Abstract
Rats with radio-frequency or ibotenic acid lesions of the hippocampus and rats with radio-frequency lesions of the fornix were tested on the visual paired comparison task (VPC), a test of recognition memory. Memory was assessed at five different delay intervals ranging from 10 sec to 24 hr. All operated groups performed normally at the shorter delays (10 sec and 1 min). Across longer delays, the two groups with hippocampal damage were impaired. Rats with fornix lesions performed well on the VPC task but were impaired on a spatial task (spontaneous alternation). The results show that the hippocampus is essential for normal recognition memory. Moreover, fornix lesions need not mimic the effects of direct damage to hippocampal tissue. The findings are discussed in the context of the contribution of the hippocampus to recognition memory.
Keywords: hippocampus, rats, visual paired comparison, fornix, ibotenic acid lesions, radio-frequency lesions, spontaneous alternation
In mammals, the formation of declarative memory depends on a system of anatomically related structures in the medial temporal lobe (Squire and Zola, 1996a;Eichenbaum, 1997). The important structures include the hippocampal region (the CA fields, dentate gyrus, and subicular complex) and the adjacent entorhinal, perirhinal, and parahippocampal cortices (Zola-Morgan and Squire, 1993). The delayed nonmatching to sample (DNMS) task, a test of visual recognition memory, was instrumental in the successful development of an animal model of human memory impairment in the monkey (Mishkin, 1978; Squire and Zola-Morgan, 1983) as well as in the work that led to identification of the brain structures in the medial temporal lobe that are important for memory (Squire and Zola-Morgan, 1991; Mishkin and Murray, 1994).
Monkeys with large bilateral lesions of the medial temporal lobe, which approximated the damage sustained by the well studied amnesic patient H.M. (Scoville and Milner, 1957; Corkin et al., 1997), exhibited severe memory impairment on the DNMS task with trial-unique objects (Mishkin, 1978; Zola-Morgan et al., 1982). When damage was limited to the hippocampal region itself, memory impairment was observed, but the deficit was less severe than when the adjacent perirhinal, entorhinal, and parahippocampal cortical regions were also damaged (Zola-Morgan et al., 1992, 1994; Alvarez et al., 1995; Bachevalier et al., 1999;Beason-Held et al., 1999; Zola et al., 2000) (but see Murray and Mishkin, 1998).
The DNMS task with trial-unique objects has also been adapted for the rodent (Mumby et al., 1990). In the rat, several studies have reported that damage to the hippocampus or fornix impairs performance on the DNMS task (Mumby et al., 1992, 1995; Wiig and Bilkey, 1995; Clark et al., 2000), but other studies of this or similar tasks have failed to find an impairment (Aggleton, et al., 1986; Rothblat and Kromer, 1991; Kesner et al., 1993; Mumby et al., 1996; Duva et al., 1997). Finding an impairment may depend on how the DNMS task has been administered to rats [e.g., using retention intervals of >30 sec and testing animals with sufficiently large hippocampal lesions (Clark et al., 2000).
The DNMS task provides one way of measuring the capacity for recognition memory. Another type of task depends on spontaneous novelty preference and assesses recognition memory by measuring an animal's tendency to explore a novel object (or location). For example, the visual paired comparison (VPC) task was used extensively with humans (Fagan, 1970) and was subsequently adapted for both the rodent (Ennaceur and Delacour, 1988) and the monkey (Gunderson and Sackett, 1984; Bachevalier, 1990). In the VPC task, two identical objects (or pictures) are presented briefly. Then, after a variable delay interval, one records how much time the animal (or human) spends exploring a new object when the recently presented object and a novel object are placed side by side. Normal animals prefer to explore the novel object more than the old object. When the animal exhibits a preference to explore the new object, it can be inferred that the animal has a memory for the familiar and now less-interesting object.
In amnesic patients with damage to the hippocampal formation, performance was spared when no delay intervened between the first and second presentations of the stimuli, but performance was markedly impaired after delay intervals of 2 min and 2 hr (McKee and Squire, 1993). Similarly, impaired performance on the VPC task has been reported for neonatal and adult monkeys with large medial temporal lobe lesions (Bachevalier, 1990; Bachevalier et al., 1993), adult monkeys with neonatal lesions of the hippocampal formation and underlying cortex (Pascalis and Bachevalier, 1999), and adult monkeys with lesions restricted to the hippocampal region (Zola et al. 2000).
The available data thus indicate that impaired object recognition performance after hippocampal lesions occurs in both humans and monkeys on the VPC task, just as it does on the DNMS task; yet the findings for rats on the VPC task have been less clear. Performance on the VPC task was impaired after ischemic damage to the hippocampus in rats (Wood and Phillips, 1991). However, it has been proposed that impaired object recognition after ischemic damage might be attributable to damage outside the hippocampus (Mumby et al., 1996; but see Squire and Zola, 1996b). In addition, several studies using the VPC task have failed to find recognition memory impairment after fornix lesions, although these same lesions did impair spatial memory (Ennaceur and Aggleton, 1994;Ennaceur et al., 1996, 1997). One possibility is that fornix lesions do not disrupt the function of the hippocampus to a sufficient extent to impair recognition memory. In any case, a clear effect of hippocampal dysfunction on the VPC task has not yet been reported in the rat. As a result, there is not yet good agreement across mammalian species with respect to recognition memory and hippocampal function.
We have assessed performance on the VPC task in rats after radio-frequency lesions of the hippocampus (dentate gyrus and CA fields), ibotenate lesions of the hippocampus, and radio-frequency lesions of the fornix. The three lesion groups, together with two control groups, were tested on the VPC task at delay intervals ranging from 10 sec to 24 hr. To optimize the sensitivity of the task, we developed a computer-assisted scoring procedure that allowed object preference to be assessed during each second of the test phase. We also tested the rats with fornix lesions on a spatial task (spontaneous alternation).
MATERIALS AND METHODS
Subjects
The subjects were 48 experimentally naive, male Long–Evans rats weighing between 300 and 350 gm at the beginning of the experiment. They were housed individually on a 12 hr light/dark cycle (behavioral testing was in the light phase) with continuous access to food and water. The animals were randomly assigned to one of five groups. There were three experimental groups: a group with radio-frequency lesions of the hippocampus (H-RF; n = 8), a group with ibotenic acid lesions of the hippocampus (H-IBO; n = 8), and a group with radio-frequency lesions of the fimbria–fornix (FX;n = 8). Radio-frequency lesions of the hippocampus damage both cell bodies and fibers of passage, but the damage can be well circumscribed. Ibotenate acid produces excitotoxic cell death but spares fibers of passage. [Note, however, that excitotoxic lesions have raised concerns about possible extrahippocampal pathology [Anagnostaras et al., 2000)]. Fornix lesions have frequently been used with the intention of disrupting hippocampal function by transection of afferent and efferent connections. There were also two control groups: a group with ibotenic acid lesions of the cortical regions immediately dorsal to the hippocampus (CTX; n = 8) and a group that underwent the same initial surgical procedures as the other groups, but no cannulas or electrodes were lowered into the brain (CON; n = 16).
Surgery
All surgery was performed using aseptic procedures. Anesthesia was initially induced with an injection of sodium pentobarbital (65 mg/kg, i.p.) mixed with 0.2 ml of atropine. The animal was positioned in a Kopf stereotaxic instrument, and the incisor bar was adjusted until bregma was level with lambda. Anesthesia was maintained throughout the surgery with isoflurane gas (0.8–2.0% isoflurane delivered in O2 at 1 l/min). The bone overlying the target site was removed using a high-speed drill. After the completion of each lesion, the wounds were closed, and the animal was allowed to recover from anesthesia on a water-circulating heating pad. Behavioral testing began ∼2 weeks after surgery. The CON group underwent the initial surgical procedures, but no lesions were made.
In two of the groups (H-IBO and CTX), excitotoxic lesions were produced by ibotenic acid (IBO), an excitatory analog of glutamic acid. Ibotenic acid (Biosearch Technologies, San Rafael, CA) was dissolved in 0.1m PBS to provide a solution with a concentration of 10 mg/ml, pH 7.4. IBO was injected at a rate of 0.1 μl/min using a 10 μl Hamilton (Reno, NV) syringe mounted on a stereotaxic frame and held with a Kopf model 5000 microinjector. The syringe needle was first lowered to the surface of the dura, and a small puncture was made in the dura just below the needle tip. The syringe needle was then lowered to the target and left in place for 1 min before beginning the injection (Table 1). After the injection, the syringe needle was left in place for 2 min to reduce the spread of IBO up the needle tract. For the H-IBO group, IBO was injected into 18 sites (total volume, 2.04 μl) on each side of the brain (modified from the method of Jarrard, 1989) and was intended to damage the dorsal and ventral hippocampus. For the CTX group, IBO was injected into nine sites on each side of the brain (total volume, 1.02 μl). This lesion was intended to damage the cortical areas immediately dorsal to the hippocampus, which are sometimes damaged when lesions are made in the hippocampus.
Table 1.
Surgical coordinates
| AP | ML | DV | |
|---|---|---|---|
| H-IBO | −2.4 | ±1.0 | −3.5 |
| −3.2 | ±1.4 | −3.2, −2.3 | |
| −3.2 | ±3.0 | −2.7 | |
| −4.0 | ±2.5 | −2.8, −1.8 | |
| −4.0 | ±3.7 | −2.7 | |
| −4.8 | ±4.9 | −7.2, −6.4 | |
| −4.8 | ±4.3 | −7.7, −7.1, −3.5 | |
| −5.4 | ±4.2 | −4.4, −3.9 | |
| −5.4 | ±5.0 | −6.6, −5.9, −5.2, −4.5 | |
| H-RF | −2.4 | ±1.0 | −3.5 |
| −3.2 | ±1.4 | −2.7 | |
| −3.2 | ±3.0 | −2.7 | |
| −4.0 | ±2.5 | −2.3 | |
| −4.0 | ±3.7 | −2.7 | |
| −4.8 | ±4.9 | −6.8 | |
| −4.8 | ±4.3 | −7.4, −3.5 | |
| 5.4 | ±4.2 | −4.2 | |
| −5.4 | ±5.0 | −6.5, −5.5, −4.5 | |
| FX | −0.1 | −1.7 | −4.71-a |
| −0.5 | −1.54 | −4.231-a | |
| −0.5 | −2.05 | −5.641-a | |
| −1.5 | ±1.8 | −3.8 |
All numbers are in millimeters and relative to bregma. AP, Anteroposterior; ML, mediolateral; DV, dorsoventral planes. ± in the ML column indicates right and left targets. The coordinates for the CTX group were identical to the H-IBO group except that the DV coordinates were 1.5 mm more dorsal than the most dorsal H-IBO target at each AP/ML location.
The target was approached at a 20° angle from the right.
In two of the groups (H-RF and FX), thermocoagulation lesions were made with a radio-frequency electrode and generator (Radionics model RF-4A). The electrode was first lowered to the surface of the dura, and a small puncture was made in the dura just below the electrode tip. The electrode was then lowered to the target and left in place for 1 min before heating the tissue to 80–90°C (depending on the target site) for 1 min. The current to the electrode was then turned off, and the electrode was removed after the tip temperature fell to 41°C. For the H-RF group, lesions were made at 12 sites on each side of the brain and were intended to damage the dorsal and ventral hippocampus. For the FX group, lesions were made at five locations (four lateral and one midline site) and were intended to completely transect the midline region of the fornix as well as large portions of the fimbria.
Behavior
Visual paired comparison task
Apparatus. Testing was performed in an open-field arena (93 × 93 × 61 cm high) constructed of black Plexiglas and illuminated by a 60 W light bulb mounted 1 m above the area.
Stimuli. The stimulus objects varied in shape and color and were made of glass, plastic, or ceramic. All the rats were tested with the same 20 objects. The sizes of the objects were no smaller than the size of the rat and no larger than ∼2.5 times the size of the rat. Most objects were heavy enough that they could not be moved by the rats, but as a safeguard, Velcro was used to secure the objects in place. The Velcro attached to the arena floor also served as fiduciary marks that ensured that the objects were always placed in the same location within the arena. Two objects were always placed in the arena together, 61 cm from the front wall, 32 cm from the back wall, 25 cm from the side walls, and 43 cm apart.
Habituation. Each rat was handled for 5 min each day for 5 consecutive days. After the handling each day, rats were allowed to explore and become familiar with the empty arena for another 5 min.
Testing. The experimenter was blind to group membership throughout testing. A single trial consisted of four phases presented in the following order:
Rehabituation. At the beginning of a trial, each rat was placed in the empty arena and allowed to explore for 1 min. Rats were then removed and placed in a transport box, and two identical stimuli were then placed in the arena.
Familiarization. Rats were then returned to the arena at the center point along the edge of the front wall and allowed to explore the two identical stimuli until they accumulated 30 sec of object exploration (see Scoring below).
Delay. Rats were removed from the box during the delay interval. For shorter delays (10 sec and 1 min), they remained in the testing room in a transport cage. For longer delays (10 min and 1 and 24 hr), they were returned to their home cages. During the delay phase, two objects were placed in the arena. One of the objects was a third copy of the two identical objects used during the familiarization phase, thus ensuring that this object had not been scent-marked during the familiarization phase. The familiar object was paired with a novel object.
Test. After completion of the delay interval, the rats were placed back in the arena, as in the familiarization phase, and allowed to explore the two objects until they accumulated 30 sec of object exploration. This sequence completed a single paired comparison trial. The entire arena and all of the objects were washed with 95% ethanol before another rat was tested.
Delay intervals. Delays of 10 sec, 1 and 10 min, and 1 and 24 hr were used. Rats were given one trial per day. They received a total of two trials at each delay (2 trials × 5 delays = 10 total trials) with the location of the novel object during the test phase (left or right) counterbalanced across the two days. For testing purposes, each group was divided into two subgroups. The objects that were used as the novel objects for one subgroup were used as the familiar objects for the other subgroup. This procedure controlled for any preferences that the rats might have had for one of the objects in each pair.
Scoring. In studies of the VPC task with humans and monkeys, memory is typically assessed during the first 5 sec of the test phase. In the rat, memory is typically assessed during the course of 3 min (Ennaceur et al., 1996). It is possible that preference for novelty is exhibited most clearly early in the test phase. Evaluating memory across a longer interval might make the VPC task less sensitive to memory impairment, because the preference for novelty is weak even in normal animals. Indeed, in one study normal rats exhibited a strong preference for the novel object during the first 2 min of the test phase, but this preference was not observed during the third and final minute of the test phase (Dix and Aggleton, 1999). Accordingly we developed a computer-assisted scoring procedure that allowed us to evaluate preference for the novel object at all points during the test phase.
Object exploration was scored only when the rat's nose was within 1 cm of the object and the vibrissae were moving. Object exploration time was not scored when the rat used the object to prop itself up to look and smell above the object or when the rat was touching the object with some part of the body but headed in another direction.
Specially designed software and a button press device were used to collect and analyze the behavioral data during both the familiarization and test phases. When a rat explored the object to the left, a button was depressed for the duration of the exploration and released when the rat stopped exploring that object. A second button was used when the object to the right was explored. When 30 sec of object exploration was accumulated, the computer automatically beeped and terminated that phase of the trial. This procedure ensured that all rats had the same amount of contact time with the objects, even though the rats were in the arena for different times. In previous studies of the VPC task, a fixed exploration time has been used, which can result in unequal amounts of object exploration between groups (Ennaceur and Aggleton, 1997).
For the test phase, the button push data were used to calculate the rat's preference for the novel object on a second-by-second basis across the 30 sec of object exploration, as well as the amount of real time required to accumulate each second of object exploration. The software also calculated how many times the subjects explored each object during the test phase and the average time per visit to the novel and familiar object. This scoring method provided the possibility for more detailed behavioral analysis than could have been obtained by relying on hand-held stopwatches.
Spontaneous alternation
After VPC testing, the FX and CON groups were given a test of spontaneous alternation.
Apparatus. Testing was performed in a T maze constructed of black Plexiglas. The stem and arms of the T maze were 40 cm long, 12 cm wide, and 15 cm high. The top of the maze was covered with clear Plexiglas. A sliding door separated a 26 cm start box from the rest of the stem. Sliding doors were also placed at the entrance to each side arm alley. A 40 W bulb was placed 1.2 m above the maze and provided the only light during testing.
Trial protocol. Each trial consisted of two runs. To begin the first run, the rat was placed in the start box with the sliding door closed for 10 sec. The sliding door was then opened, and the rat was allowed to move down the alley and choose one of the arms. A choice was recorded when all four of the rat's feet were inside the arm. A sliding door was then closed, and the rat remained in the chosen arm for 30 sec. After this confinement, the rat was removed from the arm and returned directly to the start box. Care was taken to avoid unnecessary turning of the rat as it was moved. The second run started with the rat confined in the start box for 10 sec. The sliding door was then opened, and the rat was again allowed to choose one of the arms. Each rat was given two trials, separated by ∼2 hr, each day for 5 days (10 trials total). The T maze was cleaned with 95% ethanol between the testing of each rat. The score was the percent of time that the rat chose the new arm on the second run, corrected for response bias with the formula shown below (Douglas, 1966):
where n is the number of observations, na′is the number of nonalternation responses,pR is the initial probability of a right turn, and pL is the initial probability of a left turn.
Neurohistological methods
Rats were administered an overdose of sodium pentobarbital and perfused transcardially with buffered 0.9% NaCl solution followed by 10% formaldehyde solution (in 0.1 m phosphate buffer). Brains were then removed from the skull and cryoprotected in a solution of 20% glycerol and 10% formaldehyde. Coronal sections (50 μm) were cut with a freezing microtome beginning just anterior to the hippocampus and continuing caudally through the length of the hippocampal region. Every fifth section was mounted and stained with thionin to assess the extent of the lesions.
RESULTS
Neurohistological findings
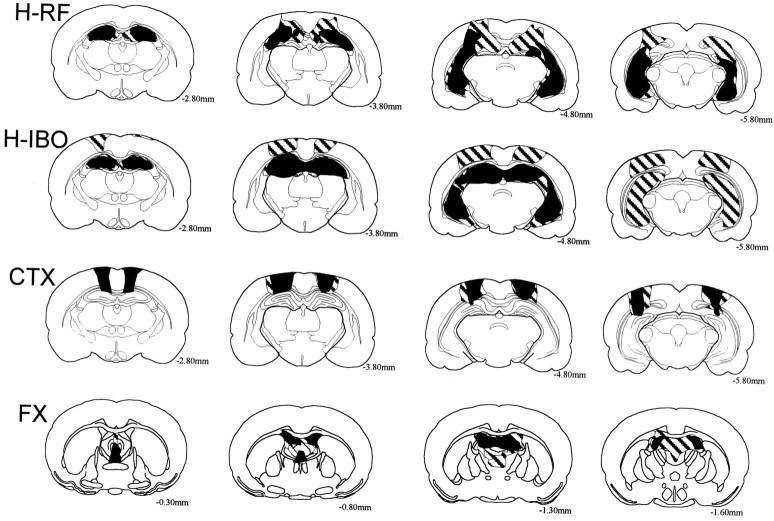
Figure 1 illustrates the extent of the largest and smallest lesion in each of the groups.
Fig. 1.
Reconstructions of coronal sections of the largest (striped) and smallest (black) lesions in the rats with radio-frequency lesions of the hippocampus (H-RF), ibotenic acid lesions of the hippocampus (H-IBO), ibotenic acid lesions of the cortex dorsal to the hippocampus (CTX), and radio-frequency lesions of the fornix (FX). Each series of sections progresses (left to right) from anterior to posterior levels. Numbers represent the distance in millimeters posterior to bregma.
H-RF group
All animals in this group sustained extensive bilateral damage to all the cell fields of the hippocampus, including the dentate gyrus. The average percent damage to the hippocampus was 71.2% (range, 59.1–87.1%). The spared hippocampal tissue involved mainly the most anterior portion of the dorsal hippocampus and the most ventromedial portion of the ventral hippocampus. All animals also had some damage to the subicular complex (<25%) as well as damage to the cortical regions dorsal to the hippocampus. The amygdala complex, entorhinal cortex, and perirhinal cortex were entirely spared in all the animals.
H-IBO group
All animals in this group sustained extensive bilateral damage to all the cell fields of the hippocampus, including the dentate gyrus. The average percent damage to the hippocampus was 89.8% (range, 67.0–98.9%). In the animal with the smallest lesion (see Fig. 1), there was considerable sparing of the ventral hippocampus. In the remaining animals the hippocampal damage was nearly complete, with only minor sparing of the most ventromedial portion of the ventral hippocampus. There was also damage to the cortical regions dorsal to the hippocampus. The animal with the smallest hippocampal lesion sustained minor bilateral damage to the anterodorsal and lateral dorsal nuclei of the thalamus. The amygdala complex, entorhinal cortex, and perirhinal cortex were entirely spared, except in one animal that sustained slight unilateral damage to the entorhinal cortex at the most posterior aspect of the hippocampus.
CTX group
All animals in this group sustained bilateral damage to the cortical regions immediately dorsal to the hippocampus that closely approximated the cortical damage in the H-RF and H-IBO groups. The damaged cortical areas included portions of the primary and secondary motor cortex, the medial regions of the primary somatosensory cortex, the parietal cortex, and the most anterior regions of the visual cortex. All animals sustained slight damage to the CA1 region of the dorsal hippocampus (<4% of hippocampal volume). In two animals, there was also slight damage to the retrosplenial cortex.
FX group
All animals had complete transection of the columns of the fornix at its most medial aspect. Additionally, the fimbria and the ventral hippocampal commissure sustained extensive damage. Except for slight damage to the most anterior aspect of the CA3 region, the hippocampus was entirely spared.
Behavioral findings
Because this is the first study to use a computer-assisted scoring method for the visual paired comparison task, we first describe the behavioral findings from the CON group in some detail.
CON group
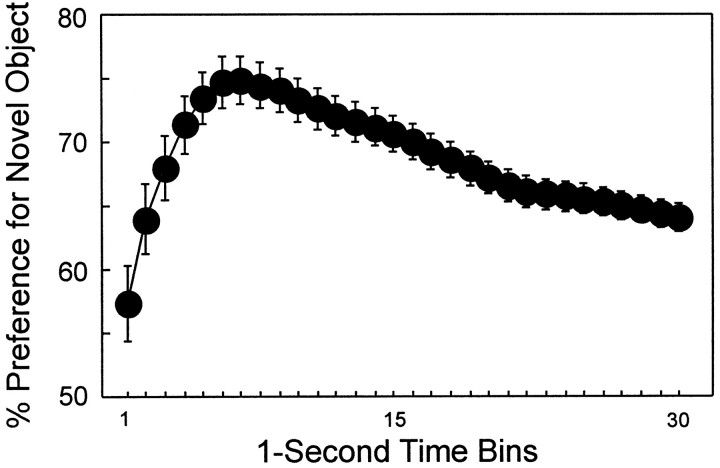
During the familiarization phase, rats in the CON group required an average of 158 sec to cumulate 30 sec of object exploration (Table2). Typically, rats explored both objects as they cumulated the 30 sec of exploration time. In the subsequent test phase, CON rats required 167 sec to cumulate 30 sec of object exploration time. Figure 2 shows the percent preference for the novel object across 30 sec of cumulative exploration during the test phase (average of two trials per delay and five delays). Note that this 30 sec score accumulated only when the rat was exploring either the novel or the familiar object. The score for each time bin represents the cumulative percent preference for the novel object in 1 sec increments across 30 sec of cumulative object exploration. Thus, the preference score for the 30 sec time bin reflects the cumulative preference for the novel object across the entire 30 sec test phase. The preference score for the novel object after 30 sec of exploration was 64.0%. This score is similar to what has been reported previously using the stopwatch technique with rats (Ennaceur and Delacour, 1988), monkeys (Bachevalier, 1990; Zola et al., 2000), and humans (McKee and Squire, 1993).
Table 2.
Total time required (seconds) to accumulate 30 sec of object exploration
Marginally different from CON group, p< 0.08.
F2-160: Significantly different from CON group, p< 0.05.
Fig. 2.
Percent preference for the novel object across 30 sec of cumulative object exploration (CON group; n= 16). The data are from the test phase (all delays combined). Preference for the novel object first increased and then gradually decreased during the 30 sec test period. Error bars indicate SEM.
The detailed analysis revealed several additional findings. First, the percent novelty preference in each time bin was significantly above chance (all p < 0.02). A preference for the novel object was apparent even during the first 1 sec of object exploration (57.3%; t(15) = 2.46;p < 0.02). Thus, rats tended first to approach and explore the novel object before they ever visited the familiar object. This suggests that the rats were being guided by the visual features of the novel objects. Second, preference for the novel objects was greater during the early portion of the test phase than during later portions. For example, the greatest preference for the novel object (74.9%) was recorded after 7 sec of cumulative object exploration. Even after 15 sec of object exploration, the preference for the novel object (70.0%) was still greater than after 30 sec of object exploration (15 sec time bin, 70.0%, vs 30 sec time bin, 64.0%;t(15) = 5.65; p < 0.0001). This pattern of performance suggests that the novel object was most attractive initially, but after some exposure it became less attractive, and preference for it steadily declined during the remainder of the test phase (Fig. 2).
Finally, with respect to performance at the different delay intervals, the CON group exhibited a significant preference for the new object at each of the five delays from 10 sec to 24 hr, but preference for the novel object at the 24 hr delay tended to be weaker than at the shorter delays. The preference scores for the five delays, from shortest to longest, were 65.8, 65.0, 62.6, 68.3, and 58.5%, respectively, and all of these preference scores were above chance (all pvalues < 0.01).
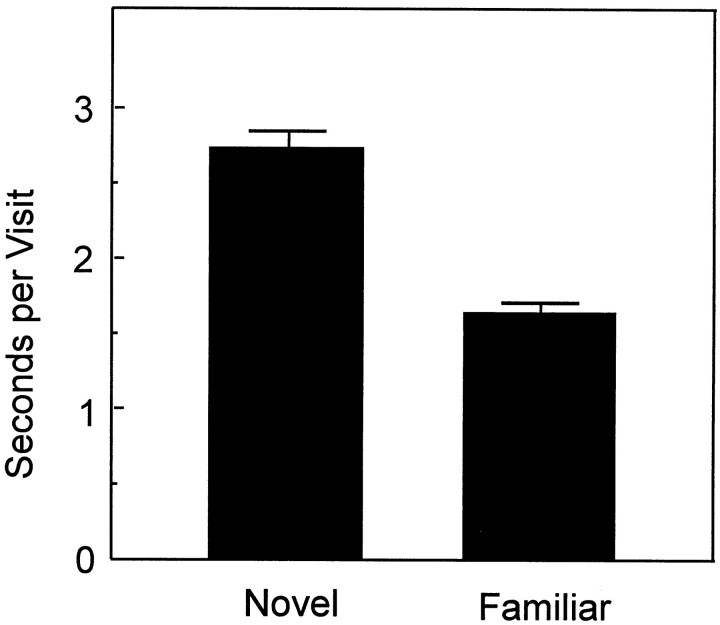
A preference for the novel object could have resulted either from exploring the novel object for longer periods each time it was approached, returning to the novel object more often, or both. We explored these alternatives by calculating the amount of time that the CON rats spent at the familiar and novel objects on each visit and the number of different times that the rats visited the familiar and novel objects. Data were combined across delays. This analysis revealed that novelty preference mainly reflected longer visits to the novel object and, to a lesser extent, more frequent visits to the novel object. First, rats spent more time on each visit to the novel object than they spent on visits to the familiar object (Fig.3; novel object, 2.7 sec; familiar object, 1.6 sec; t(15) = 11.11;p < 0.0001). The difference was apparent at each delay (p < 0.01). Second, rats also tended to visit the novel object a little more often than the familiar object (number of visits to the novel object, 8.2; number of visits to the familiar object, 7.5). Although this difference was small, the novel object was visited more often than would be expected by chance (t(15) = 4.0; p < 0.01). However, a significant difference in the number of visits was observed only at the 1 hr delay (t(15)= 2.95; p < 0.01).
Fig. 3.
Mean time that the CON group spent at the novel and familiar objects on each visit (all delays combined). Visits to the novel object lasted longer than visits to the familiar object. Error bars indicate SEM.
The CON group and CTX groups performed similarly. Two-way ANOVAs with repeated measures across delays found no effect of group at the 15th and 30th time bins (all p values > 0.10). Indeed, there were no differences between the CON and CTX groups on any of the measures described in this section (all p values > 0.10).
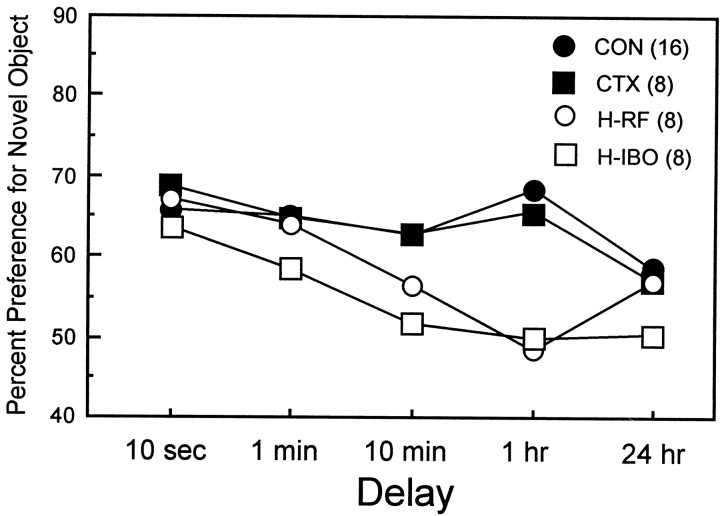
Hippocampal groups
The two groups with hippocampal lesions (H-RF and H-IBO) performed similarly overall. Although the H-IBO group performed more poorly than the H-RF group at the 24 hr delay (50.8 vs 56.9%), this difference was not significant (t(14) = 1.47;p > 0.1). Figure 4 shows the percent novelty preference score from the 30 sec time bin for the two hippocampal lesion groups (H-RF and H-IBO) and the two control groups (CON and CTX). The rats with hippocampal lesions performed well at the shorter delays and performed poorly at the longer delays. Thus, averaging across the 10 sec and 1 min delays, the two hippocampal lesion groups were not measurably impaired relative to either the CON or CTX group (t < 1.7; p > 0.10). Averaging across the longer delays (10 min, 1 hr, and 24 hr), both the H-RF and H-IBO groups were impaired relative to the CON group (H-RF,t(22) = 3.28; p < 0.01; H-IBO, t(22) = 5.10;p < 0.0001) as well as relative to the CTX group (H-RF, t(14) = 2.24; p< 0.05; H-IBO, t(14) = 4.27;p < 0.00l).
Fig. 4.
Percent preference (30 sec time bin) for the novel object across five delays for two control groups (CONand CTX) and two groups with radio-frequency or ibotenate lesions of the hippocampus (H-RF andH-IBO).
Separate comparisons at each delay indicated that the scores of the two hippocampal groups were impaired relative to both the CON and CTX groups at the 1 hr delay (p < 0.05). In addition, the H-IBO group was impaired relative to both control groups at the 10 min delay (p < 0.05) and marginally impaired at the 24 hr delay (p < 0.07 in comparison with the CON group; p < 0.11 in comparison with the CTX group). Additionally, the H-IBO group performed above chance at the 10 sec and 1 min delays (p < 0.05) but not at the 10 min, 1 hr, and 24 hr delays (p > 0.10). Similarly, the H-RF group performed above chance at the 10 sec and 1 min delays (p< 0.05) but not at the longer delays. (Performance was marginally above chance at the 24 hr delay; p = 0.06.)
The pattern of impairment after hippocampal damage was similar when preference scores were taken from the 7 and 15 sec time bins of the test phase instead of the 30 sec time bin. Thus, after 7 sec of object exploration, an impairment could be detected in both lesion groups at the 10 min and 1 hr delays (all p values < 0.05). After 15 sec of exploration, the H-IBO group was impaired at the 1 min, 10 min, and 1 hr delays (p < 0.05), and the H-RF group was impaired at the 1 hr delay (p < 0.05).
In previous studies with this task, object exploration was typically measured during a fixed period in the arena. Accordingly, we recorded preference scores after rats had been in the arena for 1 min. A similar pattern of impairment was observed. The H-IBO group was impaired relative to the CON group at the 1 min, 10 min, and 1 hr delays (p < 0.05). The H-RF group was impaired at the 1 hr delay (p < 0.01).
As noted above for the CON group, preference for the novel object resulted primarily from spending a longer time on each visit to the novel object than on each visit to the familiar object (see Fig. 3). At the 10 sec and 1 min delays, this same pattern of performance was also evident for both the H-IBO and H-RF groups; that is, when the average time per visit was averaged across the 10 sec and 1 min delays, the H-IBO group averaged longer visits to the novel object compared with the familiar object (novel object, 1.8 sec; familiar object, 1.3 sec; t(7) = 2.62; p < 0.05). The H-RF group exhibited the same pattern of performance, but the difference was marginal (t(7) = 1.96; p = 0.09). Averaging across the longer delays (10 min and 1 and 24 hr), the H-IBO and H-RF groups did not have longer visits to the novel object (p > 0.10).
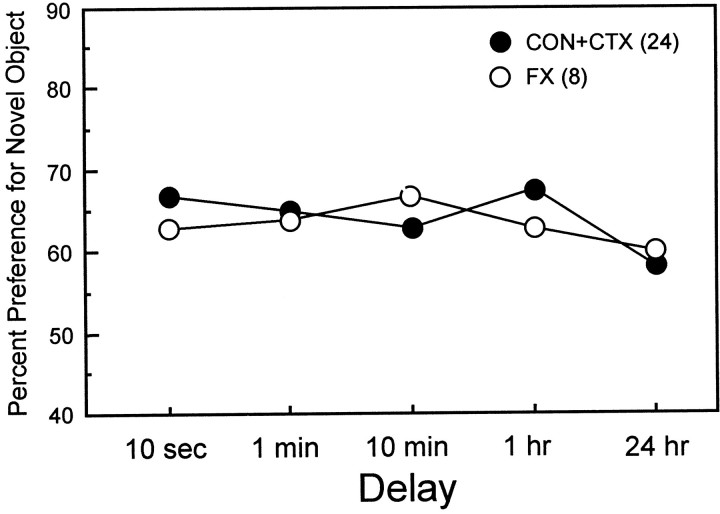
Fornix
Figure 5 shows the percent novelty preference scores from the 30 sec time bin for the two control groups (CON and CTX combined) and the FX group. A two-way ANOVA for percent novelty preference across delays for the two groups revealed no effect of group (F(1) = 0.05;p > 0.20). The same result was obtained when the 7 and 15 sec test time bins were examined (all p values > 0.20). The only difference that was found between the two control groups and the FX group was that the FX group exhibited an abnormally weak novelty preference in the early time bins at the 10 sec delay. For example, in the 15 sec time bin for the 10 sec delay, the FX group (60.4%) exhibited less of a novelty preference than the two control groups (67.7%; t(30) = 2.01;p < 0.05). However, this difference was not apparent after 30 sec of object exploration had been completed, i.e., in the 30 sec time bin for the 10 sec delay (CON, 65.8 vs 63.2%;t(30) = 0.64; p > 0.20). The FX group performed better than the H-IBO group at the 10 min delay (t(14) = 2.75; p< 0.02) and better than both the H-IBO and H-RF groups at the 1 hr delay (p <0.01). The FX group also preformed better than the H-IBO group at the 24 hr delay (t(14) = 2.21; p < 0.05).
Fig. 5.
Percent preference (30 sec time bin) for the novel object across five delays for two control groups (CON+CTX, combined) and the group with fornix lesions (FX).
Finally, like the CON and CTX groups, the FX group spent more time on each visit to the novel object than on visits to the familiar object (average across five delays: novel object, 2.7 sec; familiar object, 1.5 sec; t(7) = 6.2; p< 0.001).
Because the FX group performed well on the VPC task, we also tested the FX and CON groups on a task of spontaneous alternation, which has been reported to be sensitive to fornix damage (cf. Johnson et al., 1977). The FX group was impaired relative to the CON groups (CON, 73.2% alternation vs FX, 47.5%; t(14) = 2.52; p < 0.03) and also did not alternate at above-chance levels (t(7) = 0.30;p > 0.20).
Real-time exploration
We examined the total amount of real time that the different groups required to accumulate 30 sec of object exploration during the familiarization and test phases (Table 2). The H-RF group was slower than the CON group at accumulating 30 sec of exploration (familiarization phase, t(22) = 2.73;p < 0.05; test phase,t(22) = 2.52; p < 0.02). The CTX group was marginally slower than the CON group (familiarization phase, t(22) = 1.90;p = 0.07; test phaset(22) = 1.98; p = 0.06). The H-IBO group was numerically slower than the CON group (and faster than the CTX group), but these differences did not reach significance for either the familiarization phase (p > 0.10) or the test phase (p > 0.10).
It appears unlikely that longer exploration times could have accounted for impaired VPC performance in the rats with hippocampal lesions. First, only the H-RF group, and not the H-IBO group, had significantly elevated exploration times. Second, the CTX group had even longer exploration times than the H-IBO group, but the CTX group performed normally across all the delays. Third, an abnormality in exploration would be expected to express itself at all delay intervals. Yet, both the H-RF and the H-IBO groups performed well at the shorter delays.
DISCUSSION
Two groups of rats with direct damage to the hippocampus (H-IBO and H-RF) exhibited delay-dependent memory impairment on the VPC task, a test of spontaneous novelty preference. In both hippocampal lesion groups, performance was intact at the shorter delays (average of 10 sec and 1 min) and impaired across the longer delays (average of 10 min, 1 hr, and 24 hr). Rats with ibotenate lesions of the cortex dorsal to hippocampus (CTX) and rats with fornix lesions (FX) performed similarly to unoperated animals (CON). The fact that the performance of rats with hippocampal lesions was intact at the shortest delays tested (10 sec and 1 min) indicates that hippocampal damage did not impair the ability to appreciate novelty per se, nor did it impair sensory processing to an extent that affected the ability to discriminate between stimuli. Accordingly, the impaired novelty preference observed at longer delays is best attributed to an impairment in recognition memory.
These results are consistent with previous findings from studies that have used the VPC task to study recognition memory after hippocampal damage. First, amnesic patients with hippocampal formation damage exhibited impaired memory on the VPC task (McKee and Squire, 1993). Second, monkeys with large lesions of the medial temporal lobe, which included the hippocampus, were impaired on the VPC task when tested either as infants or as adults (Bachevalier, 1990; Bachevalier et al., 1993; Pascalis and Bachevalier, 1999). Third, impaired performance on the VPC task was observed in monkeys with radio-frequency lesions restricted to the hippocampus (Zola et al., 2000) as well as by monkeys with ibotenate acid lesions restricted to the hippocampus (Zola et al., 2000). Finally, impaired performance on the VPC task has also been reported in mice lacking the NMDAR-1 subunit in the CA1 region of the hippocampus (Rampon et al., 2000).
Radio-frequency lesions and ibotenate lesions of the hippocampus produced a similar degree of impairment, although the ibotenate lesion group achieved a numerically lower score than the radio-frequency group (percent preference for the novel object, 55.4 and 58.6% for the H-IBO and H-RF groups, respectively, averaged across all five delays;t(14) = 1.21; p > 0.10). Histological analysis, in correspondence with these observations, showed that although both groups had nearly complete damage to the hippocampus, the H-RF group had some islands of spared hippocampal tissue (see Histological analysis). In our earlier study of monkeys and the DNMS task of recognition memory, radio-frequency and ibotenic acid lesions of the hippocampus also impaired memory to a similar degree (Zola et al., 2000).
In contrast to the effects of hippocampal lesions, fornix lesions did not affect performance on the VPC task. This finding is consistent with previous studies of the VPC task in rats with fornix lesions (Ennaceur and Aggleton, 1994; Ennaceur et al., 1996, 1997). Note, though, that fornix lesions in rats have been reporred to mildly impair performance on the DNMS task (Wiig and Bilkey, 1995). Fornix and hippocampal lesions have often been found to have similar behavioral effects (cf.Gray and McNaughton, 1983; Aggleton et al., 1992). Indeed, because fornix lesions disrupt major afferent and efferent connections of the hippocampus and also produce abnormalities in hippocampal physiology (Shapiro et al., 1989; Buzsaki et al., 1989, 1991), it has seemed reasonable to suppose that fornix lesions should typically mimic the effects of hippocampal lesions.
Differences between the effects of fornix damage and direct hippocampal damage, however, have been noted. In one study, selective hippocampal lesions produced more profound spatial memory impairment as well as larger increases in locomotor activity than did lesions of the fimbria-fornix (Cassell et al., 1998). Furthermore, fornix lesions do leave intact many physiological indices of hippocampal function (Shapiro et al., 1989). For example, the waveform and the mean firing rate of complex-spike cells were not noticeably affected by fornix lesions (Shapiro et al., 1989). Furthermore, Miller and Best (1980)found that after fornix lesions the number of CA1 complex-spike cells that exhibit location-specific firing patterns was only slightly reduced. In any case, the present results emphasize that fornix lesions are not equivalent to direct damage to hippocampal tissue.
Despite the finding that rats with fornix lesions were not impaired on the VPC task, the same animals exhibited a marked deficit in spontaneous alternation. Spontaneous alternation, like the VPC task, assesses an animal's spontaneous preference for novelty. However, spontaneous alternation is a spatial task, not a visual task (Douglas, 1966). Previous studies of fornix lesions and the VPC task have also reported that the same rats that succeeded in the VPC task failed tasks of spatial memory (Ennaceur and Aggleton, 1994; Ennaceur et al., 1996,1997). In addition, a similar pattern of impairment (intact visual recognition but impaired spatial memory) has been reported in the case of small dorsal NMDA lesions of the hippocampus (Duva et al., 1997). Although this finding in isolation could be taken to mean that the hippocampus is not critical for recognition memory, we suggest that the hippocampus is critical for both spatial and recognition memory. The sensitivity of spatial memory tasks to hippocampal damage may be attributable to the fact that many spatial memory tasks resemble tests of free recall. For example, in the water maze the arbitrary location of the submerged platform must be recalled from memory. In any case, sufficiently large lesions of the hippocampus do in fact impair performance on tasks of visual recognition memory [the DNMS task (Clark et al., 2000) and the VPC task (present study)].
In summary, the present results indicate that the hippocampus is important for performance on the VPC task in the rat. Recognition memory performance after restricted hippocampal dysfunction has now been assessed in three species (humans, monkeys, and rats) and in two tasks (DNMS and VPC; Table 3). The available findings from humans, monkeys, and rats indicate that hippocampal damage impairs recognition memory in all three species, whether measured by the DNMS or the VPC task. In view of the well documented impairments in visual recognition memory after perirhinal or entorhinal cortical lesions (Mishkin and Murray, 1994; Squire and Zola, 1996a), the finding that hippocampal lesions also impair recognition memory supports the idea that recognition memory is widely dependent on the structures of the medial temporal lobe memory system (Zola and Squire, 2000). The available data from neurophysiology support a similar conclusion (Suzuki and Eichenbaum, 2000).
Table 3.
Impaired recognition memory performance after restricted hippocampal lesions
| Task | Reference | ||
|---|---|---|---|
| Humans | Monkeys | Rats | |
| DNMS | Squire et al., 1988 | Zola-Morgan et al., 1992 | Mumby et al., 1992 |
| Alvarez et al., 1995 | Mumby et al., 1995 | ||
| Beason-Held et al., 1999 | Clark et al., 2000 | ||
| Zola et al., 2000 | |||
| VPC | McKee and Squire, 1993 | Zola et al., 2000 | Present study |
DNMS, Trial-unique delayed nonmatching to sample with objects; VPC, visual paired comparison.
Although there can be little doubt that the different anatomical components of the medial temporal lobe have different functions in the support of declarative memory, it seems unlikely that there is any simple division of labor within the medial temporal lobe (based on the kind of memory task, for example) that can distinguish the function of the hippocampus from the function of medial temporal lobe structures positioned earlier in the information-processing hierarchy. Rather, it seems more likely that the functions of these structures are closely related and the hippocampus extends and combines functions performed by the adjacent cortex.
Footnotes
This research was supported by the Medical Research Service of the Department of Veterans Affairs, National Institutes of Health Grants MH24600, MH19063, and MH11154, the National Alliance for Research on Schizophrenia and Depression, and the Metropolitan Life Foundation. We thank Cecelia Manzanares, Alisha West, and Steve Burkhalter for technical assistance.
Correspondence should be addressed to Robert E. Clark, Department of Psychiatry 0603, University of California San Diego, School of Medicine, La Jolla, CA 92093. E-mail: Clark@whoville.ucsd.edu.
REFERENCES
- 1.Aggleton JP, Hunt PR, Rawlins JNP. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19:133–146. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- 2.Aggleton JP, Keith AB, Rawlins JN, Hunt PR, Sahgal A. Removal of the hippocampus and transection of the fornix produce comparable deficits on delayed non-matching to position by rats. Behav Brain Res. 1992;52:61–71. doi: 10.1016/s0166-4328(05)80325-0. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez P, Zola-Morgan S, Squire LR. Damage limited to the hippocampal region produces long-lasting memory impairment in monkeys. J Neurosci. 1995;15:3796–3807. doi: 10.1523/JNEUROSCI.15-05-03796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostaras SG, Gale GD, Fanselow MS (2000) The hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus, in press. [DOI] [PubMed]
- 5.Bachevalier J. Ontogenetic development of habit and memory formation in primates. Ann NY Acad Sci. 1990;608:457–474. doi: 10.1111/j.1749-6632.1990.tb48906.x. [DOI] [PubMed] [Google Scholar]
- 6.Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Learn Mem. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Bachevalier J, Beauregard M, Alvarado MC. Long-term effects of neonatal damage to the hippocampal formation and amygdaloid complex on object discrimination and object recognition in rhesus monkeys (Macaca mulatta). Behav Neurosci. 1999;113:1127–1151. doi: 10.1037//0735-7044.113.6.1127. [DOI] [PubMed] [Google Scholar]
- 8.Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Buzsaki G, Ponomareff GL, Ruiz BR, Gage FH. Neuronal activity in the subcortically denervated hippocampus: a chronic model for epilepsy. Neuroscience. 1989;28:527–538. doi: 10.1016/0306-4522(89)90002-x. [DOI] [PubMed] [Google Scholar]
- 10.Buzsaki G, Slamka C, Gage FH, Horvath Z. Emergence and propagation of interictal spikes in the subcortically denervated hippocampus. Hippocampus. 1991;1:163–180. doi: 10.1002/hipo.450010205. [DOI] [PubMed] [Google Scholar]
- 11.Cassell J, Cassel S, Galani R, Kelche C, Will B, Jarrard L. Fimbria-fornix vs. selective hippocampal lesions in rats: effects on locomotor activity and spatial learning and memory. Neurobiol Learn Mem. 1998;69:22–45. doi: 10.1006/nlme.1997.3807. [DOI] [PubMed] [Google Scholar]
- 12.Clark R, West A, Zola SM, Squire LR (2000) Object recognition memory is impaired in rats with lesions of the hippocampus. Hippocampus, in press.
- 13.Corkin S, Amaral DG, Gonzalez RG, Johnson KA, Hyman BT. H.M.'s medial temporal lobe lesion: findings from magnetic resonance imaging. J Neurosci. 1997;17:3964–3980. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 15.Douglas RJ. Cues for spontaneous alternation. J Comp Physiol Psychol. 1966;62:171–183. doi: 10.1037/h0023668. [DOI] [PubMed] [Google Scholar]
- 16.Duva CA, Floresco SB, Wunderlich GR, Lao TL, Pinel JPJ, Phillips AG. Disruption of spatial but not object-recognition memory by neurotoxic lesions of the dorsal hippocampus in rats. Behav Neurosci. 1997;111:1184–1196. doi: 10.1037//0735-7044.111.6.1184. [DOI] [PubMed] [Google Scholar]
- 17.Eichenbaum H. Declarative memory: insights from cognitive neurobiology. Annu Rev Psychol. 1997;48:547–572. doi: 10.1146/annurev.psych.48.1.547. [DOI] [PubMed] [Google Scholar]
- 18.Ennaceur A, Aggleton JP. Spontaneous recognition of object configurations in rats: effects of fonix lesions. Exp Brain Res. 1994;100:85–92. doi: 10.1007/BF00227281. [DOI] [PubMed] [Google Scholar]
- 19.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats: 1. Behavioural data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 20.Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- 21.Ennaceur A, Neave N, Aggleton JP. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res. 1997;88:181–193. doi: 10.1016/s0166-4328(97)02297-3. [DOI] [PubMed] [Google Scholar]
- 22.Fagan JF. Memory in the infant. J Exp Child Psychol. 1970;9:217–226. doi: 10.1016/0022-0965(70)90087-1. [DOI] [PubMed] [Google Scholar]
- 23.Gray JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions. A review. Neurosci Biobehav Rev. 1983;7:119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- 24.Gunderson VM, Sackett GP. Development of pattern recognition in infant pigtailed macaques (Macaca nemestrina). Dev Psychol. 1984;20:418–426. [Google Scholar]
- 25.Jarrard LE. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. J Neurosci Methods. 1989;29:251–259. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- 26.Johnson CT, Olton DS, Gage FH, Jenko PG. Damage to hippocampus and hippocampal connections: effects on DRL and spontaneous alternation. J Comp Physiol Psychol. 1977;91:508–522. doi: 10.1037/h0077346. [DOI] [PubMed] [Google Scholar]
- 27.Kesner RP, Bolland BL, Dakis M. Memory for spatial location, motor responses, and objects: triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Exp Brain Res. 1993;93:462–470. doi: 10.1007/BF00229361. [DOI] [PubMed] [Google Scholar]
- 28.McKee RD, Squire LR. On the development of declarative memory. J Exp Psychol Learn Mem Cognit. 1993;19:397–404. doi: 10.1037//0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- 29.Miller VM, Best PJ. Spatial correlates of hippocampal unit activity are altered by lesions of the fornix and entorhinal cortex. Brain Res. 1980;194:311–323. doi: 10.1016/0006-8993(80)91214-7. [DOI] [PubMed] [Google Scholar]
- 30.Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- 31.Mishkin M, Murray EA. Stimulus recognition. Curr Opin Neurobiol. 1994;4:200–206. doi: 10.1016/0959-4388(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 32.Mumby DG, Pinel JPJ, Wood ER. Nonrecurring-items delayed nonmatching-to-sample in rats: a new paradigm for testing nonspatial working memory. Psychobiology. 1990;18:321–326. [Google Scholar]
- 33.Mumby DG, Wood ER, Pinel JPJ. Object-recognition memory is only mildly impaired in rats with lesions of the hippocampus and amygdala. Psychobiology. 1992;20:18–27. [Google Scholar]
- 34.Mumby DG, Pinel JPJ, Kornecook TJ, Shen MJ, Redila VA. Memory deficits following lesions of hippocampus or amygdala in rat: assessment by an object-memory test battery. Psychobiology. 1995;23:26–36. [Google Scholar]
- 35.Mumby DG, Wood ER, Duva CA, Kornecook TJ, Pinel JPJ, Phillips AG. Ischemia-induced object-recognition deficits in rats are attenuated by hippocampal ablation before or soon after ischemia. Behav Neurosci. 1996;110:266–281. doi: 10.1037//0735-7044.110.2.266. [DOI] [PubMed] [Google Scholar]
- 36.Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 38.Rampon C, Ya-Ping T, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 39.Rothblat LA, Kromer LR. Object recognition memory in the rat: the role of the hippocampus. Behav Brain Res. 1991;42:25–32. doi: 10.1016/s0166-4328(05)80036-1. [DOI] [PubMed] [Google Scholar]
- 40.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapiro ML, Simon DK, Olton DS, Gage FH, Nilsson O, Bjorklund A. Intrahippocampal grafts of fetal basal forebrain tissue alter place fields in the hippocampus of rats with fimbria-fornix lesions. Neuroscience. 1989;32:1–18. doi: 10.1016/0306-4522(89)90103-6. [DOI] [PubMed] [Google Scholar]
- 42.Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci USA. 1996a;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Squire LR, Zola SM. Ischemic brain damage and memory impairment: a commentary. Hippocampus. 1996b;6:546–548. doi: 10.1002/(SICI)1098-1063(1996)6:5<546::AID-HIPO7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 44.Squire LR, Zola-Morgan S. The neurology of memory: the case for correspondence between the findings for human and nonhuman primate. In: Deutsch JA, editor. The physiological basis of memory. Academic; New York: 1983. pp. 199–268. [Google Scholar]
- 45.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki WA, Eichenbaum H. The neurophysiology of memory. Ann NY Acad Sci. 2000;911:175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- 47.Wiig KA, Bilkey DK. Lesions of rat perirhinal cortex exacerbate the memory deficit observed following damage to the fimbria-fornix. Behav Neurosci. 1995;109:620–630. doi: 10.1037//0735-7044.109.4.620. [DOI] [PubMed] [Google Scholar]
- 48.Wood ER, Phillips AG. Deficits on a one trial object recognition task by rats with hippocampal CA1 lesions produced by cerebral ischemia. Neurosci Res Commun. 1991;9:177–182. [Google Scholar]
- 49.Zola SM, Squire LR. The medial temporal lobe and the hippocampus. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. Oxford UP; New York: 2000. pp. 501–520. [Google Scholar]
- 50.Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zola-Morgan S, Squire LR. The neuroanatomy of amnesia. Annu Rev Neurosci. 1993;16:547–563. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]
- 52.Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: amygdala-hippocampus versus temporal stem. Science. 1982;218:1337–1339. doi: 10.1126/science.6890713. [DOI] [PubMed] [Google Scholar]
- 53.Zola-Morgan S, Squire LR, Rempel NL, Clower RP, Amaral DG. Enduring memory impairment in monkeys after ischemic damage to the hippocampus. J Neurosci. 1992;12:1582–2596. doi: 10.1523/JNEUROSCI.12-07-02582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus. 1994;4:483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]