Abstract
Synaptic circuitry in the rat lateral amygdala (AL) was studied in brain slices using electrophysiological recordings. Electrical stimulation of external and internal capsules evoked an EPSC followed by a sequence of GABAA and GABABreceptor-mediated IPSC in principal neurons. Paired stimulation of either afferents resulted in a significant reduction (∼45%) of the second GABAA receptor-mediated IPSC. A priming stimulation, consisting of a priming pulse to one pathway followed by a pulse to the other pathway, resulted in a strong depression of the second IPSC basically identical to that during paired stimulation. Paired- and primed-pulse depressions were largely relieved by 10 μm CGP 55845A, indicating regulation through presynaptic GABAB receptors. Furthermore, putative interneurons responded with EPSCs of constant latencies to minimal stimulation of both cortical and thalamic fibers, indicating convergent monosynaptic input. At higher stimulation strength, an ∼15% reduction of EPSCs occurred in interneurons after paired and primed stimulation, which was not sensitive to CGP 55845A. These findings indicate that a rather homogeneous population of interneurons exists in the AL with respect to their afferent connectivity, in that they receive convergent input through putative thalamic and cortical fibers, both directly and indirectly (through principal neurons), and mediate inhibitory control of postsynaptic principal neurons. This symmetrically built GABAergic circuitry can be of functional significance, given the distinctive role of the two afferent input systems for the mediation of different components of fear responses and the importance of GABAergic mechanisms for limitation of excessive neuronal activity.
Keywords: lateral amygdala, converging afferent input, inhibition, interneuron, GABAA, GABAB
The amygdaloid complex is known to be important for regulation of emotional behavior and learning (LeDoux, 2000) and to be critically involved in neurological disorders such as temporal lobe epilepsy (Gloor, 1992). A part of this group of nuclei, the lateral amygdala (AL), receives the main sensory input from cortical and subcortical fields. The information is processed through intra-amygdaloid connections and transferred toward the central amygdala, the major output station (Pitkänen et al., 1998). Cortical afferents reach the AL laterally from the external capsule. The other major sensory input reaches the AL medially from the internal capsule as subcortical thalamic afferents (LeDoux et al., 1991;Romanski and LeDoux, 1993). In the AL, as in other brain regions, principal cells and local interneurons can be classified on their electrophysiological, neurochemical, and morphological properties (Rainnie et al., 1991b; Lang and Paré, 1998; Mahanty and Sah, 1998). In vivo data demonstrated a powerful control through GABAergic inhibition over the activity of projecting principal cells (Lang and Paré, 1997, 1998), which renders a special role to the GABAergic interneurons in control of excitation in this region. Indeed, GABAergic interneurons are thought to play a crucial role in information processing in the amygdala (Lang and Paré, 1998;Mahanty and Sah, 1999) and are also thought to underlie the regulation of epileptiform activity (Callahan et al., 1991; Gloor, 1992; Washburn and Moises, 1992a). It has been suggested that these interneurons receive excitatory afferent input form cortical (Lang and Paré, 1998) as well as thalamic areas (Li et al., 1996a). It is not known, however, if these major afferents innervate different populations of interneurons or if these neurons receive converging inputs as do principal cells (Li et al., 1996b). This convergence allows the same principal cells in the amygdala to integrate information from sensory channels with different processing capacities.
Therefore, we designed electrophysiological experiments aimed at the afferent input architecture to GABAergic interneurons in the AL. One key element of our experimental paradigm was paired-pulse depression, a mechanism of presumed presynaptic origin that limits transmitter release if synaptic activation occurs within several hundred milliseconds after preceding activation (Thompson et al.,1993). During GABAergic transmission, GABA acts presynaptically to regulate further release by binding and activating GABAB receptors (Misgeld et al.,1995). In the amygdala, paired-pulse depression of NMDA receptor-mediated responses was shown to be influenced by presynaptic GABAB receptors (Huang and Gean, 1994). In our experiments, we have recorded synaptically evoked GABAA receptor-mediated IPSPs and IPSCs in putative principal neurons and studied the nature of paired-pulse depression of these responses using different activation protocols of cortical and thalamic afferent fibers. In addition, synaptic responses were recorded from interneurons that were identified through morphological and electrophysiological criteria.
MATERIALS AND METHODS
Sharp microelectrode recordings. Coronal slices of 500 μm thickness containing the amygdala and related brain areas were prepared from deeply anesthetized Long–Evans rats (halothane anesthesia; Zeneca, Plankstadt, Germany) of either sex (postnatal day 30–35), as described recently (Heinbockel and Pape, 2000). Slices were kept at 35°C in an interface type chamber during continuous superfusion with a solution containing (in mm): NaCl 126, KCl 2.5, MgSO4 2, NaHCO326, NaH2PO4 1.25, dextrose 10, and CaCl2 2, buffered to pH 7.4 with 95% O2 and 5% CO2. Intracellular recordings were performed with glass microelectrodes (TW-100F; World Precision Instruments, Sarasota, FL) and controlled with a bridge amplifier (Axon Instruments, Foster City, CA). Electrode DC resistances ranged between 60 and 80 MΩ (filled with 4m K-acetate). All membrane potential measurements were corrected for electrode offsets (typically <5 mV). Epi-illumination of the slices allowed location of the AL and other relevant brain regions. Principal neurons were identified based on electrophysiological criteria, namely the generation of slow oscillations of the membrane potential (Paré et al., 1995; Pape et al.,1998) and spike frequency adaptation (Rainnie et al.,1991a,b; Washburn and Moises, 1992b). Neurons were considered for analysis that had a stable resting membrane potential less than −60 mV, input resistances >45 MΩ at resting membrane potential (as determined from responses to hyperpolarizing current pulses, −0.1 to −0.3 nA), and overshooting action potentials. Data were stored on videotape for off-line analysis after conversion with Neurocorder DR-384 unit (Neurodata, New York, NY) and later digitized using a CED 1401 (Cambridge Electronic Design, Cambridge, UK) interface and Spike2 software.
Synaptic responses were evoked with two bipolar tungsten electrodes placed in the external capsule and in the internal capsule dorsal to the central nucleus of the amygdala for electrical stimulation (100 μsec pulse duration) of putative cortical and thalamic afferents, respectively (Mahanty and Sah, 1999; Weisskopf and LeDoux, 1999;Heinbockel and Pape, 2000). However, it should be noted that these electrical stimuli likely activated other fiber systems also. For instance, AL axons to the perirhinal cortex course through the external capsule. Stimulus intensity was adjusted to produce a synaptic response 30–50% of maximum amplitude without triggering action potentials.
Whole-cell patch-clamp recordings. Slices containing the amygdala from Long–Evans rats of either sex (postnatal day 11–16) were prepared, and recordings were performed as previously described (Meis and Pape, 1998). Briefly, rats were anesthetized with halothane and killed by decapitation. After preparation and equilibration of 300-μm-thick coronal slices, cells were obtained under visual guidance (Axioskop FS, Achroplan 40/w; Zeiss, Oberkochen, Germany) using infrared videomicroscopy (b/w camera, model C-2400; Hamamatsu, Hersching, Germany). Experiments were run at room temperature in a submerged type chamber. Extracellular solution contained the following concentration of chemicals (in mm): NaCl 125, KCl 2.5, NaH2PO4 1.25, NaHCO3 22, MgSO4 2, CaCl2 2, and dextrose 20. Acidity was adjusted with 95% O2 and 5% CO2. Electrophysiological recordings were performed using the patch-clamp technique in whole-cell mode. Patch pipettes were pulled from borosilicate glass (GC150TF-10; Clark Electromedical Instruments, Pangbourne, UK). The first set of experiments was performed with a potassium-based internal solution containing the following chemicals (in mm): K-gluconate 95, K3-citrate 20, NaCl 10, HEPES 10, EGTA 5, MgCl2 1, MgATP 3, NaGTP 0.5, and Br-QX-314 5, and pH was adjusted with 1 mKOH to 7.25. To improve space clamp and to further reduce GABAB receptor-coupled postsynaptic K+ channels, the next set of experiments was performed using an internal solution with the following concentration of chemicals (in mm): Cs-gluconate 95, Cs3-citrate 20, NaCl 10, HEPES 10, EGTA 5, MgCl2 1, MgATP 3, and NaGTP 0.5, and pH was adjusted with 1 m CsOH to 7.25. Importantly, the membrane potential was held near the reversal potential of the excitatory synaptic currents at +7 or 0 mV, depending on the internal solution (K+-based or Cs+-based pipette solution, respectively) to minimize the contribution of EPSCs to the recorded IPSCs. To stimulate afferent fibers, bipolar tungsten stimulation electrodes were placed in the external capsule and in the internal capsule dorsal to the central nucleus of the amygdala similarly as described before (Mahanty and Sah, 1999; Weisskopf and LeDoux, 1999; Heinbockel and Pape, 2000). Thirty to fifty percent of maximum responses were evoked (100 μsec pulse duration, 0.1–3 mA) to record synaptically evoked IPSCs with an Axopatch 200B amplifier (Axon Instruments). Access resistance was controlled and ranged between 5–10 MΩ. For stimulation, membrane potential control, and data acquisition pClamp 8.0 software was used (Axon Instruments). Data were low-pass filtered at 2 kHz with the integrated Bessel filter of the amplifier and digitized by a Digidata 1200 unit (Axon Instruments) at 10 kHz. Additionally, data were stored on videotape after conversion with a Neurocorder DR-890 unit (Neurodata) for off-line analysis.
Additional experiments were performed to record from interneurons in the AL using the K-gluconate-based solution described above without QX-314. Interneurons were identified according to their electrophysiological properties (Washburn and Moises, 1992b, Rainnie et al., 1993; Mahanty and Sah, 1998). After obtaining the cells in voltage-clamp mode, current-clamp mode was chosen to characterize firing properties of the interneurons. In cells that showed high-frequency firing of fast action potentials after injection of +0.1 nA current with distinct fast afterhyperpolarization after each spike and no apparent spike frequency adaptation, synaptic currents were evoked at a membrane potential of −72 mV in voltage-clamp mode. Liquid junction potentials in all voltage-clamp experiments were corrected (Neher, 1992).
Histological procedures. Biocytin labeling and light-microscopic morphological analysis were performed as described previously (Meis and Pape, 1998). Briefly, in some experiments 0.1% biocytin was added to the intracellular solution. After recording, slices were immersed in 4% paraformaldehyde in PBS. After cryoprotection with a 30% sucrose solution in PBS, slices were resectioned at 100 μm using a freeze microtome (Leica, Benzheim, Germany). Sections were treated with avidin–biotin complex horseradish peroxidase (PK 4000, 1:100; Vector Laboratories, Burlingame, CA), then treated with (NH4)2Ni(SO4)2. Finally sections were dehydrated and coverslipped.
Drug application. The following drugs were applied during the experiments: (−)-bicuculline methiodide or picrotoxin (Sigma, St. Louis, MO) were dissolved directly in the external and QX-314 (bromide salt; Sigma) in the internal K-gluconate-based solution. CGP 55845 A was diluted directly in external solution and was provided kindly by Novartis.
Statistical analysis. For statistical analysis, the two-tailed Mann–Whitney U test was applied. Populations were regarded as significantly different if p < 0.0125. Data are expressed as mean ± SEM.
RESULTS
Synaptic responses of principal cells to paired and primed stimulation of cortical and thalamic afferents
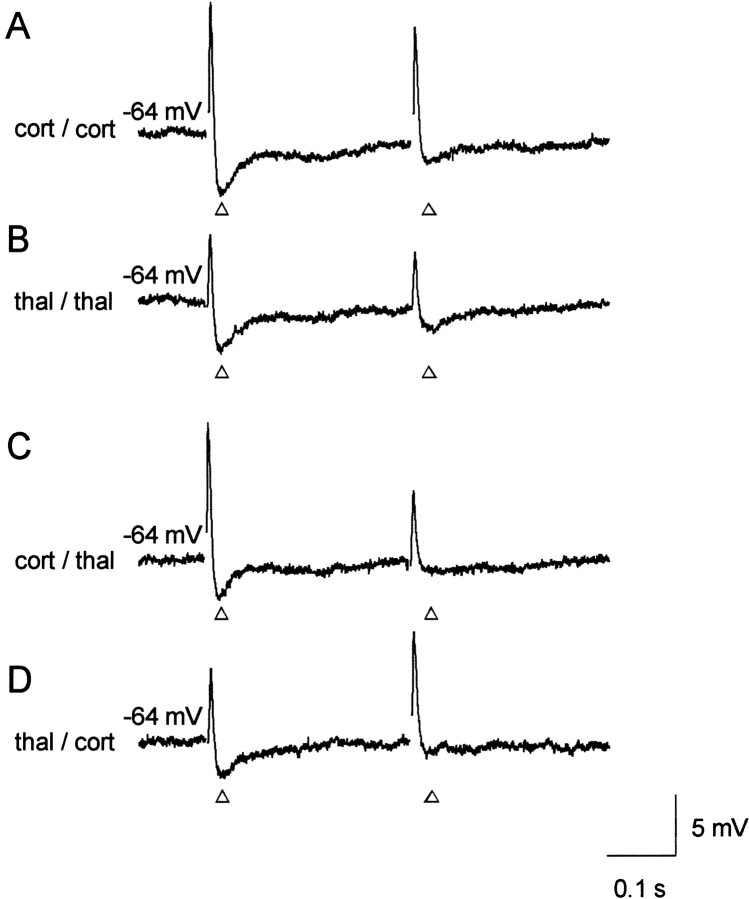
Electrical stimulation of putative cortical or thalamic afferent inputs evoked a typical triphasic synaptic response in AL principal neurons recorded at resting potential (−64.8 ± 0.37 mV;n = 5). The synaptic response consisted of an excitatory potential followed by a GABAA and GABAB receptor-mediated inhibitory potential, as described earlier (Mahanty and Sah, 1999; Weisskopf and LeDoux, 1999;Heinbockel and Pape, 2000), and as assessed in the present study using sharp electrode recordings at 35°C (Fig.1). Paired stimulation of cortical input pathways at an interstimulus interval of 0.3 sec resulted in substantially reduced inhibitory synaptic responses to the second pulse (paired-pulse depression, Fig. 1A). A very similar depression of inhibitory synaptic responses was observed after paired stimulation of thalamic input fibers in the same neuron (Fig.1B). In a next experimental step, a priming stimulation protocol was applied in the same cells, consisting of a pulse to one afferent pathway followed by a pulse to the other pathway (primed-pulse stimulation). Stimulation strengths and the interstimulus interval were maintained constant as before during the paired-pulse stimulations. Furthermore, before executing the paired- and primed-pulse stimulation paradigms, the stimulus strengths were appropriately adjusted to yield similar amplitudes of fast inhibitory potentials in response to cortical and thalamic stimulation. In cases of paired-pulse stimulations, the magnitude of depression was assessed by dividing the amplitude of the second response by the amplitude of the first response. The amount of depression in cases involving the primed-pulse depression paradigms was calculated by dividing the amplitude of the second inhibitory response by the amplitude of the first inhibitory response obtained from the corresponding paired-pulse stimulation sequence. Cortical followed by thalamic stimulation resulted in a depression of synaptic responses to the second pulse (primed-pulse depression), very similar to that observed during paired stimulation of the thalamic pathway (Fig. 1C). Vice versa, thalamic followed by cortical stimulation resulted in a primed-pulse depression, the magnitude of which was similar to that during paired stimulation of the cortical pathway (Fig. 1D). The paired- and primed-pulse depressions were observed in all tested principal neurons (n = 5). Excitatory responses also tended to show paired- and primed-pulse depressions, but at a much smaller extent compared with that of inhibitory responses (Fig. 1).
Fig. 1.
Depression of IPSPs after paired stimulation of cortical and thalamic afferents. Cortical (A, cort/cort) or thalamic (B, thal/thal) afferents were stimulated in pairs to evoke postsynaptic responses (paired-pulse stimulations). Using the same stimulation strengths, cortical or thalamic priming of afferents was followed by the activation of thalamic (C, cort/thal) or cortical (D, thal/cort) pathways, respectively (primed-pulse stimulations). Stimulus artifacts were removed for clarity, and open arrowheads indicate the investigated IPSPs. Note the depression of fast IPSPs after paired and primed stimulation. All recordings were obtained at 35°C with a sharp microelectrode from the same putative principal neuron in the AL; an average of three consecutive traces of pairs is presented in each case.
Time course of paired-pulse depressions of GABAAreceptor-mediated components
Because our interest was on GABAergic circuitries, paired and primed depression of GABAergic components were studied in more detail using the whole-cell patch-clamp technique under voltage-clamp conditions. The contribution of excitatory postsynaptic effects was minimized by holding the membrane potential close to the reversal potential of excitatory amino acid receptor-mediated responses (see Materials and Methods). To more quantitatively analyze the time course, the magnitude and the pharmacological properties of the paired and primed depression, the fast GABAAreceptor-mediated component was isolated by addition of QX-314 (5 mm) to the K-gluconate based internal solution, which blocks G-protein-dependent GABABreceptor-mediated K-channel activity (Nathan et al., 1990). Indeed, bath application of picrotoxin (100 μm;n = 5) or bicuculline (10 μm;n = 4) under these conditions completely blocked the IPSCs, indicating meditation via GABAA receptors (data not shown). Addition of QX-314 by itself had no measurable effect on the GABAA-mediated IPSCs.
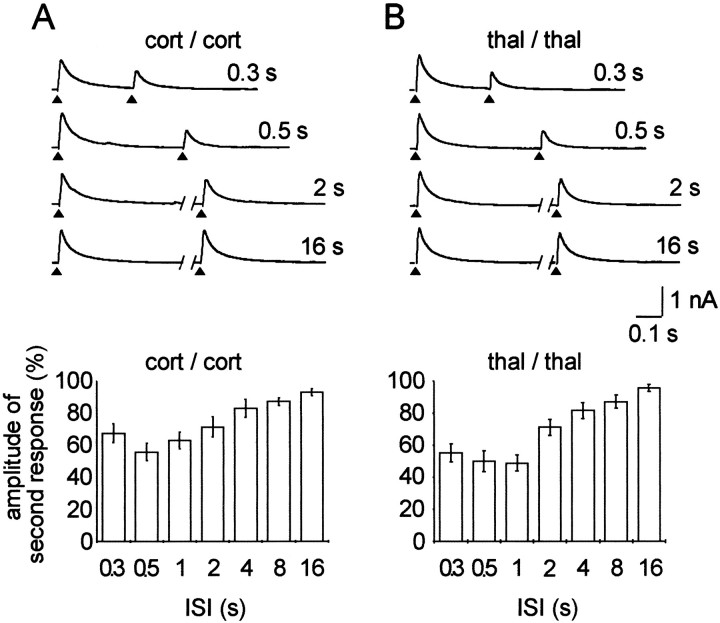
The time course of paired-pulse depression of the GABAA IPSCs was studied by delivering two stimuli of constant intensity to either thalamic or cortical input fibers and varying the interstimulus interval in a range between 16 and 0.3 sec. Shorter intervals turned out to be not feasible, because of an overlap between the synaptic currents evoked by the first and second pulses. Representative traces of responses to paired stimulation of cortical and thalamic fibers, although for clarity not all of the tested intervals, are shown in Figure 2,A and B, top panel. On thebottom panel, pooled data with all tested intervals are presented from the seven cells recorded. Cortical and thalamic paired-pulse depressions were always tested in each cell. Considering both experimental approaches, the greatest depression occurred at an interstimulus interval of 0.5 sec. The amplitude of the IPSC evoked by the second pulse was 55.6 ± 5.3% and 50 ± 6.3% compared with responses to the first pulse for cortical and thalamic activation, respectively. It should be noted that the depression at thalamic afferents at 1 sec (48.8 ± 4.9%) was not significantly different compared with that at 0.5 sec.
Fig. 2.
Time course of paired-pulse depressions of fast IPSCs. A, Top panel shows pairs of IPSCs evoked by two identical stimuli delivered at different intervals [interstimulus interval (ISI)] between 0.3 and 16 sec to the cortical afferents (cort/cort). Bottom panel shows the average response to the second stimulus calculated from seven cells. B, Top panel shows pairs of IPSCs evoked by two identical stimuli delivered between 0.3 and 16 sec to the thalamic afferents (thal/thal). Bottom panel shows the average response to the second stimulus calculated from the same cells as in A(n = 7). Please note that for clarity not all intervals are presented on the top panels. Stimulus artifacts were omitted for clarity, and arrowheads show the point of stimulation.
Magnitude and pharmacological properties of paired- and primed-pulse depressions
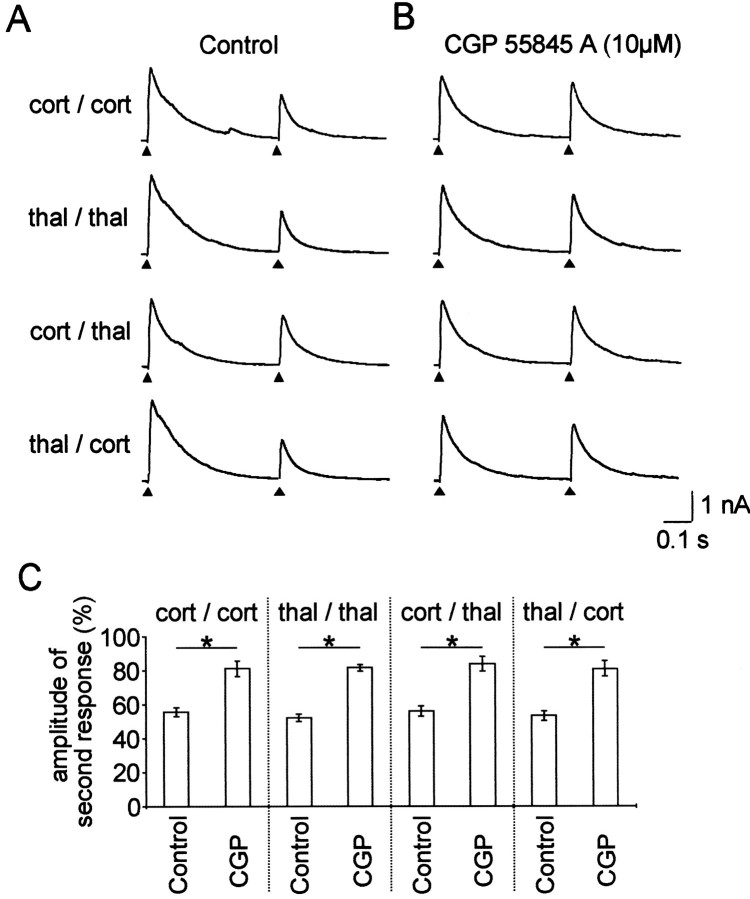
During the next experimental steps, the interstimulus interval was kept constant at 0.5 sec to obtain maximal depressant effects, and paired and primed stimulation paradigms were systematically tested in the same cells (n = 12). In addition, to improve space clamp and to further on block postsynaptic GABABreceptor-mediated outward K+ conductances, Cs-gluconate-based internal solution was used (see Materials and Methods). Representative traces and averaged data are shown in Figure3, A and C(Control), respectively. There was no significant difference in the magnitude of the depression (n = 12) that was obtained with paired stimulation of either cortical (56.6 ± 2.6%) or thalamic afferent inputs (52.1 ± 2.1%), or with primed stimulation of cortical followed by thalamic (56.1 ± 3%) or thalamic followed by cortical input stimulation (53.3 ± 2.9%). Furthermore, we examined in nine of these cells, whether presynaptic GABAB receptors were involved in paired- and primed-pulse depression by bath applying a specific GABAB receptor antagonist, CGP 55845 A (Fig.3B). The appropriate pooled experimental values were calculated from the experiments in which 10 μmCGP 55845 A was subsequently applied. These data show that the depression was significantly and similarly reduced in all experimental paradigms that were tested (Fig. 3C). The concentration of CGP 55845 A was chosen to yield maximal effects on the depression, and experimental values were collected from periods in which a steady-state level of the drug effect had been reached. During action of CGP 55845 A, depressions reached 81.3 ± 4.5% and 81.7 ± 1.9% during paired cortical and thalamic stimulation, respectively. Cortical priming followed by a thalamic stimulus and vice versa resulted in a depression to 83.9 ± 4.2% and 81.1 ± 4.6%, respectively. These values are not significantly different from each other, but significantly different from values of control experiments (p < 0.0125).
Fig. 3.
Effect of a specific GABAB receptor antagonist on paired- and primed-pulse depressions. A, Pairs of IPSCs were evoked under control conditions with stimuli delivered at an interval of 0.5 sec either twice to the cortical (cort/cort) or to the thalamic (thal/thal) afferents (paired stimulations). Afterward, using the same stimulation strengths, stimulation involved cortical followed by thalamic (cort/thal) or thalamic followed by cortical (thal/cort) afferents (primed stimulations). B, The same stimulation sequences were performed under CGP 55845 A (10 μm) treatment. Stimulus artifacts were omitted for clarity, andarrowheads show the point of stimulation.C, Averaged IPSCs in response to the second stimulus using the above described stimulation sequences either under control conditions (n = 12) or under a subsequent CGP 55845 A treatment (n = 9). Stimulus artifacts were omitted for clarity, and arrowheads show the point of stimulation. Asterisk indicates a significant difference (p < 0.0125).
Synaptic responses of identified interneurons in the AL
Firing properties of recorded neurons were tested under current-clamp conditions after injection of positive current pulses lasting for several hundred milliseconds. Neurons were considered for further analysis if they showed maintained action potential firing with little or no spike frequency accommodation during +0.1 nA depolarizing current injection from resting potential (Fig.4Aa,Ba; cf. Mahanty and Sah, 1998). Input resistance as measured from the steady-state voltage response during a −0.05 nA current injection and action potential duration at half-maximal amplitude (AP50) were 638 ± 73 MΩ and 1.22 ± 0.12 msec, respectively (n = 11). It is important to note that under the present recording conditions, the AP50 value in putative interneurons was significantly smaller than that of putative principal neurons (AP50: 1.71 ± 0.08 msec,p < 0.0125; n = 38). The increased spike duration with respect to the published results from interneurons in the basolateral complex (Mahanty and Sah, 1998) is most likely attributable to differences in recording temperatures (room temperature vs 28–30°C; cf. Volgushev et al., 2000). In any case, all 11 cells considered to be interneurons based on electrophysiological criteria, possessed aspiny dendrites emerging from rather heterogeneously shaped cell bodies, as assessed after biocytin injection, thereby corroborating the view that they represent GABAergic interneurons (Fig.4Ac,Bc; cf. McDonald 1982). On the contrary, neurons displaying typical electrophysiological properties of principal cells had spine-rich dendrites, as exemplified in Figure 4C. Six of the interneurons were tested for connectivity with thalamic and cortical input fibers. All cells responded with fast EPSCs of constant latencies to minimal (high failure rate) and low-strength stimulation of both cortical and thalamic input fibers, indicating monosynaptic convergent input. Two examples are illustrated in Figure 4,Ab and Bb.
Fig. 4.
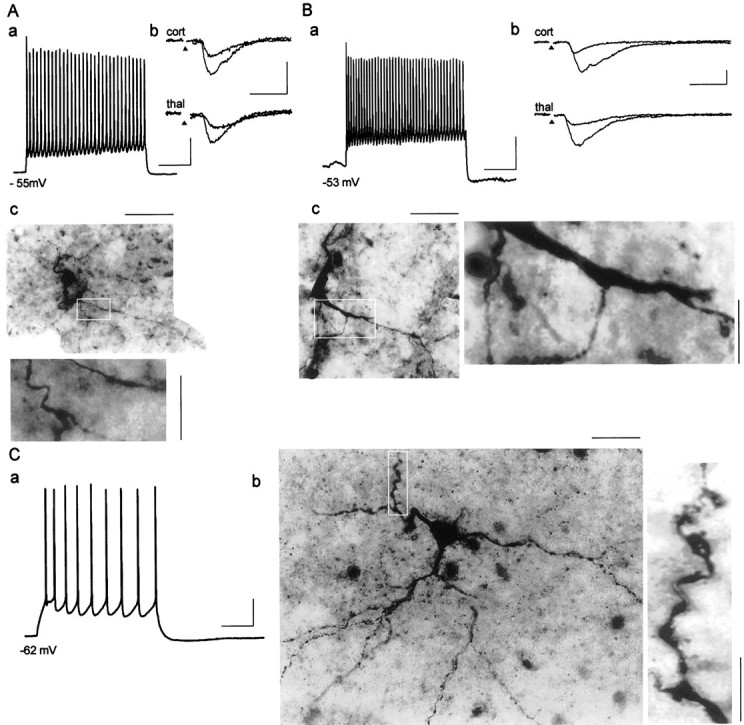
Synaptically evoked EPSCs recorded from neurons identified electrophysiologically and morphologically as interneurons.Aa,Ba, Depolarizing current injection (+0.1 nA) and resulting action potential firing at resting membrane potential recorded from two representative interneurons in current-clamp mode. Ab,Bb, Individual EPSCs after stimulation of cortical (cort) and thalamic (thal) afferents at −72 mV holding potential using two different stimulation strengths for each pathway in voltage-clamp mode. Stimulus artifacts were omitted for clarity, andarrowheads indicate the point of stimulation.Ac,Bc, Intracellular biocytin staining of these neurons revealed nonspiny dendrites of putative interneurons in the AL; regions within a white frame are shown at a higher magnification. Ca, Train of action potentials (+0.1 nA current injection) displaying spike frequency adaptation typical of a principal neuron in the AL. Cb, Intracellular staining revealed spiny dendrites of a pyramidal-like neuron (region within white frame is shown at a higher magnification). Scaling for Aa, Bb, andCa (current-clamp mode) and for Ab andBb (voltage-clamp mode) is 200 and 10 msec for horizontal and 20 mV and 50 pA for vertical bars, respectively. Scaling for Ac, Bc, and Cb (biocytin-filled cells) is 30 μm for horizontal bars (corresponding magnified regions: 10 μm, vertical bars).
Paired- and primed-pulse depressions of EPSCs in interneurons
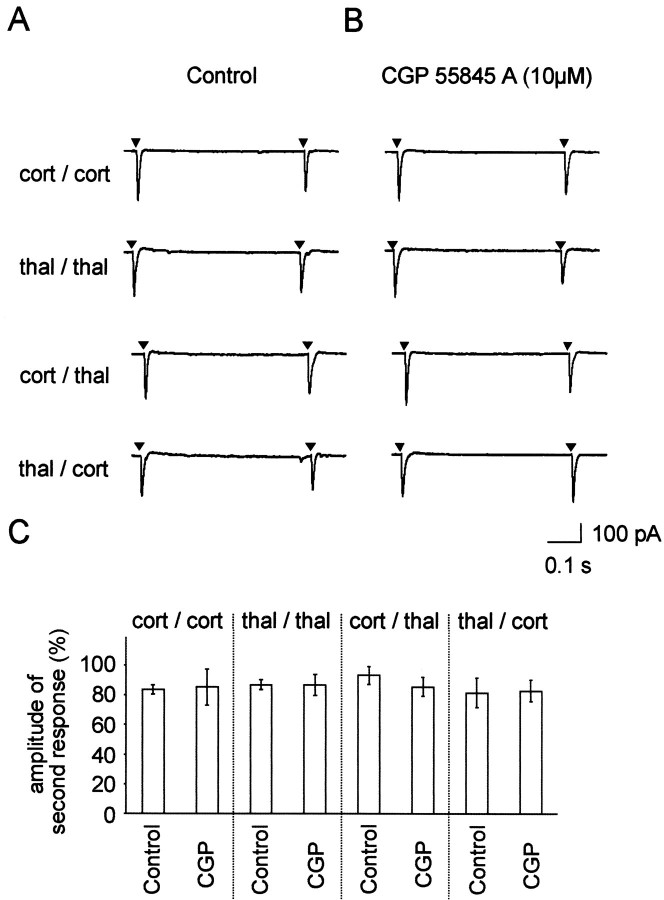
Paired- and primed-pulse depressions of evoked EPSCs were tested in 5 of the 11 interneurons, similarly as described before for IPSC recordings in principal cells. The interstimulus interval was kept at 0.5 sec, and K-gluconate based internal solution was used at a holding potential of −72 mV. Depression of the second EPSC was observed in all experimental paradigms (Fig.5A). Averaged amplitudes of the second response were 83.6 ± 2.9% and 86.9 ± 3.5% after paired stimulation of cortical and thalamic afferents, respectively, and 93.2 ± 6% and 81.5 ± 9.9% after primed cortical/thalamic and thalamic/cortical stimulation, respectively (Fig.5C). Overall, the depression of EPSCs in interneurons was small compared with the depression of IPSCs in principal cells. Furthermore, the depression of EPSCs in interneurons was not affected in any of the experimental paradigms tested during presence of 10 μm CGP 55845 A (Fig. 5B). During action of CGP 55845 A, the depressions were 85.2 ± 12.2% and 86.7 ± 6.9% during paired cortical and thalamic stimulation, respectively (Fig. 5C). Cortical priming followed by a thalamic stimulus and vice versa yielded 85.5 ± 6.4% and 82.8 ± 7.2%, respectively. These values are not significantly different from each other or from values of control experiments (Fig.5C).
Fig. 5.
Paired- and primed-pulse depressions of EPSCs in identified interneurons. A, Pairs of EPSCs were evoked under control conditions with stimuli delivered at an interval of 0.5 sec either twice to the cortical (cort/cort) or to the thalamic (thal/thal) afferents (paired stimulations). Afterward, using the same stimulation strengths stimulation involved cortical followed by thalamic (cort/thal) or thalamic followed by cortical (thal/cort) afferents (primed stimulations).B, The same stimulation sequences were performed under CGP 55845 A (10 μm) treatment. Stimulus artifacts were omitted for clarity, and arrowheads show the point of stimulation. C, Averaged EPSCs in response to the second stimulus using the above described stimulation sequences either under control conditions (n = 5) or under a subsequent CGP 55845 A treatment (n = 5).
DISCUSSION
Our results show that (1) stimulation of putative cortical and thalamic afferents to the AL using every stimulation sequence of paired- and primed-pulses results in an identical and largely GABAB receptor-dependent depression of GABAA receptor-mediated responses in principal neurons, (2) AL interneurons generate monosynaptic EPSCs after minimal stimulation of both afferents, and (3) at higher stimulation strengths, an ∼15% GABAB receptor-independent reduction of EPSCs occurs in interneurons after paired- and primed-pulse stimulation. In the following, the possibility is discussed that a rather homogeneous population of interneurons exists in the AL with respect to their afferent connectivity, in that they receive excitatory convergent input through thalamic and cortical fibers, both directly and indirectly (through principal neurons), and mediate inhibitory control of postsynaptic principal neurons.
Paired and primed depression as a tool for analyzing synaptic circuitries
To study the nature of intra-amygdaloid GABAergic circuitry, we used paired and primed activation of cortical and thalamic afferents and analyzed the GABAA receptor-mediated responses in principal neurons. The inhibitory component was isolated with masking excitatory ones by holding the membrane potential close to the reversal potential of excitatory amino acid receptor-mediated responses. Including QX-314 in the pipette solution (Nathan et al., 1990) or using Cs+ as the main positive charge carrier successfully minimized the postsynaptic GABAB receptor-mediated outward currents that could have otherwise interfered with our measurements. Application of GABAA receptor antagonists bicuculline or picrotoxin completely inhibited the postsynaptic currents, indicating that they originated from GABAA receptor activation.
Separate activation of putative cortical or thalamic afferents with paired pulses led to depressions of the second evoked IPSCs or IPSPs. The depression is thought to be modulated largely by presynaptic G-protein-coupled GABAB receptors (Misgeld et al., 1995). Application of a specific GABAB receptor antagonist CGP 55845 A and the subsequent relief of depressions confirmed GABABreceptor mediation.
Although paired-pulse depression is a widespread phenomenon in the CNS (Ziakopoulos et al., 2000), we used the paired-pulse paradigm to ask a specific question about intra-amygdaloid circuitry. Do thalamic and cortical afferents project to a common population of inhibitory interneurons in the AL, i.e., do these interneurons receive convergent excitatory input seen as GABAA IPSCs in AL principal cells? To answer this question we used an approach consisting of “classical” paired-pulse stimulation of each pathways and of primed-pulse paradigm involving the stimulation of one afferent pathways followed by the stimulation of the other. Given that paired-pulse depressions occur in both inputs as described above, we hypothesized that the primed-pulse depressions should result in the same depressions as seen with the paired-pulse depressions if one population of interneurons is activated. Furthermore, the magnitude of paired-pulse depressions of the two separate afferents should not be different from each other. Our experimental results validate our hypothesis. If, by comparison, a different population of interneurons receiving separate cortical and thalamic input existed or there were two populations of interneurons receiving convergent and nonconvergent input, then different experimental results with respect to the extent of the depressions should be obtained. In the first case one cannot expect the presence of primed-pulse depressions and in the second, the extent of primed-pulse depressions should deviate from that of paired-pulse depressions. Thus, our results show that under different stimulation conditions the same population of GABAergic synapses is engaged in inhibitory synaptic transmission. As a further evidence, the application of the specific GABAB receptor antagonist CGP 55845 A also had an identical relieving effect on the depressions in all cases. Thus, these results indirectly support the scenario that a population of interneurons exists in the AL, which receives convergent excitatory input from the sensory afferents, thereby being homogeneous with respect to the major afferents. Whether this convergence is attributable to a direct (feedforward) or indirect (feedback) mechanism, or both, will be discussed below.
Afferent synaptic connectivity of interneurons
To obtain direct evidence supporting the above described scenario, recordings from inhibitory interneurons had to be performed. The majority of neurons in the AL are spiny, often pyramidal-shaped neurons believed to be principal cells (McDonald, 1982). Their response behavior is characterized by accommodating action potentials to depolarizing current pulses followed by a large slow afterhyperpolarization (Sugita et al., 1993; Washburn and Moises, 1992b; Lang and Paré, 1998) and the generation of slow rhythmic membrane potential oscillations (Paré et al.,1995; Pape et al., 1998). The other neuronal class contains aspiny or sparsely spiny neurons, putative GABAergic interneurons (McDonald, 1982). It is accepted that these neurons in the amygdala are local-circuit interneurons (Rainnie et al., 1991b). These cells respond with regular sustained firing to depolarizing current pulses, and they possess high resting input resistance and generate fast spikes compared with those of principal cells (Washburn and Moises, 1992b; Sugita et al., 1993; Lang and Paré, 1998; Mahanty and Sah, 1998). These features allowed us to identify neurons in the AL on the basis of their morphological and electrophysiological characteristics. Furthermore, we should point out, that the recorded interneurons in our study did not belong to the intercalated cell masses that are to be found as islands of cells in the amygdala (McDonald, 1982; Royer et al., 1999) and that could be readily identified in our fresh preparations.
Stimulation of either afferents evoked postsynaptic excitatory currents in all studied interneurons. We did not find any cell that responded to only one pathway, indicating with a reasonable high probability that a population of interneurons exists in the AL that receives convergent excitatory input from the two main sensory afferents and thereby proves to be homogeneous with respect to their afferent connectivity to the tested major afferents. The constant latencies of the EPSCs using minimal stimulation range provide a strong evidence that these interneurons can be monosynaptically activated through these pathways in a feedforward manner. In support of this notion is the finding that cortical axons, although rarely, establish asymmetric synaptic contacts with GABAergic interneurons in the basolateral amygdaloid complex (Smith et al., 2000). Furthermore, in vivo data suggest the presence of feedforward inhibition in the thalamo-amygdala pathway within the AL (Li et al., 1996a). However, the testing of paired- and primed-pulse depressions in interneurons at higher stimulus intensities strongly indicated that they also receive indirect (feedback) excitation from principal cells. The rationale is as follows: the results show an ∼15% GABABreceptor-insensitive reduction of fast EPSCs in interneurons after paired and primed stimulation, and thus most likely not mediated through presynaptic GABAB receptors. The existence of primed-pulse depressions of EPSCs, in particular, points to an indirect effect mediated through axon collaterals of presynaptic principal cells receiving convergent thalamic and cortical input. In support of this are anatomical findings in the basolateral complex demonstrating that a majority of thalamic and cortical terminals contact dendritic spines (Carlsen and Heimer, 1988; LeDoux et al., 1991; Smith et al., 2000) and that intrinsic axon collaterals of principal cells contribute a significant portion of synaptic contacts onto interneurons (Smith et al., 2000). These indirect (feedback) effects, however, cannot fully explain alone the paired and primed depression of IPSCs in principal cells, mainly because the latter are substantially larger in amplitude and mediated via GABAB receptors. Therefore it appears that a population of GABAergic interneurons exists in the AL, which receives convergent input from thalamic and cortical fibers through principal neurons both directly and indirectly (in a feedforward and feedback manner, respectively). The lack of CGP 55845 A to fully antagonize the paired- or primed-pulse depressions of IPSCs in principal cells may well be explained by the remaining indirect effects on the excitatory drive of interneurons. In more general terms, the present data indicate that irrespective of the route of excitation, the interneuronal population in the AL can be regarded as homogeneous with respect to their afferent connections considering the two major input pathways. This conclusion would be valid even if the external capsule stimulation had backfired some AL principal cells, thereby indirectly evoking responses in connected interneurons. It is important to note, however, that antidromic spike initiation was never observed in the present experiments. This can be attributable to the fact that pathways different from the output pathways of the principal cells were activated and/or stimulus intensities were used that were below the threshold for antidromic spike initiation.
In conclusion, this symmetrically built GABAergic circuitry can be assumed to be of functional significance, given the distinctive role of the two input systems for the mediation of different components of fear responses (LeDoux, 2000) and the importance of GABAergic mechanisms for limitation of excessive neuronal activity (Gloor, 1992). The exact conditions under which feedforward or feedback mechanisms are preferably activated remain to be evaluated under in vivoconditions. The predominant inhibitory control of principal cells by interneurons in the AL (Lang and Paré, 1997, 1998) and the spatially organized arrangement of intercalated GABAergic neurons (Royer et al., 1999, 2000) are suggestive of the idea of polarized inhibitory interactions mediated also by the GABAergic interneurons within nuclear boundaries of the amygdaloid complex.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 426, TP B3) and by the Kultusministerium des Landes Sachsen-Anhalt (FKZ 2278A/0085). Part of this work was done in partial fulfillment of a doctoral thesis (J.M.). We thank R. Ziegler and A. Reupsch for expert technical assistance.
Correspondence should be addressed to Hans-Christian Pape, Institute of Physiology, Medical School, Otto-von-Guericke University, Leipziger Strasse 44, D-39120 Magdeburg, Germany. E-mail:Hans-Christian.Pape@Medizin.Uni-Magdeburg.de.
Dr. Heinbockel's present address: Department of Anatomy and Neurobiology, University of Maryland School of Medicine, Baltimore, MD 21201.
REFERENCES
- 1.Callahan PM, Paris JM, Cunningham KA, Shinnick-Gallagher P. Decrease of GABA-immunoreactive neurons in the amygdala after electrical kindling in the rat. Brain Res. 1991;555:335–339. doi: 10.1016/0006-8993(91)90361-x. [DOI] [PubMed] [Google Scholar]
- 2.Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- 3.Gloor P. Role of the amygdala in temporal lobe epilepsy. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory and mental dysfunction. Wiley-Liss; New York: 1992. pp. 505–538. [Google Scholar]
- 4.Heinbockel T, Pape H-C. Input-specific long-term depression in the lateral amygdala evoked by theta frequency stimulation. J Neurosci. 2000;20:RC68. doi: 10.1523/JNEUROSCI.20-07-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CC, Gean PW. Paired-pulse depression of the N-methyl-D-aspartate receptor-mediated synaptic potentials in the amygdala. Br J Pharmacol. 1994;113:1029–1035. doi: 10.1111/j.1476-5381.1994.tb17096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang EJ, Paré D. Similar inhibitory processes dominate the responses of cat lateral amygdaloid projection neurons to their various afferents. J Neurophysiol. 1997;77:341–352. doi: 10.1152/jn.1997.77.1.341. [DOI] [PubMed] [Google Scholar]
- 7.Lang EJ, Paré D. Synaptic responsiveness of interneurons of the cat lateral amygdaloid nucleus. Neuroscience. 1998;83:877–889. doi: 10.1016/s0306-4522(97)00420-x. [DOI] [PubMed] [Google Scholar]
- 8.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 9.LeDoux JE, Farb CR, Milner TA. Ultrastructure and synaptic associations of auditory thalamo-amygdala projections in the rat. Exp Brain Res. 1991;85:577–586. doi: 10.1007/BF00231742. [DOI] [PubMed] [Google Scholar]
- 10.Li XF, Armony JL, LeDoux JE. GABAA and GABAB receptors differentially regulate synaptic transmission in the auditory thalamo-amygdala pathway: an in vivo microiontophoretic study and a model. Synapse. 1996a;24:115–124. doi: 10.1002/(SICI)1098-2396(199610)24:2<115::AID-SYN3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Li XF, Stutzmann GE, LeDoux JE. Convergent but temporally separated inputs to lateral amygdala neurons from the auditory thalamus and auditory cortex use different postsynaptic receptors: in vivo intracellular and extracellular recordings in fear conditioning pathways. Learn Mem. 1996b;3:229–242. doi: 10.1101/lm.3.2-3.229. [DOI] [PubMed] [Google Scholar]
- 12.Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- 13.Mahanty NK, Sah P. Excitatory synaptic inputs to pyramidal neurons of the lateral amygdala. Eur J Neurosci. 1999;11:1217–1222. doi: 10.1046/j.1460-9568.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 14.McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- 15.Meis S, Pape H-C. Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to nociceptin/orphanin FQ. J Neurosci. 1998;18:8133–8144. doi: 10.1523/JNEUROSCI.18-20-08133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- 17.Nathan T, Jensen MS, Lambert JD. The slow inhibitory postsynaptic potential in rat hippocampal CA1 neurones is blocked by intracellular injection of QX-314. Neurosci Lett. 1990;110:309–313. doi: 10.1016/0304-3940(90)90865-7. [DOI] [PubMed] [Google Scholar]
- 18.Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- 19.Pape H-C, Paré D, Driesang RB. Two types of intrinsic oscillations in neurons of the lateral and basolateral nuclei of the amygdala. J Neurophysiol. 1998;79:205–216. doi: 10.1152/jn.1998.79.1.205. [DOI] [PubMed] [Google Scholar]
- 20.Paré D, Pape HC, Dong J. Bursting and oscillating neurons of the cat basolateral amygdaloid complex in vivo: electrophysiological properties and morphological features. J Neurophysiol. 1995;74:1179–1191. doi: 10.1152/jn.1995.74.3.1179. [DOI] [PubMed] [Google Scholar]
- 21.Pitkänen A, Savander V, Le Doux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1998;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 22.Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Excitatory transmission in the basolateral amygdala. J Neurophysiol. 1991a;66:986–998. doi: 10.1152/jn.1991.66.3.986. [DOI] [PubMed] [Google Scholar]
- 23.Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J Neurophysiol. 1991b;66:999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- 24.Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol. 1993;69:1350–1362. doi: 10.1152/jn.1993.69.4.1350. [DOI] [PubMed] [Google Scholar]
- 25.Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- 26.Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royer S, Martina M, Paré D. Polarized synaptic interactions between intercalated neurons of the amygdala. J Neurophysiol. 2000;83:3509–3518. doi: 10.1152/jn.2000.83.6.3509. [DOI] [PubMed] [Google Scholar]
- 28.Smith Y, Pare JF, Paré D. Differential innervation of parvalbumin-immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. J Comp Neurol. 2000;416:496–508. [PubMed] [Google Scholar]
- 29.Sugita S, Tanaka E, North RA. Membrane properties and synaptic potentials of three types of neurone in rat lateral amygdala. J Physiol (Lond) 1993;460:705–718. doi: 10.1113/jphysiol.1993.sp019495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson SM, Capogna M, Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- 31.Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, Eysel UT. Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J Physiol (Lond) 2000;522:59–76. doi: 10.1111/j.1469-7793.2000.0059m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Washburn MS, Moises HC. Inhibitory responses of rat basolateral amygdaloid neurons recorded in vitro. Neuroscience. 1992a;50:811–830. doi: 10.1016/0306-4522(92)90206-h. [DOI] [PubMed] [Google Scholar]
- 33.Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992b;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisskopf MG, LeDoux JE. Distinct populations of NMDA receptors at subcortical and cortical inputs to principal cells of the lateral amygdala. J Neurophysiol. 1999;81:930–934. doi: 10.1152/jn.1999.81.2.930. [DOI] [PubMed] [Google Scholar]
- 35.Ziakopoulos Z, Brown MW, Bashir ZI. GABAB receptors mediate frequency-dependent depression of excitatory potentials in rat perirhinal cortex in vitro. Eur J Neurosci. 2000;12:803–809. doi: 10.1046/j.1460-9568.2000.00965.x. [DOI] [PubMed] [Google Scholar]