Abstract
We characterized the pharmacological properties of the anandamide transport inhibitorN-(4-hydroxyphenyl)-arachidonamide (AM404) in rats and investigated the effects of this drug on behavioral responses associated with activation of dopamine D2 family receptors. Rat brain slices accumulated [3H]anandamide via a high-affinity transport mechanism that was blocked by AM404. When administered alone in vivo, AM404 caused a mild and slow-developing hypokinesia that was significant 60 min after intracerebroventricular injection of the drug and was reversed by the CB1 cannabinoid receptor antagonist SR141716A. AM404 produced no significant catalepsy or analgesia, two typical effects of direct-acting cannabinoid agonists. However, AM404 prevented the stereotypic yawning produced by systemic administration of a low dose of apomorphine, an effect that was dose-dependent and blocked by SR141716A. Furthermore, AM404 reduced the stimulation of motor behaviors elicited by the selective D2family receptor agonist quinpirole. Finally, AM404 reduced hyperactivity in juvenile spontaneously hypertensive rats, a putative model of attention deficit hyperactivity disorder. The results support a primary role of the endocannabinoid system in the regulation of psychomotor activity and point to anandamide transport as a potential target for neuropsychiatric medicines.
Keywords: AM404, anandamide transport, cannabinoid receptors, dopamine receptors, spontaneously hypertensive rats, Wistar-Kyoto rats
Cannabinoid receptors, the target of the marijuana constituent Δ9-tetrahydrocannabinol (Pertwee, 1997), are densely expressed in basal ganglia and cortex, regions of the CNS that are critical for the control of cognition, motivation, and movement (Herkenham et al., 1990; Matsuda et al., 1993; Tsou et al., 1998). This distribution provides multiple opportunities for functional interactions between endogenous cannabinoid substances, such as anandamide (Devane et al., 1992; Di Marzo et al., 1994), and ascending dopamine pathways. That these interactions may occur in vivo is indicated by several observations. First, in the striatum of freely moving rats, anandamide release is greatly increased after activation of dopamine D2 family receptors with the selective agonist quinpirole (Giuffrida et al., 1999). Second, pretreatment with the CB1 cannabinoid antagonist SR141716A enhances the stimulation of motor behavior elicited by systemic administration of quinpirole (Giuffrida et al., 1999), although it has little effect per se on basal motor activity (Rinaldi-Carmona et al., 1994; Compton et al., 1996; Navarro et al., 1997). Third, injection of D2 family agonists into basal ganglia nuclei opposes the behavioral response to locally administered CB1 receptor agonists (Sañudo-Peña et al., 1996, 1998;Sañudo-Peña and Walker, 1998). Finally, chronic treatment with D2 family antagonists results in upregulated expression of CB1 receptor mRNA in striatum (Mailleux and Vanderhaeghen, 1993). Together, these findings suggest that one of the functions of anandamide in the CNS may be to modulate dopamine D2 receptor-induced facilitation of psychomotor activity. In agreement with this possibility, anandamide and other CB1 agonists inhibit movement, produce catalepsy, and attenuated-amphetamine-induced hyperactivity and stereotypy (Pryor et al., 1978; Gorriti et al., 1999), whereas disruption of the CB1 receptor gene profoundly affects movement control (Ledent et al., 1999;Zimmer et al., 1999).
When anandamide is administered as a drug, its effects are curtailed by a two-step mechanism consisting of transport into cells, mediated by a high-affinity carrier system (Beltramo et al., 1997b; Hillard et al., 1997; Piomelli et al., 1999), followed by intracellular hydrolysis, catalyzed by a relatively nonselective amidohydrolase enzyme (Deutsch and Chin, 1993; Désarnaud et al., 1995; Cravatt et al., 1996). Consequently, the anandamide transport inhibitorN-(4-hydroxyphenyl)-arachidonamide (AM404) prolongs and enhances several responses to exogenous anandamide, including analgesia (Beltramo et al., 1997b) and vasodilatation (Calignano et al., 1997a). We hypothesized that blockade of anandamide transport, by causing this lipid to accumulate at its sites of release, may help uncover a participation of anandamide in the control of dopamine neurotransmission and might offer a pharmacological strategy to correct pathological conditions characterized by dopaminergic dysfunction. To test this hypothesis, we investigated the pharmacological properties of AM404 in the rat CNS and examined the effects of this drug on behavioral responses elicited by the activation of D2 family receptors.
MATERIALS AND METHODS
Characterization of anandamide transport. Coronal slices (0.45-mm-thick) from adult rat brain were prepared with a vibratome and split along the midline with a razor blade. Each half was collected separately and allowed to equilibrate for 2.5 hr at 37°C in Tris-Krebs' buffer (in mm: NaCl 136, KCl 5, MgCl2 1.2, CaCl2 2.5, glucose 10, and Trizma base 20, pH 7.4) aerated with 5% CO2 in O2. The slices were incubated under agitation for 10 min in Tris-Krebs' buffer containing test compounds, followed by a 5 min incubation in the presence of [3H]anandamide (30 nm, 1.8 × 105 dpm/ml, 221 Ci/mmol; NEN, Wilmington, DE) [the Michaelis constant (KM) for anandamide transport in rat brain astrocytes is 0.32 μm (Beltramo et al., 1997b)] and appropriate concentrations of vehicle or test compounds. In all experiments, SR141716A [0.1 μm; provided by Research Biochemicals (Natick, MA) as part of the Chemical Synthesis Program of the National Institute of Mental Health (Grant NO1MH30003)] was added to the incubations to prevent binding of [3H]anandamide to CB1 receptors. At the end of the incubation period, the slices were rinsed with Tris-Krebs' buffer containing fatty acid-free bovine serum albumin (0.1%) and homogenized in Tris-Krebs' buffer/methanol (1:1, v/v), and radioactivity was measured by liquid scintillation counting.
Selectivity of AM404.[3H]AM404 (arachidonyl-5,6,8,11,12,14,15-3H; 200–240 Ci/mmol; American Radiolabeled Chemicals, St. Louis, MO) was radioactively pure (>99%) by HPLC. [3H]AM404 [10 μg containing 2.8 × 106 dpm in 5 μl of dimethylsulphoxide (DMSO)] was administered by intracerebroventricular injection in cannulated rats (Taconic Farms, Germantown, NY) through a calibrated polyethylene-10 tubing. Average recovery of [3H]AM404 after passage through the tubing was 37.6 ± 2.5% (n = 4). Animals were killed 3 min after injections, and individual brain regions were dissected, weighed, and homogenized. Radioactivity in homogenates was measured by liquid scintillation counting. Identical distributions of radioactivity were obtained when measurements were performed 60 min after injection of [3H]AM404 (data not shown). AM404 had low affinity (concentration needed to produce half-maximal response, EC50 of >10 μm) for the following targets: (1) receptors: adenosine A1 (rat brain, [3H]DPCPX), α1adrenergic nonselective (rat brain, [3H]prazosin), α2 adrenergic nonselective (rat brain cortex, [3H]rauwolscine), β1 adrenergic (human, [125I]cyanopindolol), β2 adrenergic (human, [3H]CGP-12117), D1dopamine (human recombinant, [3H]SCH23390), D2Ldopamine (human recombinant, [3H]spiperone), 5-HT1 serotonin (rat brain cortex, [3H]serotonin), 5-HT2 serotonin (rat brain, [3H]ketanserin), M2 muscarinic (human recombinant, [3H]NMS), M3muscarinic (human recombinant, [3H]NMS), δ-opioid (guinea pig brain, [3H]DPDPE), κ-opioid (guinea pig brain, [3H]U-69593), μ-opioid (guinea pig brain, [3H]DAMGO), ς nonselective (guinea pig brain, [3H]DTG), NMDA glutamate (rat brain cortex), glutamate nonselective (rat brain, [3H]l-glutamate), glycine strychnine-sensitive (rat spinal cord, [3H]strychnine), H1 histamine central (guinea pig brain, [3H]pyrilamine), GABAA agonist site (rat brain, [3H]muscimol), GABAA chloride channel (rat brain cortex, [3H]TBOB), estrogen (calf uterus, [3H]estradiol), progesterone (calf uterus, [3H]R-5020), testosterone (rat ventral prostate, [3H]mibolerone), glucocorticoid (human Jurkat cells, [3H]dexamethasone), insulin (rat liver, [125I]insulin), phorbol ester (mouse brain, [3H]PDBu); (2) voltage-activated ion channels: Ca2+dihydropyridine-sensitive (rat brain cortex, [3H]nitrendipine), K+ (KATP) (syrian hamster pancreas, [3H]glyburide), Na+ site 2 (rat brain, [3H]batrachotoxin); (3) transporters: adenosine (guinea pig brain, [3H]NBTI binding), dopamine (human recombinant, [125I]RTI-55 binding), serotonin (human recombinant, [125I]RTI-55 binding), choline (rat brain, [3H]hemicholium binding), arachidonate (rat cortical neurons, [3H]arachidonate uptake); (4) ethanolamine (rat cortical neurons, [3H]ethanolamine uptake). As reported previously , AM404 displaced the binding of the cannabinoid agonist [3H]WIN-55212–2 from rat brain membranes with low affinity (EC50 of 2 μm) and did not activate CB1 cannabinoid receptors when tested in vitro at 10 μm (inhibition of forskolin-induced cAMP accumulation in cortical neurons) (Beltramo et al., 1997b) or 30 μm (stimulation of [35S]GTP-γ-S binding in rat brain membranes; data not shown). Furthermore, the administration of AM404in vivo did not mimic several key effects of CB1 receptor activation, including analgesia in mice and rats (Beltramo et al., 1997b; present study), hypotension in guinea pigs (Calignano et al., 1997a), and inhibition of intestinal motility in mice (Calignano et al., 1997b). These results indicate that AM404 does not act as an agonist at CB1 receptors either in vitro or in vivo. Moreover, AM404 did not prevent the inhibition of forskolin-induced cAMP accumulation produced in cortical neurons by the application of WIN-55212–2, indicating that the drug does not act as a partial agonist on antagonist at CB1 receptors (Beltramo et al., 1997a). CB2 receptors do not appear to be expressed in the CNS (Ledent et al., 1999; Zimmer et al., 1999); thus, the interaction of AM404 with these receptors was not investigated in the present experiments.
Surgery. Implantation of stainless steel guide cannulas and intracerebroventricular injections were performed in lateral ventricles of male Wistar rats (>8 weeks old, 300–350 gm) as described previously (Rodríguez de Fonseca et al., 1996). AM404 (dissolved in 5 μl of DMSO; Tocris Cookson, Ballwin, MO) or DMSO was injected via an 8 mm 30 gauge injector connected to a calibrated polyethylene-10 tubing. Doses were not corrected for recovery after passage through the polyethylene tubing (see above); thus, they represent an overestimate of the actual amount delivered to the tissue. Cannula placements were evaluated by injection of a blue dye, and only those rats with proper intracerebroventricular placements were included in the data analysis.
Effects of AM404 on apomorphine-induced yawning.Apomorphine-induced yawning was measured in transparent plastic boxes (35 × 30 × 17 cm) following established procedures (Yamada and Furukawa, 1980; Dourish et al., 1989). AM404 (2 μg/rat) or vehicle (DMSO, 5 μl/rat) were administered 5 min before subcutaneous injection of apomorphine (80 μg/kg) or vehicle (aqueous 0.9% NaCl containing 40% DMSO, 0.2 ml/kg). Yawning was measured for a 30 min period after apomorphine injection. Intraperitoneal injections of AM404 (10 and 20 mg/kg), anandamide (0.1, 1, and 10 mg/kg), or vehicle (0.2 ml of aqueous 0.9% NaCl containing 10% DMSO) were done 30 min before apomorphine administration. DMSO alone had no effect on yawning (data not shown).
Effects of AM404 on basal and quinpirole-induced motor behaviors. Experiments were conducted as described previously (Giuffrida et al., 1999). The animals were housed in a room with controlled photoperiod (lights on from 8:00 A.M. to 8:00 P.M.) and habituated to handling for 1 week before starting the experiments. All behavioral studies took place between 9:30 A.M. and 12:00 P.M. Locomotor activity was studied in an opaque open field (100 × 100 × 40 cm), the floor of which was marked with 20 × 20 cm squares. The field was illuminated using a ceiling halogen light that was regulated to yield 350 lux at the center of the field. Rats were habituated to the field for 10 min the day before testing. On the experimental day, the animals were placed in the center of the open field and locomotor activity (number of lines crossed) was scored during 5 min. Behavior was tested 5, 30, 60, and 120 min after the injection of either vehicle or drugs. Spontaneous motor behavior was studied in a glass observation box (40 × 30 × 30 cm, one rat per box) and tested for 5 min at 5, 30, 60, and 120 min after drug injection. The tests were conducted in a sound-isolated room, illuminated with an indirect halogen light (125 lux). The behavior was videotaped on a video cassette recorder. Animals were placed in the box 5 min before the onset of the testing period. The following behavioral acts were scored: (1) immobility (defined as complete absence of observable movement), (2) number of rearing episodes, (3) time spent grooming; (4) sniffing activity, and (5) total oral activity (yawning, vacuous chewing, and licking). We assessed catalepsy by using the bar test. At various times (0, 30, 60, or 120 min) after the injection of vehicle or drugs, the forepaws of test animals were positioned on a 10-cm-high bar, and the time spent by the animals in this position was measured. Tests were ended when the animals removed both forepaws from the bar; test cutoff time was 180 sec. All behavioral measurements were scored by trained observers, blind to experimental conditions.
Effects of AM404 in the hot plate test. The ability of AM404 to inhibit nociception was assessed by using the hot plate test. The rats were placed on a hot plate (55°C), and the latencies for the occurrence of nocifensive behaviors (paw licking or jumping) were measured by trained observers, blind to experimental conditions. Test cutoff time was 30 sec.
Effects of AM404 on juvenile spontaneously hypertensive rats. Juvenile male spontaneously hypertensive rats (SHR) (n = 10) and Wistar-Kyoto (WKY) (n = 10) rats (both from Charles River, Calco, Italy) were used. The rats were kept two per cage in standard makrolon cages with water and food pellets (Mucedola) ad libitum. Four-week-old rats were allowed a 2 week acclimatization before testing. The experimental system was a Làt-maze, a 60 × 60 × 40 cm wooden box with a 30 × 30 × 40 cm plastic transparent smaller box inserted in the middle. Rats were allowed to explore the resulting corridor (60 cm long, 15 cm wide, and 40 cm high). A set of four such boxes was located in a sound-attenuated room. The experimental box was illuminated by a white, cold 4 W lamp placed 60 cm above the floor in the center of the wooden cover, providing 0.1–0.2 μW/cm2. AM404 was dissolved in DMSO at a concentration of 1 mg/ml. Six-week-old rats were exposed for 30 min to the Làt-maze after a single subcutaneous injection of AM404 (1 mg/kg) or vehicle (DMSO, 1 ml/kg). Testing was performed at the beginning of the light phase of the circadian cycle between 9:00 AM and 2:00 P.M., and the two members of the same cage were tested simultaneously to minimize the interference with the arousal state. Behavior was monitored by a CCD camera and stored on a tape recorder for off-line analysis by blind observers. The behavioral variables, i.e., the frequency of corner crossings as index of travel distance, duration of rearings on hindlimbs, and leanings against the walls with one or both forepaws, were visually monitored in 1 min blocks (Aspide et al., 1998). The reliability index was quite high (r= 0.914; df = 198; p < 0.001). At the end of the test, the number of fecal boli was counted, and the floor was carefully cleaned with a wet sponge. Frequency of corner crossings and duration of rearings were submitted to three-way factorial ANOVA: rat line × treatment × time blocks (as dependent variable). Within-exposure changes in rearing duration were analyzed by a two-way ANOVA: rat line × time blocks, as dependent variable. Planned comparisons between group means across days within-line or between-line were made by the two-tailed t test for paired or nonpaired data, respectively. The effect of AM404 was assessed by separate two-way ANOVA: treatment × testing block (first vs second phase of the test). The rejection level was set at p > 0.05, two-sided. All animal procedures met the guidelines of the National Institutes of Health, detailed in the Guide for the Care of Laboratory Animals, and the European Community directives 86/609/EEC regulating animal research.
RESULTS
Inhibition of [3H]anandamide transport
The inhibitory effects of AM404 on [3H]anandamide transport have been characterized in primary cultures of embryonic rat brain neurons and astrocytes (Beltramo et al., 1997) and in human astrocytoma cells (Piomelli et al., 1999). To determine whether AM404 inhibits anandamide transport in the adult CNS, we examined the ability of this drug to prevent [3H]anandamide accumulation in acutely dissected rat brain slices. Brain slices incubated in the presence of [3H]anandamide and SR141716A (a cannabinoid antagonist added to prevent binding of [3H]anandamide to CB1 receptors) accumulated [3H]anandamide in a time- and temperature-dependent manner (Fig. 1and data not shown). As expected from a carrier-mediated process, the temperature-sensitive component of [3H]anandamide accumulation was prevented by nonradioactive anandamide but not by other bioactive lipids (palmitylethanolamide, arachidonate, and prostaglandin E2) or digoxin, a substrate of organic anion transport proteins (Fig. 1). Replacement of extracellular Na+ with choline chloride or incubation with the metabolic inhibitor carbonyl cyanide 3-chlorophenyl hydrazone had no effect, suggesting a Na+- and energy-independent process (data not shown). Moreover, [3H]anandamide uptake was prevented by AM404 but not by the anandamide amidohydrolase inhibitor (E)-6-(bromomethylene)tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one (Beltramo et al., 1997a) (Fig. 1). These findings indicate that [3H]anandamide accumulation in the adult rat brain is mediated by an AM404-sensitive, Na+-independent transporter analogous to that found in embryonic neurons and astrocytes and in astrocytoma cells (Beltramo et al., 1997b; Piomelli et al., 1999).
Fig. 1.
Selectivity of [3H]anandamide transport in rat brain slices. Accumulation was measured in coronal half-slices after a 5 min incubation with [3H]anandamide at 37°C in the absence (control) or presence of nonradioactive anandamide (AEA; 100 μm), palmitylethanolamide (PEA; 100 μm), arachidonic acid (AA; 100 μm), prostaglandin E2 (PGE2; 100 μm), digoxin (100 μm), AM404 (10 μm), or (E)-6-(bromomethylene)tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one (BTNP; 5 μm). Nonspecific association of [3H]anandamide to the slices was measured at 0–4°C. Results are expressed as mean ± SEM; number of independent determinations are indicated within the bars. **p < 0.01 by ANOVA, followed by Dunnett's test.
Inhibition of motor activity
Administration of AM404 (10 μg/rat, i.c.v.), but not of vehicle alone (5 μl of DMSO), caused a slow-onset reduction of motor activity that was statistically significant 60 min after drug injection (Fig.2A1). Cumulative immobility in the 120 min observation period was also significantly higher in AM404-treated animals than in controls (Fig.2A2). This response was prevented by the CB1 antagonist SR141716A (1 mg/kg, i.p., body weight, 60 min before AM404) (Figure 2A1,A2), which did not affect movement when administered alone (data not shown) (Rinaldi-Carmona et al., 1994; Compton et al., 1996; Navarro et al., 1997). The effect of AM404 was dose-dependent. No increase in immobility was observed after injection of a 0.4 μg dose of AM404, whereas the 2 and 10 μg doses were effective [times spent in immobility at 60 min: vehicle, 96.1 ± 22.7 sec (n = 12); 0.4 μg of AM404, 77.7 ± 26.2 sec (n = 8); 2 μg of AM404, 198.5 ± 37.3 sec (n = 10); 10 μg of AM404, 175 ± 18 sec (n = 12)].
Fig. 2.

Effects of the anandamide transport inhibitor AM404 on motor activity and pain threshold in rats. A1, Time course of the effects of vehicle (open bars), AM404 (filled bars), and AM404 plus SR141716A (hatched bars) on time spent in immobility.A2, Cumulative time spent in immobility. AM404 (10 μg/rat) or vehicle (DMSO, 5 μl) were administered by intracerebroventricular injection. The CB1 antagonist SR141716A (1 mg/kg) or vehicle (aqueous 0.9% NaCl containing 10% DMSO, 0.2 ml/kg) was administered intraperitoneally 60 min before intracerebroventricular injection of AM404. Values shown represent the mean ± SEM; number of experiments is indicated within the bars. *p < 0.05 compared with vehicle or AM404 plus SR141716A; one-way ANOVA, followed by Student–Newman–Keuls test for pairwise comparisons. B1–B5, Lack of effect of AM404 on various motor behaviors measured 60 min after drug injection.B6, Lack of effect of AM404 on latency to jump measured in the hot plate test 60 min after drug injection.
The hypokinetic actions of AM404 were reminiscent of those produced by administration of exogenous anandamide (Smith et al., 1994). However, in sharp contrast with the latter, AM404 had no significant inhibitory effect on a variety of motor behaviors, including grooming, oral movements, and sniffing. The lack of effect of AM404 on these behaviors is illustrated in Figure 2B1–B4 with the 10 μg/rat dose and the 60 min time point, but comparable negative results were obtained with all doses of AM404 (0.4, 2, and 10 μg/rat) and at all time points (5, 30, 60, and 120 min) (data not shown). Although we observed a trend toward decreased ambulatory activity, this trend did not reach statistical significance under the conditions of the present experiments (Fig. 2B1). Furthermore, AM404 did not elicit significant catalepsy or analgesia (Fig.2B5,B6), two hallmarks of CB1 receptor activation (Pertwee, 1997). A parsimonious interpretation of our findings, which is also in agreement with the antagonistic effect of SR141716A, is that AM404 acts by interfering with anandamide clearance and by causing this endocannabinoid substance to accumulate slowly at a restricted number of release sites within the CNS.
Distribution and selectivity of AM404
Studies on the pharmacological selectivity of AM404 support this possibility. The concentrations of AM404 reached in rat brain tissue after injection of a maximal dose of this compound (10 μg, i.c.v.) were 1.4 ± 0.5 μm in striatum and 0.4 ± 0.3 μm in cortex (n= 4; see Materials and Methods). Comparable levels were measured in thalamus, hippocampus, brainstem, and cerebellum (data not shown). At such concentrations, AM404 strongly inhibits anandamide uptake by neurons and astrocytes (Beltramo et al., 1997b; Piomelli et al., 1999), whereas it has no effect on 36 other drug targets: heterotrimeric GTP-binding protein-coupled receptors (including dopamine receptors), ligand-gated ion channels, amine uptake sites, and lipid transporters (see Materials and Methods). In particular, AM404 binds to CB1 receptors with low affinity (EC50 of 2 μm) and does not activate these receptors either in vitro or in vivo (although it markedly enhances several effects of exogenously administered anandamide; see Materials and Methods) (Beltramo et al., 1997b; Calignano et al., 1997a,b).
Inhibition of D2 family receptor responses
The observation that AM404 does not interact with D2 family receptors allowed us to test the hypothesis that inhibition of anandamide transport may affect the behavioral responses produced by activation of these receptors. We administered, therefore, AM404 in combination with either of two distinct dopamine receptor agonists: apomorphine and quinpirole.
Low doses of the nonselective dopamine agonist apomorphine elicit a stereotypic yawning response that may be mediated by D2 family receptors (Baraldi and Benassi-Benelli, 1975; Melis et al., 1987; Dourish et al., 1989). The yawning induced by apomorphine (80 μg/kg, s.c.) was strongly inhibited by AM404 (2 μg/rat, i.c.v.), an effect that was prevented by the CB1 antagonist SR141716A (0.2 mg/kg, i.v.) (Fig.3A). Similar inhibitions of the apomorphine response were observed after systemic injections of AM404 (10 and 20 mg/kg, i.p.) (Fig. 3B). The inhibitory effect of systemic AM404 was antagonized by SR141716A (Fig.3B) and mimicked by anandamide (0.1–10 mg/kg, i.p.)(Fig.3C).
Fig. 3.
Effects of AM404 on apomorphine-induced yawning.A, The effect of AM404 (2 μg/rat) or vehicle (DMSO, 5 μl), injected intracerebroventricularly 5 min before apomorphine, was prevented by intravenous administration of SR141716A (SR; 0.2 mg/kg, 15 min before apomorphine).B, Effects of systemic injections of vehicle (subcutaneously, aqueous 0.9% NaCl containing 10% DMSO), AM404 (10–20 mg/kg, s.c.), SR141716A (0.2 mg/kg, i.v.), and AM404 plus SR141716A on apomorphine-induced yawning. AM404 or vehicle were injected 30 min before and SR141716A 45 min before apomorphine.C, Effects of systemic injections of vehicle (subcutaneously, aqueous 0.9% NaCl containing 10% DMSO), anandamide (AEA; 0.1–10 mg/kg), and anandamide plus SR141716A on apomorphine-induced yawning. Values shown represent the mean ± SEM; number of experiments is indicated within the bars. **p < 0.01 compared with apomorphine.
The selective D2 family agonist quinpirole causes a biphasic motor response characterized by initial movement inhibition, which may be mediated by D2 family autoreceptors, followed by a longer lasting hyperactivity, possibly caused by activation of postsynaptic D2 family receptors (Eilam and Szechtman, 1989). Administration of AM404 30 min before quinpirole (at a low dose of 0.25 mg/kg, s.c.) significantly enhanced the initial phase of locomotor inhibition elicited by quinpirole (at time of 5 min), whereas it reduced the subsequent phase of motor stimulation (at time of 120 min) (Fig.4A). A parallel effect of AM404 was observed on the time spent in immobility (Fig.4B). This bimodal response is consistent with the finding that D2 family receptors may stimulate anandamide outflow in vivo (Giuffrida et al., 1999), because neurally released anandamide is expected to act synergistically with D2 autoreceptors (which may mediate motor inhibition) but antagonistically with postsynaptic D2 receptors (which may cause motor activation) (Picetti et al., 1997).
Fig. 4.
Effects of AM404 on the behavioral responses elicited by the dopamine D2 family agonist quinpirole. Time course of the effects of vehicle (open bars), AM404 (filled bars), quinpirole (striped bars), and AM404 plus quinpirole (hatched bars) on locomotor activity (A) and time spent in immobility (B). Quinpirole (0.25 mg/kg) or vehicle (aqueous 0.9% NaCl, 0.2 ml/kg) were administered intraperitoneally 30 min after intracerebroventricular injection of AM404 (10 μg/rat) or vehicle (DMSO, 5 μl). Values shown represent the mean ± SEM; number of experiments is indicated within the bars. *p < 0.05 compared with vehicle.
Reduction of genetic hyperactivity
Juvenile SHR are hyperactive, but not yet hypertensive, and show deficits of sustained attention in behavioral paradigms (Sagvolden et al., 1993). These abnormalities have been associated with alterations in the activity of the mesocorticolimbic dopamine systems and with changes in dopamine receptor expression (Carey et al., 1998). To determine whether inhibition of anandamide transport affects hyperactivity in SHR, we measured horizontal locomotor activity and duration of rearing episodes during exposure to a novel environment, after administration of AM404 or vehicle. In parallel tests, we examined the effects of AM404 on age-matched WKY rats, the line from which SHR were selectively bred (Okamoto, 1969).
In control WKY rats, AM404 (1 mg/kg, s.c., 30 min before testing) did not significantly affect rearing duration or horizontal locomotion in either the first (0–15 min) or the second (16–30 min) part of the testing period (Fig. 5). In contrast, the drug increased duration of rearing episodes and decreased horizontal activity in SHR during the second part of the test (Fig. 5) in which vehicle-treated SHR failed to habituate to the novel environment and maintained inordinately high activity when compared with WKY controls (Fig. 5). These results suggest that a low dose of AM404 can alleviate hyperactivity in SHR without significant effects on the normal motor behavior of progenitor WKY rats.
Fig. 5.
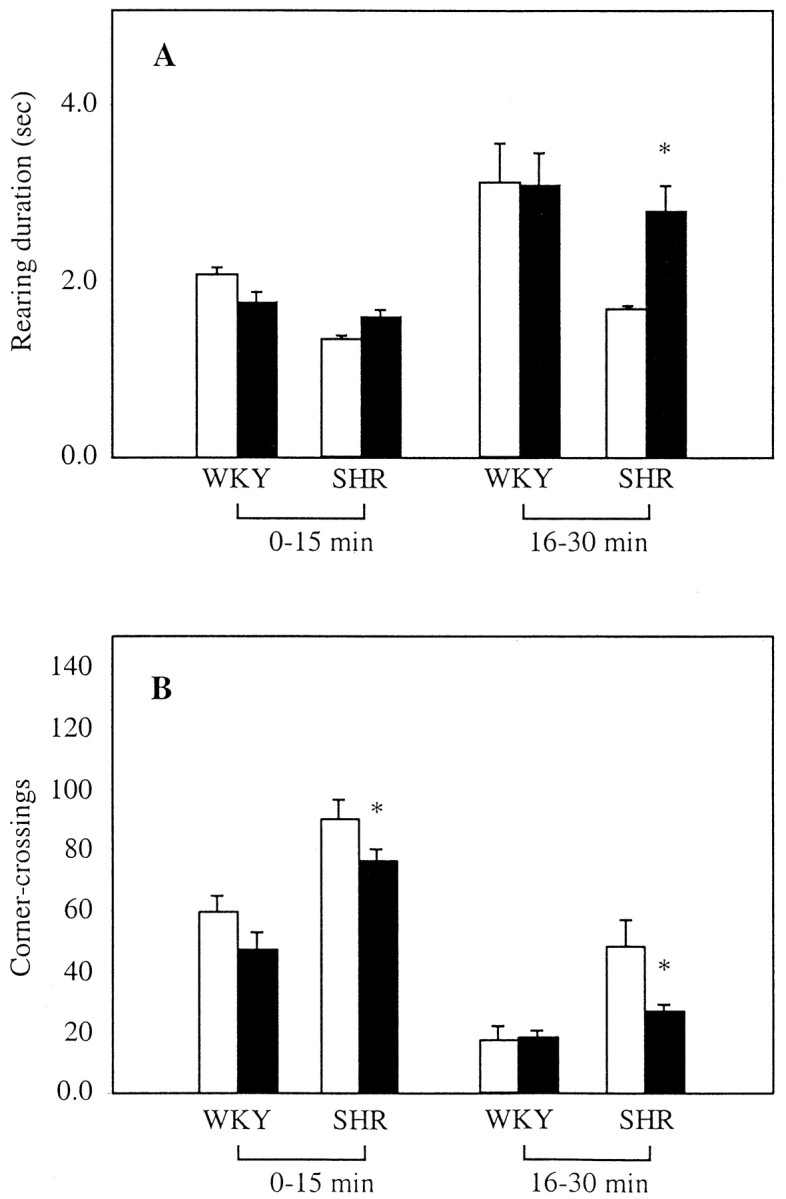
Effects of AM404 on horizontal and vertical activity in SHR and control WKY rats. Time-dependent effects of vehicle (DMSO, 1 ml/kg; open bars) or AM404 (1 mg/kg, s.c.;filled bars) on duration of rearing episodes (A) and horizontal activity (assessed as frequency of corner crossings; P < 0.001 compared with vehicle) (B) in SHR and WKY rats. Values shown represent the mean ± SEM of n = 5 per group. *p < 0.05 compared with vehicle.
DISCUSSION
There is both experimental and medical interest in developing molecules that selectively interfere with anandamide transport. Anandamide transport inhibitors may be used experimentally to uncover the functions of the endocannabinoid system, which are still essentially uncharacterized (for review, see Piomelli et al., 1998). Furthermore, anandamide transport inhibitors may offer a rational approach to a variety of disease conditions in which elevation of anandamide levels at its release sites may result in a more selective pharmacological response than direct activation of CB1 receptors by agonist drugs.
In the present study, we used the anandamide transport inhibitor AM404 to investigate functional interactions between anandamide and dopamine in the control of motor activity. The existence of such interactions was suggested by four key observations. First, in the striatum of freely moving rats, activation of D2 family receptors stimulates anandamide release (Giuffrida et al., 1999). Second, blockade of CB1 cannabinoid receptors enhances the stimulation of motor behavior elicited by D2 agonists (Giuffrida et al., 1999). Third, CB1 agonists and D2 family agonists exert opposing behavioral effects when they are administered by local injection into individual basal ganglia nuclei (Sañudo-Peña et al., 1996, 1998;Sañudo-Peña and Walker, 1998). Finally, treatment with D2 family antagonists causes an upregulation of CB1 receptor expression in striatum (Mailleux and Vanderhaeghen, 1993). In keeping with these results, we found that AM404 counteracts two characteristic responses mediated by activation of D2 family receptors: apomorphine-induced yawning and quinpirole-induced stimulation of motor behaviors. These effects are achieved at doses of AM404 that may elicit only a mild hypokinesia when the drug is administered alone and may selectively inhibit anandamide transport in vitro. In addition, doses of AM404 identical to those used in the present study are able to produce a time-dependent increase in the levels of anandamide in peripheral blood (A. Giuffrida, F. Rodríguez de Fonseca, F. Nava, J. Belluzzi, and D. Piorrelli, in preparation). Thus, our results are consistent with the hypothesis that anandamide released by stimulation of D2 family receptors participates in the control of dopamine-induced psychomotor activation.
CB1 receptor agonists elicit a broad spectrum of behavioral responses that include catalepsy, analgesia, reduced movement, and hypothermia (Pertwee, 1997). The finding that AM404 evokes only a moderate slow-onset hypokinesia when it is administered alone demarcates the pharmacological profile of this anandamide transport inhibitor from those of direct-acting cannabimimetic drugs. This distinction may result from the ability of AM404 to enhance anandamide signaling in an activity-dependent manner by causing anandamide to accumulate in discrete regions of the CNS only when release of this endocannabinoid substance is triggered by appropriate stimuli. In the absence of such stimuli, tonic anandamide release may be very low, accounting for the weak and slow-developing motor effects of AM404 in naïve animals.
We considered that the pharmacological profile of AM404 could offer an original strategy to correct behavioral abnormalities that are generally associated with dysfunction in dopamine neurotransmission. As an initial test of this hypothesis, we examined the effects of AM404 in SHR, a rat line in which hyperactivity and attention deficits have been linked to a defective regulation of mesocorticolimbic dopamine pathways (Esposito et al., 1999; Russell, 2000; Sadile, 2000). We found that administration of a low systemic dose of AM404 (1 mg/kg) normalizes motor activity in SHR with no overt motor effect in WKY controls, the strain from which SHR originate (Okamoto, 1969). These results suggest that pharmacological inhibition of anandamide inactivation may alleviate hyperactivity in SHR. Additional experiments are needed to determine whether this effect is mediated by an elevation of anandamide levels in brain regions involved in the control of movement and attention.
The multiple physiological functions served by dopamine in the control of psychomotor activity and the lack of animal models that capture the complexities of psychiatric diseases make it difficult to extrapolate from rodent models to human syndromes. Yet, the spectrum of pharmacological properties displayed by AM404 and the ability of this drug to counteract potential manifestations of dopamine dysregulation suggest that anandamide transport may be a valuable target for the development of novel neuropsychiatric medicines.
Footnotes
This work was supported by National Institute on Drug Abuse Grants DA12413 and DA12447 (to D.P.), DGICYT, Comunidad de Madrid and Plan Nacional sobre Drogas (to M.N. and F.R.F.), and Telethon Italy Grant E513 (to A.G.S.). F.R.F. and M.N. are Jaime del Amo Research Fellows at the Complutense University. We thank A. Bilbao, U. Gironi Carnevale, J. Muñoz, and M. Pignatelli for their expert assistance. We also thank Dr. L. H. Parsons for critical reading of this manuscript.
Correspondence should be addressed to Daniele Piomelli, Department of Pharmacology, 360 MSR II, University of California, Irvine, CA 92697-4625. E-mail: piomelli@uci.edu.
REFERENCES
- 1.Aspide R, Gironi Carnevale UA, Sergeant JA, Sadile AG. Non-selecive attention and nitric oxide in putative animal models of attention-deficit and hyperactivity disorder. Brain Res. 1998;95:123–133. doi: 10.1016/s0166-4328(97)00217-9. [DOI] [PubMed] [Google Scholar]
- 2.Baraldi M, Benassi-Benelli A. Apomorphine-induced penile erection in adult rats. Riv Farmacol Terapia. 1975;6:771–772. [Google Scholar]
- 3.Beltramo M, di Tomaso E, Piomelli D. Inhibition of anandamide hydrolysis in rat brain tissue by (E)-6-(bromomethylene) tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one. FEBS Lett. 1997a;403:263–267. doi: 10.1016/s0014-5793(97)00061-6. [DOI] [PubMed] [Google Scholar]
- 4.Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997b;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- 5.Calignano A, La Rana G, Beltramo M, Makriyannis A, Piomelli D. Potentiation of anandamide hypotension by the transport inhibitor, AM404. Eur J Pharmacol. 1997a;337:R1–R2. doi: 10.1016/s0014-2999(97)01297-1. [DOI] [PubMed] [Google Scholar]
- 6.Calignano A, La Rana G, Makriyannis A, Lin SY, Beltramo M, Piomelli D. Inhibition of intestinal motility by anandamide, an endogenous cannabinoid. Eur J Pharmacol. 1997b;340:R7–R8. [PubMed] [Google Scholar]
- 7.Carey MP, Diewald L, Papa M, Gironi Carnevale UA, Pellicano MP, Esposito F, Sergeant JA, Sadile AG. Differential distribution of D1 and D2 dopamine receptors in the target sites of the mesolimbic system in an animal model of ADHD. Behav Brain Res. 1998;94:173–185. doi: 10.1016/s0166-4328(97)00178-2. [DOI] [PubMed] [Google Scholar]
- 8.Compton DR, Aceto MD, Lowe J, Martin BR. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of Δ9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. [PubMed] [Google Scholar]
- 9.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 10.Désarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes: identification and partial characterization. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- 11.Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- 12.Devane W, Hanus L, Breuer A, Pertwee R, Stevenson L, Griffin G, Gibson D, Mandelbaum D, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 13.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz J-C, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 14.Dourish CT, Herbert EN, Iversen SD. Blockade of apomorphine-induced yawning in rats by the dopamine autoreceptor antagonist (+)-AJ76. Neuropharmacology. 1989;28:1423–1425. doi: 10.1016/0028-3908(89)90021-x. [DOI] [PubMed] [Google Scholar]
- 15.Eilam D, Szechtman H. Biphasic effect of D-2 agonist quinpirole on locomotion and movements. Eur J Pharmacol. 1989;161:151–157. doi: 10.1016/0014-2999(89)90837-6. [DOI] [PubMed] [Google Scholar]
- 16.Esposito FJ, Gironi Carnevale UA, Diewald LM, Carey MP, Vallone D, Sadile AG. Differential response of dopamine D-3 autoreceptors to subchronic methylphenidate treatment in anterior forebrain sites of an animal model of hyperactivity and attention deficits. Soc Neurosci Abstr. 1999;25:848. [Google Scholar]
- 17.Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 18.Gorriti MA, Rodríguez de Fonseca F, Navarro M, Palomo T. Chronic (−)-delta9-tetrahydrocannabinol treatment induces sensitization to the psychomotor effects of amphetamine in rats. Eur J Pharmacol. 1999;365:133–142. doi: 10.1016/s0014-2999(98)00851-6. [DOI] [PubMed] [Google Scholar]
- 19.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamide (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- 21.Ledent C, Valverde O, Cossu G, Petitet F, Aubert J, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 22.Mailleux P, Vanderhaeghen JJ. Dopaminergic regulation of cannabinoid receptor mRNA levels in the rat caudate-putamen: an in situ hybridization study. J Neurochem. 1993;61:1705–1712. doi: 10.1111/j.1471-4159.1993.tb09807.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- 24.Melis MR, Argiolas A, Gessa GL. Apomorphine-induced penile erection and yawning: site of action in brain. Brain Res. 1987;415:98–104. doi: 10.1016/0006-8993(87)90272-1. [DOI] [PubMed] [Google Scholar]
- 25.Navarro M, Hernández E, Munoz RM, del Arco I, Villanœa MA, Carrera MR, Rodríguez de Fonseca F. Acute administration of the CB1 cannabinoid receptor antagonist SR141716A induces anxiety-like responses in the rat. NeuroReport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto K. Spontaneous hypertension in rats. Int Rev Exp Pathol. 1969;7:227–270. [PubMed] [Google Scholar]
- 27.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 28.Picetti R, Saiardi A, Abdel Samad T, Bozzi Y, Baik JH, Borrelli E. Dopamine D2 receptors in signal transduction and behavior. Crit Rev Neurobiol. 1997;11:121–142. doi: 10.1615/critrevneurobiol.v11.i2-3.20. [DOI] [PubMed] [Google Scholar]
- 29.Piomelli D, Beltramo M, Giuffrida A, Stella N. Endogenous cannabinoid signaling. Neurobiol Dis. 1998;5:462–473. doi: 10.1006/nbdi.1998.0221. [DOI] [PubMed] [Google Scholar]
- 30.Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, Xiang-Qun Xie, Makriyannis A. Structural determinants for recognition and translocation by the anandamide transporter. Proc Natl Acad Sci USA. 1999;96:5802–5807. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pryor GT, Larsen FF, Husain S, Braude MC. Interactions of delta9-tetrahydrocannabinol with d-amphetamine, cocaine, and nicotine in rats. Pharmacol Biochem Behav. 1978;8:295–318. doi: 10.1016/0091-3057(78)90320-9. [DOI] [PubMed] [Google Scholar]
- 32.Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara P, Soubrié P, Brelière JC, Le Fur G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, Navarro M. Corticotropin-releasing factor (CRF) antagonist [d-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996;276:56–64. [PubMed] [Google Scholar]
- 34.Russell V. The nucleus accumbens motor-limbic interface of the spontaneously hypertensive rat (SHR) as studied in-vitro by the superfusion slice technique. Neurosci Biobehav Rev. 2000;24:133–136. doi: 10.1016/s0149-7634(99)00056-1. [DOI] [PubMed] [Google Scholar]
- 35.Sadile AG. Multiple evidence of a segmental defect in the anterior forebrain of an animal model of hyperactivity and attention deficit. Neurosci Biobehav Rev. 2000;24:161–169. doi: 10.1016/s0149-7634(99)00057-3. [DOI] [PubMed] [Google Scholar]
- 36.Sagvolden T, Pettersen MB, Larsen MC. Spontaneously hypertensive rats (SHR) as a putative animal model of childhood hyperkinesis: SHR behavior compared to four other rat strains. Physiol Behav. 1993;54:1047–1055. doi: 10.1016/0031-9384(93)90323-8. [DOI] [PubMed] [Google Scholar]
- 37.Sañudo-Peña MC, Walker JM. Effects of intrapallidal cannabinoids on rotational behaviour in rats: interactions with the dopaminergic system. Synapse. 1998;28:27–32. doi: 10.1002/(SICI)1098-2396(199801)28:1<27::AID-SYN4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 38.Sañudo-Peña MC, Patrick SL, Patrick RL, Walker JM. Effects of intranigral cannabinoids on rotational behavior in rats: interactions with the dopaminergic system. Neurosci Lett. 1996;206:21–24. doi: 10.1016/0304-3940(96)12436-8. [DOI] [PubMed] [Google Scholar]
- 39.Sañudo-Peña MC, Force M, Tsou K, Miller AS, Walker JM. Effects of intrastriatal cannabinoid on rotational behavior in rats: interactions with the dopaminergic system. Synapse. 1998;30:221–226. doi: 10.1002/(SICI)1098-2396(199810)30:2<221::AID-SYN12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 1994;270:219–227. [PubMed] [Google Scholar]
- 41.Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 42.Yamada K, Furukawa T. Direct evidence for involvement of dopaminergic inhibition and cholinergic activation in yawning. Psycopharmacology. 1980;67:39–43. doi: 10.1007/BF00427593. [DOI] [PubMed] [Google Scholar]
- 43.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]





