Abstract
Brain-derived neurotrophic factor (BDNF) has been postulated to be a key signaling molecule in regulating synaptic strength and overall circuit activity. In this context, we have found that BDNF dramatically increases the frequency of spontaneously initiated action potentials in hippocampal neurons in dissociated culture. Using analysis of unitary synaptic transmission and immunocytochemical methods, we determined that chronic treatment with BDNF potentiates both excitatory and inhibitory transmission, but that it does so via different mechanisms. BDNF strengthens excitation primarily by augmenting the amplitude of AMPA receptor-mediated miniature EPSCs (mEPSCs) but enhances inhibition by increasing the frequency of mIPSC and increasing the size of GABAergic synaptic terminals. In contrast to observations in other systems, BDNF-mediated increases in AMPA-receptor mediated mEPSC amplitudes did not require activity, because blocking action potentials with tetrodotoxin for the entire duration of BDNF treatment had no effect on the magnitude of this enhancement. These forms of synaptic regulations appear to be a selective action of BDNF because intrinsic excitability, synapse number, and neuronal survival are not affected in these cultures. Thus, although BDNF induces a net increase in overall circuit activity, this results from potentiation of both excitatory and inhibitory synaptic drive through distinct and selective physiological mechanisms.
Keywords: neurotrophins, BDNF, hippocampal neurons, synaptic plasticity, excitatory synaptic transmission, inhibitory synaptic transmission, AMPA receptors, NMDA receptors
Members of the neurotrophin family of peptide growth factors, particularly brain-derived neurotrophic factor (BDNF), are emerging as important mediators of activity-dependent modifications in synaptic strength. Their biological roles in such processes, however, remain uncertain. Whereas in some situations BDNF has been found to increase synaptic strength, such as at developing neuromuscular synapses and at excitatory synapses onto CA1 hippocampal neurons (Lohof et al., 1993; Vicario-Abejon et al., 1998; Sherwood and Lo, 1999), in cortical cultures, BDNF has been proposed to regulate the strength of all synapses onto a given neuron to maintain homeostasis (Rutherford et al., 1998). Consistent with both of these potential biological roles, the production and release of BDNF is activity-dependent in a variety of neuronal systems (McAllister et al., 1999).
Evidence supporting a role for BDNF in activity-dependent strengthening of specific synapses falls into two categories: acute experiments in which the effects of BDNF manifest within minutes, and chronic experiments in which effects take days to develop. Suppression of BDNF expression by gene deletion or introduction of antisense oligonucleotides results in deficiencies in hippocampal long-term potentiation (LTP), which can be rescued by acute provision of exogenous BDNF (Korte et al., 1995, 1996a,b; Patterson et al., 1996). BDNF has also been reported to enhance basal synaptic transmission at Schaffer collateral–CA1 synapses (Kang and Schuman, 1995a,b) (but see Figurov et al., 1996; Patterson et al., 1996;Frerking et al., 1998; Gottschalk et al., 1998) and to interact with tetanus-induced LTP in this region (Figurov et al., 1996;Gottschalk et al., 1998; Korte et al., 1998). Similar acute potentiative effects have been found in the hippocampal CA3 region and dentate gyrus (Scharfman, 1997, 1999). In neocortex, there is evidence that BDNF potentiates basal synaptic transmission (Akaneya et al., 1996, 1997; Carmignoto et al., 1997) (but see Huber et al., 1998;Kinoshita et al., 1999) and that BDNF increases the probability of inducing LTP rather than long-term depression for a range of stimulus paradigms (Akaneya et al., 1996, 1997; Huber et al., 1998; Kinoshita et al., 1999; Sermasi et al., 1999). Finally, rapid enhancement of excitatory transmission has also been demonstrated in dissociated hippocampal cultures (Lessmann et al., 1994; Levine et al., 1995, 1996,1998; Lessmann and Heumann, 1998; Li et al., 1998; Song et al., 1998) and at developing neuromuscular synapses in culture (Lohof et al., 1993; Wang et al., 1995; Stoop and Poo, 1996; Boulanger and Poo, 1999a,b).
More recently, long-term synaptic strengthening by BDNF has been observed in cultured hippocampal neurons. In autaptic cultures of glutamatergic CA1 neurons, chronic BDNF treatment increases quantal amplitude and the amplitude of evoked synaptic transmission in parallel (Sherwood and Lo, 1999). In dissociated cultures of early embryonic hippocampal neurons, BDNF increases the number of functional synaptic connections (Vicario-Abejon et al., 1998). In contrast, studies of long-term effects of BDNF in dissociated cortical cultures have suggested that BDNF decreases neuronal firing rate by reducing the strength of all excitatory inputs onto a given neuron (Rutherford et al., 1998; Turrigiano and Nelson, 1998; Turrigiano et al., 1998;Turrigiano, 1999).
To begin to understand this apparent pleiotropy of BDNF action, we examined the regulation of synaptic activity by BDNF in hippocampal cultures in which effects on overall action potential activity, excitation, and inhibition can be analyzed separately. In these cultures, we observed that, rather than maintaining action potential firing rates at a constant level, BDNF increased overall spontaneous firing rate by approximately threefold. We found that actions of BDNF underlying this increase in activity involved enhancement of both excitatory and inhibitory synaptic transmission in parallel but via distinct cellular mechanisms. BDNF selectively increased the quantal amplitude of AMPA receptor-mediated excitatory transmission, an increase that did not require ongoing action potential activity. In contrast, BDNF did not affect the quantal amplitude of GABAergic transmission but rather increased the frequency of spontaneous quantal inhibitory transmission. Thus, although these effects contained a homeostatic component, the overall action of BDNF was strongly stimulatory and supports a role for BDNF as a mediator of activity-dependent plasticity in vivo.
MATERIALS AND METHODS
Cell culture. Standard dissociated hippocampal cultures were prepared from postnatal day 0 rat pups using the technique of Pan et al. (1993). Briefly, rat pups were anesthetized using isofluorane, and hippocampi were dissected into HBSS (Life Technologies, Gaithersburg, MD) with 10 mmHEPES. Each hippocampus was diced and incubated at 37°C for 45 min in HBSS containing 20 U/ml papain (Worthington, Freehold, NJ), 0.5 mm EDTA, 1.5 mmCaCl2, and 10 mm HEPES (Sigma, St. Louis, MO). The papain solution was removed, and residual papain was inactivated by the addition of serum-containing medium. The cells were then triturated by passage through fire-polished Pasteur pipettes with sequentially smaller diameter openings. Cells were plated at a density of 200,000 cells per dish in 35 mm dishes that had been coated with poly-d-lysine and merosin (Life Technologies). After 1 d in culture, 25% of the medium was exchanged, and the mitotic inhibitor 5-fluoro-2-deoxyuridine was added to minimize glial proliferation.
Neurotrophin treatment. Hippocampal cultures were allowed to establish for 3 d, after which either 100 ng/ml BDNF or 5 μg/ml TrkB-IgG (both generous gifts from Regeneron Pharmaceuticals, Tarrytown, NY) was added; we have shown previously that these concentrations of BDNF and TrkB-IgG are effective over the time course of several days and are low enough to avoid cross activation of other neurotrophin receptors (Lesser and Lo, 1995; Lesser et al., 1997;Riddle et al., 1997; Sherwood et al., 1997; Sherwood and Lo, 1999). Electrophysiological recordings were made after 4–7 d of treatment.
Electrophysiology. Electrophysiological recordings were made with an Axopatch 1D patch-clamp amplifier (Axon Instruments, Foster City, CA), and data were acquired using an INDEC Systems analog-to-digital converter and custom software written in-house in Visual Basic (Microsoft, Seattle, WA). Whole-cell patch-clamp recording was done using standard methods (Hamill et al., 1981). Borosilicate patch pipettes were pulled to resistances of 3–4 MΩ. For synaptic currents, data were acquired continuously at 2.5 kHz sampling frequency and filtered at 1 kHz using a four-pole Bessel filter. Recordings with leak currents >100 pA or series resistances >20 MΩ were discarded.
For recording AMPA receptor-mediated synaptic currents, the extracellular solution contained (in mm): 137 NaCl, 5 KCl, 3 CaCl2,1 MgCl2, 10 glucose, and 5 HEPES, adjusted to 310 mOsm and pH 7.25. To block GABAA receptor-mediated and action potential-driven synaptic transmission, 25 μm bicuculline and 1 μm tetrodotoxin (TTX) were included in the extracellular solution. The intracellular solution contained (in mm): 100 gluconic acid, 0.6 EGTA, 5 MgCl2, 2 Na2-ATP, 0.3 Na2-GTP, and 40 HEPES, adjusted to 310 mOsm and pH 7.25. For recording NMDA receptor-mediated synaptic currents, identical solutions were used, except that the extracellular solution was nominally Mg2+-free and contained 3 μm2,3-dihydroxy-6-nitro-7-sulphamoylbenzo(f)-quinoxalinedione (NBQX) and 10 μm glycine. For recording GABAA receptor-mediated synaptic currents, the intracellular solution contained (in mm): 110 KCl, 10 EGTA, 5 MgCl2, 2 Na2-ATP, 0.3 Na2-GTP, and 30 HEPES, adjusted to 310 mOsm and pH 7.25. The extracellular solution was the same as described for AMPA receptor-mediated currents but containing 3 μm NBQX instead of bicuculline.
Intrinsic excitability was measured in current-clamp mode. The intracellular solution contained (in mm): 144 K-gluconate, 0.5 EGTA, 1 MgCl2, 2 Na2ATP, 0.3 Na2GTP, and 0.5 HEPES, adjusted to 310 mOsm and pH 7.25. Membrane voltages were sampled at 10 kHz and filtered at 2 kHz. To elicit action potential activity, a series of depolarizing pulses of 160 msec duration were delivered to the cells. Input–output relationships were determined by plotting the current injected versus action potential firing frequency. Action potential height, half-width, and spike threshold were measured off-line. Membrane capacitance, series resistance, and input resistance were measured under voltage clamp.
On-cell patch-clamp recording was used for noninvasive measurement of the frequency of action potential activity. Data were acquired at 5 kHz in voltage-follower recording mode and filtered at 2 kHz. The extracellular and pipette solutions were the same, containing (in mm): 137 NaCl, 5 KCl, 3 CaCl2, 1 MgCl2, 10 glucose, 0.01 glycine, and 5 HEPES, adjusted to 310 mOsm and pH 7.25.
Immunocytochemistry. To measure neuronal survival and the percentage of inhibitory neurons, parallel cultures were double-labeled using a mouse monoclonal antibody directed against the GABAergic neuronal marker glutamic acid decarboxylase (GAD) (1:500; Chemicon, Temecula, CA) and a rabbit polyclonal antibody against neuron-specific enolase (NSE) (1:500; Chemicon). GAD immunostaining was visualized with an Oregon Green 488-conjugated goat anti-mouse secondary antibody (1:400; Molecular Probes, Eugene, OR); NSE immunostaining was visualized with a Cy3-conjugated goat anti-rabbit secondary antibody (1:400; Chemicon). On the fifth day of neurotrophin treatment, cultures were fixed in 4% paraformaldehyde and 5% sucrose in PBS and then washed with PBS. Nonspecific binding was blocked with 10% goat serum in PBS, and cells were permeabilized with 0.1% Triton X-100. Cultures were then incubated overnight with primary antibody at 4°C, followed by incubation in secondary antibody for 1 hr at room temperature. Finally, cultures were rinsed with PBS, mounted in Molwiol, and coverslipped.
Cell counts were done with the experimenter blinded on an invertedZeiss (Oberkochen, Germany) microscope (Axiovert) with a 10× objective. Mean numbers of GAD- or NSE-positive neurons per field were calculated by counting the number of GAD-positive cells in a field using fluorescein filters and then switching to rhodamine filters and recounting the same field for NSE-positive cells. Fields were analyzed by randomly placing the objective and then sequentially moving the dish through a vertical strip of 10 fields.
To quantify total numbers of synapses per field and numbers of inhibitory synapses per field, cultures were double-labeled using a mouse anti-synapsin monoclonal antibody (1:500; Chemicon) and a rabbit anti-GAD polyclonal antibody (1:1000; Chemicon). GAD immunostaining was visualized with an Oregon Green 488-conjugated goat anti-rabbit secondary antibody (1:500; Molecular Probes); synapsin immunostaining was visualized with a Cy3-conjugated goat anti-mouse secondary antibody (1:500; Chemicon). The immunostaining procedure was the same as described above.
As for cell counts, synapse quantification was done with the experimenter blinded. Images were acquired and digitized using a Hamamatsu (Shizouka, Japan) chilled CCD camera on a Zeiss Axioscope using a 40× oil-immersion objective; exposure times were held constant for each fluorochrome. Images were taken by randomly placing the objective and then sequentially moving the dish through a vertical strip of 10 fields. Images were analyzed using Scion Image software (Scion Corp., Frederick, MD); briefly, a mask containing the location and area of each synapsin or GAD puncta was made using a combination of thresholding and manual highlighting. Numbers, areas, and intensities of these punctae were then determined using built-in Scion Image functions.
RESULTS
BDNF increases spontaneous activity in dissociated cultures of hippocampal neurons
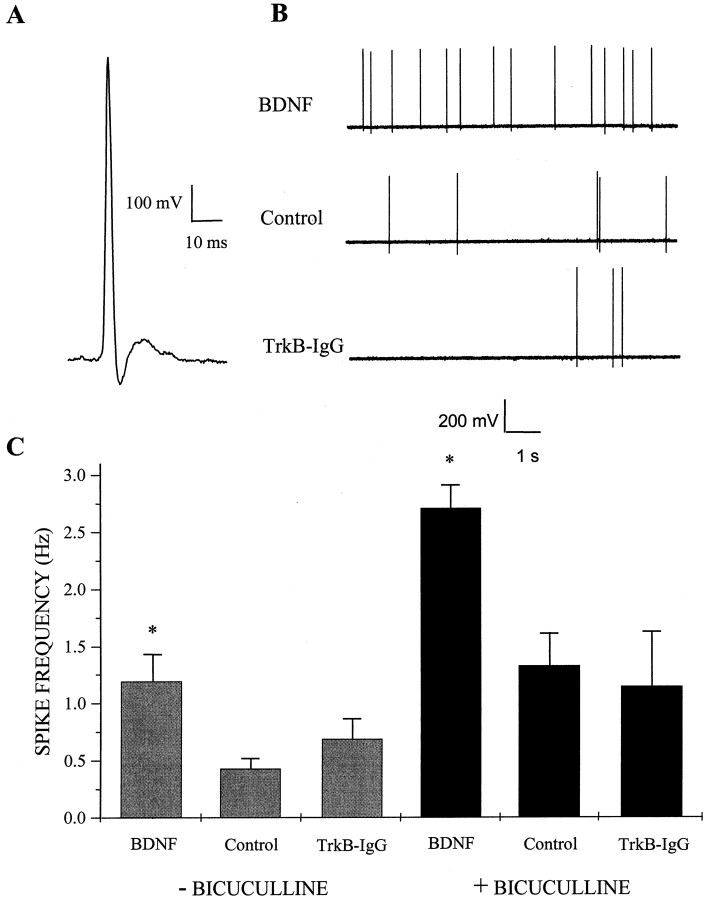
To investigate the role of BDNF in regulating the overall excitability of dissociated cultures of hippocampal neurons, cultures were treated for 4–7 d with 100 ng/ml BDNF and compared with untreated controls and cultures in which endogenous BDNF was neutralized with 5 μg/ml TrkB-IgG (Shelton et al., 1995; McAllister et al., 1996, 1997). Spontaneous action potentials in neurons with pyramidal morphology were then recorded using cell-attached patch-clamp recording as a rapid and relatively noninvasive measurement of circuit activity. BDNF treatment increased the average spontaneous action potential firing rates of neurons by approximately threefold compared with untreated controls (n = 33, 27, and 36 for BDNF, control, and TrkB-IgG cultures, respectively; BDNF vs control, p < 0.009 by ANOVA) (Fig. 1). Interestingly, there was no significant difference between untreated controls and TrkB-IgG-treated neurons with respect to firing rate (p > 0.60) or for any other electrophysiological parameter measured in this study, suggesting that levels of endogenous BDNF are below the physiological threshold required to modulate activity in these low-density cultures. This is consistent with the finding that endogenous BDNF levels are negligible in autaptic hippocampal cultures (Sherwood and Lo, 1999).
Fig. 1.
BDNF increases spontaneous action potential firing rate. A, Representative traceillustrating the waveform of an action potential recorded with an on-cell patch pipette; this configuration was used to measure action potential frequency in dissociated hippocampal cultures. Voltage traces were inverted about the vertical axis to conform to convention. Data were acquired at 5 kHz in voltage-follower recording mode and filtered at 2 kHz. B, Spontaneous action potential firing was increased in cultures treated with BDNF. Representative on-cell recordings shown at a compressed time base from cells treated for 4–7 d with 100 ng/ml BDNF (top), untreated (Control, middle), or treated with 5 μg/ml TrkB-IgG (bottom). Cultures were rinsed in recording saline several times to ensure that no BDNF or TrkB-IgG was present at the time of recording. C,Left, BDNF treatment increased the spontaneous firing rate of pyramidal neurons approximately threefold compared with untreated controls; means ± SEM are shown. *p< 0.009 indicates a significant difference by ANOVA between BDNF and either control or TrkB-IgG treatment groups;n = 33, 27, and 36 for BDNF, control, and TrkB-IgG groups, respectively. Right, Elevated action potential firing rates induced by BDNF persisted in disinhibited circuits. BDNF appeared to increase excitatory synaptic transmission directly because the increase in spontaneous firing rates of pyramidal neurons persisted after acute blockade of inhibitory transmission by bicuculline during the recording period. *p < 3 × 10−5 indicates a significant difference by ANOVA between BDNF and either control or TrkB-IgG treatment groups;n = 43, 36, and 12 for BDNF, control, and TrkB-IgG groups, respectively.
This striking increase in spontaneous firing rate could have arisen from an increase in the strength of excitation, a decrease in the strength of inhibition, or both. To evaluate the effect of BDNF on excitation directly, the contribution of inhibition to circuit activity was eliminated during the period of electrophysiological recording by pharmacologically blocking GABAA receptors acutely with bicuculline and measuring action potential firing rates in these disinhibited circuits. Under these conditions, BDNF still elevated spontaneous action potential firing rate by approximately twofold, indicating that the action of BDNF must include, at least in part, a direct potentiation of excitatory synaptic transmission (n = 43, 36, and 12 for BDNF, control, and TrkB-IgG cultures, respectively; BDNF vs control, p < 3 × 10−5 by ANOVA) (Fig. 1).
BDNF does not influence neuronal survival
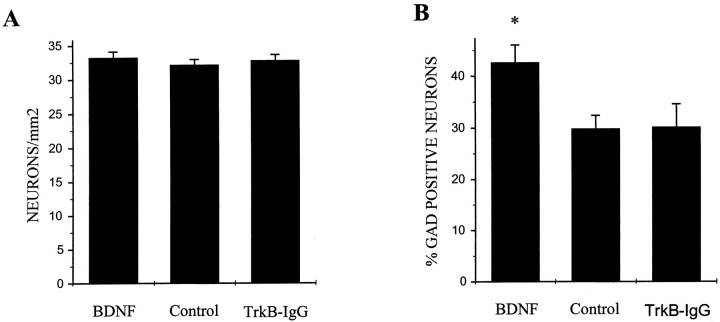
We next asked whether the action of BDNF contained a component that influenced the balance of excitation and inhibition in these cultures via differential regulation of survival of excitatory versus inhibitory neurons. We found that, by quantifying neuronal density in BDNF-treated, control, and TrkB-IgG-treated cultures, BDNF had no measurable effect on total neuronal survival (n = 161 fields counted for each condition; p > 0.35 by ANOVA for any pairwise comparison) (Fig.2A). Although there was no change in overall neuronal density, BDNF increased the percentage of neurons expressing the inhibitory neuronal marker GAD (n = 180 fields counted for each condition; BDNF vs control, p < 0.008 by ANOVA) (Fig.2B).
Fig. 2.
BDNF enhances the phenotypic differentiation of GABAergic neurons but has no effect on neuronal survival. Hippocampal cultures were treated for 5 d with either BDNF or TrkB-IgG or were left untreated; cultures were then double-labeled for the neuronal marker NSE and the GABAergic neuronal marker GAD. Numbers of NSE-positive and GAD-positive neurons were counted independently; all counts were done blind. A, BDNF treatment did not affect neuronal survival. Means ± SEM are shown; n = 9 dishes per condition with 20 fields counted and averaged per dish; p > 0.35 for any pairwise comparison by ANOVA. B, BDNF increased the percentage of GAD-positive neurons. *p < 0.008 indicates a significant difference by ANOVA between BDNF and either control or TrkB-IgG treatment groups; n = 9 dishes per condition with 20 fields counted and averaged per dish.
There are two possible interpretations of this result. First, BDNF may have caused a decrease in the survival of non-GABAergic neurons that was exactly balanced by an increase in survival of GABAergic neurons. Second, BDNF may have promoted the neurochemical maturation of inhibitory neurons such that the level of GAD expression was increased to detectable levels in a greater percentage of inhibitory neurons, with no effect on neuronal survival per se. The latter interpretation is consistent with previous work suggesting that BDNF potentiates phenotypic differentiation of GABAergic neurons (Ip et al., 1993; Nawa et al., 1993; Mizuno et al., 1994; Ventimiglia et al., 1995;Marty et al., 1996a,b; Rutherford et al., 1997; Vicario-Abejon et al., 1998). Furthermore, other studies have shown that BDNF-mediated increases in GABA and neuropeptide expression levels are reversible, thus arguing against differential survival as a mechanism for BDNF action (Marty and Onteniente, 1997; Rutherford et al., 1997).
Because BDNF did not affect overall neuronal density and its enhancement of inhibitory neuronal differentiation would be predicted to decrease circuit activity, these factors were not likely to contribute positively to the observed increase of circuit activity induced by BDNF. Thus, these findings suggested that BDNF must increase circuit activity by other mechanisms, such as enhanced synaptic drive or by increased intrinsic membrane excitability.
BDNF does not regulate intrinsic excitability
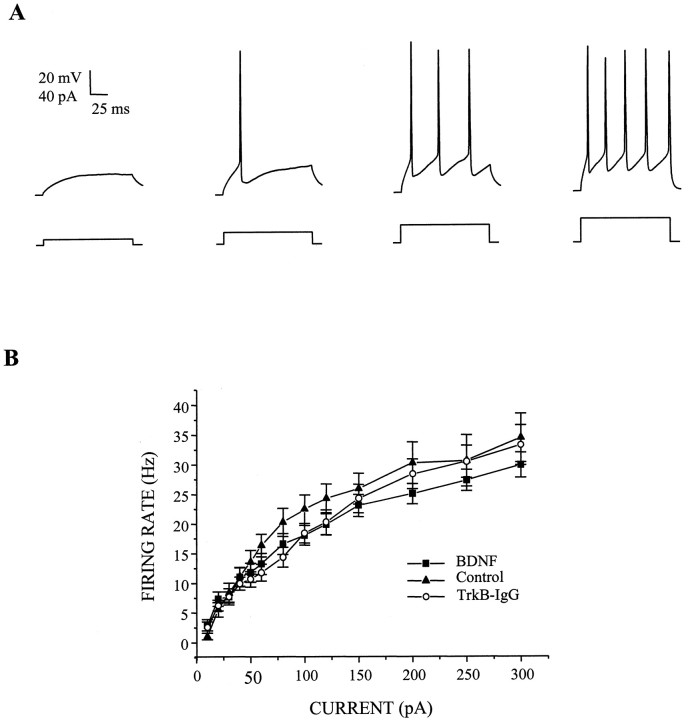
Because the action potential firing properties of a network depend on the intrinsic excitability of its neuronal elements as well as the synaptic connectivity of the ensemble, we next investigated whether BDNF regulated intrinsic membrane excitability in these cultures. Accordingly, we measured several parameters of action potential activity that was generated by injecting a series of current pulses of increasing amplitude into current-clamped neurons; voltage responses to these current pulses were measured while all synaptic transmission was blocked pharmacologically. Most importantly, we found that BDNF did not change the firing rate of neurons at any of the current injection amplitudes examined (Fig. 3). Additionally, we found that BDNF affected neither action potential shape, as measured by action potential height and half-width (Table1), nor the voltage threshold at which the regenerative action potentials were first observed.
Fig. 3.
BDNF does not affect intrinsic membrane excitability. A, Representative whole-cell current-clamp traces of action potential trains elicited in a control cell by depolarizing the membrane by a sequence of inward current pulses (left to right: 10, 20, 30, and 40 pA).Top traces show voltage responses to 160 msec depolarizing current pulses (bottom traces) relative to resting potential; synaptic transmission was blocked pharmacologically during the recording period as described in Materials and Methods. Data were sampled at 10 kHz and filtered at 2 kHz. B, BDNF treatment did not alter input–output relationships for current injection versus action potential firing rate. Neither augmentation (squares) nor depletion (circles) of BDNF affected action potential firing rate at any of the current injection amplitudes examined compared with untreated controls (triangles). Means ± SEM are shown;n = 27, 23, and 24 for BDNF, control, and TrkB-IgG groups, respectively.
Table 1.
BDNF does not alter intrinsic excitability
| BDNF | Control | TrkB-IgG | |
|---|---|---|---|
| Vm(mV) | −50.7 ± 1.3, n = 24 | −50.8 ± 1.3, n = 20 | −49.3 ± 1.1, n = 24 |
| Rin(MΩ) | 175.8 ± 16.1, n = 27 | 213.4 ± 16.9, n = 22 | 220.7 ± 20.2, n = 24 |
| Cm (pF) | 69.1 ± 3.6, n = 27* | 58.2 ± 3.3, n = 22 | 55.5 ± 2.8, n = 24 |
| VT (mV) | −35.1 ± 1.0, n = 24 | −33.0 ± 1.2, n = 20 | −33.3 ± 0.9, n = 24 |
| AP ½-width (msec) | 2.1 ± 0.2, n = 24 | 2.1 ± 0.2, n = 20 | 1.8 ± 0.2, n = 24 |
| AP height (mV) | 93.2 ± 3.05, n = 24 | 101.4 ± 3.6, n = 20 | 96.7 ± 2.8, n = 24 |
Neither long-term augmentation or depletion of BDNF affected the shape of action potentials or the voltage threshold for action potential initiation (Vm, resting potential; Rin, input resistance; Cm, membrane capacitance; VT, voltage threshold for action potential initiation; AP ½-width, width of action potential at half-height; AP height, height of action potential; procedures as described in Materials and Methods). A small increase in capacitance, however, suggested the possibility of minor morphological changes; asterisk denotes significant differences between BDNF and control groups (p < 0.03 by ANOVA) and between BDNF and TrkB-IgG groups (p < 0.005 by ANOVA) with respect to cell capacitance. No other differences were statistically significant at the 0.05 level by ANOVA for any parameter measured.
Similarly, there were no differences between BDNF-treated and control or TrkB-IgG-treated neurons in resting membrane potential (Table 1), but BDNF did cause a small increase in capacitance and a decrease in input resistance (Table 1). These minor differences in particular membrane properties suggested that BDNF may have had small effects on the morphology of these neurons, but it is unlikely that these differences contributed significantly to increasing spontaneous circuit activity for two reasons: first, neither the shape, frequency, or voltage-threshold of action potentials, nor any other aspect of intrinsic excitability measured, was changed by BDNF; second, the rise and decay kinetics of AMPA receptor-mediated synaptic currents were not affected by BDNF (see following sections).
BDNF selectively increases the amplitude of AMPA receptor-mediated miniature EPSCs
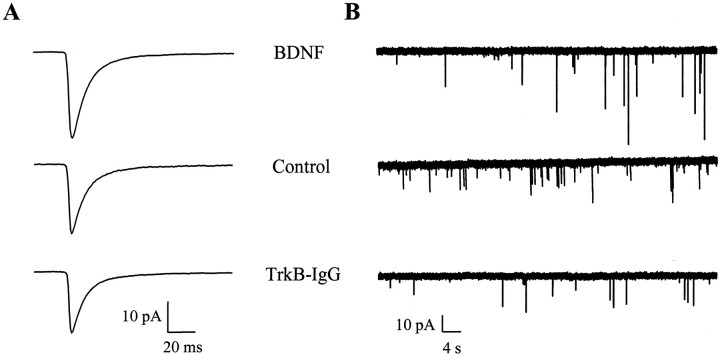
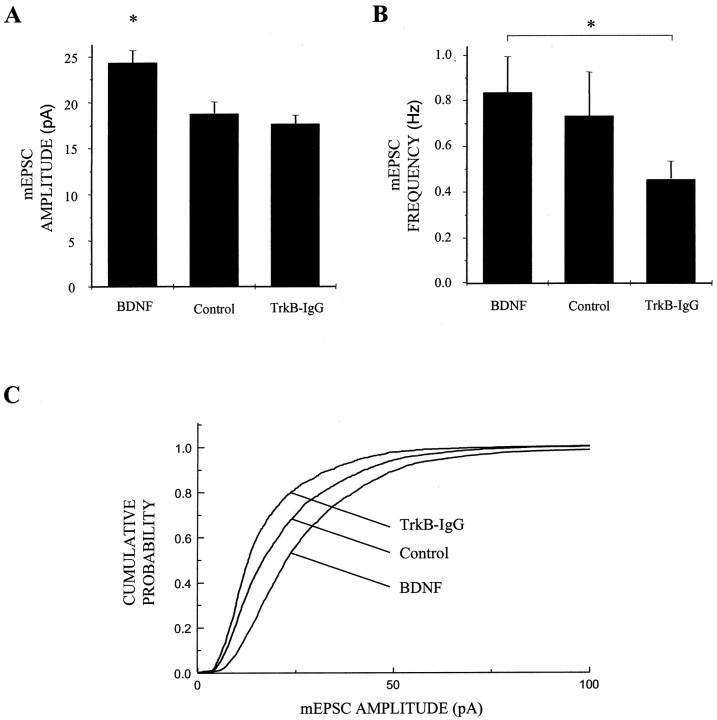
Because the firing rate of neurons is determined, in part, by the number, strength and temporal characteristics of its individual excitatory and inhibitory synaptic inputs, we next examined the effects of BDNF on unitary synaptic transmission. We first measured the behavior of miniature EPSCs (mEPSCs) by blocking sodium channel-mediated action potential activity with TTX and recording spontaneous mEPSCs under whole-cell voltage clamp (Fig.4). We found that BDNF significantly increased the amplitude of AMPA receptor-mediated mEPSCs by ∼30% (n = 82, 44, and 69 for BDNF, control, and TrkB-IgG cultures, respectively; BDNF vs control, p < 0.007 by ANOVA) (Fig. 5A). BDNF shifted the cumulative probability distribution of AMPA receptor-mediated mEPSC amplitudes uniformly toward larger amplitudes (Fig. 5C), suggesting that the action of BDNF was not limited to a subset of synapses of a particular quantal size and that there was no saturation of mEPSC sizes at higher amplitudes. This increase in the amplitude of unitary synaptic transmission by BDNF was accompanied by a significant increase in mEPSC frequency in BDNF-treated compared with TrkB-IgG-treated cells, but not compared with control cells (n = 83, 45, and 73 for BDNF, control, and TrkB-IgG cultures, respectively; BDNF vs TrkB-IgG, p < 0.04 by ANOVA; BDNF vs control, p > 0.7 by ANOVA) (Fig.5B). BDNF had no effect on the decay rates of either AMPA or NMDA receptor-mediated synaptic currents (Table2). Together, these findings suggested that BDNF increased excitation in these cultures predominantly by selectively increasing the amplitude of AMPA receptor-mediated synaptic currents without substantially affecting other aspects of excitatory synaptic transmission.
Fig. 4.
BDNF increases the amplitude but does not change the kinetics of AMPA receptor-mediated mEPSCs.A, Population averages of pharmacologically isolated AMPA receptor-mediated mEPSCs from all cells in each treatment group show the increase in mEPSC amplitude induced by BDNF;n = 82, 44, and 69 for BDNF (top), control (middle), and TrkB-IgG (bottom) groups, respectively. Note that the time courses of these averaged mEPSCs are similar; data were acquired continuously under voltage clamp at 2.5 kHz and filtered at 1 kHz. B, Representative recordings of AMPA receptor-mediated mEPSCs on a compressed time base illustrate the lack of effect of BDNF treatment on mEPSC frequency;traces from neurons in BDNF (top), control (middle), and TrkB-IgG (bottom) groups are shown.
Fig. 5.
BDNF increases AMPA receptor-mediated mEPSC amplitude. A, BDNF increased mEPSC amplitude by ∼30% compared with control cells and by ∼40% compared with TrkB-IgG treated neurons (n = 82, 44, and 69 for BDNF, control, and TrkB-IgG groups, respectively). *p < 0.007 indicates a significant difference by ANOVA between BDNF and either control or TrkB-IgG groups; means ± SEM are shown. Data were acquired continuously under voltage clamp at 2.5 kHz and filtered at 1 kHz. B, mEPSC frequency was elevated by BDNF- compared with TrkB-IgG-treated cells but not significantly so compared with control cells; n = 83, 45, and 73 for BDNF, control, and TrkB-IgG groups, respectively. *p < 0.042 and line indicate a significant difference by ANOVA between the BDNF and TrkB-IgG groups; p > 0.69 by ANOVA between BDNF and control groups. C, BDNF treatment shifted mEPSC amplitudes uniformly toward higher amplitudes as shown in cumulative probability distributions; all mEPSCs recorded in a 3 min interval from 40 randomly chosen neurons in each treatment condition were grouped and analyzed.
Table 2.
BDNF does not alter decay kinetics of either excitatory or inhibitory synaptic currents
| AMPA | NMDA | GABAA | |
|---|---|---|---|
| BDNF | 6.4 ± 0.4, n = 63 | 76.6 ± 9.2, n = 13 | 31.9 ± 1.2, n = 35 |
| Control | 5.9 ± 0.4, n = 31 | — | 29.8 ± 1.1, n = 31 |
| TrkB-IgG | 5.8 ± 0.3, n = 65 | 84.1 ± 13, n = 13 | 33.5 ± 1.4, n = 33 |
Neither long-term augmentation nor depletion of BDNF affected the mean decay time constants (in milliseconds) for AMPA receptor-mediated mEPSCs (“AMPA”), NMDA receptor-mediated mEPSCs (“NMDA”), or GABAA receptor-mediated mIPSCs (“GABAA”); isolation and recording of these synaptic currents as described in Materials and Methods. Single exponential decay time constants were derived by iterative minimization of least squares differences; this was done on “true minis” in the presence of TTX for AMPA receptor-mediated mEPSCs and GABAA receptor-mediated mIPSCs but on all spontaneous NMDA receptor-mediated mEPSCs to enhance signal-to-noise.
BDNF induction of AMPA receptor-mediated mEPSC amplitude does not require activity
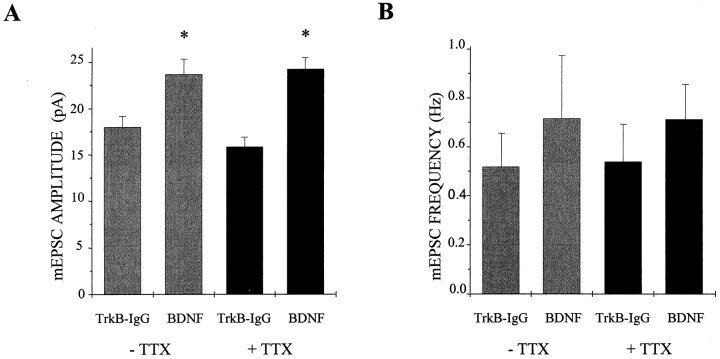
Several previous studies have reported that the actions of neurotrophins are activity-dependent or that activity and neurotrophins interact in their regulation of the physiological properties of neurons and neuronal circuits (Marty et al., 1996a; McAllister et al., 1996; Rutherford et al., 1997). We therefore asked whether the potentiative effect of BDNF on excitation observed here was dependent on ongoing electrical activity by blocking action potential activity with 5 μm TTX for the entire duration of BDNF or TrkB-IgG treatment. We found, surprisingly, that TTX had no effect on the enhancement of mEPSC amplitude by BDNF (n = 38, 30, 39, and 29 for BDNF, TrkB-IgG, BDNF plus TTX, and TrkB-IgG plus TTX cultures, respectively; BDNF vs TrkB-IgG, p < 0.01 by ANOVA; BDNF plus TTX vs TrkB-IgG plus TTX, p < 10−4 by ANOVA) (Fig.6A). In fact, TTX appeared to increase slightly the magnitude of enhancement by BDNF, from 31 to 53% in the presence of TTX. We verified that the TTX used remained active for the duration of the experiment by applying media containing the TTX to naïve cells and observing complete sodium channel blockade (data not shown). Thus, the regulation of AMPA receptor-mediated mEPSC amplitude by BDNF did not require concurrent action potential activity and thus may differ from other reported actions of BDNF.
Fig. 6.
BDNF regulation of mEPSC amplitude does not require activity. A, The addition of 5 μmTTX to block action potential activity for the entire duration of the treatment period did not block the enhancement of mEPSC amplitude by BDNF; mEPSC amplitudes increased by 31 and 53% with and without TTX treatment, respectively. Means ± SEM are shown; without TTX,n = 38 and 30 for BDNF and TrkB-IgG groups, respectively, p < 0.01 by ANOVA; with TTX,n = 39 and 29 for BDNF and TrkB-IgG groups, respectively, p < 10−4 by ANOVA. B, BDNF, TTX, or BDNF and TTX treatment together did not significantly alter mEPSC frequency. Means ± SEM are shown; without TTX, n = 38 and 30 for BDNF and TrkB-IgG groups, respectively, p > 0.99 by ANOVA; with TTX, n = 39 and 29 for BDNF and TrkB-IgG groups, respectively, p > 0.93 by ANOVA.
BDNF selectively increases the frequency of GABAA-mediated mIPSCs
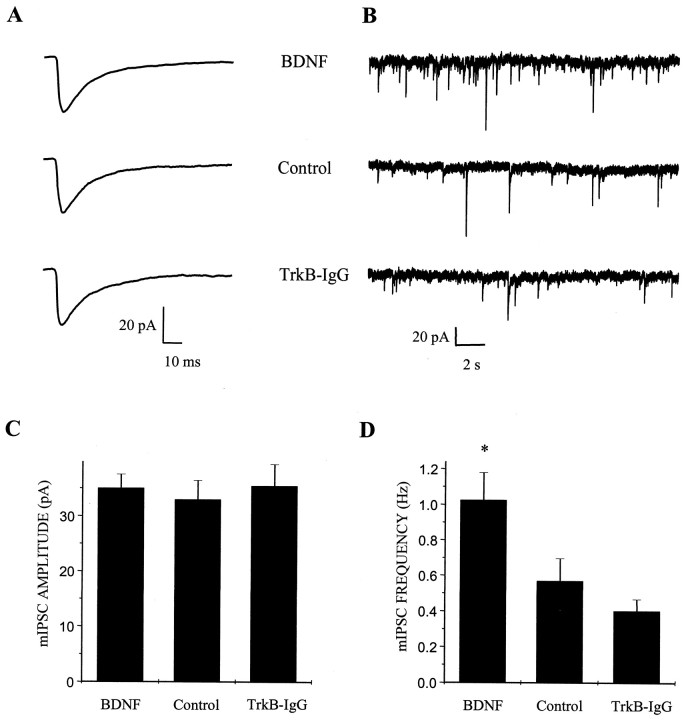
To determine whether BDNF also regulated the unitary properties of inhibitory synaptic transmission, we next recorded mIPSCs arising from GABAergic inputs onto neurons with pyramidal morphology. During the recording period, TTX and NBQX were added to the extracellular solution to block action potentials and glutamatergic inputs, respectively. We found that BDNF treatment increased the frequency of mIPSCs by almost twofold (n = 35, 31, and 35 for BDNF, control, and TrkB-IgG cultures, respectively; BDNF vs control, p < 0.028 by ANOVA) (Fig. 7D). In contrast to the increase in AMPA receptor-mediated mEPSC amplitude described above, GABAA receptor-mediated mIPSC amplitudes were not affected by BDNF (n = 35, 31, and 35 for BDNF, control, and TrkB-IgG cultures, respectively; BDNF vs control, p > 0.6 by ANOVA) (Fig. 7C). As with AMPA- and NMDA-mediated synaptic currents, BDNF did not alter the kinetics of synaptic currents mediated by GABAAreceptors (Table 2). These findings indicated that, although BDNF potentiated both excitatory and inhibitory synaptic transmission in these cultures, it did so through distinct physiological mechanisms.
Fig. 7.
BDNF increases GABAA receptor-mediated mIPSC frequency but not amplitude. A, Population averages of pharmacologically isolated GABAAreceptor-mediated mIPSCs from all cells in each treatment group show similar amplitude and time courses; n = 35, 31, and 33 for BDNF (top), control (middle), and TrkB-IgG (bottom) groups, respectively. Data were acquired continuously under voltage clamp at 2.5 kHz and filtered at 1 kHz. B, Representative recordings of GABAA receptor-mediated mIPSCs on a compressed time base show the elevation of mIPSC frequency induced by BDNF treatment;traces from neurons in BDNF (top), control (middle), and TrkB-IgG (bottom) groups are shown. C, In contrast, BDNF treatment had no effect on GABAA receptor-mediated mIPSC amplitude. Means ± SEM are shown; n = 35, 31, and 33 for BDNF, control, and TrkB-IgG groups, respectively. D, BDNF treatment increased mIPSC frequency by ∼1.8 fold compared with controls. *p < 0.028 indicates a significant difference by ANOVA between BDNF and either control or TrkB-IgG treatment groups; n = 35, 31, and 35 for BDNF, control, and TrkB-IgG groups, respectively.
BDNF does not change synapse number but enhances differentiation of GABAergic terminals
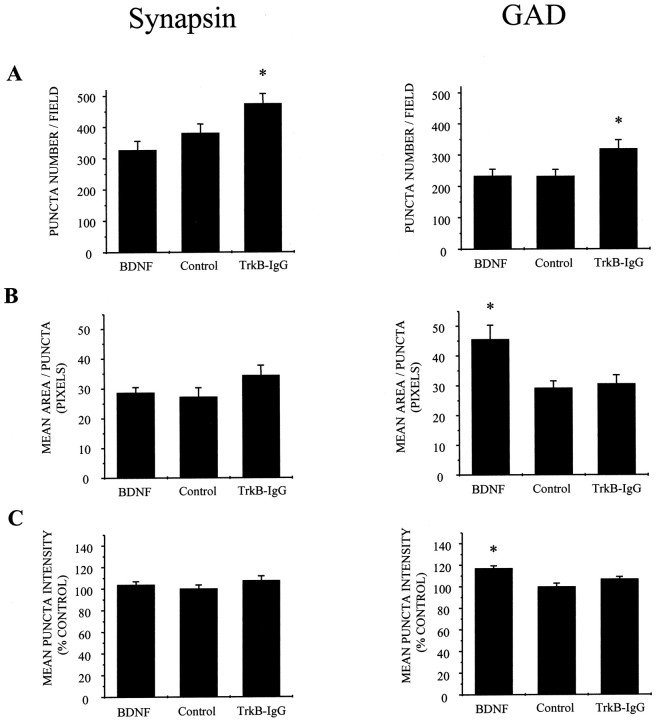
Finally, we asked whether BDNF regulated inhibition by regulating numbers of GABAergic inputs because we observed that BDNF increased the frequency of mIPSCs. First, we quantified total numbers of presumptive synapses by immunostaining with antibodies against the synaptic vesicle protein synapsin I (Fig.8A). Because synapsin I is present in the terminals of both excitatory and inhibitory neurons, these initial measurements reflected the combined number of glutamatergic and GABAergic synapses. BDNF did not increase numbers of synapses per field, terminal size, or intensity of synapsin labeling; in fact, there was a slight increase in numbers of synapses in cultures in which endogenous BDNF had been antagonized with TrkB-IgG (Fig.9, left).
Fig. 8.
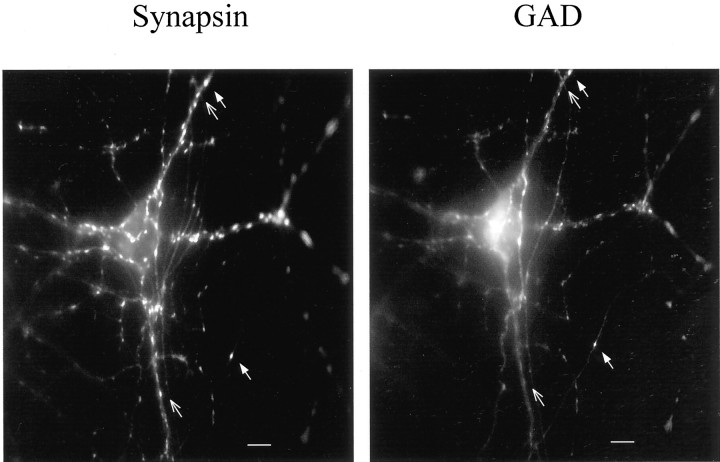
Immunostaining against synaptic markers in hippocampal neuronal cultures. Cultures were double-labeled with anti-synapsin and anti-GAD antibodies to visualize all and only GABAergic synaptic terminals, respectively. Synaptic punctae staining with both antibodies represented inhibitory presynaptic terminals (filled arrow), whereas those staining with the anti-synapsin antibody only were considered to be excitatory (open arrow). Scale bar, 10 μm.
Fig. 9.
BDNF does not affect synaptogenesis but does enhance GABAergic phenotype. A, BDNF treatment did not increase synaptic density as quantified by either anti-synapsin (left) or anti-GAD (right) staining; in fact, synaptic density was slightly increased by TrkB-IgG treatment by both measures. *p < 0.03 indicates significant differences by ANOVA (anti-synapsin) andp < 0.02 by ANOVA (anti-GAD) between TrkB-IgG and either control or BDNF groups; n = 23 (anti-synapsin) and n = 24 (anti-GAD) dishes scored in each treatment group. B, BDNF treatment did not increase synaptic terminal size significantly in the population as a whole (anti-synapsin staining, left) but did increase average GAD-positive terminal size by ∼50%. *p< 0.01 indicates a significant difference by ANOVA between BDNF and either control or TrkB-IgG groups; n = 22, 23, and 21 dishes scored for BDNF, control, and TrkB-IgG treatment groups, respectively. C, Similarly, BDNF treatment did not affect the intensity of synapsin labeling (left) but did increase the average staining intensity of GAD-positive terminals (right). *p < 0.002 indicates a significant difference by ANOVA between BDNF and either control or TrkB-IgG groups; n = 22, 23, and 24 dishes scored for BDNF, control, and TrkB-IgG treatment groups, respectively.
We next examined GABAergic terminals specifically with an antibody directed against GAD (Fig. 8B). BDNF did not increase the number of GAD-positive synapses; as found for synapsin staining, however, there was a slight increase in the number of GAD punctae with TrkB-IgG treatment (Fig. 9A, right). Strikingly, BDNF increased average GABA terminal size by 50% and concomitantly increased the fluorescence intensity of these punctae, presumably reflecting an increase in the level of GAD expression (Fig.9B, right). Together with the increased mIPSC frequency described above, these observations suggest that BDNF increased inhibition by increasing the efficacy and/or probability of transmission at GABAergic synapses.
DISCUSSION
We have found that BDNF strongly regulates both excitation and inhibition in dissociated cultures of hippocampal neurons. Although the increase in excitation was dominant in that overall levels of spontaneous activity in these cultures were increased after BDNF treatment, this parallel enhancement of excitation and inhibition also resulted in partial homeostasis in terms of circuit activity. The increase in circuit activity produced by BDNF arose principally from changes in excitatory and inhibitory synaptic drive, both relative and absolute, but via distinct physiological mechanisms, with no significant changes in intrinsic neuronal excitability or neuronal survival.
BDNF regulation of excitation
We found that long-term treatment of hippocampal cultures with BDNF led to a selective increase in the quantal size of AMPA receptor-mediated mEPSCs. Such a change is consistent with a postsynaptic mechanism. Moreover, the absence of kinetic changes in AMPA receptor-mediated synaptic currents limits possible cellular and molecular mechanisms to those that do not alter the kinetic profile of postsynaptic currents. For example, an increase in numbers of functional AMPA receptors at synaptic sites would be expected to increase mEPSC amplitude without affecting kinetics; in fact, changes in the half-life of AMPA receptors can result in changes in receptor density that have been shown to correlate with quantal size (O'Brien et al., 1998). Recently, rapid insertion and removal of AMPA receptors from potentiated and depressed synapses has been proposed to underlie changes in synaptic efficacy (Carroll et al., 1999; Lissin et al., 1999; Shi et al., 1999), suggesting that longer term action of BDNF such as those reported here may similarly involve increases in the density of AMPA receptors at synapses. BDNF has, for example, recently been demonstrated to increase the expression of AMPA receptor subunit 1 and 2/3 protein in neocortical neurons (Narisawa-Saito et al., 1999a,b). Alternatively, BDNF may induce the accumulation of AMPA receptors at synapses previously devoid of these receptors as has been proposed for “silent” synapses in neonatal hippocampus (Isaac et al., 1995; Liao et al., 1995; Durand et al., 1996; Wu et al., 1996).
Changes in the composition or functional states of AMPA receptors are also possible that have minimal effects on the overall time course of mEPSCs. For example, induction of LTP in CA1 hippocampus and Ca2+/calmodulin kinase II phosphorylation of the glutamate receptor subunit GluR1 have been shown to produce increases in AMPA receptor single-channel conductance (Tan et al., 1994; Barria et al., 1997a,b; Mammen et al., 1997; Benke et al., 1998; Derkach et al., 1999). Our results are also consistent, however, with an increase in the amount of glutamate packaged per vesicle (for review, see Reimer et al., 1998) as has been reported recently for catecholamines (Pothos et al., 1998). In this context, it is interesting that there was increased mEPSC frequency in BDNF-treated neurons because changes in the frequency of mEPSCs is often associated with presynaptic alterations, such as in probability of release.
Interestingly, BDNF did not affect the kinetics of the synaptic currents measured in this study (neither AMPA, NMDA, nor GABAA receptor-mediated), a finding that has several implications. First, any effects BDNF may have had on neuronal morphology, spatial distribution of synaptic inputs, or input resistance did not alter the passive membrane properties of the neuron sufficiently to affect the time course of such fast synaptic currents (Mennerick et al., 1995; Bekkers and Stevens, 1996). Second, in the case of AMPA receptor-mediated mEPSCs, the potential cellular mechanisms responsible for the quantal amplitude increase induced by BDNF are limited to those that do not affect the rate of decay of synaptic currents, as discussed above; however, our experiments do not specifically address mechanisms such as regulation of glutamate transporter function, whose contribution to synaptic current kinetics remains uncertain (for review, see Clements, 1996; Diamond and Jahr, 1997; Mennerick et al., 1999). Finally, that BDNF did not regulate the decay time course of NMDA receptor-mediated synaptic currents suggests that BDNF does not alter the subunit composition of the NMDA receptors under these conditions because changes in the relative expression of NR2A–NR2D subunits have been shown, for example, to alter NMDA receptor decay times significantly (Carmignoto and Vicini, 1992;Hestrin, 1992).
A previous study using hippocampal cultures from embryonic day 16 rats found similar BDNF-mediated increases in the amplitude of sucrose-evoked mEPSCs, but at this early developmental stage, the dominant effect was an increase in the number of functional synapses (Vicario-Abejon et al., 1998). Our results are more consistent with studies in CA1 hippocampal autapses in which BDNF induces a 1.7-fold increase in quantal amplitude of AMPA receptor-mediated mEPSCs and a parallel increase in the amplitude of evoked synaptic currents (Sherwood and Lo, 1999). Interestingly, the effects of BDNF described here and by Vicario-Abejon et al. (1998) and Sherwood and Lo (1999) are quite different from those reported recently in dissociated cultures of visual cortical neurons (Rutherford et al., 1998; Turrigiano et al., 1998). In these studies, the quantal amplitude of AMPA-mediated synaptic currents was scaled by activity; Rutherford et al. (1998)reported that BDNF prevents this increase in AMPA receptor-mediated quantal amplitude in response to activity blockade, the opposite of the action of BDNF found here. Interestingly, however, bipolar interneurons as described by Rutherford et al. (1998) responded to BDNF similarly to the hippocampal pyramidal neurons in the present study.
Such differences in BDNF regulation of synaptic transmission presumably arise from the pleiotropy and cell context-dependence of BDNF action and emphasize the diversity of roles BDNF may transpire to play in synaptic development and plasticity. In this context, it is notable that there are developmental differences in the relative abundance of GluR1–GluR4 subunits and the alternative splicing variants, GluR Flip and GluR Flop, between pyramidal cells of the hippocampus and cortex (Boulter et al., 1990; Keinanen et al., 1990;Sommer et al., 1990; Monyer et al., 1991; Pellegrini-Giampietro et al., 1991; Petralia and Wenthold, 1992; Craig et al., 1993; Eshhar et al., 1993; Conti et al., 1994). Such differences in subunit expression may contribute to the apparent regional difference in regulation of quantal amplitude by BDNF in hippocampus versus cortex.
BDNF regulation of inhibition
We found that inhibitory synaptic transmission was also enhanced by chronic BDNF treatment but that the physiological mechanisms underlying this potentiation were different from those that enhanced excitatory synaptic drive. In this case, BDNF selectively increased the frequency of mIPSCs with no effects on their quantal amplitude or kinetic properties. Such frequency changes could have arisen from changes in probability of transmitter release, numbers of inhibitory synaptic contacts, or both. Our finding that the number of GABAergic terminals was not affected by BDNF but that the size of inhibitory terminals and intensity of GAD immunostaining was increased suggests that BDNF is likely to have enhanced the probability of transmitter release presynaptically.
The 40% increase in the ratio of neurons that showed detectable anti-GAD staining we observed after BDNF treatment was similar to previous reports of increases in GABAergic phenotypic differentiation, but not inhibitory neuronal numbers, after treatment with BDNF in vitro and in vivo (Ip et al., 1993; Nawa et al., 1993,1994; Croll et al., 1994; Marty et al., 1996a,b). Interestingly, BDNF is not synthesized by GABAergic interneurons (Cellerino et al., 1996; Rocamora et al., 1996; Schmidt-Kastner et al., 1996), but rather their source of BDNF is thought to be neighboring glutamatergic neurons (Nawa et al., 1995; Marty et al., 1996a). Such observations continue to support a general role for BDNF in regulating inhibitory synaptic transmission and are consistent with BDNF acting as an activity-dependent, target-derived differentiation factor for GABAergic interneurons. In turn, such regulation of GABAergic transmission by BDNF and activity would be expected to have major ramifications for neural development and function (Hendry and Jones, 1988).
In summary, our experiments have shown that BDNF can have profound effects on the function of neural circuits and that it can do so via regulation of selective aspects of excitatory and inhibitory synaptic function. In combination with activity-dependent production and localized release of neurotrophins (Wetmore et al., 1994; Blochl and Thoenen, 1995, 1996; Goodman et al., 1996; Fawcett et al., 1997, 1998;Smith et al., 1997; Wang and Poo, 1997; Moller et al., 1998), such effects of BDNF provide powerful mechanisms of action in their increasingly apparent roles in regulating synaptic development and plasticity.
Footnotes
This work was supported by National Institutes of Health Grants NS32742 (to D.L.) and MH11519 (to M.B.) and The McKnight Endowment Fund for Neuroscience (D.L.). We thank D. Fitzpatrick, S. Lesser, N. Tang Sherwood, and T. Yacoubian for their helpful comments on this manuscript and valuable discussions on experimental design. We also thank Regeneron Pharmaceuticals for their generous provision of recombinant BDNF and TrkB-IgG.
Correspondence should be addressed to Donald C. Lo, Department of Neurobiology Box 3209, Duke University Medical Center, Durham, NC 27710. E-mail: lo@neuro.duke.edu.
REFERENCES
- 1.Akaneya Y, Tsumoto T, Hatanaka H. Brain-derived neurotrophic factor blocks long-term depression in rat visual cortex. J Neurophysiol. 1996;76:4198–4201. doi: 10.1152/jn.1996.76.6.4198. [DOI] [PubMed] [Google Scholar]
- 2.Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997a;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- 4.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997b;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 5.Bekkers JM, Stevens CF. Cable properties of cultured hippocampal neurons determined from sucrose-evoked miniature EPSCs. J Neurophysiol. 1996;75:1250–1255. doi: 10.1152/jn.1996.75.3.1250. [DOI] [PubMed] [Google Scholar]
- 6.Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- 7.Blochl A, Thoenen H. Characterization of nerve growth factor (NGF) release from hippocampal neurons: evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 8.Blochl A, Thoenen H. Localization of cellular storage compartments and sites of constitutive and activity-dependent release of nerve growth factor (NGF) in primary cultures of hippocampal neurons. Mol Cell Neurosci. 1996;7:173–190. doi: 10.1006/mcne.1996.0014. [DOI] [PubMed] [Google Scholar]
- 9.Boulanger L, Poo M. Gating of BDNF-induced synaptic potentiation by cAMP. Science. 1999a;284:1982–1984. doi: 10.1126/science.284.5422.1982. [DOI] [PubMed] [Google Scholar]
- 10.Boulanger L, Poo MM. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat Neurosci. 1999b;2:346–351. doi: 10.1038/7258. [DOI] [PubMed] [Google Scholar]
- 11.Boulter J, Hollmann M, O'Shea-Greenfield A, Hartley M, Deneris E, Maron C, Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990;249:1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- 12.Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 13.Carmignoto G, Pizzorusso T, Tia S, Vicini S. Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. J Physiol (Lond) 1997;498:153–164. doi: 10.1113/jphysiol.1997.sp021848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- 15.Cellerino A, Maffei L, Domenici L. The distribution of brain-derived neurotrophic factor and its receptor trkB in parvalbumin-containing neurons of the rat visual cortex. Eur J Neurosci. 1996;8:1190–1197. doi: 10.1111/j.1460-9568.1996.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 16.Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- 17.Conti F, Minelli A, Brecha NC. Cellular localization and laminar distribution of AMPA glutamate receptor subunits mRNAs and proteins in the rat cerebral cortex. J Comp Neurol. 1994;350:241–259. doi: 10.1002/cne.903500208. [DOI] [PubMed] [Google Scholar]
- 18.Craig AM, Blackstone CD, Huganir RL, Banker G. The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron. 1993;10:1055–1068. doi: 10.1016/0896-6273(93)90054-u. [DOI] [PubMed] [Google Scholar]
- 19.Croll SD, Wiegand SJ, Anderson KD, Lindsay RM, Nawa H. Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur J Neurosci. 1994;6:1343–1353. doi: 10.1111/j.1460-9568.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 20.Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 23.Eshhar N, Petralia RS, Winters CA, Niedzielski AS, Wenthold RJ. The segregation and expression of glutamate receptor subunits in cultured hippocampal neurons. Neuroscience. 1993;57:943–964. doi: 10.1016/0306-4522(93)90040-m. [DOI] [PubMed] [Google Scholar]
- 24.Fawcett JP, Aloyz R, McLean JH, Pareek S, Miller FD, McPherson PS, Murphy RA. Detection of brain-derived neurotrophic factor in a vesicular fraction of brain synaptosomes. J Biol Chem. 1997;272:8837–8840. doi: 10.1074/jbc.272.14.8837. [DOI] [PubMed] [Google Scholar]
- 25.Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, McLean JH, Miller FD. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci. 1998;18:2808–2821. doi: 10.1523/JNEUROSCI.18-08-02808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 27.Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- 28.Goodman LJ, Valverde J, Lim F, Geschwind MD, Federoff HJ, Geller AI, Hefti F. Regulated release and polarized localization of brain-derived neurotrophic factor in hippocampal neurons. Mol Cell Neurosci. 1996;7:222–238. doi: 10.1006/mcne.1996.0017. [DOI] [PubMed] [Google Scholar]
- 29.Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 31.Hendry SH, Jones EG. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1988;1:701–712. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
- 32.Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- 33.Huber KM, Sawtell NB, Bear MF. Brain-derived neurotrophic factor alters the synaptic modification threshold in visual cortex. Neuropharmacology. 1998;37:571–579. doi: 10.1016/s0028-3908(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 34.Ip NY, Li Y, Yancopoulos GD, Lindsay RM. Cultured hippocampal neurons show responses to BDNF, NT-3, and NT-4, but not NGF. J Neurosci. 1993;13:3394–3405. doi: 10.1523/JNEUROSCI.13-08-03394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995a;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 37.Kang HJ, Schuman EM. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J Physiol (Paris) 1995b;89:11–22. doi: 10.1016/0928-4257(96)80547-x. [DOI] [PubMed] [Google Scholar]
- 38.Keinanen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science. 1990;249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- 39.Kinoshita S, Yasuda H, Taniguchi N, Katoh-Semba R, Hatanaka H, Tsumoto T. Brain-derived neurotrophic factor prevents low-frequency inputs from inducing long-term depression in the developing visual cortex. J Neurosci. 1999;19:2122–2130. doi: 10.1523/JNEUROSCI.19-06-02122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci USA. 1996a;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korte M, Staiger V, Griesbeck O, Thoenen H, Bonhoeffer T. The involvement of brain-derived neurotrophic factor in hippocampal long-term potentiation revealed by gene targeting experiments. J Physiol (Paris) 1996b;90:157–164. doi: 10.1016/s0928-4257(97)81415-5. [DOI] [PubMed] [Google Scholar]
- 43.Korte M, Kang H, Bonhoeffer T, Schuman E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 44.Lesser SS, Lo DC. Regulation of voltage-gated ion channels by NGF and ciliary neurotrophic factor in SK-N-SH neuroblastoma cells. J Neurosci. 1995;15:253–261. doi: 10.1523/JNEUROSCI.15-01-00253.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lesser SS, Sherwood NT, Lo DC. Neurotrophins differentially regulate voltage-gated ion channels. Mol Cell Neurosci. 1997;10:173–183. doi: 10.1006/mcne.1997.0656. [DOI] [PubMed] [Google Scholar]
- 46.Lessmann V, Heumann R. Modulation of unitary glutamatergic synapses by neurotrophin-4/5 or brain-derived neurotrophic factor in hippocampal microcultures: presynaptic enhancement depends on pre-established paired-pulse facilitation. Neuroscience. 1998;86:399–413. doi: 10.1016/s0306-4522(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 47.Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. NeuroReport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 48.Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine ES, Dreyfus CF, Black IB, Plummer MR. Selective role for trkB neurotrophin receptors in rapid modulation of hippocampal synaptic transmission. Brain Research. Mol Brain Res. 1996;38:300–303. doi: 10.1016/0169-328x(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 50.Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-d-aspartic acid receptor activity. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li YX, Xu Y, Ju D, Lester HA, Davidson N, Schuman EM. Expression of a dominant negative TrkB receptor, T1, reveals a requirement for presynaptic signaling in BDNF-induced synaptic potentiation in cultured hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:10884–10889. doi: 10.1073/pnas.95.18.10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 53.Lissin DV, Carroll RC, Nicoll RA, Malenka RC, von Zastrow M. Rapid, activation-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J Neurosci. 1999;19:1263–1272. doi: 10.1523/JNEUROSCI.19-04-01263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 55.Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 56.Marty S, Onteniente B. The expression pattern of somatostatin and calretinin by postnatal hippocampal interneurons is regulated by activity-dependent and -independent determinants. Neuroscience. 1997;80:79–88. doi: 10.1016/s0306-4522(97)00134-6. [DOI] [PubMed] [Google Scholar]
- 57.Marty S, Berninger B, Carroll P, Thoenen H. GABAergic stimulation regulates the phenotype of hippocampal interneurons through the regulation of brain-derived neurotrophic factor. Neuron. 1996a;16:565–570. doi: 10.1016/s0896-6273(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 58.Marty S, Carroll P, Cellerino A, Castren E, Staiger V, Thoenen H, Lindholm D. Brain-derived neurotrophic factor promotes the differentiation of various hippocampal nonpyramidal neurons, including Cajal-Retzius cells, in organotypic slice cultures. J Neurosci. 1996b;16:675–687. doi: 10.1523/JNEUROSCI.16-02-00675.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 60.McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 61.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 62.Mennerick S, Que J, Benz A, Zorumski CF. Passive and synaptic properties of hippocampal neurons grown in microcultures and in mass cultures. J Neurophysiol. 1995;73:320–332. doi: 10.1152/jn.1995.73.1.320. [DOI] [PubMed] [Google Scholar]
- 63.Mennerick S, Shen W, Xu W, Benz A, Tanaka K, Shimamoto K, Isenberg KE, Krause JE, Zorumski CF. Substrate turnover by transporters curtails synaptic glutamate transients. J Neurosci. 1999;19:9242–9251. doi: 10.1523/JNEUROSCI.19-21-09242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizuno K, Carnahan J, Nawa H. Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol. 1994;165:243–256. doi: 10.1006/dbio.1994.1250. [DOI] [PubMed] [Google Scholar]
- 65.Moller JC, Kruttgen A, Heymach JV, Jr, Ghori N, Shooter EM. Subcellular localization of epitope-tagged neurotrophins in neuroendocrine cells. J Neurosci Res. 1998;51:463–472. doi: 10.1002/(SICI)1097-4547(19980215)51:4<463::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 66.Monyer H, Seeburg PH, Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron. 1991;6:799–810. doi: 10.1016/0896-6273(91)90176-z. [DOI] [PubMed] [Google Scholar]
- 67.Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Brain-derived neurotrophic factor regulates the expression of AMPA receptor proteins in neocortical neurons. Neuroscience. 1999a;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- 68.Narisawa-Saito M, Silva AJ, Yamaguchi T, Hayashi T, Yamamoto T, Nawa H. Growth factor-mediated Fyn signaling regulates alpha-amino-3- hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression in rodent neocortical neurons. Proc Natl Acad Sci USA. 1999b;96:2461–2466. doi: 10.1073/pnas.96.5.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nawa H, Bessho Y, Carnahan J, Nakanishi S, Mizuno K. Regulation of neuropeptide expression in cultured cerebral cortical neurons by brain-derived neurotrophic factor. J Neurochem. 1993;60:772–775. doi: 10.1111/j.1471-4159.1993.tb03216.x. [DOI] [PubMed] [Google Scholar]
- 70.Nawa H, Pelleymounter MA, Carnahan J. Intraventricular administration of BDNF increases neuropeptide expression in newborn rat brain. J Neurosci. 1994;14:3751–3765. doi: 10.1523/JNEUROSCI.14-06-03751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 72.O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 73.Pan ZZ, Tong G, Jahr CE. A false transmitter at excitatory synapses. Neuron. 1993;11:85–91. doi: 10.1016/0896-6273(93)90273-t. [DOI] [PubMed] [Google Scholar]
- 74.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 75.Pellegrini-Giampietro DE, Bennett MV, Zukin RS. Differential expression of three glutamate receptor genes in developing rat brain: an in situ hybridization study. Proc Natl Acad Sci USA. 1991;88:4157–4161. doi: 10.1073/pnas.88.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- 77.Pothos EN, Davila V, Sulzer D. Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reimer RJ, Fon EA, Edwards RH. Vesicular neurotransmitter transport and the presynaptic regulation of quantal size. Curr Opin Neurobiol. 1998;8:405–412. doi: 10.1016/s0959-4388(98)80068-8. [DOI] [PubMed] [Google Scholar]
- 79. Riddle DR, Katz LC, Lo DC. Focal delivery of neurotrophins into the central nervous system using fluorescent latex microspheres. Biotechniques 23 1997. 928 934, 936–937. [DOI] [PubMed] [Google Scholar]
- 80.Rocamora N, Pascual M, Acsady L, de Lecea L, Freund TF, Soriano E. Expression of NGF and NT3 mRNAs in hippocampal interneurons innervated by the GABAergic septohippocampal pathway. J Neurosci. 1996;16:3991–4004. doi: 10.1523/JNEUROSCI.16-12-03991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- 83.Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol. 1997;78:1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- 84.Scharfman HE, Goodman JH, Sollas AL. Actions of brain-derived neurotrophic factor in slices from rats with spontaneous seizures and mossy fiber sprouting in the dentate gyrus. J Neurosci. 1999;19:5619–5631. doi: 10.1523/JNEUROSCI.19-13-05619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmidt-Kastner R, Wetmore C, Olson L. Comparative study of brain-derived neurotrophic factor messenger RNA and protein at the cellular level suggests multiple roles in hippocampus, striatum and cortex. Neuroscience. 1996;74:161–183. doi: 10.1016/0306-4522(96)00093-0. [DOI] [PubMed] [Google Scholar]
- 86.Sermasi E, Tropea D, Domenici L. A new form of synaptic plasticity is transiently expressed in the developing rat visual cortex: a modulatory role for visual experience and brain-derived neurotrophic factor. Neuroscience. 1999;91:163–173. doi: 10.1016/s0306-4522(98)00598-3. [DOI] [PubMed] [Google Scholar]
- 87.Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sherwood NT, Lo DC. Long-term enhancement of central synaptic transmission by chronic brain-derived neurotrophic factor treatment. J Neurosci. 1999;19:7025–7036. doi: 10.1523/JNEUROSCI.19-16-07025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sherwood NT, Lesser SS, Lo DC. Neurotrophin regulation of ionic currents and cell size depends on cell context. Proc Natl Acad Sci USA. 1997;94:5917–5922. doi: 10.1073/pnas.94.11.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 91.Smith MA, Zhang LX, Lyons WE, Mamounas LA. Anterograde transport of endogenous brain-derived neurotrophic factor in hippocampal mossy fibers. NeuroReport. 1997;8:1829–1834. doi: 10.1097/00001756-199705260-00008. [DOI] [PubMed] [Google Scholar]
- 92.Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- 93.Song DK, Choe B, Bae JH, Park WK, Han IS, Ho WK, Earm YE. Brain-derived neurotrophic factor rapidly potentiates synaptic transmission through NMDA, but suppresses it through non-NMDA receptors in rat hippocampal neuron. Brain Res. 1998;799:176–179. doi: 10.1016/s0006-8993(98)00474-0. [DOI] [PubMed] [Google Scholar]
- 94.Stoop R, Poo MM. Synaptic modulation by neurotrophic factors: differential and synergistic effects of brain-derived neurotrophic factor and ciliary neurotrophic factor. J Neurosci. 1996;16:3256–3264. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tan SE, Wenthold RJ, Soderling TR. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J Neurosci. 1994;14:1123–1129. doi: 10.1523/JNEUROSCI.14-03-01123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- 97.Turrigiano GG, Nelson SB. Thinking globally, acting locally: AMPA receptor turnover and synaptic strength. Neuron. 1998;21:933–935. doi: 10.1016/s0896-6273(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 98.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 99.Ventimiglia R, Mather PE, Jones BE, Lindsay RM. The neurotrophins BDNF, NT-3 and NT-4/5 promote survival and morphological and biochemical differentiation of striatal neurons in vitro. Eur J Neurosci. 1995;7:213–222. doi: 10.1111/j.1460-9568.1995.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 100.Vicario-Abejon C, Collin C, McKay RD, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang T, Xie K, Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang XH, Poo MM. Potentiation of developing synapses by postsynaptic release of neurotrophin-4. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 103.Wetmore C, Olson L, Bean AJ. Regulation of brain-derived neurotrophic factor (BDNF) expression and release from hippocampal neurons is mediated by non-NMDA type glutamate receptors. J Neurosci. 1994;14:1688–1700. doi: 10.1523/JNEUROSCI.14-03-01688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]