Abstract
Using in situ hybridization and immunoblot analysis, the present studies identified Gz mRNA and Gz-protein in the hypothalamic paraventricular nucleus. The role of Gz-proteins in hypothalamic 5-HT1Areceptor signaling was examined in vivo. Activation of 5-HT1A receptors increases the secretion of oxytocin and ACTH, but not prolactin. Intracerebroventricular infusion (3–4 d) of Gz antisense oligodeoxynucleotides, with different sequences and different phosphorothioate modification patterns, reduced the levels of Gz-protein in the hypothalamic paraventricular nucleus, whereas missense oligodeoxynucleotides had no effect. Neither antisense nor missense oligodeoxynucleotide treatment altered basal plasma levels of ACTH, oxytocin, or prolactin, when compared with untreated controls. An antisense-induced decrease in hypothalamic Gz-protein levels was paralleled by a significant decrease in the oxytocin and ACTH responses to the 5-HT1A agonist 8-hydroxy-dipropylamino-tetralin (8-OH-DPAT). In contrast, the prolactin response to 8-OH-DPAT (which cannot be blocked by 5-HT1A antagonists) was not inhibited by Gz antisense oligodeoxynucleotides. Gz-proteins are the only members of the Gi/Go-protein family that are not inactivated by pertussis toxin. In a control experiment, pertussis toxin treatment (1μg/5 μl, i.c.v.; 48 hr before the 8-OH-DPAT challenge) did not inhibit the ACTH response, potentiated the oxytocin response, and eliminated the prolactin response to 8-OH-DPAT. Thus, pertussis toxin-sensitive Gi/Go-proteins do not mediate the 5-HT1A receptor-mediated increase in ACTH and oxytocin secretion. Combined, these studies provide the firstin vivo evidence for a key role of Gz-proteins in coupling hypothalamic 5-HT1Areceptors to effector mechanisms.
Keywords: serotonin, receptor, oxytocin, ACTH, prolactin, G–protein, signaling, signal transduction, hormones, neuroendocrine, hypothalamus, paraventricular
Heterotrimeric guanine-nucleotide-binding proteins (G-proteins) transduce extracellular signals to intracellular messengers. Gz-protein is a member of the Gi-protein family with a 41–67% of sequence homology with Gi- and Go-proteins (Fong et al., 1988; Matsuoka et al., 1988). Unlike the other members of the Gi-protein family, Gz-protein lacks the cysteine residue in the C terminus that serves as the substrate for pertussis toxin-catalyzed ADP ribosylation (Casey et al., 1990). This lack of a cysteine residue makes Gz-protein the only member of the Gi/o-protein family known to be insensitive to pertussis toxin and provides a valuable means to identify the involvement of Gz-protein in physiological responses. In vitro studies indicate that 5-HT1A receptors are coupled to members of the Gi/Go-protein family, including the pertussis toxin-sensitive Gi1-, Gi2-, Gi3-, and Go-proteins, and the pertussis toxin-insensitive Gz-protein (Butkerait et al., 1995; Albert et al., 1996; Barr et al., 1997). Gz-protein is involved in 5-HT1A receptor-mediated inhibition of adenylyl cyclase in Sf9 cells (Butkerait et al., 1995; Barr et al., 1997). In spite of extensive in vitro studies, little is known about the nature of the receptors that couple to Gz-proteins in vivo. It is still unknown which G-proteins couple hypothalamic 5-HT1A receptors to the secretion of ACTH and oxytocin. The present studies investigated the possible coupling of Gz-proteins to 5-HT1Areceptor signaling in the hypothalamus in vivo.
Neuroendocrine challenges provide a noninvasive approach to study the responsivity of hypothalamic postsynaptic 5-HT1Areceptors in vivo (Cowen, 1998). Serotonergic nerve terminals make synaptic connections with oxytocin and corticotropin releasing hormone (CRH)-containing neurons in the paraventricular nucleus of the hypothalamus (Liposits et al., 1987; Saphier, 1991;Kawano et al., 1992). Neurons in the paraventricular nucleus express 5-HT1A receptors (Li et al., 1997a). Activation of 5-HT1A receptors by a 5-HT1A agonist, such as 8-hydroxy-dipropylamino-tetralin (8-OH-DPAT), induces an increase in oxytocin and CRH secretion (Calogero et al., 1989; Bagdy, 1996). CRH subsequently stimulates the secretion of ACTH from the pituitary gland into the circulation. The ACTH and oxytocin responses to 8-OH-DPAT can be inhibited by the selective 5-HT1A antagonist WAY-100,635 (Vicentic et al., 1998). In contrast with ACTH and oxytocin, the prolactin response to 8-OH-DPAT is not blocked by 5-HT1A antagonists, indicating that prolactin secretion induced by 8-OH-DPAT is mediated by other receptors (Aulakh et al., 1988; Vicentic et al., 1998). Therefore, in the present studies, plasma ACTH and oxytocin levels were used as peripheral indices of hypothalamic 5-HT1Areceptor function. As a negative control, ACTH and oxytocin responses to 8-OH-DPAT were compared with that of prolactin.
Initially, the expression of Gz mRNA and Gz-protein in the hypothalamic paraventricular nucleus was examined using in situ hybridization and immunoblot analysis, respectively. Subsequently, two approaches were used to examine the functional role of Gz-proteins in 5-HT1Areceptor-mediated hormone secretion: (1) an antisense strategy to specifically reduce the level of Gz-protein in the hypothalamic paraventricular nucleus and (2) intracerebroventricular pretreatment with pertussis toxin. In both experiments, rats were challenged with the 5-HT1Aagonist 8-OH-DPAT to test whether the physiological responses triggered by activation of hypothalamic 5-HT1A receptors are mediated by Gz-protein.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats (225–275 gm) were purchased from Harlan (Indianapolis, IN). The rats were housed two per cage in lighting- (12 hr light/dark: lights on at 7:00 A.M.), humidity-, and temperature-controlled conditions. Food and water were availablead libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by the Loyola University Institutional Animal Care and Use Committee.
Drugs
HPLC-purified oligodeoxynucleotides were purchased from Genosys Biotechnologies (The Woodlands, TX). Pertussis toxin was purchased from Sigma (St. Louis, MO). (±)8-OH-DPAT HBr was purchased from Research Biochemicals (Natick, MA). All drugs were dissolved in saline. Pertussis toxin was intracerebroventricularly injected in a volume of 5 μl.
Two experiments with antisense oligodeoxynucleotides were designed because the first experiment revealed a nonspecific toxicity of the phosphorothioate Gz antisense oligodeoxynucleotides. In the first experiment, the sequence of Gαz antisense corresponding to nucleotide sequence 308–323 was 5′-TCGTAGGCACGGTCA, and the sequence for missense was 5′-CTGCAGGTATGGCTA. These 15 mer oligodeoxynucleotides were phosphorothioate-modified at all positions to delay their degradation and were infused using Alzet osmotic minipumps for 3 consecutive days. Because the first batch of antisense oligodeoxynucleotide produced a reduction in Gz-, Gi1- and Gi2-protein levels in the paraventricular nucleus, in the second experiment, the (33 base) Gαz antisense oligodeoxynucleotide was modified to correspond to nucleotide sequence 330–363. The sequence was 5′-CGTGATCTCACCCTTGCTCTCTGCCGGGCCAGT-3′, and the oligodeoxynucleotide was only phosphorothioate-modified at the two bases of each end (5′-CG and GT-3′) (Sanchez-Blazquez et al., 1995). The sequence of the missense oligodeoxynucleotide was 5′-CCCTTATTTACTTTCGCC-3′, and it was phosphorothioate modified at positions 5′-CC and GC-3′ (Sanchez-Blazquez et al., 1995). In all experiments, saline or 8-OH-DPAT were injected subcutaneously in a volume of 1 ml/kg.
Surgery
After several days of adaptation to their home cage, intracerebroventricular cannulae were implanted in the rats by stereotaxic surgery. For the Gz antisense experiment, rats were anesthetized with xylazine (6.7 mg/kg)-ketamine (100 mg/kg). An L-shaped cannula (Plastics One, Roanoke, VA) was implanted into the third cerebral ventricle at stereotaxic coordinates 1.5 mm caudal and 0.0 mm lateral with respect to bregma and 9.5 mm ventral from the skull surface. Positioning the tip of the cannula in the third ventricle instead of inside the paraventricular nucleus was intended to spare the neurons from mechanical damage. Damage to hypothalamic neurons would produce a reduced hormone response to the 5-HT1A agonist, which could be misinterpreted as a reduction in receptor coupling. A stainless steel obturator ensured that the cannula would stay patent. The cannulae were kept in place by dental cement attached to miniscrews embedded in the skull. The rats received a subcutaneous injection of saline (1 ml) and ampicillin (50 mg/kg, s.c.) after surgery to prevent dehydration and infection, respectively, and were returned to their home cage. The position of the cannula tracks was carefully verified in each animal when the brains were subsequently sectioned in a cryostat. The procedures for the pertussis toxin experiment were similar except that a cannula was implanted in the lateral cerebral ventricle (0.5 mm caudal, 1.4 mm lateral from bregma and 4.5 mm ventral to the surface of the skull).
Experimental protocols
Gz antisense experiment
At least 10 d after cannula implantation, rats were randomly assigned to groups that received either missense or antisense oligodeoxynucleotides. Untreated animals were used as controls to verify for nonselective toxicity of the nucleotides.
First antisense experiment. Osmotic mini-pumps (Alzet model 1003; Alza, Palo Alto, CA) were used for chronic microinfusions of the 15 mer phosphorothioate modified Gαzantisense oligodeoxynucleotides (0.426 μg/μl; 0.093 nmol/μl), or missense oligodeoxynucleotides (0.426 μg/μl; 0.095 nmol/μl) into the third ventricle, allowing a sustained delivery at a rate of 1 μl/hr for 3 d. The osmotic minipumps were implanted subcutaneously between the scapulae under halothane anesthesia and connected to the cannulae with silicone rubber tubing (Plastics One). The incisions were closed with sterile clips, and the rats were returned to their home cage. On the fourth day, the rats received an injection of either saline (1 ml/kg, s.c.) or 8-OH-DPAT (50 μg/kg, s.c.) and were decapitated 15 min after the injection. Trunk blood was collected in tubes containing 0.5 ml of a 0.3 m EDTA, pH 7.4, solution and centrifuged at 4°C. Plasma aliquots were stored at −70°C until they were used for hormone radioimmunoassays. The brains were removed and rapidly frozen with dry ice, then stored at −70°C for the microdissection of the hypothalamic paraventricular nucleus.
Second antisense experiment. Osmotic minipumps (Alzet model 2001; Alza) were used for chronic microinfusions of Gαz antisense oligodeoxynucleotides or missense oligodeoxynucleotides (0.2083 nmol/μl) into the third ventricle, allowing a sustained delivery at a rate of 1 μl/hr for 4 d. The dose was increased approximately twofold (compared with the first experiment) to compensate for the higher vulnerability to degradation of the nucleotides, which are only modified at the two bases on each end of the molecule. The osmotic minipumps were implanted between the scapulae under halothane anesthesia and connected to the cannulae with silicone rubber tubing (Plastics One). The incisions were closed with sterile clips, and the rats were returned to their home cage. On the fifth day, the rats received an injection of either saline (1 ml/kg, s.c.) or 8-OH-DPAT (50 μg/kg, s.c.) and were decapitated 15 min after the injection. Trunk blood was collected as described for experiment 1, and plasma aliquots were stored at −70°C until they were used for hormone radioimmunoassays. The brains were removed and rapidly frozen with dry ice, then stored at −70°C for the microdissection of the hypothalamic paraventricular nucleus.
Pertussis toxin experiment
At least 10 d after surgical implantation of the cannulae, the rats received 5 μl (intracerebroventricularly) of either saline or pertussis toxin (0.2 μg/μl; total of 1 μg/5 μl) through the cannulae and were returned to their home cage. Forty-eight hours later, the rats were challenged with saline or 8-OH-DPAT (50 μg/kg, s.c.) and were decapitated 15 min after the injection. Trunk blood was collected in tubes containing 0.5 ml of a 0.3 m EDTA, pH 7.4, solution and centrifuged at 4°C. Plasma aliquots were stored at −70°C until they were used for hormone radioimmunoassays.
Molecular and biochemical assays
In situ hybridization
Gz mRNA was examined in cells in the hypothalamic paraventricular nucleus using in situhybridization. The brain was removed and blocked, frozen, and stored at −70°C. Ten-micrometer-thick sections were cut on a cryostat and then fixed in 4% paraformaldehyde at 4°C. Using previously described methods (Muma et al., 1990), tissue sections (10 μm) were rinsed and then acetylated in 0.25% acetic anhydride in 0.1m triethanolamine hydrochloride. After dehydration, tissue sections were hybridized with35S-labeled cDNA probes overnight at 37°C. The cDNA probe for Gz was a generous gift from Dr. Yoshio Kaziro (Tokyo Institute of Technology, Tokyo, Japan). The hybridization buffer consisted of 50% formamide, 4× SSPE, Denhardt's solution, 200 μg/ml ssDNA and tRNA, and 20 mm dithiothreitol. Probes were labeled using a Nick translation kit (Boehringer Mannheim, Mannheim, Germany). Slides were washed in 1× SSC (150 mm sodium chloride and 15 mm sodium citrate) at 50°C, dried, and dipped in NTB2 (Eastman Kodak, Rochester, NY) nuclear emulsion. After incubation for 3–4 weeks, silver grains were developed, fixed, and sections were stained with cresyl violet.
Immunoblot analysis
Tissue dissection. Gαz-, Gαi1-, and Gαi2-protein levels were measured in micropunches of the paraventricular nucleus of the hypothalamus. Rat brains were placed in a cryostat at −10°C, and coronal sections were cut to obtain a 700 μm section containing the paraventricular nucleus. Using the diagram in Figure1, the paraventricular nucleus was microdissected from this section with the aid of a stereomicroscope.
Fig. 1.
Diagrammatic representation of the microdissection of the hypothalamic paraventricular nucleus from coronal sections (700 μm). The landmarks used to identify the coronal section are those seen at 1.8 mm caudal to bregma according to Paxinos and Watson (1986).F, Fornix; PVN, paraventricular nucleus of the hypothalamus.
Protein fractionation. All procedures were conducted at 4°C unless otherwise indicated. Briefly, the tissues were homogenized in 0.5 ml of a 50 mm Tris buffer, pH 7.4, containing 150 mm NaCl, 10% sucrose, and 0.5 mmphenylmethanesulfonyl fluoride (PMSF). After centrifugation at 20,000 × g for 60 min, the pellets (containing the membrane-bound proteins) were solubilized in a 20 mm Tris buffer, pH 8, containing 1 mm EDTA, 100 mm NaCl, 0.1% sodium cholate, and 1 mm dithiothreitol in a ratio of 3 μl of buffer per milligram of tissue. The resuspended homogenates were incubated and shaken for 1 hr, followed by centrifugation at 100,000 × g for 60 min. The supernatant (containing the membrane-bound G-proteins) was collected and stored for the determination of membrane-bound G-protein levels. Protein concentrations were measured according to Lowry et al. (1951)using bovine serum albumin as a standard.
Quantification of G-proteins. The solubilized proteins (1.6 μg/lane) were resolved by SDS-PAGE, using 0.75-mm-thick Tris-glycine denaturing reducing gels, containing 0.1% SDS, 12.5% acrylamide/bisacrylamide (30:0.2), 4.6 m urea, and 375 mm Tris, pH 8.7 (Mullaney and Miligan, 1990). The proteins were then electrophoretically transferred for 2 hr to nitrocellulose membranes, which were then allowed to dry. The membranes were incubated at room temperature in a solution containing 5% nonfat dry milk, 0.05% NP-40, 50 mm Tris, and 150 mmNaCl, pH 7.4, for 1 hr and were then washed. The membranes were incubated with polyclonal antisera for Gz (I-20; Santa Cruz Biotechnology, Santa Cruz, CA; 1:6000 dilution) or Gi1/2 (AS/7; DuPont NEN, Boston, MA; 1:2500 dilution), at 4°C overnight. Membranes were then incubated at room temperature with a secondary antibody (goat anti-rabbit serum; Organon Teknika Cappel, Durham, NC; 1:5000 dilution) for 60 min. After four washes with 0.05% NP-40 in 50 mm Tris and 150 mm NaCl, the membranes were incubated with rabbit peroxidase–antiperoxidase (Organon Teknika Cappel; 1:10,000) for 1 hr. The membranes were incubated with the ECL chemiluminescence substrate solution (Amersham, Arlington Heights, IL) for 1 min and then exposed to Kodak x-ray film for 10–60 sec.
G-protein data analysis. Films were analyzed densitometrically using NIH Image (version 1.57) for Macintosh computers. The gray scale density readings were calibrated using a transmission step wedge standard. The integrated optical density (IOD) of each band was calculated as the sum of optical densities of all the pixels within the area of the band outlined. An area adjacent to the G-protein bands was used to calculate the background optical density of the film. The IOD for the film background was subtracted from the IOD for each band. The resulting IOD for each G-protein band was then divided by the amount of protein loaded on the corresponding lane. Three samples from each treatment group and three randomly selected control samples were loaded on each gel. Each sample was measured on three independent gels. To control for intergel variability, a mean IOD per microgram of protein was obtained from the three controls on each gel. To determine the relative amounts of the G-proteins per sample, the IOD per microgram of protein value for each sample was divided by the mean IOD per microgram of protein value obtained from control samples on the same gel. Data for each rat were expressed as “% of control.” Because tissue samples were measured on three independent gels, the mean % of control obtained from the three gels represented the data for each rat.
Radioimmunoassays
Plasma ACTH and prolactin were measured by radioimmunoassays as detailed previously (Li et al., 1993). ACTH antiserum was purchased from IgG Corporation (Nashville, TN). ACTH (1–39) standards were obtained from Calbiochem (San Diego, CA). Bovine serum albumin and aprotinin were purchased from Sigma. Normal rabbit serum and goat anti-rabbit-γ-globulin were purchased from Calbiochem.125I-ACTH was obtained from DiaSorin (Stillwater, MN). Kits for prolactin radioimmunoassay were provided by the National Institute of Arthritis, Diabetes, Digestive, and Kidney Disorders (NIADDK). Plasma oxytocin was assayed as detailed in our previous paper (Li et al., 1997b). For oxytocin extraction from plasma, acetone (Spectranalyzed A-19) and petroleum ether were obtained from Fisher Scientific (Pittsburgh, PA). The oxytocin antiserum was a generous donation by Dr. Lanny Keil (Ames Research Center, Sunnyvale CA). 125I-oxytocin was purchased from DuPont NEN at a specific activity of 135 Ci/mmol.
Statistics
The data are presented as group means with the SEM values. The G-protein data were analyzed by a one-way ANOVA, and group means were compared by Newman–Keuls' multiple range test (Steel and Torrie, 1960). The hormone data were analyzed by a two-way ANOVA, and group means were compared by Newman–Keuls' multiple range test (Steel and Torrie, 1960). GB-STAT software (Dynamic Microsystems, Silver Spring, MD) was used for all the statistical analyses.
RESULTS
In situ hybridization
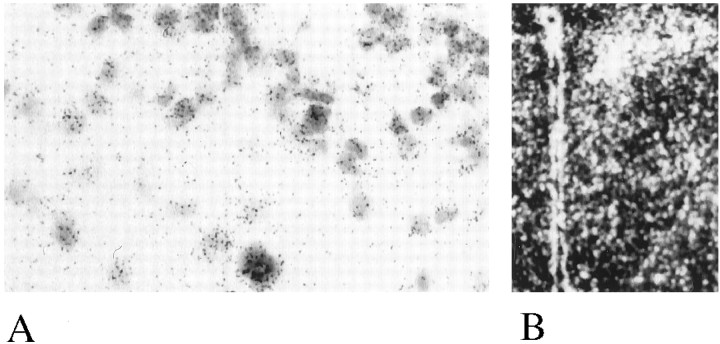
Figure 2 shows a coronal section through the hypothalamus at a high magnification of 330× (A) and a dark-field picture of a lower magnification (B). High grain density was observed in cells of the hypothalamic paraventricular nucleus, indicating the expression of mRNA encoding Gαz.
Fig. 2.
In situ hybridization of Gz mRNA in the hypothalamic paraventricular nucleus.A, High magnification (330×) of cells in the paraventricular nucleus expressing Gz mRNA; B,dark-field low level magnification of the hypothalamus.
Gz antisense experiments
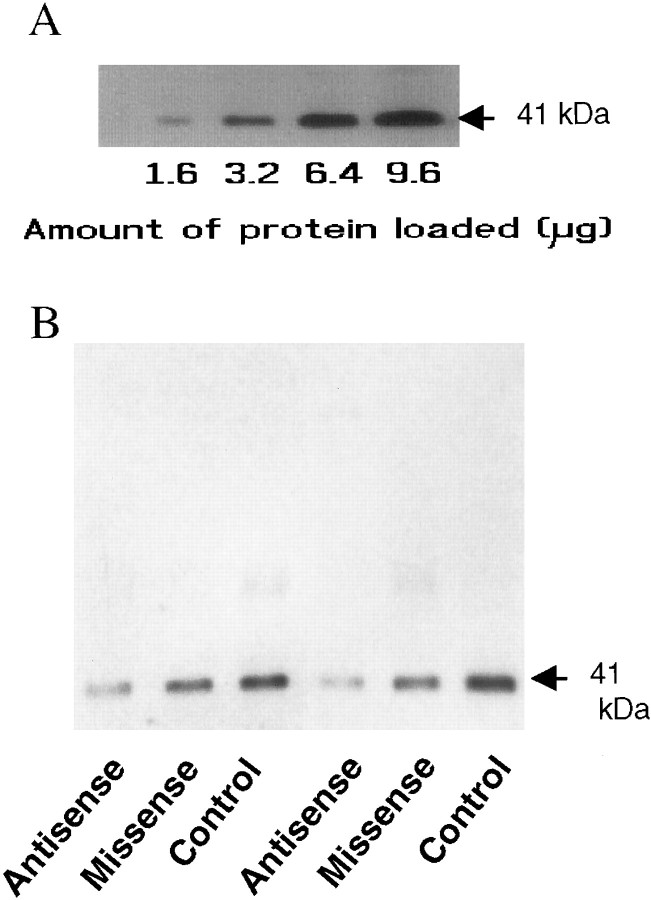
Figure 3A displays immunoblots of different amounts of homogenate obtained from microdissected tissue containing the hypothalamic paraventricular nucleus. Loading increasing amounts of protein resulted in increased IOD of the bands on the immunoblots. The levels of Gz-protein in the paraventricular nucleus are sufficiently high to allow detection when 1.6 μg of total protein was loaded on the gel.
Fig. 3.
A, Immunoblots showing the expression of Gz-proteins in the hypothalamic paraventricular nucleus. The numbers below indicate the amount of protein loaded on each lane of the gel. B,Immunoblots demonstrating the changes in Gz-proteins in the hypothalamic paraventricular nucleus after treatment with missense or Gz antisense oligodeoxynucleotides.
Levels of Gz-protein
Figure 3B illustrates immunoblots of Gz-proteins obtained from control rats and from rats treated with missense or antisense oligodeoxynucleotides. Missense treatment did not result in a substantial reduction in the Gz-protein band, whereas treatment with antisense oligodeoxynucleotides substantially decreased the integrated optical density of the Gz band (Fig. 3B). In the first experiment, treatment with 15 mer antisense oligodeoxynucleotides that were phosphorothioate-modified at all positions produced a decrease (by 41%) in Gz-protein levels, compared with untreated rats (F(2,23) = 6.076; p < 0.01), whereas treatment with missense oligodeoxynucleotides did not significantly reduce the levels of Gz-proteins in the paraventricular nucleus (Table 1). However, this antisense treatment also reduced the levels of Gi1 and Gi2-proteins (40–50%; Table 1), suggesting nonspecific changes.
Table 1.
Effects of completely phosphorothioated, 15 mer missense and Gz antisense oligodeoxynucleotides on the levels of Gz, Gi1, and Gi2 proteins in hypothalamic paraventricular nucleus “punches”
| G-protein | Uninjected | Missense | Antisense |
|---|---|---|---|
| Gz | 101.3 ± 8.8 | 76.7 ± 12.6 | 52.4 ± 8.7* |
| Gi1 | 101.8 ± 22.6 | 101.0 ± 28.1 | 45.1 ± 8.9* |
| Gi2 | 107.6 ± 15.1 | 102.3 ± 21.1 | 46.3 ± 4.8* |
The data represent the mean ± SEM (IOD/μg proteins as percentage of control) obtained from 7–10 rats. *Significant reduction compared with the missense and uninjected groups (one-way ANOVA and Newman–Keuls' test).
In a second experiment, we tested longer oligodeoxynucleotides that were only phosphorothioate-modified at the two bases on each end. Figure 4 shows the mean percent reduction in Gz-, Gi1-, and Gi2-protein levels in the paraventricular nucleus. Antisense treatment produced a decrease of 38.5% in Gz-protein levels, compared with missense, and 41.2% compared with untreated rats (F(2,19) = 4.1423; p< 0.05). Treatment with missense oligodeoxynucleotides did not reduce the levels of Gz-proteins in the paraventricular nucleus (Fig. 4). The levels of Gi1 and Gi2-proteins were not reduced by either antisense or missense treatments (Fig. 4).
Fig. 4.

Effect of Gz antisense oligodeoxynucleotides on the levels of Gz-, Gi1-, and Gi2-proteins in the hypothalamic paraventricular nucleus. The data represent the mean ± SEM of seven or eight rats per group. *Significant difference from the missense and uninjected group, p < 0.05 (one-way ANOVA and Newman–Keuls' test).
Hormone responses to 8-OH-DPAT
In both experiments, basal levels of oxytocin, ACTH, and prolactin were not significantly different among untreated, missense-treated, and antisense-treated rats.
In the first antisense experiment, administration of 8-OH-DPAT (50 μg/kg, s.c.) increased plasma oxytocin by 888% (Fig.5A), ACTH by 693% (Fig.5B), and prolactin by 118% (Fig. 5C) in untreated rats. Treatment with 15 mer phosphorothioate-modified Gz antisense oligodeoxynucleotides produced a significant inhibition of the oxytocin response to 8-OH-DPAT, by 43% compared with untreated controls, and by 35% compared with missense-treated rats (Fig. 5A). The two-way ANOVA indicated a significant main effect of treatment (F(2,46) = 3.38; p < 0.05), a significant main effect of 8-OH-DPAT (F(1,46) = 101.8; p < 0.001), and a significant interaction between 8-OH-DPAT and oligodeoxynucleotide treatment (F(2,46) = 3.796; p < 0.05). Post hoc Newman–Keuls' test indicated that the oxytocin response to 8-OH-DPAT is significantly decreased in antisense-treated rats compared to missense-treated rats (p < 0.05) and untreated rats (p < 0.01).
Fig. 5.
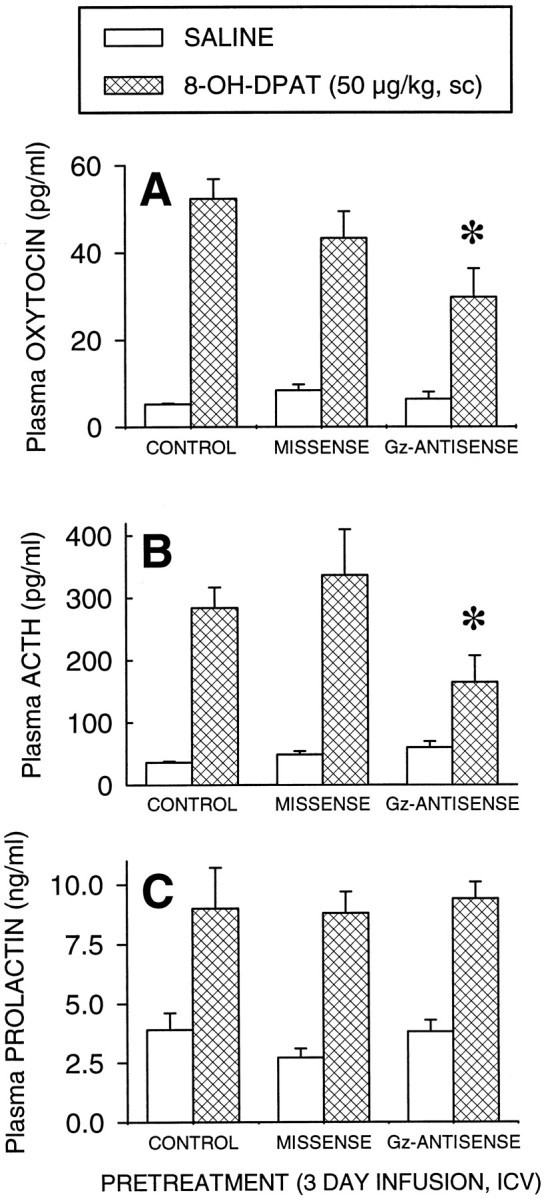
Effect of completely phosphorothioate-modified 15 mer Gz antisense oligodeoxynucleotides on the oxytocin (A), ACTH (B), and prolactin (C) responses to 8-OH-DPAT (50 μg/kg, s.c.). The data represent the mean ± SEM of seven or eight rats per group. *Significant effect of Gz antisense oligodeoxynucleotides, p < 0.01 (Newman–Keuls' test).
Treatment with Gz antisense oligodeoxynucleotides also significantly decreased the ACTH response to 8-OH-DPAT, by 51% compared to the missense group and by 42% compared with untreated rats (Fig. 5B). The two-way ANOVA indicated no main effect of antisense treatment (F(2,45) = 2.30;p > 0.10), a significant main effect of 8-OH-DPAT (F(1,45) = 48.1; p < 0.0001), and a significant interaction between oligodeoxynucleotide treatment and 8-OH-DPAT (F(2,45) = 3.27; p < 0.05). A post hoc Newman–Keuls' test indicated that the response to 8-OH-DPAT is significantly decreased in Gz antisense-treated rats compared with missense-treated rats (p < 0.05).
8-OH-DPAT significantly increased the plasma levels of prolactin in untreated rats, missense-, and Gzantisense-treated rats (Fig. 5C). The two-way ANOVA indicated no significant main effect of the treatment (F(2,46) = 0.29; p > 0.10), a main effect of 8-OH-DPAT (F(1,46) = 30.929; p< 0.01), and no significant interaction between oligonucleotide treatment and 8-OH-DPAT (F(2,46) = 0.0783; p > 0.10).
In the second antisense experiment, administration of 8-OH-DPAT (50 μg/kg, s.c.), increased plasma oxytocin by 1274% (Fig.6A), ACTH by 860% (Fig. 6B), and prolactin by 300% (Fig.6C) in untreated rats. The oxytocin response to 8-OH-DPAT was not significantly different in missense-treated rats when compared to untreated rats. However, treatment with the partly phosphorothioate-modified 33 mer Gz antisense oligodeoxynucleotides reduced the oxytocin response to 8-OH-DPAT by 39% compared with untreated controls and by 25% compared with missense-treated rats (Fig. 6A). The two-way ANOVA indicated a significant main effect of 8-OH-DPAT (F(1,31) = 170.5; p < 0.001), a significant main effect of treatment (F(2,31) = 3.73; p < 0.05), and a significant interaction between 8-OH-DPAT and oligodeoxynucleotide treatment (F(2,31) = 4.09; p < 0.05). A post hoc Newman–Keuls' test indicated that the oxytocin response to 8-OH-DPAT is significantly decreased in antisense-treated rats compared to control rats (p < 0.05).
Fig. 6.
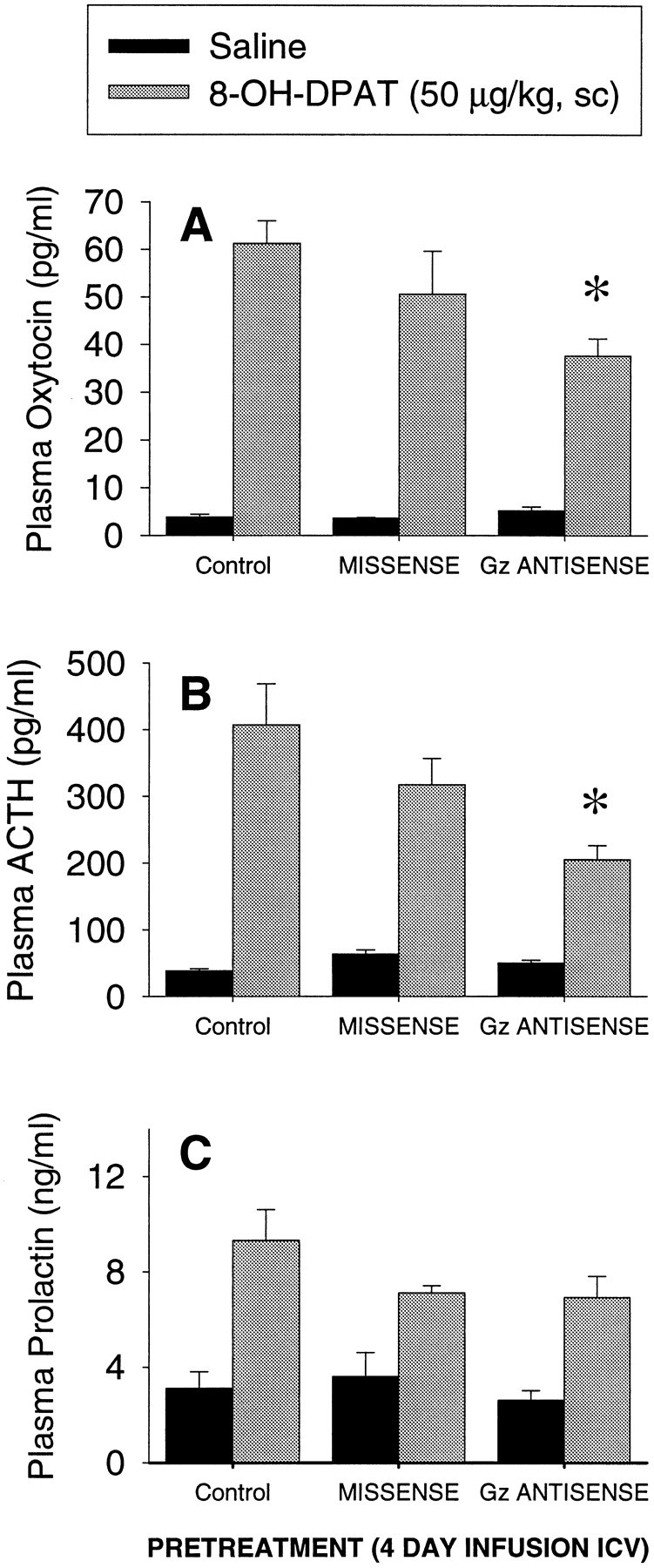
Effect of partially phosphorothioate-modified 33 mer Gz antisense oligodeoxynucleotides on the oxytocin (A), ACTH (B), and prolactin (C) responses to 8-OH-DPAT (50 μg/kg, s.c.). The data represent the mean ± SEM of seven or eight rats per group. *Significant effect of Gz antisense oligodeoxynucleotides, p < 0.01 (Newman–Keuls' test).
Treatment with Gz antisense oligodeoxynucleotides also significantly decreased the ACTH response to 8-OH-DPAT, by 35% compared to the missense group, and by 49% compared with untreated rats (Fig. 6B). The two-way ANOVA indicated a significant main effect of antisense treatment (F(2,31) = 5.23; p < 0.05), a significant main effect of 8-OH-DPAT (F(1,31) = 109.8; p < 0.0001), and a significant interaction between oligodeoxynucleotide treatment and 8-OH-DPAT (F(2,31) = 5.45; p < 0.01). A post hoc Newman–Keuls' test indicated that the response to 8-OH-DPAT is significantly decreased in Gz antisense-treated rats compared with missense-treated (p < 0.05) and control rats (p < 0.01).
8-OH-DPAT significantly increased the plasma levels of prolactin in untreated, missense-treated, and Gzantisense-treated rats (Fig. 6C). The two-way ANOVA indicated no significant main effect of the treatment (F(2,30) = 2.18; p > 0.1), a significant main effect of 8-OH-DPAT (F(1,31) = 41.51; p < 0.01), and no significant interaction between oligodeoxynucleotide treatment and 8-OH-DPAT (F(2,31) = 2.09; p > 0.1).
Pertussis toxin experiment
Treatment with pertussis toxin also did not alter the basal plasma levels of oxytocin (Fig. 7A). Injection of 8-OH-DPAT to vehicle-pretreated rats increased plasma levels of oxytocin (by 963%; Fig. 7A). Pretreatment with pertussis toxin significantly potentiated the oxytocin response to 8-OH-DPAT (by 206.4%; Fig. 7A). The two-way ANOVA indicated no significant main effect of pertussis toxin (F(1,31) = 2.94; p > 0.05), a significant main effect of 8-OH-DPAT (F(1,31) = 22.02; p < 0.0001), and no significant interaction between pertussis toxin and 8-OH-DPAT (F(1,31)= 2.53;p > 0.1). However, a post hocNewman–Keuls' test indicated that the oxytocin response to 8-OH-DPAT was significantly potentiated in rats pretreated with pertussis toxin, when compared with rats pretreated with vehicle (p < 0.05).
Fig. 7.

Effect of pretreatment with pertussis toxin (1 μg, i.c.v.) on the oxytocin (A), ACTH (B), and prolactin (C) responses to 8-OH-DPAT (50 μg/kg, s.c.). The data represent the mean ± SEM of 8–10 rats per group. *Significant effect of pertussis toxin, p < 0.01 (Newman–Keuls' test).
Pretreatment with pertussis toxin did not alter the basal levels of ACTH (Fig. 7B). Administration of 8-OH-DPAT to rats that were pretreated with an intracerebroventricular injection of saline significantly elevated plasma levels of ACTH (by 1031%). The ACTH response to 8-OH-DPAT was not significantly affected by treatment with pertussis toxin (Fig. 7B). The two-way ANOVA indicated no significant main effect of pertussis toxin (F(1,32) = 0.95; p > 0.1), a significant main effect of 8-OH-DPAT (F(1,32) = 39.1; p < 0.0001), and no significant interaction between pertussis toxin and 8-OH-DPAT (F(1,32) = 1.27;p > 0.1).
In contrast with its effects on ACTH and oxytocin, pertussis toxin treatment significantly reduced the basal plasma levels of prolactin (to 38.2% of control; Fig. 7C). 8-OH-DPAT also significantly increased the plasma levels of prolactin in rats that were pretreated with vehicle (by 162%; Fig. 7C). Pretreatment with pertussis toxin significantly blocked the 8-OH-DPAT-induced elevation of plasma prolactin (Fig. 7C). The two-way ANOVA indicated a significant main effect of pertussis toxin (F(1,30) = 22.82;p < 0.0001), a significant main effect of 8-OH-DPAT (F(1,30) = 6.75; p < 0.05), but no significant interaction between pertussis toxin and 8-OH-DPAT (F(1,30) = 1.23;p > 0.1). The post hoc Newman–Keuls' test indicated that the prolactin response to 8-OH-DPAT was significantly inhibited in rats pretreated with pertussis toxin when compared with rats pretreated with vehicle (p < 0.01).
DISCUSSION
This study provides functional in vivo evidence for the coupling of Gz-proteins to 5-HT1A receptors in the hypothalamic paraventricular nucleus. We have established that Gz mRNA and Gz-protein are expressed in the hypothalamic paraventricular nucleus and that an antisense oligodeoxynucleotide-induced reduction in the levels of Gz-protein inhibits the ACTH and oxytocin responses to a 5-HT1A agonist. These observations suggest that Gz-proteins transduce signals from 5-HT1A receptors to the secretion of ACTH and oxytocin.
Cotransfection experiments in cell lines indicate that Gz-proteins can couple to a variety of receptors such as μ- and δ-opioid, α2-adrenergic, dopamine-D2, adenosine-A1, and 5-HT1A receptors (Ho and Wong, 1998). However, because Gz-protein is expressed in large quantities in these cell lines, coupling of Gz-protein to a receptor subtype might not be of physiological significance. Such studies have to be validated byin vivo approaches.
8-OH-DPAT is the prototypical 5-HT1A agonist used in neuroendocrine research. It has a high affinity for 5-HT1A receptors and a 10- to 100-fold lower affinity for other 5-HT receptor subtypes (Hoyer et al., 1994; Jasper et al., 1997). The dose of 8-OH-DPAT was selected to be submaximal and close to its ED50 value to minimize activation of other receptors (Li et al., 1993). Activation of 5-HT1A receptors stimulates the secretion of oxytocin and CRH (Pan and Gilbert, 1992; Bagdy, 1998). Increased release of CRH stimulates the secretion of ACTH from the pituitary gland (Bagdy, 1998). The 8-OH-DPAT-induced elevation in plasma ACTH and oxytocin levels is inhibited by the selective 5-HT1A antagonist WAY-100,635 (Vicentic et al., 1998). In contrast, the prolactin response to 8-OH-DPAT is not antagonized by WAY-100,635, indicating that it is not mediated by 5-HT1A receptors (Vicentic et al., 1998). Therefore, prolactin measurements provided a control for the selectivity of effects on 5-HT1A receptor signaling.
Antisense oligodeoxynucleotides are readily degraded (Morris and Li, 1998). To increase their cellular stability, we used phosphorothioate-modified oligodeoxynucleotides. Our initial experiment, using oligodeoxynucleotides in which all bases were phosphorothioate modified, resulted in a reduction in the levels of Gz and a 40–50% inhibition of the oxytocin and ACTH responses to 8-OH-DPAT, compared with the missense and uninjected control groups. However, this sequence of phosphorothioate-modified Gz antisense oligodeoxynucleotides produced a reduction in both Gi1- and Gi2-protein levels. The nonspecific effect of this antisense oligodeoxynucleotide is likely attributable to its sequence, because the phosphorothioate-modified missense oligodeoxynucleotide did not reduce the levels of Gi1- or Gi2-proteins. Therefore, in a subsequent experiment, we changed the sequence of the oligodeoxynucleotides, and only the two bases at each end were phosphorothioate-modified. These longer and less modified oligodeoxynucleotides were more selective for Gz-protein because the levels of Gi1- and Gi2-proteins in the paraventricular nucleus were not reduced. Furthermore, the baseline levels of hormones were not different in missense- or antisense oligodeoxynucleotide-treated groups, compared with uninjected controls. Thus, both Gz antisense oligodeoxynucleotides have a common effect on the levels of Gz-proteins and on the ACTH and oxytocin response to the 5-HT1A agonist. The lack of significant reduction in Gz-protein level, in missense-treated rats, was further confirmed by the lack of a significant inhibition of the hormone responses to 8-OH-DPAT, compared with the uninjected controls.
The decrease in Gz-protein levels, after antisense treatment, induced a proportional inhibition of the oxytocin and ACTH responses to 8-OH-DPAT. Oxytocin synthesizing cells are located in the paraventricular nucleus and release oxytocin directly into the bloodstream from the neurohypophysis. Therefore, changes in plasma levels of oxytocin provide a direct and sensitive indicator of changes in Gz-protein-coupled 5-HT1A receptor function in the hypothalamus.
The data obtained using both antisense oligodeoxynucleotides and pertussis toxin suggest that pertussis toxin-sensitive Gi/o-proteins do not mediate 5-HT1A receptor-induced ACTH secretion and that ACTH secretion is under the control of the pertussis toxin-insensitive Gz-protein. ACTH secretion is amplified because it is mediated by activation of CRH receptors, which are coupled via G-proteins to effector enzymes. Therefore, any decrease in 5-HT1A receptor signaling could be masked by the amplification by CRH of ACTH release. Consequently, the observed decrease in ACTH would not be expected to reflect a proportional decrease in 5-HT1A receptor systems in the paraventricular nucleus. The fact that Gzantisense oligodeoxynucleotide treatment produced an ∼40% decrease of the ACTH response to 8-OH-DPAT suggests a possibility of a much larger decrease of 5-HT1A receptor function in CRH cells.
Pertussis toxin-sensitive G-proteins include members of the Gi-protein family such as Gi1-, Gi2-, Gi3-, and Go-proteins (Hamm and Gilchrist, 1996; Fields and Casey, 1997). Proteins insensitive to pertussis toxin include Gq/11-, Gs-, and Gz-proteins (Fields and Casey, 1997). 5-HT1A receptors have a very low affinity for Gs and Gq/11-proteins (Butkerait et al., 1995; Albert et al., 1996), and it is not likely that these G-proteins mediate the effect of 5-HT1A receptors on the secretion of ACTH and oxytocin. To our knowledge, all in vivo evidence reported to date suggests that, in other brain regions such as the dorsal raphe and hippocampus, the physiological effects of 5-HT1A receptor activation are mediated by pertussis toxin-sensitive Gi/o-proteins (Clarke et al., 1987; Innis and Aghajanian, 1987; Blier et al., 1993; Romero et al., 1994).
In the present study, we used a relatively low dose (1 μg, i.c.v.) of pertussis toxin to prevent its toxic side effects. However, this pertussis toxin dose (1 μg, i.c.v.) is equal to or higher than doses that were previously shown to affect physiological responses to activation of other receptors that are coupled to Gi- and/or Go-proteins, such as μ-opioid receptors (Parolaro et al., 1990; Lin and Pan, 1996), dopamine-D2 receptors (Okada et al., 1994), and substance P receptors (Bot and Chahl, 1993). The failure of pertussis toxin to inhibit the ACTH and oxytocin responses to 8-OH-DPAT, combined with the inhibition of the ACTH and oxytocin responses to 8-OH-DPAT by Gz antisense oligodeoxynucleotides, suggests that Gz-proteins are the key component in 5-HT1A receptor signaling in the hypothalamic paraventricular nucleus.
An unexpected finding was the oxytocin response to 8-OH-DPAT, which was potentiated instead of reduced by pertussis toxin. One explanation for this potentiated response is that other receptors that are coupled to Gi- and/or Go-proteins inhibit the secretion of oxytocin. For example, μ-opioid receptors inhibit the secretion of oxytocin (Pumford et al., 1993; Ingram et al., 1996). Similar to our results, the opioid antagonist naloxone potentiated the oxytocin response to another stimulus (systemic injection of cholecystokinin) without altering basal oxytocin levels (Leng et al., 1992). Another possible explanation is that hypothalamic Gi/o-proteins compete with Gz-proteins for 5-HT1Areceptor coupling, but only Gz-proteins would mediate the effects on oxytocin secretion. Inactivation of Gi/o-proteins by pertussis toxin would thus allow an increased coupling efficiency of Gz-protein to 5-HT1A receptors, resulting in an enhanced secretion to the same 8-OH-DPAT dose.
We measured plasma prolactin levels as a control measure on the specificity and effectiveness of our treatments. As mentioned earlier, the effect of 8-OH-DPAT on the secretion of prolactin is not exclusively mediated by 5-HT1A receptors and involves other, as yet uncharacterized, mechanisms (Aulakh et al., 1988; Vicentic et al., 1998). The injection of Gzantisense oligodeoxynucleotides did not inhibit the prolactin response to 8-OH-DPAT, supporting our conclusion that Gz-proteins specifically couple 5-HT1A receptors to the stimulation of oxytocin and ACTH secretion.
The pertussis toxin-induced inhibition of basal as well as 8-OH-DPAT-induced increase of prolactin release could be explained as a consequence of increased activity of tuberoinfundibular dopamine neurons. For example, receptors that are coupled to pertussis toxin-sensitive Gi-proteins, such as μ-opioid receptors (Parolaro et al., 1990; Chan et al., 1995), mediate a tonic inhibition of the activity of tuberoinfundibular dopamine neurons in the hypothalamus (Callahan et al., 1996). Therefore, pertussis toxin-induced inactivation of Gi/o-proteins coupled to the μ-opioid receptors in the hypothalamus could produce a disinhibition of dopaminergic neurons and result in increased release of dopamine. Increased dopamine levels in the anterior pituitary gland inhibit the secretion of prolactin, regardless of the presence of other stimuli that increase its secretion (Pilotte and Porter, 1981; Callahan et al., 1996). This explanation is consistent with the observation that pertussis toxin treatment reduced basal prolactin levels.
In conclusion, the present study provides the first in vivoevidence demonstrating that Gz-proteins couple 5-HT1A receptors in the hypothalamus to effector systems that trigger the secretion of ACTH and oxytocin. These data suggest that the ACTH and oxytocin responses to 5-HT1A agonists can be used as peripheral markers of alterations in hypothalamic 5-HT1A receptors signal transduction via altered coupling of Gz-protein.
Footnotes
This work was supported in part by United States Public Health Service Grants NS34153 and MH58448 (L.D.V.), Loyola Neuroscience and Aging Institute (F.S.), and National Alliance for Research on Schizophrenia and Depression (D.K.R.).
Correspondence should be addressed to Dr. Louis D. Van de Kar, Department of Pharmacology, Stritch School of Medicine, Loyola University Chicago, 2160 South First Avenue, Maywood, IL 60153. E-mail:lvandek@luc.edu.
Dr. Serres' present address: Lilly Research Center, Erl Wood Manor, Sunninghill Road, Windlesham Surrey GU20 6PH, UK.
Dr. Raap's present address: Department of Psychology, University of Alaska Fairbanks, Fairbanks, Alaska 99775.
REFERENCES
- 1.Albert PR, Lembo P, Storring JM, Charest A, Saucier C. The 5HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;14:19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- 2.Aulakh CS, Wozniak KM, Haas M, Hill JL, Zohar J, Murphy DL. Food intake, neuroendocrine and temperature effects of 8-OHDPAT in the rat. Eur J Pharmacol. 1988;146:253–259. doi: 10.1016/0014-2999(88)90300-7. [DOI] [PubMed] [Google Scholar]
- 3.Bagdy G. Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav Brain Res. 1996;73:277–280. doi: 10.1016/0166-4328(96)00112-x. [DOI] [PubMed] [Google Scholar]
- 4.Bagdy G. The role of biogenic amines in neuroendocrine regulation in conscious rats. In: Van de Kar LD, editor. Methods in neuroendocrinology. CRC; Boca Raton: 1998. pp. 145–161. [Google Scholar]
- 5.Barr AJ, Brass LF, Manning DR. Reconstitution of receptors and GTP-binding regulatory proteins (G proteins) in Sf9 cells. A direct evaluation of selectivity in receptor G protein coupling. J Biol Chem. 1997;272:2223–2229. doi: 10.1074/jbc.272.4.2223. [DOI] [PubMed] [Google Scholar]
- 6.Blier P, Lista A, de Montigny C. Differential properties of pre- and postsynaptic 5-hydroxytryptamine1A receptors in the dorsal raphe and hippocampus: I. Effect of spiperone. J Pharmacol Exp Ther. 1993;265:7–15. [PubMed] [Google Scholar]
- 7.Bot G, Chahl LA. Effects of pertussis toxin on behavioural responses of guinea-pigs to centrally administered substance P, quinpirol, carbachol, U-50,488H, morphine and morphine withdrawal. Eur J Pharmacol. 1993;231:53–60. doi: 10.1016/0014-2999(93)90683-9. [DOI] [PubMed] [Google Scholar]
- 8.Butkerait P, Zheng Y, Hallak H, Graham TE, Miller HA, Burris KD, Molinoff PB, Manning DR. Expression of the human 5-hydroxytryptamine1A receptor in Sf9 cells. Reconstitution of a coupled phenotype by co-expression of mammalian G protein subunits. J Biol Chem. 1995;270:18691–18699. doi: 10.1074/jbc.270.31.18691. [DOI] [PubMed] [Google Scholar]
- 9.Callahan P, Baumann MH, Rabii J. Inhibition of tuberoinfundibular dopaminergic neural activity during suckling: involvement of mu and kappa opiate receptor subtypes. J Neuroendocrinol. 1996;8:771–776. doi: 10.1046/j.1365-2826.1996.05207.x. [DOI] [PubMed] [Google Scholar]
- 10.Calogero AE, Bernardini R, Margioris AN, Bagdy G, Gallucci WT, Tamarkin L, Tomai TP. Effect of serotonergic agonists and antagonists on corticotropin-releasing hormone secretion by explanted rat hypothalami. Peptides. 1989;10:189–200. doi: 10.1016/0196-9781(89)90096-x. [DOI] [PubMed] [Google Scholar]
- 11.Casey PJ, Fong HK, Simon MI, Gilman AG. Gz, a guanine nucleotide-binding protein with unique biochemical properties. J Biol Chem. 1990;265:2383–2390. [PubMed] [Google Scholar]
- 12.Chan JS, Chiu TT, Wong YH. Activation of type II adenylyl cyclase by the cloned mu-opioid receptor: coupling to multiple G proteins. J Neurochem. 1995;65:2682–2689. doi: 10.1046/j.1471-4159.1995.65062682.x. [DOI] [PubMed] [Google Scholar]
- 13.Clarke WP, De Vivo M, Beck SG, Maayani S, Goldfarb J. Serotonin decreases population spike amplitude in hippocampal cells through a pertussis toxin substrate. Brain Res. 1987;410:357–361. doi: 10.1016/0006-8993(87)90338-6. [DOI] [PubMed] [Google Scholar]
- 14.Cowen PJ. Neuroendocrine challenge tests: what can we learn from them? In: Van de Kar LD, editor. Methods in neuroendocrinology. CRC; Boca Raton: 1998. pp. 205–223. [Google Scholar]
- 15.Fields TA, Casey PJ. Signaling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem J. 1997;321:561–571. doi: 10.1042/bj3210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fong HK, Yoshimoto KK, Eversole-Cire P, Simon MI. Identification of a GTP-binding protein alpha subunit that lacks an apparent ADP-ribosylation site for pertussis toxin. Proc Natl Acad Sci USA. 1988;85:3066–3070. doi: 10.1073/pnas.85.9.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamm HE, Gilchrist A. Heterotrimeric G proteins. Curr Opin Cell Biol. 1996;8:189–196. doi: 10.1016/s0955-0674(96)80065-2. [DOI] [PubMed] [Google Scholar]
- 18.Ho MKC, Wong YH. Structure and function of the pertussis-toxin-insensitive Gz protein. Biol Signals. 1998;7:80–89. doi: 10.1159/000014533. [DOI] [PubMed] [Google Scholar]
- 19.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PPA. VII. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–204. [PubMed] [Google Scholar]
- 20.Ingram CD, Kavadas V, Thomas MRM, Threapleton JD. Endogenous opioid control of somatodendritic oxytocin release from the hypothalamic supraoptic and paraventricular nuclei in vitro. Neurosci Res. 1996;25:17–24. doi: 10.1016/0168-0102(96)01027-9. [DOI] [PubMed] [Google Scholar]
- 21.Innis RB, Aghajanian GK. Pertussis toxin blocks 5-HT1A and GABAB receptor-mediated inhibition of serotonergic neurons. Eur J Pharmacol. 1987;143:195–204. doi: 10.1016/0014-2999(87)90533-4. [DOI] [PubMed] [Google Scholar]
- 22.Jasper JR, Kosaka A, To ZP, Chang DJ, Eglen RM. Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7(b)). Br J Pharmacol. 1997;122:126–132. doi: 10.1038/sj.bjp.0701336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawano S, Osaka T, Kannan H, Yamashita H. Excitation of hypothalamic paraventricular neurons by stimulation of the raphe nuclei. Brain Res Bull. 1992;28:573–579. doi: 10.1016/0361-9230(92)90105-7. [DOI] [PubMed] [Google Scholar]
- 24.Leng G, Dyball REJ, Way SA. Naloxone potentiates the release of oxytocin induced by systemic administration of cholecystokinin without enhancing the electrical activity of supraoptic oxytocin neurones. Exp Brain Res. 1992;88:321–325. doi: 10.1007/BF02259107. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Levy AD, Cabrera TM, Brownfield MS, Battaglia G, Van de Kar LD. Long-term fluoxetine, but not desipramine, inhibits the ACTH and oxytocin responses to the 5-HT1A agonist 8-OH-DPAT in male rats. Brain Res. 1993;630:148–156. doi: 10.1016/0006-8993(93)90652-4. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Battaglia G, Van de Kar LD. Autoradiographic evidence for differential G-protein coupling of 5-HT1A receptors in the rat brain: lack of effect by repeated injections of fluoxetine. Brain Res. 1997a;769:141–151. doi: 10.1016/s0006-8993(97)00693-8. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Muma NA, Battaglia G, Van de Kar LD. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of Gi and Go proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther. 1997b;282:1581–1590. [PubMed] [Google Scholar]
- 28.Lin JY, Pan JT. Prolonged pertussis toxin treatment affects morphine's action on tuberoinfundibular dopaminergic neuron activity and on prolactin secretion. Brain Res. 1996;727:182–186. doi: 10.1016/0006-8993(96)00377-0. [DOI] [PubMed] [Google Scholar]
- 29.Liposits Z, Phelix C, Paull WK. Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry. 1987;86:541–549. doi: 10.1007/BF00489545. [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough WY, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Matsuoka M, Itoh H, Kozasa T, Kaziro Y. Sequence analysis of cDNA and genomic DNA for a putative pertussis toxin-insensitive guanine nucleotide-binding regulatory protein alpha subunit. Proc Natl Acad Sci USA. 1988;85:5384–5388. doi: 10.1073/pnas.85.15.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris M, Li P. Antisense oligonucleotides as tools in neuroendocrinology. In: Van de Kar LD, editor. Methods in neuroendocrinology. CRC; Boca Raton: 1998. pp. 131–144. [Google Scholar]
- 33.Mullaney I, Miligan G. Identification of two distinct isoforms of the guanine nucleotide binding protein Go in neuroblastoma X glioma hybrid cells: independent regulation during cyclic AMP-induced differentiation. J Neurochem. 1990;55:1890–1898. doi: 10.1111/j.1471-4159.1990.tb05773.x. [DOI] [PubMed] [Google Scholar]
- 34.Muma NA, Hoffman PN, Slunt HH, Applegate MD, Lieberburg I, Price DL. Alterations in levels of mRNAs coding for neurofilament protein subunits during regeneration. Exp Neurol. 1990;107:230–235. doi: 10.1016/0014-4886(90)90140-n. [DOI] [PubMed] [Google Scholar]
- 35.Okada F, Takahashi N, Ito A, Tokumitsu Y, Nomura Y. Acceleration of desipramine-induced changes on the dopamine receptor-coupled adenylate cyclase system by pertussis toxin. J Neural Transm. 1994;98:133–142. doi: 10.1007/BF01277016. [DOI] [PubMed] [Google Scholar]
- 36.Pan L, Gilbert F. Activation of 5-HT1A receptor subtype in the paraventricular nuclei of the hypothalamus induces CRH ACTH release in the rat. Neuroendocrinology. 1992;56:797–802. doi: 10.1159/000126332. [DOI] [PubMed] [Google Scholar]
- 37.Parolaro D, Patrini G, Giagnoni G, Massi P, Groppetti A, Parenti M. Pertussis toxin inhibits morphine analgesia and prevents opiate dependence. Pharmacol Biochem Behav. 1990;35:137–141. doi: 10.1016/0091-3057(90)90218-7. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; Sydney: 1986. [DOI] [PubMed] [Google Scholar]
- 39.Pilotte NS, Porter JC. Dopamine in hypophysial portal plasma and prolactin in systemic plasma of rats treated with 5-hydroxytryptamine. Endocrinology. 1981;108:2137–2141. doi: 10.1210/endo-108-6-2137. [DOI] [PubMed] [Google Scholar]
- 40.Pumford KM, Leng G, Russell JA. A pertussis toxin-sensitive G protein mediates inhibition by morphine of spontaneous electrical activity of oxytocin neurons in anaesthetized rats. Exp Brain Res. 1993;94:247–251. doi: 10.1007/BF00230292. [DOI] [PubMed] [Google Scholar]
- 41.Romero L, Celada P, Artigas F. Reduction of in vivo striatal 5-hydroxytryptamine release by 8-OH-DPAT after inactivation of Gi/Go proteins in dorsal raphe nucleus. Eur J Pharmacol. 1994;265:103–106. doi: 10.1016/0014-2999(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Blazquez P, Garcia-Espana A, Garzon J. In vivo injection of antisense oligodeoxynucleotides to G alpha subunits and supraspinal analgesia evoked by mu and delta opioid agonists. J Pharmacol Exp Ther. 1995;275:1590–1596. [PubMed] [Google Scholar]
- 43.Saphier D. Paraventricular nucleus magnocellular neuronal responses following electrical stimulation of the midbrain dorsal raphe. Exp Brain Res. 1991;85:359–363. doi: 10.1007/BF00229413. [DOI] [PubMed] [Google Scholar]
- 44.Steel RGD, Torrie JH. Principles and procedures of statistics with special reference to the biological sciences. McGraw-Hill; New York: 1960. [Google Scholar]
- 45.Vicentic A, Li Q, Battaglia G, Van de Kar LD. WAY-100635 inhibits 8-OH-DPAT stimulated oxytocin, ACTH, and corticosterone, but not prolactin secretion. Eur J Pharmacol. 1998;346:261–266. doi: 10.1016/s0014-2999(97)01607-5. [DOI] [PubMed] [Google Scholar]