Abstract
The 5-HT1A and 5-HT1B receptors for serotonin exhibit a different membrane localization to either soma and dendrites (5-HT1AR) or axons and terminals (5-HT1BR) of neurons in the CNS. The mechanisms responsible for their differential targeting were investigated previously by transfecting various 5-HT1AR/5-HT1BR chimeras in the epithelial Lilly pork kidney (LLC-PK1) cell line. This first study suggested that a specific targeting signal is located in the C-terminal portion (comprising the last two transmembrane and the cytoplasmic C-terminal domains) of the 5-HT1Aand/or 5-HT1Breceptors. In the present study, the role of the cytosolic C-terminal tail of the receptors was further investigated by transfecting truncated receptors and 5-HT1AR/5-HT1BR chimeras in both the epithelial LLC-PK1 cells and rat hippocampal neurons in primary culture. Confocal microscopic analysis of immunofluorescence with specific anti-5-HTR antibodies and anti-microtubule-associated protein 2 or anti-neurofilament 200k antibodies showed that substitution of the cytosolic C-terminal tail of the 5-HT1BR in the 5-HT1AR addressed the resulting chimera to the axon of neurons and to the apical domain of LLC-PK1 cells. Therefore, the short tail of the 5-HT1BR presents an apical targeting signal that can also act as an axonal targeting signal. In addition, a domain within the third intracytoplasmic loop of the 5-HT1BR, responsible for its Golgi sequestration in LLC-PK1 cells, appeared to act as another axonal targeting signal in hippocampal neurons.
Keywords: cell polarity, addressing mechanisms, axons, dendrites, serotonin receptors, 5-HT1AR, 5-HT1BR
The serotonin-1A (5-HT1AR) and serotonin-1B (5-HT1BR) receptors are two G-protein-coupled receptors. These serotonin (5-hydroxytryptamine, 5-HT) receptors belong to the 5-HT1 family showing a high affinity for serotonin, are negatively coupled with adenylyl cyclase, and share 43% identity in their amino acid sequences, mainly within the transmembrane domains (Barnes and Sharp, 1999). Neuronal functions of these receptors depend on their localization. Whereas the 5-HT1AR modulates the firing of serotonergic neurons in the raphe nuclei (Haj-Dahmane et al., 1991), the 5-HT1BR participates in a local control of serotonin release from axon terminals in their projection areas (Engel et al., 1986). Their distribution, investigated by specific radioligand binding, in situ hybridization, and immunohistochemistry, showed a good colocalization of the mRNA and the protein for the 5-HT1AR (Miquel et al., 1991; Pompeiano et al., 1992), whereas in contrast, the 5-HT1BR appeared to be localized in different areas compared with its mRNA (Boschert et al., 1994; Doucet et al., 1995). Immunocytochemistry at the electron microscope level confirmed that the 5-HT1AR is localized on the soma and dendrites of neurons (Kia et al., 1996; Riad et al., 2000), whereas the 5-HT1BR is in preterminal unmyelinated axons (Sari et al., 1999; Riad et al., 2000) throughout the rat CNS.
We first investigated the origin of the differential targeting of the 5-HT1A and 5-HT1B receptors by expressing them in polarized epithelial Lilly Pork Kidney (LLC-PK1) cells. Indeed, Dotti and Simons (1990) made the hypothesis that epithelial cells and neurons share common mechanisms of protein targeting, with the apical domain being the equivalent of axons and the basolateral domain corresponding to the soma and dendrites, respectively. Previous studies showed that the 5-HT1BR stayed in a Golgi-like intracellular compartment in both LLC-PK1 cells (Langlois et al., 1996) and Madin-Darby canine kidney II (MDCKII) cells (Ghavami et al., 1999). In contrast, the 5-HT1AR was targeted mainly to the basolateral domain of the plasma membrane in LLC-PK1 cells and to both its apical and basolateral domains in MDCKII cells. Subsequent analysis of the targeting of chimeras of 5-HT1AR and 5-HT1BR in LLC-PK1 cells revealed that the 5-HT1BR and all the chimeras containing its third intracellular domain (I3) were localized in the Golgi apparatus (Darmon et al., 1998), suggesting that this domain was responsible for intra-Golgi sequestration. In addition, the different localization of two chimeras that differ in their C-terminal portion suggested that a specific targeting signal was located in the C-terminal portion of the 5-HT1AR and/or 5-HT1BR.
In the present study, we have constructed new chimeras and truncated receptors to identify the targeting signals of the 5-HT1AR and/or 5-HT1BR. Chimeras were expressed by stable transfection in LLC-PK1 cells. We have, in addition, compared their targeting with that observed in another expression system: primary cultures of rat hippocampal neurons cocultured with glial cells. These neurons were transfected with plasmids containing the cDNAs of the 5-HT1AR, 5-HT1BR, or 5-HT1AR/5-HT1BR chimeras. Their targeting in axons and/or dendrites was visualized by immunofluorescence and analyzed by confocal microscopy.
MATERIALS AND METHODS
Materials. Antibodies used to detect rat 5-HT1AR (El Mestikawy et al., 1990) and 5-HT1BR (Langlois et al., 1995) have been described previously. Both are polyclonal rabbit antibodies directed against peptides located within the third intracellular domain of the receptors. Mouse monoclonal microtubule-associated protein 2 (MAP2) antibody is the AP20 clone (Roche, Meylan, France), and mouse monoclonal neurofilament 200k (NF200k) antibody corresponds to the RT97 clone (Roche) that recognizes a phosphorylated form of NF200k.
LLC-PK1 cell culture and primary cultures of hippocampal neurons. LLC-PK1 cells were grown essentially as described previously (Darmon et al., 1998) in DMEM supplemented with 10% fetal bovine serum. Stably transfected clones were selected in the presence of 1.25 mg/ml G418 and maintained in 0.4 mg/ml G418 (Life Technologies, Cergy Pontoise, France). Neuronal cultures were performed essentially as described by Goslin et al. (1998) with some modifications. Hippocampi of rat embryos were dissected at day 17–18. Dissociation was achieved after trypsinization, with a Pasteur pipette. Cells were counted and plated on poly-l-lysine-coated 12-mm-diameter coverslips, at a density of 60,000–75,000 cells per 16 mm dish (300–375 per square millimeter), in complete Neurobasal medium supplemented with B27 (Life Technologies), containing 1 mml-glutamine, 25 μm β-mercaptoethanol, and penicillin G (10 U/ml)–streptomycin (10 mg/ml). Four hours after plating, the coverslips were transferred to a confluent plate of glial cells and maintained for 24 hr in complete Neurobasal medium. The medium was partially changed every 3–4 d.
Construction of chimeric receptors. The chimeras A to F are those described by Darmon et al. (1998). Truncated receptors deleted in the C-terminal tail were constructed by insertion of a stop codon, by PCR using two complementary oligonucleotides containing the mutation. Only the sense oligonucleotides are listed below, with the mutated nucleotides as bold letters and the stop codonunderlined. The name of each oligonucleotide contains the position of the stop codon in the nucleotide sequence of the rat 5-HT1AR (Albert et al., 1990) and 5-HT1BR (Hamblin et al., 1992). The chimera 1ActB was constructed by PCR by using a hybrid oligonucleotide (Oligo 1ActB) containing a 3′ priming sequence corresponding to the 5-HT1BR (italics) and the 5′ sequence corresponding to the 5-HT1AR (bold), and conversely for the other chimera 1BctA. The corresponding amino acid sequences are indicated in parentheses: Oligo 1AΔ1320, CCGGTTATTTGAGCTTATTTCAAC (PVIstop) for the truncated 1AΔ400; Oligo 1AΔ1345, TTCAACAAAGACTAGCAAAACGC (FNKDstop) for the truncated 1AΔ407; Oligo 1BΔ1580, CCCCATCATCTGAACCATGTCCAATG (PIIstop) for the truncated 1BΔ365; Oligo 1BΔ1605, CAATGAGGACTAGAAACAAGCATT (NEDstop) for the truncated 1BΔ372; Oligo 1ActB,GCTTATTTCAACAAAGACTTTAAACAAGCAGCATTCCATAAAC(AYFNKDKQAFHKLI); and Oligo 1BctA,ACCATGTCCAATGAGGACTTCCAAAACGCTTTTAAGAAG(TMSNEDFQNAFKK).
The mutations were confirmed by sequencing the whole coding sequence. Insertion was made in the same pCB6 vector as that used for the former chimeras (Darmon et al., 1998).
Transfection. Transfection was performed with the cationic polymer polyethyleneimine (PEI) (25 kDa; Aldrich, Saint Quentin Fallavier, France), which complexes the anionic charges of the DNA (Lambert et al., 1996). In the present case, 1 μl of 0.1m PEI solution contained 100 nmol of amine charges and complexed 33 μg of DNA. In our hands, neurons were not transfectable until 3 d after plating. Neutralization of the anionic charges of the different DNA preparations by the cationic polymer PEI was tested by complexation with different amounts of PEI, to determine the amount of PEI that completely complexed 1 μg of DNA and prevented its migration in agarose gel (Martres et al., 1998). This amount was named the PEI charge equivalent. An efficient transfection occurred only when a threefold to sixfold PEI charge equivalent was used for complexation. For our DNA constructs, four different transfections were performed with a threefold, fourfold, fivefold, or sixfold PEI charge equivalent, and the best conditions of transfection were analyzed by immunofluorescence and confocal microscopy. DNA (1 μg) and PEI were each diluted in 50 μl of Neurobasal medium without B27 supplement. The PEI solution was added to the DNA dilution, mixed, and left for 10 min at room temperature. The complex was then mixed with 900 μl of complete Neurobasal medium, and 450 μl of the resulting dilution was immediately added to the wells containing the coverslip of neurons that had been removed from the glial plate. Transfection lasted 4 hr at 37°C. The previous medium removed from the glial plate was added back to the neurons and left for 2 d until the immunofluorescence experiments.
Indirect immunofluorescence. For neurons, immunofluorescence was performed from 8 to 15 d after plating and always 2 d after transfection. For LLC-PK1 cells, stably transfected clones were grown on coverslips up to 7 d after confluency. Coverslips were washed twice with PBS+ (PBS containing 0.1 mm CaCl2 and 0.1 mm MgCl2) and fixed with 4% paraformaldehyde in PBS+, and, after three washes of 10 min in PBS+, they were incubated for 1 hr in antibody buffer [3% bovine serum albumin, 2% normal donkey serum, 2% normal goat serum (Interchim, Montluçon, France), and 0.3% Triton X-100 in PBS+]. Incubation with the primary antibodies was performed in the antibody buffer for 2 hr at room temperature. After three washes of 10 min with PBS+, incubation with the secondary antibodies proceeded for 1 hr. The secondary antibodies used were CY3-conjugated donkey anti-rabbit Ig (1:1000 dilution; Interchim) and either CY2-conjugated (1:400; Amersham, Les Ulis, France) or Alexa 488-conjugated (1:1000; Interchim) goat anti-mouse Ig. The coverslips were finally mounted in Fluoromount-G solution (Clinisciences, Montrouge, France). Immunofluorescent images were generated using a Leica (Rueil-Malmaison, France) TCS-400 laser scanning confocal microscope. Contrast and brightness were chosen to ensure that all pixels were within linear range. Images are the product of eightfold line average. Thex–z sections were produced using a 0.2 μm motor step. For double-labeling experiments, pictures were generated using Adobe Photoshop 5.0 (Adobe Systems, San Jose, CA). For each construct, the transfection in neurons was performed at least three times, and at least 10 neurons were analyzed with confocal microscopy each time. One representative neuron was chosen for the figures.
Cell surface biotinylation experiments. Transfected cells were grown for 7 d after confluency on 24-mm-diameter filter inserts (Transwell; pore size, 0.45 μm; Costar, Cambridge, MA) and biotinylated at either side as described previously (Darmon et al., 1998). Proteins were separated by electrophoresis, transferred to nitrocellulose, and probed with 5-HT1AR antibodies at 1:1000. After incubation with anti-rabbit antibodies coupled to horseradish peroxidase, revelation was performed with the ECL+ kit (Amersham), and detection of the fluorescent product was performed using a Fuji (Raytest, Courbevoie, France) FLA 2000 Phosphoimager, with excitation at 473 nm and emission at 520 nm. Linearity of detection is within a range of 0–100 with this apparatus.
RESULTS
Truncated 5-HT1AR and 5-HT1BR are confined intracellularly in LLC-PK1 cells
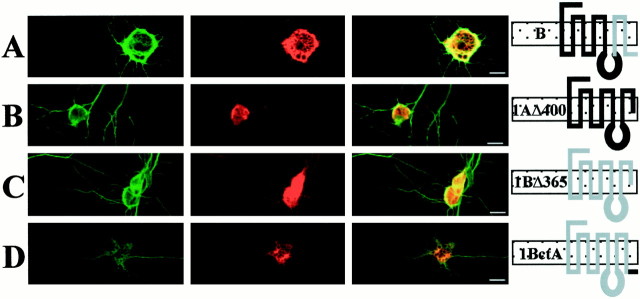
Previous studies on the mechanisms responsible for the differential targeting of 5-HT1AR/5-HT1BR chimeras in LLC-PK1 cells suggested that inclusion of the last two transmembrane domains and the cytosolic tail of the 5-HT1AR (chimera D) (Table 1) or of the 5-HT1BR (chimera E) in their sequence led to a basolateral or an apical targeting, respectively (Darmon et al., 1998). First, we determined whether truncation of the cytosolic C-tail after the seventh transmembrane domain of 5-HT1AR or 5-HT1BR could redirect by default their apical or basolateral localization because of the deletion of a targeting signal. In contrast to the basolateral localization of the 5-HT1AR (Fig.1A) or the Golgi-like intracellular localization of the 5-HT1BR (Fig.1B), large amounts of the truncated 5-HT1AR and 5-HT1BR (Table1) were detected intracellularly in LLC-PK1 cells transfected with the corresponding plasmids. Figure 1C shows that truncated 5-HT1AR (1AΔ400) is retained in the endoplasmic reticulum (ER) of transfected cells and proceeds no further. Similarly, the truncated 5-HT1BR, 1BΔ365, failed to reach the cell surface and remained intracellularly in a compartment that resembles the ER (Fig. 1D) but, in addition, seems to be located in large vesicles. This truncated receptor could not yield stable transfection, and the transient transfected cells might synthesize excessive amounts of the protein that are stored in lysosomes for degradation. Indeed, double labeling with a Golgi marker did not show colocalization (data not shown), suggesting that these vesicles did not belong to the Golgi apparatus. Previously, chimera B was found to be localized in the endoplasmic reticulum and did not exhibit any binding properties in experiments with selective radioligands of each receptor. On Western blot, the corresponding protein appeared to be nonglycosylated (Darmon et al., 1998). Apparently, the truncated receptors had the same localization as chimera B. The intracellular localization of truncated 5-HT1AR and 5-HT1BR may reflect an unstable structure that does not exit from the ER. These results suggest that 5-HT1AR and 5-HT1BR are excluded from the plasma membrane in the absence of their cytosolic tail. Because truncation was made at the very limit of the seventh transmembrane domain, some destabilization of the latter domain might have occurred. Accordingly, new deletion mutants of 5-HT1AR (1AΔ407) and 5-HT1BR (1BΔ372) were constructed (Table 1). These mutants lacked the C-terminal domain but kept a few amino acids beyond the seventh transmembrane domain. In that case too, the resulting truncated receptors failed to reach the plasma membrane (Fig.1E,F).
Table 1.
Targeting of the chimeras in LLC-PK1 cells and neurons
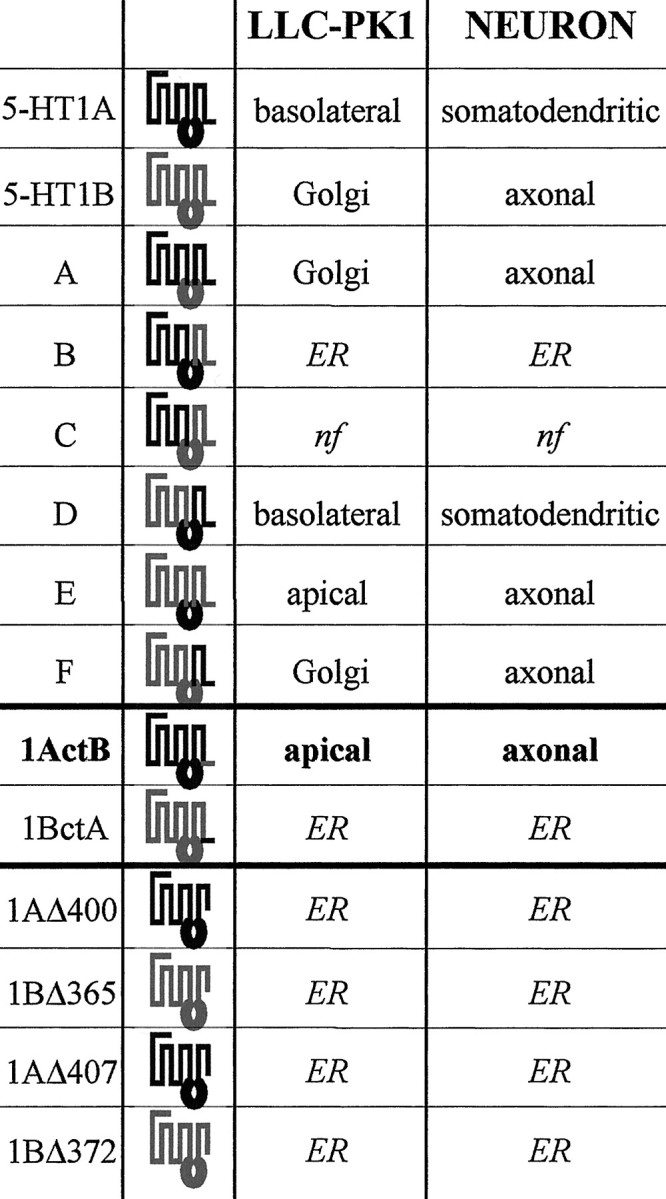 |
Schematic diagrams of 5-HT1AR/5-HT1BR chimeras primary structures with 5-HT1AR sequence shown in black and 5-HT1BR sequence shown in gray. Chimeras A–F were previously constructed by combining three cassettes corresponding to (1) the first five TM domains, (2) the third intracellular domain, and (3) the last two TM domains and the cytoplasmic tail (Darmon et al., 1998). The truncated receptors were constructed by replacing the triplet of the amino acid indicated in the truncated receptor name by a stop codon. The chimeras 1ActB and 1BctA correspond to the entire receptors in which the small cytoplasmic tails have been exchanged between the two receptors. The targeting is resumed as it appeared predominantly in: LLC-PK1 cells, apical, basolateral, or Golgi; ER, endoplasmic reticulum; nf, nonfunctional, i.e., do not yield either stable or transient transfection. For neurons, somatodendritic means that the localization was restricted to the soma and dendrites, and axonal means that in addition to dendrites, the axon was labeled.
Fig. 1.
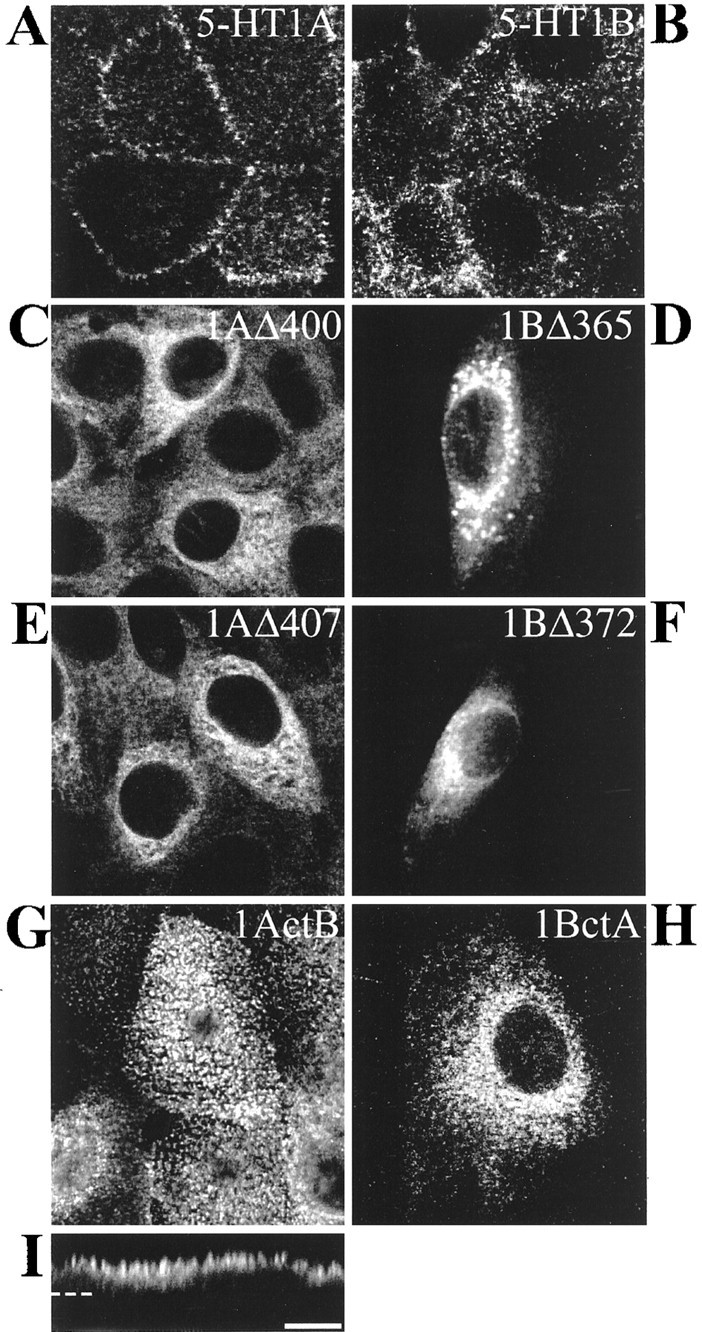
Localization of 5-HT1AR, 5-HT1BR, and chimeras of receptors in transfected LLC-PK1 cells. Confocal immunofluorescence detection of labeling performed with anti-5-HT1AR and anti-5-HT1BR antibodies and revealed by CY3-conjugated anti-rabbit antibodies. 5-HT1AR (A), 5-HT1BR (B), 1AΔ400 (C), and 1AΔ407 (E) (truncated receptors) and 1ActB (G, I) are stably transfected in LLC-PK1 cells. The truncated 1BΔ365 (D), 1BΔ372 (F), and the chimera 1BctA (H) are visualized in transiently transfected LLC-PK1 cells. In contrast to the basolateral localization of 5-HT1AR (A), the truncated 1AΔ400 (C) and 1AΔ407 (E) are localized in an intracellular compartment that resembles the endoplasmic reticulum. The 5-HT1BR, truncated 1BΔ365 (D) and 1BΔ372 (F), and 1BctA (H) are also confined intracellularly. The chimera 1ActB exhibits a punctate staining in a confocal analysis of an apical plane of 0.2 μm (x–y; G), typical of an apical localization visualized also in z-cut detection (I). The horizontal dashed line indicates the surface of the coverslip. Scale bar, 10 μm.
The cytosolic C-terminal domain of 5-HT1BR addresses the 5-HT1AR/5-HT1BR chimeras to the apical domain in LLC-PK1 cells
We substituted the cytosolic tail of 5-HT1BR in the 5-HT1AR and vice versa. The first type of 5-HT1AR/5-HT1BR chimera was constructed in such a way that the 15 amino acids of the 5-HT1BR tail were joined just after the 5-HT1A seventh transmembrane domain. The resulting chimeric receptor 1ActB was then stably transfected in LLC-PK1 cells. Immunofluorescence of 1ActB-expressing cells visualized in the x–y plane revealed a punctate immunostaining (Fig.1G). This typical staining characterizes epithelial microvilli and indicates that 1ActB chimera was localized at the apical domain. Confocal z-cut analysis confirmed a predominant apical localization of chimeric 1ActB receptor (Fig.1I). This result showed that substitution of the 5-HT1AR tail for that of 5-HT1BR redirected the basolateral 5-HT1AR to the apical pole. Binding experiments with [3H]alnespirone (Fabre et al., 1997) showed that this chimera could bind the specific agonist of 5-HT1AR (data not shown).
In the same way, we explored the existence of a putative sorting signal in the cytosolic tail of 5-HT1AR by creating the reverse mutant 1BctA consisting of the native 5-HT1BR fused to the 5-HT1AR cytosolic tail (Table 1). However, 1BctA chimera gave no stable transfected clone, despite several transfection experiments. This chimera presented a clearly intracellular retention in LLC-PK1 cells transiently transfected with the 1BctA encoding sequence (Fig. 1H). Probably, synthesis of 1BctA is immediately followed by removal and degradation before any plasma membrane insertion.
To quantify the targeting of 1ActB chimera, the same clonal cell line as that used in immunofluorescence experiments was grown on Transwell filters and biotinylated at either apical or basolateral surface. Biotinylated proteins were precipitated by streptavidin agarose, separated by PAGE, and detected on a nitrocellulose membrane with 5-HT1AR-specific antibodies. As expected, 1ActB appeared to be targeted predominantly to the apical surface of LLC-PK1 cells (Fig.2A). Quantification yielded values three times higher for apical than basolateral biotinylation (Fig. 2B). In contrast, biotinylation of the 5-HT1AR was larger at the basolateral surface (Fig. 2A,B), whereas tail-out (1AΔ400, 1AΔ407, 1BΔ365, and 1BΔ372) and 1BctA tail-swapped mutants having no plasma membrane localization could not be labeled by the biotinylation procedure (data not shown).
Fig. 2.
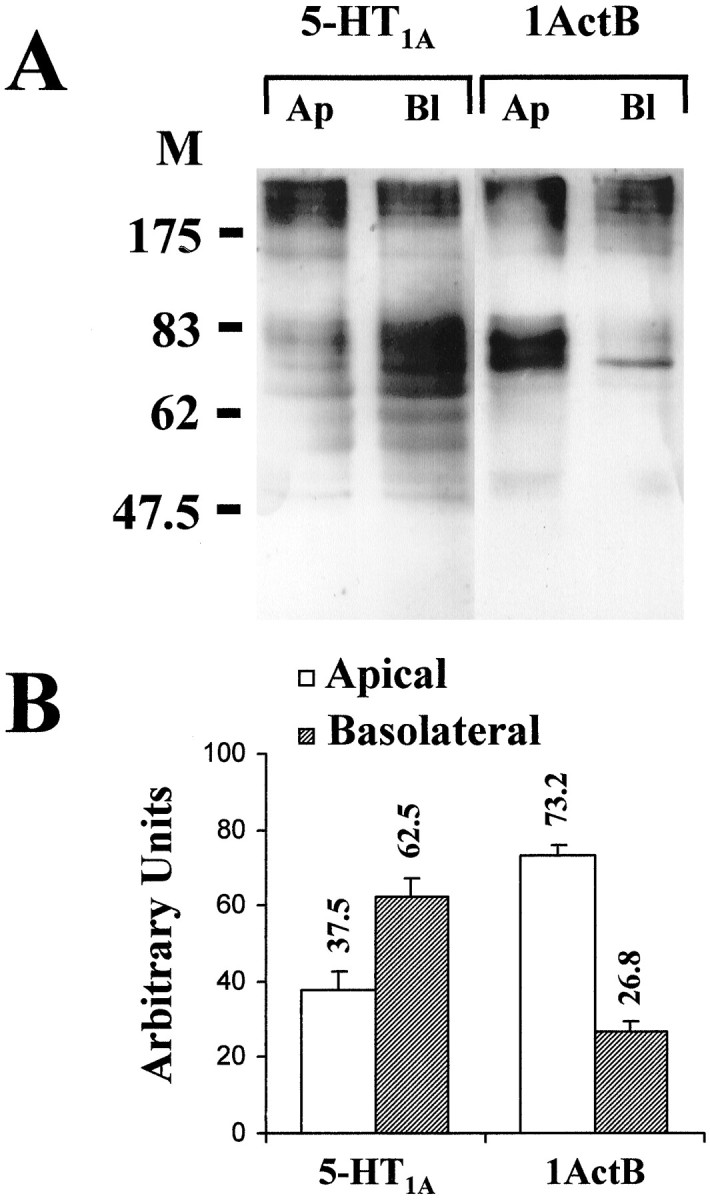
Apical predominance of 1ActB chimera in stably transfected LLC-PK1 cells. A, After apical (Ap) and basolateral (Bl) biotinylation of membrane proteins of stably transfected cells cultured on Transwell filters, extracts were separated by PAGE. Detection was performed on nitrocellulose membrane with 5-HT1AR antibodies and ECL+ Western blotting detection system (Amersham). Molecular weights of markers are indicated in kilodaltons.B, Bars represent the apical or basolateral labeling compared with the total receptor content on the cell surface (in percent, mean ± SD). Three quantifications were performed on one or two different immunoblots from three independent biotinylation experiments. The ECL+ fluorescence was quantified using a Fuji FLA-2000 Phosphoimager apparatus.
Polarized targeting of 5-HT1AR and 5-HT1BR in hippocampal neurons
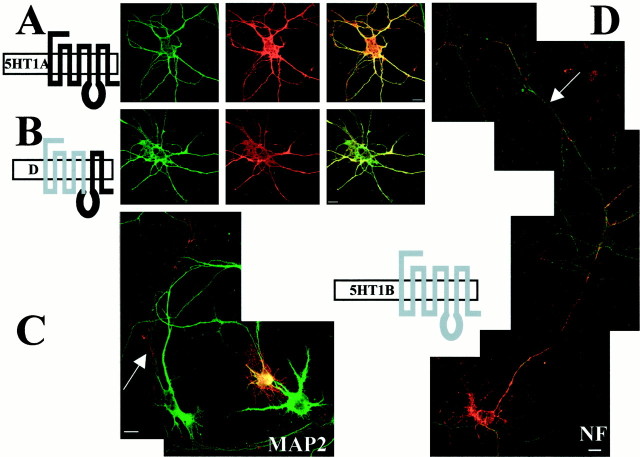
Hippocampal neurons were transfected with the same plasmids as those used in LLC-PK1 cells. In this cell culture derived from immature pyramidal cells of embryos at embryonic day 18 (E18), our 5-HT1BR antibodies did not detect any labeling in nontransfected neurons, suggesting that, at this stage, pyramidal neurons do not yet express the 5-HT1BR. A weak signal in the soma of few neurons was detected with our 5-HT1AR antibodies, suggesting that the expression of 5-HT1AR may begin earlier than that of 5-HT1BR when pyramidal neurons are not yet differentiated into CA1, CA2, and CA3 cell types. Figure3 shows the detection of 5-HT1AR and 5-HT1BR 2 d after transfection (i.e., 15 d after plating), using a double-labeling procedure. The labeling of 5-HT1AR (Fig. 3A, red) showed a good colocalization with MAP2 labeling (green), in accordance with its predominant localization in dendrites. Accordingly, this expression system enabled us to visualize an appropriate targeting of the 5-HT1AR with respect to its localization in the CNS, i.e., a dendritic targeting and an axonal exclusion. Neurons transfected with the 5-HT1BR showed a labeling of the axon, as revealed by the double labeling with NF200k antibody (Fig.3D). The double labeling with MAP2 antibody (Fig.3C) confirmed that, in addition to the axon, dendrites were labeled with 5-HT1BR antibodies. These observations indicate that the 5-HT1BR followed an axonal targeting, without dendritic exclusion. These results are in accordance with those obtained for metabotropic glutamate receptors expressed from recombinant viral vector in primary cultures of hippocampal neurons (Stowell and Craig, 1999). Indeed, metabotropic glutamate receptor 1 (mGluR1) and mGluR2 (like the 5-HT1AR) were found to exhibit a dendritic targeting with an axonal exclusion, whereas mGluR7 (like the 5-HT1BR) was characterized by a dominant axonal targeting in addition to a dendritic localization. Accordingly, this culture system reproduces the differential targeting of receptors in axons but not in dendrites and can be used to identify axonal but not somatodendritic targeting signals. Dendritic localization of the 5-HT1BR could be attributable to overexpression resulting from the strong promoter present in the plasmid used for transfection. However, Jareb and Banker (1998)addressed this question about neurons infected by recombinant viruses and showed that there was no significant correlation between the level of expression and the degree of polarization for any of the apical proteins that they studied.
Fig. 3.
Somatodendritictargetingof 5-HT1AR and chimera D and axonal targeting of 5-HT1BR in cultured hippocampal neurons. Confocal immunofluorescence detection of 5-HT1AR (A), chimera D (B), and 5-HT1BR (C, D) appearing inred with CY3-conjugated anti-rabbit antibodies and either MAP2 (A–C) or NF200k (D), detected in green with Alexa 488-conjugated anti-mouse antibodies, in transfected hippocampal neurons. Colocalization of 5-HT1AR and chimera D with the dendritic marker MAP2 is shown on the right with a superposition of the two labels (red andgreen) in a yellow/orangecolor. The 5-HT1BR exhibits an axonal location that is visualized as a thin red labeling around the cell body and a distal labeling of the axon (C), in addition to a dendritic location detected by a yellowcolocalization with MAP2. The double labeling of the 5-HT1BR and NF200k in the axon is shown inD. Scale bars, 10 μm.
The third intracellular domain of the 5-HT1BR plays different roles in receptor targeting in epithelial cells and neurons
To better characterize the targeting signals of 5-HT1AR and 5-HT1BR in hippocampal neurons, we tested the targeting of some chimeras of these receptors that were already used in our study using LLC-PK1 cells (Darmon et al., 1998). Table 1 shows a summary of the chimeras and their previous localization in LLC-PK1 cells, as well as some new chimeras, which we have constructed to delineate some potential targeting signals. Our previous study had shown that the third intracellular domain was responsible for the 5-HT1BR localization in the Golgi apparatus (chimeras A and F) of LLC-PK1 cells. In addition, a chimera containing the C-terminal domain of the 5-HT1BR (chimera E) was targeted to the apical domain of these cells, suggesting the existence of an apical targeting signal in the 5-HT1BR. On the other hand, the chimera D that contains the C-terminal of the 5-HT1AR was targeted to the basolateral domain of LLC-PK1 cells.
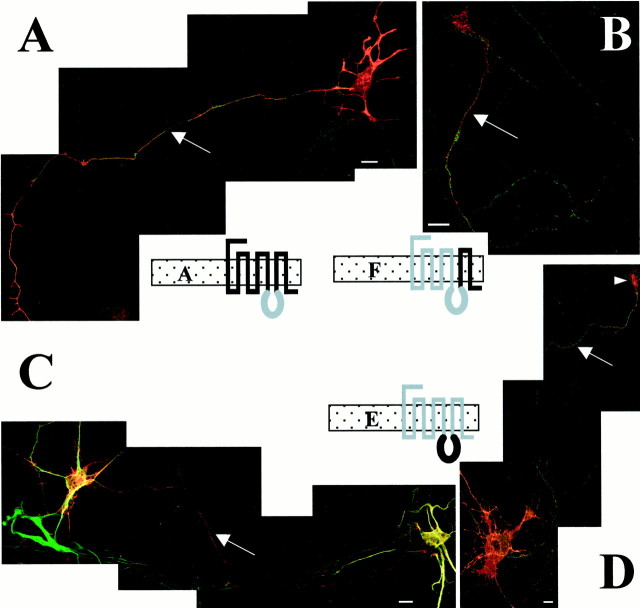
The targeting of chimeras A, E, and F in neurons is illustrated in Figure 4. In contrast to what was observed in LLC-PK1 cells, the three chimeras A, E, and F exhibited the same targeting in neurons. For the three chimeras, the dendrites were labeled (illustrated here only for the chimera E), as revealed by the double labeling with MAP2 antibody (Fig. 4C). In addition, a long axon labeled with the 5-HT1BR and NF200k antibodies, but not with MAP2 antibody, confirmed the targeting of these chimeras to the axon (Fig.4A,B,D). These observations showed that these three chimeras presented an axonal targeting in addition to a dendritic localization.
Fig. 4.
Axonal targeting of chimera A, chimera F, and chimera E in cultured hippocampal neurons. Confocal immunofluorescence detection of chimera A (A), chimera F (B), and chimera E (C,D) in transfected hippocampal neurons. These three chimeras exhibit an axonal targeting visualized by a long and thin neurite in red (CY3-conjugated anti-rabbit antibodies) and labeled by NF200k antibody in green(arrow) (A, B,D, Alexa 488-conjugated anti-mouse antibodies). This axonal targeting is confirmed in a double-labeling experiment with the MAP2 dendritic marker, which shows in addition to a double labeling of dendrites, a long and thin neurite labeled only with the antibodies directed against the 5-HT1BR for the chimeras A and F and against the 5-HT1AR for the chimera E (C). An arrowhead shows a labeled terminal or a growth cone that is frequently detected with the chimera E (D). Scale bars, 10 μm.
For the chimera D (Fig. 3B), we observed the same type of labeling as for the 5-HT1AR: a dendritic targeting with an axonal exclusion. The labeling of the dendrites matched perfectly the labeling by MAP2 antibody. The two constructs that were either nonfunctional in LLC-PK1 cells, chimera C, or restricted to the ER, chimera B, were also nonfunctional in neurons or were restricted to an inner compartment within the cell body (Fig.5A), respectively.
Fig. 5.
Intracellular retention of chimeras B and 1BctA and truncated receptors in hippocampal neurons. The chimeric and truncated receptors B and 1AΔ400 are labeled with 5-HT1AR antibodies (A, B) and 1BΔ365 and 1BctA with 5-HT1BR antibodies (C,D) and detected in red with CY3-conjugated anti-rabbit antibodies. Each chimera (illustrated on theright) exhibits an intracellular localization in the soma of neurons with no labeling of neurites characterized by the dendritic marker MAP2 (detected in green with Alexa 488-conjugated anti-mouse antibodies). Scale bars, 10 μm.
The cytoplasmic C-terminal domain of the 5-HT1BR addresses the 5-HT1AR/5-HT1BR chimeras to the axon of hippocampal neurons
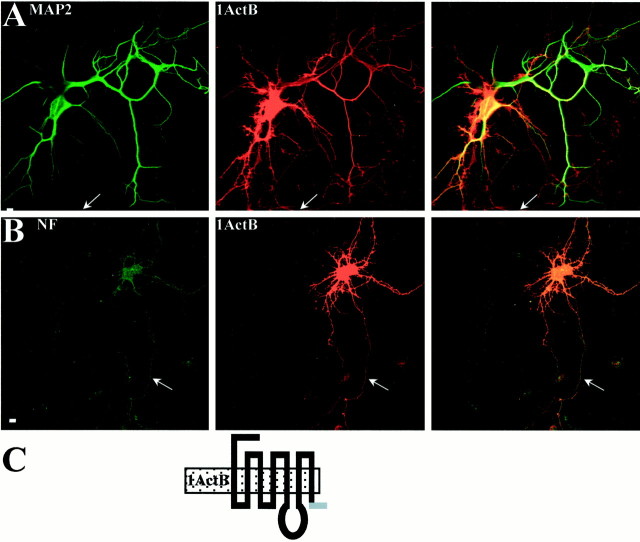
To investigate the respective roles of the last two transmembrane segments and the intracellular C-terminal domain, we first studied the targeting of the different constructs of 5-HT1AR and 5-HT1BR deleted in their C-terminal moiety. In Figure 5 are shown the chimeras B and 1BctA, as well as the truncated receptors 1AΔ400 and 1BΔ365. All these constructs, like the 1BctA chimera, were restricted to an intracellular compartment within the cell body of neurons similar to what was observed in LLC-PK1 cells. The truncated 1BΔ365 showed a weak signal in the proximal dendrites (Fig. 5C), which can correspond to its localization in the endoplasmic reticulum in dendrites. This result strengthens the hypothesis of a misfolding of the protein when the truncation in the cytosolic C-terminal domain is too close to the exit of the seventh transmembrane domain from the plasma membrane. In contrast, the chimera 1ActB exhibited an axonal localization as shown in Figure 6. Indeed, the axon, clearly identified as a thin and long neurite labeled by 5-HT1AR antibodies, was not labeled by MAP2 antibodies (Fig. 6A) but was labeled by NF200k antibodies (Fig. 6B), therefore suggesting that an axonal targeting signal was located in the small cytoplasmic C-terminal domain of the 5-HT1BR. Interestingly, this same sequence allowed an apical targeting in LLC-PK1 cells (Fig.1G,I). Together, these results suggest the existence of two axonal targeting signals in the 5-HT1BR, one in the C-terminal domain and the other one in the third intracellular domain. The comparison of the results obtained in LLC-PK1 cells suggests that these signals do not function in the same way in epithelial and neuronal cells.
Fig. 6.
Axonal localization of the chimera 1ActB in neuronal cultures. Confocal immunofluorescence detection of 1ActB chimera detected in red with 5-HT1AR antibodies and CY3-conjugated anti-rabbit antibodies in transfected cultured hippocampal cells (middle panels). MAP2 (A) and NF200k (B) antibodies are detected in green with Alexa 488-conjugated anti-mouse secondary antibodies. In the right panels of A and B, the superimposition of the two labelings is shown. Arrowspoint at 5-HT1AR-immunoreactive axons devoid of MAP2 labeling (A) but labeled by NF200k antibody (B). C, Schematic description of the chimera 1ActB; sequence originating from the 5-HT1AR is in black and from the 5-HT1BR ingray. Scale bars, 10 μm.
DISCUSSION
All of these experiments showed that the cytosolic C-terminal domain of the 5-HT1BR, when substituted for that of the 5-HT1AR, can address the 5-HT1AR to the apical domain of LLC-PK1 cells and to the axon of hippocampal neurons, suggesting that the 5-HT1BR exhibits an apical–axonal targeting signal. In addition, comparison of the targeting of various chimeras in LLC-PK1 cells and neurons suggests that the second axonal targeting signal located in the third intracellular domain of the 5-HT1BR is not recognized as an apical targeting signal in LLC-PK1 cells.
In the present study, the 5-HT1AR and 5-HT1BR without their C-terminal domains were confined intracellularly in both epithelial cells and neurons. These observations suggest that recombinant receptors require their short C-terminal tail for the preservation of their three-dimensional structure and correct folding. Many natural and artificial mutations have already been observed to result in ER retention because they affect protein folding or oligomerization (Klausner and Sitia, 1990). For example, the betaine transporter with a short deletion in C-terminal fails to reach the cell surface (Perego et al., 1997). The E-cadherin protein when truncated in its C-terminal domain also exhibits an ER localization (Chen et al., 1999).
Previous studies on 5-HT1AR/5-HT1BR chimeras (Darmon et al., 1998) showed that the last two transmembrane domains and the cytosolic C-terminal tail of either 5-HT1AR or 5-HT1BR include a targeting signal. The apical localization of the chimera 1ActB in LLC-PK1 cells suggests that this signal is restricted to the cytosolic C-terminal tail of one or the other receptor. Unfortunately, the intracellular localization of truncated receptors does not help to understand the apical localization of the chimera 1ActB. Is it attributable to a dominant apical targeting signal in 5-HT1BR or to a dominant basolateral targeting signal in 5-HT1AR, disrupted by the substitution and leading to the use of an apical default pathway? Limitations of this epithelial model prompted us to study the targeting of these polytopic neuronal proteins in cultured neurons.
Transfections of the 5-HT1AR and 5-HT1BR in primary cultures of hippocampal neurons showed that the 5-HT1AR was targeted to the dendrites with an axonal exclusion, whereas the 5-HT1BR showed an axonal targeting in addition to a dendritic localization. The fact that a membrane-bound protein such as the 5-HT1BR was present in dendrites in addition to its axonal targeting has also been shown in the same culture system for the mGluR7 receptor (Stowell and Craig, 1999) and for the GABA transporter GAT-3 (Ahn et al., 1996), which are both localized in axons in the CNS. For the targeting of the 5-HT1BR, this culture system is more appropriate than the LLC-PK1 cell line because the receptor is actually inserted within the plasma membrane of cultured neurons, whereas it is confined to intracellular compartments in the latter cells. However, in contrast to that expected from the subcellular localization of 5-HT1BR in the CNS, its axonal localization is not accompanied by a dendritic exclusion in transfected neurons. At the adult stage, pyramidal neurons from CA1, CA2, and CA3 areas express naturally the 5-HT1AR mRNA (Miquel et al., 1991), but only CA1 pyramidal neurons express the 5-HT1BR mRNA (Neumaier et al., 1996). However, at E18, when fetal tissues are taken for setting up the primary cultures, the CA1 and CA3 neurons are not yet differentiated and may not possess the machinery for a dendritic exclusion of the 5-HT1BR. This would explain why 5-HT1BR in transfected hippocampal neurons was addressed in both the axon and the somatodendritic compartment under our experimental conditions. Furthermore, because all types of neurons expressing 5-HT1AR and/or 5-HT1BR throughout the CNS have obviously not yet been examined at a subcellular level, it cannot be excluded that the sorting of these receptors might be different in different types of neurons and at different stages of differentiation.
Interestingly, the chimera D, which exhibited a basolateral localization in the LLC-PK1 cells, shows a dendritic localization like that observed for the 5-HT1AR. This chimera, which contains the third intracellular and C-terminal domains of the 5-HT1AR, could exhibit a targeting signal in one or the other domain that restricts the localization to the somatodendritic compartment. However, the chimeras A and F that have the C-terminal domain of 5-HT1AR or the chimera E that has the I3 domain of the 5-HT1AR are targeted to the axon, thus ruling out the hypothesis of a dominant somatodendritic targeting restriction signal in either domain. Data concerning the chimeras E and 1ActB show that they are both targeted to the axon of hippocampal neurons. These results support the idea that the localization of the 5-HT1BR in the axon is probably attributable to a dominant targeting signal located in the C-terminal tail of the 5-HT1BR. Both chimeras are also apical in LLC-PK1 cells, thus strengthening the hypothesis that the apical targeting signal can also be implicated in axonal targeting. In addition, the two constructs that were restricted to the Golgi apparatus in LLC-PK1 cells, i.e., chimeras A and F, are now targeted to the axon. These two chimeras have in common the third intracellular loop of the 5-HT1BR, thereby suggesting the existence of another axonal targeting signal in this portion of the sequence. Interestingly, this second axonal targeting signal is revealed when the C-terminal one is absent. In LLC-PK1 cells, this signal was not recognized as an apical one and led to an intracellular localization, probably because of the lack of a specific targeting protein present in neurons and absent in LLC-PK1 cells.
The existence of two targeting signals in the same protein has already been reported for the low-density lipoprotein receptor (Matter et al., 1992), suggesting that proteins can have redundant signals. Here the difference of targeting between epithelial cells and neurons concerning the third intracellular domain further emphasizes the limits of the use of epithelial cells as a model for studying the targeting of polytopic neuronal proteins. The previous hypothesis that LLC-PK1 cells lack addressing proteins that interact with the targeting signal located in the third intracellular domain is therefore strengthened by these novel data. All of these results together suggest that the machinery responsible for the apical targeting may be different from that responsible for the axonal targeting. This hypothesis has already been suggested by Jareb and Banker (1998), who concluded from the targeting of various monotopic epithelial proteins expressed in neurons that the targeting of basolateral and dendritic proteins depends on common mechanisms, whereas the sorting of proteins to the axon requires signals that are not present in apical proteins. Indeed, the epithelial system appears to be useful to underline some important features in a given sequence (for example, the cytosolic tail of 5-HT1BR). However, the Golgi sequestration of the 5-HT1BR would not have been emphasized without the comparison with the axonal localization in neurons. In any case, comparison of the targeting between neurons and epithelial cells (Dotti and Simons, 1990) has some limits, and we could have to shift to another model as suggested by Colman (1999), who proposed that the axons and dendrites correspond in fact to the same epithelial compartment, the basolateral one, and that the differential sorting between axons and dendrites relies on mechanisms different from those used between the apical and the basolateral domain.
The role of the C-terminal sequence in the targeting of polytopic proteins in neurons has already been suggested by other studies. Indeed, Poyatos et al. (2000) have shown that two dileucine motifs in the cytosolic C-terminal tail of the glycine transporter GLYT1 can play a role in its basolateral–somatodendritic localization. An axonal targeting signal has been described in the C-terminal sequence of polytopic proteins such as the GABA transporter GAT-3 (Muth et al., 1998) and the metabotropic glutamate receptor mGluR7 (Stowell and Craig, 1999). The existence of an apical sorting signal in the cytoplasmic tail of rhodopsin has also been shown (Chuang and Sung, 1998). However, our study is the first one to show that an apical targeting signal can also be used as an axonal signal. No identity could be detected between the peptidic sequence of the cytoplasmic tail of the 5-HT1BR and those of mGluR7, GAT-3, or rhodopsin. No target motif for a PDZ (postsynaptic density/Discs large/zona occludens-1) protein (Niethammer et al., 1996) could be found either. Indeed, the 5-HT1BR is a presynaptic receptor that modulates the release of neurotransmitters (5-HT, ACh, and GABA … ), and we can suggest that the mechanisms of targeting differ for presynaptic versus postsynaptic receptors or transporters. PDZ proteins play a role in the clustering of postsynaptic proteins, and we can imagine that another family of proteins is involved in the axonal and/or presynaptic targeting. Cytoplasmic domains may play a major role in the targeting of polytopic proteins expressed in neurons by interacting with some specific targeting proteins specialized for the differential targeting in axons and terminals, in opposition with dendrites and postsynaptic densities. One important step in these studies will be to isolate specific sorting proteins that interact with the C-terminal sequence of the rat 5-HT1BR.
Footnotes
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale, the European Community (Biotech Bio4 CT 960752), and Bristol-Myers Squibb Foundation (unrestricted biomedical research grant). N.J. was the recipient of a fellowship from the Conseil Général de la Vendée during the performance of this study. We thank Dr. G. Barbin for assistance in initial experiments and Drs. M. B. Emerit and S. Vyas for judicious comments on this manuscript.
Correspondence should be addressed to Michèle Darmon, Institut National de la Santé et de la Recherche Médicale U288, Faculté de Médecine Pitié-Salpêtrière, 91 Boulevard de l'Hôpital, 75634 Paris Cedex 13, France. E-mail: darmon@ext.jussieu.fr.
Dr. Langlois's present address: Department of Biochemical Pharmacology, Janssen Research Foundation, B-2340 Beerse, Belgium.
REFERENCES
- 1.Ahn J, Mundig O, Muth TR, Rudnick G, Caplan MJ. Polarized expression of GABA transporter in Madin-Darby Canine Kidney cells and cultured hippocampal neurons. J Biol Chem. 1996;271:6917–6924. doi: 10.1074/jbc.271.12.6917. [DOI] [PubMed] [Google Scholar]
- 2.Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O. Cloning, functional expression and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990;265:5825–5832. [PubMed] [Google Scholar]
- 3.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Boschert U, Amara DA, Segu L, Hen R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience. 1994;58:167–182. doi: 10.1016/0306-4522(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 5.Chen YT, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol. 1999;144:687–699. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang JZ, Sung CH. The cytoplasmic tail of rhodopsin acts as a novel apical sorting signal in polarized MDCK cells. J Cell Biol. 1998;142:1245–1256. doi: 10.1083/jcb.142.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colman DR. Neuronal polarity and the epithelial metaphor. Neuron. 1999;23:649–651. doi: 10.1016/s0896-6273(01)80024-6. [DOI] [PubMed] [Google Scholar]
- 8.Darmon M, Langlois X, Suffisseau L, Fattaccini CM, Hamon M. Differential membrane targeting and pharmacological characterization of chimeras of rat serotonin 5-HT1A and 5-HT1B receptors expressed in epithelial LLC-PK1 cells. J Neurochem. 1998;71:2294–2303. doi: 10.1046/j.1471-4159.1998.71062294.x. [DOI] [PubMed] [Google Scholar]
- 9.Dotti CG, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- 10.Doucet E, Riad M, Laporte AM, Vergé D, Daval G, Gozlan H, Hamon M. In situ hybridization evidence for the synthesis of the 5-HT1B receptor in serotoninergic neurones of anterior raphe nuclei in the rat brain. Synapse. 1995;19:18–28. doi: 10.1002/syn.890190104. [DOI] [PubMed] [Google Scholar]
- 11.El Mestikawy S, Riad M, Laporte AM, Vergé D, Daval G, Gozlan H, Hamon M. Production of specific anti-rat 5-HT1A receptor antibodies in rabbits injected with a synthetic peptide. Neurosci Lett. 1990;118:189–192. doi: 10.1016/0304-3940(90)90623-h. [DOI] [PubMed] [Google Scholar]
- 12.Engel G, Göthert M, Hoyer D, Schlicker E, Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- 13.Fabre V, Boni C, Mocaër E, Lesourd M, Hamon M, Laporte AM. [3H]Alnespirone: a novel specific radioligand of serotonin 5-HT1A receptors in the rat brain. Eur J Pharmacol. 1997;337:297–308. doi: 10.1016/s0014-2999(97)01288-0. [DOI] [PubMed] [Google Scholar]
- 14.Ghavami A, Stark KL, Jareb M, Ramboz S, Segu L, Hen R. Differential addressing of 5-HT1A and 5-HT1B receptors in epithelial cells and neurons. J Cell Sci. 1999;112:967–976. doi: 10.1242/jcs.112.6.967. [DOI] [PubMed] [Google Scholar]
- 15.Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing nerve cells. MIT; Cambridge, MA: 1998. pp. 339–370. [Google Scholar]
- 16.Haj-Dahmane S, Hamon M, Lanfumey L. K+ channel and 5-hydroxytryptamine1A autoreceptor interactions in the rat dorsal raphe nucleus: an in vitro electrophysiological study. Neuroscience. 1991;41:495–505. doi: 10.1016/0306-4522(91)90344-n. [DOI] [PubMed] [Google Scholar]
- 17.Hamblin MW, McGuffin RW, Metcalf MA, Dorsa DM, Merchant KM. Distinct 5-HT1B and 5-HT1D serotonin receptors in rat: structural and pharmacological comparison of the two cloned receptors. Mol Cell Neurosci. 1992;3:578–587. doi: 10.1016/1044-7431(92)90070-i. [DOI] [PubMed] [Google Scholar]
- 18.Jareb M, Banker G. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron. 1998;20:855–867. doi: 10.1016/s0896-6273(00)80468-7. [DOI] [PubMed] [Google Scholar]
- 19.Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Vergé D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Klausner RD, Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990;62:611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- 21.Lambert RC, Maulet Y, Dupont JL, Mykita S, Craig P, Volsen S, Feltz A. Polyethylenimine-mediated DNA transfection of peripheral and central neurons in primary culture: probing Ca2+ channel structure and function with antisense oligonucleotides. Mol Cell Neurosci. 1996;7:239–246. doi: 10.1006/mcne.1996.0018. [DOI] [PubMed] [Google Scholar]
- 22.Langlois X, Gérard C, Darmon M, Chauveau J, Hamon M, El Mestikawy S. Immunolabelling of central 5-HT1Dβ receptors in the rat, mouse and guinea-pig with a specific anti-peptide antiserum. J Neurochem. 1995;65:2671–2681. doi: 10.1046/j.1471-4159.1995.65062671.x. [DOI] [PubMed] [Google Scholar]
- 23.Langlois X, El Mestikawy S, Arpin M, Triller A, Hamon M, Darmon M. Differential addressing of 5-HT1A and 5-HT1B receptors in transfected LLC-PK1 epithelial cells: a model of receptor targeting in neurons. Neuroscience. 1996;74:297–302. doi: 10.1016/0306-4522(96)00234-5. [DOI] [PubMed] [Google Scholar]
- 24.Martres MP, Demeneix B, Hanoun N, Hamon M, Giros B. Up- and down-expression of the dopamine transporter by plasmid DNA transfer in the rat brain. Eur J Neurosci. 1998;10:3607–3616. doi: 10.1046/j.1460-9568.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- 25.Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- 26.Miquel MC, Doucet E, Boni C, El Mestikawy S, Matthiessen L, Daval G, Hamon M. Central serotonin1A receptors: respective distribution of encoding mRNA, receptor protein and binding sites by in situ hybridization histochemistry, radioimmunohistochemistry and autoradiographic mapping in the rat brain. Neurochem Int. 1991;19:453–465. [Google Scholar]
- 27.Muth TR, Ahn J, Caplan M. Identification of sorting determinants in the C-terminal cytoplasmic tails of the gamma-aminobutyric acid transporters GAT-2 and GAT-3. J Biol Chem. 1998;273:25616–25627. doi: 10.1074/jbc.273.40.25616. [DOI] [PubMed] [Google Scholar]
- 28.Neumaier JF, Szot P, Peskind ER, Dorsa DM, Hamblin MW. Serotonergic lesioning differentially affects presynaptic and postsynaptic 5-HT1B receptor mRNA levels in rat brain. Brain Res. 1996;722:50–58. doi: 10.1016/0006-8993(96)00178-3. [DOI] [PubMed] [Google Scholar]
- 29.Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perego C, Bulbarelli A, Longhi R, Caimi M, Villa A, Caplan MJ, Pietrini G. Sorting of two polytopic proteins, the γ-aminobutyric acid and betaine transporters, in polarized epithelial cells. J Biol Chem. 1997;272:6584–6592. doi: 10.1074/jbc.272.10.6584. [DOI] [PubMed] [Google Scholar]
- 31.Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poyatos I, Ruberti F, Martinez-Maza R, Gimenez C, Dotti CG, Zafra F. Polarized distribution of glycine transporter isoforms in epithelial and neuronal cells. Mol Cell Neurosci. 2000;15:99–111. doi: 10.1006/mcne.1999.0807. [DOI] [PubMed] [Google Scholar]
- 33.Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, El Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- 34.Sari Y, Miquel MC, Brisorgueil MJ, Ruiz G, Doucet E, Hamon M, Vergé D. Cellular and subcellular localization of 5-hydroxytryptamine1B receptors in the rat central nervous system: immunocytochemical, autoradiographic and lesion studies. Neuroscience. 1999;88:899–915. doi: 10.1016/s0306-4522(98)00256-5. [DOI] [PubMed] [Google Scholar]
- 35.Stowell JN, Craig AM. Axon/dendrite targeting of metabotropic glutamate receptors by their cytoplasmic carboxy-terminal domains. Neuron. 1999;22:525–536. doi: 10.1016/s0896-6273(00)80707-2. [DOI] [PubMed] [Google Scholar]