Abstract
Administration of the hallucinogenic 5-HT2A/2C agonist 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) induces expression of Fos protein in the cerebral cortex. To understand the mechanisms subserving this action of DOI, we examined the consequences of pharmacological and surgical manipulations on DOI-elicited Fos expression in the somatosensory cortex of the rat. DOI dose-dependently increased cortical Fos expression. Pretreatment with the selective 5-HT2A antagonist MDL 100,907 completely blocked DOI-elicited Fos expression, but pretreatment with the 5-HT2C antagonist SB 206,553 did not modify DOI-elicited Fos expression. These data suggest that DOI acts through 5-HT2A receptors to increase cortical Fos expression. However, we found that DOI did not induce Fos in cortical 5-HT2A immunoreactive neurons but did increase expression in a band of neurons spanning superficial layer V to deep III, within the apical dendritic fields of layer V 5-HT2A-immunoreactive cells. This band of Fos immunoreactive neurons was in register with anterogradely labeled axons from the ventrobasal thalamus, which have previously been shown to be glutamatergic and express the 5-HT2A transcript. The effects of DOI were markedly reduced in animals pretreated with the AMPA/KA antagonist GYKI 52466, and lesions of the ventrobasal thalamus attenuated DOI-elicited Fos expression in the cortex. These data suggest that DOI activates 5-HT2A receptors on thalamocortical neurons and thereby increases glutamate release, which in turn drives Fos expression in cortical neurons through an AMPA receptor-dependent mechanism. These data cast new light on the mechanisms of action of hallucinogens.
Keywords: AMPA receptor, DOI, Fos, glutamate, hallucinogen, pyramidal cell, serotonin, somatosensory cortex, thalamus, 5-HT2A receptor
The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) is a 5-HT2A/2C partial agonist (McClue et al., 1989;Smith et al., 1998). Acute administration of DOI and lysergic acid diethylamide (LSD), another 5-HT2A/2Cagonist, induces expression of the immediate-early gene c-fos and its protein product Fos in the cortex of the rat (Leslie et al., 1993; Tilakaratne et al., 1995; Moorman and Leslie, 1996, 1998; Tilakaratne and Friedman, 1996; Abi-Saab et al., 1999; Erdtmann-Vourliotis et al., 1999). The mechanisms subserving this effect of DOI, including the specific type of 5-HT2 receptor involved, are poorly understood. DOI has high affinities for 5-HT2A and 5-HT2C receptors, both of which are widely distributed in the rat brain (Molineaux et al., 1989; Appel et al., 1990; Pompeiano et al., 1994; Wright et al., 1995; Cornea-Hebert et al., 1999a). 5-HT2A-immunoreactive pyramidal cells are especially prominent in layer Va (Wright et al., 1995;Willins et al., 1997; Jakab and Goldman-Rakic, 1998; Bubser et al., 2000). Neurons expressing the 5-HT2C transcript have a more restricted laminar cortical distribution and are predominantly found in layer VI (Mengod et al., 1990; Pompeiano et al., 1994; Wright et al., 1995). DOI administration induces Fos in a band of neurons that have been described as being in superficial layer V (Moorman et al., 1996; Moorman and Leslie, 1998; Maćkowiak et al., 1999), consistent with the suggestion that DOI targets 5-HT2A-expressing neurons.
In a series of studies examining serotonergic regulation of cortical neurons, Aghajanian and Marek (Aghajanian and Marek, 1997, 1999; Marek and Aghajanian, 1998, 1999) noted that application of serotonin to the area of the distal apical dendrites of layer V pyramidal cells results in EPSCs in these neurons. In contrast, the effects of serotonin applied to the proximal apical or basilar dendrites were much weaker or absent. The excitatory action of serotonin on pyramidal cells was mediated through 5-HT2A receptors (Aghajanian and Marek, 1997; Marek and Aghajanian, 1999), suggesting that the ability of serotonin to influence layer V pyramidal cells may occur by actions on some element in the superficial cortical layers.
We undertook a series of studies to determine the mechanisms through which DOI activates cortical neurons, as reflected by Fos induction. Although DOI induces Fos across most cortical areas (Moorman and Leslie, 1996; Tilakaratne and Friedman, 1996; Abi-Saab et al., 1999), we focused our studies on the somatosensory cortex (SSC). The SSC displays a strong Fos response to acute DOI challenge (Moorman and Leslie, 1996) and has a well characterized anatomical organization (Woolsey, 1978; Chapin and Lin, 1990; Ebner and Armstrong-James, 1990), including serotonergic afferents from the pons and afferents from cells expressing either the 5-HT2A or 5-HT2C transcripts (Wright et al., 1995;Bennett-Clarke et al., 1997; Mansour-Robaey et al., 1998).
Some of this work has been published previously in abstract form (Patel et al., 1999).
MATERIALS AND METHODS
Animals and drug treatments. Adult male Sprague Dawley rats (Harlan) were used as subjects. Rats were group-housed on a 12 hr light/dark cycle with lights on at 6 A.M. Food and water were available ad libitum. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals as promulgated by National Institutes of Health.
To determine the effects of DOI on Fos expression in the SSC, rats received a subcutaneous injection of 1.0, 5.0, or 10.0 mg/kg DOI (RBI, Natick, MA) or vehicle, and 2 hr later were deeply anesthetized with isofluorane and decapitated. The brain was removed, and the SSC was dissected from a 1.0-mm-thick coronal slice. Tissue was stored at −75° C until assayed by Western blots to reveal Fos protein.
On the basis of the outcome of the Western blot assessment, all subsequent studies examined rats treated with 5.0 mg/kg DOI, which we found elicited the maximal Fos response in the SSC. Unless noted otherwise, animals were deeply anesthetized 2 hr after DOI challenge and transcardially perfused with 4% paraformaldehyde; the tissue was post-fixed for 3–4 hr and then cryoprotected in 24% sucrose in PBS before 42 μm coronal sections were cut through the SSC.
To determine whether the effects of DOI were mediated by 5-HT2A receptors, rats (n = 4 per group) were pretreated with the specific 5-HT2A antagonist MDL 100,907 (0.5 mg/kg, s.c.) (Kehne et al., 1996) or vehicle, and 30 min later they received DOI; animals were killed 2 hr thereafter. To assess the contribution of 5-HT2C receptors, rats (n = 4 per group) were pretreated with SB 206,553 (7.5 mg/kg, s.c.; RBI), a specific 5-HT2C/B antagonist (Kennett et al., 1996), and 30 min thereafter they were challenged with DOI.
To assess the involvement of glutamatergic mechanisms in DOI-elicited cortical Fos expression, rats (n = 4 per group) were injected with GYKI 52466 (15 mg/kg, i.p.; RBI), a noncompetitive AMPA/kainate receptor antagonist (Bleakman et al., 1996). Because of the short duration of action of this antagonist, animals were injected with GYKI 52466 15 min before administration of DOI (5.0 mg/kg, s.c.) and then again 15 min after DOI challenge; animals were killed 90 min later.
Anatomical studies. To determine the distribution and numbers of Fos-like immunoreactive (-li) neurons after DOI treatment, animals (n = 4 per group) were injected with DOI (5.0 mg/kg, s.c.), and 2 hr later they were deeply anesthetized and perfused with PBS followed by 4% paraformaldehyde in PBS. Coronal frozen sections (42 μm) were cut through the forebrain, and free-floating sections were processed immunohistochemically for the demonstration of Fos-li following our previously described methods (Deutch et al., 1991,1998). We also determined the ability of DOI (n = 3 per group) to induce Zif/268, another immediate-early gene protein product. The numbers of immunoreactive nuclei within a 500-μm-wide column of the SI region of the SSC, extending from the white matter to the pial surface were counted by a person blind to the treatment condition of the animal. The numbers of Fos-li neurons per millimeter squared in various treatment groups were compared by ANOVAs and subsequent Bonferonni t tests when indicated.
We determined whether DOI administration induced Fos in neurons expressing the 5-HT2A receptor by first processing tissue sections to reveal Fos-li using an avidin–biotin method with heavy metal intensification, which resulted in a blue-black diaminobenzidine (DAB) reaction product in the nucleus of the cell. Sections were subsequently washed extensively and treated with methanolic-peroxide and then incubated in a mouse monoclonal 5-HT2A antibody (PharMingen, San Diego, CA; 1:2000). Sections were developed using a peroxidase anti-peroxidase (PAP) procedure with plain DAB as the chromogen to yield a brown reaction product marking 5-HT2A-li neurons. In some cases, adjacent sections were incubated in a mixture of the Fos and 5-HT2A antibodies, then washed extensively and incubated in Cy2- and Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA; 1:1500) in preparation for examination by confocal microscopy.
Six rats were injected with the anterograde tracer biotinylated dextran amine (BDA). A 10–15% solution of BDA (10,000 MW BDA; Molecular Probes, Eugene, OR) in 0.1 m phosphate buffer, pH 7.6, was iontophoretically deposited into the ventroposteromedial nucleus (VPM) (n = 3) or ventroposterolateral nucleus (VPL) (n = 3) through glass micropipettes (18–25 μm tips) using a pulsed positive current (5.0 μA, 7 sec on/off) for 10–20 min; these nuclei provide thalamic inputs to the SSC (Saporta and Kruger, 1977). Five to seven days after the operation, the animals were injected with DOI and perfused 2 hr later. Tissue was processed to reveal BDA-labeled axons in the SSC using peroxidase-labeled streptavidin and cobalt–nickel-intensified DAB as the chromogen, and subsequently processed to reveal Fos-li neurons using PAP immunohistochemistry.
Tissue from DOI-treated animals was also processed to reveal cytochrome oxidase (CO) staining, a high density of which marks layer IV and allows one to visualize the barrel fields of the SSC (Wong-Riley and Welt, 1980). Sections were processed for CO activity following the protocol of Wong-Riley and Welt (1980), and subsequently developed to reveal Fos-li neurons.
Immunoblot methods. Fos protein levels were also determined by Western blot methods, using total protein isolated from tissue homogenates of brain samples. Tissue samples were sonicated in 2% SDS, and an aliquot was removed for measurement of protein levels (Lowry et al., 1951). Each lane of 10% acrylamide/0.27% methylenebisacrylamide gels were loaded with 20 μg of protein and run overnight at 65 V, and the protein was then transferred to nitrocellulose; the gel was then stained with Coomassie Blue. The blots were incubated 4 [times] 15 min in 3% nonfat dry milk in a Tris-buffered saline solution (TBS+; 10 mm Tris, pH 7.4, containing 150 mm NaCl, and 0.1% Tween 20) and then incubated for ∼48 hr at 4°C in the Fos antibody (1:5000) in TBS/Blotto. The blots were washed in blocking buffer, incubated for 2 hr in HRP-conjugated secondary antibody (1:500; Vector Labs, Burlingame, CA), and then washed in TBS+before being developed using enhanced chemiluminescence and exposed to Hyperfilm-ECl (Amersham, Arlington Heights, IL).
Levels of Fos protein were determined using a previously characterized rabbit anti-Fos antibody generated against the M peptide (Quinn et al., 1989), which recognizes Fos-related antigens (Fras) as well as Fos. Fos protein levels were quantitated by measuring band optical densities using computer-assisted densitometry with the public domain NIH Image program (developed at National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image) after calibration with a Kodak optical step chart. Depending on electrophoretic resolution, an apparent doublet was seen at ∼58 kDa; this probably represents a post-translational modification of Fos.
Lesion studies. To assess the contribution of thalamocortical glutamatergic projections to DOI-elicited increases in SSC Fos expression, rats were subjected to NMDA lesions of the thalamic ventrobasal complex. Animals were anesthetized with pentobarbital, and 50 nmol NMDA in 1.5 μl PBS pH 7.4, or PBS vehicle was injected into each of two sites in the thalamus [coordinates (Paxinos and Watson, 1986): anteroposterior (AP): −2.9 from bregma; lateral (L): −2.4; dorsoventral (DV): −6.2 from skull surface and AP: −3.7; L: −2.4; DV: −5.8) at a rate of 0.5 μl/min. Animals were allowed to recover for 10–13 d and then injected with DOI and killed 2 hr later. The extent of the thalamic lesion was assessed by examination of serial toluidine blue-stained sections through the thalamus.
RESULTS
Distribution of Fos-li neurons in DOI-treated animals
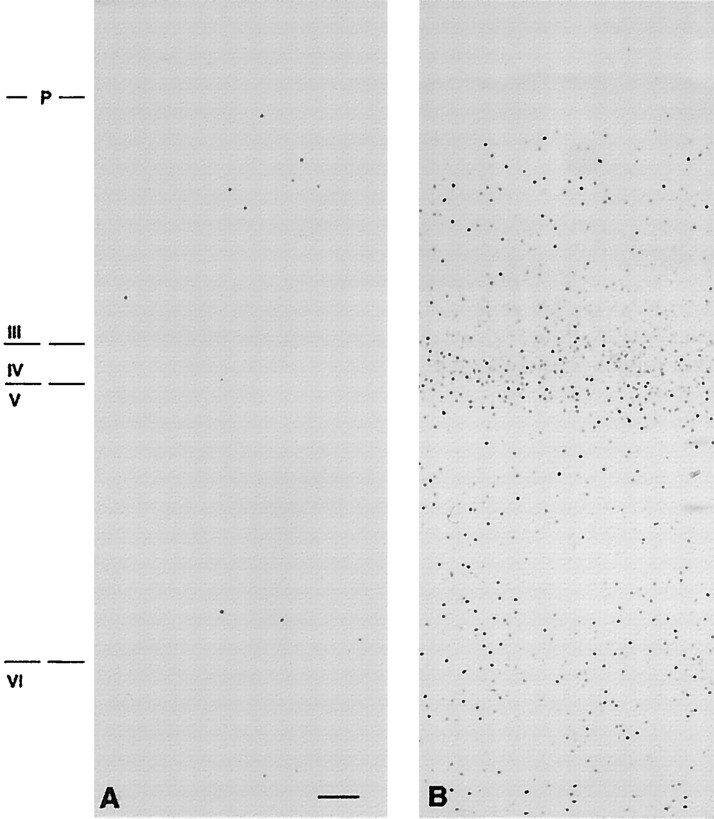
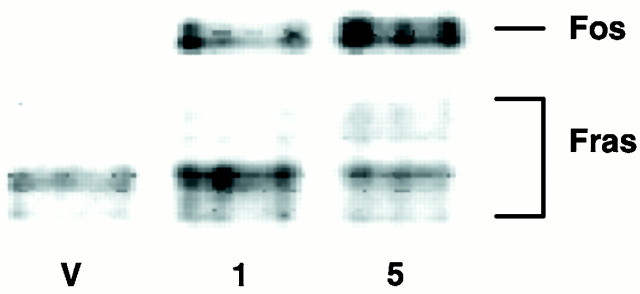
DOI treatment markedly induced Fos in the SSC of the rat, as revealed by both immunohistochemical (Fig.1) and immunoblot (Fig.2) methods. Immunoblot analysis showed that DOI dose-dependently increased Fos protein as well as several Fos-related antigens (Fig. 2). The anatomical studies revealed that Fos-li neurons were present throughout the SSC, including SI, and were most abundant in a dense band of cells in the most superficial aspects of layer V, layer IV, and deep layer III (Fig. 1). Very densely stained Fos-li neurons in layer VI, adjacent to the white matter, were observed. A similar pattern of Zif/268 induction was also seen (data not shown).
Fig. 1.
Immunohistochemical localization of Fos-li neurons in the somatosensory cortex of vehicle (A) and DOI-treated (B) rats. The digitized photomicrograph shows a strong band of immunoreactive cells spanning superficial layer V to layer III in animals challenged with 5.0 mg/kg DOI. Scale bar, 50 μm.
Fig. 2.
A representative immunoblot demonstrating that DOI increases expression of both Fos and several Fos-related antigens (Fras) in the SSC of the rat. Both 1.0 and 5.0 mg/kg DOI increased Fos expression relative to vehicle (V), with a greater effect seen at the higher dose. Fras were also increased slightly in response to DOI challenge.
The Fos-li cells that comprised the “band” in layers V–III appeared subjectively to be of two types: intensely immunoreactive Fos cells, which were located in superficial layer V, neurons with lightly stained Fos nuclei, which were mainly seen in layers IV and deep III (Fig. 1). Injections of the anterograde tracer BDA into the VPM resulted in a densely labeled plexus of fibers in the cortex that was largely in register with the lightly stained Fos-li neurons, whereas those cells exhibiting intense Fos immunoreactivity were usually beneath the dense plexus of BDA-labeled fibers, in layer Va (Fig. 3A).
Fig. 3.
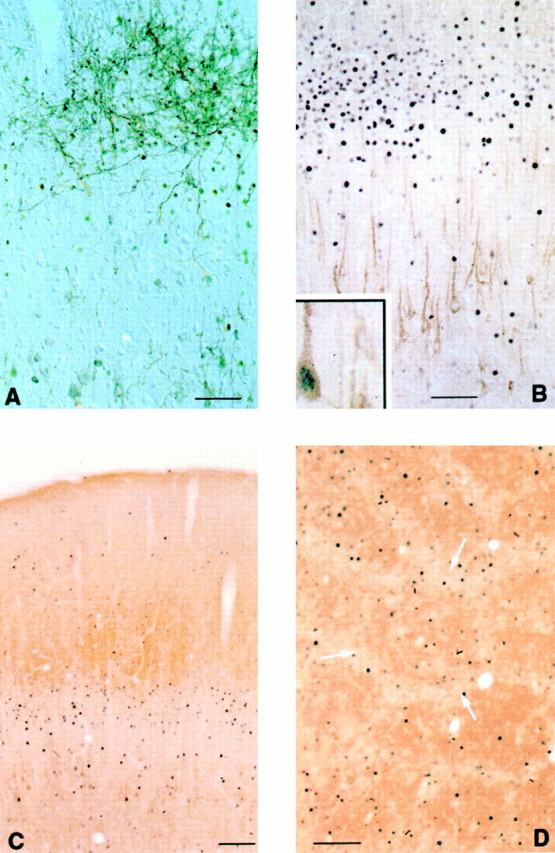
The effects of acute DOI challenge (5.0 mg/kg) on Fos expression in the SSC. A, Fos-li neurons can be seen in register with the distribution of a cluster of anterogradely labeled axons after BDA injection of the VPM. The neurons with more densely stained nuclei are just inferior to the BDA-labeled fibers, in superficial layer V. The lightly stained cells in layer IV are somewhat obscured by the dense plexus of BDA-labeled fibers. B, Dual staining for 5-HT2A- and Fos-like immunoreactivities reveals that DOI does not induce Fos in the 5-HT2A-li neurons of layer V, but instead in neurons located in the apical dendritic fields of the 5-HT2A-li neurons. These Fos-li neurons are present in the superficial-most aspect of layer V (intensely Fos-immunoreactive nuclei) and in layers IV and III (less densely stained cells). Rarely, a 5-HT2A-li pyramidal cell that does express Fos can be observed (B,inset). C, DOI-induced Fos appears in a band of cells that overlaps layer IV as visualized by dense CO staining. The intensely labeled Fos-li neurons in superficial aspects of layer Va are ventral to the dense CO staining, whereas the lightly stained Fos-li nuclei in layer IV are difficult to see. These lightly stained cells can be visualized more clearly in sections cut tangential to the pial surface (D), where Fos-li neurons are seen both in the barrels of the SSC (more densely reactive CO staining) and in interbarrel septa (arrows), but Fos-li neurons are more concentrated in the septa. Scale bars, 100 μm.
In sections histochemically stained for CO, a similar picture emerged: many of the more lightly stained Fos-li neurons were seen in layer IV (as revealed by dense CO staining), with the more densely immunoreactive neurons being seen in superficial layer V, just ventral to the dense CO-positive neuropil marking layer IV (Fig.3C). Sections of flat mounts of the SSC that were stained for Fos and CO revealed that Fos-li neurons were found in both barrels and interbarrel septa, but were most prominent in the septa (Fig.3D). The ratio of Fos-li cells in the septa/barrels was 3.26, with the density of Fos-li neurons being significantly greater in the septa (t12 = 5.062; p≤ 0.0005).
DOI-elicited Fos expression in 5-HT2A-li neurons
Dual staining for Fos- and 5-HT2A-li neurons revealed that DOI treatment did not induce Fos in most cortical 5-HT2A-li neurons. Instead, the band of Fos-li neurons in layers V–III were superficial to the 5-HT2A-li pyramidal cells of layer V, being located in the fields of the apical dendrites of the 5-HT2A-li neurons (Fig. 3B). Although the great majority of 5-HT2A-li neurons did not express detectable Fos immunoreactivity, rare double-labeled cells were seen (Fig. 3B, inset).
Effects of 5-HT2 antagonists on DOI-elicited Fos expression
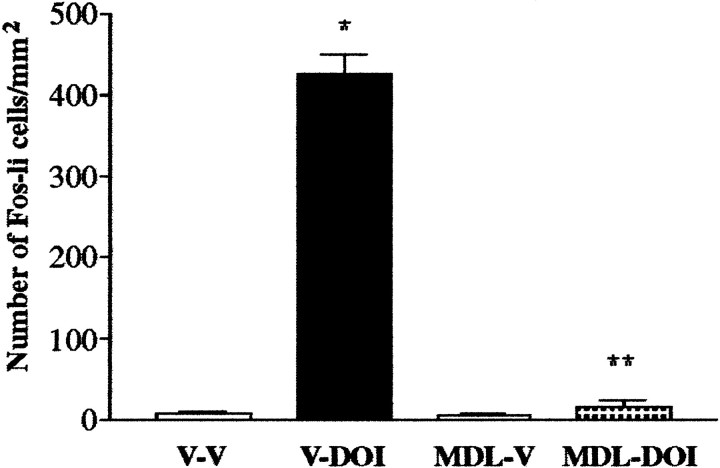
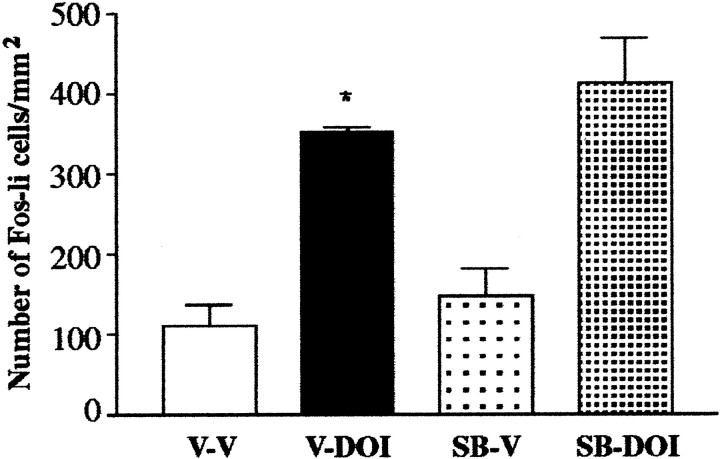
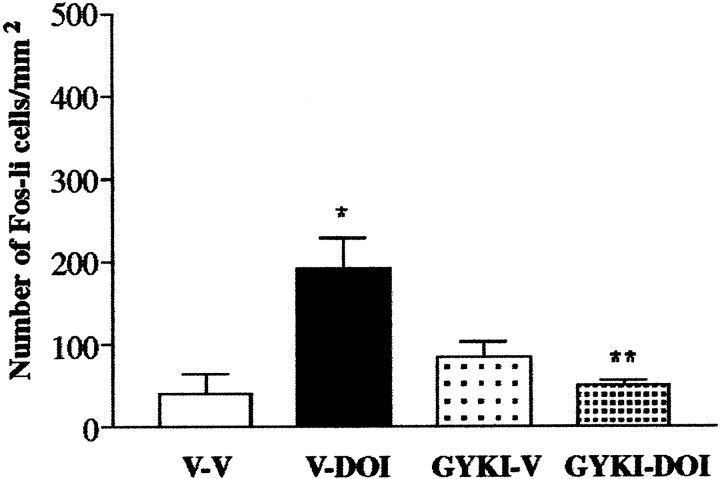
ANOVA revealed a significant treatment effect when the effects of MDL 100,907 on DOI-elicited Fos expression (F(3,15) = 303.2; p < 0.0001) were examined. Post hoc tests revealed that the 5-HT2A antagonist MDL 100,907 blocked the ability of DOI to elicit an increase in the number of Fos-li neurons in the SSC (Fig. 4); the antagonist by itself did not change the number of Fos-li neurons relative to vehicle-injected control subjects. The 5-HT2C antagonist SB 206,553 did not attenuate DOI-elicited Fos expression in SSC neurons (Fig. 5).
Fig. 4.
Pretreatment with the selective 5-HT2Aantagonist MDL 100,907 completely blocks DOI-elicited Fos induction in the SSC. V-V, Animals pretreated with vehicle followed 30 min later by vehicle; V-DOI, vehicle-pretreated rats injected with DOI; MDL-V, pretreatment with MDL 100,907 with subsequent vehicle administration; MDL-DOI, pretreatment with MDL 100,907 followed 30 min later by administration of DOI. *p ≤ 0.0001 relative to V-V; **p≤ 0.001 relative to V-DOI.
Fig. 5.
Pretreatment with the 5-HT2Cantagonist SB 206,553 does not attenuate DOI-elicited Fos expression in the SSC. SB 206,553 neither blunted the effect of DOI nor had any effect of its own. Abbreviations as in Figure 4, except that the pretreatment condition is SB 205,553 (SB). *p ≤ 0.005 relative to V-V.
Effects of AMPA antagonist pretreatment on DOI-induced Fos expression
One-way ANOVA revealed a significant (F(3,11) = 8.07; p = 0.0084) treatment effect, with post hoc tests showing that pretreatment with the AMPA/KA antagonist GYKI 52466 blocked the ability of DOI to induce Fos in the SSC (Fig. 6). Administration of GYKI 52466 followed by vehicle did not result in any significant changes in Fos expression in the cortex nor did the animals treated with GYKI 52466 and DOI differ significantly from vehicle-treated animals.
Fig. 6.
Treatment with the AMPA/KA antagonist GYKI 52466 completely blocks DOI-induced Fos expression in the SSC.p ≤ 0.0001 relative to V-V; **p ≤ 0.001 relative to V-DOI.
Effects of thalamic lesions on DOI-elicited Fos expression
The large NMDA lesions typically involved most of the VPM, the VPL, and the medial posterior nucleus (Pom). In addition, the laterodorsal and lateroposterior nuclei were frequently involved, as was the ventroanterolateral nucleus (Fig.7). However, the most posterior aspects of the VPM and Pom, as well as the central lateral nucleus, which also projects to the SSC (Jones and Leavitt, 1974; Nothias et al., 1988), were often spared. Almost all lesions resulted in some neuronal loss in the CA3 field of the dorsal hippocampus, which does not project to the SSC.
Fig. 7.
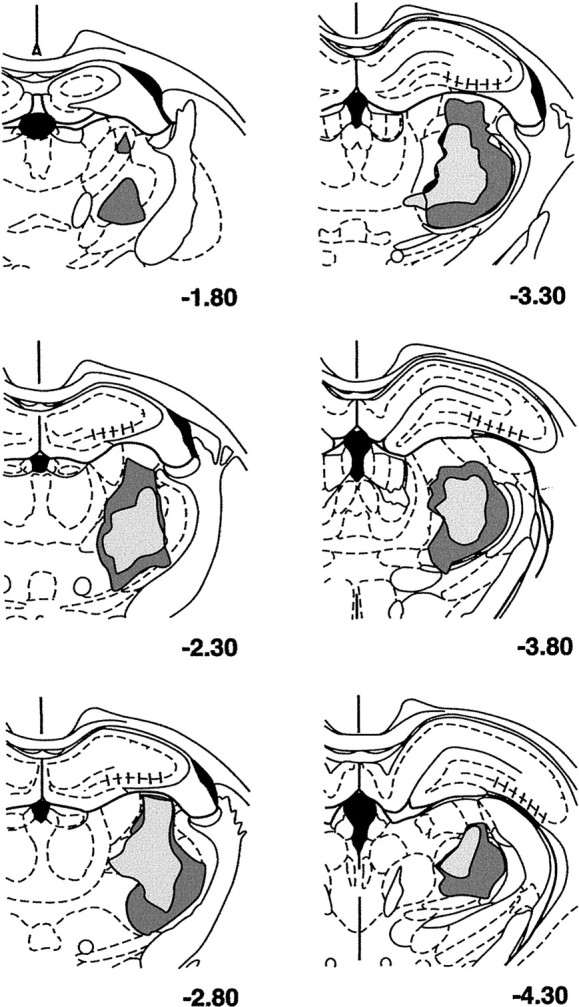
Reconstruction of largest (dark stipple) and smallest (light stipple) thalamic lesions, as charted on plates of the atlas of Paxinos and Watson (1986); numbers refer to the atlas plates. The smallest lesions involved most of the Pom and much of the VPM, as well as other dorsal thalamic nuclei. The largest lesions involved a greater proportion of the VPM, and also typically damaged most of VPL and encroached on the thalamic reticular nucleus. Almost all lesions resulted in neuronal loss in the CA3 field of the dorsal hippocampus (indicated by hatching).
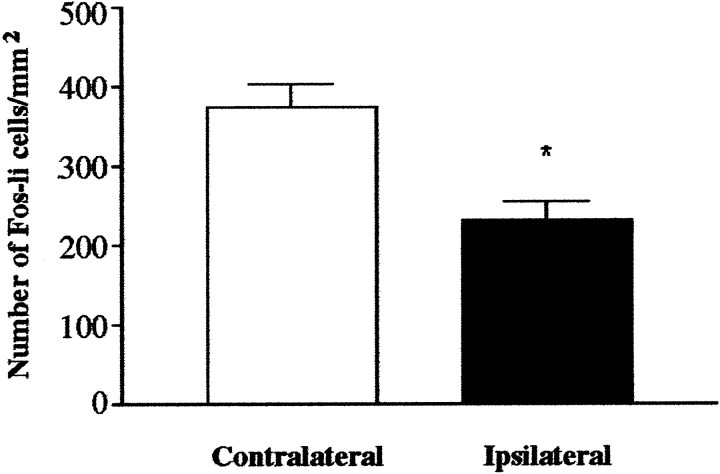
The thalamic lesion attenuated the ability of DOI challenge to induce Fos, with a significant decrease in the number of Fos-li neurons in the ipsilateral SSC relative to the contralateral (intact) SSC (Fig.8). The magnitude of DOI-elicited Fos expression in the intact (nonlesioned) side of lesioned animals and those animals receiving vehicle infusions was the same. The lesion effect appeared to be more pronounced in the supragranular than infragranular layers of the SSC.
Fig. 8.
NMDA lesions of the thalamus decrease the number of Fos-li neurons in the cortex in response to DOI challenge. The number of Fos-li neurons in the SSC on the side of the lesion drops sharply relative to the intact side. There was no significant difference (p = 0.200) between the number of Fos-li neurons in the contralateral (intact) SSC of sham- and lesioned-treated rats. *p 163 ≤ 0.001 relative to intact (contralateral) side.
DISCUSSION
Anatomical organization of DOI-elicited Fos induction
Our findings agree with earlier studies reporting that DOI induces Fos throughout the parietal cortex but most prominently in a band of cells (Moorman and Leslie, 1996, 1998; Maćkowiak et al., 1999). Previous studies reported that this band of Fos-li cells is in layer V. We found that the dense aggregation of Fos-li neurons extends into layers IV and III, as defined by the presence of anterogradely labeled axons of ventrobasal thalamic origin and dense CO staining. This band of cells includes two cell types: those with intensely immunoreactive Fos nuclei that are mainly in superficial Va, and less densely stained cells concentrated in layers III/IV.
Defining the barrels and septa of SI with CO histochemistry revealed that DOI induced Fos in both SSC compartments, but activated neurons of the septa to a significantly greater degree. Thalamic projections to the septa and barrels originate in the Pom and VPM, respectively (Koralek et al., 1988; Lu and Lin, 1993), and there are physiological differences between the compartments (Chiaia et al., 1991). Neurons of the septa and barrel also differ in their projections: axons of barrel cells are confined locally, whereas neurons in the septa project to other cortical regions (Akers and Killackey, 1978; Kim and Ebner, 1999). Because DOI predominantly induces Fos in the septa, it appears likely that DOI activates intercortical projections.
5-HT2A receptors subserve DOI-induced Fos expression
Our anatomical data indicate that DOI induces Fos in cortical neurons that typically do not express 5-HT2A-li, yet our pharmacological data suggest that DOI-elicited Fos expression depends on 5-HT2A but not 5-HT2C receptors. Maćkowiak et al. (1999)recently reported that DOI never induced Fos in 5-HT2A-li neurons. We observed Fos in a few 5-HT2A-li neurons, suggesting that the lack of Fos in most cortical 5-HT2A-li neurons does not reflect an inability of these cells to express Fos.
Several factors argue that the ability of DOI to induce cortical Fos depends on 5-HT2A receptors. Neurons in layer V express 5-HT2A mRNA (Wright et al., 1995), where we observed 5-HT2A-li neurons. We found previously that different 5-HT2A antibodies generated against different domains of the receptor stain the same cortical neurons (Willins et al., 1997; Bubser et al., 2000). MDL100,907 pretreatment blocked the effects of DOI; the affinity of MDL 100,907 for the 5-HT2A receptor is ∼100-fold higher than for the 5-HT2C receptor (Kehne et al., 1996). Moreover, a high dose of the 5-HT2Cantagonist SB 206553, well above the maximal dose for blocking diverse 5-HT2C-mediated events (Kennett et al., 1996; Griebel et al., 1997; Millan et al., 1997a,b), did not attenuate DOI-elicited Fos expression. Consistent with our data,Gingrich et al. (1999) noted that DOI does not induce Fos in 5-HT2A knockout mice.
The lack of DOI-elicited Fos in cortical 5-HT2A-li cells may be attributable to a lack of access of the agonist to the receptor. Pyramidal cell 5-HT2A immunoreactivity is prominently localized to the apical dendrite, where recent immunoelectron microscopic studies have concluded that most 5-HT2A is intracellular and not membrane associated (Jakab and Goldman-Rakic, 1998;Cornea-Hebert et al., 1999a,b). The degree to which the receptor is present and accessible (membrane-associated) in axon terminals is unclear.
Glutamate release determines DOI-induced cortical Fos expression
Consistent with electrophysiological data indicating that suppression of glutamate release or blockade of AMPA receptors attenuates serotonin-elicited EPSCs in layer V pyramidal cells (Aghajanian and Marek, 1999; Marek and Aghajanian, 1999; Marek et al., 2000), we found that pretreatment with an AMPA/KA antagonist completely blocked DOI-elicited cortical Fos expression. Among the sources of glutamatergic projections to the SSC are neurons in the ventrobasal thalamus, which express 5-HT2A immunoreactivity and mRNA (Cornea-Hebert et al., 1999a; Cyr et al., 2000). Lesions of the ventrobasal thalamus significantly attenuated DOI-induced Fos expression, consistent with our hypothesis that DOI interacts with 5-HT2A receptors on thalamocortical neurons.
Administration of GYKI 52466 completely blocked DOI-elicited Fos expression, whereas thalamic lesions significantly attenuated but did not eliminate the response to DOI. The failure of the thalamic lesions to completely block DOI effects is probably attributable to the incomplete lesions that we obtained. Alternatively, DOI may also act at other SSC afferents, including cortical sites, the claustrum, and the basal forebrain. Cortical projection neurons are glutamatergic, whereas basal forebrain projections to the SSC include cholinergic and GABAergic cells and a third group of cells in which the transmitter is unknown (Gritti et al., 1997).
Recent studies have concluded that activation of glutamatergic afferents to the cortex may dampen the activity of nearby GABAergic interneurons via activation of metabotropic glutamatergic (type III) receptors on the interneurons (Mitchell and Silver, 2000). This presumably allows for a greater effect in the cortex, including a broadening of stimulus-elicited changes in activity (Moore et al., 1999). Accordingly, DOI may drive thalamic projections to the SSC and also trans-synaptically activate cortical areas that are the targets of SSC projection neurons, thereby contributing to the widespread cortical Fos expression seen in response to 5-HT2Ahallucinogens.
Neural circuitry of DOI-elicited cortical activation
Electrophysiological studies examining the effects of serotonin on pyramidal cells have yielded results that are strikingly congruent with our data. Aghajanian and Marek (1997) found that serotonin and DOI evoke EPSCs in layer V pyramidal cells if the agonist is applied near the “hot spot” on the apical dendrite, but not when applied to the proximal apical or basilar dendrites or soma. We found that the DOI-induced band of Fos-li neurons amid the apical dendrites of layer V 5-HT2A-li neurons corresponds to the laminar position of the hot spot.
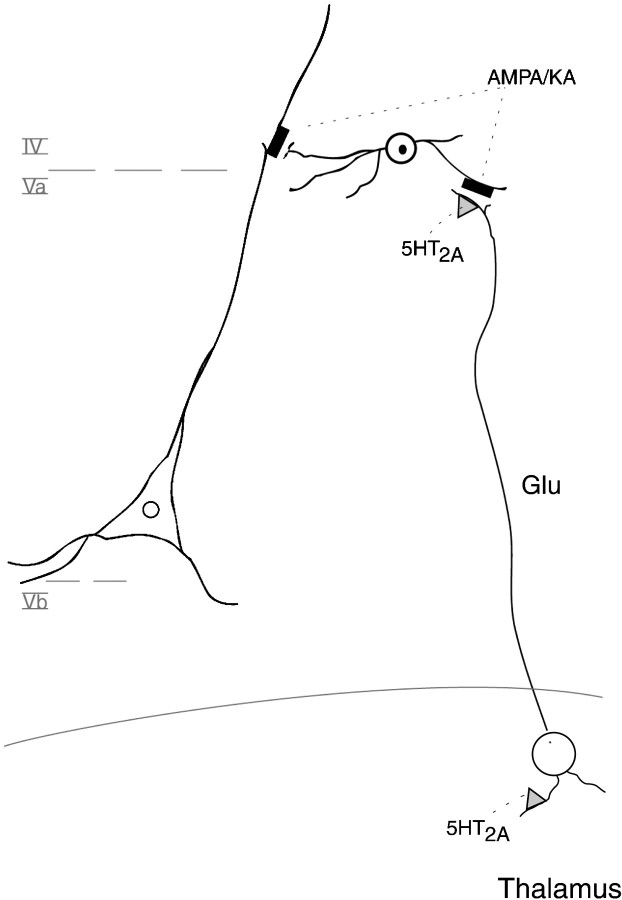
We interpret our data to indicate that DOI induces Fos in cortical neurons by acting on 5-HT2A receptors located on glutamatergic thalamic afferents to the SSC. Marek and Aghajanian arrived at the same conclusion (Aghajanian and Marek, 1999; Marek and Gewirtz, 1999; Marek et al., 2000). Serotonin-elicited EPSCs in layer V pyramidal cells do not appear to be sufficient to generate either Fos expression or an action potential. Because disruption of glutamatergic transmission blocks 5-HT2A-elicited EPSCs, Marek et al. (2000) suggested that axons with 5-HT2Aheteroceptors release glutamate, which then targets AMPA receptors on the apical dendrites of pyramidal cells. Our data, although consistent with this interpretation, suggest another possible scenario (Fig.9). DOI may interact with 5-HT2A receptors on thalamocortical neurons to activate (as reflected by Fos expression) certain cortical glutamatergic neurons, which in turn synapse asymmetrically onto apical dendrites of 5-HT2A-li pyramidal cells. This model fits well with the observation of Jakab and Goldman-Rakic (1998), who noted that axons that make asymmetric (excitatory) synapses with pyramidal cell dendrites rarely express 5-HT2A-li. In view of the low abundance of membrane-associated 5-HT2A-li in axons, DOI may activate 5-HT2A receptors on somatodendritic regions of thalamocortical neurons.
Fig. 9.
A hypothetical model of the mechanisms underlying DOI-induced cortical Fos expression. DOI does not directly target the layer V 5-HT2A-li pyramidal neuron, but instead acts on a 5-HT2A receptor on thalamocortical neurons, which impinge on excitatory (glutamatergic) neurons in the apical dendritic field of the pyramidal cell. Activation of this 5-HT2A receptor leads to glutamate release, which results in Fos expression in excitatory neurons of layers V–III. In turn, the glutamate released by these neurons, which form synapses onto the apical dendritic fields of the pyramidal cell, effects EPSCs (but not action potentials) in the pyramidal cell. The ability of an AMPA/KA antagonist to block the effects of DOI indicates the presence of a crucial glutamatergic receptor, which may be on both of the layer V–III neurons that express Fos and the pyramidal cell.
Functional considerations
DOI and LSD are 5-HT2A partial agonists (McClue et al., 1989; Glennon, 1992; Marek and Aghajanian, 1996), and DOI substitutes for LSD in drug discrimination studies (Arnt, 1989;Chojnacka-Wojcik and Klodzinska, 1997). The hallucinogen psilocybin, which results in extensive cortical activation in humans (Vollenweider et al., 1997, 1998), induces psychotic symptoms in humans by acting at 5-HT2A receptors (Vollenweider et al., 1998). The magnitude of psilocybin-induced increases in cortical glucose metabolism differs somewhat in different brain regions, but activation is seen across the cortex. Such global activation resembles that seen in response to DOI, as reflected by Fos expression, and suggests that hallucinogens may drive the cortex indirectly, by acting on thalamocortical neurons. However, the fact that 5-HT2A receptors are not present in many thalamic nuclei (Wright et al., 1995) suggests that hallucinogens may preferentially activate distinct parts of the cortex, which in turn drive other cortical sites. Our data suggest that DOI activates intercortical projections, because Fos was increased in the projection (septa) neurons of the SSC. It is possible that the synesthesia reported in response to hallucinogens may be related to alterations in intercortical activation.
The 5-HT2A receptor is the major target of DOI, LSD, and related hallucinogens, although some actions of LSD may be mediated through the 5-HT2C receptor (Fiorella et al., 1995a,b; Krebs-Thomson et al., 1998; Smith et al., 1998). We found that DOI induces cortical Fos through a 5-HT2Areceptor mechanism. However, the mechanisms subserving DOI effects on neuronal firing differ somewhat between the orbitofrontal and medial prefrontal cortices (Bergqvist et al., 1999). It will be important to determine whether the actions of DOI, LSD, and other hallucinogens are subserved by the same mechanisms in different cortical regions (Gresch and Sanders-Bush, 2000).
The 5-HT2A receptor is an important target of atypical antipsychotic drugs (Fatemi et al., 1996). The role of 5-HT2A receptors in schizophrenia and antipsychotic drug actions has been studied extensively (Meltzer and Deutch, 1998; Meltzer, 1999), but 5-HT2A–glutamate interactions have received relatively little attention in studies of schizophrenia (Aghajanian and Marek, 2000). Recent studies have suggested that suppression of glutamate release may be a novel treatment strategy for schizophrenia (Moghaddam and Adams, 1998; Pellicciari and Costantino, 1999;Aghajanian and Marek, 2000). Our findings provide a mechanism for understanding how 5-HT2A antagonists and suppression of glutamate transmission may be useful in treating schizophrenia.
Footnotes
This work was supported in part by National Institutes of Health Grants MH-45124 and MH-57995 (A.Y.D.) and T32 MH-19732 (J.L.S.), by a NARSAD Young Investigator Award (M.B.), and by the National Parkinson Foundation Center of Excellence at Vanderbilt University. We appreciate the technical assistance of Tara Signoracci. We are indebted to Drs. Gerard Marek and George Aghajanian for extended discussions of their data, and Drs. Ford Ebner, Peter Melzer, and Elaine Sanders-Bush for helpful comments and advice. We acknowledge Dr. Christopher Schmidt (Marrion-Merrill Dow) for providing MDL 100,907.
REFERENCES
- 1.Abi-Saab W, Bubser M, Roth RH, Deutch AY. 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology. 1999;20:92–96. doi: 10.1016/S0893-133X(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 3.Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- 4.Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 5.Akers RM, Killackey HP. Organization of corticocortical connections in the parietal cortex of the rat. J Comp Neurol. 1978;181:513–537. doi: 10.1002/cne.901810305. [DOI] [PubMed] [Google Scholar]
- 6.Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teitler M, DeSouza EB. Autoradiographic characterization of (±)-1-(2,5-dimethoxy-4-[125I]iodophenyl)-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1c receptors in rat brain. J Pharmacol Exp Ther. 1990;255:843–857. [PubMed] [Google Scholar]
- 7.Arnt J. Characterization of the discriminative stimulus properties induced by 5-HT1 and 5-HT2 agonists in rats. Pharmacol Toxicol. 1989;64:165–172. doi: 10.1111/j.1600-0773.1989.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 8.Bennett-Clarke CA, Chiaia NL, Rhoades RW. Contributions of raphe-cortical and thalamocortical axons to the transient somatotopic pattern of serotonin immunoreactivity in rat cortex. Somatosens Mot Res. 1997;14:27–33. doi: 10.1080/08990229771196. [DOI] [PubMed] [Google Scholar]
- 9.Bergqvist PB, Dong J, Blier P. Effect of atypical antipsychotic drugs on 5-HT2 receptors in the rat orbito-frontal cortex: an in vivo electrophysiological study. Psychopharmacology. 1999;143:89–96. doi: 10.1007/s002130050923. [DOI] [PubMed] [Google Scholar]
- 10.Bleakman D, Ballyk BA, Schoepp DD, Palmer AJ, Bath CP, Sharpe EF, Woolley ML, Bufton HR, Kamboj RK, Tarnawa I, Lodge D. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology. 1996;35:1689–1702. doi: 10.1016/s0028-3908(96)00156-6. [DOI] [PubMed] [Google Scholar]
- 11.Bubser M, Backstrom JR, Sanders-Bush E, Roth B, Deutch AY (2000) Localization of the serotonin 5-HT2A receptor in striatal afferents. Synapse, in press. [DOI] [PubMed]
- 12.Chapin JK, Lin C-S. The somatic sensory cortex of the rat. In: Kolb B, Tees RC, editors. The cerebral cortex of the rat. MIT; Cambridge, MA: 1990. pp. 341–379. [Google Scholar]
- 13.Chiaia NL, Rhoades RW, Fish SE, Killackey HP. Thalamic processing of vibrissal information in the rat. II. Morphological and functional properties of medial ventral posterior nucleus and posterior nucleus neurons. J Comp Neurol. 1991;314:217–236. doi: 10.1002/cne.903140203. [DOI] [PubMed] [Google Scholar]
- 14.Chojnacka-Wojcik E, Klodzinska A. Involvement of 5-hydroxytryptamine 2A (5-HT2A) receptors in the mediation of the discriminative stimulus properties of (+/−)DOI in rats. Pol J Pharmacol. 1997;49:299–304. [PubMed] [Google Scholar]
- 15.Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999a;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Cornea-Hebert V, Watkins KC, Willins DL, Roth BL, Descarries L. Intracellular distribution of the 5-HT2A serotonin receptor in rat cortical neurons. Soc Neurosci Abstr. 1999b;25:1204. [Google Scholar]
- 17.Cyr M, Landry M, Di Paolo T. Modulation by estrogen-receptor directed drugs of 5-hydroxytryptamine-2A receptors in rat brain. Neuropsychopharmacology. 2000;23:69–78. doi: 10.1016/S0893-133X(00)00085-3. [DOI] [PubMed] [Google Scholar]
- 18.Deutch AY, Lee MC, Gillham MH, Cameron D, Goldstein M, Iadorola MJ. Stress selectively increases Fos protein in dopamine neurons innervating the prefrontal cortex. Cereb Cortex. 1991;1:273–292. doi: 10.1093/cercor/1.4.273. [DOI] [PubMed] [Google Scholar]
- 19.Deutch AY, Bubser M, Young CD. Psychostimulant-elicited Fos protein induction in the thalamic paraventricular nucleus. J Neurosci. 1998;18:10680–10687. doi: 10.1523/JNEUROSCI.18-24-10680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebner FF, Armstrong-James MA. Intracortical processes regulating the integration of sensory information. Prog Brain Res. 1990;86:129–141. doi: 10.1016/s0079-6123(08)63172-6. [DOI] [PubMed] [Google Scholar]
- 21.Erdtmann-Vourliotis M, Mayer P, Riechert U, Hollt V. Acute injections of drugs with low addictive potential (delta(9)tetrahydrocannabinol, 3,4-methylenedioxymethamphetamine, lysergic acid diamide) causes a much higher c-fos expression in limbic brain areas than high addicting drugs (cocaine and morphine). Mol Brain Res. 1999;71:313–324. doi: 10.1016/s0169-328x(99)00207-7. [DOI] [PubMed] [Google Scholar]
- 22.Fatemi HS, Meltzer HY, Roth BL. Atypical antipsychotic drugs: clinical and preclinical studies. Handb Exp Pharmacol. 1996;120:77–115. [Google Scholar]
- 23.Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology. 1995a;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- 24.Fiorella D, Helsley S, Lorrain DS, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. III: The mechanistic basis for supersensitivity to the LSD stimulus following serotonin depletion. Psychopharmacology. 1995b;121:364–372. doi: 10.1007/BF02246076. [DOI] [PubMed] [Google Scholar]
- 25.Gingrich JA, Zhou M, Sealfon S, Hen R. Mice lacking the 5-HT2A receptor are insensitive to many of the behavioral and physiological effects of hallucinogens. Soc Neurosci Abstr. 1999;25:799. [Google Scholar]
- 26.Glennon RA. Central serotonin receptors as targets for drug research. J Med Chem. 1992;30:1–12. doi: 10.1021/jm00384a001. [DOI] [PubMed] [Google Scholar]
- 27.Gresch PJ, Sanders-Bush E. LSD induced c-fos expression in rat brain: role of serotonin-2A and serotonin-2C receptors. Soc Neurosci Abstr. 2000;26:119. doi: 10.1016/s0306-4522(02)00349-4. [DOI] [PubMed] [Google Scholar]
- 28.Griebel G, Perrault G, Sanger DJ. A comparative study of the effects of selective and nonselective 5-HT2 receptor subtype antagonists in rat and mouse models of anxiety. Neuropharmacology. 1997;36:793–802. doi: 10.1016/s0028-3908(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 29.Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- 30.Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones EG, Leavitt RY. Retrograde axonal transport and the demonstration of non-specific projections to the cerebral cortex and striatum from thalamic intralaminar nuclei in the rat, cat, and monkey. J Comp Neurol. 1974;154:349–378. doi: 10.1002/cne.901540402. [DOI] [PubMed] [Google Scholar]
- 32.Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, Van Giersbergen PLM, McCloskey TC, Johnson MP, McCarty DA, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- 33.Kennett GA, Wood MD, Bright F, Cilia J, Piper DC, Gager T, Thomas D, Baxter GS, Forbes IT, Ham P, Blackburn TP. In vitro and in vivo profile of SB 200553, a potent 5-HT2A/5-HT2C receptor antagonist with anxiolytic-like properties. Br J Pharmacol. 1996;117:427–434. doi: 10.1111/j.1476-5381.1996.tb15208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim U, Ebner FF. Barrels and septa: separate circuits in rat barrels field cortex. J Comp Neurol. 1999;408:489–505. [PubMed] [Google Scholar]
- 35.Koralek KA, Jensen KF, Killackey HP. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res. 1988;453:346–351. doi: 10.1016/0006-8993(88)90408-8. [DOI] [PubMed] [Google Scholar]
- 36.Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- 37.Leslie RA, Moorman JM, Grahame-Smith DG. Lithium enhances 5-HT2A receptor-mediated c-fos expression in rat cerebral cortex. NeuroReport. 1993;5:241–244. doi: 10.1097/00001756-199312000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 39.Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res. 1993;10:1–16. doi: 10.3109/08990229309028819. [DOI] [PubMed] [Google Scholar]
- 40.Maćkowiak M, Chocyk A, Fijal K, Czyrak A, Wȩdzony K. c-Fos proteins, induced by the serotonin receptor agonist DOI, are not expressed in 5-HT2A positive cortical neurons. Mol Brain Res. 1999;71:358–363. doi: 10.1016/s0169-328x(99)00195-3. [DOI] [PubMed] [Google Scholar]
- 41.Mansour-Robaey S, Mechawar N, Radja F, Beaulieu C, Descarries L. Quantified distribution of serotonin transporter and receptors during the postnatal development of the rat barrel field cortex. Dev Brain Res. 1998;107:159–163. doi: 10.1016/s0165-3806(98)00016-9. [DOI] [PubMed] [Google Scholar]
- 42.Marek GJ, Aghajanian GK. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J Pharmacol Exp Ther. 1996;278:1373–1382. [PubMed] [Google Scholar]
- 43.Marek GJ, Aghajanian GK. 5-Hydroxytryptamine-induced excitatory postsynaptic currents in neocortical layer V pyramidal cells: suppression by mu-opiate receptor activation. Neuroscience. 1998;86:485–497. doi: 10.1016/s0306-4522(98)00043-8. [DOI] [PubMed] [Google Scholar]
- 44.Marek GJ, Aghajanian GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- 45.Marek GJ, Gewirtz JC. Serotonin2A receptor-induced EPSCs in layer V pyramidal cells of prefrontal cortex: block by lesions of the medial thalamus. Soc Neurosci Abstr. 1999;25:438. [Google Scholar]
- 46.Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine2A and group II metabotrophic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- 47.McClue SJ, Brazell C, Stahl SM. Hallucinogenic drugs are partial agonists of the human platelet shape change response: a physiological model of the 5-HT2 receptor. Biol Psychiatry. 1989;26:297–302. doi: 10.1016/0006-3223(89)90042-5. [DOI] [PubMed] [Google Scholar]
- 48.Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106.S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 49.Meltzer HY, Deutch AY. Neurochemistry and neurobiology of schizophrenia. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic neurochemistry: molecular, cellular, and medical aspects, Ed 6. Lippincott-Raven; New York: 1998. pp. 1053–1072. [Google Scholar]
- 50.Mengod G, Pompeiano M, Martinez-Mir MI, Palacios JM. Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 1990;524:139–143. doi: 10.1016/0006-8993(90)90502-3. [DOI] [PubMed] [Google Scholar]
- 51.Millan MJ, Girardon S, Bervoets K. 8-OH-DPAT-induced spontaneous tail-flicks in the rat are facilitated by the selective serotonin (5-HT)2C agonist, RO 60–0175: blockade of its actions of the novel 5-HT2C receptor antagonist SB 206,553. Neuropharmacology. 1997a;36:743–745. doi: 10.1016/s0028-3908(97)00071-3. [DOI] [PubMed] [Google Scholar]
- 52.Millan MJ, Peglion J-L, Lavielle G, Perrin-Monneyrom S. 5-HT2C receptors mediate penile erections in rats: actions of novel and selective agonists and antagonists. Eur J Pharmacol. 1997b;325:9–12. doi: 10.1016/s0014-2999(97)89962-1. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell SJ, Silver RA. Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature. 2000;404:498–505. doi: 10.1038/35006649. [DOI] [PubMed] [Google Scholar]
- 54.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 55.Molineaux SM, Jessell TM, Axel R, Julius D. 5-HT1c receptor is a prominent serotonin receptor subtype in the central nervous system. Proc Natl Acad Sci USA. 1989;86:6793–6797. doi: 10.1073/pnas.86.17.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore CI, Nelson SB, Sur M. Dynamics of neuronal processing in rat somatosensory cortex. Trends Neurosci. 1999;22:513–520. doi: 10.1016/s0166-2236(99)01452-6. [DOI] [PubMed] [Google Scholar]
- 57.Moorman J, Leslie RA. P-chloroamphetamine induces c-fos in rat brain: a study of serotonin2A/2C receptor function. Neuroscience. 1996;72:129–139. doi: 10.1016/0306-4522(95)00553-6. [DOI] [PubMed] [Google Scholar]
- 58.Moorman JM, Leslie RA. Paradoxical effects of lithium on serotonergic receptor function: an immunocytochemical, behavioural and autoradiographic study. Neuropharmacology. 1998;37:357–374. doi: 10.1016/s0028-3908(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 59.Moorman JM, Grahame-Smith DG, Smith SE, Leslie RA. Chronic electroconvulsive shock enhances 5-HT2 receptor-mediated head shakes but not brain C-fos induction. Neuropharmacology. 1996;35:303–313. doi: 10.1016/0028-3908(95)00167-0. [DOI] [PubMed] [Google Scholar]
- 60.Nothias F, Peschanski M, Besson M-J. Somatotopic reciprocal connections between the somatosensory cortex and the thalamic Po nucleus in the rat. Brain Res. 1988;447:169–174. doi: 10.1016/0006-8993(88)90980-8. [DOI] [PubMed] [Google Scholar]
- 61.Patel S, Scruggs JL, Abi-Saab W, Bubser M, Deutch AY. DOI elicits cortical Fos expression via 5-HT2A receptors but does not do so in 5-HT2A-expressing cells. Soc Neurosci Abstr. 1999;25:1660. [Google Scholar]
- 62.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 2. Academic; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- 63.Pellicciari R, Costantino G. Metabotropic G-protein-coupled glutamate receptors as therapeutic targets. Curr Opin Chem Biol. 1999;3:433–440. doi: 10.1016/S1367-5931(99)80064-7. [DOI] [PubMed] [Google Scholar]
- 64.Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 65.Quinn JP, Takimoto M, Iadarola MJ, Holbrook N, Levens D. Distinct factors bind the AP-1 consensus sites in gibbon ape leukemia virum and simian virus 40 enhancers. J Virol. 1989;63:1737–1742. doi: 10.1128/jvi.63.4.1737-1742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saporta S, Kruger L. The organisation of thalamocortical relay neurons in the ventrobasal complex studied by the retrograde transport of horseradish peroxidase. J Comp Neurol. 1977;174:187–208. doi: 10.1002/cne.901740202. [DOI] [PubMed] [Google Scholar]
- 67.Smith RL, Canton H, Barrett RJ, Sanders-Bush E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav. 1998;61:323–330. doi: 10.1016/s0091-3057(98)00110-5. [DOI] [PubMed] [Google Scholar]
- 68.Tilakaratne N, Yang Z, Friedman E. Chronic fluoxetine or desmethylimipramine treatment alters 5-HT2 receptor mediated c-fos gene expression. Eur J Pharmacol. 1995;290:263–266. doi: 10.1016/0922-4106(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 69.Tilakaratne N, Friedman E. Genomic responses to 5-HT1A or 5-HT2A/2C receptor activation is differentially regulated in four regions of rat brain. Eur J Pharmacol. 1996;307:211–217. doi: 10.1016/0014-2999(96)00233-6. [DOI] [PubMed] [Google Scholar]
- 70.Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 71.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 72.Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2a receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 73.Wong-Riley MT, Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci USA. 1980;77:2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woolsey TA. Some anatomical bases of cortical somatotopic organization. Brain Behav Evol. 1978;15:325–371. doi: 10.1159/000123786. [DOI] [PubMed] [Google Scholar]
- 75.Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]