Abstract
A training protocol was developed to classically condition feeding behavior in Aplysia californica using tactile stimulation of the lips as the conditional stimulus (CS) and food as the unconditional stimulus (US). Paired training induced a greater increase in the number of bites to the CS than unpaired training or US-only stimulation. Memory for classical conditioning was retained for at least 24 hr. The organization of the reinforcement pathway that supports classical conditioning was analyzed in additional behavioral experiments. No evidence was found for the contribution to appetitive reinforcement of US-mediating pathways originating in the lips of the animals. Bilateral lesions of the anterior branch of the esophageal nerve, which innervates parts of the foregut, however, were found to attenuate classical conditioning. Thus, it appears likely that reinforcement during appetitive classical conditioning of feeding was mediated by afferent pathways that originate in the foregut. The companion paper (Lechner et al., 2000) describes two neurophysiological correlates of the classical conditioning.
Keywords: learning and memory, classical conditioning, feeding behavior, Aplysia, sensory pathway, long-term memory
Feeding behavior inAplysia consists of a sequence of coordinated appetitive and consummatory components (Kupfermann, 1974a,b). The appetitive phase involves locomotion, rearing of the head, and head waving, which serve to bring the animal in contact with food. Once food is centered on the mouth, the consummatory phase is initiated (Kupfermann, 1974b; Susswein et al., 1976). Consummatory feeding consists of a series of rhythmic bite movements. Because biting behavior occurs in an all-or-nothing fashion, it can be easily quantified, and thus, it is well suited for behavioral observations. In the present study, biting was used as a behavioral assay to develop an appetitive classical conditioning procedure for feeding behavior, in which the cellular mechanisms underlying associative learning and memory could be analyzed (see companion paper).
In classical conditioning, an animal learns to associate a conditional stimulus (CS) and an unconditional stimulus (US). The US is chosen to elicit a quantifiable behavioral response in naïve animals [unconditional response (UR)], whereas the CS, before training, is typically neutral with respect to the UR. Repeated paired CS–US presentations result in the development of an adaptive response to the CS [conditional response (CR)], in anticipation of the US.
Early attempts to classically condition feeding behavior inAplysia using appetitive paradigms (Lickey, 1968;Jahan-Parwar, 1970) produced variable results that were called into question after others were unable to replicate them (Kupfermann, 1974a). Recent classical conditioning procedures that used chemical or tactile stimuli as the CS have proven more successful. For example, a differential conditioning procedure for feeding behavior has been developed (Colwill et al., 1997) in which a chemical stimulus (CS+) was repeatedly paired with food reinforcement (US). One hour after the last of 14 training sessions, presentation of the CS+ resulted in a higher number of bites than presentation of another chemical stimulus (CS−), which had not been paired with the US during training. Similar results were obtained with tactile stimuli that differed in texture. Although, this study demonstrated the potential of Aplysia for appetitive associative learning, it is not well suited for further, electrophysiological analyses. One problem arises from the current lack of available information on the organization of sensory pathways for chemical stimuli inAplysia (Xin et al., 1995). Even for tactile stimuli, the neural representation of which is better understood (Anderson, 1967;Rosen et al., 1979; Fredman and Jahan-Parwar, 1980), it is unlikely that stimulus qualities representing different textures can be simulated in vitro.
Here we report the development of an appetitive classical conditioning procedure that was designed to facilitate subsequent cellular analyses of associative memory in vitro. It differs from the procedure described above, mainly in the use of a single tactile stimulus as CS and in the temporal parameters of the training protocol. The experiments reported here document short-term and long-term associative memory induced by this training procedure. The gross organization of the sensory pathways mediating the US during classical conditioning was analyzed using behavioral manipulations and surgical lesions. Finally, we show that correlates of this classical conditioning can be identified in vitro (see companion paper).
Some of the results presented here have been presented previously in abstract form (Lechner et al., 1997).
MATERIALS AND METHODS
General. Aplysia californica (80–220 gm) were obtained from Alacrity Marine Biological Specimens (Redondo Beach, CA), Marine Specimens Unlimited (Pacific Palisades, CA), and Marinus (Long Beach, CA). They were housed individually in perforated plastic cages, floating in aerated seawater tanks (150 l) at a temperature of 12–15°C. Animals were fed ∼1 gm of dried laver, 3 times a week. Before the experiments, animals were food-deprived for a period of 2–7 d, during which time the animals were motivated to feed and remained active and healthy. The experiments reported here were performed in the months of January to March and May to September.
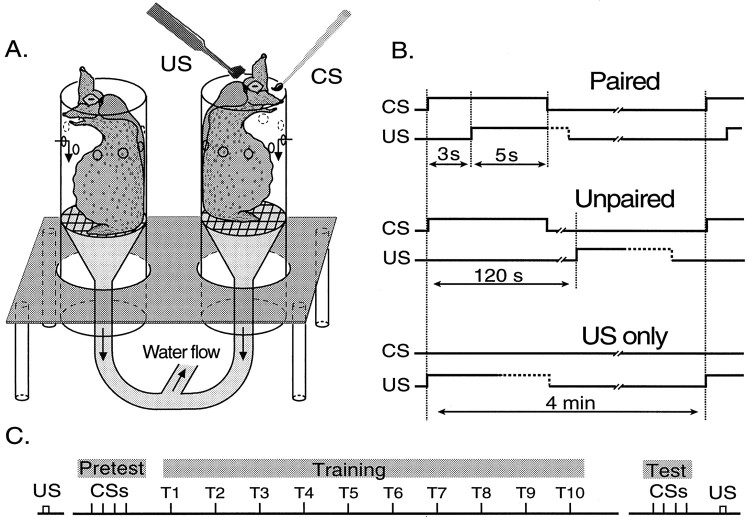
Training apparatus. Training took place in a separate aquarium that contained a set of clear plastic tubes. The tubes helped to restrain animals in a head-up position (Fig.1A). The tubes (20 × 6.5 cm) were held in place by a submerged platform, so that the upper rim extended ∼2 cm above the water surface. Below the water surface, perforations in the tubes allowed for the influx of water. A constant flow of water was created by a filter/pump (Eheim type 2213; Houston, TX) attached to the bottom of the tubes. A grid within the tubes could be adjusted in height to keep animals near the surface of the water.
Fig. 1.
Protocol for classical conditioning of feeding behavior. A, Animals were restrained in clear plastic tubes, which facilitated stimulation of the mouth and lips (see Materials and Methods for details). Tactile stimulation of the lips with a paintbrush served as the conditional stimulus (CS) and food as the unconditional stimulus (US). B, A delayed pairing procedure was used in which the CS onset preceded the US onset by 3 sec. CS and US overlapped for 5 sec, after which the CS was terminated. The US was presented until the animal ingested it, but no longer than 60 sec. Unpaired training consisted of CS and US presentations at an ISI of 120 sec. An additional control group received US presentations only. The ITI was 4 min in all experiments. C, Animals were tested for feeding behavior (biting) by offering them a piece of seaweed (US) before the experiment. Immediately before training, animals received four CS presentations (pretest). The number of bites during this stimulation period was counted. Subsequently, animals received 10 trials of paired, unpaired, or US-only training. After training, the number of bites elicited by four CSs (test) was determined and compared to the score obtained during the pretest. Finally, animals were tested once again for feeding behavior by offering a piece of food (US).
Behavioral procedures. Small pieces of laver (∼15 mg, dry weight) were used as USs. The food was presented directly to the lips of the animal with blunt forceps (Fig. 1A). This stimulation reliably elicited consummatory feeding behavior (i.e., bites) in food-deprived animals. The US presentation lasted either until the food was ingested, or for a maximum of 60 sec, when ingestion failed to occur. In some experiments (see Fig. 5), the US was brought in contact with the lips for 5 sec and then removed without permitting the animal to ingest it. Tactile stimulation of the lips with a paintbrush (size, number 3) for 8 sec was used as the CS. For paired training (Fig. 1B), the interstimulus interval (ISI) between the onset of CS and food presentation (US) was 3 sec, resulting in an overlap of 5 sec (delayed classical conditioning). The correct timing of the presentation of stimuli was guided by the sound of a metronome set at 1 Hz, which kept the variability of stimulus presentations <1 sec. A number of control procedures were used in different experiments. In unpaired training, the ISI was 120 sec, which prevented overlap between the CS and the presentation of food. Animals of other control groups received only US presentations (Fig.1B) or no stimulus presentations.
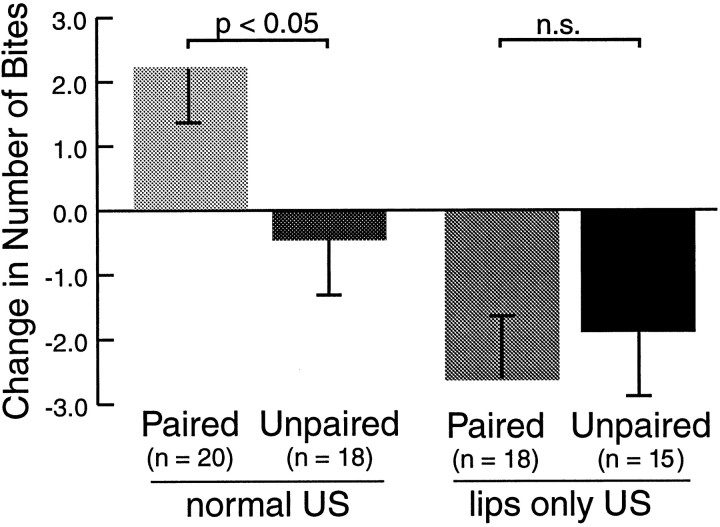
Fig. 5.
Behavioral dissection of the reinforcing pathways. Groups of animals were conditioned by pairing the CS with food that could be ingested as in previous experiments (normal US) or food that was brought in contact with the lips, but was not made available for ingestion (lips-only US). Unpaired controls received the same treatment at the longer ISI. Only paired training with the normal US resulted in associative learning, as measured by the number of bites during CS presentation before and after training. Thus, cerebral afferents originating in the lips were not sufficient to mediate reinforcement during classical conditioning, despite the fact that they reliably elicit biting behavior. US presentation to only the lips resulted in a decrease in the number of bites to the CS 1 hr after training. This decrease was not pairing-specific and was examined in more detail in the experiment below (Fig. 6).
The training protocol is illustrated in Figure 1C. After the food-deprived animals were placed in the tubes, biting behavior was tested by feeding each animal a piece of laver (US). A 10 min interval was interposed between this test and the remaining procedure. Before training, animals received four CS presentations at 60 sec intervals (pretest). The total number of bites that occurred during this 4 min period was determined. To control for individual differences in the naïve response to the CS, pretest scores were balanced among groups. Biting behavior was defined as a sequence of movements involving the opening of the jaws and the protraction of the odontophore (a white, tongue-like structure) from within the buccal cavity, followed by the retraction of the odontophore and the closing of the jaws. As an additional criterion, the odontophore had to be extended beyond the level of the jaws at the peak of protraction.
Training consisted of 10 trials at an intertrial interval (ITI) of 4 min. After training, animals received another four presentations of the CS (Fig. 1C). As in the pretest, the total number of bites that occurred during this 4 min interval was counted. Testing was done blindly, without knowledge of the training history of the animal. Finally, animals received a piece of food (US) to test for intact feeding behavior. Animals that did not eat at this point were excluded from analysis. Animals were also excluded if they failed to ingest the US within 60 sec, before, or more than once during training, or if they inked at any time during the handling or training. Approximately 15% of animals failed to meet these criteria.
Surgery. Animals were tested for good feeding responses before surgery, injected with 20 ml of isotonic MgCl2, and placed in a small tank containing seawater and ice cubes (also prepared from seawater). After animals had ceased to contract in response to strong tactile stimulation, two hooks shaped from epidermic needles (30G1/2; Becton Dickinson, Franklin Lakes, NJ) were placed in the skin along the dorsal midline of the animal and fastened on either end of the tank by strings. The anterior hook was placed just caudal of the base of the rhinophores, and the posterior hook was placed ∼2 cm caudal of the anterior hook. By adjusting the length of the strings, the skin between the hooks was stretched along the dorsal midline and raised above the water surface. An incision was cut along the dorsal midline between the hooks and pulled open using a second pair of hooks arranged perpendicularly to the first pair. Using forceps, the esophagus was pulled up until the esophageal nerve (En2) (see Nargeot et al., 1997, for nomenclature) of the buccal ganglia became visible at the junction between the buccal mass and the esophagus. The anterior branch of the esophageal nerve was cut on both sides of the animal. The esophagus was also handled in sham-operated animals, but no nerves were cut. Subsequently, the second pair of hooks was removed, and the wound margins were stitched together. Using nonabsorbable surgical silk suture (4–0; Ethicon, Somerville, NJ) a triple-knot stitch was made at one end of the incision, and continuous single-knot stitches were made approximately every 2 mm to close the wound. The final stitch was fastened with a triple knot, the ends of the suture were cut short, and the remaining hooks were removed from the skin. Animals were then returned to 15°C seawater and left to recover for a minimum of 7 d before behavioral training and testing. All animals resumed eating seaweed within 1 d of surgery. After the behavioral experiments, the lesions were verified in all animals.
Scoring and data analysis. To examine the effects of training, difference scores were calculated by subtracting the total number of bites observed during the pretest from the total number of bites observed during the test. Thus, positive difference scores indicated an increased responsiveness to the CS. Data were analyzed with either the Mann–Whitney test (U), the Kruskal–Wallis test (H), or the Scheirer–Ray–Hare extension of the Kruskal–Wallis test (Hx). A nonparametric version of the Newman–Keuls test (Q), was used for post hoc analyses, and pairwise comparisons were done using the Wilcoxon signed-rank test (W). All statistics are two-tailed.
RESULTS
Feeding behavior can be classically conditioned
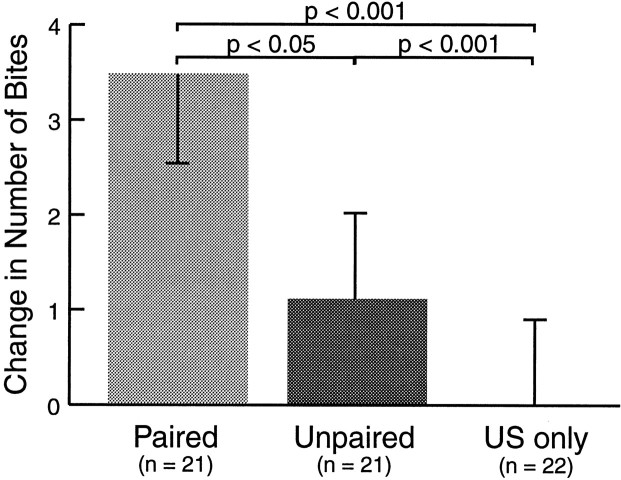
Three groups of animals were trained using the protocol outlined above (Fig. 1). Animals in the paired group were trained with a delay classical conditioning procedure. To assess whether learning depended on the contiguity of the stimuli, an unpaired control group received CS and US presentations with an ISI of 2 min. A second control group received only US presentations, to determine whether the response to the test CS could be modified by context conditioning and/or nonassociative food arousal (Fig. 1B). The effect of these training procedures on the number of bites in response to the CS was tested 1 hr after the last conditioning trial and compared to the pretest score (Fig. 2). Paired training resulted in a greater increase in the number of bites to the CS (3.48 ± 0.93; n = 21) as compared to unpaired training (1.10 ± 0.94; n = 21) and US-only presentations (0.00 ± 0.87, n = 22,p < 0.05, H = 7.206; paired vs unpaired: p < 0.05, Q = 2.151; paired vs US-only: p < 0.001, Q = 5.270). A comparison between the difference scores in the paired and unpaired groups suggested that ∼70% of the increase in biting after paired training was indeed pairing-specific. Thus, in subsequent classical conditioning experiments the use of an unpaired control group was deemed sufficient.
Fig. 2.
One hour retention for classical conditioning of feeding behavior. Conditioning was measured by a change in the number of bites elicited by the CS before (pretest) and after (test) training. Paired training resulted in a greater increase than unpaired training and US presentations only. In this and subsequent illustrations, data are displayed as means + SEM.
These results indicate that feeding behavior can be classically conditioned using the paired training protocol outlined above, and that memory for the CS–US association was retained for at least 1 hr. Other protocols that differed in the type of tactile CS (glass probe) and the intertrial interval (10 min) were also effective in inducing classical conditioning (Lechner et al., 1997). In all these protocols, classical conditioning resulted in an increased probability of biting behavior in response to the CS (see also companion paper).
Long-term memory for classical conditioning of feeding behavior
Memory storage is thought to be a continuously ongoing process of stabilizing, transforming, and updating neural substrates that represent learned information. Evidence from multiple animal models for memory storage suggests that this process involves distinct molecular mechanisms and occurs in temporally distinct phases. For example, whereas short-term memory is likely to depend on rapid, covalent modifications of proteins involved in synaptic release, postsynaptic receptivity, and/or intrinsic properties of neurons, long-term memory is generally thought to depend on protein synthesis (Flexner et al., 1963; Agranoff and Klinger, 1964; Barondes and Cohen, 1965) (for review, see Davis and Squire, 1984) and morphological modifications of existing neurons (Bailey and Chen, 1983; Patel et al., 1988). A model system that exhibits both short-term and long-term memory is therefore likely to contain a more complex system of molecular signaling cascades than one that exhibits only short-term storage. To determine whether classical conditioning of feeding was able to induce long-term associative memory, retention was tested 1 d after training. At this interval, memory in analogs of nonassociative (Castellucci et al., 1986; Montarolo et al., 1986; Dale et al., 1987; Schacher et al., 1988;Zhang, 1997) and associative (Schacher et al., 1997) memory inAplysia have been shown to depend on protein synthesis.
Two groups of animals received either paired or unpaired training as outlined above (Fig. 1). In these experiments, however, animals were returned to their home cages after training, and retention was tested 24 hr later. To mimic the pretest conditions of the previous day, animals were fed one piece of seaweed 10 min before testing. The increase in the paired group (5.42 ± 1.38; n = 19) was significantly greater than the increase in the unpaired group (2.90 ± 1.01; n = 20; p < 0.05;U = 117; Fig. 3), suggesting that the associative memory for classical conditioning of feeding was retained for at least 24 hr after training. Furthermore, this result raises the possibility that classical conditioning of feeding can serve as a model system for studying the cellular and molecular mechanisms underlying short-term and long-term forms of associative plasticity.
Fig. 3.
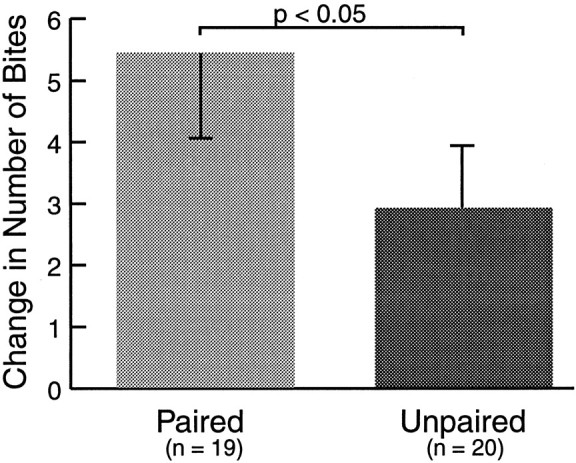
Long-term memory for classical conditioning of feeding. Associative memory for the CS, induced by paired training was retained for a minimum of 24 hr. Paired training resulted in a significantly greater increase in the number of bites to the CS than unpaired training. Before testing, animals were fed a piece of seaweed to mimic the conditions of the pretest. This procedure may account for the higher scores in both groups, compared to those reported in other experiments.
Pathways of reinforcement in classical conditioning of feeding
One goal in the behavioral analysis of learning and memory is to determine the boundary conditions for the successful acquisition and retention of information. The temporal dynamics of the learning process, for example, can be addressed experimentally by determining the rate of acquisition that is achieved when the temporal features of the training protocol are altered systematically. Likewise, information about the organization of sensory pathways that mediate information during learning can be gained by manipulating certain features of the stimulus presentation. Insights gained from such behavioral manipulations can then be used to guide a reductionist approach to studying learning and memory in greater detail.
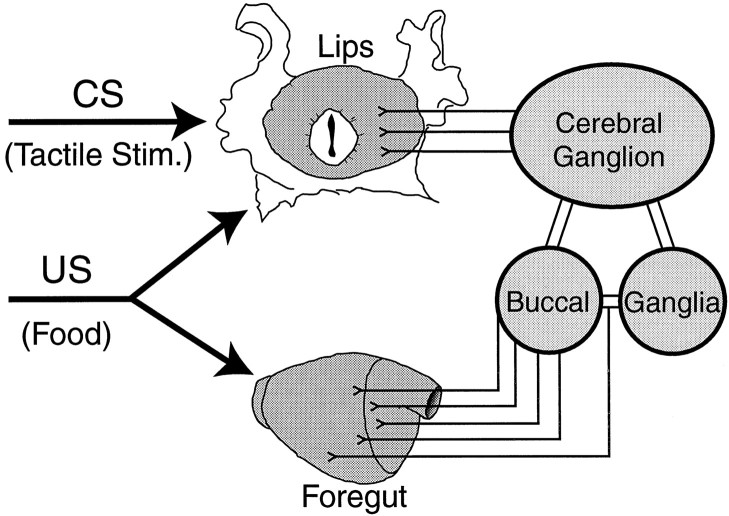
In the classical conditioning procedure for feeding behavior presented here, the US comes in contact with external epithelia (e.g., lips) and the internal epithelia (e.g., foregut) of the animal. These receptive fields are innervated by the peripheral nerves of the cerebral and buccal ganglia, respectively (Fig. 4). To gain more insight into the organization of the US pathway, the US presentation was manipulated to selectively activate afferent pathways originating in the lips. Lip stimulation was achieved by bringing food in contact with the lips for 5 sec without allowing the animals to ingest it. The duration of the US presentation was based on the mean latency for food-induced bites, at which time contact between food and lips is terminated naturally.
Fig. 4.
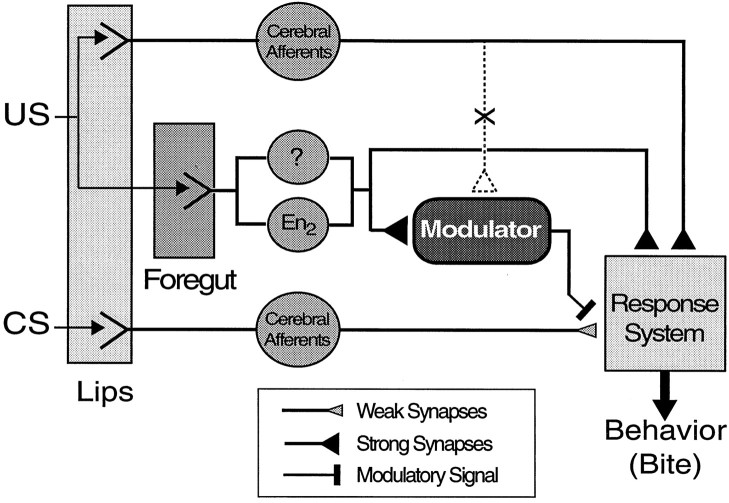
Organization of US-mediating afferents. In the training protocol outlined above, the CS is limited to the tactile stimulation of the lips. In contrast, the US (food) comes in contact with the sensory epithelia of the lips and the foregut. The lips and other regions of the head are innervated by nerves of the cerebral ganglion. The foregut is exclusively innervated by nerves of the buccal ganglia. Although food-induced activation of cerebral afferents reliably elicits biting behavior, it is not known whether these afferents also mediate reinforcement during classical conditioning. An alternative hypothesis is that buccal afferents mediate this reinforcement instead or contribute to reinforcement.
Two groups of animals received paired or unpaired training during which they were allowed to ingest the US presented to the lips (i.e., normal). Another two groups received paired or unpaired training with US presentations to the lips only (i.e., they were not allowed to ingest food; lips only; Fig. 5). Only paired training with the normal US led to an increase in the number of bites in response to the CS 1 hr after training (pairednormal: 2.20 ± 0.85;n = 20). In contrast, paired training with US presentation to the lips only resulted in a decrease in CS responsiveness (pairedlips-only: −2.61 ± 0.97; n = 18). Decreases in the responsiveness to the CS were also observed in the controls (unpairednormal: −0.44 ± 0.86,n = 18; and unpairedlips-only: −1.87 ± 0.98, n = 15). A post hocanalysis after a Kruskal–Wallis test (p < 0.0001; Hx = 20.89) between the groups that received the normal US revealed a pairing-specific increase in responsiveness to the CS (pairednormal vs unpairednormal: p < 0.05;Q = 1.981), indicative of associative learning. In contrast, there was no significant difference between paired and unpaired groups of animals that had received US presentation to the lips only (pairedlips-only vs unpairedlips-only: NS, Q = 0.887), suggesting that these animals failed to form CS–US associations. Finally, paired training with the normal US resulted in more bites in response to the CS than paired training with US presentation to the lips only (pairednormal vs pairedlips-only: p < 0.001,Q = 4.225). These findings suggest that US presentation to the lips only was not sufficient to reinforce CS-mediating pathways during classical conditioning.
Unexpectedly, repeated US presentations to only the lips resulted in a decrease in the response to the CS in both paired and unpaired groups. Although this decrease was not pairing-specific, it was significant with respect to the pretest scores (p < 0.001;W = 66.0; n = 33) when data from both groups were pooled. This decrease raised the possibility that repeated US presentation to the lips only had an inhibitory effect on feeding behavior, which is normally overcome by food stimulation of epithelia in the foregut, during ingestion. However, in another associative training proto- col that was reported by Susswein and Schwarz (1983), inhibition of feeding behavior by food that was prevented from entering the buccal cavity was not observed. Thus, a more parsimonious explanation for the decrease in the number of bites 1 hr after training may be a time-dependent decrement in food arousal over the course of training. In this case, the failure of lip stimulation to support CS–US associations may indicate that US-mediating afferent information from the foregut is necessary for associative reinforcement of the CS pathway, whereas afferents from the lips are able to elicit biting behavior, but are neutral with respect to reinforcement.
To test this possibility, another behavioral experiment was performed. A group of animals that received 10 trials of US presentation to the lips only was compared to a group that received no food stimulation after the pretest. Bite responses to the CS were tested 1 hr after training or after a period equivalent to the training and retention interval (Fig. 6). In both groups, the number of bites to the CS was lower after training than before (lip only: −2.32 ± 1.20, n = 21; no training: −2.93 ± 1.13; n = 16). This result, in the absence of any CS during training, suggests that the decrease in the responsiveness to the CS in the previous experiment was not specific to the CS presentation. Furthermore, the similarity of the scores between the groups that received US presentation to the lips only and the group that received no training suggested that food stimulation of only the lips was not effective in sustaining food arousal over the course of a 1 hr retention interval. Thus, it is possible that the decrease in biting responses after paired and unpaired training with food stimulation of the lips only as reinforcement reflected a time-dependent decrease in food arousal, rather than a US-specific inhibitory effect.
Fig. 6.
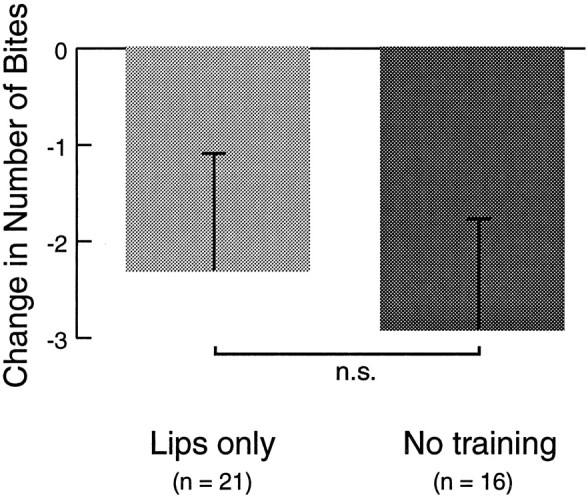
Time-dependent decrements in CS-evoked biting. To determine whether food presented only to the lips caused an inhibition of feeding behavior, the effect of US presentation to the lips only was compared to the time-dependent decrement in food arousal over an interval equivalent to training and 1 hr retention. Ten trials of food stimulation of only the lips resulted in a decrease in responsiveness to the CS 1 hr after the last food presentation. This decrease, which was similar in magnitude to the decrease seen after paired training with US presentation to the lips only, however, was not distinguishable from the time-dependent decrease in biting responses in control animals that did not receive any stimulation after the pretest. This result suggests that food stimulation of the lips only does not have an inhibitory effect on feeding, but rather fails to support food arousal over the duration of the retention interval.
Involvement of the esophageal nerve in appetitive reinforcement
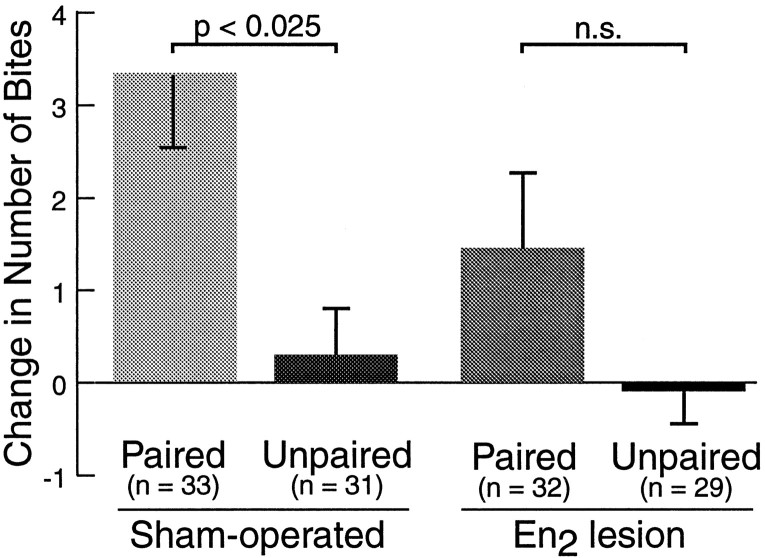
To examine the potential role of buccal afferent pathways in appetitive classical conditioning more directly, the effect of surgically lesioning selected buccal nerves before training was determined. Bilateral lesions of the anterior branch of the esophageal nerve (En2) were performed by severing these nerve branches in anesthetized animals (see Materials and Methods). These lesions had no apparent effects on feeding behavior at the time of training, 7–14 d after surgery. Sham-operated animals that did not sustain injury to the nervous system during surgery served as controls. Lesioned and sham-operated animals received either paired or unpaired training. In this experiment, all animals were allowed to ingest food during training and were tested for conditioned responses to the CS 1 hr after training. Sham-operated animals that received paired training showed a pairing-specific increase in the number of bites to the CS (pairedsham: 3.33 ± 0.79;n = 33) compared to unpaired sham-operated animals (unpairedsham: 0.29 ± 0.50;n = 31; p < 0.025; U = 343). In contrast, difference scores of paired animals with bilateral En2 lesions (pairedlesion: 1.44 ± 0.83; n = 32), were not significantly different from those of unpaired animals with En2 lesions (unpairedlesion: −0.07 ± 0.37;n = 29; NS, U = 397.5; Fig.7). These observations are consistent with the possibility that afferent pathways originating in the foregut and mediated by the anterior branch of the esophageal nerve contributed to appetitive reinforcement during classical conditioning of feeding inAplysia.
Fig. 7.
Effect of esophageal nerve lesions on classical conditioning. Animals with bilateral lesions of the anterior branch of the esophageal nerve (En2) did not show a significant increase in the number of bites to the CS 1 hr after training when compared with unpaired control animals. In contrast, sham-operated animals that were trained concurrently showed normal conditioning.
DISCUSSION
Appetitive reinforcement can induce classical conditioning in Aplysia
This series of experiments demonstrated that components of feeding behavior (i.e., biting) in Aplysia can be brought under the control of a tactile stimulus as a result of appetitive classical conditioning. Because the tactile stimulus could occasionally elicit biting behavior before training, this example of classical conditioning may be referred to as α conditioning (Byrne, 1987).
Thus far, most training protocols that have been developed to study the cellular and molecular mechanisms of associative plasticity in mammals and invertebrates have relied on classical conditioning procedures involving aversive reinforcement to induce associative learning (Estes and Skinner, 1941; Gormezano et al., 1962; Garcia et al., 1966; Crow and Alkon, 1978; Carew et al., 1981; Tully and Quinn, 1985). Although this approach has proven very productive, the predominance of a particular type of associative learning and memory in studies of learning and memory does not reveal to what extent the cellular and molecular mechanisms identified in these cases generalize to other forms of associative learning and memory, such as appetitive conditioning or operant forms of learning. To attain a broader understanding of the mechanisms underlying associative learning and memory, it will be necessary to include other examples of associative learning in future analyses. The appetitive classical conditioning procedure described here could be a first step to such a comparative approach. Classical conditioning of feeding inAplysia is a reliable phenomenon that has been replicated several times using the protocol above, or slightly different protocols (Colwill et al., 1997; Lechner et al., 1997). Moreover, training produced an associative memory that persisted for at least 24 hr, which allows for subsequent cellular analyses (see companion paper), and may thus prove a useful model system in which to extend the analysis of mechanisms for associative learning and memory to appetitive classical conditioning. Finally, evidence that feeding behavior inAplysia could be modified by operant conditioning is accumulating (Susswein and Schwarz, 1983; Nargeot et al., 1997), which would further broaden the spectrum of analysis.
The experiments presented here also provide the first insights into the organization of the US-mediating pathways that contribute to appetitive associative learning in Aplysia. The information gathered thus far can be summarized in a simple model circuit (Fig.8). In this model, biting behavior is produced by a response system that comprises command-like neurons in the cerebral ganglion and central pattern generators in the cerebral and buccal ganglia. Cerebral mechanosensory pathways originating in the lips have been shown to make connections to the response system (Rosen et al., 1979, 1982), but are presumably too weak to drive the response system reliably before conditioning. The effectiveness of mechanosensory activity to drive the response system can increase if CS and US presentations are made contingent on each other during paired training. Sensory fibers that originate in the lips as well as in the foregut mediate the US and reliably drive the response system by means of strong excitatory synapses. According to the results shown in Figures 5 and 6, however, afferents from the lips are unable to modulate the ability of CS-mediating pathways to elicit biting behavior, and thus, fail to produce classical conditioning. Afferent pathways originating in the foregut, on the other hand, may be sufficient to modulate the CS pathway. The impairment of classical conditioning in animals that sustained bilateral lesions of En2 (Fig. 7) is consistent with the idea that En2 contributes to the increase in CS-induced biting during training by modulating CS-mediating pathways. This hypothesis is supported by independent evidence for a role of the esophageal nerve in associative learning in a paradigm developed bySusswein and Schwarz (1983). In this paradigm, animals were presented with food sewn into small nets that the animals could pull into the buccal cavity but not swallow. After repeated attempts to swallow netted food, animals ceased responding to netted food, and made fewer attempts to swallow netted food when presented with it on subsequent days. This protocol shares features with operant conditioning in that negative reinforcement (inability to ingest food) is made contingent on attempts to eat netted food (Susswein et al., 1986; Nargeot et al., 1997). Yet, because the conditioned decrease in response to netted food is specific to the particular kind of food used during training, it is likely that animals also form an association between a chemical stimulus characteristic for the food and negative reinforcement. Thus, this associative learning paradigm may contain components of classical conditioning. Moreover, bilateral lesions of the esophageal nerve, which mediates sensory information from the foregut, abolished this form of learning (Schwarz and Susswein, 1986). A comparison between the effect of nerve lesions (Fig. 7) and of US presentations to the lips only (Fig. 5), however, suggests that En2 lesions attenuated appetitive classical conditioning to a lesser extent than the lips-only US, which prevented the activation of all buccal afferents. Thus, it is likely that appetitive modulation in classical conditioning of feeding is not exclusively mediated by En2. Other nerves that innervate the sensory epithelia of the foregut, such as branches of buccal nerve 2, may contribute to this modulation, in addition to En2. Future lesion studies will be necessary to explore the reinforcement-mediating pathways for classical conditioning of feeding in greater detail.
Fig. 8.
Model circuit for classical conditioning of feeding behavior in Aplysia. Information about the CS is mediated by cerebral mechanosensory afferents that originate in the lips. These afferents make weak synaptic connections to a response system consisting of command neurons, located in the cerebral ganglion, and central pattern generators in the cerebral and buccal ganglia that produce consummatory feeding behavior (bites). As a result of classical conditioning, these weak connections and/or the response system itself undergo modulation that leads to an increased probability for feeding behavior to occur in response to mechanosensory stimulation of the lips. The modulatory system is activated by sensory afferents mediating the US (food). Although US-mediating cerebral afferents from the lips reliably activate the response system, no evidence was found that they activate the modulatory system (dashed lines), and thus do not contribute to appetitive reinforcement. In contrast, experimental evidence is consistent with the idea that afferents from the foregut, including the En2, mediate the reinforcing component of the US by activating the modulatory system during conditioning.
Other invertebrate models of classical conditioning using appetitive training
Appetitive protocols for classical conditioning have been developed in other invertebrates. Proboscis extension, for example, has been successfully conditioned in flies (Nelson, 1971) and honeybees (Bitterman et al., 1983), using food as reinforcement and chemical or olfactory stimuli as CSs. Although insect brains are challenging preparations for electrophysiological techniques, neurons in the honeybee brain have been identified that mediate appetitive reinforcement (Hammer, 1993) or correlate with associative learning (Mauelshagen, 1993). In addition, octopamine has been identified as a modulatory transmitter for appetitive reinforcement (Menzel et al., 1993; Kreissl et al., 1994; Hammer and Menzel, 1998), and the molecular mechanisms underlying classical conditioning in the honeybee have been characterized in some detail (Hildebrandt and Müller, 1995;Menzel and Müller, 1996; Müller, 1996, 1997). Classical conditioning protocols for molluscan preparations, such as the pond snail Lymnea stagnalis that are more accessible to cellular analyses, have also been developed (Alexander et al., 1982, 1984;Audesirk et al., 1982; Kemenes and Benjamin, 1989, 1994). The molecular mechanisms underlying this example of associative learning, however, have not been studied. Reward learning in Lymnea could be induced by making the presence of sucrose solution (US) in the water surrounding the animal contingent on a chemical or tactile stimulus (CS). Training results in an increase in feeding movements in response to tactile stimulation that can persist for several weeks (Alexander et al., 1984; Kemenes and Benjamin, 1994). Recently, extracellular and cellular correlates of classical conditioning in Lymnea have been identified (Staras et al., 1998, 1999) in elements of the circuitry that produces feeding behavior (Benjamin and Elliott, 1989), and the neural representation of the CS and US have been characterized (Kemenes and Benjamin, 1994; Staras et al., 1999).
An analysis of the cellular and molecular mechanisms underlying classical conditioning in the currently studied invertebrate models of appetitive learning may elucidate mechanisms of neural function that specifically mediate appetitive forms of learning and memory. Such analyses could then provide the foundation for expanding and refining the known principles that apply to associative learning and memory in general.
Footnotes
This work was supported by National Institute of Mental Health Grant RØ1 MH 58321 and Grant 011618–048 from the Texas Higher Education Coordinating Board. We thank Hanh N. Nguyen for training many of the animals included in this study.
Correspondence should be addressed to John H. Byrne, Department of Neurobiology and Anatomy, The University of Texas, Houston Medical School, 6431 Fannin Street, Houston, TX 77030. E-mail:jbyrne@nba19.med.uth.tmc.edu.
REFERENCES
- 1.Agranoff BW, Klinger PD. Puromycin effect on memory fixation in the goldfish. Science. 1964;146:952–953. doi: 10.1126/science.146.3646.952. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JE, Audesirk TE, Audesirk GJ. Rapid, nonaversive conditioning in a freshwater gastropod. II. Effects of temporal relationships in learning. Behav Neural Biol. 1982;36:391–402. doi: 10.1016/s0163-1047(82)90792-0. [DOI] [PubMed] [Google Scholar]
- 3.Alexander JE, Audesirk TE, Audesirk GJ. One-trial reward learning in the snail Lymnea stagnalis. J Neurobiol. 1984;15:67–72. doi: 10.1002/neu.480150107. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JA. Patterns of response of neurons in the cerebral ganglion of Aplysia californica. Exp Neurol. 1967;19:65–77. doi: 10.1016/0014-4886(67)90007-6. [DOI] [PubMed] [Google Scholar]
- 5.Audesirk TE, Alexander JE, Audesirk GJ, Moyer CM. Rapid, nonaversive conditioning in a freshwater gastropod. I. Effects of age and motivation. Behav Neural Biol. 1982;36:379–390. doi: 10.1016/s0163-1047(82)90782-8. [DOI] [PubMed] [Google Scholar]
- 6.Bailey CH, Chen M. Morphological basis of long-term habituation and sensitization in Aplysia. Science. 1983;220:91–93. doi: 10.1126/science.6828885. [DOI] [PubMed] [Google Scholar]
- 7.Barondes SH, Cohen HD. Puromycin effect on successive phases of memory storage. Science. 1965;151:594–595. doi: 10.1126/science.151.3710.594. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin PR, Elliott JH. Snail feeding oscillator: the central pattern generator and its control by modulatory interneurons. In: Jacklet JW, editor. Neuronal and cellular oscillators. Marcel Dekker; New York: 1989. pp. 173–214. [Google Scholar]
- 9.Bitterman ME, Menzel R, Fietz A, Schaefer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol. 1983;97:107–119. [PubMed] [Google Scholar]
- 10.Byrne JH. Cellular analysis of associative learning. Physiol Rev. 1987;67:329–439. doi: 10.1152/physrev.1987.67.2.329. [DOI] [PubMed] [Google Scholar]
- 11.Carew TJ, Walters ET, Kandel ER. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981;1:1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellucci VF, Frost WN, Goelet P, Montarolo PG, Schacher S, Morgan JA, Blumenfeld H, Kandel ER. Cell and molecular analysis of long-term sensitization in Aplysia. J Physiol (Paris) 1986;81:349–357. [PubMed] [Google Scholar]
- 13.Colwill RM, Goodrum K, Martin A. Pavlovian appetitive discriminative conditioning in Aplysia californica. Anim Learn Behav. 1997;25:268–276. [Google Scholar]
- 14.Crow T, Alkon DL. Retention of an associative behavioral change in Hermissenda. Science. 1978;201:1239–1241. doi: 10.1126/science.694512. [DOI] [PubMed] [Google Scholar]
- 15.Dale N, Kandel ER, Schacher S. Serotonin produces long-term changes in the excitability of Aplysia sensory neurons in culture that depend on new protein synthesis. J Neurosci. 1987;7:2232–2238. doi: 10.1523/JNEUROSCI.07-07-02232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- 17.Estes WK, Skinner BF. Some quantitative properties of anxiety. J Exp Psychol. 1941;39:390–400. [Google Scholar]
- 18.Flexner JB, Flexner LB, Stellar E. Memory in mice as affected by intracerebral puromycin. Science. 1963;141:57–59. doi: 10.1126/science.141.3575.57. [DOI] [PubMed] [Google Scholar]
- 19.Fredman SM, Jahan-Parwar B. Processing of chemosensory and mechanosensory information in identifiable Aplysia neurons. Comp Biochem Physiol. 1980;66A:25–34. [Google Scholar]
- 20.Garcia J, Ervin FR, Koelling RA. Learning with prolonged delay of reinforcement. Psychonom Sci. 1966;5:121–122. [Google Scholar]
- 21.Gormezano I, Schneiderman N, Deaux E, Fuentes I. Nictitating membrane: classical conditioning and extinction in the albino rabbit. Science. 1962;138:33–34. doi: 10.1126/science.138.3536.33. [DOI] [PubMed] [Google Scholar]
- 22.Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- 23.Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn Mem. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- 24.Hildebrandt H, Müller U. Octopamine mediates rapid stimulation of protein kinase A in the antennal lobe of honeybees. J Neurobiol. 1995;27:44–50. doi: 10.1002/neu.480270105. [DOI] [PubMed] [Google Scholar]
- 25.Jahan-Parwar B. Conditioned responses in Aplysia californica. Am Zool. 1970;10:287–287. [Google Scholar]
- 26.Kemenes G, Benjamin PR. Appetitive learning in snails shows characteristics of conditioning in vertebrates. Brain Res. 1989;489:163–166. doi: 10.1016/0006-8993(89)90019-x. [DOI] [PubMed] [Google Scholar]
- 27.Kemenes G, Benjamin PR. Training in a novel environment improves the appetitive learning performance of the snail, Lymnea stagnalis. Behav Neural Biol. 1994;61:139–149. doi: 10.1016/s0163-1047(05)80067-6. [DOI] [PubMed] [Google Scholar]
- 28.Kreissl S, Eichmueller S, Bicker G, Rapus J, Eckert M. Octopamine-like immunoreactivity in the brain and subesophageal ganglion in the honeybee. J Comp Neurol. 1994;348:583–595. doi: 10.1002/cne.903480408. [DOI] [PubMed] [Google Scholar]
- 29.Kupfermann I. Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol. 1974a;10:1–26. doi: 10.1016/s0091-6773(74)91644-7. [DOI] [PubMed] [Google Scholar]
- 30.Kupfermann I. Dissociation of the appetitive and consummatory phases of feeding behavior in Aplysia: a lesion study. Behav Biol. 1974b;10:89–97. doi: 10.1016/s0091-6773(74)91694-0. [DOI] [PubMed] [Google Scholar]
- 31.Lechner HA, Baxter DA, Byrne JH. Classical conditioning of feeding behavior in Aplysia. Soc Neurosci Abstr. 1997;23:1334–1334. [Google Scholar]
- 32.Lechner HA, Baxter DA, Byrne JH. Classical conditioning of feeding in Aplysia: II. Neurophysiological correlates. J Neurosci. 2000;20:3377–3386. doi: 10.1523/JNEUROSCI.20-09-03377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lickey ME. Learned behavior in Aplysia vacaria. J Comp Physiol Psychol. 1968;66:712–718. [Google Scholar]
- 34.Mauelshagen J. Neural correlates of olfactory learning in an identified neuron in the honeybee brain. J Neurophysiol. 1993;69:609–625. doi: 10.1152/jn.1993.69.2.609. [DOI] [PubMed] [Google Scholar]
- 35.Menzel R, Müller U. Learning and memory in honeybees: from behavior to neural substrates. Annu Rev Neurosci. 1996;19:379–404. doi: 10.1146/annurev.ne.19.030196.002115. [DOI] [PubMed] [Google Scholar]
- 36.Menzel R, Hammer M, Schneider U, Heyne M, Durst C. Aminergic modulation of associative and non-associative plasticity in honeybees. In: Elsner N, Heisenberg M, editors. Gene—Brain—Behaviour. Thieme Verlag; Stuttgart: 1993. pp. 843–862. [Google Scholar]
- 37.Montarolo P, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- 38.Müller U. Inhibition of nitric oxide synthase impairs a distinct form of long-term memory in the honeybee, Apis mellifera. Neuron. 1996;16:541–549. doi: 10.1016/s0896-6273(00)80073-2. [DOI] [PubMed] [Google Scholar]
- 39.Müller U. Neuronal cAMP-dependent protein kinase type II is concentrated in mushroom bodies of Drosophila melanogaster and the honeybee Apis mellifera. J Neurobiol. 1997;33:33–44. doi: 10.1002/(sici)1097-4695(199707)33:1<33::aid-neu4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 40.Nargeot R, Baxter DA, Byrne JH. Contingent-dependent enhancement of rhythmic motor patterns: an in vitro analog of operant conditioning. J Neurosci. 1997;17:8093–8105. doi: 10.1523/JNEUROSCI.17-21-08093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson MC. Classical conditioning in the blowfly (Phormia re- gina): associative and excitatory factors. J Comp Physiol Psychol. 1971;77:353–368. doi: 10.1037/h0031882. [DOI] [PubMed] [Google Scholar]
- 42.Patel SN, Rose SC, Stewart MG. Training-induced dendritic spine density changes are specifically related to memory formation processes in the chick, Gallus domesticus. Brain Res. 1988;463:168–173. doi: 10.1016/0006-8993(88)90542-2. [DOI] [PubMed] [Google Scholar]
- 43.Rosen SC, Weiss KR, Kupfermann I. Response properties and synaptic connections of mechanoafferent neurons in cerebral ganglion of Aplysia. J Neurophysiol. 1979;42:954–974. doi: 10.1152/jn.1979.42.4.954. [DOI] [PubMed] [Google Scholar]
- 44.Rosen SC, Weiss KR, Cohen JL, Kupfermann I. Interganglionic cerebral-buccal mechanoafferents of Aplysia: receptive fields and synaptic connections to different classes of neurons involved in feeding behavior. J Neurophysiol. 1982;48:271–288. doi: 10.1152/jn.1982.48.1.271. [DOI] [PubMed] [Google Scholar]
- 45.Schacher S, Castellucci VF, Kandel ER. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science. 1988;240:1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- 46.Schacher S, Wu F, Sun Z-Y. Pathway-specific synaptic plasticity: activity-dependent enhancement and suppression of long-term heterosynaptic facilitation at converging inputs on a single target. J Neurosci. 1997;17:597–606. doi: 10.1523/JNEUROSCI.17-02-00597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz M, Susswein AJ. Identification of the neural pathway for reinforcement of feeding when Aplysia learn that food is inedible. J Neurosci. 1986;6:1528–1536. doi: 10.1523/JNEUROSCI.06-05-01528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staras K, Kemenes G, Benjamin PR. Neurophysiological correlates of unconditioned and conditioned feeding behavior in the pond snail Lymnaea stagnalis. J Neurophysiol. 1998;79:3030–3040. doi: 10.1152/jn.1998.79.6.3030. [DOI] [PubMed] [Google Scholar]
- 49.Staras K, Kemenes G, Benjamin PR. Cellular traces of behavioral classical conditioning can be recorded at several specific sites in a simple nervous system. J Neurosci. 1999;19:347–357. doi: 10.1523/JNEUROSCI.19-01-00347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Susswein AJ, Schwarz M. A learned change of response to inedible food in Aplysia. Behav Neural Biol. 1983;39:1–6. doi: 10.1016/s0163-1047(83)90535-6. [DOI] [PubMed] [Google Scholar]
- 51.Susswein AJ, Kupfermann I, Weiss KR. The stimulus control of biting in Aplysia. J Comp Physiol [A] 1976;108:75–96. [Google Scholar]
- 52.Susswein AJ, Schwarz M, Feldman E. Learned changes of feeding behavior in Aplysia in response to edible and inedible foods. J Neurosci. 1986;6:1513–1527. doi: 10.1523/JNEUROSCI.06-05-01513.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 54.Xin Y, Weiss KR, Kupfermann I. Distribution in the central nervous system of Aplysia of afferent fibers arising from cell bodies located in the periphery. J Comp Neurol. 1995;359:627–643. doi: 10.1002/cne.903590409. [DOI] [PubMed] [Google Scholar]
- 55.Zhang F. PhD dissertation. University of Texas–Houston, Health Science Center, Graduate School of Biomedical Sciences; 1997. Long-term synaptic facilitation of tail sensorimotor connections in Aplysia. [Google Scholar]