Abstract
A circadian clock is located in the retinal photoreceptors of the African clawed frog Xenopus laevis. These photoreceptor clocks are thought to govern a wide variety of output rhythms, including melatonin release and gene expression. Both light and dopamine phase shift the retinal clock in a phase-dependent manner. Two homologs of the Drosophila period gene have been cloned in Xenopus, and one of these (xPer2) is acutely regulated by light. Light and dopamine inducexPer2 mRNA in a similar manner. In addition, the increase of xPer2 mRNA in response to light and dopamine is the same at all times of day tested. In contrast,xPer1 mRNA exhibits circadian oscillations but is relatively insensitive to phase-shifting treatments of light or dopamine. Our data suggest that xPer2 functions as the molecular link between the light/dark cycle and the circadian clock.
Keywords: circadian rhythms, circadian clock, periodgenes, phase shifting, light induction, retina, photoreceptors, dopamine, D2 receptors, Xenopus
Information about time of day is provided to organisms by external stimuli such as light and by circadian clocks. Circadian clocks consist of a set of genes that govern rhythmic events in the absence of external timing cues such as light (Green, 1998). Clocks are modulated by environmental light via daily resetting in response to the imposed light/dark cycle. Resetting (or phase shifting) of the clock is initiated when light causes acute changes in the activity or abundance of central clock gene products (Pittendrigh, 1993).
Circadian rhythms are generated by activating the transcription of a set of genes that ultimately enter the nucleus and inhibit their own transcription. This negative feedback mechanism is conserved in all species (Dunlap, 1999). In Drosophila, the period(per) gene is a clock gene that negatively regulates its own transcription. PER only enters the nucleus with a protein partner called TIMELESS (TIM). Light applied at night results in the rapid degradation of TIM protein (Hunter-Ensor et al., 1996; Lee et al., 1996; Myers et al., 1996; Zeng et al., 1996). Thus, light changes the abundance of both per and tim mRNA, by acutely affecting TIM and by interrupting the normal negative feedback of per. The degradation of TIM by light resets the clock to an earlier or later time, depending on the time of day light is delivered.
A different mechanism exists for phase shifting the mammalian suprachiasmatic nucleus (SCN) involving period gene products. There are three period gene homologs in mice (mPer1, mPer2, and mPer3) that are rhythmically expressed (Zylka et al., 1998) and are able to function as transcriptional inhibitors (Jin et al., 1999; Kume et al., 1999). Light causes an increase in the abundance of the mRNA of two of these genes,mPer1 and mPer2 (Albrecht et al., 1997; Shearman et al., 1997; Shigeyoshi et al., 1997; Takumi et al., 1998; Zylka et al., 1998; Field et al., 2000). Treatment of mice with antisensemPer1 oligonucleotides blocks phase shifts induced by light, providing direct evidence that changes in mPer1 mRNA contribute to the phase-shifting mechanism (Akiyama et al., 1999).
Phase shifting of the retinal circadian clock has been studied primarily in Xenopus by measuring how light affects the rhythm of melatonin release by cultured eyecups (Besharse and Iuvone, 1983; Cahill and Besharse, 1991). Both light and dopamine are able to phase shift the retinal melatonin rhythm (Cahill and Besharse, 1991). Our previous work demonstrated that xPer2 mRNA was rhythmic in a light/dark cycle (LD) but was maintained at constitutively low levels in constant darkness (DD), indicating that light plays a major role in the regulation of xPer2 (Zhuang et al., 2000). In this report using the eyecup system, we show that light exposure increases xPer2 mRNA at all times of day tested. In a similar manner, xPer2 mRNA is increased by dopamine. Our data suggest that in Xenopus retina, changes inxPer2 mRNA play a critical role in phase shifting.
MATERIALS AND METHODS
Animals. Mature male Xenopus laevispurchased from Nasco (Ft. Atkinson, WI) were maintained at 21°C on a 12/12 hr light/dark cyclic lighting schedule (LD) for at least 2 weeks before all experiments. Zeitgeber time (ZT) is used by convention to denote the time of day relative to the normal lighting condition. ZT 0 is when lights are turned on, and ZT 12 is when lights are turned off. Animal care and experimental protocols involving these animals were performed in full compliance with the institutional and federal guidelines.
In vivo collections. Retinas were collected fromXenopus maintained in LD beginning at ZT 0 and proceeding for 6 hr after lights were turned on (normal room lights). For the ZT 0 group, animals were killed, and retinas were dissected using infrared illumination (FJW Optical, St. Palatine, IL). Normal lighting conditions were used for all other collections. Retinas were immediately frozen on dry ice and stored at −80°C.
Eyecup culture. A defined medium gassed with 95% O2/5% CO2 was used for dissection and culturing of the eyecups as described previously (Cahill and Besharse, 1991). In several experiments (see Figs. 4,5c–f, 6c–f, 7), the defined medium was supplemented with 20% Wolf and Quimby's amphibian tissue culture medium (Life Technologies, Gaithersburg, MD). In all experiments (for exception, see Fig. 3b), 100 μm5-hydroxy-l-tryptophan was added to increase overall melatonin to measurable levels (Cahill and Besharse, 1990). Melatonin assays of culture medium (Cahill and Besharse, 1991) from each experiment showed that light and dopamine agonists inhibited melatonin release as expected (data not shown). This concentration of 5-hydroxy-l-tryptophan does not alter the phasing of the circadian oscillator when applied in a cyclic manner (Cahill et al., 1991).
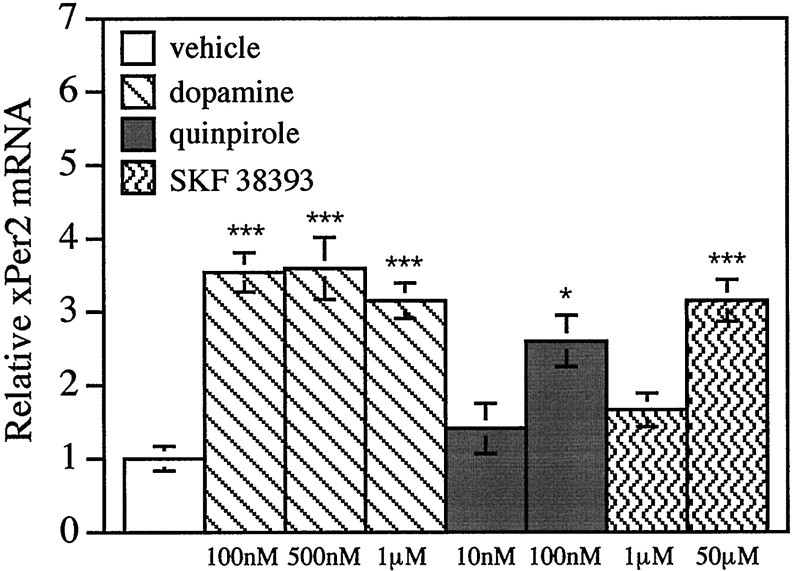
Fig. 4.
Dopamine increases xPer2 by activating D2-like dopamine receptors. Three hour treatments with various concentrations of vehicle (white bars), dopamine (bars with diagonal lines), the D2-like agonist quinpirole (gray bars), or the D1-like agonist SKF 38393 (bars with wavy lines) were delivered to eyecups at ZT 10. Eyecups were maintained in constant darkness throughout the experiment. The abundance of xPer2 mRNA was quantitated as in Figure2c, and values were normalized to the vehicle-alone group. The mean value of each group was calculated from five retinas from individual animals. Error bars indicate SEM. Dopamine (100 nm, 500 nm, and 1 μm), quinpirole (100 nm), and SKF 38393 (50 μm) groups were significantly different from the vehicle-alone group (***p < 0.001, *p < 0.05, ANOVA).
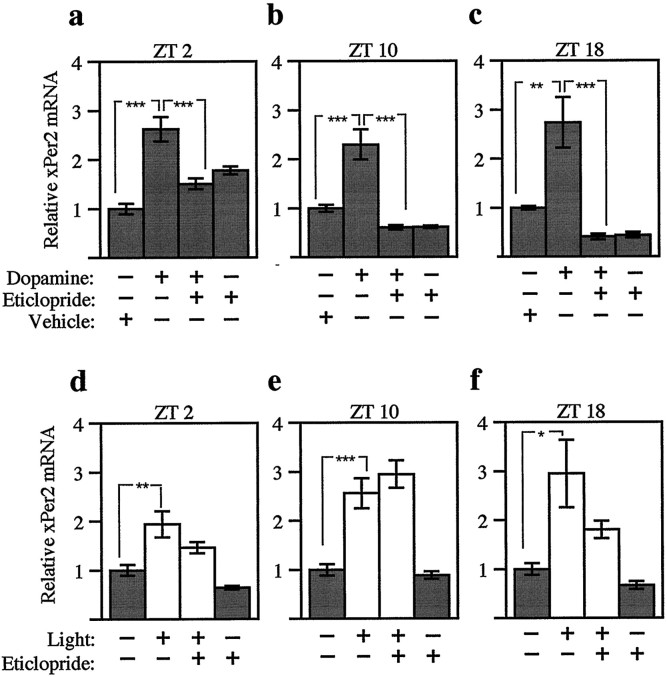
Fig. 5.
Light and dopamine act in parallel pathways to increase xPer2 mRNA at three times of day. Three hours of dopamine (a–c; 500 nm) or light (d–f; 3–5 × 10−4 W/cm2) were delivered to eyecups beginning at early subjective day (a, d; ZT 2), late subjective day (b, e; ZT 10), or subjective night (c, f; ZT 18) after previous treatment with 50 μm eticlopride, a D2-like dopamine antagonist. The abundance of xPer2 mRNA was quantitated as in Figure2c, and values were normalized to the vehicle-alone group for each experiment. The mean value of each group was calculated from four to five retinas from individual animals. Error bars indicate SEM. White bars indicate those groups receiving light;graybars indicate those groups maintained in constant darkness. At all three times of day (a–c), the dopamine-treated groups were significantly different from both the vehicle-alone groups and the dopamine plus eticlopride groups (***p < 0.001, **p < 0.01, ANOVA). At all three times of day (d–f), the light-treated groups were significantly different from the vehicle-alone groups (***p < 0.001, **p < 0.01, *p < 0.05, ANOVA). No significant differences were detected between the light and the light plus eticlopride groups at any time of day (ANOVA).
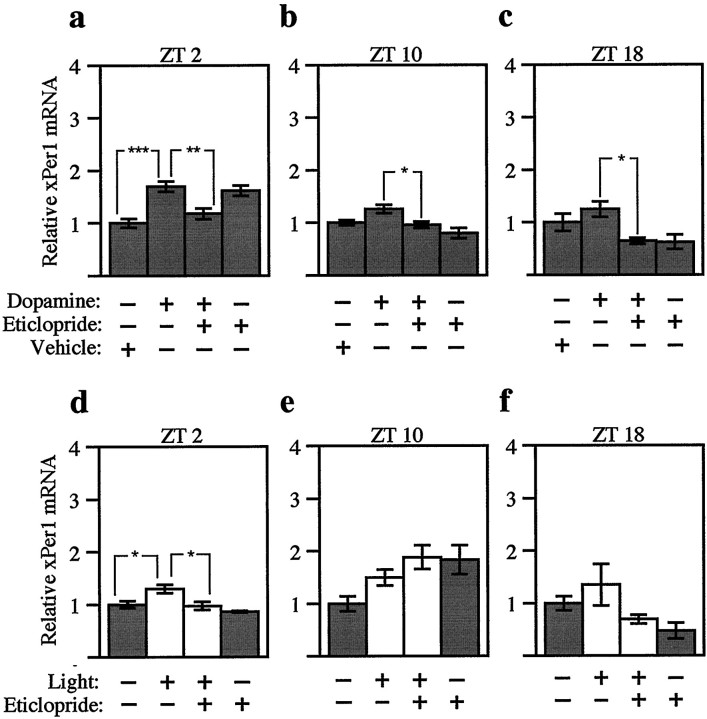
Fig. 6.
Three hour pulses of dopamine and light have modest effects on xPer1. Northern blots from the experiments described in Figure 5 were hybridized with anxPer1 probe. Three hours of dopamine (a–c; 500 nm) or light (d–f; 3–5 × 10−4 W/cm2) were delivered to eyecups beginning at early subjective day (a, d; ZT 2), late subjective day (b, e; ZT 10), or subjective night (c, f; ZT 18) after previous treatment with 50 μm eticlopride, a D2-like dopamine antagonist. The abundance of xPer1 mRNA was quantitated as in Figure2c, and values were normalized to the vehicle-alone group for each experiment. The mean value of each group was calculated from four to five retinas from individual animals. Error bars indicate SEM. White bars indicate those groups receiving light;graybars indicate those groups maintained in constant darkness. At ZT 2 (a), the dopamine-treated group was significantly different from the vehicle-alone group (***p < 0.001, ANOVA). At all three times of day (a–c), the dopamine-treated groups were significantly different from the dopamine plus eticlopride groups (**p < 0.01, *p < 0.05, ANOVA). At ZT 2 (d), the light-treated group was significantly different from both the control group and the light plus eticlopride group (*p < 0.05, ANOVA). No significant differences were detected between the light-treated groups and either the control groups or the light plus eticlopride groups at ZT 10 or ZT 18 (e, f; ANOVA).
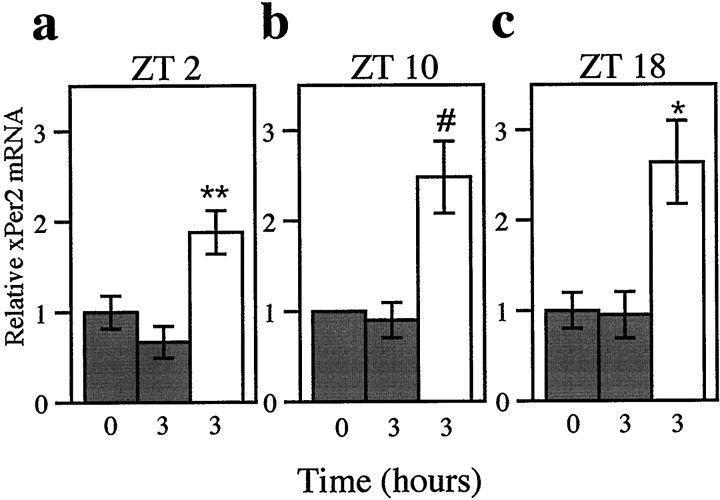
Fig. 3.
Light induces xPer2 mRNA at three different times of day. Three hour pulses of light (3–5 × 10−4 W/cm2) were delivered to eyecups beginning at early subjective day (a; ZT 2), late subjective day (b; ZT 10), or subjective night (c; ZT 18). The abundance ofxPer2 mRNA was quantitated as in Figure2b, and values were normalized to the time 0 hr dark control. The mean value of each group was calculated from four retinas from individual animals, except in b wheren = 1 for the 0 hr time. Error bars indicate SEM. At all three times of day (a–c), the light-treated groups (white bars) were significantly different from the dark controls (3 hr; gray bars) (**p < 0.01, *p < 0.05, ANOVA; #p < 0.05, t test).
Dissections were done 1–2 hr before normal dark onset. Eyecups were prepared by removing the anterior portion of the eye (cornea, lens, and vitreous) by a circular cut following the boundary of the iris. In most experiments, paired experimental and control eyecups from an individual animal were compared to reduce variability. For light induction experiments, single eyecups were set into baskets mounted in 35 mm culture dishes to orient the eyecup toward the light source (Pierce and Besharse, 1988). In experiments involving only drug pulses, five eyecups were cultured per 60 mm culture dish. Eyecups were maintained in darkness in a rotating incubator (60 rpm). Fresh medium was exchanged at 6 or 12 hr intervals. Light and dopamine treatments were delivered the following day beginning at three different times (ZT 2, ZT 10, and ZT 18). These times were chosen because we have studied previously phase resetting by light and dopamine at these times (Cahill and Besharse, 1991). All manipulations during the experiment were conducted under infrared illumination.
Light. A tungsten–halogen lamp (Oriel Corporation, Stanford, CT) at constant voltage (12 V) delivered light via mirrors to the eyecups in the rotating incubator at an intensity of 3–5 × 10−4W/cm2 (measured using a radiometer from International Light, Newburyport, MA).
Drugs. Dopamine, quinpirole, and eticlopride were purchased from Research Biochemicals (Natick, MA). SKF 38393 was donated by Smith Kline and French Laboratories (SmithKline Beecham, Philadelphia, PA). Drug stocks (100×) were prepared immediately before each experiment. Dopamine was solubilized in 100 mg/ml ascorbic acid. Quinpirole, eticlopride, and SKF 38393 were solubilized in water. Final drug concentrations were obtained by diluting stocks into culture medium that was exchanged to begin the drug pulse. Vehicle-alone groups received the same final concentration of ascorbic acid. The antagonist eticlopride was delivered for 15 min before adding additional drugs or light.
Retina collections. Retinas were harvested from the eyecup at indicated times after light or drug treatment using a dissecting microscope and either overhead room lights (light groups) or infrared lights (dark groups). Retinas were immediately frozen on dry ice and were stored at −80°C.
RNA isolation and Northern blot analysis. Total RNA was isolated from individual retinas using Trizol (Life Technologies). RNA was separated on an agarose-formaldehyde gel and was transferred to a Bright Star positively charged nylon membrane (Ambion, Austin, TX). Hybridizations were done using Ultrahyb solution (Ambion) according to the manufacturer's instructions. Blots were hybridized overnight at 42°C with 32P-labeled probes (1 × 106 cpm/ml). Final washes were at 42°C with 0.1× SSC and 0.1% SDS.
Probes. Single-strand DNA probes were generated by asymmetric PCR labeled with [32P]dCTP (New England Nuclear, Boston, MA) and were purified using DyeEx spin columns (Qiagen, Hilden, Germany). Blots were sequentially hybridized to xPer2 (either nucleotides 36–182 or 575–1066 of GenBank AF199499), xPer1 (GenBank AF250547), and either actin (nucleotides 171–680 of GenBank AF079161) or random-primed glyceraldehyde-3-phosphate dehydrogenase (GAPDH; GenBank J02642) probes for normalization.
Quantitative analysis. Four or five retinas from individual animals were analyzed in each group, and mean per gene responses are reported ± SEM. The signal of xPer1 orxPer2 mRNA from an individual retina was quantified using the Storm PhosphorImaging system (Molecular Dynamics, Sunnyvale, CA) and was normalized to the signal of either actin or GAPDH internal controls. The means of all groups are normalized to the untreated control. ANOVA with Tukey–Kramer multiple comparisons ort test were done using InStat software (version 2.03).
RESULTS
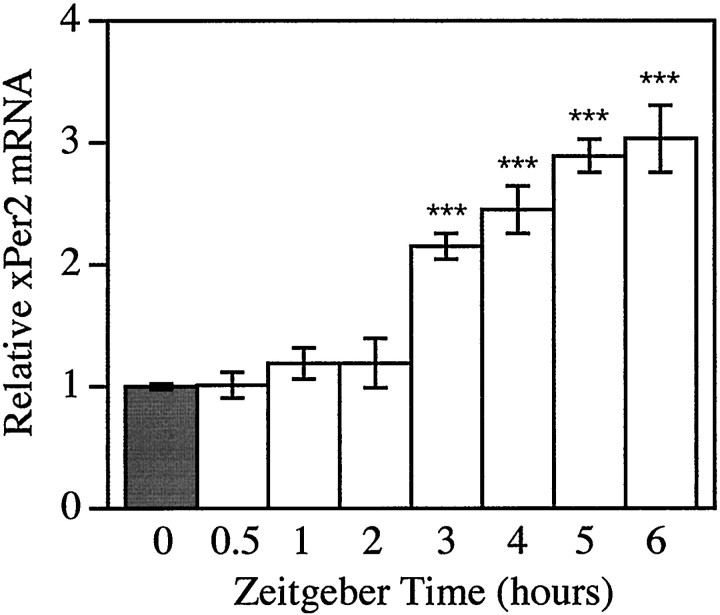
In DD, xPer2 mRNA levels in retina remain low and arrhythmic. However, in LD, xPer2 mRNA exhibits a robust rhythm with higher levels during the light phase (Zhuang et al., 2000). In those experiments, samples were collected at 4 hr intervals. To determine the extent of light induction occurring between 0 and 4 hr, frogs were exposed to continuous light beginning at the normal dark-to-light transition (ZT 0). Retinas were collected at various times after the onset of light, and the abundance of xPer2mRNA was quantified by Northern blotting (Fig.1). At 3 hr after light onset, significant differences were seen in the abundance of xPer2mRNA. The level of xPer2 mRNA continued to increase between 3 and 6 hr to a level approximately threefold higher than that at ZT 0.
Fig. 1.
Three hours of light increase xPer2mRNA in vivo. Total retinal RNA was prepared fromXenopus killed at various times after the onset of a 6 hr continuous light treatment delivered at ZT 0. The abundance ofxPer2 mRNA was quantitated by Northern blotting, and the signal was normalized to an internal control (actin). The mean value was calculated from five retinas from individual animals and was normalized to the 0 hr dark group (graybar). Error bars indicate SEM. Significant differences were detected between the 3, 4, 5, and 6 hr light-treated groups (white bars) compared with the 0 hr dark control group (***p < 0.001, ANOVA). No significant differences were detected between the 0, 0.5, 1, or 2 hr groups (ANOVA).
To verify that light increases xPer2 mRNA in a similar manner in cultured retinas, a time course was repeated usingXenopus eyecups (Besharse and Dunis, 1983; Cahill and Besharse, 1991). Eyecups were dissected on the afternoon before the experiment and were cultured in constant darkness until a continuous bright white light (3–5 × 10−4W/cm2) was delivered for 3 hr (Fig.2a,b) or for 6 hr (Fig.2c). Retinas were collected at various times after the beginning of the light treatment. In agreement with the in vivo data, cultured retinas required 3 hr of light exposure to achieve a significant increase in the abundance of xPer2mRNA compared with dark controls. Both the 10 and 13 kbxPer2 mRNAs were increased by light and were quantified together for all experiments (Fig. 2a). On the basis of these data, we chose to assay for changes in xPer2 mRNA after 3 hr of light in subsequent experiments.
Fig. 2.
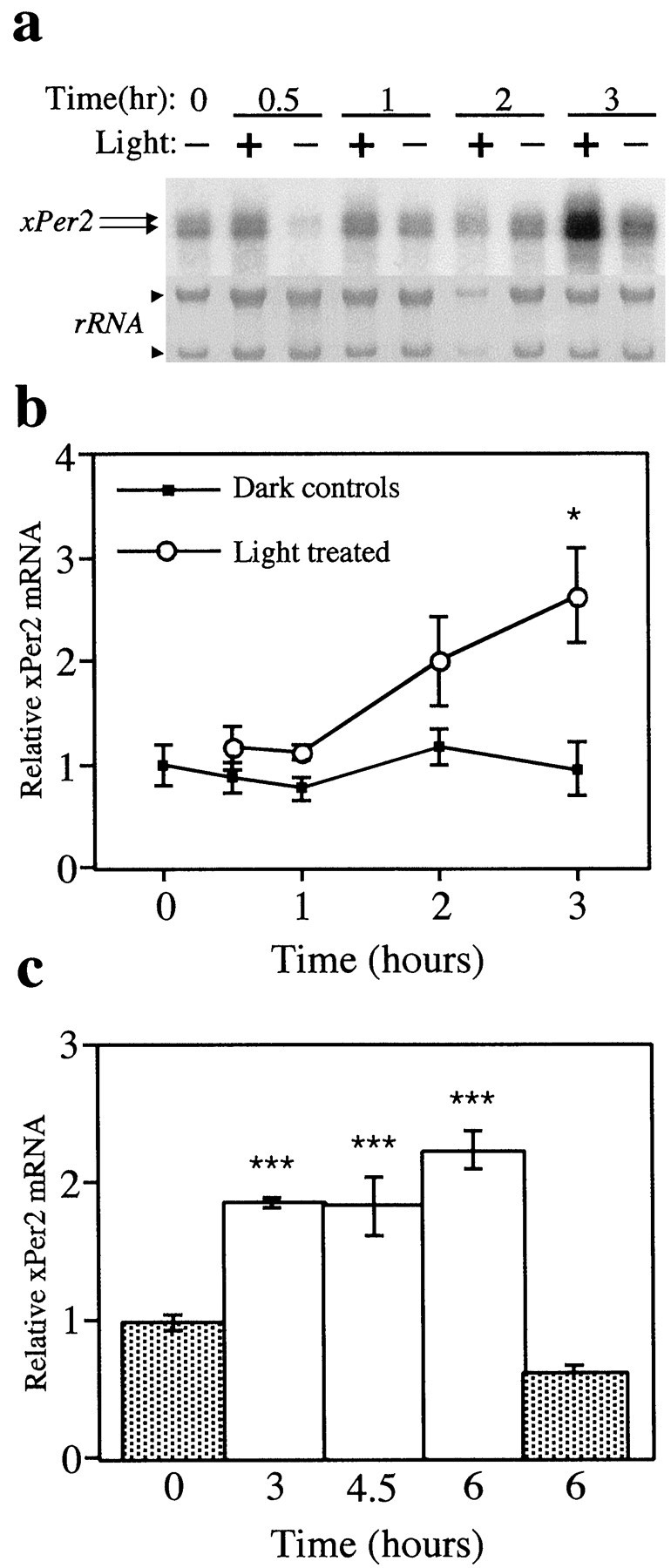
Three hours of light increase xPer2mRNA in vitro. Eyecups were dissected the afternoon before the experiment and were cultured in constant darkness until a continuous light pulse (3–5 × 10−4W/cm2) was delivered at either ZT 18 (a, b) or ZT 2 (c). a, Top,xPer2 mRNA for all experiments was measured by Northern blotting. Each lane contains the total RNA from a single retina of an individual animal. Two xPer2 bands (arrows) at 10 and 13 kb were increased after 3 hr of light. Both bands were quantitated in all experiments.Bottom, The abundance of the ribosomal RNA (arrowheads) was visualized by methylene blue staining of the blot. b, One eyecup from each animal received light (white circles), and the paired eyecup from the same animal was maintained in darkness (black squares). Retinas were collected at 0, 0.5, 1, 2, or 3 hr after the beginning of the light pulse. The abundance of xPer2 mRNA was quantitated by Northern blotting, and the xPer2 signal was normalized to the signal of an internal control (GAPDH) for each individual retina. Mean values (4–5 retinas from individual animals) for each group were normalized to the 0 hr dark control. The 3 hr light-treated group was significantly different from the 3 hr dark group (*p < 0.05, ANOVA). No significant differences were detected between any of the dark control groups (ANOVA). c, Retinas were collected after 3, 4.5, or 6 hr of light (white bars) or after 0 or 6 hr of dark (gray bars). The abundance ofxPer2 mRNA was quantitated by Northern blotting, and thexPer2 signal was normalized to the signal of an internal control (actin) for each individual retina. Mean values (4–5 retinas from individual animals) for each group were normalized to the 0 hr dark control. The light groups were significantly different from both the 0 and 6 hr dark controls (***p < 0.001, ANOVA). No significant difference was detected between the 0 and 6 hr dark groups (ANOVA).
If xPer2 is solely under the control of light, then its response to light should remain the same regardless of what time of day the light is delivered. Retinas were collected after 3 hr of light exposure that began at early subjective day (Fig.3a), late subjective day (Fig.3b), or subjective night (Fig. 3c). These times correspond to times for which phase-shifting data are available (Cahill and Besharse, 1991). At all three times of day, retinas receiving light have twofold to threefold more xPer2 mRNA than do dark controls.
The increase in xPer2 mRNA after a light pulse may serve to phase shift the retinal clock. In addition to light, dopamine phase shifts the retinal clock (Cahill and Besharse, 1991). To test whether dopamine would also increase xPer2 mRNA, quinpirole (1 μm), a D2-like dopamine agonist, was delivered to eyecups for 3 hr. Retinas were collected at various times after the beginning of the treatment, and xPer2 mRNA levels were measured. The level of xPer2 mRNA was significantly increased after 3 hr of quinpirole treatment (data not shown). Therefore, we chose to assay for changes in xPer2 mRNA levels after 3 hr of drug treatments. Three hours of various concentrations of dopamine, quinpirole, or SKF 38393 (a D1-like agonist) were delivered to eyecups at ZT 10 (Fig.4). Both dopamine and the D2-like agonist (quinpirole) in the nanomolar concentration range caused increases inxPer2 mRNA. The D1-like agonist SKF 38393 inducedxPer2 mRNA, but only at high micromolar concentrations (50 μm). These high concentrations of the D1 agonist likely cross-react with D2-like receptors (Munemura et al., 1980).
Dopamine (500 nm) increased xPer2 mRNA at all three times of day (Fig.5a–c). The increase inxPer2 mRNA was blocked by previous treatment with the D2-like antagonist eticlopride (50 μm). Because light is known to stimulate retinal dopamine release (Boatright et al., 1989), the effect of light on xPer2 mRNA could be mediated via the release of dopamine. However, light-induced phase shifts are not blocked by previous treatment with eticlopride (Cahill and Besharse, 1991). In a similar manner, the light-induced increase inxPer2 mRNA was not blocked by treating eyecups with eticlopride before light exposure at ZT 10 (Fig. 5e). There may be a partial block by eticlopride in the light-induced increase ofxPer2 mRNA at ZT 2 and ZT 18 (Fig. 5d,f). There was no significant difference, however, between the light and the light plus eticlopride groups at any time of day (ANOVA). Thus, it appears that light and dopamine increase xPer2 mRNA via parallel pathways as seen for phase shifting.
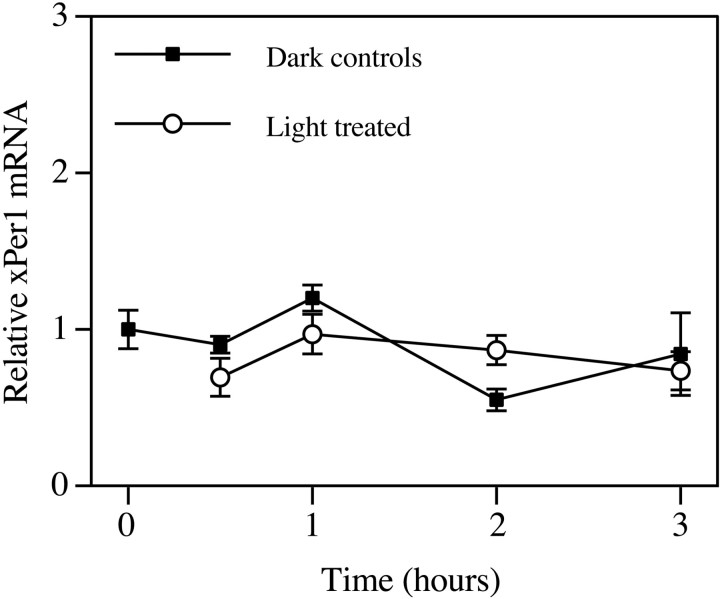
In contrast to xPer2, xPer1 mRNA is rhythmically expressed in LD and DD with peak levels at ZT 0 (Zhuang et al., 2000). To determine whether xPer1 mRNA was sensitive to light or dopamine treatments, blots from the previous experiments were probed with xPer1 cDNA. At ZT 2, both dopamine and light treatment induced xPer1 mRNA to a modest but significant extent compared with the vehicle control group (Fig.6a,d). Previous treatment with eticlopride blocked the effect of both light and dopamine at this time of day. However, there was no effect of light on xPer1 mRNA levels at ZT 10 or ZT 18 (Fig. 6e,f). A significant difference was detected between dopamine groups and the dopamine plus eticlopride groups at ZT 10 and ZT 18, but the induction was modest at best, and xPer1 mRNA values after dopamine treatment were not significantly different from vehicle controls (Fig.6b,c). These data suggest that xPer1 mRNA is not induced by light or dopamine at times of day known to phase shift the retinal clock (Cahill and Besharse, 1991).
In the mouse SCN, mPer1 mRNA is acutely increased by light and returns to control levels within 3 hr (Zylka et al., 1998). To exclude the possibility that light induces xPer1 mRNA with faster kinetics as seen in mouse SCN, retinas were sampled at various times during 3 hr of continuous light beginning at ZT 10 (Fig.7). No significant differences were seen in xPer1 mRNA levels between the light and dark groups at any time during the treatment. After 3 hr of light, xPer2mRNA was increased compared with dark controls as expected (data not shown). Therefore, xPer1 mRNA is relatively insensitive to light and dopamine, whereas xPer2 mRNA is induced by both phase-shifting agents.
Fig. 7.
xPer1 mRNA is not responsive to light at ZT 10. Three hours of continuous light (3–5 × 10−4 W/cm2) were delivered to eyecups beginning at ZT 10. At indicated times after the beginning of the pulse, retinas were collected from either light-treated groups (white circles) or from dark controls (black squares). The abundance ofxPer1 mRNA was quantitated as in Figure2c, and values were normalized to the 0 hr dark group. The mean value of each group was calculated from four to five retinas from individual animals. Error bars indicate SEM. No significant differences were detected between any of the groups (ANOVA).
DISCUSSION
Our observations suggest that phase shifting inXenopus retina involves the light- and dopamine-induciblexPer2 gene. Three hours of light or dopamine increased the levels of xPer2 mRNA at all times of day, and the effect of dopamine was shown to be mediated by activation of D2-like dopamine receptors. Eticlopride, a D2 antagonist, blocked the effects of dopamine but not the effects of light, indicating that light increasesxPer2 mRNA in a dopamine-independent manner. These features of acute stimulation of xPer2 mRNA are remarkably similar to the features of light- and dopamine-induced phase shifts of melatonin outflow shown in previous work on this system (Cahill and Besharse, 1991). Thus, increased xPER2 in daytime would be expected to reinforce the existing circadian phase, whereas increased xPER2 at night would lead to delay or advance phase shifts.
The mouse homolog of xPer2 has been shown to be an indispensable part of the clock (Zheng et al., 1999). BothmPer1 and mPer2 mRNA levels increase in SCN after a phase-shifting light treatment. In good agreement with the retinalxPer2 induction shown here, mPer2 in the SCN responds more slowly to light with maximal mRNA levels seen after 2–3 hr (Albrecht et al., 1997; Shearman et al., 1997; Takumi et al., 1998;Zylka et al., 1998). A recent study in quail retina shows thatqPer2 mRNA is induced by light at both ZT 10 and ZT 16 with a similar time course to that of xPer2 (Yoshimura et al., 2000). The per genes are thought to be negative elements in the transcription feedback loop. Positive elements in the feedback loop are CLOCK and BMAL1, which are basic helix–loop–helix transcription factors that activate transcription at E box elements within the promoters of the per genes (Gekakis et al., 1998;Hogenesch et al., 1998). In transient transfection assays, mPER2 inhibits CLOCK- and BMAL1-mediated transcription (Griffin et al., 1999; Jin et al., 1999; Kume et al., 1999). Recent evidence suggests that mPER2 also positively regulates bmal1transcription (Shearman et al., 2000). Thus, changes in the level ofPer2 are expected to both increase and decrease the levels of other clock genes, which will change the phasing of the clock.
We could not detect light induction of xPer1 mRNA in retina at times of day known to phase shift the retinal circadian clock. This is strikingly different from the light response shown formPer1 in mouse SCN, in which mPer1 mRNA levels are maximally induced at 30–60 min and return to baseline levels by 3 hr (Albrecht et al., 1997; Shearman et al., 1997; Shigeyoshi et al., 1997; Zylka et al., 1998). Injection of mPer1 antisense oligonucleotides into SCN causes inhibition of light-induced phase shifts of behavior (Akiyama et al., 1999). This suggests a major role for mPer1 in phase shifting within the mouse SCN. The disparity in the light induction of Per1 betweenXenopus retina and mouse SCN may reflect a fundamental difference in the mechanism of phase shifting between the retina and SCN. In the SCN, the light signal received in the retina is transmitted via the retinohypothalamic tract, causing neurotransmitter release onto SCN neurons. In the retina, a large proportion of clock cells are directly photosensitive (Cahill and Besharse, 1993).
The induction of xPer2 mRNA by dopamine inXenopus retina is one of the first reports of a member of the per family being acutely responsive to a stimulus other than light (Balsalobre et al., 1998; Maywood et al., 1999). For many retinal processes, the effect of light is mimicked by dopamine. For example, light-evoked cone contraction is blocked by a D2-like dopamine antagonist (Pierce and Besharse, 1985), suggesting that dopamine release mediates light-evoked cone contraction (Besharse and Witkovsky, 1992). In contrast, light and dopamine act in parallel, convergent pathways to cause phase shifts in the melatonin rhythm (Cahill and Besharse, 1991). In those studies, a D2-like antagonist was able to block the effects of exogenous dopamine but was ineffective in blocking phase shifts induced by light. D2-like receptor activation causes a decrease in cAMP levels (Vallone et al., 2000). Increasing cAMP inXenopus retina blocks phase shifting by dopamine but does not block phase shifting by light (Hasegawa and Cahill, 1999). Thus, light and dopamine cause phase shifts via different second messenger systems. We show that light and dopamine act in parallel to increasexPer2 mRNA. This may be the first point at which the light and dopamine pathways converge on the retinal clock.
Whether the induction of xPer2 mRNA is the initial event leading to phase shifting in Xenopus retina is an important question. Light may cause increases in xPer2 mRNA by stabilizing existing xPer2 mRNA or by activating new transcription of xPer2. Both possibilities may require the synthesis or recruitment of an additional protein. Evidence from transgenic mice in which green fluorescent protein (GFP) is driven by the mPer1 promoter shows that acute light exposure increases GFP signal above controls, indicating a transcriptional mechanism for mPer1 induction (Kuhlman et al., 2000). Three hours of light are required to increase xPer2 mRNA inXenopus retina, similar to the induction of mPer2in SCN (Albrecht et al., 1997; Shearman et al., 1997; Takumi et al., 1998; Zylka et al., 1998). In the SCN, however, there are other genes that respond to phase-shifting pulses of light with faster kinetics such as mPer1 (Shigeyoshi et al., 1997) and the immediate-early gene c-fos (Ikonomov and Stoynev, 1994;Shearman and Weaver, 1999). We do not believe that xPer1 is involved in phase shifting in Xenopus retina becausexPer1 mRNA is insensitive to phase-shifting pulses of light and dopamine. Fos induction by light is seen in amacrine and ganglion cells of the mammalian retina (Sagar and Sharp, 1990;Chambille et al., 1993; Koistinaho and Sagar, 1995; Huerta et al., 1997). It is possible that fos or other immediate-early genes induced by light could play a role in phase shifting the retinal clock.
A unique circadian organization exists in Xenopus retina. We found that xPer1 mRNA levels are regulated by an endogenous clock and that xPer2 mRNA levels are regulated by light and dopamine. It appears that xPer2 provides the molecular link between light and the circadian clock in retina. Further analysis will provide an understanding of how xPer2 regulates the cycling of other clock components and alters rhythmic physiology downstream of the clock.
Footnotes
This work was supported by National Institutes of Health Grant EY 02414 to J.C.B. We would like to thank Pam Megaw, Sheila Baker, Minhong Zhuang, Carla Green, and Greg Cahill for many valuable discussions concerning this work. A special thanks goes to Pam Megaw for doing the melatonin measurements. We also thank Cheryl Stucky and Maureen Neitz for critical reading of this manuscript.
Correspondence should be addressed to Dr. Joseph C. Besharse, Department of Cell Biology, Neurobiology, and Anatomy, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226. E-mail:jbeshars@mcw.edu.
REFERENCES
- 1.Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci. 1999;19:1115–1121. doi: 10.1523/JNEUROSCI.19-03-01115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 3.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 4.Besharse JC, Dunis DA. Rod photoreceptor disc shedding in eye cups: relationship to bicarbonate and amino acids. Exp Eye Res. 1983;36:567–579. doi: 10.1016/0014-4835(83)90051-9. [DOI] [PubMed] [Google Scholar]
- 5.Besharse JC, Iuvone PM. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983;305:133–135. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- 6.Besharse JC, Witkovsky P. Light-evoked contraction of red absorbing cones in the Xenopus retina is maximally sensitive to green light. Vis Neurosci. 1992;8:243–249. doi: 10.1017/s0952523800002893. [DOI] [PubMed] [Google Scholar]
- 7.Boatright JH, Hoel MJ, Iuvone PM. Stimulation of endogenous dopamine release and metabolism in amphibian retina by light- and K+-evoked depolarization. Brain Res. 1989;482:164–168. doi: 10.1016/0006-8993(89)90555-6. [DOI] [PubMed] [Google Scholar]
- 8.Cahill GM, Besharse JC. Circadian regulation of melatonin in the retina of Xenopus laevis: limitation by serotonin availability. J Neurochem. 1990;54:716–719. doi: 10.1111/j.1471-4159.1990.tb01932.x. [DOI] [PubMed] [Google Scholar]
- 9.Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahill GM, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- 11.Cahill GM, Grace MS, Besharse JC. Rhythmic regulation of retinal melatonin: metabolic pathways, neurochemical mechanisms, and the ocular circadian clock. Cell Mol Neurobiol. 1991;11:529–559. doi: 10.1007/BF00734814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambille I, Doyle S, Serviere J. Photic induction and circadian expression of Fos-like protein. Immunohistochemical study in the retina and suprachiasmatic nuclei of hamster. Brain Res. 1993;612:138–150. doi: 10.1016/0006-8993(93)91654-b. [DOI] [PubMed] [Google Scholar]
- 13.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 14.Field MD, Maywood ES, O'Brien JA, Weaver DR, Reppert SM, Hastings MH. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron. 2000;25:437–447. doi: 10.1016/s0896-6273(00)80906-x. [DOI] [PubMed] [Google Scholar]
- 15.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 16.Green CB. How cells tell time. Trends Cell Biol. 1998;8:224–230. doi: 10.1016/s0962-8924(98)01269-0. [DOI] [PubMed] [Google Scholar]
- 17.Griffin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, Cahill GM. A role for cyclic AMP in entrainment of the circadian oscillator in Xenopus retinal photoreceptors by dopamine but not by light. J Neurochem. 1999;72:1812–1820. doi: 10.1046/j.1471-4159.1999.0721812.x. [DOI] [PubMed] [Google Scholar]
- 19.Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci USA. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huerta JJ, Llamosas MM, Cernuda-Cernuda R, Garcia-Fernandez JM. Fos expression in the retina of rd/rd mice during the light/dark cycle. Neurosci Lett. 1997;232:143–146. doi: 10.1016/s0304-3940(97)00595-8. [DOI] [PubMed] [Google Scholar]
- 21.Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996;84:677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- 22.Ikonomov OC, Stoynev AG. Gene expression in suprachiasmatic nucleus and circadian rhythms. Neurosci Biobehav Rev. 1994;18:305–312. doi: 10.1016/0149-7634(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 23.Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 24.Koistinaho J, Sagar SM. Light-induced c-fos expression in amacrine cells in the rabbit retina. Brain Res Mol Brain Res. 1995;29:53–63. doi: 10.1016/0169-328x(94)00218-4. [DOI] [PubMed] [Google Scholar]
- 25.Kuhlman SJ, Quintero JE, McMahon DG. GFP fluorescence reports Period1 circadian gene regulation in the mammalian biological clock. NeuroReport. 2000;11:1479–1482. [PubMed] [Google Scholar]
- 26.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 27.Lee C, Parikh V, Itsukaichi T, Bae K, Edery I. Resetting the Drosophila clock by photic regulation of PER and a PER-TIM complex. Science. 1996;271:1740–1744. doi: 10.1126/science.271.5256.1740. [DOI] [PubMed] [Google Scholar]
- 28.Maywood ES, Mrosovsky N, Field MD, Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci USA. 1999;96:15211–15216. doi: 10.1073/pnas.96.26.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munemura M, Cote TE, Tsuruta K, Eskay RL, Kebabian JW. The dopamine receptor in the intermediate lobe of the rat pituitary gland: pharmacological characterization. Endocrinology. 1980;107:1676–1683. doi: 10.1210/endo-107-6-1676. [DOI] [PubMed] [Google Scholar]
- 30.Myers MP, Wager-Smith K, Rothenfluh-Hilfiker A, Young MW. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- 31.Pierce ME, Besharse JC. Circadian regulation of retinomotor movements. I. Interaction of melatonin and dopamine in the control of cone length. J Gen Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce ME, Besharse JC. Circadian regulation of retinomotor movements. II. The role of GABA in the regulation of cone position. J Comp Neurol. 1988;270:279–287. doi: 10.1002/cne.902700208. [DOI] [PubMed] [Google Scholar]
- 33.Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 34.Sagar SM, Sharp FR. Light induces a Fos-like nuclear antigen in retinal neurons. Brain Res Mol Brain Res. 1990;7:17–21. doi: 10.1016/0169-328x(90)90068-o. [DOI] [PubMed] [Google Scholar]
- 35.Shearman LP, Weaver DR. Photic induction of Period gene expression is reduced in Clock mutant mice. NeuroReport. 1999;10:613–618. doi: 10.1097/00001756-199902250-00031. [DOI] [PubMed] [Google Scholar]
- 36.Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 37.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 38.Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- 39.Takumi T, Matsubara C, Shigeyoshi Y, Taguchi K, Yagita K, Maebayashi Y, Sakakida Y, Okumura K, Takashima N, Okamura H. A new mammalian period gene predominantly expressed in the suprachiasmatic nucleus. Genes Cells. 1998;3:167–176. doi: 10.1046/j.1365-2443.1998.00178.x. [DOI] [PubMed] [Google Scholar]
- 40.Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimura T, Suzuki Y, Makino E, Suzuki T, Kuroiwa A, Matsuda Y, Namikawa T, Ebihara S. Molecular analysis of avian circadian clock genes. Brain Res Mol Brain Res. 2000;78:207–215. doi: 10.1016/s0169-328x(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 42.Zeng H, Qian Z, Myers MP, Rosbash M. A light-entrainment mechanism for the Drosophila circadian clock. Nature. 1996;380:129–135. doi: 10.1038/380129a0. [DOI] [PubMed] [Google Scholar]
- 43.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 44.Zhuang M, Wang Y, Steenhard BM, Besharse JC. Differential regulation of two period genes in the Xenopus eye. Brain Res Mol Brain Res. 2000;82:52–64. doi: 10.1016/s0169-328x(00)00177-7. [DOI] [PubMed] [Google Scholar]
- 45.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]