Abstract
Voila1, an enhancer-trap strain in Drosophila melanogaster, expresses GAL4 in most gustatory neurons, both before and after metamorphosis.Voila1 expression starts at embryonic stage 10. In the periphery, it labels larval gustatory sensilla in the antennomaxillary complex as well as in the pharynx. GAL4 is also expressed in the CNS in a manner that prefigures expression in adult flies. Most Voila1/1 homozygotes die between second larval instar and early adulthood. Moreover, escapingVoila1/1 larvae do not show gustatory responses to NaCl and sucrose. The simultaneous rescue of normal larval gustation together with adult viability after removal of the transposable PGAL4 element suggests that both these phenotypes are caused by the same inserted element.
Keywords: Drosophila, taste, gustatory nervous system, development, larval behavior, enhancer-trap PGAL4 strain
The study of the taste sensory system requires the availability of a reliable behavioral phenotype (or phenotypes) related to the genetic alteration of a restricted number of neurons. In the fruit fly Drosophila melanogaster, the recent engineering of PGAL4 enhancer-trap strains expressed in a specific subset of neurons (Brand and Perrimon, 1993) has made it possible to begin unraveling the function of neurons by studying behavior in parallel with genetic misexpression (Ferveur et al., 1995;Sweeney et al., 1995; Connolly et al., 1996).
The major components of the chemosensory system ofDrosophila larvae are the dorsal organ (DO), the terminal organ (TO), and a number of pharyngeal sensilla (for review, seeStocker, 1994). The structural features of the DO and TO, which together form the antennomaxillary complex (AMC), suggest that they are involved in olfaction and taste, respectively (Singh and Singh, 1984). This has recently been confirmed by electrophysiological recording (Oppliger et al., 2000) and chemosensory preference assays after toxin-induced silencing of larval chemosensory neurons (Heimbeck et al., 1999).
During metamorphosis of holometabolous insects, most of the larval motor neurons and many interneurons persist and combine with new imaginal neurons to form the adult CNS (Truman et al., 1993). Conversely, almost all larval sensory neurons degenerate, and adult sensory neurons form de novo (Jan and Jan, 1993). Exceptions are specialized subsets of larval sensory neurons that may act as a scaffold during peripheral neuronal reorganization (Williams and Shepherd, 1999). Apart from cellular persistence, other precise sensory functions appear to be conserved through metamorphosis. In the visual system, some opsin pigments are expressed in both the larval photoreceptor organ and the adult compound eye (for review, seeMeinertzhagen and Hanson, 1993). A mutant study has also indicated that larval and adult visual transduction systems share several proteins (Busto et al., 1999). Such a two-stage screening has allowedRiesgo-Escovar et al. (1992) to isolate enhancer-trap lines showing very restricted expression patterns in both larval and adult olfactory organs. Among the candidate genes, acj6 showed altered larval and adult olfaction in response to specific chemicals (McKenna et al., 1989; Ayer and Carlson, 1991). acj6 was subsequently used to characterize the first Drosophila olfactory receptors (Clyne et al., 1999).
Similarly, we show here that theVoila1–PGAL4 strain, which was found to express GAL4 specifically in adult taste sensilla (Balakireva et al., 1998), also specifically labels gustatory organs during larval and pupal stages. Not surprisingly, homozygousVoila1/1 larvae exhibit serious gustatory defects. Moreover, most homozygous individuals, which are smaller than controls, die between second larval instar and late pupal stages. On the basis of genetic and behavioral experiments, we suggest that Voila plays a crucial role during the development and/or maturation of the gustatory system.
MATERIALS AND METHODS
Fly stocks and genetics. Strains were kept at 25°C (unless otherwise noted) in a 12 hr dark/light cycle on standard cornmeal food. A description of the chromosomes and mutations used in this study can be found in Lindsley and Zimm (1992). As a control strain, we chose Canton-S (CS), which is a laboratory strain that has been studied for several decades. The PGAL4 enhancer-trap line DB345 was isolated in a screen for expression in the adult chemosensory system (Balmer, 1994) and was subsequently namedVoila1 because of its dominant bisexual courtship phenotype in heterozygote males (Balakireva et al., 1998). TheVoila1 variant is a recessive lethal that has been maintained balanced over the chromosomes TM3, Sb Ser, or TM6C, Sb Tb, or TM6, Ubx (a gift of P. Santamaria, Gif-sur-Yvette, France).
Complementation analysis ofVoila1 was performed with a set of deficiencies uncovering the chromosomal region 86C-87C [for breakpoints and origin, see Reuter et al. (1987)]. Each deficiency was tested in a trans-heterozygous combination against the homologous chromosome 3 carryingVoila1.
Derivative lines of Voila1 were produced according to the standard procedure (Cooley et al., 1988). Mobilization of theVoila1–PGAL4 transposon was performed by mating males carrying the transposase-producing Δ2–3 chromosome (Robertson et al., 1988) together withVoila1/TM3 females. Individual F1 male progeny carrying both chromosomes 3 (one withVoila1 and one with the Δ2–3 transposase) were mated to w;+/TM3 females. Each F2 w− male was used to establish a derivative line containing an independentVoila excision event (Voilaexc) balanced over the TM3, Sb Ser chromosome (the white eye color indicates that at least the part of the PGAL4 transposon containing thew+ minigene sequence has been excised from the genome of theVoila1 strain).
Developmental lethality. For measuring developmental lethality, eggs were collected for a period of 24 hr at 25°C (for experiments performed at 25 and 29°C) or at 20°C (for experiments at 20°C) and deposited in vials at the experimental temperature. Thirty to forty hours after the end of egg-laying, the number of dead embryos was counted. Strains carrying a balancer chromosome are expected to yield an average of 25% dead embryos (homozygous for the balancer chromosome). Adults emerging from the pupal case were counted according to their genotype (nA), and the frequency of adult survival was estimated relative to the number of surviving embryos (nA/nE). The frequency of lethality during pupal life was also directly measured (nP/nE). The occurrence of lethality during larval stages is thus the difference between the number of hatching embryos minus the number of individuals that reach (and die during) pupariation and adulthood (nL = nE − [nP + nA]).
The respective lethality of both homozygous and heterozygousVoila1 genotypes was assessed with the dominant marker Tubby (Tb) carried on the balancer chromosome TM6C, Sb Tb. The Tbmarker makes it possible to distinguish both genotypes during larval and pupal stages. The lethality of the different adult genotypes was based on our estimation carried out with bothVoila1/TM3 andVoila1/TM6 strains. A similar protocol was used to estimate the lethality of flies carrying derivative Voilaexc chromosomes or deficiencies.
Food renewal was performed by transferring first instar larvae (once) on fresh food medium. For all experiments, the number of larvae was roughly controlled (200–300) to prevent competition for food resources.
Gustatory tests. Petri dishes divided into halves (Falcon 1003) were filled with 1% agarose/water (control) and 1% agarose/test solution (test) on opposite halves (Heimbeck et al., 1999). Chemicals tested were sucrose (Fluka 84100) and NaCl (Fluka 71380). Thirty to fifty late second–early third instar larvae were placed on the center of the dish and allowed to move freely. The number of larvae found on control (Nc) and test (Ns) halves was counted after 10, 15, 30, and 60 min. Larvae found at <0.5 cm from the separating line were not included in the calculation. A response index (RI) was calculated for each time point (RI = [Ns − Nc]/[Ns + Nc]).
The RI values were relatively stable between 15 and 60 min of the test period. This is the reason why we have shown theRI values that were yielded after 30 min. However, theRI values toward sucrose that were very significantly different between strains at 15 min are also described in Results. We used two-way ANOVA to compare the difference between our data that were normally distributed within most samples (for each genotype, for a given concentration, and time of observation). Statistical significance was tested with least significant difference and Newman–Keulspost hoc tests.
Reporter gene expression. Voila1/TM6 was crossed with either UAS–lacZ (Brand and Perrimon, 1993) or UAS-green fluorescent protein (GFP) (Yeh et al., 1995). For visualization of β-galactosidase, embryos were stained with X-Gal according to Ghysen and O'Kane (1989). Larvae and pupae were dissected in Millonig's buffer, fixed in 1% glutaraldehyde (in Millonig's), and stained for β-galactosidase activity with a solution containing 5–10 mg X-Gal/ml DMSO (Brand and Perrimon, 1993). Embryos and the dissected parts of larvae and pupae were mounted in Faure's solution (Ashburner, 1989).
For visualization of GFP through the confocal microscope, larvae and pupae were dissected in Drosophila Ringer's solution and fixed in 4% paraformaldehyde [for details, see Laissue et al. (1999)]. Counterstaining of the neuropil was performed by mAb nc82 and the Cy3 fluorophore (Laissue et al., 1999). The dissected tissues were embedded in Vectashield medium (Vector Laboratories, Burlingame, CA) and viewed with a Bio-Rad MRC 1024 confocal microscope equipped with a Kr/Ar laser. Multiple series of optical sections of 0.9 μm were taken with 512 × 512 pixel resolution.
RESULTS
Preimaginal lethality
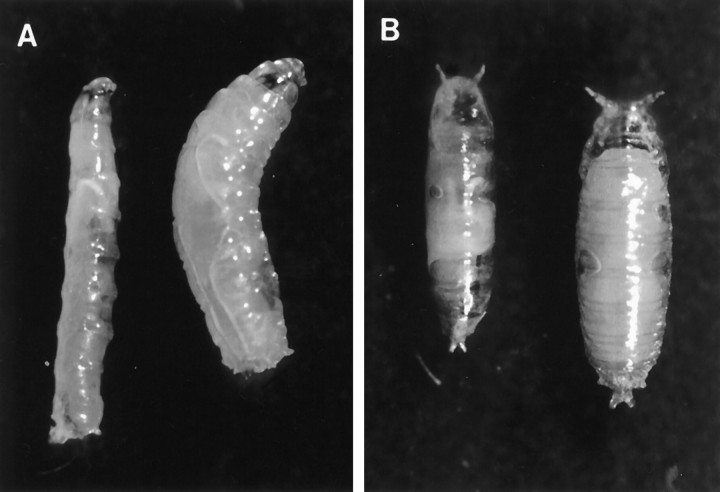
The PGAL4 enhancer trap lineVoila1, which was isolated in a screen for expression in the adult chemosensory system, yielded only viable heterozygous adult flies (Voila1/+). Voila1/1 exhibited developmental lethality at various stages (see below). Furthermore, homozygousVoila1 larvae and pupae remained much smaller than Voila1/+ or wild-type genotypes (Fig. 1). We do not know yet whether this effect is caused by starvation. We have not noted obvious abnormal movements, digging, or feeding behaviors of mutant larvae.
Fig. 1.
A, B, HomozygousVoila1/1 larvae and pupae (left side in each panel) are significantly smaller than the corresponding wild-type stages (right side).A, Third instar larvae. B, Pupae after head eversion.
The lethality of Voila1/1homozygotes was clearly postembryonic: individuals died between the second larval instar and the late pupal stage. The developmental lethality that occurred during embryonic, larval, pupal, and early imaginal stages was measured by counting the frequency of individuals surviving at the end of each of these developmental phases. TheVoila1/1 genotype showed a highly reproducible pattern of lethality during development (Fig.2A).
Fig. 2.
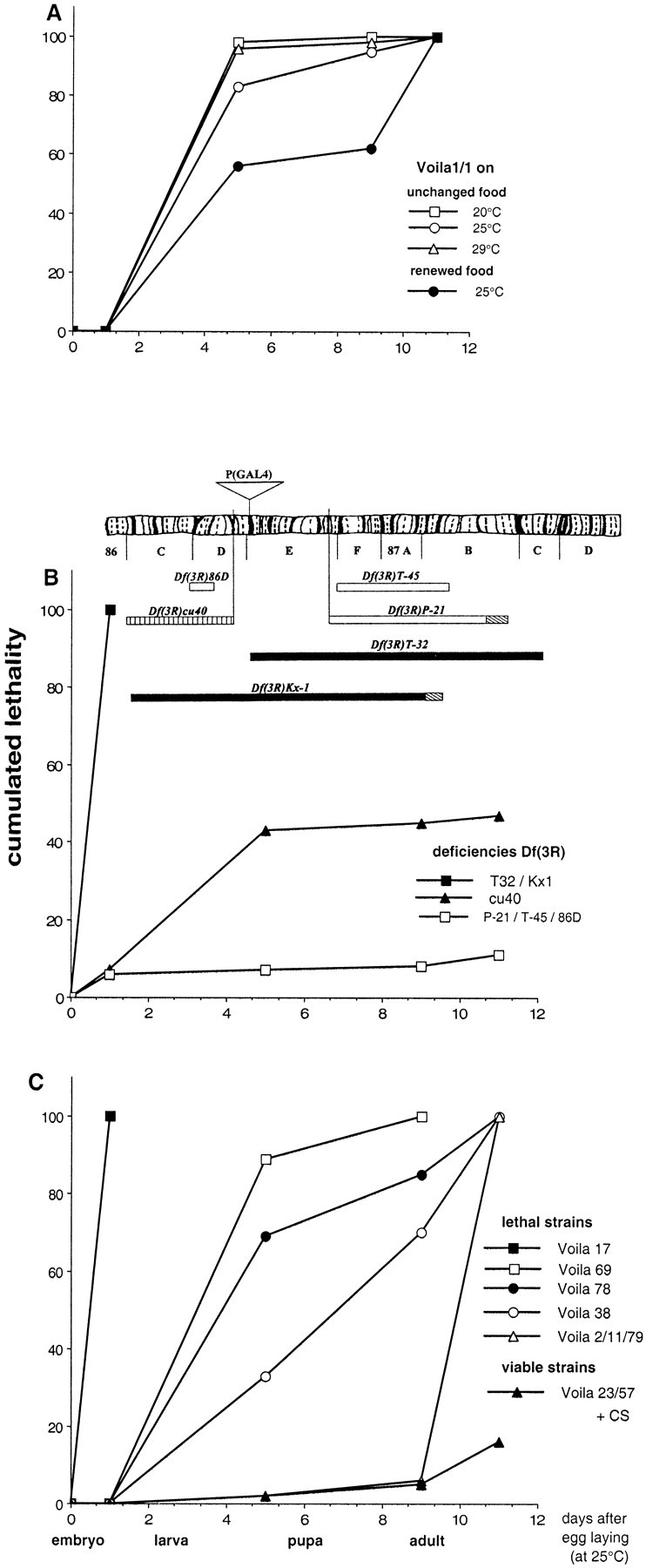
A–C, Cumulative lethality during different phases of development of (A) homozygousVoila1/1 in various environmental conditions and (B, C) of various genotypes. A, Effect of temperature and food quality on the lethality rate for Voila1/1genotype. B, Complementation analysis ofVoila1 with different genetic deficiencies. Deficiencies shown above the graph are aligned with the salivary gland chromosome map (Lindsley and Zimm, 1992). Bars represent the extent of the deficiencies, and hatched regions indicate uncertainty as to breakpoint position. The density of bars represents developmental viability (black, lethal;striped, semi-lethal; white, viable). The position for the insertion of the PGAL4 transposon is shown above the chromosome. C, Genetic analysis with various homozygousVoilaexc/exc excision alleles. For all strains, the genetic background included the TM3 balancer carrying either Sb Ser or Sb Tb. The main phases of development are indicated in days (as measured after egg-laying at 25°C). Time scale was readjusted as for the other developmental temperatures. Each value corresponds to one or two experiments; each experiment was performed with more than 250 embryos.
Temperature and feeding conditions, but not genetic background, affect developmental lethality
At 25°C, ∼83% of Voila1/1 homozygotes died before reaching puparium formation (Fig. 2A). We tested the influence of different genetic backgrounds (each one including a different balancer for chromosome 3: TM3, Sb Ser, or TM6,Ubx, or TM6C, Sb Tb). At 25°C, the lethality profile between these three strains showed only slight quantitative variations: 5–12% of individuals died during pupal life, and the 5–12% of adult escapers (of both sexes) died during their first 2 d of adult life (data not shown). The surviving imagoes had difficulties standing up and therefore showed no visible locomotor activity.
We also tested the influence of temperature and food on developmental lethality. When the developmental temperature was either shifted down to 20°C or raised to 29°C, noVoila1/1 adult flies eclosed (Fig. 2A). In both cases, the percentage of dying larvae increased dramatically (95–98%); 25°C is thus the most favorable temperature for prolonging the survival ofVoila1/1 homozygotes. Food quality also largely influenced the lethality profile (Fig.2A). Larvae that were transferred to fresh food medium during their first instar showed an increased probability of reaching puparium formation (43%) as compared with siblings held on the same medium throughout larval development (17%). As a consequence, the larvae that were raised on renewed food more frequently yielded adult flies (38%) than larvae held on unchanged medium (12%). Nevertheless, no Voila1/1 imago survived for more than 48 hr, regardless of rearing conditions.
For the rest of our study and to standardize our measurements, these experimental conditions were kept constant. Strains were always raised at 25°C and held on the same food medium during their entire preimaginal development.
Genetic mapping of developmental lethality
Voila1 was originally mapped to chromosome 3, at 86E1–2 (Balakireva et al., 1998). To confirm that developmental lethality was caused by the PGAL4 transposon inserted at the Voila locus, we performed two series of genetic experiments. First, a complementation analysis was made with several strains carrying a deficiency in the chromosomal region surrounding Voila (86D4-E19) (Fig.2B). We found that neither Df(3R)Kx1 nor Df(3R)T-32 deficiency could complement the defect caused byVoila1 on the homologous chromosome: both double heterozygotes (Df(3R)/Voila1) showed embryonic lethality. This was not the case with the other deficiencies tested here. It should be remembered that both Kx1 and T-32 deficiencies were previously found to yield abnormal adult male courtship behavior (Balakireva et al., 1998). The Df(3R)cu40 deficiency, which was not previously tested, induced semi-lethality during development when paired with theVoila1 chromosome (Fig.2B). This defect indicates that cu40 deficiency partially uncovers the genomic region involved in adult viability.
The second genetic experiment was performed to rescue adult viability by remobilizing the PGAL4 transposon. Each remobilization event was subsequently maintained in aVoilaexc strain. We obtained 61Voilaexc strains, each of which was characterized for the profile of developmental lethality of its homozygous Voilaexc/exc flies (M. Balakireva, unpublished data). Out of theseVoilaexc strains, adult viability was completely rescued in 35 cases (viable strains =Voilaexc-Vb lines). Data are shown for the Voila23 andVoila57 strains that exhibited mortality curves that were very similar to that of the control CS strain (Fig. 2C). This rescue indicates that the PGAL4 transposon is responsible for the developmental lethality ofVoila1/1 homozygotes.
Remobilization can often yield imprecise excisions of the transposon, thus producing new alleles at the same locus (Wilson et al., 1989; Deak et al., 1997). The profile of developmental lethality of the 26 otherVoilaexc lines that showed no rescue of adult viability was thus examined in detail. TheseVoilaexc-Lt lines (Fig.2C, lethal strains) exhibited various patterns of lethality. The most dramatic case was found in theVoila17 strain in which all homozygous Voila17/17 embryos died. On the other hand,Voila2,Voila11, andVoila79 strains exhibited only a slight lethality: only a few homozygotes died during their preimaginal development, with a high proportion (>85%) of eclosing adult flies. However, even in these strains, homozygousVoilaexc/exc imagoes never survived for more than 48 hr. Furthermore, they behave very poorly, like Voila1/1 adult escapers (see above). Between these two extreme cases, otherVoilaexc-Lt lines (such asVoila69,Voila78, andVoila38) showed an intermediate profile of developmental lethality that was somewhat similar to theVoila1/1 genotype.
Gustatory defects in Voila larvae tested with sodium chloride
Voila1/1 andVoila1/TM3 larvae were compared with larvae from the control strain (CS) and with homozygous larvae from two Voilaexc-Vb(Voila23/23 andVoila57/57) strains with rescued developmental viability. Gustatory responses after 30 min were measured as RI, which indicates the relative number of larvae choosing agar mixed with the test solution versus neutral agar (see Materials and Methods).
Voila1/1 homozygotes showedRI values that were significantly different from the four other genotypes (0.00001 < p < 0.019; except for both Voilaexc-Vb strains at 0.1m). The values shown on Figure3A indicate thatVoila1/1 larvae are unable to choose between neutral agar and agar mixed with NaCl, at the three concentrations tested here. Conversely, larvae of all other genotypes were clearly repelled by the higher concentration (0.5–0.3m) of salt. Interestingly, bothVoilaexc-Vb strains of larvae showed RI values that were similar to the RIvalues of the CS control genotype. Furthermore,Voila1/TM3 showed RIvalues that were significantly different from bothVoilaexc-Vb strains (p = 0.002–0.007) at 0.5m. If RI values were relatively stable during the entire 60 min test period for most data points,Voilaexc-Vb—but notVoila1/TM3 heterozygotes and CS—larvae were slightly attracted by NaCl during the first 15 min (data not shown).
Fig. 3.
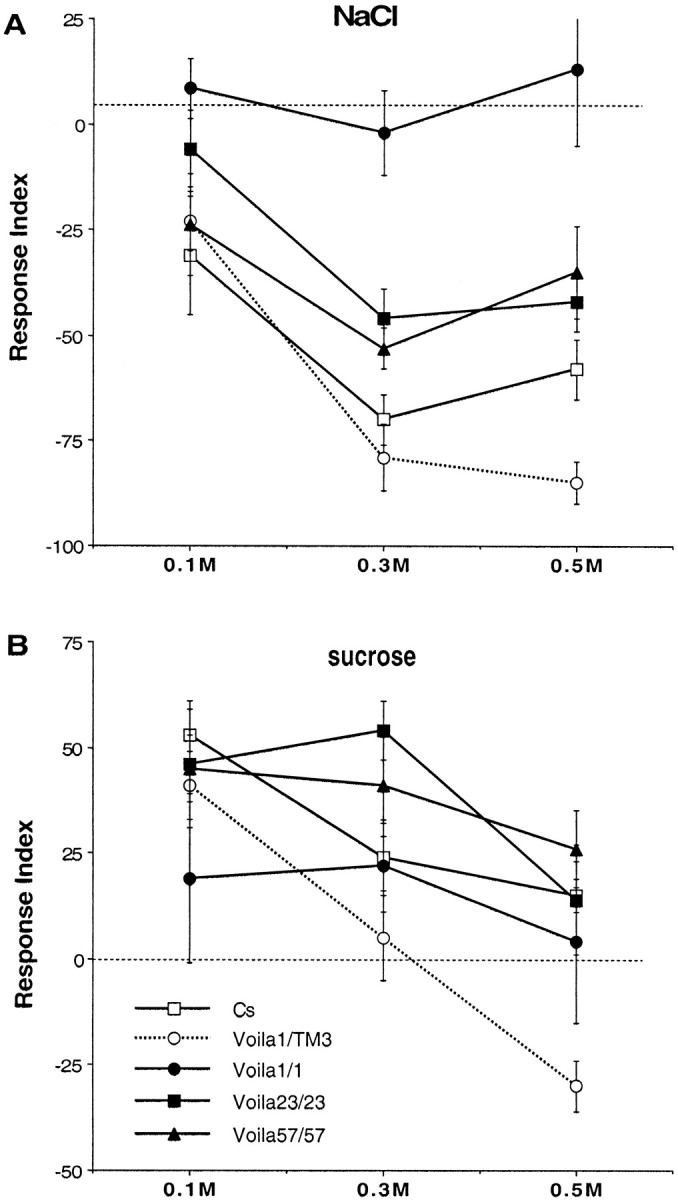
A, B, Mean (and SE) of larval gustatory responses in tests involving different concentrations of NaCl (A) and sucrose (B). For each test, ∼50 second instar larvae were put in the middle of a Petri dish that was divided into two halves containing either neutral agar or agar mixed with the substance to be tested. The numbers of larvae present on each side were noted after 30 min. The response index represents their relative number on each side. Larvae that did not move from the starting point were not taken into account (see Materials and Methods). The dashed linerepresents indifference (RI = 0); negativeRI values on the y-axis represent repulsion, and positive values represent attraction. Each data point represents the mean of 5–16 replicate experiments. Two-way ANOVA revealed a significant effect of genotype and concentration for NaCl and sucrose (respectively, df = 113 and 132;Fgenotype = 21.28 and 6.29;Fconcentration = 16.25 and 14.50; 0.000001 < p < 0.0001), but not of their interaction (F = 1.68 and 1.72;p = 0.10–0.11).
These results show thatVoila1/1 larvae are impaired for their response to salt because, unlike the four other genotypes, they cannot discriminate between NaCl mixed with agar and neutral agar. The PGAL4 transposon is clearly responsible for the gustatory defect ofVoila1/1 larvae because homozygous larvae of bothVoilaexc-Vb strains showed rescued RI values in response to NaCl. Gustatory indifference to salt appears to be recessively controlled by the mutation. However, Voila1/TM3 larvae seem to be more sensitive to 0.5 m NaCl than the larvae from the three other strains. This effect is not caused by the TM3 balancer because TM3/+ larvae did not show abnormal gustatory response (data not shown).
Gustatory behavior toward sucrose
The tests performed with 0.1–0.5 m sucrose suggest that Voila1/1 larvae are barely attracted toward this substance (Fig. 3B). In contrast to the data obtained with NaCl (see above), few significant differences were noted between Voila1/1 and larvae of other genotypes (withVoila1/TM3, at 0.5m: p = 0.011; and with CS, at 0.1m: p = 0.036). In contrast, heterozygous Voila1/TM3 larvae showed very contrasted responses depending on the dose of sucrose: they were repelled by 0.5 m and yielded significant difference with CS and bothVoilaexc-Vb strains (0.00001 < p < 0.002).Voila1/TM3 larvae were indifferent at 0.3 m sucrose, and theirRI values were still significantly lower than that obtained with both Voilaexc-Vb strains (p = 0.001–0.014).
After 15 min of test, significant differences were noted betweenVoila1/1 and the four other genotypes at 0.1 m (0.00001 <p < 0.006), and with bothVoilaexc-Vb strains at 0.3m (p = 0.0001). On the other hand, Voila1/TM3 showed a slight difference with CS at 0.5 m(p = 0.041) but more substantial difference with both Voilaexc-Vb strains at 0.3 and 0.5 m (0.00001< p < 0.006). If the gustatory defect ofVoila1/1 larvae on sucrose was not as strong as that observed with NaCl, the reaction of heterozygoteVoila1/TM3 larvae was more spectacular (if compared with the other genotypes), especially after 30 min of test. The strong aversive response noted with 0.5m was likely caused by a single copy ofVoila1 because TM3/+ larvae were slightly attracted by that concentration of sucrose (data not shown).
Embryonic expression of Voila1
To better understand the developmental lethality and the lack of gustatory discrimination inVoila1/1 larvae, we studied the developmental expression of the PGAL4 lineVoila1, using lacZ and GFP reporter products for visualization.
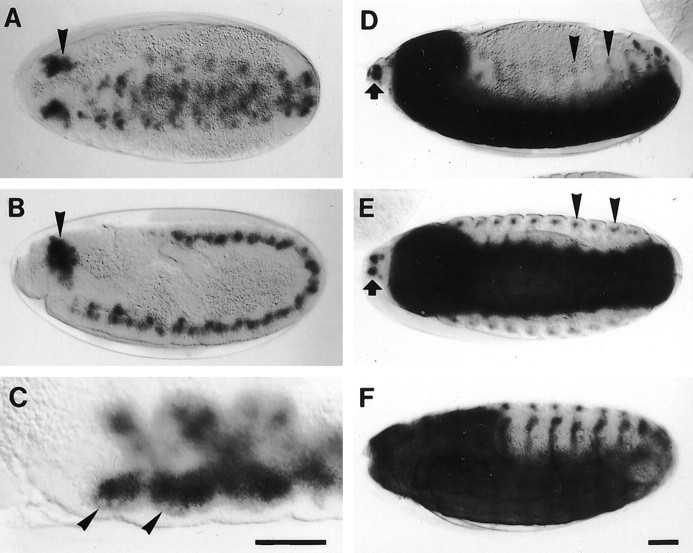
Embryonic lacZ expression inVoila1 was observed for the first time at stage 10, when the stomodeum invaginates (cf.Campos-Ortega and Hartenstein, 1985; Hartenstein, 1993). Staining consisted of diffuse patches in the entire germ band, with the highest intensity in a paired cluster of cells in the head region (Fig.4A,B). At stage 11, large neuroblast-like cells were seen at the periphery of the patches (Fig. 4C). During germ band retraction, the patches became segmental, and staining intensity increased dramatically. From stage 13 onward, strong labeling included three different components: the CNS, twin spots at the anterior tip of the head, and a dorsoventral stripe of cells in each body segment (Fig.4D,E). Whereas the twin spots represent the precursors of the AMC complex, the dorsoventral stripes correspond to developing sensory elements of the body wall. Staining of dorsal, lateral, and ventral cells in both thoracic and abdominal segments suggests that the expression includes different types of sensilla (cf. Campos-Ortega and Hartenstein, 1985). During late stages, staining intensity in the CNS faded significantly (Fig.4F).
Fig. 4.
A–F, Embryonic expression pattern of Voila1visualized by the lacZ reporter. A, B, At embryonic stage 10, staining appears in the entire germ band, with a strongly expressing cluster of cells in the head region (arrowheads). C, At stage 11, large neuroblast-like cells (arrowheads) are seen in each segment. D, E, At subsequent stages (D, stage 13; E, stage 14), strong label appears at three sites: in the CNS, in a segmental, dorsoventral stripe of cells (arrowheads), probably part of the PNS, and in twin spots at the anterior tip of the head (arrows), very likely the precursors of the antennomaxillary complex.F, At stage 16, staining intensity in the CNS begins to fade. Scale bar, 50 μm.
Larval expression
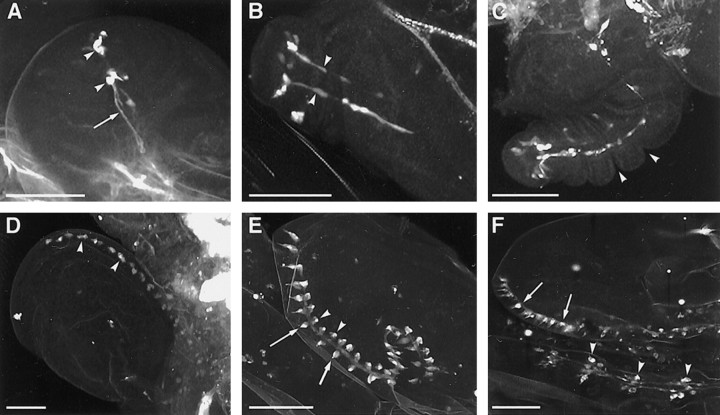
Reporter gene expression in second and third instar larvae ofVoila1 was restricted almost exclusively to chemosensilla and CNS elements. In the periphery, label resided in the AMC and an anterior and a posterior group of pharyngeal gustatory sensilla (Fig.5A,D). In the AMC, dendritic staining extended to the cuticular portion of the gustatory TO (Fig.5B,E,F), suggesting that expression is neuronal. Label was particularly strong in the dorsolateral group of TO sensilla, the afferents of which reach the brain via the larval antennal nerve (Fig. 5E) (Kankel et al., 1980). Weaker staining was sometimes also observed in the second component of the AMC, the olfactory DO, but no dendrites were labeled (Fig. 5B). The third putative gustatory AMC component, the ventral organ (Chu-Wang and Axtell, 1972; Singh and Singh, 1984), did not reveal any expression. On the other hand, many pharyngeal sensilla were stained, including an anterior group behind the mouth hooks (Kankel et al., 1980; Singh and Singh, 1984) and a paired sensillum in the posterior pharyngeal wall (Fig.5A,D). In all of these cases, the label extended to the cuticular pores and also for some distance in the axons. In summary, we interpret GAL4 expression in these gustatory sensilla as neuronal but cannot exclude additional glial or sheath cell expression. In the olfactory DO, expression may be either neuronal, although weak, or restricted to sheath and/or glial cells. In the second larval instar, weak expression occurred in subsets of chordotonal organs.
Fig. 5.
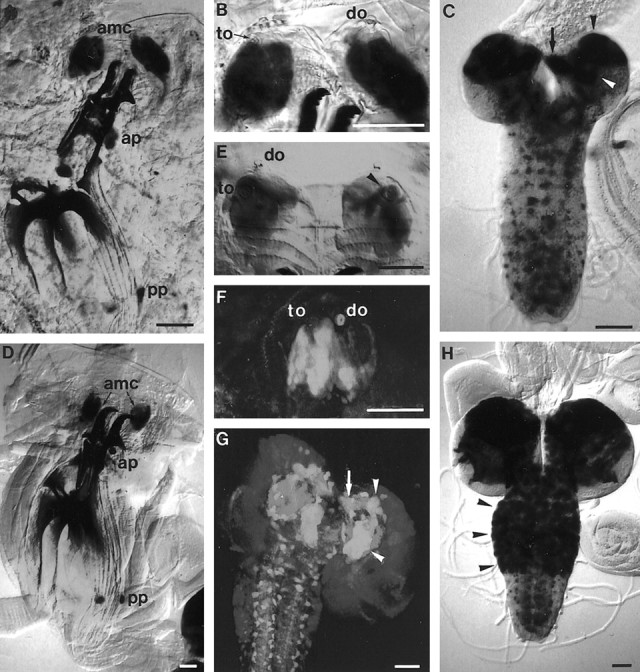
A–H, LarvalVoila1 expression pattern shown with lacZ (A–E, H) and GFP reporters (F, G).A–C, Second larval instar. Expression is seen in the antennomaxillary complex (A,amc) and in an anterior and posterior group of pharyngeal sensilla (A, ap,pp). In the AMC, dendritic staining extends to the gustatory terminal organ (B, to) but not the olfactory dorsal organ (B, do). Intense labeling occurs in the mushroom bodies (C,arrowheads; β/γ lobes, arrow) and in additional scattered cells in the brain and ventral nerve cord.D–H, Third larval instar. Peripheral expression is similar as in second instar (D). Neurons are labeled exclusively in the terminal organ (E, F), including the so-called dorsolateral group of sensilla (E,arrowhead), but not in the dorsal organ. Massive staining occurs in the mushroom bodies (G,arrowheads; β/γ lobes, arrow) and in many cells of the brain and thoracic ganglia (H,arrowheads). The neuropil in G was counterstained by mAb nc82. Scale bar, 50 μm.
Larval GAL4 expression was present also in the brain, subesophageal ganglion (SOG), and ventral nerve cord (Fig.5C,G,H). In the brain, massive expression occurred in the mushroom bodies. The pattern consisted of a large cluster of cells in the dorsal hemispheres, presumably Kenyon cells, and of several distinctive tracts, such as the pedunculus and the α and β/γ lobes (Fig. 5C,G). In the second larval instar, a number of additional cells showed reporter expression in the brain, SOG, and ventral ganglia (Fig. 5C). They extended short processes, suggesting that they might be interneurons. In third instar larvae, the pattern in abdominal ganglia remained unchanged, whereas numbers and staining intensity of elements in the brain and thoracic ganglia increased dramatically (Fig.5H). The labeling of all of these cells prevented tracing of gustatory afferents up to their central target regions.
Pupal expression
Expression in the developing adult peripheral nervous system (PNS) was first observed at puparium formation in leg imaginal disks (Fig.6A). The pattern consisted of two distal clusters of cells with axonal processes. From 2 hr after puparium formation (APF), these processes assembled in two nerves in the elongating leg (Fig.6B,C). In the wing disk, initial expression was seen at 8 hr APF in evenly spaced cell clusters at the anterior wing margin (Fig. 6D). At 16 hr APF, the clusters were clearly revealed as wing sensilla, characterized by dendritic-like processes. The clusters coincided with the known pattern of approximately 30 chemosensilla on the dorsal triple row and approximately 12 chemosensilla on the ventral triple row (cf. Stocker, 1994) (Fig. 6E). Nerve staining in the marginal wing vein indicated that the expression in the sensilla was neuronal. Additional expression in sheath cells remains possible. At 24 hr APF, slightly more than 40 stained elements with dendritic and axonal processes were present on the wing margin, corresponding to the numbers of adult taste bristles (Figs. 6F,7B). In the legs, both strongly and moderately expressing elements were present (Fig.6F). Strongly labeled clusters were similar in numbers to known chemosensory bristles; for example, 10 were within the two distal tarsal segments of mesothoracic or metathoracic legs (Fig.7A) (cf. Nayak and Singh, 1983). Moderate expression was present in future mechanosensory bristles (Fig. 7A), but in contrast to chemosensilla, these components did not exhibit axon-like processes. An additional, weakly stained element was cells on certain wing veins, at the sites of prospective campaniform sensilla (Fig.7B). GAL4 expression in taste bristles and campaniform sensilla was shown to persist during adulthood, whereas expression in mechanosensory bristles is lost (Balakireva et al., 1998).
Fig. 6.
A–F, Confocal images of early pupal Voila1/UAS–GFP expression pattern. A, At puparium formation, expression appears in the third leg disks in two distal clusters of cells (arrowheads) with axon-like processes (arrow). B, At 2 hr APF, two nerves are visible in the elongating leg (arrowheads).C, At 4 hr APF, leg segmentation begins (arrowheads). D, In wing disks, initial expression is seen at 8 hr APF in regularly spaced groups of cells along the anterior margin (arrowheads).E, At 16 hr APF, the clusters of cells are clearly revealed as wing sensilla. Their distribution coincides with the adult pattern of approximately 30 chemosensilla on the dorsal triple row (arrowheads) and approximately 12 chemosensilla on the ventral triple row (arrows). F, At 24 hr APF, the strong staining pattern in legs (arrowheads) and wings (arrows) is reminiscent of the pattern of chemosensilla. Scale bar, 100 μm.
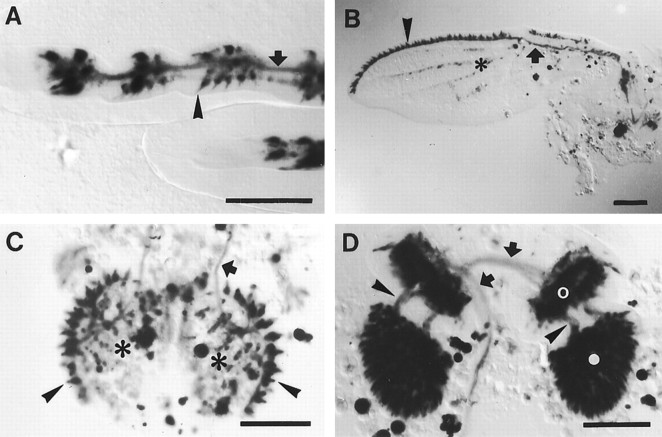
Fig. 7.
A–D,Voila1/UAS–lacZ expression pattern 24 hr after puparium formation. In legs (A), wings (B), and labial palps (C), strong expression is seen in developing gustatory sensilla (arrowheads) and afferent nerves (arrows). In the legs, strongly and moderately stained elements can be distinguished. For example, in the metathoracic leg shown in A, approximately 10 intensely labeled cell clusters are present in the two distalmost tarsal segments (left), corresponding to known numbers of taste bristles. In addition, future mechanosensory bristles exhibit moderate expression (arrowhead). On the wing margin (B), approximately 40 bristle sensilla are intensely stained (arrowhead), reflecting the numbers of adult taste bristles. Weak expression is also present in certain wing veins (B, asterisk) and in the fat body of the palps (C, asterisks).D, The entire second and third antennal segments (open and filled circles, respectively) are strongly labeled. The transient staining of nerves between the antennal segments (arrowheads) and toward the brain (arrows) suggests that at least some olfactory neurons express GAL4 at this stage. Scale bar, 100 μm.
Massive expression occurred also in the developing labial palps and antennae. At 24 hr APF, all prospective taste bristles in the labial palps, including their afferent nerves, were stained (Fig.7C). This pattern is identical to the expression in adult palps (Balakireva et al., 1998). In the antenna, the entire third segment and a ring of cells in the second segment, very likely the developing Johnston's organ, were strongly labeled (Fig.7D). In the adult, weak labeling was reported from the Johnston's organ (Balakireva et al., 1998). Staining of nerves between the antennal segments and further toward the brain suggests that in the mid-pupa at least some of the cells expressing GAL4, including those in olfactory sensilla, are neurons (Fig. 7D). At 48 hr APF, antennal nerve staining disappeared completely (data not shown). In contrast, cellular labeling in the third segment was shown to persist during adulthood, which was interpreted as expression in sheath cells (Balakireva et al., 1998). These observations suggest that inVoila1, differentiating olfactory neurons transiently express GAL4, whereas sheath cell expression in olfactory sensilla persists in the adult fly. Apart from the PNS, numerous cells in the CNS and the fat body were labeled during pupal stages.
DISCUSSION
Reporter gene expression
The expression pattern ofVoila1 is almost identical when comparing lacZ and GFP reporter labels, suggesting that the pattern described here includes all cells that express GAL4 in significant amounts. The most striking attribute ofVoila1 expression in the PNS is its almost complete restriction to gustatory sensilla (for exceptions, see below). This applies to larval and pupal life as well as to the adult stage (Balakireva et al., 1998).
Invariable staining of dendritic and axonal portions in these sensilla shows that GAL4 expression is neuronal, although additional expression in sheath cells cannot be excluded. The intensity of staining in the TO as well as in leg and wing chemosensilla suggests that most, if not all, of the receptor neurons comprising these sensilla express GAL4. However, a small subset of known or suspected taste sensilla remain unlabeled, for example the ventral organ in the larva (Chu-Wang and Axtell, 1972; Singh and Singh, 1984) or, in the adult, labellar taste pegs, a subset of labral sensilla and the dorsal cibarial sense organ (Balakireva et al., 1998). This implies genetic and/or functional differences despite the “common” gustatory function.
In addition to taste sensilla, a number of other sensilla express GAL4 in Voila1, such as the larval DO, mechanosensory bristles during leg formation, olfactory sensilla in the developing antenna, and a few wing campaniform sensilla. However, except in the latter two cases, these elements do not exhibit axonal labeling, which suggests an expression in sheath cells rather than neurons. Alternatively, low level neuronal GAL4 expression remains possible even in nongustatory sensilla. It will be interesting to combine Voila1 with mutant genes involved in the specification of the sensory system [such aspoxn (Nottebohm et al., 1994)] to obtain clues about the possible developmental role(s) ofVoila1.
GAL4 expression in sensory neurons begins very early during differentiation. For example, in leg disks, expression in two distal clusters of neurons and in corresponding nerves extending toward the leg base is already visible at pupariation. This pattern is reminiscent of a set of premetamorphic neurons that may serve as afferent pioneers (Jan et al., 1985; Tix et al., 1989). Whether they are still functional in the adult is not known. In the wing margin, we observe labeled cell clusters at 8 hr APF and axons at 16 hr APF, a time course that corresponds to earlier reports of neuronal differentiation (Murray et al., 1984). The expression pattern corresponds from the very beginning to the known pattern of taste sensilla. Mechanosensory wing bristles are never stained, but those on the legs show transient moderate staining, which disappears again during late pupal life. Also, the second and third antennal segments contain many stained cells, especially during development. At 24 hr APF, labeling of nerves between the antennal segments and toward the brain implies that at least some of the expression in the third segment (and perhaps in the second) is neuronal. Later on, axonal staining disappears again, but intense expression in the third antennal segment persists in the adult (Balakireva et al., 1998).
In conclusion, with the exception of a few wing campaniform sensilla (see above), the only mature sensilla that show neuronal expression inVoila1 are gustatory. In mature olfactory sensilla on the antenna and maxillary palp, GAL4 expression appears to be localized in sheath cells or glial cells (or in neurons at low levels). This spatiotemporal pattern of expression suggests a bifunctional neuronal role of an underlying gene or genes, apart from possible roles in associated cells. On the one hand, such a gene may be involved in the maturation of gustatory and olfactory neurons, and on the other it may directly assist in gustation. The latter role is convincingly demonstrated by the recessive gustatory mutant effects ofVoila1 and is further supported by dominant courtship effects that may be caused by defects in pheromonal detection (Balakireva et al., 1998).
The CNS expression of Voila1 is widespread, and it remains unclear whether the labeled neural tissues correspond to taste function. In the larval CNS, the structures that show the strongest expression include the mushroom bodies, the SOG, and the ventral ganglia. Interestingly, adult flies show a particularly strong expression in the same three structures. It is possible that part of the larval and adult CNS staining corresponds to the primary projection of gustatory afferents, yet the widespread GAL4 expression prevented us from further analyzingVoila1 patterns at that level.
Possible function of Voila in gustation during development
Among the Drosophila chemosensory mutants that have been described, the great majority exhibit olfactory defects. In some cases, olfactory anomalies have been shown to affect both larval and adult development (Carlson, 1996). Conversely, mutations that specifically alter gustation have been described much less frequently (Isono and Kikuchi, 1974; Falk and Atidia, 1975; Tompkins et al., 1979), and very few of them are directly related to a defect in the gustatory nervous system (Rodrigues et al., 1995).Voila1 is a rare example of a genetic variant that alters gustation and is simultaneously expressed in the gustatory system at both premetamorphic and postmetamorphic stages. We have not yet tested the olfactory response ofVoila1.
We previously hypothesized that the hyperexcitability observed in adult heterozygous Voila1/TM3 males was caused by defective expression of Voila in a neural center (mushroom bodies), whereas the ectopic expression of the UAS-transformer transgene in the gustatory sensory neurons was responsible for altered male pheromonal perception (Balakireva et al., 1998). The present study indicates thatVoila1 is also involved in larval gustation of NaCl and sucrose.Voila1 creates a dose-dependent effect on larval gustation because homozygous larvae were indifferent to NaCl, whereas heterozygousVoila1/TM3 larvae showed increased avoidance response toward both substances at 0.5m, as compared with the CS strain. Rescue of the gustatory defect toward NaCl was obtained after removal of the PGAL4 transposon: homozygous larvae from bothVoilaexc-Vb strains were repelled by NaCl. We need to investigate the relationship between the number of Voila1 copies and the specificity of defective gustatory phenotype.
The fact that remobilization of PGAL4 simultaneously rescued larval gustation and adult viability suggests that both anomalies are caused by the same transposon. We are currently investigating the causal relation between both phenotypes. Two preliminary observations support a causal link: (1) the reduced size ofVoila1/1 larvae and pupae (Fig.1), which could be caused by abnormal gustation and starvation of homozygous wandering larvae, and (2) their frequency of reaching, but not of surviving, during adulthood that can be slightly increased by selecting more favorable environmental conditions (25°C, transfer to fresh food). Cloning and characterization of the gene(s) responsible will help elucidate the correct hypothesis.
The fact that the differentVoilaexc-Lt strains exhibit very different patterns of developmental lethality suggests thatVoila has a complex effect, rather than an all-or-none function, on survival during development. After the genetic approach presented here, we are currently performing the molecular dissection of the Voila locus with a set ofVoilaexc strains. Preliminary data indicate that rescue of developmental defects does not overlap with the rescue of behavioral anomalies observed in adult male flies (Y. Grosjean, M. Balakireva, and J-F. Ferveur, unpublished observations). It will be very interesting to determine whether preimaginal and adult phenotypes are encoded and regulated by different molecular sequences. However, severalVoilaexc-Lt strains that were surveyed for their GAL4 pattern of expression did not show any qualitative difference, although changes in intensity cannot be formally excluded (N. Gendre and R. Stocker, unpublished observations).
This study shows that theVoila1 strain is very useful for specifically manipulating taste sensory organs during preimaginal development because of its limited GAL4 expression in the periphery. New PGAL4 strains make it possible to manipulate different subsets of chemosensory neurons in living larvae and flies (Heimbeck et al., 1999). Simultaneous use of various secondary reporters, in particular vital transgenic markers (such as UAS–GFP or UAS–GFPS65T) (Brand, 1995; Cubitt et al., 1995; Yeh et al., 1995), makes it possible to visualize the patterns of expression in these groups of neurons during all phases of development. The combined use of these genetic tools, together with the molecular elucidation of the identity of Voila, will help us to better understand how taste sensitivity arises during development and how it differs from olfaction.
Footnotes
This work was supported by grants from the Human Frontier Science Program (RG-93/94 B) to J.F.F. and R.S., from the Burgundy Region to M.B., and from the Swiss National Funds (31-42053.94 and 31-52639.97) to R.S. We are very grateful to Dr. Gertrud Heimbeck for help with embryonic staging, to Yaël Grosjean for unpublished data, and to Laurence Dartevelle for technical help. Christine Dambly-Chaudière, Matthew Cobb, and two anonymous reviewers are thanked for their comments on this manuscript.
M.B. and N.G. contributed equally to this work.
Correspondence should be addressed to Jean-François Ferveur, Unité de Recherche 5548 Associée au Centre National de la Recherche Scientifique, Faculté des Sciences, Université de Bourgogne, 6 Boulevard Gabriel, 21 000 Dijon, France. E-mail:jean-francois.ferveur@u-bourgogne.fr.
REFERENCES
- 1.Ashburner M. Drosophila. A laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 2.Ayer R, Carlson J. acj6: a gene affecting olfactory physiology and behavior in Drosophila. Proc Natl Acad Sci USA. 1991;88:5467–5471. doi: 10.1073/pnas.88.12.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balakireva M, Stocker RF, Gendre N, Ferveur JF. Voila, a new Drosophila courtship variant that affects the nervous system: behavioral, neural and genetic characterization. J Neurosci. 1998;18:4335–4343. doi: 10.1523/JNEUROSCI.18-11-04335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balmer D. Isolation von zellspezifisch exprimierenden Gal4-Insertionslinien bei Drosophila melanogaster, unter besonderer Berücksichtigung des chemosensorischen Systems. Diploma thesis. University of Fribourg; 1994. [Google Scholar]
- 5.Brand AH. GFP in Drosophila. Trends Genet. 1995;11:324–325. doi: 10.1016/s0168-9525(00)89091-5. [DOI] [PubMed] [Google Scholar]
- 6.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 7.Busto M, Iyengar B, Campos AR. Genetic dissection of behavior: modulation of locomotion by light in the Drosophila melanogaster larva requires genetically distinct visual system functions. J Neurosci. 1999;19:3337–3344. doi: 10.1523/JNEUROSCI.19-09-03337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. Springer; New York: 1985. [Google Scholar]
- 9.Carlson JR. Olfaction in Drosophila: from odor to behavior. Trends Genet. 1996;12:175–180. doi: 10.1016/0168-9525(96)10015-9. [DOI] [PubMed] [Google Scholar]
- 10.Chu-Wang IW, Axtell RC. Fine structure of the ventral organ of the house fly larva, Musca domestica L. Z Zellforsch. 1972;130:489–495. doi: 10.1007/BF00307003. [DOI] [PubMed] [Google Scholar]
- 11.Clyne PJ, Certel SJ, de Bruyne M, Zaslavsky L, Johnson WA, Carlson JR. The odor specificities of a subset of olfactory receptor neurons are governed by acj6, a POU-domain transcription factor. Neuron. 1999;22:339–347. doi: 10.1016/s0896-6273(00)81094-6. [DOI] [PubMed] [Google Scholar]
- 12.Connolly JB, Roberts IJH, Armstrong JD, Kaiser K, Forte M, Tully T, O'Kane CJ. Associative learning disrupted by impaired GS signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- 13.Cooley L, Kelley R, Spradling A. Insertional mutagenesis of the Drosophila genome with single P elements. Science. 1988;239:1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- 14.Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY. Understanding, improving and using green fluorescent proteins. Trends Biochem Sci. 1995;20:448–455. doi: 10.1016/s0968-0004(00)89099-4. [DOI] [PubMed] [Google Scholar]
- 15.Deak P, Omar MM, Saunders RD, Pal M, Komonyi O, Szidonya J, Maroy P, Zhang Y, Ashburner M, Benos P, Savakis C, Siden-Kiamos I, Louis C, Bolshakov VN, Kafatos FC, Madueno E, Modolell J, Glover DM. P-element insertion alleles of essential genes on the third chromosome of Drosophila melanogaster: correlation of physical and cytogenetic maps in chromosomal region 86E–87F. Genetics. 1997;147:1697–1722. doi: 10.1093/genetics/147.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falk R, Atidia J. A mutation affecting taste perception in Drosophila melanogaster. Nature. 1975;254:325–326. doi: 10.1038/254325a0. [DOI] [PubMed] [Google Scholar]
- 17.Ferveur JF, Störtkuhl KF, Stocker RF, Greenspan RJ. Genetic feminization of brain structures and changed sexual orientation in male Drosophila melanogaster. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- 18.Ghysen A, O'Kane C. Neural enhancer-like elements as specific cell markers in Drosophila. Development. 1989;105:35–52. doi: 10.1242/dev.105.1.35. [DOI] [PubMed] [Google Scholar]
- 19.Hartenstein V. Atlas of Drosophila development. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1993. [Google Scholar]
- 20.Heimbeck G, Bugnon V, Gendre N, Häberlin C, Stocker RF. Smell and taste perception in Drosophila melanogaster larva: toxin expression studies in chemosensory neurons. J Neurosci. 1999;19:6599–6609. doi: 10.1523/JNEUROSCI.19-15-06599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isono K, Kikuchi T. Autosomal recessive mutation in sugar response of Drosophila. Nature. 1974;24:243–244. doi: 10.1038/248243a0. [DOI] [PubMed] [Google Scholar]
- 22.Jan YN, Jan LY. The peripheral nervous system. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1993. pp. 1207–1244. [Google Scholar]
- 23.Jan YN, Ghysen A, Barbel CI, Jan LY. Formation of neuronal pathways in the imaginal discs of Drosophila melanogaster. J Neurosci. 1985;5:2453–2464. doi: 10.1523/JNEUROSCI.05-09-02453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kankel DR, Ferrus A, Garen SH, Harte PJ, Lewis PE. The structure and development of the nervous system. In: Ashburner M, Wright TRF, editors. The genetics and biology of Drosophila, Vol 2. Academic; New York: 1980. pp. 295–368. [Google Scholar]
- 25.Laissue PP, Reiter C, Hiesinger PR, Halter S, Fischbach KF, Stocker RF. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J Comp Neurol. 1999;405:543–552. [PubMed] [Google Scholar]
- 26.Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. Academic; New York: 1992. [Google Scholar]
- 27.McKenna M, Monte P, Helfand S, Woodard C, Carlson J. A simple chemosensory response in Drosophila and the isolation of acj mutants in which it is affected. Proc Natl Acad Sci USA. 1989;86:8118–8122. doi: 10.1073/pnas.86.20.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meinertzhagen IA, Hanson TA. The development of the optic lobe. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1993. pp. 1363–1492. [Google Scholar]
- 29.Murray MA, Schubiger M, Palka J. Neuron differentiation and axon growth in the developing wing of Drosophila melanogaster. Dev Biol. 1984;104:259–273. doi: 10.1016/0012-1606(84)90082-4. [DOI] [PubMed] [Google Scholar]
- 30.Nayak SV, Singh RN. Sensilla on the tarsal segments and mouth parts of adult Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int J Insect Morphol Embryol. 1983;12:273–291. [Google Scholar]
- 31.Nottebohm E, Usui A, Therianos S, Kimura KI, Dambly-Chaudière C, Ghysen A. The gene poxn controls different steps of the formation of chemosensory organs in Drosophila. Neuron. 1994;12:25–34. doi: 10.1016/0896-6273(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 32.Oppliger FY, Guerin PM, Vlimant M. Neurophysiological and behavioural evidence for an olfactory function for the dorsal organ and a gustatory one for the terminal organ in Drosophila melanogaster larvae. J Insect Physiol. 2000;46:135–144. doi: 10.1016/s0022-1910(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 33.Reuter G, Gausz J, Gyurkovics H, Friede B, Bang R, Spierer A, Hall LMC, Spierer P. Modifiers of position-effect variegation in the region from 86C to 88B of the Drosophila melanogaster third chromosome. Mol Gen Genet. 1987;210:429–436. doi: 10.1007/BF00327193. [DOI] [PubMed] [Google Scholar]
- 34.Riesgo-Escovar J, Woodard C, Gaines P, Carlson J. Development and organization of the Drosophila olfactory system: an analysis using enhancer traps. J Neurobiol. 1992;23:947–964. doi: 10.1002/neu.480230803. [DOI] [PubMed] [Google Scholar]
- 35.Robertson HM, Preston CR, Phillis RW, Johnson-Schiltz DM, Benz WK, Engels WR. A stable source of P-element transposase in Drosophila melanogaster. Genetics. 1988;118:461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigues V, Cheah PY, Ray K, Chia W. malvolio, the Drosophila homologue of mouse NRAMP-1 (Bcg), is expressed in macrophages and in the nervous system and is required for normal taste behavior. EMBO J. 1995;14:3007–3020. doi: 10.1002/j.1460-2075.1995.tb07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh RN, Singh K. Fine structure of the sensory organs of Drosophila melanogaster Meigen larva (Diptera: Drosophilidae). Int J Insect Morphol Embryol. 1984;13:255–273. [Google Scholar]
- 38.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 39.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 40.Tix S, Bate M, Technau GM. Pre-existing neuronal pathways in the leg imaginal discs of Drosophila. Development. 1989;107:855–862. doi: 10.1242/dev.105.4.739. [DOI] [PubMed] [Google Scholar]
- 41.Tompkins L, Cardosa M, White FV, Sanders TG. Isolation and analysis of chemosensory behavior mutant in Drosophila melanogaster. Proc Natl Acad Sci USA. 1979;76:884–887. doi: 10.1073/pnas.76.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Truman JW, Taylor BJ, Awad TA. Formation of the adult nervous system. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1993. pp. 1245–1276. [Google Scholar]
- 43.Williams DW, Shepherd D. Persistent larval sensory neurons in adult Drosophila melanogaster. J Neurobiol. 1999;39:275–286. [PubMed] [Google Scholar]
- 44.Wilson C, Pearson RK, Bellen HJ, O'Kane CJ, Grossniklaus U, Gehring WJ. P-element-mediated enhancer detection: an efficient method for isolation and characterizing developmentally regulated genes in Drosophila. Genes Dev. 1989;3:1301–1313. doi: 10.1101/gad.3.9.1301. [DOI] [PubMed] [Google Scholar]
- 45.Yeh E, Gustafson K, Boulianne GL. Green fluorescent protein as a vital marker and reporter of gene expression in Drosophila. Proc Natl Acad Sci USA. 1995;92:7036–7040. doi: 10.1073/pnas.92.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]