Abstract
Feeding behavior in Aplysia californica can be classically conditioned using tactile stimulation of the lips as conditional stimulus (CS) and food as unconditional stimulus (US) [Lechner et al., 2000 (companion paper)]. Conditioning resulted in an increase in the number of CS-evoked bites that persisted for at least 24 hr after training. In this study, neurophysiological correlates of classical conditioning training were identified and characterized in anin vitro preparation of the cerebral and buccal ganglia. Stimulation of a lip nerve (AT4), which mediates mechanosensory information, resulted in a greater number of buccal motor patterns (BMPs) in ganglia isolated from animals that had received paired training than in ganglia from control animals. The majority of the evoked BMPs were classified as ingestion-like patterns. Intracellular recordings from pattern-initiating neuron B31/32 revealed that stimulation of AT4 evoked greater excitatory input in B31/32 in preparations from animals that had received paired training than from control animals. In contrast, excitatory input to buccal neuron B4/5 in response to stimulation of AT4 was not significantly increased by paired training. Moreover, correlates of classical conditioning were specific to stimulation of AT4. The number of spontaneously occurring BMPs and the intrinsic properties of two buccal neurons (B4/5 and B31/32) did not differ between groups. These results suggest that appetitive classical conditioning of feeding resulted in the pairing-specific strengthening of the polysynaptic pathway between afferent fibers and pattern-initiating neurons of the buccal central pattern generator.
Keywords: neural correlates, classical conditioning, feeding behavior, Aplysia, learning and memory, buccal motor patterns
The identification of mechanisms for neural plasticity and learning and memory has been facilitated by using electrophysiological techniques to assay the biophysical properties of individual neurons and their synaptic connections in in vitro preparations. One approach involves developing correlate preparations, in which plasticity is induced by behavioral training of the intact animal and subsequent electrophysiological analyses in vitro. Neural correlates of learning and memory can thus help to establish a link between behavioral learning and neural plasticity and can reveal the nature of the plasticity induced by learning.
The identification of individual neurons and synaptic connections that undergo plasticity in response to associative learning is greatly facilitated by pre-existing knowledge of the neural networks that are active during training. Here, feeding behavior in Aplysiahas important advantages. Feeding behavior can be classically conditioned using tactile stimuli as conditional stimulus (CS) and food as unconditional stimulus (US) (Colwill et al., 1997) (see also Lechner et al., 2000), and the neural circuitry that underlies its control has been studied extensively (Kupfermann, 1974a,b; Gardner, 1977; Cohen et al., 1978; Rosen et al., 1979, 1982, 1991; Jahan-Parwar et al., 1983;Weiss et al., 1986a–c; Susswein and Byrne, 1988; Kirk, 1989; Chiel et al., 1990; Cropper et al., 1990a,b; Plummer and Kirk, 1990; Teyke et al., 1990, 1991; Morton and Chiel, 1993a,b; Church and Lloyd, 1994;Hurwitz et al., 1994, 1996; Evans et al., 1996; Hurwitz and Susswein, 1996; Perrins and Weiss, 1996, 1998; Baxter et al., 1997;Kabotyanski et al., 1998; Nargeot et al., 1999a,b). The picture that emerges (Fig. 1) from this continuing analysis of the feeding circuitry is that of a mainly hierarchical organization. The cerebral ganglia contain sensory afferents that mediate tactile information [e.g., cerebral mechanoafferents (CM), Rosen et al., 1979; and interganglionic cerebral-buccal mechanoafferents (ICBM), Rosen et al., 1982], and the cerebral ganglia receive chemosensory information from the lips and other regions of the head (Xin et al., 1995). Mechanosensory and chemosensory inputs converge onto cerebral–buccal interneurons (e.g., CBI-1 and CBI-2; Rosen et al., 1991), some of which can elicit neural activity for feeding behavior and are therefore referred to as command-like interneurons. The motor activity that controls the rhythmic movements of the odontophore and radula during consummatory feeding behavior is generated by a central pattern generator (CPG;Teyke et al., 1993; Ziv et al., 1994; Hurwitz et al., 1996, 1997;Baxter et al., 1997) within the buccal ganglia. Importantly, preparations of isolated buccal ganglia continue to express patterned activity that correlates with feeding movements in the intact animal (Cropper et al., 1990b; Morton and Chiel, 1993a,b; Scott et al., 1995;Warman and Chiel, 1995; Hurwitz et al., 1996; Nargeot et al., 1997,1999a–c; Kabotyanski et al., 2000).
Fig. 1.
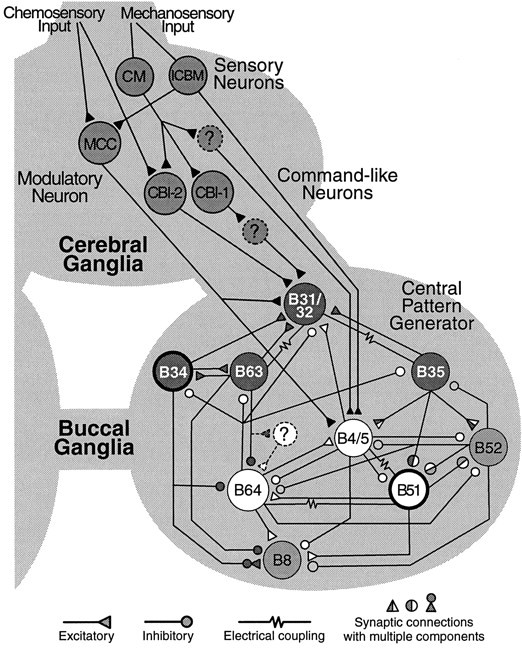
Selected elements of the neural circuit for feeding behavior. Sensory neurons in the cerebral ganglion, such as the cerebral mechanoafferents (CM) mediate tactile information from the lips and other head regions to the buccal ganglion via polysynaptic pathways. A subclass of CM cells, the interganglionic cerebral buccal mechanoafferents (ICBM) project directly to the buccal ganglion. Primary chemosensory neurons are thought to have cell bodies in the periphery and project to interneurons within the cerebral ganglia. Cerebral-to-buccal interneurons (CBI) that receive mechanosensory and chemosensory input, project to the CPG in the buccal ganglia. Some CBI neurons (e.g., CBI-1 and CBI-2) can evoke patterned activity in the CPG and are therefore referred to as command-like neurons. The CPG consists of a network of premotor and motor neurons that give rise to the rhythmic movements of the odontophore and radula during ingestion and rejection behavior. According to the phase of the behavior during which these neurons are active, they can be grouped into protraction neurons (dark circles), such as B31/32, B63, B35, and B34, and retraction neurons (white circles), such as B64, B4/5, and B51. The expression of activity for ingestion or rejection is determined by the phase relationship of activity in closure motor neurons (e.g., B8) and the protraction/retraction cycle. Gating neurons (bold circles) shift the closure activity either toward the protraction phase (B34), to produce rejection movements, or toward the retraction phase (B51) to produce ingestion movements. Patterned activity is terminated, in part, by activity in neuron B52. The phasic motor activity of the CPG can be recorded extracellularly from buccal nerves in isolated ganglia. The circuitry for feeding behavior is under the control of a number of modulatory transmitters that are released by modulatory neurons, such as the serotonergic metacerebral cell (MCC). Note that this diagram is not a comprehensive description of available data on identified neurons and synaptic connections involved in the control, expression, and modulation of feeding behavior.
The present study exploited these advantages and identified the first neurophysiological correlates of classical conditioning of feeding behavior in Aplysia. Specifically, the effects of classical conditioning on fictive feeding, on the intrinsic properties of identified elements of the buccal CPG, and on their synaptic input were examined. We report the identification of extracellular and cellular correlates of appetitive classical conditioning of feeding behavior inAplysia, the expression of which was specific to the stimulation of an afferent pathway, which is likely to mediate information about the CS in intact animals.
MATERIALS AND METHODS
General methods. Aplysia californica were obtained from Alacrity Marine Biological Specimens (Redondo Beach, CA), Marine Specimens Unlimited (Pacific Palisades, CA), and Marinus (Long Beach, CA). They were housed individually in perforated plastic cages, floating in aerated seawater tanks (150 l) at a temperature of 12–15°C. Animals were fed ∼1 gm of dried laver 3 times a week. Before behavioral experiments, animals were food-deprived (see companion paper). To characterize the population of animals used in the experiments reported here, animals were weighed, and their ages were determined by measuring the length of the shell (Peretz and Adkins, 1982). The average weight (± SEM) of the animals used in this study was 140 ± 8.1 gm, and the average age was 116 ± 3.2 d. Experiments were conducted in the months of February, March, July, and August.
Behavioral training. The protocol for classical conditioning of feeding has been described in greater detail by Lechner et al. (2000). Briefly, tactile stimulation of the lips with a paintbrush served as the CS, and food (dried laver) served as the US. Animals received 10 trials of either paired or unpaired presentation of CS and US over the course of 40 min. The total number of bites in response to four CS presentations before training was subtracted from the total number of bites in response to four CSs 1 hr after training. The change in the number of bites was determined in paired and unpaired groups.
Dissection. Within an average of 6 hr after training, animals were injected with 60 ml of isotonic MgCl2 solution, while they were eating a piece of seaweed. This procedure minimizes aversive reactions to the injection, such as respiratory pumping, defensive withdrawal, and the release of mucus and/or ink. An incision was made along the anterior dorsal midline to expose the buccal mass and the esophagus. The most medial and ventral branch (designated branch 4) of the right anterior tentacle nerve (AT) (for nomenclature, see Jahan-Parwar and Fredman, 1976), which terminates in the lip region of the animal, was retained. All other peripheral nerves of the cerebral ganglion were cut short. Then, the esophagus was cut, and the buccal mass together with the buccal and cerebral ganglia was removed and transferred to high divalent solution (see below) for further dissection and desheathing.
Selected peripheral nerves of the right buccal ganglion were retained for extracellular recording and stimulation (Fig.2). The cerebral and buccal ganglia were pinned out with the ventral and caudal surfaces pointing up, and the right buccal hemiganglion was desheathed. To monitor buccal motor patterns (BMPs), which are an in vitro correlate of consummatory feeding behavior (Cropper et al., 1990b; Morton and Chiel, 1993a,b; Scott et al., 1995; Warman and Chiel, 1995; Hurwitz et al., 1996), extracellular electrodes were placed on nerves Rn1, I2, and Bn2.1 or Bn3 (for nomenclature, see Nargeot et al., 1997) of the right hemiganglion. Signals were amplified with a differential AC amplifier (model 1700; A-M Systems, Everett, WA). For stimulation, extracellular bipolar platinum electrodes were placed on nerves AT4 and En2. All extracellular electrodes were isolated from the surrounding bath using Vaseline. The high divalent solution was then exchanged for normal artificial seawater (ASW). Intracellular recordings were made from the right hemiganglion using conventional two-electrode current-clamp techniques. Electrode resistances varied between 10 and 20 MΩ. The temperature of the bath was maintained at 15°C with a feedback-controlled peltier cooling device (model SE 5010; Marlow Industries, Dallas, TX).
Fig. 2.
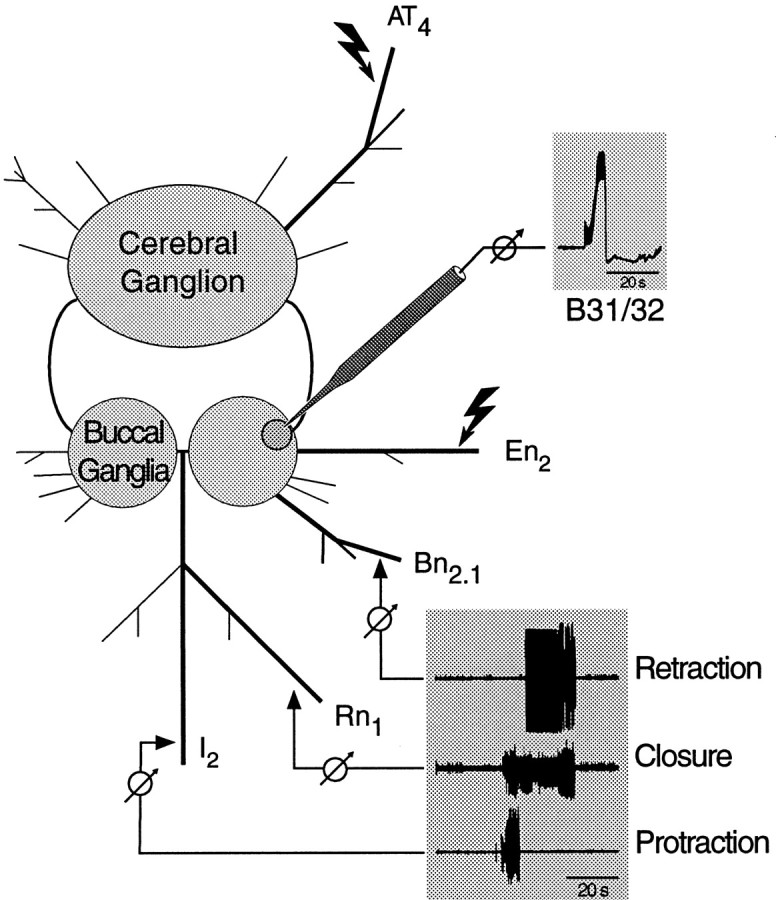
In vitro preparation. After behavioral training, the cerebral and buccal ganglia were isolated together with selected peripheral nerves. BMPs (bottom inset) were recorded extracellularly from buccal nerves that innervate buccal muscles for protraction (I2), closure (Rn1), and retraction (e.g.,Bn2.1) of the odontophore and radula. Intracellular recordings from identified buccal neurons (e.g., B31/32) were done simultaneously (top inset). Bipolar stimulating electrodes were placed along the most medial and ventral branch of the AT nerve (AT4) and the esophageal nerve (En2). Stimulation of AT4 was used to activate afferent fibers that innervate the region of the lip that was targeted for CS presentation during behavioral training. Stimulation of En2 was used to determine whether a preparation was capable of expressing BMPs (see Materials and Methods for details).
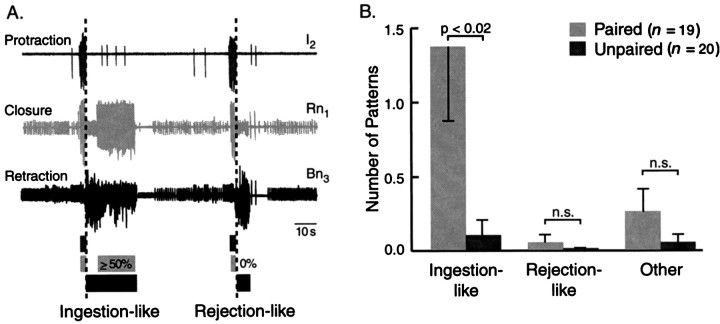
Classification of BMPs. Feeding behavior can result in the ingestion of food into the buccal cavity or the expulsion of inedible material (rejection), dependent on the relative timing between the protraction/retraction cycle of the odontophore and the closure of the radula. Ingestion results when the radula is open during protraction of the odontophore and closed during retraction, whereas rejection results when radula closure shifts from the retraction to the protraction phase. Phasic large-unit activity that represents efferent activity for the protraction/retraction cycle of the odontophore and for radula closure (Morton and Chiel, 1993a) can be recorded in vitrofrom nerves of isolated buccal ganglia (Morton and Chiel, 1993b). Phasic activity in all three buccal nerves (see above) was considered a buccal motor pattern. BMPs were classified as being ingestion-like or rejection-like using the criteria described by Nargeot et al. (1997). Briefly, patterns were classified as ingestion-like if ≥50% of closure activity (i.e., large-unit activity in Rn1) occurred after the termination of protraction activity (i.e., large-unit activity in I2) at which point retraction activity (i.e., large-unit activity in Bn2.1or Bn3) begins (see Fig. 7A). The criterion for rejection-like activity was no overlap between closure and retraction activity. Patterns that did not meet either of these criteria were classified as “other BMPs”.
Fig. 7.
Classification of AT4stimulation-evoked BMPs. A, Patterned large-unit activity recorded from buccal nerves I2(Protraction), Rn1 (Closure), and Bn3 (Retraction) was classified as ingestion-like or rejection-like on the basis of the relative overlap of closure activity and the protraction/retraction cycle. The relative duration of large-unit activity for protraction (dark gray), closure (light gray), and retraction (black) is diagrammed by shaded boxesunderneath the recorded traces. Patterns were classified as ingestion-like if ≥50% of large-unit closure activity occurred after the end of large-unit protraction activity (dashed line). Patterns were classified as rejection-like if there was no overlap between large-unit closure activity and large-unit protraction activity (Nargeot et al., 1997, 1999a,b). (Examples shown here are spontaneously expressed BMPs.) B, Using the criteria described above, AT4 stimulation-evoked BMPs were classified. Patterns that did not fit either of the above criteria were labeled “other.” Ingestion-like BMPs were evoked most frequently. A comparison of the number of ingestion-like BMPs after paired and unpaired training yielded a significant difference. Thus, the increased number of AT4 stimulation-evoked BMPs was almost entirely attributable to ingestion-like BMPs.
Solutions. Normal ASW (in mm): 450 NaCl, 10 KCl, 30 MgCl2, 20 MgSO4, 10 CaCl2, and 10 HEPES/NaOH, pH 7.5. High divalent ASW (in mm): 330 NaCl, 10 KCl, 90 MgCl2, 20 MgSO4, 30 CaCl2, and 10 HEPES/NaOH, pH 7.5.
Correlates in neuron B4/5. After dissection (see above) neuron B4/5 was impaled with two microelectrodes (one for passing current, and the other for monitoring the membrane potential) and identified by antidromic spikes in response to extracellular stimulation of Bn3, in addition to its relative position within the ganglion and its characteristic bursting activity during the retraction phase of BMPs. Spontaneous BMPs were recorded extracellularly for 30 min. B4/5 was then hyperpolarized to −80 mV, and the stimulation threshold for eliciting EPSPs by stimulation of AT4 (0.5 msec pulses; 0.2 Hz; starting at 3 V in 0.2 V increments) was determined. While B4/5 was held at −80 mV, the peak amplitude of the EPSP in response to stimulation of AT4 at a fixed intensity (0.5 sec pulses, 0.2 Hz; 6 V) and the integral of the EPSP (over the duration of 250 msec) was determined. After these tests, B4/5 was current-clamped at −70 mV, and the input resistance and excitability were determined by injecting hyperpolarizing and depolarizing current pulses (5 sec pulses at 20 sec intervals; −3 to +20 nA). Subsequently, B4/5 was released from current clamp, and four trains of stimulation of AT4 (5 sec, 5 Hz, 0.5 msec pulses; 6 V) were delivered at 60 sec intervals to mimic the tactile CS used for classical conditioning. These values were chosen from pilot studies performed to determine effective stimulation parameters that mimicked the known responses of mechanoafferents to tactile stimulation (Anderson, 1967; Rosen et al., 1979; Fredman and Jahan-Parwar, 1980), yet minimized fatigue with repeated stimulation. The pilot studies also determined that the intensity of AT4 stimulation (single pulses) was above the mean threshold (4.2 ± 0.5 V) for eliciting antidromic spikes in neurons of the lateral and medial mechanosensory clusters of the cerebral ganglion. The number of BMPs that occurred during this stimulation period was scored. Finally, a single train of stimulation of En2 (4 sec, 10 Hz, 0.5 msec pulses; 8 V) was used to determine whether the preparation was capable of producing BMPs. Preparations that did not produce BMPs in response to stimulation of En2 (∼5%) were discarded.
Correlates in neuron B31/32. Procedures were identical to the methods described above, with the following exceptions. After desheathing the right hemisphere of the buccal ganglion, two large motor neurons (B1 and B2) involved in the regulation of gut motility (Lloyd et al., 1988) were removed with sharp forceps to provide access to the soma of neuron B31/32. This procedure had no obvious effects on activity within the CPG. To monitor retraction activity, Bn2.1 (instead of Bn3) was recorded. Neuron B31/32 was identified by an antidromic potential in response to I2 stimulation and its characteristic plateau potential with nonovershooting axonal spikes during the protraction phase of the BMP.
After identification of B31/32 a 10 min baseline was recorded, after which B31/32 was current-clamped at −70 mV to determine its input resistance and excitability using a series of hyperpolarizing and depolarizing pulses (10 sec pulses at 60 sec intervals; −3 to +20 nA). Because the soma of B31/32 does not support action potentials, excitability was determined as the stimulation threshold for eliciting extracellular potentials recorded in I2, that coincided with depolarizing potentials in the soma of B31/32. Finally, the amplitude and integral of complex PSPs (cPSPs) elicited by four trains of stimulation of AT4 (5 sec, 5 Hz, 0.5 msec pulses; 6 V) delivered every 60 sec was determined at a potential of −80 mV.
Statistical analyses and blind procedures. The Mann–WhitneyU test (U) was used to compare behavioral scores and the number of BMPs. The peak amplitudes and integrals of cPSPs were analyzed using unpaired t tests (t). All statistics are two-tailed. Behavioral testing, electrophysiological recordings, and scoring of all data were done blindly, i.e., without knowledge of the experimental history of each animal or preparation.
RESULTS
Lip nerve stimulation evokes more BMPs after paired training
Two groups of animals received 10 trials of either paired or unpaired training (see Materials and Methods). This protocol reliably induces associative memory, which can last for at least 24 hr (Lechner et al., 1997, 2000). Memory was quantified by comparing the number of bites during CS presentations before and 1 hr after training (Fig.3). Paired training resulted in an increase in biting responses (2.68 ± 1.09; n = 19), whereas unpaired training resulted in a small decrease (−0.20 ± 0.37; n = 20; p < 0.02; U = 102.5).
Fig. 3.
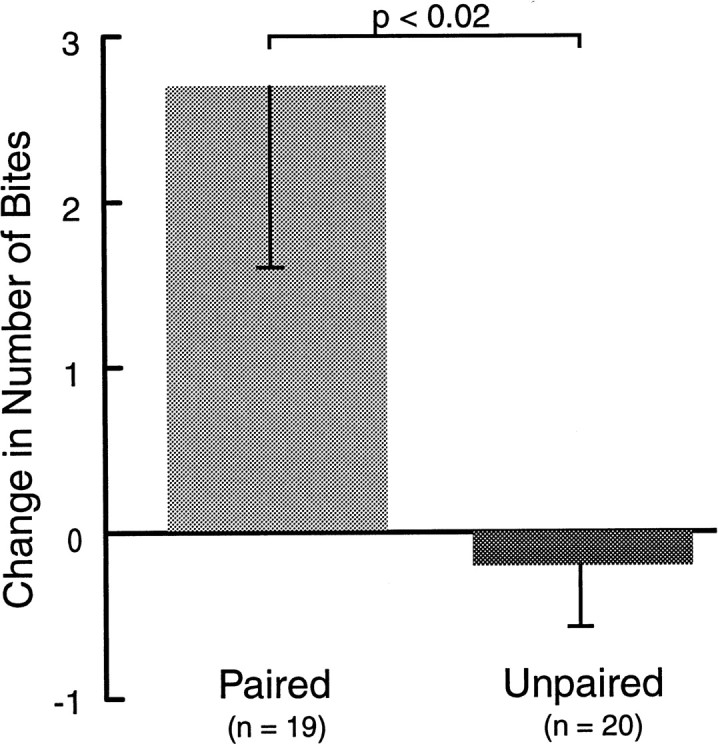
Classical conditioning for extracellular correlates. Two groups of animals received either paired or unpaired presentations of CS and US. Only paired training resulted in an increased number of bites in response to the CS 1 hr after training. In this and subsequent illustrations, data are displayed as means + SEM.
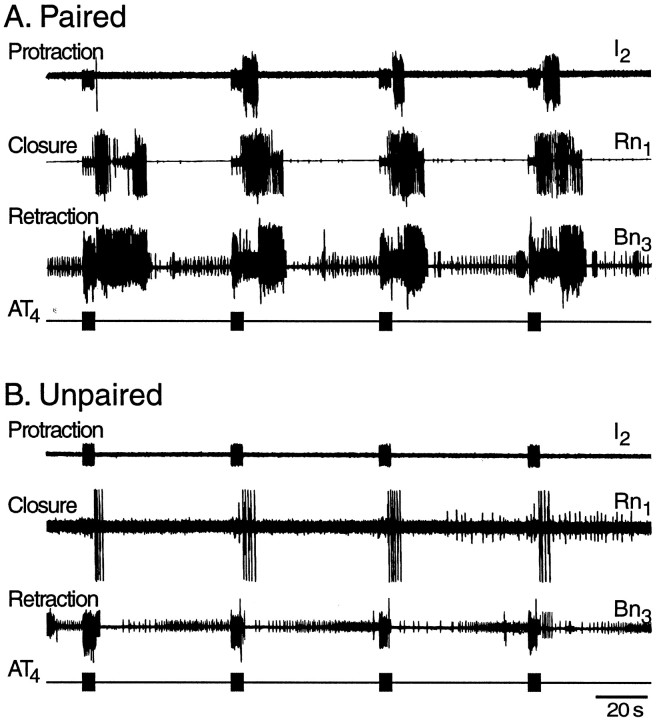
After the 1 hr retention test, the cerebral and buccal ganglia from paired and unpaired animals were prepared for intracellular recording from buccal neuron B4/5. In addition, extracellular recordings were made from three buccal nerves representing the protraction (I2), retraction (Bn3), and closure (Rn1) phases of radula movement. Patterned activity from some of these nerves (i.e., BMPs, see Materials and Methods) has been recorded in behaving animals and correlated with different types of feeding behavior (Cropper et al., 1990b; Morton and Chiel, 1993a,b; Scott et al., 1995; Warman and Chiel, 1995; Hurwitz et al., 1996). Therefore, these nerve recordings provide a means of monitoring a correlate of feeding behavior in vitro. The effect of AT4 stimulation on the generation of BMPs was examined. To mimic the behavioral test, AT4 was stimulated four times at intervals of 60 sec. Each nerve stimulation consisted of a 5 sec train of 0.5 msec depolarizing pulses at a frequency of 5 Hz and an intensity of 6 V. The number of BMPs occurring during this 4 min period was counted. Representative examples are shown in Figure4. Stimulation of AT4 elicited significantly more BMPs after paired training (1.68 ± 0.61; n = 19) than after unpaired training (0.15 ± 0.11; n = 20;p < 0.02; U = 106; Fig.5). This effect was only evident when BMPs were elicited by nerve stimulation. The number of spontaneously occurring BMPs, counted over a 30 min period before the experiment, was not different in preparations from animals that had received paired (6.63 ± 1.38; n = 19) and unpaired (7.20 ± 2.04; n = 20; U = 174.5) training (Fig.6).
Fig. 4.
The effect of stimulation of AT4 on the expression of BMPs after paired and unpaired training. Repeated stimulation of AT4 (four trains at intervals of 60 sec;A, B, bottom traces) evoked a higher number of BMPs in preparations from animals that received paired training than in preparations from animals that had received unpaired training.A, The total number of complete BMPs in the paired example was three (evoked by trains 2, 3, and 4). The first train failed to evoke a complete BMP (lack of protraction activity).B, A representative example from the unpaired group shows that no complete BMPs were evoked by the same stimulation protocol used in A. See Figure 5 for summary data.
Fig. 5.
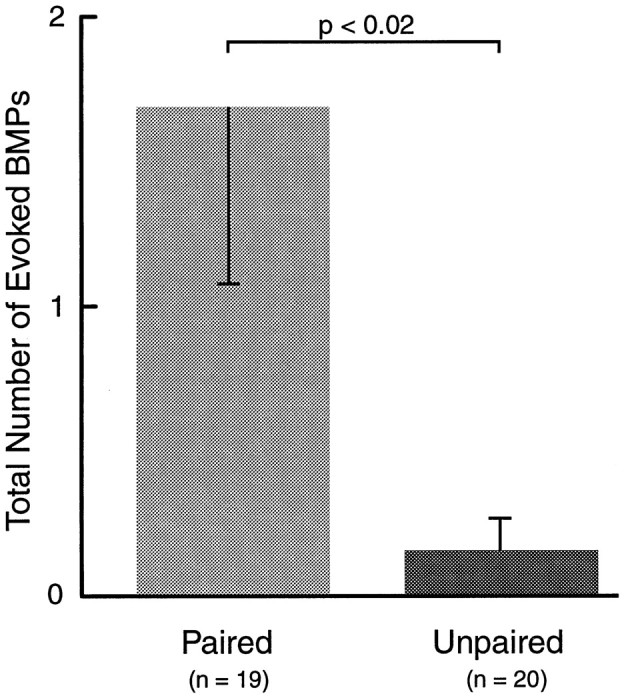
Extracellular correlates of classical conditioning. Within ∼6 hr after training, the cerebral and buccal ganglia were dissected from paired and unpaired animals and prepared for extracellular recording. Four trains of stimulation of AT4 (5 sec, 5 Hz) were used to mimic the CS in vitro. Patterned activity in the buccal ganglion (BMPs) evoked by this stimulation was monitored. Stimulation of AT4elicited a greater number of BMPs in preparations from animals that had received paired training than in preparations from animals that had received unpaired training.
Fig. 6.
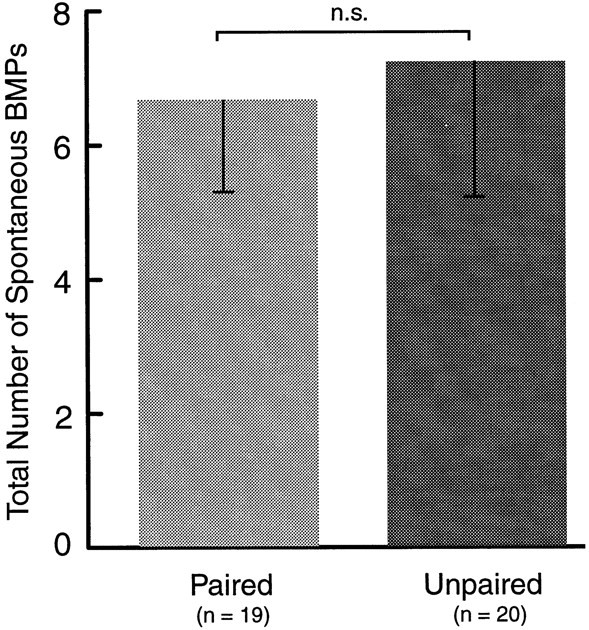
Spontaneous BMPs. The number of spontaneously occurring BMPs over the course of a 30 min observation period before stimulation of AT4 was not different after paired and unpaired training. Thus, associative memory was not manifest as an increased baseline activity of the CPG.
Together these results suggest that a correlate of associative memory, which was induced by classically conditioning intact animals, can be observed and studied in preparations of isolated ganglia. Moreover, the effect of paired training appears to strengthen the CS-mediating pathways selectively, without affecting the baseline activity of the CPG.
The majority of BMPs were ingestion-like
Patterned motor activity in buccal nerves has been recordedin vivo and correlated directly with behavioral ingestion and rejection (Cropper et al., 1990b; Morton and Chiel, 1993a,b; Scott et al., 1995; Warman and Chiel, 1995; Hurwitz et al., 1996). A key feature distinguishing ingestion and rejection in the in vivo recordings was the relative overlap between activity in nerves that mediate radula closure and nerves that mediate the retraction or protraction of the odontophore (see Materials and Methods). Because these phase relationships are maintained in the isolated buccal ganglion, BMPs recorded in vitro can be classified as ingestion-like or rejection-like BMPs (Fig.7A). A classification of BMPs evoked by AT4 stimulation revealed that most of them were ingestion-like (Fig. 7B). Comparing the number of ingestion-like BMPs in preparations after paired (1.37 ± 0.50;n = 19) and unpaired (0.10 ± 0.10;n = 20) training found that the pairing-specific effect described above was almost exclusively attributable to an increase in the number of ingestion-like BMPs (p < 0.02;U = 109.5). In contrast, the number of spontaneous BMPs that were classified as ingestion-like did not differ after paired (4.53 ± 1.2; n = 19) and unpaired (3.15 ± 1.09; n = 20) training (U = 156). The increase in the number of AT4-evoked ingestion-like BMPs reported here closely resembles the effect of behavioral training, i.e., a pairing-specific increase in the number of bites in response to tactile stimulation of the lips.
Together, these results suggest that classical conditioning of feeding induces pairing-specific changes in the neural circuitry that controls and produces feeding behavior. These changes resulted in increased ingestion-like motor activity in the CPG of the buccal ganglia. Moreover, these changes were not manifest as an increased baseline activity of the CPG, but were specific to the activation of CS-mediating pathways. Although these data indicate that neural correlates of appetitive classical conditioning survive into isolated ganglia preparations and can be recorded extracellularly, they do not point to the sites within the nervous system at which associative plasticity for classical conditioning may take place. Thus, further experiments were conducted with the goal to identify specific sites of neural plasticity that correlate with conditioned feeding behavior.
Paired training correlated with a greater synaptic input to B31/32 than unpaired training
Based on the observation that paired training resulted in a higher number of BMPs in response to AT4 stimulationin vitro, buccal neuron B31/32 was examined in paired and unpaired preparations. Activity in B31/32 has been shown to initiate BMPs, and hyperpolarizing B31/32 can prevent the expression of BMPs (Susswein and Byrne, 1988). Thus, B31/32 plays a key role in the expression of BMPs and possibly the initiation of feeding behavior.
To study the effects of classical conditioning on B31/32, two groups of animals received either paired or unpaired training (Fig.8). Paired training resulted in a greater increase in biting behavior in response to the CS (4.35 ± 1.07;n = 17) than unpaired training (1.94 ± 0.81;n = 17; p < 0.025; U = 79), 1 hr after training.
Fig. 8.
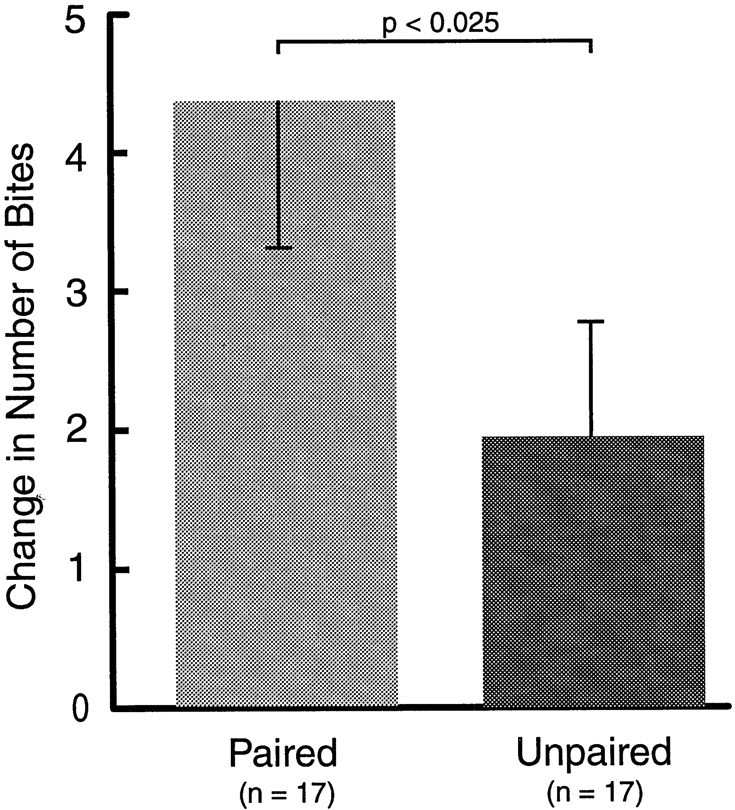
Classical conditioning for cellular correlates. Two groups of animals were trained using either paired or unpaired CS–US presentations. Paired training resulted in a greater increase in the number of bites in response to the CS 1 hr after training.
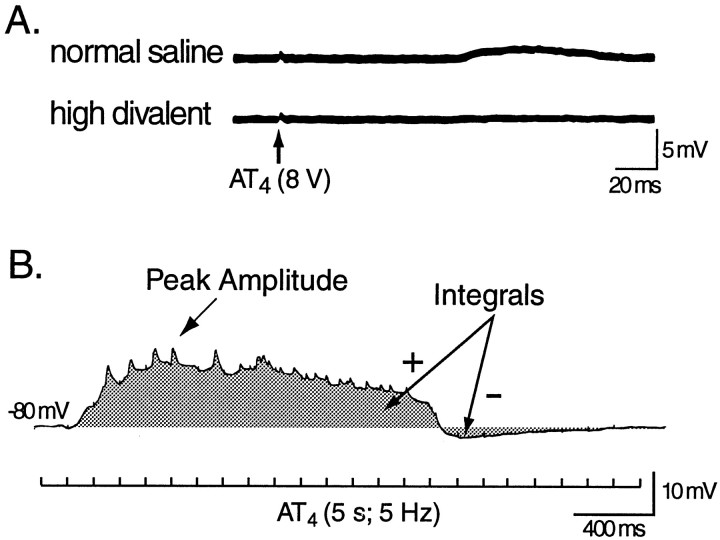
Within 6 hr of behavioral testing, cerebral and buccal ganglion were prepared for intracellular and extracellular recording and stimulation (see Materials and Methods). The intrinsic properties and synaptic responses of neuron B31/32 were examined. In response to single pulses (0.5 msec, 8 V) of AT4 stimulation, B31/32 received only weak excitatory input (Fig.9A), which disappeared in high-divalent saline. In response to trains of AT4 stimulation (5 sec, 5 Hz, 6 V), B31/32 received complex synaptic input (Fig. 9B). This cPSP was reduced in high-divalent solution (data not shown), which indicated that most of the connections between the AT4fibers and B31/32 were polysynaptic. To examine the effect of classical conditioning on these connections, B31/32 was current-clamped at −80 mV, and the magnitude of the cPSPs in B31/32 evoked by trains of stimulation of AT4 was determined in preparations from animals that had received paired or unpaired training. The peak amplitude and the net depolarization (i.e., the integral of the cPSP) over the 5 sec duration of the stimulation were measured (Fig.9B) and averaged across the four stimulations of AT4.
Fig. 9.
Synaptic input to B31/32 in response to stimulation of AT4. A, Single pulses of AT4 stimulation (0.5 msec, 8 V) evoked only weak excitation in B31/32, which disappeared in the presence of high-divalent solution.B, In normal saline, trains of AT4stimulation (5 sec, 5 Hz) elicited cPSPs in B31/32. To quantify the magnitude of the synaptic input B31/32 received in response to 5 sec trains of AT4 stimulation, the cell was hyperpolarized to −80 mV, and the peak depolarizing amplitude as well as the integral (shaded region) of the cPSP were determined. To calculate the net excitatory component of the cPSP, negative integrals (i.e., hyperpolarizations; minus symbol) were subtracted from the depolarizing component (plussymbol) of the cPSP.
The peak depolarization during the cPSP in B31/32 evoked by trains of stimulation of AT4 was significantly greater after paired (12.29 ± 1.44 mV; n = 17) than after unpaired (7.90 ± 1.51 mV; n = 17) training (Fig.10A; p < 0.05; df = 32; t = 2.103). Although this result suggests a strengthening of cerebral input to B31/32, the peak depolarization may not be the best measure of the ability of presynaptic input evoked by AT4 stimulation to effectively depolarize B31/32. Because the soma of B31/32 does not support action potentials (Susswein and Byrne, 1988; Hurwitz et al., 1994), it was not possible to accurately determine the number of spikes evoked by AT4 stimulation. Instead, the magnitude of the integral of the cPSP was determined (Fig. 10B) as a measure of the ability of AT4-evoked input to depolarize B31/32. The average cPSP integral was significantly larger after paired (19.61 ± 2.53 arbitrary units; n = 17) than after unpaired (11.99 ± 2.62 arbitrary units;n = 17) training (p < 0.05; df = 32; t = 2.093). This result indicated that AT4 stimulation exerts a greater depolarizing effect on the pattern-initiating neuron B31/32 after appetitive classical conditioning. In contrast to the presynaptic input to B31/32, measurements of intrinsic properties of B31/32, such as resting membrane potential, input resistance, and the threshold for evoking extracellularly recorded potentials in nerve I2by depolarizing B31/32, yielded no pairing-specific differences (Table1).
Fig. 10.
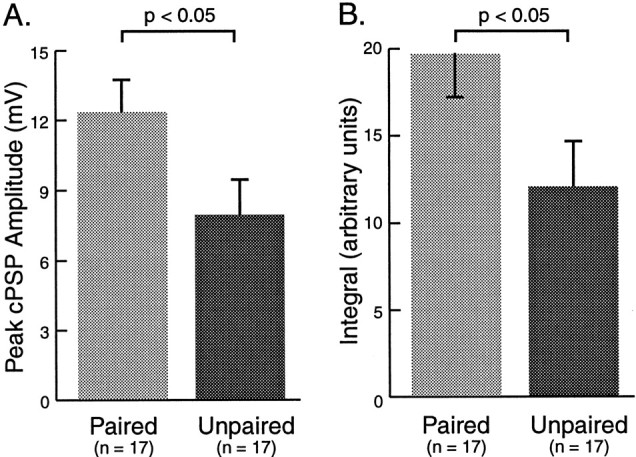
Cellular correlates of classical conditioning in the BMP-initiating neuron B31/32. A, The average peak depolarizing amplitude of cPSPs elicited by four trains of AT4 stimulation was determined after paired and after unpaired training. Paired training correlated with a larger amplitude of cPSPs than unpaired training. B, The average magnitude of excitation was also measured by integrating the area underneath the PSP over the 5 sec train of AT4 stimulation (Fig. 9B). The cPSP integral after paired training was larger than after unpaired training. These results are consistent with a potentiation of the CS-mediating pathway, upstream of neuron B31/32, as a result classical conditioning of feeding behavior.
Table 1.
Intrinsic properties of identified neurons in the buccal CPG
| Paired | Unpaired | t= | p< | ||
|---|---|---|---|---|---|
| B4/5: Intrinsic properties and evoked | Resting membrane potential (mV) | −68.2 ± 1.3 | −67.8 ± 1.1 | 0.208 | 0.50 |
| potentials (P = 19; UP = 20) | Input resistance (MΩ) | 3.1 ± 0.2 | 3.4 ± 0.2 | −0.904 | 0.20 |
| Excitability: spike threshold (nA) | 6.1 ± 1.1 | 5.7 ± 1 | 0.239 | 0.50 | |
| Stimulation threshold for EPSPs (V) | 3.3 ± 0.1 | 3.5 ± 0.2 | −0.77 | 0.20 | |
| Excitability: number of spikes | 30.6 ± 6.98 | 17.6 ± 4.16 | −1.625 | 0.20 | |
| Excitability: initial firing frequency (Hz) | 10.9 ± 2.3 | 7.2 ± 1.77 | −1.3 | 0.20 | |
| B31/32: Intrinsic properties | Resting membrane potential (mV) | −68.6 ± 2.1 | −68.8 ± 2 | −0.089 | 0.50 |
| (P = 17; UP = 17) | Input resistance (MΩ) | 3.4 ± 0.1 | 3.5 ± 0.2 | −0.492 | 0.50 |
| Excitability: threshold for extracell. spikes (nA) | 4.4 ± 0.8 | 3.8 ± 0.5 | 0.599 | 0.50 |
Intrinsic properties, such as membrane potential, input resistance, and spike threshold of buccal neurons B31/32 and B4/5 were examined in preparations from animals that had received paired (P) or unpaired (UP) training. No evidence was found that the intrinsic properties of these neurons undergo changes in response to classical conditioning. See Results for details.
Together, these results suggest that classical conditioning results in a potentiation of the net excitatory synaptic input to B31/32 evoked by stimulation of AT4, but does not affect the intrinsic properties of a pattern-initiating element of the CPG, which mediates aspects of feeding behavior. Because activation of B31/32 has been shown to be crucial for the expression of patterned activity in the buccal CPG (Susswein and Byrne, 1988), it is conceivable that the increase in synaptic input to B31/32 contributes to the larger number of BMPs evoked by stimulation of AT4 after classical conditioning and possibly to the increased number of biting responses during CS presentation after paired training.
Synaptic input and intrinsic properties of B4/5 did not differ after paired and unpaired training
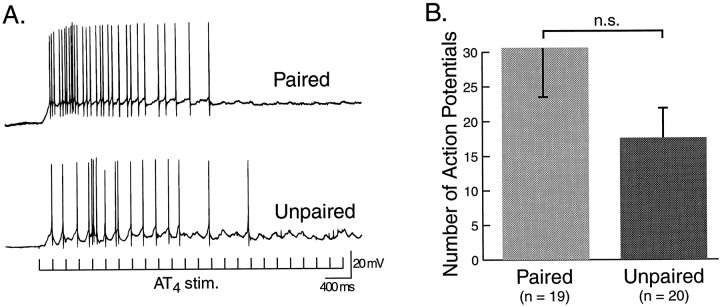
In addition to monitoring AT4-evoked BMPs and cPSPs in neuron B31/32, the intrinsic properties of neuron B4/5 and its synaptic input in response to single pulses and trains of stimulation of AT4 were examined in preparations from paired and unpaired animals. B4/5 is a multifunctional neuron of the CPG that fires during the retraction phase of BMPs (Fig. 1). It has been shown to receive excitatory input from mechanosensory neurons (e.g., ICBMs and other CM cells; Rosen et al., 1979, 1982) in the cerebral ganglion. A comparison between the peak amplitude of the cEPSP evoked by single pulses of AT4 stimulation, while B4/5 was current-clamped at −80 mV (Fig.11A), yielded slightly higher values after paired (8.54 ± 1.44 mV;n = 19) than unpaired (7.54 ± 1.07 mV;n = 20) training. However, this difference was not statistically significant (df = 37; t = 0.558; Fig. 11B). Similar results were obtained when the integrals of cEPSPs evoked in B4/5 by single pulses of AT4 stimulation were measured after paired (31.3 ± 1.07 arbitrary units) and unpaired (23.6 ± 3.9 arbitrary units) training were determined (df = 37;t = 1.019; NS; Fig. 11C). As an additional measure of the ability of afferent input from AT4to drive activity in B4/5 after paired and unpaired training, the cell was released from current clamp, and the average number of action potentials in B4/5, evoked by four trains of AT4stimulation, delivered at intervals of 60 sec, was determined (Fig.12A). The average number of spikes recorded in preparations from animals that received paired training (30.64 ± 6.98; n = 19) was higher than in preparations from animals that had received unpaired training (17.60 ± 4.16; n = 20), but this difference was not statistically significant (df = 37; t = 1.625; Fig. 12). Similarly, the number of spikes evoked in B4/5 during the first second of AT4 stimulation (initial firing rate) was not significantly higher in paired preparations (10.89 ± 2.3 Hz) than in unpaired preparations (7.15 ± 1.77 Hz; NS, df = 37; t = 1.3). Finally, intrinsic properties of B4/5, such as resting membrane potential, spike threshold, and input resistance, were not different in preparations from animals that had received paired versus unpaired training (Table 1). Thus, although neuron B4/5 ap- peared to receive slightly larger excitatory synaptic input in response to AT4 stimulation after paired training, the neuron did not appear to be the primary target of these inputs, compared to neuron B31/32.
Fig. 11.
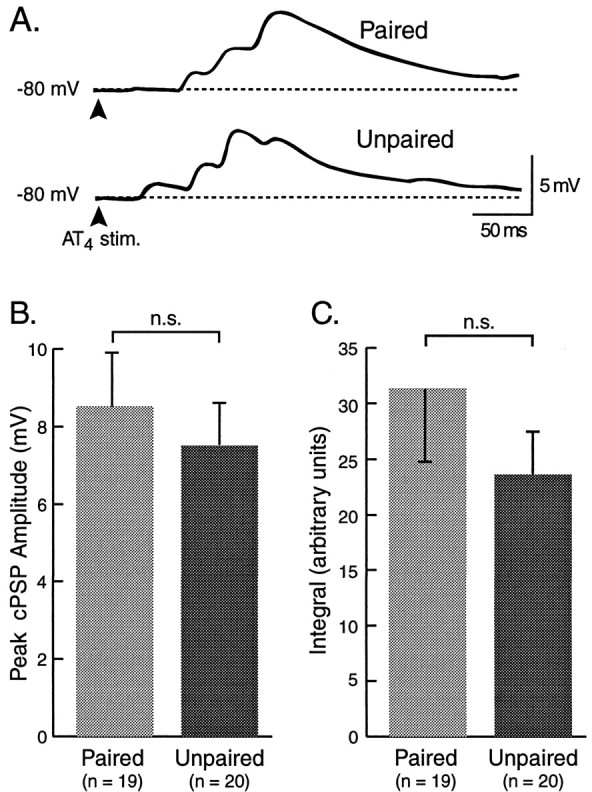
Synaptic input to neuron B4/5. The magnitude of cEPSPs in identified neuron B4/5 was determined by measuring the peak amplitude and the integral of the cEPSP over a duration of 250 msec, while the membrane potential was current-clamped to −80 mV.A, Examples of cEPSPs evoked by single pulses of stimulation of AT4 in preparations from animals that had received paired or unpaired training. B, C, Although the average peak amplitude and the integrals of cEPSPs were slightly greater after paired than after unpaired training, the effect was not statistically significant.
Fig. 12.
Evoked spiking activity in neuron B4/5. The average number of spikes, evoked during four successive trains of stimulation of AT4, was determined as a measure of the strength of afferent pathways originating in the lips to drive elements of the buccal CPG. A, Examples of evoked spikes by trains of stimulation of AT4 in preparations from animals that had received paired or unpaired training.B, Although the average number of AT4-evoked spikes was somewhat greater after paired than after unpaired training, the effect was not statistically significant.
DISCUSSION
The present experiments suggest that appetitive classical conditioning of feeding induced pairing-specific changes in the circuitry that controls and produces feeding behavior. Paired training using tactile stimulation of the lips as CS and food as US resulted in an increased probability of the CS to elicit biting behavior. In vitro, stimulation of a lip nerve (AT4) that carries the majority of mechanosensory fibers (Rosen et al., 1979,1982) resulted in a greater probability of ingestion-like BMPs occurring after paired training. Moreover, appetitive classical conditioning enhanced the polysynaptic pathway between afferents in AT4 and a neuron of the buccal CPG (i.e., B31/32), which is thought to initiate the protraction phase of consummatory feeding behavior. Thus, the behavioral and physiological effects produced by classical conditioning are in close register.
Importantly, all correlates of classical conditioning were expressed selectively in response to stimulation of the putative CS-mediating pathway. Classical conditioning did not result in an increased number of BMPs in the absence of stimulation of AT4 and did not affect the intrinsic properties of two key elements of the CPG (i.e., B4/5 and B31/32). This specificity further supports the notion that the neural correlates of classical conditioning identified in this study are related to the conditioned response.
The results of this and the companion paper can be summarized in a simple model circuit for classical conditioning (Fig.13). In this model, tactile stimulation of the lips is mediated by cerebral mechanosensory (CM) cells that make monosynaptic connections to command-like interneurons, such as CBI-1 and CBI-2. These neurons in turn, make monosynaptic and polysynaptic excitatory connections to BMP-initiating neurons of the CPG, such as B31/32 (Rosen et al., 1991; Fig. 1). In naïve animals, tactile stimulation is inefficient at activating the CPG. Although CBI-1 is strongly activated by tactile lip stimulation, its activity drives the CPG only weakly through a polysynaptic pathway. CBI-2 drives the CPG reliably through a monosynaptic excitatory connection with B31/32, but tactile stimulation does not activate this command neuron as efficiently as chemical stimuli (e.g., food; Rosen et al., 1991). As a result of paired presentation of CS and US, the synaptic connections and/or the intrinsic properties of CS-mediating neurons upstream of B31/32 undergo associative facilitation, which enhances their ability to drive patterned activity in the CPG in response to subsequent CS presentations (i.e., CR). Patterned activity in the CPG, in turn, produces aspects of feeding movements (bites). The facilitation of mechanosensory pathways depends on the activation of a modulatory system, or mechanism, by sensory afferents mediating the US (food). Behavioral experiments suggest that afferents from the buccal epithelia, rather than from the lips, mediate the modulatory component of the US (Lechner et al., 2000). It is important to note that because only two elements of the CPG (B31/32 and B4/5) have been examined for correlates of classical conditioning thus far, the sites of plasticity proposed in this model represent only a few of several possibilities.
Fig. 13.
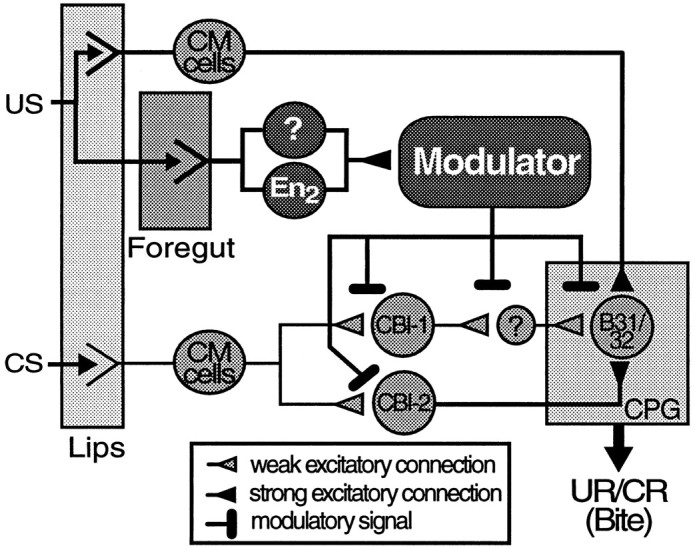
Hypothetical model circuit for classical conditioning of feeding behavior. Information about the tactile CS that was used for classical conditioning in the experiments reported in this study is mediated by cerebral mechanosensory afferents (CM cells) that innervate in the lips. These afferents make synaptic connections to command-like neurons located in the cerebral ganglion, such as CBI-1 and CBI-2. CBI-2, which receives weak mechanosensory input from CM cells, is able to reliably elicit patterned activity in a buccal CPG by means of a strong monosynaptic, excitatory connection to buccal neuron B31/32. Activity in B31/32 is critical for the expression of BMPs and may also initiate biting behavior in the intact animal. A second, polysynaptic pathway involving command-like neuron CBI-1 also mediates mechanosensory input to B31/32. In naïve animals, both pathways are inefficient at driving the CPG. As a result of classical conditioning, however, excitatory mechanoafferent input to B31/32 is facilitated by modifying synaptic connections and/or the intrinsic properties of neurons along the mechanosensory pathways. This facilitation is caused by the activation of a yet unidentified modulatory system or mechanism, by food stimulation (US). The modulator may alter synaptic transmission and/or the intrinsic properties of neurons at multiple sites along the mechanosensory pathways when activity representing the CS and US coincide (paired training). Based on results from Lechner et al. (2000), buccal afferents from the foregut, not from the lips, mediate the reinforcing component of the US. Additional sites of plasticity to the ones suggested by this model may exist.
Other sites of plasticity
It is likely that the associative plasticity induced by classical conditioning is not limited to facilitated excitatory input to neuron B31/32. Associative plasticity at sites within the CPG, other than those examined in this study, may contribute to the effect of classical conditioning. For example, the expression of ingestion-like BMPs is also under the control of neurons B63 and B35, which are electrically coupled to B31/32 (Fig. 1). Thus, associative facilitation of mechanoafferent pathways that may provide input to these cells may have similar effects to the plasticity described above. Another interesting candidate for associative modulation is neuron B51, the activity of which has been found to correlate strongly with the expression of BMPs (Nargeot et al., 1999a; Fig. 1). Moreover, B51 has been identified as a target for associative plasticity induced by an in vitroanalog of operant conditioning (Nargeot et al., 1999a,b).
An obvious but deliberate omission in this study was the analysis of appetitive components of feeding behavior. Although this study and the companion paper focused on biting behavior as a readily quantifiable component of feeding, the expression of biting as the conditional response is typically preceded by appetitive behaviors (Kupfermann, 1974a). It is possible that the neural circuitry for appetitive feeding behaviors also undergo pairing-specific plasticity during classical conditioning. Finally, it is possible that paired training increases the probability of feeding behavior by lowering the threshold for its spontaneous expression, in response to mechanoafferent inputs. In other words, the CS would induce a conditioned behavioral state (Carew et al., 1981; Walters et al., 1981) that would increase the probability of biting behavior. The expression of consummatory feeding behavior inAplysia is strongly modulated by the behavioral states of the animal (Kupfermann, 1974a), and neural substrates that mediate behavioral states, such as food arousal, have been identified. Activity in the serotonergic metacerebral cells (MCC) (Fig. 1), for example, is induced by food stimulation and has been found to correlate with food arousal in intact animals (Kupfermann and Weiss, 1982). Modulatory cells, such as MCC, are potential targets for associative plasticity, in addition to the direct connections between mechanoafferent pathways and command-like neurons or elements of the buccal CPG.
Correlates of classical conditioning in invertebrates
Correlates of conditioned feeding behavior have also been found in the pond snail Lymnea stagnalis. CS presentation to semi-intact preparations resulted in higher numbers of fictive feeding cycles (i.e., patterned activity recorded in vitro) after paired training than after random CS and US presentations (Staras et al., 1998). Moreover, CS-evoked EPSPs and the number of action potentials in B3 recorded in Lymnea were greater after paired in vitro training than after random CS–US presentations (Staras et al., 1999). Because B3 activity contributes to the retraction of the odontophore (Rose and Benjamin, 1981), however, it is unclear how the increased excitatory input to B3 relates to the increase in the number of fictive feeding cycles. The study did not report data on the CS-evoked activity in a protraction motor neuron (B1) that was monitored simultaneously with B3 or pattern-initiating neurons, comparable to neuron B31/32. The increased excitatory input to B3 may have been the result of an increased number of spikes in cerebral afferents because the frequency of CS-evoked spikes, recorded from the connective between the cerebral and buccal ganglia, was slightly higher after paired than after unpaired training. Because the intrinsic properties of B3 were not measured, however, it is possible that changes in the input resistance and excitability of B3 contribute to the pairing-specific difference in synaptic potentials. Although an exact comparison between the findings in Lymnea andAplysia is not feasible for these reasons, the correlates of classical conditioning reported by Staras et al. (1998, 1999) are nevertheless similar to the correlates in Aplysia reported here, in that both suggest an increase in the CS-evoked excitatory input to the buccal CPG as a result of classical conditioning. An intriguing possibility is therefore, that the cellular mechanisms for classical conditioning of feeding behavior in these gastropods share similar features, and that further, more detailed analyses of the cellular and molecular mechanisms underlying associative plasticity in these model systems will complement each other.
Future directions
The series of experiments performed in the present study represents a first step toward developing and analyzing a simple animal model of an appetitive form of associative learning and memory. The potential of this preparation will be more fully realized in future studies. Among the objectives that can be pursued in this preparation is the development of an analog preparation, in which the cellular and molecular mechanisms underlying the induction and retention of an appetitive form associative plasticity can be studied in greater detail.
An analysis of the mechanisms underlying appetitive forms of classical conditioning in an analog preparation could contribute importantly to the understanding of associative learning. Current models of associative plasticity are mainly based on analog preparations of aversive classical conditioning. It is not clear, however, to what extent these changes contribute to learning in the intact animal and to what extent these models generalize to other forms of associative learning. Recently, a cellular analysis of associative plasticity has been performed in an analog preparation of operant conditioning of feeding in Aplysia (Nargeot et al., 1997, 1999a–c). Thus, the feeding system of Aplysia may provide the first simple circuitry, in which both operant and classical forms of plasticity can be directly compared on the level of individual cells and synaptic connections. Already, our results suggest a fundamental distinction between classical and operant conditioning. Contingent reinforcement of ingestion-like BMPs results in changes in the intrinsic properties of cell B51 (Nargeot et al., 1999a,b), which is part of the CPG, and may be involved in biasing the output of the CPG toward ingestion-like activity (Baxter et al., 1999; Nargeot et al., 1999b). In contrast, plasticity induced by classical conditioning seemed to be restricted to synaptic input from putative CS-mediating pathways. These findings suggest that the procedural distinction between operant conditioning (reinforcing an emitted behavior) and classical conditioning (reinforcing a stimulus) may be reflected in the nervous system. Whereas operant conditioning caused an increase in the occurrence of the operant by modifying the neural circuits that produce this behavior, classical conditioning led to the associative reinforcement of the CS-mediating afferents that control (or drive) the behavior. These insights indicate the potential of a comparative approach, for identifying key principles underlying two forms of learning and memory.
Footnotes
This work was supported by National Institute of Mental Health Grant RØ1 MH 58321 and Grant 011618–048 from the Texas Higher Education Board. We thank Hanh N. Nguyen for training most of the animals included in this study.
Correspondence should be addressed to John H. Byrne, Department of Neurobiology and Anatomy, The University of Texas, Houston Medical School, 6431 Fannin Street, Houston, TX 77030. E-mail:jbyrne@nba19.med.uth.tmc.edu.
REFERENCES
- 1.Anderson JA. Patterns of response of neurons in the cerebral ganglion of Aplysia californica. Exp Neurol. 1967;19:65–77. doi: 10.1016/0014-4886(67)90007-6. [DOI] [PubMed] [Google Scholar]
- 2.Baxter DA, Patel VC, Byrne JH. Computational model of a multifunctional central pattern generator (CPG) that underlies consummatory feeding behavior of Aplysia. Soc Neurosci Abstr. 1997;23:1044. [Google Scholar]
- 3.Baxter DA, Nargeot R, Byrne JH. Examining the role of contingency-dependent modulation in a model of operant conditioning. Soc Neurosci Abstr. 1999;25:1314. [Google Scholar]
- 4.Carew TJ, Walters ET, Kandel ER. Associative learning in Aplysia: Cellular correlates supporting a conditioned fear hypothesis. Science. 1981;211:501–504. doi: 10.1126/science.7455692. [DOI] [PubMed] [Google Scholar]
- 5.Chiel HJ, Weiss KR, Kupfermann I. Multiple roles of a histaminergic afferent neuron in the feeding behavior of Aplysia. Trends Neurosci. 1990;13:223–227. doi: 10.1016/0166-2236(90)90164-6. [DOI] [PubMed] [Google Scholar]
- 6.Church PJ, Lloyd PE. Activity of multiple identified motor neurons recorded intracellularly during evoked feedinglike motor programs in Aplysia. J Neurophysiol. 1994;72:1794–1809. doi: 10.1152/jn.1994.72.4.1794. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JL, Weiss KR, Kupfermann I. Motor control of buccal muscles in Aplysia. J Neurophysiol. 1978;41:157–180. doi: 10.1152/jn.1978.41.1.157. [DOI] [PubMed] [Google Scholar]
- 8.Colwill RM, Goodrum K, Martin A. Pavlovian appetitive discriminative conditioning in Aplysia californica. Anim Learn Behav. 1997;25:268–276. [Google Scholar]
- 9.Cropper EC, Kupfermann I, Weiss A. Differential firing patterns of the peptide-containing cholinergic motor neurons B15 and B16 during feeding behavior in Aplysia. Brain Res. 1990a;522:176–179. doi: 10.1016/0006-8993(90)91598-b. [DOI] [PubMed] [Google Scholar]
- 10.Cropper EC, Miller MW, Vilim FS, Tenenbaum R, Kupfermann I, Weiss KR. Bucalin is present in the cholinergic motor neuron B16 of Aplysia and it depresses accessory radula closer muscle contractions evoked by stimulation of B16. Brain Res. 1990b;512:175–179. doi: 10.1016/0006-8993(90)91189-n. [DOI] [PubMed] [Google Scholar]
- 11.Evans CG, Rosen SC, Kupfermann I, Weiss KR, Cropper EC. Characterization of a radula opener neuromuscular system in Aplysia. J Neurophysiol. 1996;76:1267–1281. doi: 10.1152/jn.1996.76.2.1267. [DOI] [PubMed] [Google Scholar]
- 12.Fredman SM, Jahan-Parwar B. Processing of chemosensory and mechanosensory information in identifiable Aplysia neurons. Comp Biochem Physiol. 1980;66A:25–34. [Google Scholar]
- 13.Gardner D. Interconnections of identified multiaction interneurons in buccal ganglia of Aplysia. J Neurophysiol. 1977;40:349–361. doi: 10.1152/jn.1977.40.2.349. [DOI] [PubMed] [Google Scholar]
- 14.Hurwitz I, Susswein AJ. B64, a newly identified central pattern generatory element producing a phase switch from protraction to retraction in buccal motor programs of Aplysia californica. J Neurophysiol. 1996;75:1327–1344. doi: 10.1152/jn.1996.75.4.1327. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz I, Goldstein RS, Susswein AJ. Compartmentalization of pattern-initiating and motor functions in the B31 and B32 Neurons of the buccal ganglia of Aplysia californica. J Neurophysiol. 1994;71:1514–1527. doi: 10.1152/jn.1994.71.4.1514. [DOI] [PubMed] [Google Scholar]
- 16.Hurwitz I, Neustadter D, Morton DW, Chiel HJ, Susswein AJ. Activity patterns of the B31/32 pattern initiators innervating the I2 muscle of the buccal mass during normal feeding movements in Aplysia californica. J Neurophysiol. 1996;75:1309–1326. doi: 10.1152/jn.1996.75.4.1309. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz I, Kupfermann I, Susswein AJ. Different roles of neurons B63 and B34 that are active during the protraction phase of buccal motor programs in Aplysia californica. J Neurophysiol. 1997;78:1305–1319. doi: 10.1152/jn.1997.78.3.1305. [DOI] [PubMed] [Google Scholar]
- 18.Jahan-Parwar B, Fredman SM. Cerebral ganglion of Aplysia: cellular organization and origin of nerves. Comp Biochem Physiol. 1976;54A:347–357. doi: 10.1016/s0300-9629(76)80124-7. [DOI] [PubMed] [Google Scholar]
- 19.Jahan-Parwar B, Wilson AH, Jr, Fredman SM. Role of proprioceptive reflexes in control of feeding muscles of Aplysia. J Neurophysiol. 1983;49:1469–1480. doi: 10.1152/jn.1983.49.6.1469. [DOI] [PubMed] [Google Scholar]
- 20.Kabotyanski EA, Baxter DA, Byrne JH. Identification and characterization of catecholaminergic neuron B65, which initiates and modifies patterned activity in the buccal ganglia of Aplysia. J Neurophysiol. 1998;79:605–621. doi: 10.1152/jn.1998.79.2.605. [DOI] [PubMed] [Google Scholar]
- 21.Kabotyanski EA, Baxter DA, Cushman SJ, Byrne JH. Modulation of fictive feeding by dopamine and serotonin in Aplysia. J Neurophysiol. 2000;83:374–392. doi: 10.1152/jn.2000.83.1.374. [DOI] [PubMed] [Google Scholar]
- 22.Kirk MD. Premotor neurons in the feeding system of Aplysia californica. J Neurobiol. 1989;20:497–512. doi: 10.1002/neu.480200516. [DOI] [PubMed] [Google Scholar]
- 23.Kupfermann I. Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol. 1974a;10:1–26. doi: 10.1016/s0091-6773(74)91644-7. [DOI] [PubMed] [Google Scholar]
- 24.Kupfermann I. Dissociation of the appetitive and consummatory phases of feeding behavior in Aplysia: a lesion study. Behav Biol. 1974b;10:89–97. doi: 10.1016/s0091-6773(74)91694-0. [DOI] [PubMed] [Google Scholar]
- 25.Kupfermann I, Weiss KR. Activity of an identified serotonergic neuron in free moving Aplysia correlates with behavioral arousal. Brain Res. 1982;241:334–337. doi: 10.1016/0006-8993(82)91072-1. [DOI] [PubMed] [Google Scholar]
- 26.Lechner HA, Baxter DA, Byrne JH. Classical conditioning of feeding behavior in Aplysia. Soc Neurosci Abstr. 1997;23:1334–1334. [Google Scholar]
- 27.Lechner HA, Baxter DA, Byrne JH. Classical conditioning of feeding in Aplysia: I. Behavioral analysis. J Neurosci. 2000;20:3369–3376. doi: 10.1523/JNEUROSCI.20-09-03369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd PE, Kupfermann I, Weiss KR. Central peptidergic neurons regulate gut motility in Aplysia. J Neurophysiol. 1988;59:1613–1626. doi: 10.1152/jn.1988.59.5.1613. [DOI] [PubMed] [Google Scholar]
- 29.Morton DW, Chiel HJ. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J Comp Physiol [A] 1993a;172:17–32. doi: 10.1007/BF00214712. [DOI] [PubMed] [Google Scholar]
- 30.Morton DW, Chiel HJ. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol [A] 1993b;173:519–536. doi: 10.1007/BF00197761. [DOI] [PubMed] [Google Scholar]
- 31.Nargeot R, Baxter DA, Byrne JH. Contingent-dependent enhancement of rhythmic motor patterns: an in vitro analog of operant conditioning. J Neurosci. 1997;17:8093–8105. doi: 10.1523/JNEUROSCI.17-21-08093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nargeot R, Baxter DA, Byrne JH. In vitro analog of operant conditioning in Aplysia. I. Contingent reinforcement modifies the functional dynamics of an identified neuron. J Neurosci. 1999a;19:2247–2260. doi: 10.1523/JNEUROSCI.19-06-02247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nargeot R, Baxter DA, Byrne JH. In vitro analog of operant conditioning in Aplysia. II. Modifications of the functional dynamics of an identified neuron contribute to motor pattern selection. J Neurosci. 1999b;19:2261–2272. doi: 10.1523/JNEUROSCI.19-06-02261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nargeot R, Baxter DA, Patterson GW, Byrne JH. Dopaminergic synapses mediate neuronal changes in an analog of operant conditioning. J Neurophysiol. 1999c;81:1983–1987. doi: 10.1152/jn.1999.81.4.1983. [DOI] [PubMed] [Google Scholar]
- 35.Peretz B, Adkins L. An index of age when birthdate is unknown in Aplysia californica: shell size and growth in long-term maricultured animals. Biol Bull. 1982;162:333–344. [Google Scholar]
- 36.Perrins R, Weiss KR. A cerebral central pattern generator in Aplysia and its connections with buccal feeding circuitry. J Neurosci. 1996;16:7030–7045. doi: 10.1523/JNEUROSCI.16-21-07030.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrins R, Weiss KR. Compartmentalization of information processing in an Aplysia feeding circuit interneuron through membrane properties and synaptic interactions. J Neurosci. 1998;18:3977–3989. doi: 10.1523/JNEUROSCI.18-10-03977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plummer MR, Kirk MD. Premotor neurons B51 and B52 in the buccal ganglia of Aplysia californica: synaptic connections, effects on ongoing motor rhythms, and peptide modulation. J Neurophysiol. 1990;63:539–558. doi: 10.1152/jn.1990.63.3.539. [DOI] [PubMed] [Google Scholar]
- 39.Rose RM, Benjamin PR. Interneuronal control of feeding in Lymnea stagnalis. II. The interneuronal mechanisms generating feeding cycles. J Exp Biol. 1981;29:203–228. [Google Scholar]
- 40.Rosen SC, Weiss KR, Kupfermann I. Response properties and synaptic connections of mechanoafferent neurons in cerebral ganglion of Aplysia. J Neurophysiol. 1979;42:954–974. doi: 10.1152/jn.1979.42.4.954. [DOI] [PubMed] [Google Scholar]
- 41.Rosen SC, Weiss KR, Cohen JL, Kupfermann I. Interganglionic cerebral-buccal mechanoafferents of Aplysia: receptive fields and synaptic connections to different classes of neurons involved in feeding behavior. J Neurophysiol. 1982;48:271–288. doi: 10.1152/jn.1982.48.1.271. [DOI] [PubMed] [Google Scholar]
- 42.Rosen SC, Teyke T, Miller MW, Weiss KR, Kupfermann I. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J Neurosci. 1991;11:3630–3655. doi: 10.1523/JNEUROSCI.11-11-03630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott ML, Li Y, Kirk MD. Functional neural regeneration in the feeding system of Aplysia: behavioral recovery correlated with changes in buccal motor output. J Neurophysiol. 1995;73:39–55. doi: 10.1152/jn.1995.73.1.39. [DOI] [PubMed] [Google Scholar]
- 44.Staras K, Kemenes G, Benjamin PR. Neurophysiological correlates of unconditioned and conditioned feeding behavior in the pond snail Lymnaea stagnalis. J Neurophysiol. 1998;79:3030–3040. doi: 10.1152/jn.1998.79.6.3030. [DOI] [PubMed] [Google Scholar]
- 45.Staras K, Kemenes G, Benjamin PR. Cellular traces of behavioral classical conditioning can be recorded at several specific sites in a simple nervous system. J Neurosci. 1999;19:347–357. doi: 10.1523/JNEUROSCI.19-01-00347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Susswein AJ, Byrne JH. Identification and characterization of neurons initiating patterned neural activity in the buccal ganglia of Aplysia. J Neurosci. 1988;8:2049–2061. doi: 10.1523/JNEUROSCI.08-06-02049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teyke T, Weiss KR, Kupfermann I. An identified neuron (CPR) evokes neuronal responses reflecting food arousal in Aplysia. Science. 1990;247:85–87. doi: 10.1126/science.2294596. [DOI] [PubMed] [Google Scholar]
- 48.Teyke T, Weiss KR, Kupfermann I. Activity of identified cerebral neuron correlates with food-induced arousal in Aplysia. Neurosci Lett. 1991;133:307–310. doi: 10.1016/0304-3940(91)90595-k. [DOI] [PubMed] [Google Scholar]
- 49.Teyke T, Rosen SC, Weiss KR, Kupfermann I. Dopaminergic neuron B20 generates rhythmic neuronal activity in the feeding motor circuitry of Aplysia. Brain Res. 1993;630:226–237. doi: 10.1016/0006-8993(93)90661-6. [DOI] [PubMed] [Google Scholar]
- 50.Walters ET, Carew TJ, Kandel ER. Associative learning in Aplysia: evidence for conditioned fear in an invertebrate. Science. 1981;211:504–506. doi: 10.1126/science.7192881. [DOI] [PubMed] [Google Scholar]
- 51.Warman EN, Chiel HJ. A new technique for chronic single-unit extracellular recordings in freely behaving animals using pipette electrodes. J Neurosci Methods. 1995;57:161–169. doi: 10.1016/0165-0270(94)00144-6. [DOI] [PubMed] [Google Scholar]
- 52.Weiss KR, Shapiro E, Kupfermann I. Modulatory synaptic actions of an identified histaminergic neuron on the serotonergic metacerebral cell of Aplysia. J Neurosci. 1986a;6:2393–2402. doi: 10.1523/JNEUROSCI.06-08-02393.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss KR, Chiel HJ, Koch U, Kupfermann I. Activity of an identified histaminergic neuron, and its possible role in arousal of feeding behavior in semi-intact Aplysia. J Neurosci. 1986b;6:2403–2415. doi: 10.1523/JNEUROSCI.06-08-02403.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss KR, Chiel HJ, Kupfermann I. Sensory function and gating of histaminergic neuron C2 in Aplysia. J Neurosci. 1986c;6:2416–2426. doi: 10.1523/JNEUROSCI.06-08-02416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xin Y, Weiss KR, Kupfermann I. Distribution in the central nervous system of Aplysia of afferent fibers arising from cell bodies located in the periphery. J Comp Neurol. 1995;359:627–643. doi: 10.1002/cne.903590409. [DOI] [PubMed] [Google Scholar]
- 56.Ziv I, Baxter DA, Byrne JH. Simulator for neural networks and action potentials: description and application. J Neurophysiol. 1994;71:294–308. doi: 10.1152/jn.1994.71.1.294. [DOI] [PubMed] [Google Scholar]