Abstract
Insulysin (EC. 3.4.22.11) has been implicated in the clearance of β amyloid peptides through hydrolytic cleavage. To further study the action of insulysin on Aβ peptides recombinant rat insulysin was used. Cleavage of both Aβ1–40 and Aβ1–42by the recombinant enzyme was shown to initially occur at the His13-His14, His14-Gln15, and Phe19-Phe20 bonds. This was followed by a slower cleavage at the Lys28-Gly29, Val18-Phe19, and Phe20-Ala21 positions. None of the products appeared to be further metabolized by insulysin. Using a rat cortical cell system, the action of insulysin on Aβ1–40 and Aβ1–42 was shown to eliminate the neurotoxic effects of these peptides. Insulysin was further shown to prevent the deposition of Aβ1–40 onto a synthetic amyloid. Taken together these results suggest that the use of insulysin to hydrolyze Aβ peptides represents an alternative gene therapeutic approach to the treatment of Alzheimer's disease.
Keywords: amyloid peptide metabolism, metallopeptidase, insulysin, Aβ neurotoxicity, Aβ deposition, Aβ cleavage
The major pathological feature of Alzheimer's disease is the presence of senile plaques in the brain of affected individuals. Although controversy exists whether the formation of amyloid deposits is the primary cause of Alzheimer's disease, they contribute to its etiology and progression (Selkoe, 1994). These insoluble amyloid deposits contain as a major constituent amyloid β peptides (Aβ peptides) derived by processing of the amyloid precursor protein (Goldgaber et al., 1987) by β and γ secretases (Sinha et al., 1999; Vassar et al., 1999). Considerable effort has been expended in identifying these secretases, the goal being the development of specific inhibitors that would block the formation of amyloid plaques. The recent report of an aspartyl protease that appears to be a true β secretase (Vassar et al., 1999) provides optimism that this approach can soon be tested.
Tseng et al. (1999) showed that amyloid formation involves the deposition of monomeric Aβ. Thus, inhibition of monomeric Aβ aggregation or deposition represents an alternative strategy for the treatment of Alzheimer's disease. Compounds that prevent Aβ aggregation have been reported (Soto et al., 1996; Tjernberg et al., 1996; Tomiyama et al., 1996; Wood et al., 1996), and a high throughput screen has been developed (Esler et al., 1997). Another approach is to hydrolyze Aβ peptides before they deposit onto amyloid plaques.Howell et al. (1995) showed that the zinc metallopeptidase neprilysin (neutral endopeptidase, enkephalinase; EC 3.4.24.11) degraded Aβ1–40, whereas Iwata et al. (2000) showed that inhibition of neprilysin in rat brain produces an increase in Aβ1–42 concentration and the formation of diffuse amyloid plaques. However, it was observed that neprilysin inhibitors were less effective in altering the Aβ1–40 concentration, suggesting that Aβ1–40 might be cleared by a different mechanism or peptidase (Iwata et al., 2000).
Kurochkin and Goto (1994) showed that another zinc metallopeptidase insulysin (insulin degrading enzyme, insulinase, EC 3.4.22.11) also cleaved Aβ1–40, although the products of the reaction were not identified. In a subsequent study McDermott and Gibson (1997) confirmed the degradation of Aβ1–40 by insulysin, identified a number of putative reaction products, and showed that Aβ1–40 displayed an IC50in the low micromolar range. Because this study used partially purified enzyme, the contribution of contaminating peptidases cannot be ruled out. Qiu et al. (1998) showed that a secreted form of insulysin was produced from microglial cells (BV-2) and provided evidence that primary rat brain cultures and differentiated rat adrenal pheochromocytoma (PC12) cells expressed a cell surface form of insulysin (Vekrellis et al., 2000). Recently Perez et al. (2000) showed that insulysin represents an abundant Aβ degrading activity in human brain soluble extracts.
In this study we have used homogeneous recombinant rat insulysin to study the reaction of this peptidase with Aβ1–40 and Aβ1–42. We have identified cleavage sites and studied the cleavage reaction and its effect on the neurotoxic properties of the Aβ peptides and the ability of Aβ1–40 to deposit onto preformed synthetic amyloid fibrils. The results of this study suggest that insulysin may represent an alternative therapeutic approach for the treatment of Alzheimer's disease.
MATERIALS AND METHODS
Materials. Aβ1–40 and Aβ1–42 were obtained from Bachem (Torrance, CA). Solutions were prepared by dissolving the peptide in dimethylsulfoxide (DMSO) to give a stock concentration of 200 μm. The peptide stock was lyophilized and stored at −80°C until use. The aggregation state of Aβ peptide stock solutions was checked by electron microscopy (Ray et al., 2000) and found to be predominantly, if not exclusively, monomeric. For thein vitro reactions with insulysin, a final concentration of 25 μm Aβ1–40 was obtained after bringing the lyophilized peptide into solution with double-distilled water. For cytotoxicity studies Aβ1–40 and Aβ1–42peptides were dissolved in sterile N2 medium (Life Technologies, Rockville, MD). Human β-endorphin1–31, obtained from the National Institute on Drug Abuse drug supply system, was dissolved in water to give a stock solution of 300 μm. Trifluoroacetic acid (Sigma, St. Louis, MO) was diluted into water to produce a 5% working solution.
Expression and purification of recombinant insulysin. A rat insulysin cDNA, (pECE-IDE), kindly provided by Dr. Richard Roth of Stanford University (Stanford, CA), was subcloned into the baculovirus-derived vector pFASTBAC (Life Technologies) throughBamHI and XhoI restriction sites such that a His6-affinity tag was attached to the N terminus of the protein. Generation of recombinant virus and expression of the recombinant protein in Sf9 cells was performed according to the manufacturer's directions. For the purification of recombinant insulysin, a 1:10 (w/v) suspension derived from a 50 ml culture of viral infected Sf9 cells was prepared in 100 mmpotassium phosphate buffer, pH 7.2, containing 1 mm dithiothreitol (K-PO4/DTE buffer). The suspension was sonicated 10 times, each burst for 1 sec, using a Branson sonifier (setting 3 at 30%) and then centrifuged at 75,000 × g for 30 min to pellet cell debris and membranes. The supernatant containing recombinant rat insulysin was loaded onto a 0.5 ml nickel-nitrilotriacetic acid (Ni-NTA) column (Qiagen, Valencia, CA) that had been equilibrated with the K-PO4/DTE buffer. After extensive washing of the column with starting buffer, and then with 20 mm Imidazole-HCl, pH 7.2, the enzyme was eluted with 0.1 m Imidazole-HCl, pH 7.2. The enzyme was further purified over a 1 ml Mono-Q anion exchange column (Pharmacia Biotech, Piscataway, NJ) in 20 mmphosphate buffer, pH 7.2. A linear salt gradient of 0–0.6m KCl, equivalent to 60 column volumes, was applied to the column with the enzyme eluted at 0.28m KCl. SDS-PAGE of the insulysin was conducted on a 7.5% gel.
Insulysin activity determination. Insulysin activity was assayed by measuring the disappearance of β-endorphin by isocratic reverse-phase HPLC (Safavi et al., 1996). A 100 μl reaction mixture containing 40 mm potassium phosphate buffer, pH 7.2, 30 μm β-endorphin, and enzyme was incubated for 15 min at 37°C. The reaction was stopped by the addition of 10 μl of 5% trifluoroacetic acid to give a final concentration of 0.5%. The reaction mix was loaded onto a C4 reverse-phase HPLC column (Vydac, Hisperia, CA), and products were resolved isocratically at 32% acetonitrile. The β-endorphin peak was detected by absorbance at 214 nm using a Waters 484 detector. The reaction was quantitated by measuring the decrease in the β-endorphin peak area.
Determination of sites of cleavage of Aβ peptides.Purified insulysin was incubated with 25 μmAβ1–40 in 40 mm potassium phosphate buffer, pH 7.2, at 37°C for 1 hr. The reaction products were loaded onto a C4 reverse-phase HPLC column and products resolved using a linear gradient of 5–75% acetonitrile over 65 min. Products were detected by absorbance at 214 nm using a Waters 484 detector, and individual product peaks were collected manually. Product analysis was also conducted on an intact reaction mixture in which products were not resolved by HPLC. Products were identified by matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS). The reaction of insulysin with Aβ1–42 was conducted in a similar manner with products identified by MALDI-TOF-MS directly from reaction mixtures.
AB1–40 deposition assay. β amyloid deposition assays were conducted as described by Esler et al. (1999). Briefly, 96 well microtiter plates precoated with aggregated amyloid β1–40 (QCB/Biosource, Hopkinton, MA) were additionally coated with 200 μl of a 0.1% bovine serum albumin solution in 50 mm Tris-HCl, pH 7.5, for 20 min to prevent nonspecific binding. For measuring Aβ1–40deposition in the presence or absence of insulysin, a 150 μl solution of 0.1 nm125I labeled Aβ1–40 in 50 mm Tris-HCl, pH 7.5, was added to the precoated well and incubated for 4 hr. When added, insulysin (0.5–500 ng) was placed directly in the well at zero time. The reaction was stopped by washing off excess undeposited radiolabeled Aβ1–40 with 50 mm Tris-HCl, pH 7.5. The radiolabel deposited onto the washed well was counted in a gamma counter. In a variation of this protocol, insulysin was preincubated with 1 nm125I-Aβ1–40 for 60 min and then added to the deposition assay.
Neuoroprotection assays. Neurotoxicity assays were performed as described by Estus et al. (1997) using embryonic day 18 rat fetuses to establish primary rat cortical neuron cultures. Rat brain cortical cells were initially cultured in AM0 media for 3–5 hr in 16 well chamber slides (Nalge Nunc International, Rochester, NY) precoated with polyethyleneimine at a density of ∼1 × 105 cells per well. The culture was enriched in neurons by replacement of the AM0media with DMEM (Life Technologies) containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 2% B27 serum supplement (Life Technologies).
Cells were treated with Aβ peptides and then fixed with 4% paraformaldehyde for 15 min at room temperature. After washing the cells with PBS, they were then stained with Hoechst 33258 at 1 μg/ml for 10 min. Neurons were then visualized by fluorescence microscopy. Those cells with uniformly dispersed chromatin were scored as survivors, whereas those cells containing condensed chromatin were scored as nonsurvivors. Readings were typically taken in triplicate with a minimum of 250 neurons scored from each well. Cells treated as described above were visualized using a Nikon microscope equipped with a Hoffman modulation contrast lens. Statistical analysis was performed on the samples using ANOVA.
Immunofluorescence. The presence of aggregated Aβ1–40 was detected in the neuronal cultures using the monoclonal antibody 10D5 (Walker et al., 1994) at a 1:100 dilution in 5% goat serum in PBS. After an overnight incubation at 4°C with this primary antibody, the wells were rinsed with PBS and incubated with a goat anti-mouse secondary antibody conjugated to Cy3 (Jackson ImmunoResearch, West Grove, PA) at a dilution of 1:250 in 5% goat serum in PBS. The wells were incubated at room temperature for 60 min and then after further washing with PBS, cells were examined under a fluorescence microscope.
RESULTS
To characterize the reaction of insulysin with the Aβ peptides, recombinant rat enzyme containing an N-terminal His6 affinity tag was expressed in baculovirus-infected Sf9 cells. Expression of the enzyme in this system was high, as evidenced by the ability to see insulysin protein in a crude extract by SDS-PAGE (Fig. 1). Purification of the recombinant enzyme was achieved by chromatography on a Ni-NTA-agarose column producing highly purified enzyme followed by chromatography on a Mono-Q column, which produced homogeneous enzyme (Fig. 1). The specific activity of the recombinant enzyme (2.6 μmol · min−1 · mg−1) was comparable to enzyme purified from a thymoma cell line, EL-4 (3.3 μmol · min−1 · mg−1), and thus the presence of the His6 affinity tag had no discernable effect on enzyme activity.
Fig. 1.
Purification of recombinant rat insulysin. Insulysin was purified as described in Materials and Methods, and 15 μg aliquots from various stages of purification were analyzed by SDS-PAGE on a 7.5% gel stained with Coomassie Blue. A,Sf9 cell extract. B, Nonbound proteins from the Ni-NTA-agarose column. C, Protein eluted from the Ni-NTA-agarose column with 20 mm imidazole.D, Protein eluted from the Ni-NTA-agarose column with 100 mm imidazole. E, Protein eluted from the Mono-Q column. The position of molecular weight markers (myosin, 200 kDa; β-galactosidase, 116 kDa; phosphorylase B, 97.4 kDa; bovine serum albumin, 66 kDa; and ovalbumin, 45 kDa) is shown on theleft.
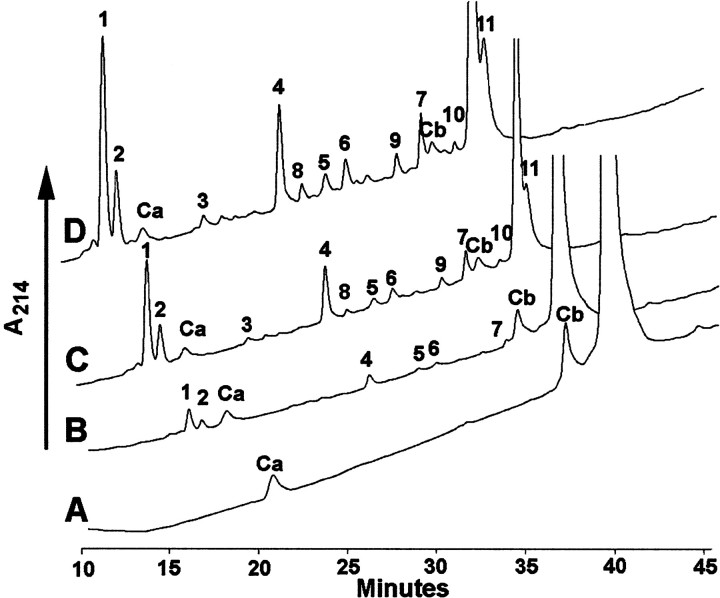
To delineate the sites of cleavage of the Aβ1–40 peptide by insulysin, the peptide was incubated with varying concentrations of the enzyme for 1 hr at 37°C, and then products were resolved by gradient reverse-phase HPLC. With 50 ng of insulysin, the lowest enzyme concentration used, three major cleavage sites at His14-Gln15(peak 1), His13-His14(peak 2), and Phe19-Phe20(peak 4 and peak 7) were discernible (Table1, Fig. 2). In addition, minor cleavage sites at Lys28-Gly29(peak 5) and Phe20-Ala21(peak 6) were observed. When the amount of insulysin was increased to 250 ng, each of the products seen with 50 ng of enzyme increased, and an additional product corresponding to cleavage at Val18-Phe19(peak 3) was observed. Further increasing insulysin to 500 ng showed a continued increase in each of the products. The same products were seen when Aβ1–40 was treated with 500 ng of insulysin and analyzed by MALDI-TOF-MS without separation of the reaction products. It is interesting to note that one product peak Aβ14–40 was not observed, whereas other product peaks were not apparent until after substantial metabolism had occurred. For example, Aβ1–14 can be seen in the digest using 50 ng of insulysin, whereas the product corresponding to the C-terminal half of this cleavage, Aβ15–40, is not seen in the 50 ng reaction, but is observed with the 250 ng of enzyme. This is in part attributed to the hydrophobic nature of the C-terminal peptides and their greater retention times, which produces HPLC peak broadening and decreased sensitivity. The overall cleavage profile is illustrated in Figure3.
Table 1.
Identification of products from insulysin cleavage of Aβ1–40
| Peak no. | Aβ1–40 Fragment | Sequence |
|---|---|---|
| 1 | 1–14 | DAEFRHDSGYEVHH |
| 2 | 1–13 | DAEFRHDSGYEVH |
| 3 | 1–18 | DAEFRHDSGYEVHHQKLV |
| 4 | 1–19 | DAEFRHDSGYEVHHQKLVF |
| 5 | 1–28 | DAEFRHDSGYEVHHQKLVFFAEDVGSNK |
| 6 | 1–20 | DAEFRHDSGYEVHHQKLVFF |
| 7 | 20–40 | FAEDVGSNKGAIIGLMVGGVV |
| 8 | 29–40 | GAIIGLMVGGVV |
| 9 | 21–40 | AEDVGSNKGAIIGLMVGGVV |
| 10 | 19–40 | FFAEDVGSNKGAIIGLMVGGVV |
| 11 | 15–40 | QKLVFFAEDVGSNKGAIIGLMVGGVV |
Fig. 2.
HPLC profile of products generated from the cleavage of Aβ1–40 by insulysin. Varying amounts of recombinant rat insulysin were incubated with 25 μmAβ1–40 for 30 min at 37°C. Cleavage products were separated by a 5–75% gradient of acetonitrile on a C4reverse-phase HPLC column. Product peaks are numbered according to their order of elution. The peaks designated Ca andCb refer to contaminants in the Aβ1–40solution. These are not reacted on by insulysin, as is seen by their invariant peak areas in all the traces. A,Aβ1–40 alone. B, Aβ1–40incubated with 50 ng of insulysin. C,Aβ1–40 incubated with 250 ng of insulysin.D, Aβ1–40 incubated with 500 ng of insulysin. The HPLC scans are skewed ∼2 min to the left to permit overlapping peaks to be viewed. The time scale refers to traceA.
Fig. 3.

Positions of cleavage within the Aβ1–40 and Aβ1–42 sequences. Primary cleavage sites are noted with the thick arrows.
The Aβ1–42 peptide was incubated with insulysin in an identical manner as with Aβ1–40, and the products were analyzed by MALDI-TOF mass spectrometry without prior separation by HPLC. Product peaks corresponding to cleavage at the His13-His14, His14-Gln15, Phe19-Phe20, and Phe20-Ala21positions were observed. These results indicate that both Aβ1–40 and Aβ1–42 are cleaved at the same sites. The rate of cleavage of 25 μmAβ1–40 was measured as 1.2 μmol · min−1 · mg−1enzyme, which indicates that the Aβ peptides are good substrates for insulysin.
The products of the action of insulysin on the Aβ peptides produces relatively large fragments. Because the peptide Aβ25–35, which is derived from Aβ1–40, is neurotoxic, it is possible that the products of insulysin action on the Aβ peptides could be toxic to neurons. To test this, rat cortical neurons were treated with Aβ peptides in the presence and absence of insulysin. Preliminary experiments were performed to obtain a suitable Aβ peptide concentration that would show a significant cytotoxic effect, as there are batch to batch variations in the ability of the Aβ peptides to mediate cytotoxic effects on cells in culture. These experiments established 30 μm Aβ1–40 and 25 μm Aβ1–42 as reasonable peptide concentrations that produce ∼70 and 80% loss of cortical neurons, respectively, in 48 hr.
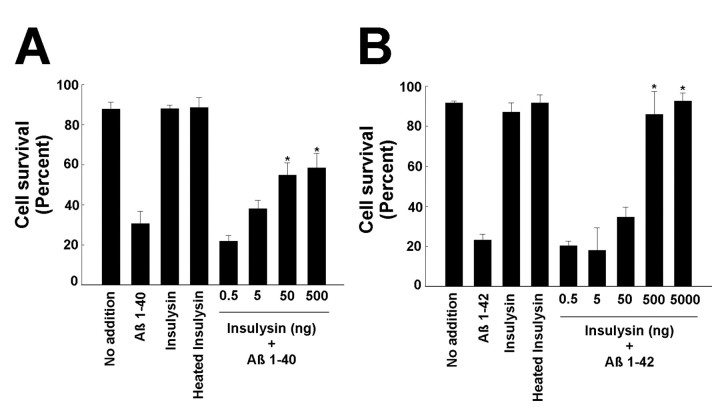
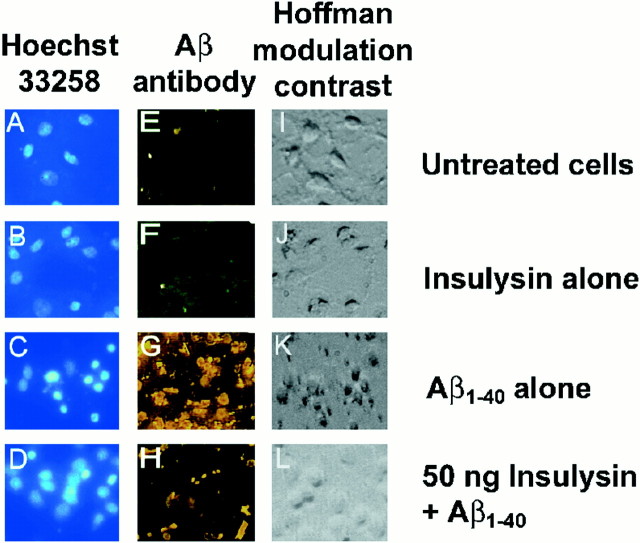
The cell-based assay using primary rat cortical neurons was used to determine whether the insulysin cleavage products of the Aβ peptides were themselves neurotoxic. Recombinant insulysin at concentrations ranging from 0.5 to 5000 ng was added simultaneously with the Aβ peptides to the cortical cultures. When added directly to the cultures, as little as 50 ng of insulysin was effective in sparing the neurotoxic effects of Aβ1–40 (Fig.4A), whereas 500 ng of insulysin was effective in sparing the neurotoxic effects of Aβ1–42 (Fig. 4B). This effect of insulysin is illustrated in Figure5 in which cells were either stained with Hoechst 33258 to visualize DNA (A–D), with the Aβ antibody 10D5 to visualize cell-associated Aβ (E–H), or visualized directly by Hoffman modulation microscopy (I–L). Using this phase-contrast microscopy, it can be seen that Aβ1–40 caused the cells to appear shrunken (K) as compared to control cells, which appear rounded (I). Aβ1–40 induced chromatin condensation, which appears as small rounded nuclei (C), and Aβ cellular accumulation, which appears as a bright layering over the cells (G), is not evident in untreated cells (A, E). Cells to which insulysin was added along with Aβ1–40 more closely resembled untreated cells (D, H, L). Also shown in Figure 5 are controls in which cells were treated with insulysin alone (B, F, J).
Fig. 4.
Effect of insulysin on the neurotoxic effects of Aβ peptides. Purified insulysin was added with Aβ1–40(30 μm) or Aβ1–42 (25 μm) to primary cortical neurons, and incubation continued for an additional 48 hr. The neurotoxic effect of the Aβ peptides was determined as described in Materials and Methods. The insulysin and heat-inactivated insulysin controls used 5000 ng of enzyme. A, Effect of incubation with insulysin on the neurotoxic effects of Aβ1–40. B, Effect of incubation with insulysin on the neurotoxic effects of Aβ1–42. *p < 0.01 relative to the Aβ-treated sample as determined by ANOVA.
Fig. 5.
Insulysin protects against Aβ1–40mediated neurotoxicity. Rat cortical neurons were treated as described in Figure 4 in the presence or absence of 50 ng of insulysin. Cells were stained with Hoechst 33258 (A–D) or with the Aβ antibody 10D5 (E–H). Hoffman modulation contrast micrographs are shown in I–L.A, E, and I show untreated neurons. B, F, and J show neurons with 50 ng of insulysin added. C,G, and K show neurons treated with 30 μm Aβ1–40. D,H, and L show neurons treated with 50 ng of insulysin and 30 μm Aβ1–40.
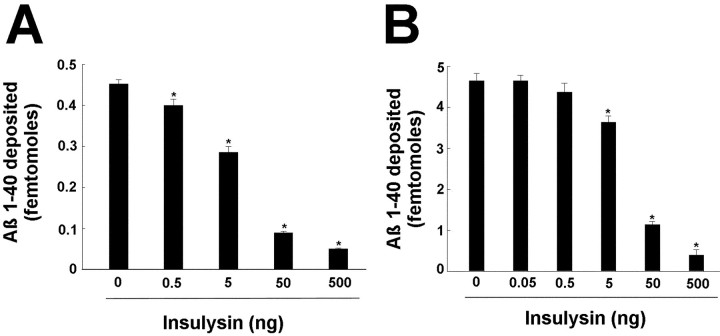
During the progression of Alzheimer's disease, monomeric Aβ peptides are deposited onto senile plaques. To test whether insulysin is able to prevent the deposition of the Aβ1–40 peptide, a model system was used in which the deposition of radiolabeled Aβ1–40 onto a synthetic amyloid plaque (synthaloid) is followed (Esler et al., 1999). As seen in Figure6A, addition of insulysin at 0.5–500 ng with radiolabeled [125I]Aβ1–40shows that 50 ng of insulysin is able to prevent the deposition of radiolabeled Aβ1–40. Figure6B shows that preincubation of insulysin with radiolabeled [125I]Aβ1–40for 60 min before adding it to the wells also shows that 50 ng of insulysin is able to prevent the deposition of radiolabeled Aβ1–40 onto the synthetic amyloid. We also conducted an experiment in which [125I]Aβ1–40was first deposited onto the synthetic amyloid and then treated with insulysin to see if the enzyme could degrade preaggregated Aβ1–40. After a 24 hr incubation with 5 μg of insulysin, no radioactivity was released, indicating that insulysin does not degrade aggregated Aβ peptides.
Fig. 6.
Insulysin inhibits the deposition of Aβ1–40 onto synthetic amyloid plaques. A,Effect of incubation with insulysin on the deposition of Aβ1–40. Aβ1–40 (0.1 nm) was mixed with the indicated amount of purified insulysin and then added to synthaloid in 96 well plates. Deposition was permitted to occur over a 4 hr time period. B, Effect of preincubation with insulysin on the deposition of Aβ1–40. Aβ1–40 (1 nm) was preincubated for 60 min with the indicated amount of purified insulysin. The incubation mixtures were then added to synthaloid in 96 well plates, and deposition was permitted to occur over a 4 hr time period. *p < 0.01 as determined by ANOVA.
DISCUSSION
The balance between the anabolic and catabolic pathways in the metabolism of the Aβ peptides is a delicate one. Although considerable effort has focused on the generation of the Aβ peptides, until recently considerably less emphasis has been placed on the clearance of these peptides. Removal of extracellular Aβ peptide appears to proceed through two general mechanisms: cellular internalization and extracellular degradation by neuropeptidases. Apparently neither of these mechanisms is adequate in Alzheimer's disease. Interest in the mechanism of cellular internalization stems from the apparent involvement of apolipoprotein E and α-2-macroglobulin in this process (Narita et al., 1997; Kang et al., 1997; Hughes et al., 1998; Blacker et al., 1998).
A number of neuropeptidases have been suggested to be involved in the extracellular degradation of Aβ peptides, and these include neprilysin (Howell et al., 1995), insulysin (Kurochkin and Goto, 1994;McDermott and Gibson, 1997; Qiu et al., 1998), and the plasmin system (Tucker et al., 2000). Studies by Iwata et al. (2000) showed that inhibition of neprilysin in rat brain led to increased levels of Aβ1–42 and the formation of diffuse plaques. Interestingly, Aβ1–40 levels did not increase as a consequence of neprilysin inhibition, suggesting that a different peptidase may be responsible for Aβ1–40metabolism. Previous studies have shown that the zinc metalloprotease insulysin (insulin degrading enzyme) is able to cleave Aβ1–40 (Kurochkin and Goto, 1994; McDermott and Gibson, 1997; Qiu et al., 1998), making this a candidate enzyme for its clearance. Perez et al. (2000) showed that the insulysin activity was decreased in the soluble fraction derived from human Alzheimer brains compared to aged matched controls. They suggested this decrease could contribute to increased Aβ accumulation in Alzheimer's disease.
The use of neuropeptidases such as neprilysin or insulysin to remove extracellular Aβ peptides represents a potential treatment for Alzheimer's disease. However, for a peptidase to be useful, it must be established that its action eliminates the amyloidogenic properties of Aβ peptides. In this study we have shown that insulysin cleaves both Aβ1–40 and Aβ1–42 at the His13-His14, His14-Gln15, and Phe19-Phe20bonds as initial cleavage sites. Although the exact substrate specificity of insulysin is still unclear, it has been observed that insulysin can cleave at the C terminus of basic and hydrophobic amino acid residues. Thus, the cleavage pattern obtained with Aβ1–40 and Aβ1–42 is consistent with this specificity. Other cleavage sites that appear using higher concentrations of insulysin are at the Lys28-Gly29, Val18-Phe19, and Phe20-Ala21positions. These cleavage sites are also consistent with the known substrate specificity of the enzyme.
The cleavage products observed with insulysin indicate distinct cleavage events and not products derived from secondary cleavage of an initial product. That is, no fragment was observed lacking an intact N terminus, the C-terminal fragment corresponding to each N-terminal fragment was seen in all but one case, and products increased with an increasing concentration of insulysin.
Neuronal cell cultures are susceptible to the toxic effects mediated by Aβ1–40 and Aβ1–42. We have used this neuronal cell culture system to establish that the products of the insulysin-dependent cleavage of Aβ1–40 and Aβ1–42produces products that are not in themselves neurotoxic. This is an important point if one were to consider the use of insulysin in the treatment of Alzheimer's disease.
Related to cellular toxicity, Aβ peptides are able to deposit onto an existing matrix of peptides in what is thought to lead to an increase in the size of senile plaques and consequently to the progression of Alzheimer's disease. In a model system, Esler et al. (1997) have shown that the deposition of Aβ1–40 onto a preformed synthaloid matrix mimics the in vivo deposition of Aβ peptides onto the brain cortex. Using this model, we have shown that insulysin cleavage of Aβ1–40 prevents the deposition of the Aβ peptides onto the synthaloid. This suggests that insulysin may be able to prevent the formation and growth of senile plaques in Alzheimer's disease patients.
In summary we have established that the insulysin-dependent cleavage of the Aβ peptides leads to the loss of both their neurotoxic properties as well as their ability to contribute to plaque formation and growth. The use of insulysin and other peptidases to degrade extracellular Aβ peptides represents a new approach toward the treatment of Alzheimer's disease.
Footnotes
This research was supported in part by National Institute on Drug Abuse Grants DA 02243 and DA 07062 and National Institute on Aging Grant AG 05893. We thank Dr. Richard Roth of Stanford University for providing us with the cDNA clone to rat insulysin and for insulysin antisera, Dr. John Maggio and Jeffrey R. Marshall of the University of Cincinnati for helping us establish the Aβ deposition assay, and Drs. Mark Lovell, Chengsong Xie, and William Markesbery of the Sanders-Brown Center on Aging, University of Kentucky for helping us in preliminary neuronal toxicity studies.
Correspondence should be addressed to Dr. Louis B. Hersh, Department of Biochemistry, University of Kentucky, Chandler Medical Center, 800 Rose Street, Lexington, KY 40536-0298. E-mail: lhersh@pop.uky.edu.
REFERENCES
- 1.Blacker D, Wilcox MA, Laird NM, Rodes L, Horvath SM, Go RC, Perry R, Watson B, Jr, Bassett SS, McInnis MG, Albert MS, Hyman BT, Tanzi RE. Alpha-2 macroglobulin is genetically associated with Alzheimer disease. Nat Genet. 1998;19:357–360. doi: 10.1038/1243. [DOI] [PubMed] [Google Scholar]
- 2.Esler WP, Stimson ER, Ghilardi JR, Felix AM, Lu Y-A, Vinters HV, Mantyh PW, Maggio JE. A beta deposition inhibitor screen using synthetic amyloid. Nat Biotech. 1997;15:268–263. doi: 10.1038/nbt0397-258. [DOI] [PubMed] [Google Scholar]
- 3.Esler WP, Stimson ER, Mantyh PW, Maggio JE. Deposition of soluble amyloid-beta onto amyloid templates: with application for the identification of amyloid fibril extension inhibitors. Methods Enzymol. 1999;309:350–374. doi: 10.1016/s0076-6879(99)09025-4. [DOI] [PubMed] [Google Scholar]
- 4.Estus S, Tucker HM, van Rooyen C, Wright S, Brigham E, Wogulis M, Rydel R. Aggregated amyloid-beta protein induces cortical neuronal apoptosis and concomitant “apoptotic” pattern of gene induction. J Neurosci. 1997;17:7736–7745. doi: 10.1523/JNEUROSCI.17-20-07736.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987;235:877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- 6.Howell S, Nalbantoglu J, Crine P. Neutral endopeptidase can hydrolyze beta-amyloid(1–40) but shows no effect on beta-amyloid precursor protein metabolism. Peptides. 1995;16:647–652. doi: 10.1016/0196-9781(95)00021-b. [DOI] [PubMed] [Google Scholar]
- 7.Hughes SR, Khorkova O, Goyal S, Knaeblein J, Heroux J, Riedel NG, Sahasrabudhe S. Alpha2-macroglobulin associates with beta-amyloid peptide and prevents fibril formation. Proc Natl Acad Sci USA. 1998;95:3275–3280. doi: 10.1073/pnas.95.6.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- 9.Kang DE, Saitoh T, Chen X, Xia Y, Masliah E, Hansen LA, Thomas RG, Thal LJ, Katzman R. Genetic association of the low-density lipoprotein receptor-related protein gene (LRP) an apolipoprotein E receptor, with late-onset Alzheimer's disease. Neurology. 1997;49:56–61. doi: 10.1212/wnl.49.1.56. [DOI] [PubMed] [Google Scholar]
- 10.Kurochkin IV, Goto S. Alzheimer's beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett. 1994;345:33–37. doi: 10.1016/0014-5793(94)00387-4. [DOI] [PubMed] [Google Scholar]
- 11.McDermott JR, Gibson AM. Degradation of Alzheimer's beta-amyloid protein by human and rat brain peptidases: involvement of insulin-degrading enzyme. Neurochem Res. 1997;22:49–56. doi: 10.1023/a:1027325304203. [DOI] [PubMed] [Google Scholar]
- 12.Narita M, Holtzmann DM, Schwartz AL, Bu G. Alpha2 macroglobulin complexes with and mediates the endocytosis of beta amyloid peptide via cell surface low-density lipoprotein receptor related protein. J Neurochem. 1997;69:1904–1911. doi: 10.1046/j.1471-4159.1997.69051904.x. [DOI] [PubMed] [Google Scholar]
- 13.Perez A, Morekki L, Cresto JC, Castano EM. Degradation of soluble amyloid b-peptides 1–40, 1–42, and the Dutch variant 1–40Q by Insulin-degrading enzyme from Alzheimer disease and control brains. Neurochem Res. 2000;25:247–255. doi: 10.1023/a:1007527721160. [DOI] [PubMed] [Google Scholar]
- 14.Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 15.Ray I, Chauhan A, Wegiel J, Chauhan VP. Gelsolin inhibits the fibrillization of amyloid beta-protein, and also defibrillizes its preformed fibrils. Brain Res. 2000;853:344–351. doi: 10.1016/s0006-8993(99)02315-x. [DOI] [PubMed] [Google Scholar]
- 16.Safavi A, Miller BC, Cottam L, Hersh LB. Identification of gamma-endorphin-generating enzyme as insulin-degrading enzyme. Biochemistry. 1996;35:14318–14325. doi: 10.1021/bi960582q. [DOI] [PubMed] [Google Scholar]
- 17.Selkoe DJ. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 19.Soto C, Kindy MS, Baumann M, Frangione B. Inhibition of Alzheimer's amyloidosis by peptides that prevent beta-sheet conformation. Biochem Biophys Res Commun. 1996;226:672–680. doi: 10.1006/bbrc.1996.1413. [DOI] [PubMed] [Google Scholar]
- 20.Tjernberg LO, Naslund J, Lindqvist F, Johansson J, Karlstrom AR, Thyberg J, Terenius L, Nordstedt C. Arrest of beta-amyloid fibril formation by a pentapeptide ligand. J Biochem. 1996;271:8545–8548. doi: 10.1074/jbc.271.15.8545. [DOI] [PubMed] [Google Scholar]
- 21.Tomiyama T, Shoji A, Kataoka K-I, Suwa Y, Asano S, Kaneko H, Endo N. Inhibition of amyloid beta protein aggregation and neurotoxicity by rifampicin. Its possible function as a hydroxyl radical scavenger. J Biol Chem. 1996;271:6839–6884. doi: 10.1074/jbc.271.12.6839. [DOI] [PubMed] [Google Scholar]
- 22.Tseng BP, Esler WP, Clish CB, Stimson ER, Ghilardi JR, Vinters HV, Mantyh PW, Lee JP, Maggio JE. Deposition of monomeric, not oligomeric, Abeta mediates growth of Alzheimer's disease amyloid plaques in human brain preparations. Biochemistry. 1999;38:10424–10431. doi: 10.1021/bi990718v. [DOI] [PubMed] [Google Scholar]
- 23.Tucker HM, Kihiko M, Caldwell JN, Wright S, Kawarabayashi T, Price D, Walker D, Scheff S, McGillis JP, Rydel RE, Estus S. The plasmin system is induced by and degrades amyloid-beta aggregates. J Neurosci. 2000;20:3937–3946. doi: 10.1523/JNEUROSCI.20-11-03937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 25.Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000;20:1657–1665. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker LC, Price DL, Voytko ML, Schenk DB. Labeling of cerebral amyloid in vivo with a monoclonal antibody. J Neuropathol Exp Neurol. 1994;53:377–383. doi: 10.1097/00005072-199407000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood SJ, MacKenzie L, Maleeff B, Hurle MR, Wetzel R. Selective inhibition of Abeta fibril formation. J Biol Chem. 1996;271:4086–4092. doi: 10.1074/jbc.271.8.4086. [DOI] [PubMed] [Google Scholar]