through the Trk receptors are well characterized (Klesse et al., 1999; Kaplan and Miller, 2000), those through p75NTR remain relatively uncharacterized. Elevation of intracellular ceramide levels and activation of c-jun N-terminal kinase (JNK), NF-kB, and caspases, have been reported to occur as the result of p75NTR activation (Casaccia-Bonnefil et al., 1999; Barrett, 2000; Kaplan and Miller, 2000).
Whereas the initial attention was placed on neurotrophic effects of p75NTR, an increasing body of evidence now indicates that p75NTR can mediate cell death under certain conditions. For example,p75NTR-mediated apoptosis has been implicated in cell death of the developing retina and spinal cord, mature oligodendrocytes, and postnatal sympathetic neurons (Rabizadeh et al., 1993; Frade et al., 1996; Bamji et al., 1998). The role of neurotrophins, especially NGF, in the p75NTR-mediated apoptosis appears variable (Kaplan and Miller, 1997; Casaccia-Bonnefil et al., 1999; Barrett, 2000). In some cells such as oligodendrocytes, the binding of NGF to p75NTR seems to promote cell death (Casaccia-Bonnefil et al., 1996; Gu et al., 1999; Soilu-Hanninen et al., 1999). On the other hand, in PC12 and SK-N-BE neuroblastoma cells, NGF-unoccupied p75NTR may mediate cell death (Barrett and Georgiou, 1996; Lievremont et al., 1999).
p75NTR is structurally analogous to TNF-α receptor and contains the death domain motif in its cytoplasmic tail (Chapman, 1995; Liepinsh et al., 1997). The cytoplasmic domain is considered to interact with various mediators that transmit signals to other downstream proteins. Several candidate molecules such as TRAFs, SC-1, RhoA, and NRIF (Casademunt et al., 1999; Chittka and Chao, 1999; Khursigara et al., 1999; Yamashita et al., 1999; Ye at al., 1999) have been proposed as mediators of p75NTRsignaling. Of those, NRIF and TRAF2 have been reported to function in p75NTR-mediated apoptotic pathway. However, definite proof causally linking these to p75NTR-mediated apoptosis in neurons is not available. Recently, a 22 kDa cytosolic protein termed p75NTR-associated death executor (NADE), was identified as a necessary death effector that interacts with the death domain of p75NTR in PC12 and oligodendrocytes (Mukai et al., 2000). The NADE-dependent apoptosis pathway seems to require the binding of NGF to p75NTR.
NGF and p75NTR are known to be induced in neurons after brain insults such as ischemia or seizures (Lee et al., 1995; Kokaia et al., 1998; Roux et al., 1999). However, there is no consensus yet whether the induction of NGF or p75NTR plays a harmful or a beneficial role. Because NADE has been recently identified as a p75NTR-associated death effector, we examined the possibility that p75NTR/NADE system is activated in degenerating neurons after insults. Here we report that p75NTR and NADE are co-induced in degenerating neurons after zinc exposure in cortical culture and transient forebrain ischemia in the rat. Furthermore, in cortical culture, blockade of p75NTR signaling or downregulation of NADE attenuates zinc-mediated neuronal death.
MATERIALS AND METHODS
Cortical cell cultures, exposure to toxins, and estimation of neuronal death. Mixed mouse cortical cultures containing both neurons and astrocytes were prepared from neonatal mice at 15 d of gestation as previously described (Y. H. Kim et al., 1999). Cultures were used for experiments between 10 and 13 d in vitro (DIV).
Fifteen minutes of exposure to zinc (as ZnCl2) was done in HBSS supplemented with 1.8 mmCaCl2 and 0.813 mmMgSO4. After the exposure, Eagle's minimal essential medium (MEM), supplied glutamine-free (Life Technologies, Grand Island, NY) was reintroduced, and cultures were placed back into the CO2 incubator. REX antibody (1:400 dilution) (Weskamp and Reichardt, 1991) or NGF (Life Technologies) was added to MEM immediately after 15 min zinc exposure. Caspase inhibitors were added from 4 hr before 15 min zinc exposure until the estimation of neuronal death.
Continuous exposure to low concentrations of zinc was done in MEM for the hours indicated. When needed, cultures were pretreated for 12 hr with caspase inhibitors or cycloheximide before zinc exposure.
Neuronal death was quantitatively estimated by measuring lactate dehydrogenase (LDH) released from damaged cells into the bathing medium, as previously described (Koh and Choi, 1987). All LDH values were scaled to the mean value in sister cultures exposed for 16 hr to 300 μm NMDA, which resulted in complete yet selective neuronal death, after subtraction of mean background values in sham wash controls.
Western blot analysis. Cortical cells were lysed in Triton X-100 buffer (50 mm Tris-HCl, pH 7.5, 10% glycerol, 1% Triton X-100, 150 mm NaCl, 25 mm NaF, 1 mmNa3VO4, 1 mm EGTA, 100 μm DTT, 2 mm phenylmethylsulfonyl fluoride, 0.5 μg/ml aprotinin, 0.5 μg/ml leupeptin, and 0.5 μg/ml pepstatin). Lysates were normalized for protein by modified Lowry method (Bio-Rad, Hercules, CA). Proteins were separated by SDS-PAGE, followed by transferring onto polyvinylidene difluoride membrane. Membrane was blocked with 5% nonfat dry milk for 1 hr and incubated with respective primary antibody for overnight. Enhanced chemiluminescence (Amersham Life Science, Buckinghamshire, UK) was used for detection. Anti-human p75NTR polyclonal antibody was from Promega (Madison, WI). Anti-mouse NADE polyclonal antibody was prepared from rabbits by immunizing glutathioneS-transferase-NADE fusion protein (Mukai et al., 2000).
Immnocytochemistry. Cortical cultures and sections were fixed with 4% paraformaldehyde for 1 hr, permeabilized with 0.2% Triton X-100 in PBS. After blocking with 5% BSA in PBS, cultures and sections were incubated overnight with anti-mouse NeuN monoclonal antibody (Chemicon, Temecula, CA), anti-human p75NTR polyclonal antibody (from rabbit, Promega; from goat, Santa Cruz Biotechnology, Santa Cruz, CA), or anti-mouse NADE polyclonal antibody (Mukai et al., 2000). After washing, cortical cultures were treated for 1 hr with FITC- or rhodamine-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA), and examined under fluorescence microscope equipped with a digital camera (Olympus, Tokyo, Japan). Alternatively, after incubation with biotinylated secondary antibody, cortical cultures and brain sections were treated with avidin-horseradish peroxide solution to develop the chromogenic signal (Vector Laboratories, Burlingame, CA).
RT-PCR analysis. After total RNA was prepared with Trizol (Life Technologies) from cortical culture cells, complementary DNA was synthesized and reverse-transcribed using Oligo(dT)15 primers (Promega). With this cDNA as a template, PCR was performed with primer pairs. The primer pairs forNADE were 5′-CATTCCCAACAGGCAGATG −3′ and 5′-GGCATAAGGCAGAATTCATC-3′; and those for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were 5′-CTCAAGATTGTCAGCAATGC-3′ and 5′-AGACAACCTGGTCCTCAGTG-3′. PCR was performed for 35 (NADE) or 30 (GAPDH) cycles at 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min.
ELISA for NGF. NGF in the bathing medium was quantified using the NGF Emax ImmunoAssay system kit (Promega). Flat-bottom 96 well plates were coated with anti-NGF polyclonal antibody and incubated overnight with 100 μl of medium. The captured NGF was incubated with anti-NGF monoclonal antibody overnight. After washing, NGF was detected using secondary antibody conjugated to horseradish peroxidase and TMB substrate. The color change was measured at 450 nm on a microplate reader. The concentration of soluble NGF was calculated from the NGF standard curve.
Co-immnunoprecipitation of p75NTR with NADE.Cortical neuronal cells were lysed for immunoprecipitation in NETN buffer (20 mm Tris-HCl, pH 8.0, 100 mm NaCl, 1 mm EDTA, 0.2% NP-40, and 10 μm proteasome inhibitor I) with protease inhibitors, as described (Mukai et al., 2000). The supernatants were incubated with anti-NADE polyclonal antibody for 14 hr and 50% Protein A-agarose bead for 2 hr at 4°C. The beads were resolved on SDS-polyacrylamide gel and analyzed by Western blot using anti-human p75NTR polyclonal antibody.
Antisense oligonucleotides for NADE. The phosphorothioated antisense oligonucleotides for NADE were targeted against the 3′ untranslated region of murine NADE mRNA. The antisense oligonucleotides were tried in several different experiments, and those found to be effective in reducing NADE expression were chosen for experiments. Unrelated nonsense oligonucleotides were used as control. The sequences for NADE antisense oligonucleotides were 5′-CAGCGGGAGTCACAGTATGG-3′ (AS #1) and 5′-GGGCTACAGCGGGAGTCACA-3′ (AS #2), and the sequence for nonsense oligonucleotides (NS) was 5′-TGCTACTCGCGTCTAACACT-3′. Antisense or nonsense oligonucleotides were introduced into cortical cells usingN-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP) liposomal transfection reagent (Roche Products, Germany).
Transient forebrain global ischemia in the rat. All animal experiments were performed in accordance with the Guide of Ulsan University for Care and Use of Laboratory Animals. Male Sprague Dawley rats weighing 300–350 gm were used. Global forebrain ischemia was induced as previously described (Smith et al., 1984; Koh et al., 1996). Core body temperatures were continuously monitored and maintained between 36.5 and 37.5°C, during and for 1 hr after ischemia. To examine the effect of zinc chelation on ischemic damage, 3 μl of saline or 300 mm CaEDTA in saline was injected stereotaxically into the lateral ventricle under halothane anesthesia 30 min before the ischemia surgery.
Tissue preparation and zinc fluorescence of brain sections. At 24, 48, and 72 hr after ischemic insult, rats were killed by cervical dislocation, and brains were moved rapidly to dry ice or liquid nitrogen. Coronal brain sections were prepared with cryostat (thickness, 10 μm) and put onto the slide glass coated with poly-l-lysine.
To examine zinc accumulation, brain sections were rinsed in saline and incubated inN-(6-methoxy-8-quinolyl)-p-carboxybenzoylsulphonamide (TFL-Zn, 0.1 mm in PBS; Calbiochem, La Jolla, CA) for 90 sec. After washing with normal saline, TFL-Zn fluorescence was examined under fluorescence microscope with an ultraviolet filter (excitation 355–375 nm; dichroic, 380 nm; barrier, 420 nm) (Budde et al., 1997).
Terminal deoxynucleotidyl transferase-mediated biotinylated dUTP nick end-labeling. To identify neuronal cell death in the rat brain, terminal deoxynucleotidyl transferase-mediated biotinylated dUTP nick end labeling (TUNEL) staining was performed with the in situ cell death detection kit, following the manufacturer's instruction manual (Boehringer Mannheim, Mannheim, Germany). After fixation in 4% paraformaldehyde, the sections were incubated in 0.3% H2O2 and in permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate). After TUNEL reaction, the sections were examined under a fluorescent microscope and photographed.
RESULTS
Induction of p75NTR and NADE specifically by zinc
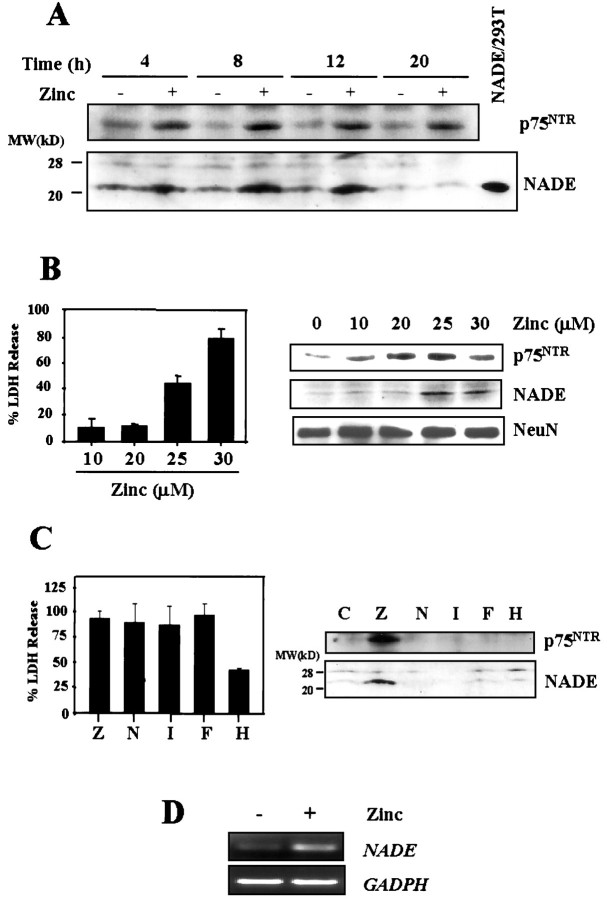
When cultured cortical neurons were exposed for 15 min to 300 μm zinc, neurons degenerated over the following day, as previously reported (E. Y. Kim et al., 1999). Western blot analysis revealed that p75NTR was induced in cortical cultures beginning 4 hr after the 15 min zinc exposure (Fig. 1A, top), when signs of neuronal degeneration were not yet evident. Concurrently, NADE was also induced in the zinc-exposed cortical cultures (Fig.1A, bottom). NADE expression decreased 20 hr after the zinc exposure, when neurons exhibited signs of irreversible neuronal damage.
Fig. 1.
Co-induction of p75NTR and NADE in cultured cortical neurons after zinc exposure. A, Western blots for p75NTR and NADE (22 kDa). Samples were prepared from cortical cultures at indicated hour after 15 min exposure to 300 μm zinc. Positive control for NADE was prepared from 293T cells transfected with NADE (NADE/293T) (Mukai et al., 2000). B, Left, Bars represent LDH release (mean + SEM; n = 3) in cortical cultures, after 24 hr exposure to indicated concentrations of zinc.Right, Western blots for p75NTR and NADE (22 kDa) in cortical cultures after 12 hr exposure to indicated concentrations of zinc. Western blots for the neuronal marker NeuN were used as control. C, Left, Bars represent LDH release (mean + SEM; n = 4–8), 16 hr after 15 min exposure to 300 μm zinc (Z) or continuous 16 hr exposure to 30 μm NMDA (N), 500 nm ionomycin (I), 100 μmFeCl2 (F), or 50 μmH2O2 (H). All but H2O2 induced near complete neuronal death.Right, Western blots for p75NTR and NADE 4 hr after 15 min exposure to zinc or after 4 hr exposure to other toxicants as above. Whereas zinc (300 μm, Z) induced both, NMDA, ionomycin, FeCl2, or H2O2 induced neither. D, RT-PCR assays for NADE mRNA in cortical cultures, sham-washed, or 8 hr after 15 min exposure to 300 μm zinc. RT-PCR assay for GAPDH mRNA is presented as control.
More prolonged, less intense zinc exposure also increased the expression of p75NTR and NADE (Fig.1B). Whereas 12 hr exposure to 10–20 μm zinc induced p75NTR, little induction of NADE was seen at this concentration. On the other hand, 12 hr exposure to 25–30 μm zinc induced both p75NTR and NADE. Because 25–30 μm but not 10–20 μmzinc induced neuronal death with 24 hr exposure (Fig. 1B, bars), there appears to be correlation between p75NTR/NADE co-induction and neuronal death.
Other than zinc neurotoxicity, several mechanisms are currently implicated in pathological neuronal death. Major ones include calcium-overload excitotoxicity and oxidative injury. Hence, we compared zinc neurotoxicity with NMDA, ionomycin (a calcium ionophore), FeCl2, and H2O2 neurotoxicity. NMDA and ionomycin induce calcium overload toxicity (Choi, 1994; Gwag et al., 1999), whereas FeCl2 and H2O2 induce mainly oxidative injury (Halliwell, 1992). As shown in Figure 1C, 15 min of exposure to 300 μm zinc, or continuous exposure to 30 μm NMDA, 500 nm ionomycin, or 100 μmFeCl2, induced neuronal death to similar extents 16 hr after the onset of exposure. Although H2O2 (50 μm) was not as neurotoxic as the others, because using higher concentrations tended to induce additional astroglial injury (J. A. Park and J.-Y. Koh, unpublished observation), this concentration was used for comparison. Western blots revealed that none but zinc exposure was effective for the induction of both p75NTR and NADE (Fig. 1C); other toxicants induced neither. RT-PCR assay revealed that zinc exposure increased mRNA levels of NADE (Fig.1D), indicating that the induction occurs at least partly at the transcription level.
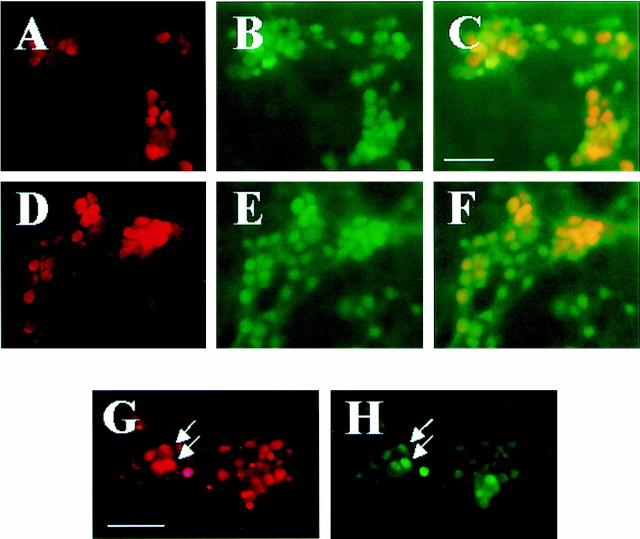
Because cortical cultures contained both neurons and astrocytes, we examined which cell type expresses p75NTRand/or NADE after zinc exposure. In this culture, astrocytes form the confluent monolayer of flat cells, on which neuronal cells lie. Cultures were immunocytochemically stained with anti-p75NTR (Fig.2A, rhodamine) or anti-NADE antibody (Fig. 2D,rhodamine), and then double-stained with anti-NeuN antibody (Fig. 2B,E, FITC). Composite photographs of Figure 2, A and B (Fig.2C), and of Figure 2, D and E (Fig.2F), show that both p75NTR and NADE were exclusively expressed in Neu-N(+) neurons (yellow). In addition, double staining of zinc-exposed cultures with anti-p75NTR (Fig. 2G,rhodamine, red) and anti-NADE antibodies (Fig.2H, FITC, green) revealed that a subset of p75NTR-expressing neurons also expressed NADE.
Fig. 2.
p75NTR and NADE induction in neurons. A–F, Fluorescent photomicrographs of cortical cultures containing both neurons and astrocytes, immunocytochemically stained with anti-p75NTR antibody (A), anti-NADE antibody (D), or anti-NeuN antibody (B,E) 4 hr after 15 min exposure to 300 μm zinc. p75NTR and NADE immunoreactivity was visualized with rhodamine-labeled secondary antibody (red) and NeuN immunoreactivity (a specific marker for neurons) with FITC-labeled secondary antibody (green). Composite images ofA and B (C) andD and E (F) revealed that p75NTR and NADE were expressed almost exclusively in neurons (yellow). Scale bar, 200 μm. G,H, Fluorescent photomicrographs of identical field of cortical culture 4 hr after 15 min exposure to 300 μm zinc, stained with anti-p75NTRantibody (G) or anti-NADE antibody (H). Arrows denote same neurons. NADE expression appeared to occur in a subset of p75NTR-expressing neurons. Scale bars, 200 μm.
NGF and zinc neurotoxicity
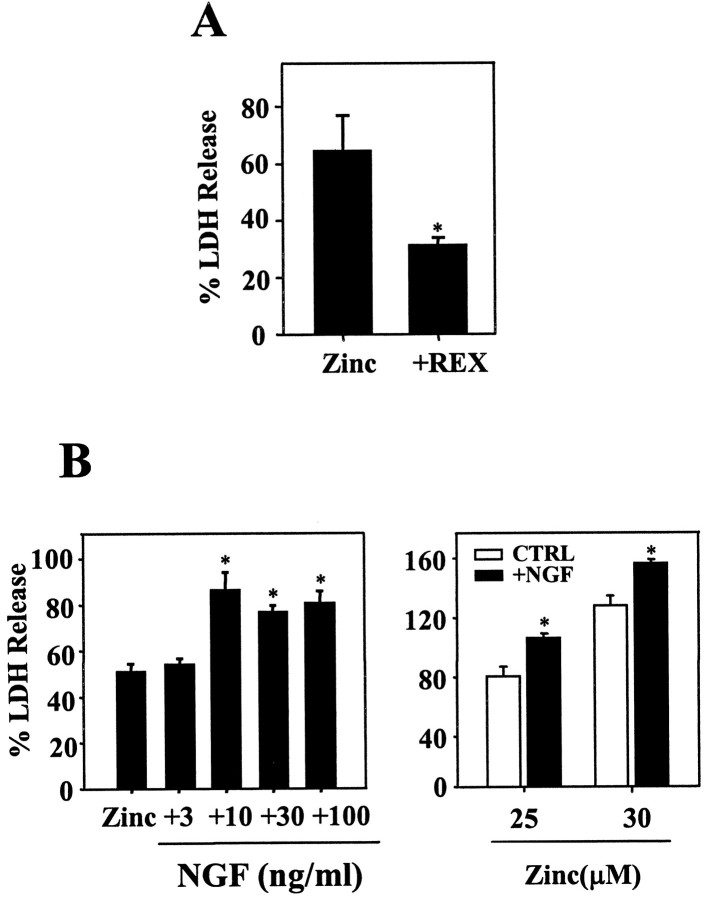
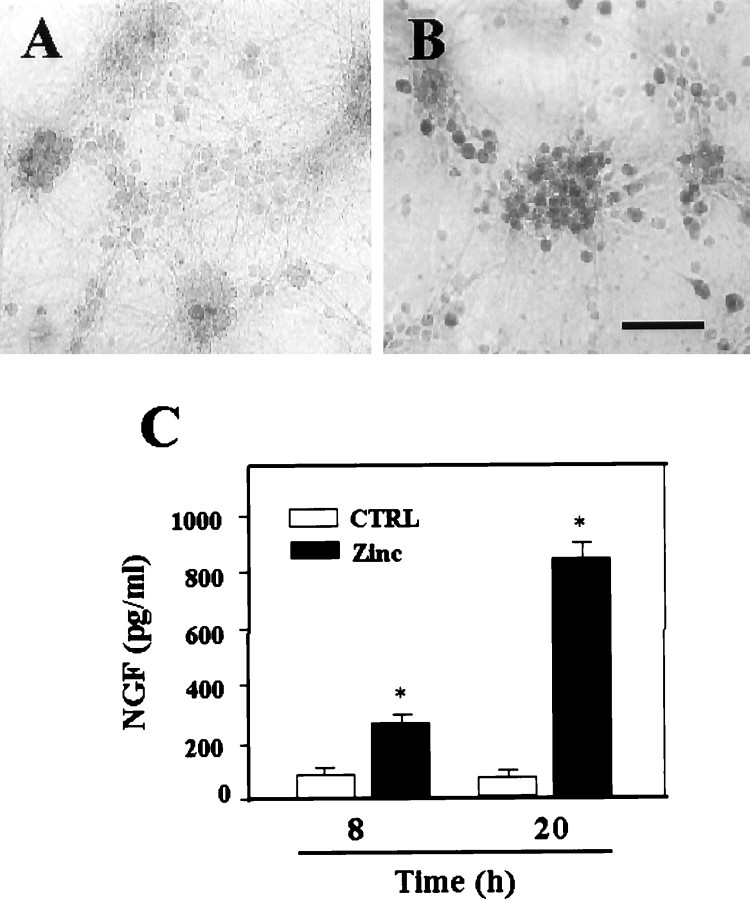
Suggesting that signaling through p75NTR contributed to zinc neurotoxicity, a p75NTR function-blocking antibody (REX) attenuated zinc neurotoxicity (Fig.3A). Conversely, addition of 10–100 ng/ml of NGF significantly potentiated zinc neurotoxicity in both brief and continuous exposure paradigms (Fig. 3B). These results suggest that NGF binding to p75NTR promotes p75NTR-mediated neuronal death. Thus, next we examined whether zinc exposure also increases NGF expression in cortical neurons. Exposure of cortical cultures for 15 min to 300 μm zinc increased NGF immunoreactivity selectively in neurons (Fig.4A,B). Furthermore, ELISA revealed that levels of NGF in the bathing medium markedly increased after exposure to zinc at 8 and 20 hr after the 15 min zinc exposure (Fig. 4C).
Fig. 3.
Effects of NGF and REX on zinc-induced neuronal death. A, Bars denote LDH release in cortical cultures (mean + SEM; n = 4), 18 hr after 15 min exposure to 300 μm zinc without (Zinc) or with addition of REX (1:400) (+REX). B,LDH release (n = 4–8) in cortical cultures, 18 hr after 15 min exposure to 300 μm zinc in the presence of indicated concentrations (nanograms per milliliter) of NGF (left), and after 24 hr exposure to 25 or 30 μm zinc without (CTRL) or with 100 ng/ml NGF (+NGF) (right).Asterisks denote difference from zinc (p < 0.05; two-tailed t test with Bonferroni correction for multiple comparisons).
Fig. 4.
Induction of NGF after zinc exposure. Immunocytochemical staining with anti-NGF antibody of a sham-washed control culture (A) and a sister culture 8 hr after 15 min exposure to 300 μm zinc (B). After zinc exposure, NGF levels were increased in the majority of neurons. Scale bar, 200 μm.C, Bars represent NGF concentrations (picograms per milliliter) attained in the bathing medium (n = 4; mean + SEM) in cortical cultures 8 or 20 hr after sham wash or 15 min exposure to 300 μm zinc. Asterisk denotes difference from control (p < 0.01; two-tailed t test with Bonferroni correction for two comparisons).
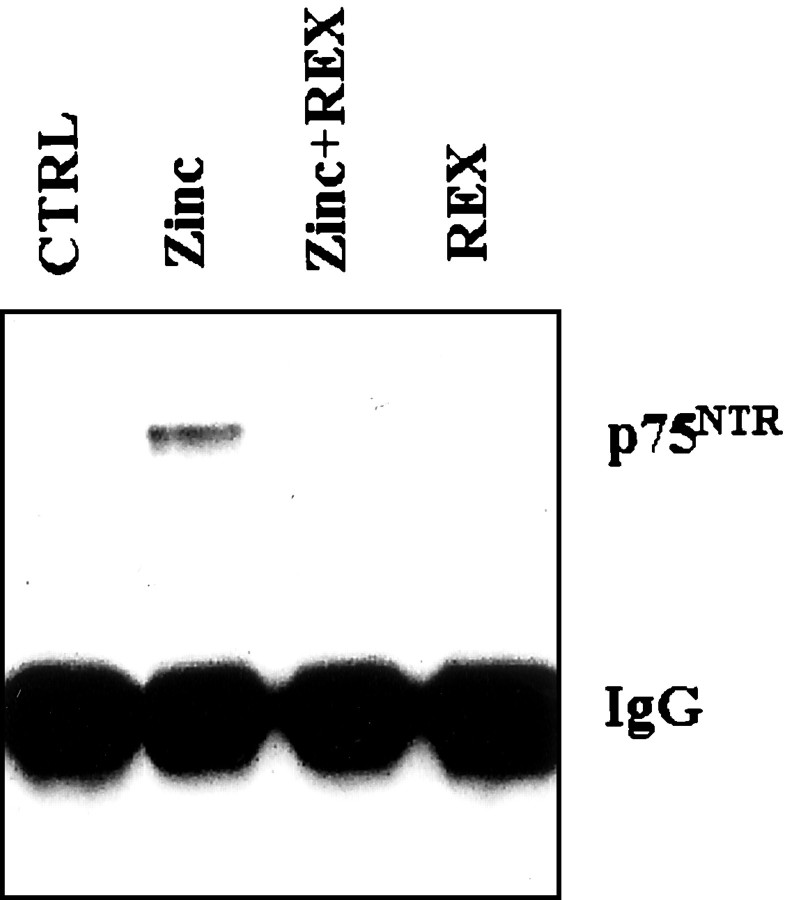
It was shown that p75NTR and NADE associate in PC12 cells and oligodendrocytes undergoing p75NTR-triggered apoptosis (Mukai et al., 2000). Also in zinc-exposed cortical culture, physical association between p75NTR and NADE was demonstrated by their co-immunoprecipitation (Fig. 5). Consistent with the idea that p75NTR and NADE interaction depends on NGF binding to p75NTR, addition of the p75NTR-blocking antibody REX inhibited the association (Fig. 5)
Fig. 5.
Co-immunoprecipitation of p75NTR with anti-NADE antibody. Western blots for p75NTR were done on immunoprecipitates with anti-NADE antibody. Proteins were isolated from cortical cultures 8 hr after 15 min zinc exposure. Here, p75NTRco-immumoprecipitated with NADE. Addition of REX (1:400) inhibited the co-immunoprecipitation.
Caspase inhibitors attenuate zinc neurotoxicity
The death signaling through the p75NTR/NADE system culminates in caspase activation (Gu et al., 1999; Mukai et al., 2000). Consistently, addition of caspase inhibitors (zVAD-fmk, zDEVD-fmk, zYVAD-fmk) attenuated cortical neuronal death induced by brief (15 min, Fig.6A) or continuous (20 hr, Fig. 6B) zinc exposure; the protective effect was more prominent in the latter.
Fig. 6.
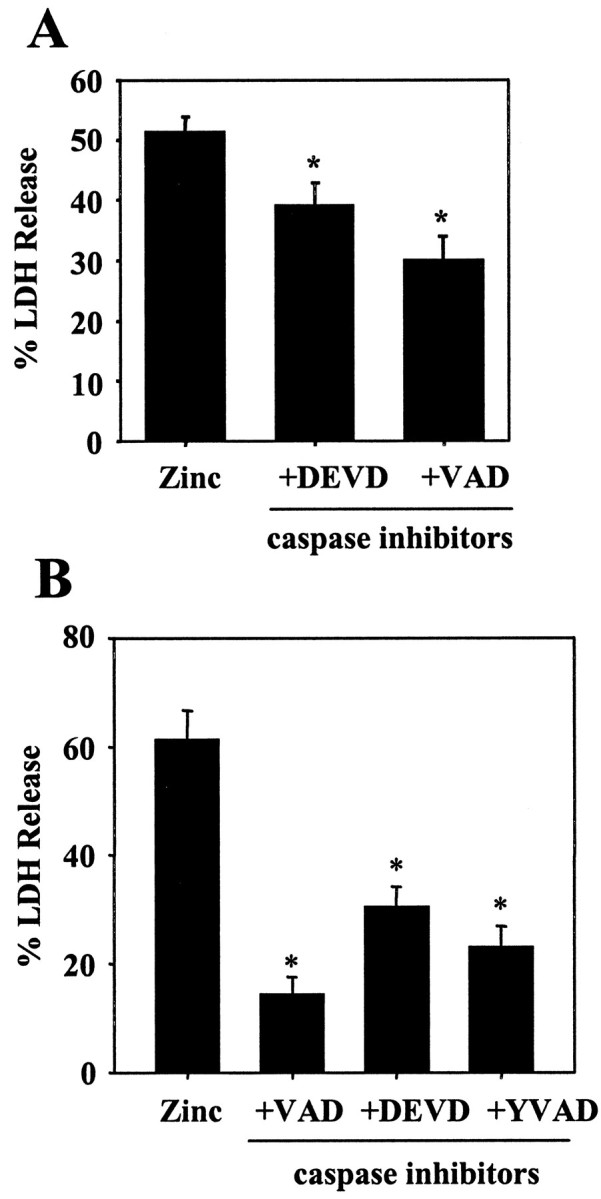
Caspase inhibitors on zinc neurotoxicity.A, LDH release (n = 4; mean + SEM) 16 hr after 15 min zinc exposure done in the absence (Zinc) or presence of 100 μm zVAD-fmk (+VAD) or zDEVD-fmk (+DEVD).B, LDH release (n = 3; mean + SEM) after 20 hr exposure to 25 μm zinc in the absence or presence of 100 μm zVAD-fmk (100 μm), zDEVD-fmk (100 μm), or 50 μm zYVAD-fmk.Asterisks denote difference from respective zinc exposure alone (p < 0.05; two-tailedt test).
Downregulation of NADE attenuates zinc neurotoxicity
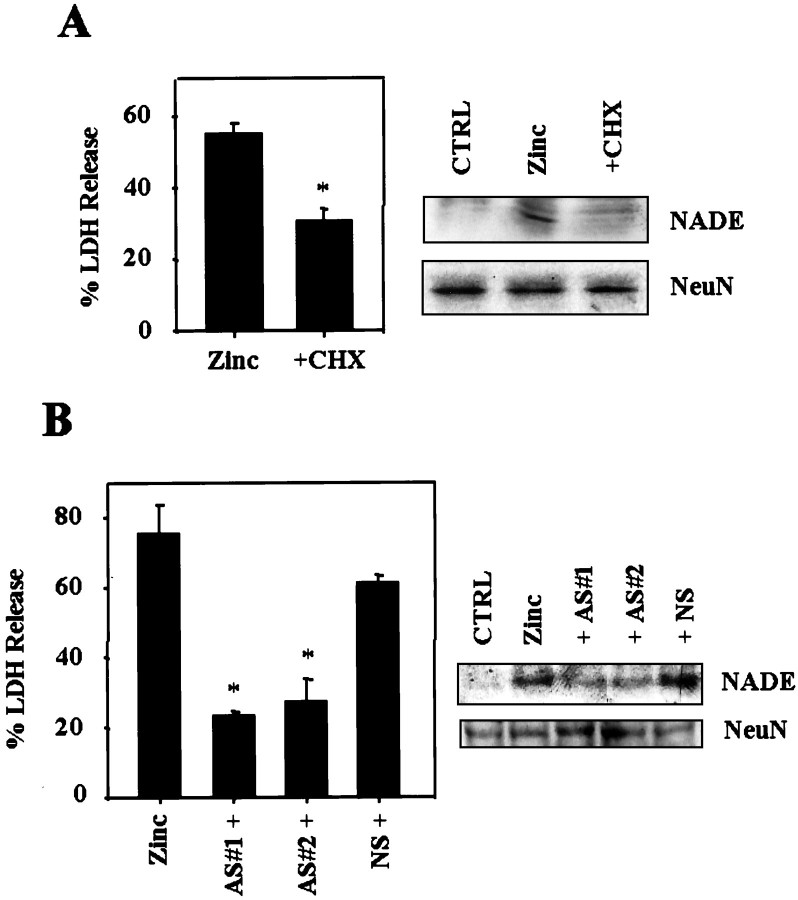
To investigate the direct role of NADE induction in zinc neurotoxicity, we examined effects of a protein synthesis inhibitor cycloheximide and NADE antisense oligonucleotides. The reduction of NADE expression with cycloheximide resulted in partial reduction of zinc-induced neuronal death (Fig.7A). Furthermore, two different kinds of NADE antisense oligonucleotides that substantially downregulated NADE expression, also partially attenuated zinc-induced neuronal death (Fig. 7B). Nonsense oligonucleotides had little effect on NADE expression or neuronal death induced by zinc. These results suggest that NADE induction partly contributes to zinc-induced neuronal death.
Fig. 7.
Downregulated NADE attenuates zinc neurotoxicity.A, Left, Bars represent LDH release in cortical cultures after 20 hr exposure to 25 μm zinc alone (Zinc) or zinc in the presence of 1 μg/ml cycloheximide (+CHX). Asteriskdenotes difference from Zinc (p < 0.05; two-tailed t test). Right, Western blots for p75NTR and NADE in control (CTRL) or after 12 hr exposure to 25 μm zinc without (Zinc) or with 1 μg/ml cycloheximide (+CHX). Western blots for NeuN are presented as control. B, Left, Bars represent LDH release in cortical cultures by additional 20 hr exposure to zinc 25 μm without (Zinc) or with 10 μm of NADE antisense (AS#1+or AS#2+) or 10 μm nonsense (NS+) oligonucleotides. Oligonucleotides were transfected into cortical cells using DOTAP liposomal reagents 3 hr before zinc exposure. Asterisks denote difference from Zinc (p < 0.05; two-tailed ttest with Bonferroni correction for three comparisons).Right, Western blots for NADE in cortical cultures treated with 10 μm of each antisense or nonsense oligonucleotides.
Induction of p75NTR and NADE in degenerating CA1 neurons after transient forebrain ischemia: its blockade by Ca EDTA
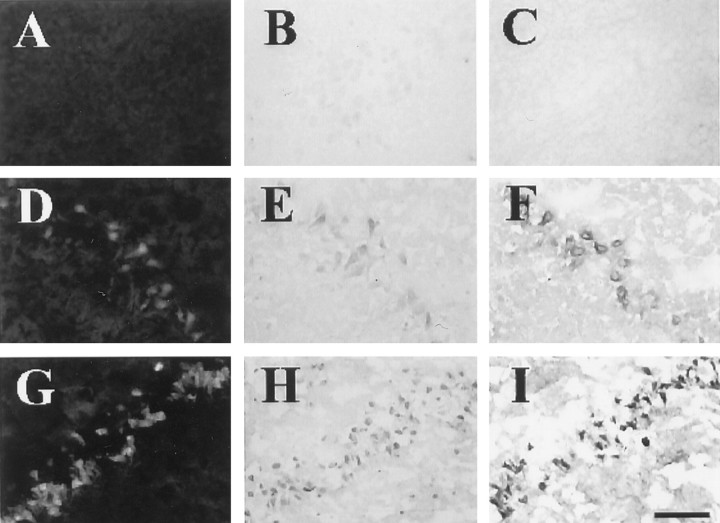
Previously, we have demonstrated that toxicity mediated by endogenous zinc contributes to selective neuronal death after transient cerebral ischemia (Koh et al., 1996). Hence, we examined the possibility that p75NTR and/or NADE induction occurs also in this case. Examination of the rat hippocampus after 15 min ischemia revealed that p75NTRand NADE immunoreactivity began to show up in the CA1 pyramidal cell layer at 48 hr after the ischemia (Fig. 9E,F) and became more prominent at 72 hr (Figs.8D,E,9H,I; five of five rats). At 24 hr time point, neither p75NTR nor NADE immunoreactivity was seen (data not shown). Based on the findings from adjacent sections, it appeared that NADE induction occurred in a subset of p75NTR-immunoreactive neurons. For example, dentate granule neurons exhibited p75NTR, but they did not develop NADE immunoreactivity (Fig. 8D,E, arrowheads). TUNEL staining of adjacent sections showed that a close correlation existed between p75NTR/NADE co-immunoreactivity and irreversible neuronal damage in CA1 pyramidal neurons (Fig. 8F). By contrast, as well documented in the literature (Roux et al., 1999), the dentate granule neurons and CA3 pyramidal neurons that expressed only p75NTR, were not stained with TUNEL (Fig.8F, arrowheads). Injection of CaEDTA into the lateral ventricle completely blocked the development of both p75NTR and NADE immunoreactivity throughout the hippocampus (Fig. 8G,H). As shown previously (Koh et al., 1996), this maneuver markedly reduced CA1 neuronal death of transient global ischemia as demonstrated here by the lack of TUNEL staining (Fig. 8I). In sham-operated animals, neither p75NTR/NADE expression nor TUNEL staining was seen (Fig.8A--C)
Fig. 9.
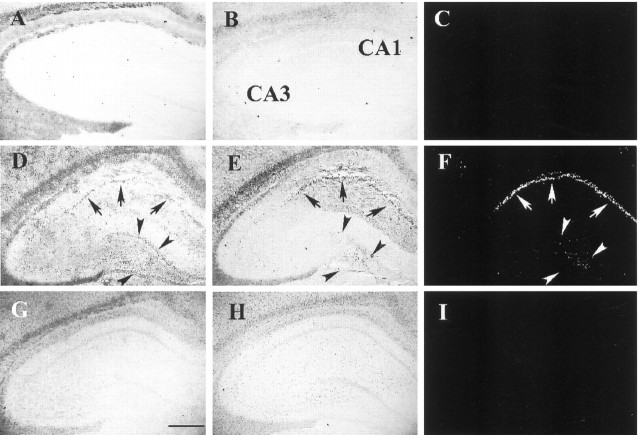
Correlation between zinc accumulation and p75NTR/NADE induction. Higher power photomicrographs of the hippocampal CA1 region, sham-operated (top row), 48 hr (middle row), and 72 hr (bottom row) after 15 min ischemia, stained with TFL-Zn (A, D, G), anti-p75NTRantibody (B, E, H), and anti-NADE antibody (C, F, I). It appeared that zinc accumulation reached its maximum at 48 hr, whereas p75NTR and NADE immunoreactivity increased between 48 and 72 hr. The NADE immunoreactivity first appeared in the cytoplasm (F), then spread throughout the cell including nuclei (I). In contrast, none of zinc accumulation (A), p75NTRimmunoreactivity (B), or NADE immunoreactivity (C) was detected in a sham-operated animal. Scale bar, 100 μm.
Fig. 8.
Induction of p75NTR and NADE in hippocampal neurons after transient global ischemia. Low-power photomicrographs of anti-p75NTR antibody-stained (A,D,G), anti-NADE antibody-stained (B,E,H), or TUNEL-stained (C,F,I) brain sections of a sham-operated rat (top row) and of rats 72 hr after 15 min ischemia with intraventricular injection of saline (middle row) or CaEDTA (bottom row). p75NTRimmunoreactivity, NADE immunoreactivity, and TUNEL reactivity were conspicuous in the CA1 pyramidal cell layer 72 hr after ischemia (D–F, arrows). Interestingly, whereas p75NTR immunoreactivity developed in dentate granule cells (arrowheads), neither NADE immunoreactivity nor TUNEL reactivity developed in them. Intraventricular injection of CaEDTA almost completely blocked the emergence of all three (G–I). Scale bar, 1 mm.
Staining brain sections with a zinc-specific fluorescent dye, TFL-Zn, revealed dense zinc accumulation in CA1 pyramidal neurons at both 48 and 72 hr after 15 min ischemia (Fig.9D,G). Immunocytochemical staining of adjacent brain sections with anti-p75NTR antibody revealed faint staining at 48 hr after ischemia (Fig. 9E), which became conspicuous at 72 hr (Fig. 9H). Similarly, immunocytochemical staining with anti-NADE antibody showed faint, mostly cytoplasmic staining at 48 hr (Fig. 9F) and dense staining throughout cell bodies at 72 hr (Fig.9I). This time course is consistent with that of CA1 neuronal degeneration in transient global ischemia (Smith et al., 1984;Koh et al., 1996) and appears preceded by accumulation of zinc in neuronal cell bodies that starts ∼24 hr after ischemia (Koh et al., 1996).
DISCUSSION
Conventional wisdom has been that actions by neurotrophic factors should be always beneficial to neuronal cells (Lewin and Barde, 1996;Klesse and Parada, 1999; Kaplan and Miller, 2000). However, a recent discovery that p75NTR mediates cell death (Bredesen and Rabizadeh, 1997; Kaplan and Miller, 1997;Casaccia-Bonnefil et al., 1999) argues against the idea. Although phenomenon of p75NTR-mediated apoptosis is now well established, the signaling molecules involved had not been elucidated. Recently the concurrence of apoptosis and p75NTR induction in neurons was demonstrated in a seizure model (Roux et al., 1999). However, whether p75NTR induction contributes causally to neuronal apoptosis in this case has not been determined. Because NADE was recently identified as a necessary factor for p75NTR-mediated apoptosis in oligodendrocytes (Mukai et al., 2000), we asked whether the p75NTR/NADE system might play a significant role in various models of pathological neuronal death.
The present study has demonstrated that p75NTR and NADE are indeed co-induced in degenerating neurons in cortical cultures. Intriguingly, this co-induction occurred only in association with zinc neurotoxicity, but not with calcium or oxidant toxicity, arguing against the possibility that the induction is a general response to neuronal injury. Furthermore, signaling through the induced p75NTR and NADE may partly contribute to neuronal death in zinc neurotoxicity, because blockade of the p75NTR signaling with REX or reduction of NADE expression with cycloheximide or antisense oligonucleotides substantially attenuated zinc-induced neuronal death.
Zinc-triggered neuronal death exhibits features of both apoptosis and necrosis (Y. H. Kim et al., 1999; Lobner et al., 2000). Consistently, in the present study, caspase inhibitors attenuated zinc-induced neuronal death substantially but not completely. In oligodendrocytes, p75NTR/NADE activation results in caspase activation and apoptosis (Mukai et al., 2000). Although the present study was unable to directly demonstrate that p75NTR/NADE is responsible for most of the apoptosis component in zinc neurotoxicity, the fact that similar degree of protection against zinc toxicity was obtained with caspase inhibitors, REX, cycloheximide, or NADE antisense oligonucleotides, seems to favor this possibility.
Another interesting finding is the role for NGF in this setting. Addition of NGF at trophic concentrations (10–100 ng/ml) augmented zinc-induced neuronal death. Combined with the inhibitory effect of REX, it seems likely that NGF binding is required for the p75NTR/NADE system to carry through its apoptosis signaling in cortical neurons, as shown in oligodendrocytes (Mukai et al., 2000). Normally, the level of NGF in medium or cells of cortical culture is quite low. However, NGF is also induced in cortical neurons, and moreover, gets released into the bathing medium. Therefore, in zinc neurotoxicity, all three necessary components of p75NTR apoptosis, which are NGF, p75NTR, and NADE, get induced and contribute to neuronal death.
The co-induction of p75NTR and NADE is not unique to cultured neurons. After transient global ischemia, both are induced in degenerating CA1 neurons before neuronal degeneration. Suggesting that toxic zinc accumulation is a key mechanism for the induction of p75NTR and NADE also in thisin vivo model, injection of a metal chelator CaEDTA completely blocked induction of p75NTR and NADE. The induction of p75NTR has been reported in degenerating neurons after seizures and ischemia (Lee et al., 1995; Kokaia et al., 1998; Roux et al., 1999). Hence, p75NTR-mediated apoptosis may play a significant role in diverse models of brain injury. Interestingly, we have found that p75NTR is also induced in dentate granule neurons. However, NADE is not induced in these neurons, consistent with the fact that dentate granule neurons usually do not degenerate after transient ischemia.
In cultures, zinc influx into cells occurs immediately after the exposure to zinc (Koh and Choi, 1994). Consistently, the time course of p75NTR and NADE induction in cortical culture appears quite fast, already apparent at 4 hr after zinc exposure. In contrast, in the rat with transient global ischemia, zinc accumulation gradually occurs in CA1 regions ∼24–48 hr after transient global ischemia (Koh et al., 1996). Probably reflecting the delayed time course of zinc influx, induction of p75NTR and NADE in CA1 neurons that underwent transient global ischemia, took much slower time course (apparent at 48 hr after ischemia) than that in zinc-exposed cortical cultures.
The present study for the first time has demonstrated that p75NTR and newly discovered death executor NADE are co-induced in degenerating neurons after zinc exposure in cortical culture. Furthermore, thus induced p75NTR and NADE may play an active role in neuronal death. These insights obtained in cortical culture seem relevant in vivo, because the co-induction of p75NTR and NADE is also observed in degenerating CA1 neurons after ischemia, which is inhibited by zinc chelation. Future studies should examine whether the induced p75NTR and NADE in ischemia play an active role in neuronal degeneration as in cortical culture.
Footnotes
This work was supported by Creative Research Initiatives of the Korean Ministry of Science and Technology (J.-Y.K.) and in part by National Institutes of Health R01-GM55147 (T.-A.S.) and the Ribosome Engineering Project of Organized Research Combination System (Japanese Science and Technology Agency) (T.-A.S.). We thank Dr. L. F. Reichardt (University of California, San Francisco, CA) for REX antibody.
Correspondence should be addressed to Jae-Young Koh, National Creative Research Initiative Center for the Study of CNS Zinc and Department of Neurology, University of Ulsan College of Medicine, 388-1 Poongnap-Dong Songpa-Gu, Seoul 138-736, Korea. E-mail: jkko@www.amc.seoul.kr.
REFERENCES
- 1.Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911–923. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbacid M. Structural and functional properties of the TRK family of neurotrophin receptors. Ann NY Acad Sci. 1995;766:442–458. doi: 10.1111/j.1749-6632.1995.tb26693.x. [DOI] [PubMed] [Google Scholar]
- 3.Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog Neurol. 2000;61:205–229. doi: 10.1016/s0301-0082(99)00056-8. [DOI] [PubMed] [Google Scholar]
- 4.Barrett GL, Georgiou A. The low-affinity nerve growth factor receptor p75NGFR mediates death of PC12 cells after nerve growth factor withdrawal. J Neurosci Res. 1996;45:117–128. doi: 10.1002/(SICI)1097-4547(19960715)45:2<117::AID-JNR4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Bredesen DE, Rabizadeh S. p75NTR and apoptosis: Trk-dependent and Trk-independent effects. Trends Neurosci. 1997;20:287–290. doi: 10.1016/s0166-2236(96)01049-1. [DOI] [PubMed] [Google Scholar]
- 6.Budde T, Minta A, White JA, Kay AR. Imaging free zinc in synaptic terminals in live hippocampal slices. Neuroscience. 1997;79:347–358. doi: 10.1016/s0306-4522(96)00695-1. [DOI] [PubMed] [Google Scholar]
- 7.Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 8.Casaccia-Bonnefil P, Gu C, Khursigara G, Chao MV. p75 neurotrophin receptor as a modulator of survival and death decisions. Microsc Res Tech. 1999;45:217–224. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<217::AID-JEMT5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Casademunt E, Carter BD, Benzel I, Frade JM, Dechant G, Barde YA. The zinc finger protein NRIF interacts with the neurotrophin receptor p75(NTR) and participates in programmed cell death. EMBO J. 1999;18:6050–6061. doi: 10.1093/emboj/18.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman BS. A region of the 75 kDa neurotrophin receptor homologous to the death domains of TNFR-I and Fas. FEBS Lett. 1995;374:216–220. doi: 10.1016/0014-5793(95)01113-s. [DOI] [PubMed] [Google Scholar]
- 11.Chittka A, Chao MV. Identification of a zinc finger protein whose subcellular distribution is regulated by serum and nerve growth factor. Proc Natl Acad Sci USA. 1999;96:10705–10710. doi: 10.1073/pnas.96.19.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi DW. Calcium and excitotoxic neuronal injury. Ann NY Acad Sci. 1994;747:162–171. doi: 10.1111/j.1749-6632.1994.tb44407.x. [DOI] [PubMed] [Google Scholar]
- 13.Frade JM, Rodriguez-Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- 14.Gu C, Casaccia-Bonnefil P, Srinivasan A, Chao MV. Oligodendrocyte apoptosis mediated by caspase activation. J Neurosci. 1999;19:3043–3049. doi: 10.1523/JNEUROSCI.19-08-03043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwag BJ, Canzoniero LM, Sensi SL, Demaro JA, Koh JY, Goldberg MP, Jacquin M, Choi DW. Calcium ionophores can induce either apoptosis or necrosis in cultured cortical neurons. Neuroscience. 1999;90:1339–1348. doi: 10.1016/s0306-4522(98)00508-9. [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan DR, Miller FD. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;20:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 19.Khursigara G, Orlinick JR, Chao MV. Association of the p75 neurotrophin receptor with TRAF6. J Biol Chem. 1999;274:2597–2600. doi: 10.1074/jbc.274.5.2597. [DOI] [PubMed] [Google Scholar]
- 20.Kim EY, Koh JY, Kim YH, Sohn S, Joe E, Gwag BJ. Zn2+ entry produces oxidative neuronal necrosis in cortical cell cultures. Eur J Neurosci. 1999;11:327–334. doi: 10.1046/j.1460-9568.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim YH, Kim EY, Gwag BJ, Sohn S, Koh JY. Zinc-induced cortical neuronal death with features of apoptosis and necrosis: mediation by free radicals. Neuroscience. 1999;89:175–182. doi: 10.1016/s0306-4522(98)00313-3. [DOI] [PubMed] [Google Scholar]
- 22.Klesse LJ, Parada LF. Trks: signal transduction and intracellular pathways. Microsc Res Tech. 1999;45:210–216. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<210::AID-JEMT4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 23.Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 24.Koh JY, Choi DW. Zinc toxicity on cultured cortical neurons: involvement of N-methyl-d-aspartate receptors. Neuroscience. 1994;60:1049–1057. doi: 10.1016/0306-4522(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 25.Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 26.Kokaia Z, Andsberg G, Martinez-Serrano A, Lindvall O. Focal cerebral ischemia in rats induces expression of p75 neurotrophin receptor in resistant striatal cholinergic neurons. Neuroscience. 1998;84:1113–1125. doi: 10.1016/s0306-4522(97)00579-4. [DOI] [PubMed] [Google Scholar]
- 27.Lee TH, Abe K, Kogure K, Itoyama Y. Expression of nerve growth factor and p75 low affinity receptor after transient forebrain ischemia in gerbil hippocampal CA1 neurons. J Neurosci Res. 1995;41:684–695. doi: 10.1002/jnr.490410515. [DOI] [PubMed] [Google Scholar]
- 28.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 29.Liepinsh E, Ilag LL, Otting G, Ibanez CF. NMR structure of the death domain of the p75 neurotrophin receptor. EMBO J. 1997;16:4999–5005. doi: 10.1093/emboj/16.16.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lievremont JP, Sciorati C, Morandi E, Paolucci C, Bunone G, Valle GD, Meldolesi J, Clementi E. The p75NTR-induced apoptotic program develops through a ceramide-caspase pathway negatively regulated by nitric oxide. J Biol Chem. 1999;274:15466–15472. doi: 10.1074/jbc.274.22.15466. [DOI] [PubMed] [Google Scholar]
- 31.Lobner D, Canzoniero LM, Manzerra P, Gottron F, Ying H, Knuson M, Tian M, Dugan LL, Kerchner GA, Sheline CT, Korsmeyer SJ, Choi DW. Zinc-induced neuronal death in cortical neurons. Cell Mol Biol. 2000;46:797–806. [PubMed] [Google Scholar]
- 32.Mukai J, Hachiya T, Shoji-Hoshino S, Kimura M, Nadano D, Suvanto P, Hanaoka T, Li Y, Irie S, Greene LA, Sato TA. NADE, a p75NTR-associated cell death executor, is involved in signal transduction mediated by the common neurotrophin receptor p75NTR. J Biol Chem. 2000;275:17566–17570. doi: 10.1074/jbc.C000140200. [DOI] [PubMed] [Google Scholar]
- 33.Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 34.Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19:6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith ML, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjo BK. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand. 1984;69:385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. [DOI] [PubMed] [Google Scholar]
- 36.Soilu-Hanninen M, Ekert P, Bucci T, Syroid D, Bartlett PF, Kilpatrick TJ. Nerve growth factor signaling through p75 induces apoptosis in Schwann cells via a bcl-2-independent pathway. J Neurosci. 1999;19:4828–4838. doi: 10.1523/JNEUROSCI.19-12-04828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weskamp G, Reichardt LF. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 39.Ye X, Mehlen P, Rabizadeh S, VanArsdale T, Zhang H, Shin H, Wang JJ, Leo E, Zapata J, Hauser CA, Reed JC, Bredesen DE. TRAF family proteins interact with the common neurotrophin receptor and modulate apoptosis induction. J Biol Chem. 1999;274:30202–30208. doi: 10.1074/jbc.274.42.30202. [DOI] [PubMed] [Google Scholar]