Abstract
Activation of the pontine inhibitory area (PIA) including the middle portion of the pontine reticular nucleus, oral part (PnO), or the gigantocellular reticular nucleus (Gi) suppresses muscle tone in decerebrate animals. The locus coeruleus (LC) and midbrain locomotor region (MLR) have been implicated in the facilitation of muscle tone. In the current study we investigated whether PIA and Gi stimulation causes changes in activity in these brainstem motor facilitatory systems. PIA stimulation evoked bilateral muscle tone suppression and inhibited 26 of 28 LC units and 33 of 36 tonically active units located in the anatomical equivalent of the MLR (caudal half of the cuneiform nucleus and the pedunculopontine tegmental nucleus). Gi stimulation evoked bilateral suppression of hindlimb muscle tone and inhibited 20 of 35 LC units and 24 of 24 neurons located in the MLR as well as facilitated 11 of 35 LC units. GABA and glycine release in the vicinity of LC was increased by 20–40% during ipsilateral PnO stimulation inducing hindlimb muscle tone suppression on the same side of the body. We conclude that activation of pontine and medullary inhibitory regions produces a coordinated reduction in the activity of the LC units and neurons located in the MLR related to muscle tone facilitation. The linkage between activation of brainstem motor inhibitory systems and inactivation of brainstem facilitatory systems may underlie the reduction in muscle tone in sleep as well as the modulation of muscle tone in the isolated brainstem.
Keywords: muscle tone, gigantocellular reticular nucleus, pontine inhibitory area, locus coeruleus, subcoeruleus nucleus, midbrain locomotor region, REM sleep, GABA, glycine, microdialysis
Previous studies performed in decerebrate cats have shown that electrical stimulation and chemical excitation of the medial pontine reticular formation and magnocellularis and paramedianus nuclei of the medulla induced bilateral muscle atonia (Lai and Siegel, 1988, 1990a,b, 1991). In decerebrate rats, stimulation sites inducing bilateral muscle tone inhibition are located in middle portions of the oral and caudal pontine reticular nuclei and gigantocellular and dorsal gigantocellular reticular nuclei (Hajnik et al., 2000). These pontine and medullary inhibitory regions are elements of a brainstem inhibitory system hyperpolarizing spinal motoneurons (Magoun and Rhines, 1946; Jankowska et al., 1968; Takakusaki et al., 1994) and participating in rapid eye movement (REM) sleep atonia (Sakai et al., 1979; Chase and Morales, 1990; Lai and Siegel, 1990a,b). Lesions of the pontine inhibitory area (PIA) (Jouvet and Delorme, 1965; Henley and Morrison, 1974) and medial medulla (Schenkel and Siegel, 1989; Holmes and Jones, 1994) prevent the normal suppression of muscle tone in REM sleep, resulting in REM sleep without atonia. The REM sleep behavior disorder (Schenck and Mahowald, 1991) seen particularly in elderly males is the human analog of REM sleep without atonia.
Pharmacological, electrophysiological, and behavioral studies indicate an overall facilitatory action of noradrenaline on spinal motoneurons (White and Neuman, 1980, 1983; Holstege and Kuypers, 1987). Electrical stimulation of the locus coeruleus (LC) nucleus evokes a depolarizing response in spinal motoneurons (Fung and Barnes, 1981, 1987) and has a biphasic effect on lumbar monosynaptic reflex amplitude with facilitation followed by inhibition (Fung et al., 1994). It is well known that LC units reduce their activity during non-REM sleep and cease discharge for an extended period during REM sleep muscle atonia (Hobson et al., 1975; Jacobs, 1986; Aston-Jones et al., 1991b). It has been shown recently (Wu et al., 1999) that noradrenergic LC neurons cease discharge during cataplectic episodes (loss of muscle tone) in narcoleptic dogs.
The midbrain locomotor region (MLR) in the cat and rat brain includes the caudal half of the cuneiform nucleus and the pedunculopontine tegmental nucleus (Garcia-Rill et al., 1983, 1986; Skinner and Garcia-Rill, 1984; Coles et al., 1989). Stimulation of this region induces locomotion on a treadmill in cats and rats in which the brainstem has been transected at the precollicular–postmamillary level (Shik et al., 1966; Garcia-Rill et al., 1983; Skinner and Garcia-Rill, 1984). Garcia-Rill and Skinner (1988) reported that neurons in the MLR showed either bursting activity related to cyclic hindlimb neurogram activity or an “on/off” tonic pattern related to the onset or termination of locomotor episodes in the “fictive spontaneous locomotion preparation.” The firing rate of some of the off neurons correlated with the tonic activity of the hindlimb neurograms between locomotor cycles. Electrical stimulation of the pedunculopontine tegmental and cuneiform nuclei increases the tonic muscle tone in decerebrate cats (Lai and Siegel, 1990a) and anesthetized rats (Juch et al., 1992). These results suggest that some tonically active MLR neurons may participate in muscle tone facilitation. In the current study we have determined the responses of LC neurons and tonically active units located in the caudal half of the cuneiform nucleus and pedunculopontine nucleus to the PIA and gigantocellular reticular nucleus (Gi) stimulation. Because we have not elicited locomotion to confirm physiologically that we were recording from the MLR at every site we studied in the current investigation, we have used the term “anatomic equivalent of the MLR” rather than simply using the term MLR to refer to this area.
MATERIALS AND METHODS
All procedures were approved by the Animal Studies Committee of the Sepulveda Veterans Administration Medical Center/University of California, Los Angeles, in accordance with United States Public Health Service guidelines. Experiments were done using 32 male Wistar rats weighing 250–300 gm.
Surgery. Animals were anesthetized with fluothane followed by ketamine HCL (Ketalar; 70 mg/kg, i.p.) for cannulation of the trachea and decerebration. Four holes (diameter, 1.5 mm) were symmetrically drilled over the skull above the PIA (Hajnik et al., 2000) and LC nuclei at 8.0 and 9.5 mm posterior to bregma and 1.3 mm lateral to the midline for insertion of stimulating and unit recording electrodes. One hole (diameter, 1.5 mm) was drilled over the skull above the Gi nucleus at 11.0 mm posterior to bregma for insertion of stimulating electrodes. Guide cannulae with 0.6 mm in diameter were fixed on the skull bone to reach the fourth ventricle (for clonidine microinjections during LC recordings) and LC nuclei (for microdialysis sampling). Coordinates of all structures were based on the rat brain atlas of Paxinos and Watson (1997). Two rectangular holes were cut in each parietal bone in preparation for decerebration. The transverse anterior and posterior borders of these holes were located 1 and 5 mm posterior to bregma, with the longitudinal sagittal border 0.5 mm from the midline. Precollicular–postmamillary decerebration was performed 4.0–5.0 mm posterior to bregma with a stainless steel spatula, taking care not to injure the sagittal vein. Excess fluid was aspirated by syringe and absorbed with Gelfoam (Pharmacia, Piscataway, NJ/Upjohn, Kalamazoo, MI). Then anesthesia was discontinued. Rectal temperature was continuously monitored with an electrical thermometer (model BAT-12; Physitemp Instruments, Clifton, NJ) and maintained between 37 and 38C° with the Heat Therapy Pump (model TP-500; Gaymar Industries, Inc., Orchard Park, NY). Experiments were begun when bilateral postdecerebrate muscle rigidity appeared.
Stimulation and recording. Constant-current square-wave pulses (0.2 msec; 30–50 Hz; 50–200 μA; continuous stimulation for 15–30 sec) were delivered through bipolar electrodes (stainless steel; 100 μm) by the use of an S88 stimulator (Grass Instruments) coupled with a stimulus isolation unit (TF5S21ZZ). Bipolar electrode tips were separated by ∼100 μm. Extracellular unit recordings were performed using monopolar tungsten microelectrodes (A-M Systems, Carlsborg, WA). Spikes were amplified with a model 1700 A-M Systems amplifier. Bipolar stimulating electrodes were inserted in the PIA at 8.3–8.8 mm posterior to bregma, 1.0–2.0 mm lateral to the midline, and 8.0–9.0 mm below the skull and in the Gi at 10.5–11.5 mm posterior to bregma, ±0.8 mm lateral to the midline, and 8.5–10.0 mm below the skull. Stimulation artifact was determined to have a duration of <1.3 msec during PIA and Gi stimulation. Therefore, antidromic responses should have been recorded if they had arisen (Guyenet, 1980).
Monopolar tungsten microelectrodes (A-M Systems) were used to stimulate the subcoeruleus region (SubCR) after unit recordings. Cathodal stimuli (0.2 msec; 50 Hz; 50–200 μA) were delivered by the use of a Grass SIU5 stimulus isolation unit. The anode, a screw electrode, was placed in the frontal cranial bone. Electromyograms (EMG) were recorded from the gastrocnemius and tibialis anterior muscles bilaterally with stainless steel wires (100 μm) and amplified using a Grass polygraph (model 78D). Unit pulses and EMG were recorded on a personal computer using the 1401plus interface and Spike2 program (Cambridge Electronic Design, Ltd., Cambridge, UK). The rate of digitization was 372 Hz for EMG and 21 kHz for unit activity.
Microinjections. Clonidine hydrochloride (Sigma, St. Louis, MO), dissolved in 0.9% saline, was injected into the fourth ventricle to identify noradrenergic LC neurons. Clonidine injections (20 mm; 2.0 μl; pH 4.0; 20 sec) were performed using a 10 μl Hamilton microsyringe connected to an injecting cannulae with an outside diameter of 0.25 mm. Clonidine microinjections and control saline microinjections (n = 4) were made after PIA or Gi electrical stimulations.
Identification of LC cells. Cells were classified as noradrenergic using the criteria reported for LC cells in rats and cats (Foote et al., 1980; Guyenet, 1980; Aston-Jones and Bloom, 1981; Fung and Barnes, 1987). These criteria include the following: (1) slow and regular firing, (2) long-duration (>2 msec) action potentials, (3) suppression of firing by intraventricular administration of the α2-agonist clonidine, (4) fast activation followed by inhibition in response to a pinch stimulus applied to the hindlimb paw, and (5) histological location within the LC tyrosine hydroxylase-positive cell cluster.
Identification of tonically active neurons located in the anatomic equivalent of the MLR and the SubCR. Identification of neurons located in the anatomical equivalent of the MLR and related to muscle tone facilitation was performed using criteria reported for cat and rat. These criteria include the following: (1) location of cells inside of the cuneiform and pedunculopontine tegmental nuclei, which are components of the MLR (Garcia-Rill, 1983; Milner and Mogenson, 1988; Coles et al., 1989), (2) long duration (>1 msec) of action potential (Garcia-Rill et al., 1983; Garcia-Rill and Skinner, 1988), (3) regular firing frequency during postdecerebrate rigidity (Pompeiano and Hoshino, 1976), and (4) unit activity decrease during lumbar perivertebral pressure evoking motor depression similar to motor depression induced by stimulation of pontine and medulla inhibitory regions (Hisamitsu et al., 1987).
SubCR neurons participating in muscle tone inhibition were identified using three criteria as follows: (1) location of cells inside of the pontine inhibitory region (Hajnik et al., 2000), (2) slow and regular firing frequency during postdecerebrate rigidity (Hoshino and Pompeiano, 1976), and (3) cell excitation during stimulation of brainstem inhibitory regions (Mileikovskii et al., 1991; Mileikovsky and Nozdrachev, 1997).
Microdialysis sampling. Microdialysis probes were inserted bilaterally into the LC through the implanted guide cannulae. Microdialysis probes (type A-I-01; EICOM) were perfused with artificial CSF (Harvard, Hill Road, MA) at a flow rate of 4 μl/min using an infusion pump (EPS-64; EICOM). Dialysates were collected for 30 sec intervals during the prestimulation, stimulation, and poststimulation period, with electrical excitation in the pontine reticular nucleus, oral part (PnO), inducing ipsilateral hindlimb muscle tone suppression and contralateral hindlimb muscle tone facilitation. The stimulation sites in the PnO were chosen to measure GABA and glycine release in ipsilateral LC and contralateral LC (as a control site in this case). For the baseline determination, we used seven samples for ipsilateral and nine samples for contralateral stimulation sites. Samples were stored at −80°C until analysis. Dialysis experiments were performed with hindlimb EMG recordings. The records were visually inspected.
Amino acid assay. The concentration of amino acids in the perfusates was detected by a high-performance liquid chromatograph (EDT-300; EICOM) with a fluorescent detector (Soma S-3350; excitation at 340 nm; emission at 440 nm) and quantified with a PowerChrom (AD Instruments) using external amino acid standards (Sigma). Precolumn derivatization was performed witho-phthaldialdehyde/2-mercaptoethanol by an autosampler (model 231XL; Gilson Medical Electric, Middleton, WI) at 5°C for 3 min. The derivatives were then separated in a liquid chromatography column (MA-5ODS; EICOM; 4.6 mm in diameter; 150 mm in length) at 30°C with 30% methanol in 0.1 m phosphate buffer, pH 6.0, being degassed by an on-line degasser (DG-100; EICOM). The detection limits for GABA and glycine were 20 and 10 fmol, respectively.
Histology. Cathodal current (0.1–0.3 mA; 3–6 sec) was passed through the microelectrodes at the end of each recording track. The location of recorded neurons was determined by using the track made by the microelectrode, the depth of the marking lesion, and the depth measurements on the microdrive. Rats were deeply anesthetized by pentobarbital (70 mg/kg, i.p.) and perfused transcardially with 0.01m PBS, pH 7.4, followed by 4% paraformaldehyde in 0.1 m PBS. Brains were removed, cut into 60-μm-thick sections, and processed for tyrosine hydroxylase (TH) immunostaining. Sections were rinsed three times in 0.05 m Trizma-buffered saline, pH 7.4, followed by a 24 hr incubation in a 1:400 dilution of primary mouse antiserum to TH (Chemicon, Temecula, CA) with a solution of 2% normal goat serum in 0.05 m Trizma-buffered saline, pH 7.4, at 4°C. This was followed by a 2.5 hr incubation in a 1:200 dilution of biotinylated goat anti-mouse IgG (Vector Laboratories, Burlingame, CA). The tissue was then incubated for 2 hr with the avidin–biotin complex (Vector Laboratories). Each incubation was followed by three rinses. Dilutions and rinses were prepared with 2% normal goat serum in 0.05 m Trizma-buffered saline. The sections were then treated with a 0.05% solution of 3,3′-diaminobenzidine and 0.01% hydrogen peroxide. The electrode tracks and the TH-positive cells were visualized on a Nikon microscope and plotted with a Neurolucida interface according to the rat brain atlas (Paxinos and Watson, 1997).
Data analysis. Unit firing rates were analyzed by the Wilcoxon matched-pairs test. The average unit firing rates were calculated for 10 sec before and during electrical stimulation. Three electrical stimulations were performed for each neuron. The time period between stimulations was 3–4 min. Microdialysis data were analyzed by paired t tests. The perfusates obtained during stimulation and 30, 60, 90, 120, and 150 sec after stimulation were compared with the prestimulation level.
The latency of muscle tone inhibition was measured from the start of the microinjection to a 30% decrease in muscle tone compared with baseline. The duration was calculated from the time of onset of a sustained decrease in muscle tone until the return to baseline levels. The latencies and durations were averaged for right and left hindlimbs. The latency and duration were calculated for the first microinjection into each site of the PIA and Gi. All average values are means ± SEM.
RESULTS
Identification of recorded LC neurons
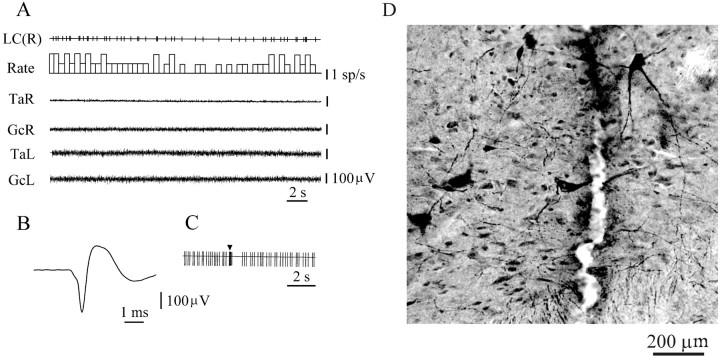
A total of 206 neurons with slow (0.5–20 spikes/sec) and regular firing frequencies were recorded from the dorsolateral pontine area. Seventy-eight of these neurons met the criteria for noradrenergic cells. These neurons showed a slow (4.4 ± 0.4 spikes/sec;n = 78) regular firing rate when hindlimb muscle rigidity was observed (Fig.1A) and had an average spike duration of 2.44 ± 0.03 msec (Fig. 1B). Pinching of the contralateral or ipsilateral hindlimb paw evoked an initial burst of activity (0.3–0.8 sec) followed by an interval of inhibition (Fig. 1C). These cells were in regions with TH-positive immunostaining (Fig. 1D). Clonidine microinjections into the fourth ventricle completely inhibited the activity of all of these neurons for 20–40 sec after the drug administration and also suppressed muscle tone. Unit activity and muscle tone returned in parallel to the baseline level by 50–60 min after clonidine microinjection. Control microinjections of saline into the fourth ventricle (n = 4) did not produce LC unit inhibition.
Fig. 1.
Electrophysiological and histological identification of LC neurons. A, Slow and regular spontaneous firing during muscle rigidity. B, Long duration of noradrenergic spike. C, Fast activation followed by inhibition in response to a pinch stimulus (arrowhead) applied to the hindlimb paw.D, Histological location of tyrosine hydroxylase-positive cells near the recording track in the pontine region. GcR, GcL, EMG of gastrocnemius muscle (right side, left side); LC(R), locus coeruleus unit (right side); sp, spike; TaR,TaL, EMG of tibialis anterior muscle (right side, left side). LC(R) and unit traces in subsequent figures are computer-generated pulses marking discriminated action potentials.
Identification of tonically active neurons recorded in the anatomical equivalent of the MLR and the SubCR
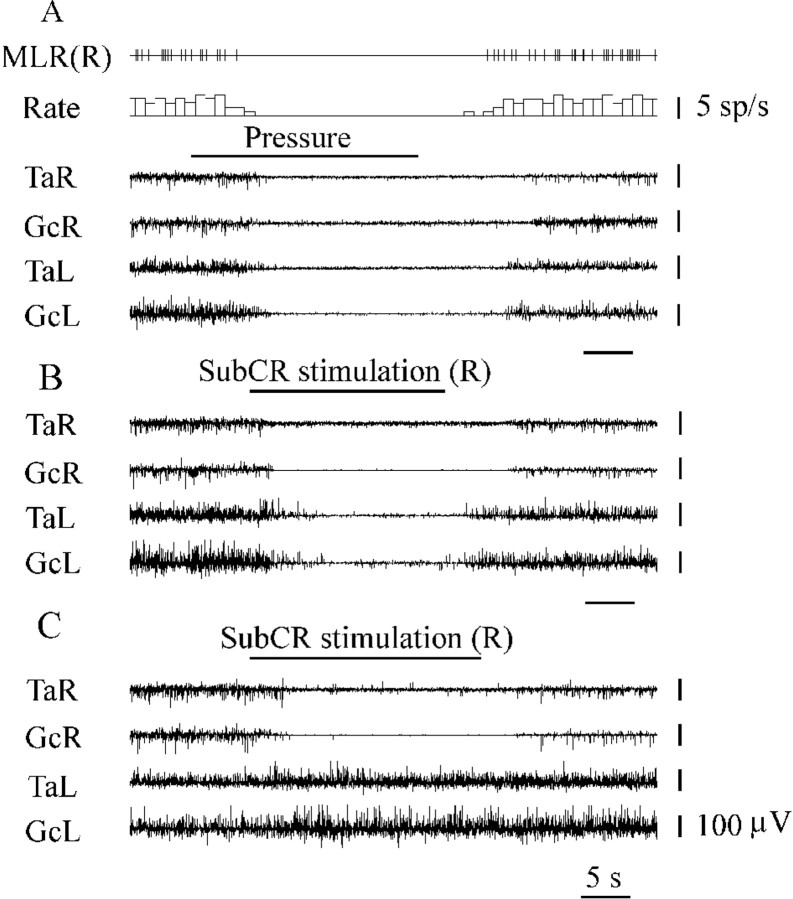
A total of 76 neurons with a regular firing rate during hindlimb muscle rigidity were recorded from the region including cuneiform and pedunculopontine tegmental nuclei. These neurons had an average firing discharge of 10.2 ± 0.7 spikes/sec (n = 76) and a spike duration of 2.27 ± 0.03 msec. Pressure applied by squeezing the lumbar perivertebral region with the thumb and index finger evoked bilateral hindlimb muscle tone suppression and reduction or complete inhibition of discharges in these neurons (Fig.2A).
Fig. 2.
Identification of tonically active neurons located in the anatomical equivalent of the MLR and muscle tone suppression during SubCR stimulation. A, Inhibition of MLR tonically active neuron during lumbar perivertebral pressure evoking bilateral hindlimb muscle tone suppression. B, Bilateral hindlimb muscle tone inhibition during SubCR electrical stimulation (R, right side). C, Ipsilateral hindlimb muscle tone inhibition during SubCR electrical stimulation (right side). MLR(R), Tonically active MLR unit (right side). See abbreviations in Figure 1.
A total of 52 neurons with a regular firing rate (4.7 ± 0.8 spikes/sec) were recorded in the SubCR. These neurons were excited by stimulation of the PIA and Gi sites inducing bilateral hindlimb muscle tone inhibition (see Figs. 6B, 9B) and had a spike duration of 1.68 ± 0.04 msec (n = 52). Threshold electrical stimulation (92 ± 21 μA; 50 Hz;n = 9) of this region induced bilateral, ipsilateral, or contralateral muscle tone inhibition (Fig.2B,C).
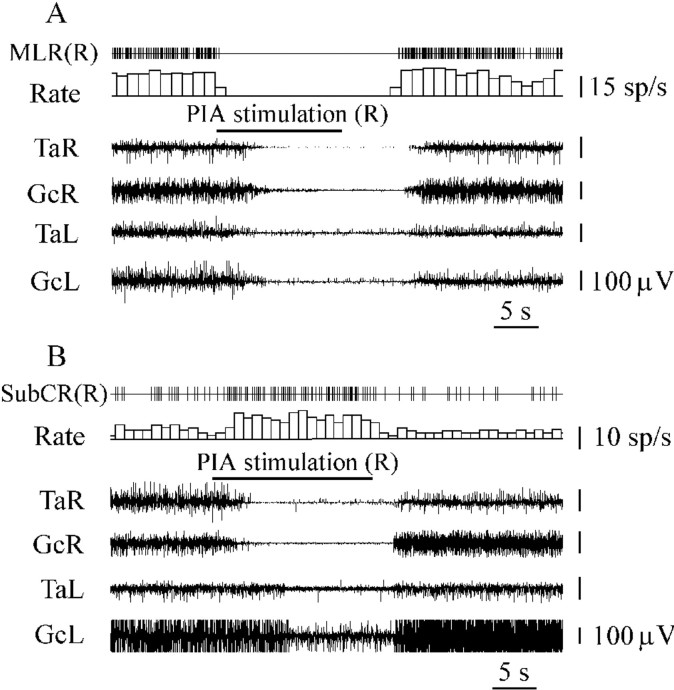
Fig. 6.
Inhibition of a unit located in the anatomical equivalent of the MLR (A) and SubCR unit excitation (B) during PIA electrical stimulation inducing bilateral hindlimb muscle tone suppression.SubCR(R), SubCR units (right side). See abbreviations in Figures 1 and 2.
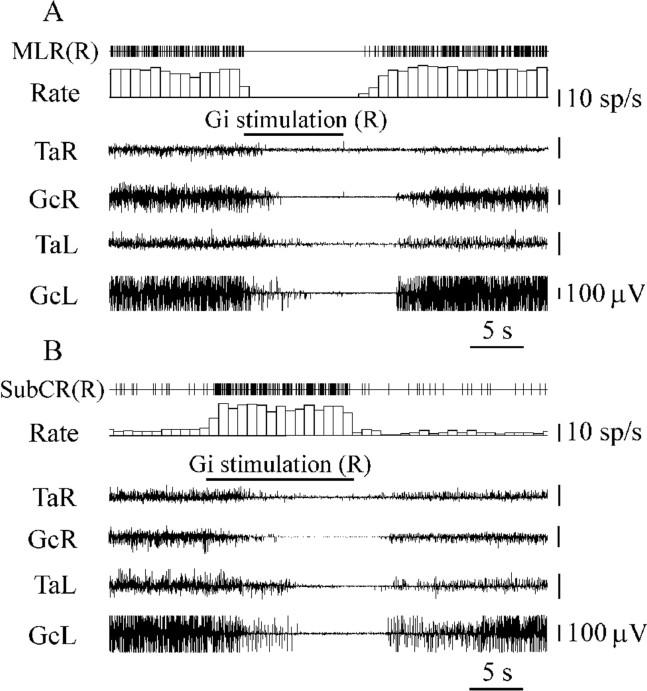
Fig. 9.
Inhibition of a unit located in the anatomical equivalent of the MLR (A) and SubCR unit excitation (B) during Gi electrical stimulation inducing bilateral muscle tone suppression. See abbreviations in Figures 1, 2, and 6.
Pontine inhibitory area stimulation
LC neurons
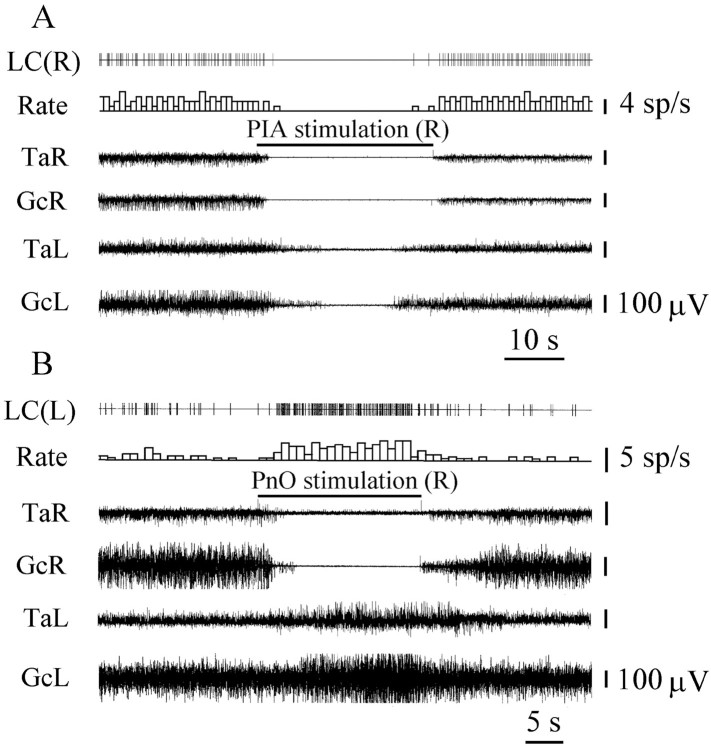
The activity of 28 LC units was analyzed during electrical stimulation of 12 PIA sites located inside of the PnO and inducing bilateral hindlimb muscle tone suppression (Fig.3A). Fourteen LC units located ipsilateral and 12 LC units located contralateral to PIA stimulation sites showed reduced activity (Fig. 4). Twenty of these LC units were completely inhibited (Fig.5A), and the discharge of 6 cells was reduced by 64 ± 7% (n = 6;T = 0; p < 0.05). Two ipsilateral LC units increased the firing rate by 100–300% compared with the baseline during PIA stimulation.
Fig. 3.
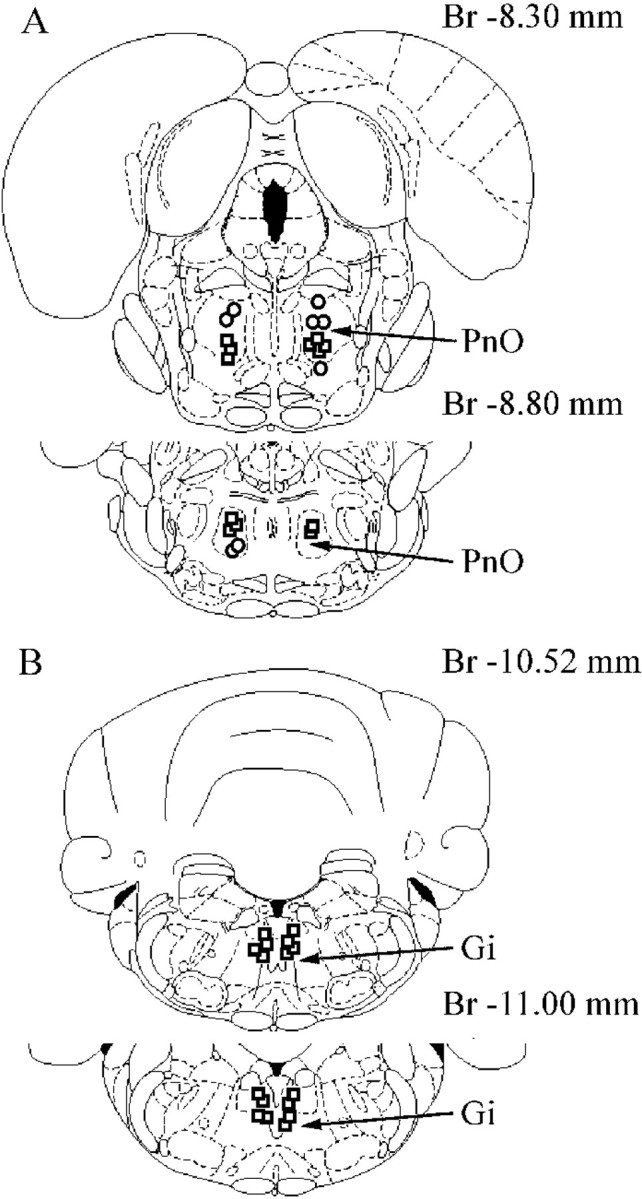
Location of PnO (A) and Gi (B) sites inducing bilateral hindlimb muscle tone suppression (squares) and ipsilateral hindlimb muscle tone suppression with contralateral hindlimb muscle tone facilitation (circles) during electrical stimulation. Br, Bregma.
Fig. 4.
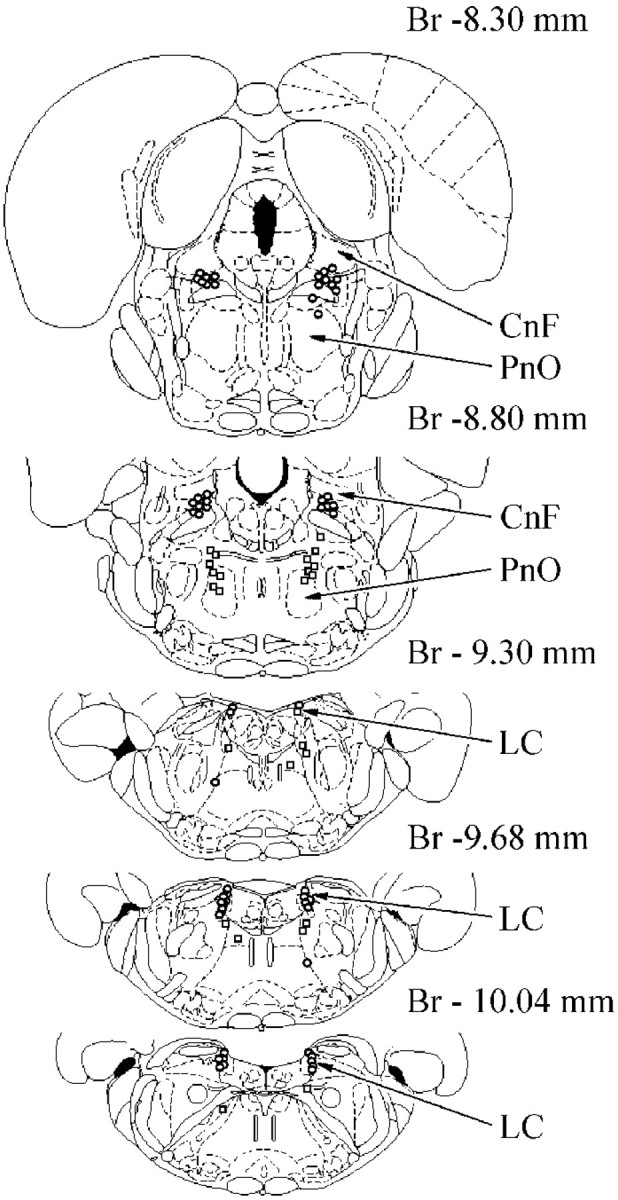
Location of inhibited (circles) and excited (squares) pontine reticular units during PIA electrical stimulation. CnF, Cuneiform nucleus. See abbreviation in Figure 3.
Fig. 5.
LC unit inhibition (A) during PIA electrical stimulation inducing bilateral muscle tone suppression and LC unit excitation (B) during PnO electrical stimulation inducing ipsilateral hindlimb muscle tone suppression with contralateral hindlimb muscle tone facilitation.LC(L), Locus coeruleus unit (left side). See abbreviations in Figure 1.
Stimulation of five sites into the dorsal and three sites into ventral part of the oral pontine reticular nucleus (Fig. 3A) induced ipsilateral hindlimb muscle tone suppression and contralateral hindlimb muscle tone facilitation. The firing rate of 15 LC units was analyzed during stimulation of these pontine sites. Nine LC units located ipsilateral to stimulation sites were completely inhibited, and 5 contralateral LC units were excited by an average of 242 ± 43% (n = 5; T = 0;p < 0.05) compared with the background (Fig.5B). One ipsilateral LC unit increased activity during PnO stimulation.
Tonically active neurons located in the anatomical equivalent of the MLR
Thirty-six neurons located in the cuneiform nucleus, pedunculopontine tegmental nucleus, and adjacent reticular formation and firing with a regular discharge rate were recorded during stimulation of 12 PIA sites inducing bilateral hindlimb muscle tone suppression. Thirty-three units were completely silenced by PIA stimulation (Fig. 6A). Nineteen of these neurons were located in the ipsilateral MLR, and 14 units were situated in the contralateral MLR (Fig. 4). Three neurons did not change firing frequency when contralateral PIA stimulation was applied.
Sixteen neurons located in the anatomical equivalent of the MLR were recorded during stimulation of PnO sites inducing ipsilateral hindlimb muscle tone inhibition and contralateral hindlimb muscle tone facilitation. Five cells were completely silenced by ipsilateral PnO stimulation that inhibited the hindlimb muscle tone on the same side of the body. Nine neurons located in the contralateral MLR increased the firing rate by an average of 324 ± 34% (n = 9;T = 0; p < 0.01) compared with the baseline. This unit excitation was correlated with hindlimb muscle tone elevation on the same side of the body. Two cells located in the MLR increased firing frequency during ipsilateral PnO stimulation.
Tonically active SubCR neurons
Thirty SubCR neurons (Fig. 4) firing with regular and slow frequency were recorded during stimulation of seven PIA sites producing bilateral muscle tone inhibition. Twenty-four SubCR units (16 units located ipsilaterally and 8 units located contralaterally to PIA stimulation sites) increased the firing rate by an average of 381 ± 20% (n = 24; T = 0;p < 0.01) compared with the baseline (Fig.6B). Two ipsilateral SubCR units were inhibited, and four contralateral SubCR neurons did not change firing rate during PIA stimulation.
Medulla inhibitory area stimulation
LC neurons
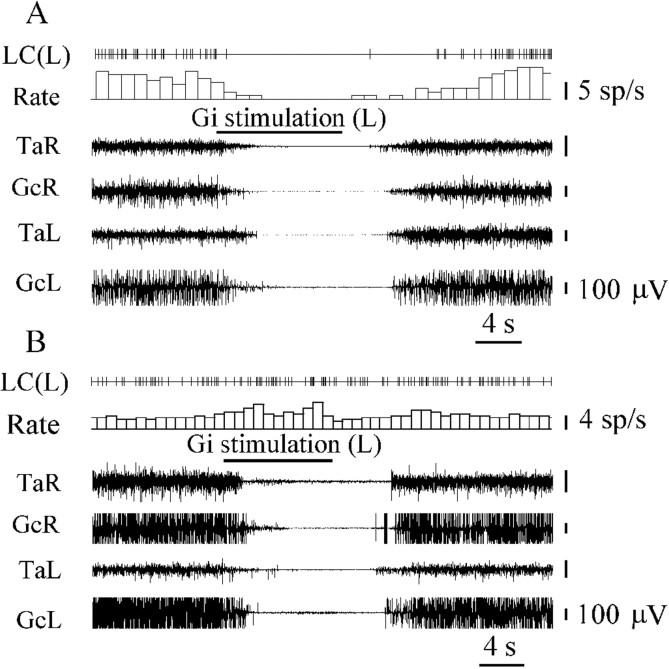
The activity of 35 LC units was analyzed during stimulation of 16 Gi sites that induced bilateral hindlimb muscle atonia (Fig.3B). The noradrenergic LC cells could be divided into three groups. The first group (n = 20) consisted of LC neurons that were depressed by Gi stimulation. Eleven LC units were located ipsilateral and 9 LC units were located contralateral to Gi stimulation sites (Fig. 7). The discharge rate of 8 of these cells was reduced by an average of 63 ± 7% (n = 8; T = 0; p < 0.05), and 12 cells were completely inhibited (Fig.8A).
Fig. 7.
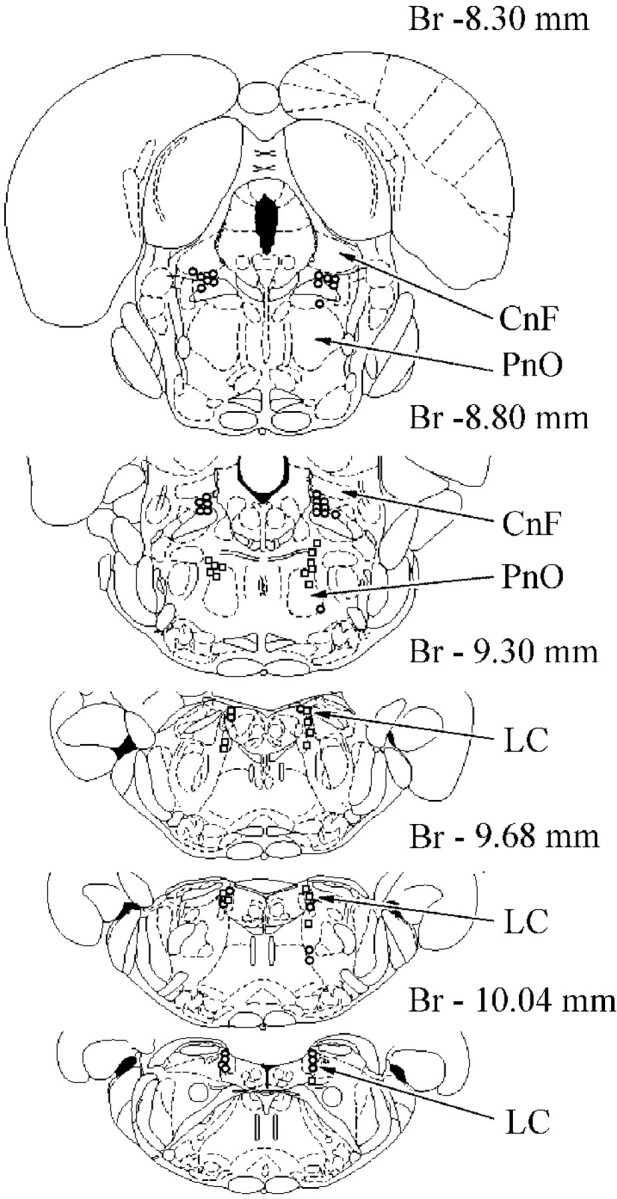
Location of inhibited (circles) and excited (squares) pontine reticular units during Gi electrical stimulation. See abbreviations in Figures 3 and 4.
Fig. 8.
LC unit inhibition (A) and LC unit excitation (B) during Gi stimulation inducing bilateral hindlimb muscle tone suppression. See abbreviations in Figures 1 and 5.
Neurons in the second group (n = 11) were excited by stimulation of the Gi (Fig. 8B). Four LC units were located ipsilaterally and 7 LC units were located contralaterally to Gi stimulation sites. The discharge rate of these cells increased by an average of 47 ± 4% (n = 11; T = 0; p < 0.01) compared with the baseline. LC neurons in the third group (n = 4) did not change firing frequency when Gi stimulation was applied.
Tonically active neurons located in the anatomical equivalent of the MLR
The responses of 24 neurons located in the cuneiform nucleus, pedunculopontine tegmental nucleus, and adjacent reticular formation were analyzed during stimulation of six Gi sites inducing bilateral hindlimb muscle tone suppression. All neurons (15 units located ipsilaterally and 9 units located contralaterally to Gi stimulation sites) reduced discharge during Gi stimulation. Twenty of these cells were completely silenced by Gi stimulation (Fig.9A), and 4 cells decreased the firing rate by 50–70%.
Tonically active SubCR neurons
The activity of 22 SubCR units (Fig. 7) with a slow and regular firing rate was analyzed during Gi stimulation inducing bilateral hindlimb muscle tone inhibition. Eighteen SubCR units (11 units located ipsilaterally and 7 located contralaterally to Gi stimulation sites) were excited (339 ± 30%; n = 18;T = 0; p < 0.01) by Gi stimulation (Fig. 9B). Four SubCR units became completely silent during Gi stimulation.
Microdialysis investigations
Microdialysis was used to investigate amino acid release during PnO stimulation inducing ipsilateral hindlimb muscle tone inhibition and contralateral hindlimb muscle tone facilitation. One hundred twelve perfusates from 16 experiments (9 with contralateral and 7 with ipsilateral electrical stimulation) were obtained from the LC. The mean level (±SEM) of GABA release in the ipsilateral and the contralateral LC during the prestimulation control period was 156.8 ± 13.8 and 160.0 ± 16.3 fmol/sample, respectively. Glycine release in the ipsilateral and the contralateral LC during the prestimulation control periods was 12.5 ± 1.8 and 13.0 ± 2.0 pmol/sample, respectively.
GABA release in the LC was significantly increased during the 30 sec period of electrical stimulation in the ipsilateral PnO (t = 3.04; df = 13; p < 0.01). On the other hand, the GABA release was not significantly increased during contralateral PnO stimulation but started to increase at 60 sec after stimulation, with the increase lasting for 90 sec (Fig.10A). Similarly, the glycine release in the LC was significantly increased during 30 sec of electrical stimulation in the ipsilateral PnO (t = 3.52; df = 13; p < 0.01). The glycine release in the contralateral LC was not increased during PnO stimulation, but it started to increase at 60 sec after stimulation, and the increase lasted for 90 sec (Fig. 10B).
Fig. 10.
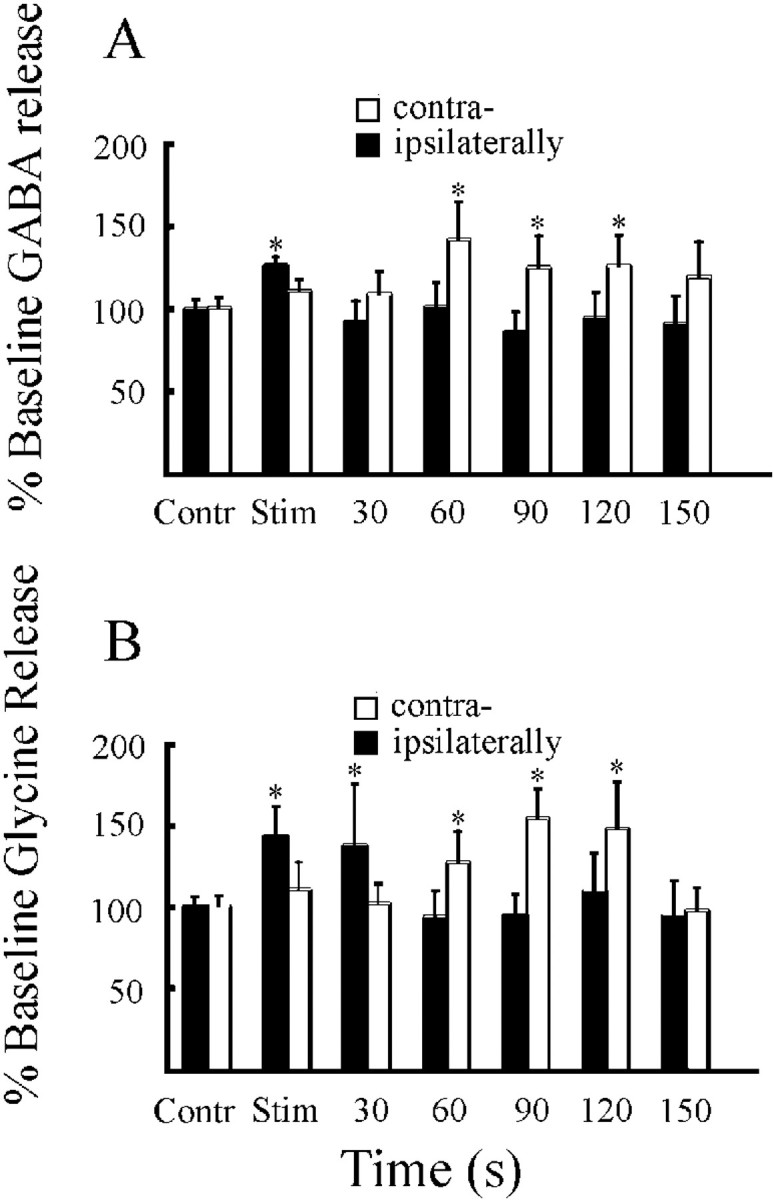
GABA (A) and glycine (B) release in the vicinity of ipsilateral and contralateral LC nuclei during PnO stimulation inducing ipsilateral hindlimb muscle tone suppression with contralateral hindlimb muscle tone facilitation. Contr, Control; Stim, stimulation. *p < 0.01.
DISCUSSION
Since the discovery of the stimulation-induced atonia phenomenon in decerebrate cats (Magoun and Rhines, 1946), the decerebrate preparation has been extremely useful in analyzing the connections and neurochemistry of the brainstem muscle tone control circuits. Our results have shown that electrical stimulation of the PIA and Gi excited neurons located in the SubCR, an area participating in descending inhibition of spinal motoneurons (Lai and Siegel, 1990a,b), and markedly reduced discharge of LC units and neurons located in the anatomical equivalent of the MLR linked to muscle tone facilitation and locomotion (Garcia-Rill et al., 1983; Skinner and Garcia-Rill, 1984;Garcia-Rill and Skinner, 1988; Juch et al., 1992). We have shown previously that electrical and chemical stimulation of the inhibitory sites in the PnO depressed hindlimb muscle tone and reduced the activity of medullary reticulospinal neurons related to muscle tone facilitation induced by hypothalamic stimulation (Mileykovskiy et al., 1999). Iwakiri et al. (1995) reported that stimulation of inhibitory points in the medial pontine reticular formation reduced the activity of medullary reticulospinal neurons excited by MLR stimulation. In previous work it has been shown that the SubCR is part of an inhibitory brainstem–reticulospinal system hyperpolarizing α-motoneurons via inhibitory interneurons (Jankowska et al., 1968; Morales et al., 1987;Kawahara et al., 1988; Chase et al., 1989; Takakusaki et al., 1989,1994). These results suggest that brainstem inhibitory area stimulation activates reticulospinal inhibitory mechanisms and simultaneously inhibits the activity of several systems inducing muscle tone facilitation and movement at the pontine and medullary levels. The observed inhibition of the LC neurons and neurons located in the MLR was of orthodromic origin because short-latency antidromic spikes were not recorded in our experiments and because a gradual increase in inhibition of the neurons was observed as the stimulating currents were raised.
Inhibition of pontine and reticulospinal neurons linked with muscle tone facilitation and movement may be realized by direct GABA or glycinergic projections from inhibitory structures or by local interneurons with the same mediators. Our microdialysis studies indicated that LC unit inhibition during PnO stimulation was correlated with GABA and glycine release onto LC neurons. In previous work it was shown that cessation of discharge in LC neurons during REM sleep was correlated with increased GABA release (Nitz and Siegel, 1997). Subsequently, Gervasoni et al. (1998) found that iontophoretic application of bicuculline, a specific GABAAreceptor antagonist, disrupted LC unit inhibition during REM and slow wave sleep and increased the discharge rate during wakefulness. Intracellular recordings performed by Shefner and Osmanovic (1991)indicated that GABA application inhibited spontaneous firing and increased the conductance of LC neurons primarily via activation of GABAA receptors. GABAB-selective agonists inhibited spontaneous LC unit firing more weakly.
It has been reported that glycine tonically inhibits noradrenergic LC neurons. In particular, Darracq et al. (1996) observed that iontophoretically applied strychnine, a specific glycine antagonist, induced strong excitation of LC neurons across the sleep–waking cycle and during wakefulness. The main origin of glycinergic inputs to rat LC neurons is the ventral and ventrolateral periaqueductal gray including the adjacent deep mesencephalic reticular nucleus, paragigantocellular nucleus, and rostral ventromedial medullary reticular formation (Rampon et al., 1999).
In the cat, electrical stimulation of the PnO generally yields bilateral inhibition of muscle tone, whereas in the rat, similar stimulation frequently produces ipsilateral inhibition and contralateral facilitation. It is possible that the different influences of PnO stimulation on the ipsilateral and contralateral hindlimb muscle tone and neurons located in the ipsilateral and contralateral LC and MLR are related to the small dimension of the inhibitory area in the rat's PnO. Stimulating currents can spread into adjacent areas, in particular, into a pontine region inducing rotation (Mileikovskii et al., 1991). The excitatory influences of PIA and PnO stimulation on a few ipsilateral LC units are probably linked with direct activation of LC dendrites that extend preferentially into the pericoeruleus region (Aston-Jones et al., 1991a).
LC units receive direct projections from the nucleus prepositus hypoglossi and dorsal paragigantocellular nucleus (Luppi et al., 1995). Within the nucleus prepositus hypoglossi a large proportion of LC-projecting neurons stained for GABA (Aston-Jones et al., 1991a). It is possible that LC unit inhibition during stimulation of the dorsomedial part of the medulla is related to excitation of GABAergic prepositus hypoglossi units or local inhibitory mechanisms in the LC nucleus. An excitation of 30% of LC units, which we observed during Gi electrical stimulation, may be evoked by oligosynaptic and polysynaptic ascending influences from the surrounding excitatory reticular formation. Takakusaki et al. (1994) reported that motor facilitatory and inhibitory brainstem areas partially overlap.
Our results also show that inhibition of rostral brainstem structures facilitating muscle tone and excitation of the inhibitory subcoeruleus region contribute to the inhibition of muscle tone induced by medullary stimulation (Magoun and Rhines, 1946; Lai and Siegel, 1988). These data are consistent with findings that brainstem transection at the pontomedullary junction and pontine lidocaine injections block muscle atonia induced by medullary stimulation (Siegel et al., 1983; Kohyama et al., 1998).
Although a correlation between LC unit activity and muscle tone has been shown in numerous studies (D'Ascanio et al., 1985, 1989; Fung et al., 1987, 1988, 1991; Stampacchia et al., 1987; Andre et al., 1995), it is worth noting that firing rate changes in response to brainstem activation in some LC neurons may be also related to the regulation of other functional brain systems. Katayama et al. (1984) reported that microinjection of the cholinergic agonist carbachol into the PIA suppressed somatosensory, somatomotor, and sympathetic visceromotor functions as well as orienting behavior. They conclude that the PIA is a system, which regulates animal responsiveness to external stimuli. Furthermore, inhibitory reactions were recorded in medial medullary cells receiving tactile, proprioceptive, and nociceptive information during stimulation of the inhibitory regions in the pons (Mileikovskii, 1994). PIA stimulation would also be expected to attenuate LC unit responses to somatosensory stimuli.
We propose that cataplexy represents a triggering of the REM sleep atonia mechanisms (activation of brainstem neurons linked to inhibition of muscle tone) with a simultaneous disfacilitation of neurons related to increasing muscle tone and movement activity. Siegel et al. (1991), studying unit activity during REM sleep and cataplectic episodes in the narcoleptic dog, showed that a population of neurons located in the ventromedial part of the medulla was activated during both REM sleep atonia and cataplexy. Wu et al. (1999) reported that cessation of LC discharge is tightly linked to cataplectic episodes. In addition to the activation of a population of presumed motor inhibitory neurons in the brainstem and the simultaneous inactivation of facilitatory neurons in the locus coeruleus during cataplexy, Siegel et al. (1992) have reported that many wake-active neurons cease discharge during cataplexy. Thus cataplexy seems to be the result of coordinated activation and inactivation of several postural inhibitory and excitatory systems, respectively.
Previous studies have emphasized active inhibitory processes linked to the loss of muscle tone that can be elicited in the decerebrate animal and that occur in REM sleep and cataplexy. More recent studies have indicated that LC neurons cease discharge during both cataplexy and REM sleep. The current studies demonstrate that electrical stimulation of the PIA and Gi that induce atonia cause a reduction in activity of the LC and in the anatomical equivalent of the MLR, which are linked to the facilitation of muscle tone. The same stimulations simultaneously cause an increase of neuronal activity in the SubCR which is linked to muscle tone suppression. Brainstem inhibitory systems act by suppression of activity in motor excitatory systems as well as by direct inhibition of spinal motoneurons, and this coordinated activity pattern contributes to the normal regulation of muscle tone across the sleep–wake cycle. Pathologies of muscle tone regulation may result from disruptions of this coordinated inhibitory–disfacilitatory pattern.
Footnotes
Correspondence should be addressed to Dr. Jerome Siegel, Los Angeles, Neurobiology Research 151A3, Veterans Administration Medical Center, 16111 Plummer Street, North Hills, CA 91343. E-mail:JSiegel@UCLA.edu.
This work was supported by United States Public Health Service Grants HL 41370 and HL 60296 and by the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- 1.Andre P, Horn E, Pompeiano O. Microinjections of GABAergic agents in the locus coeruleus modify the gain of vestibulospinal reflexes in decerebrate cats. Arch Ital Biol. 1995;133:47–75. [PubMed] [Google Scholar]
- 2.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991a;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- 4.Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991b;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- 5.Chase MH, Morales FR. The atonia and myoclonia of active (REM) sleep. Annu Rev Psychol. 1990;209:557–584. doi: 10.1146/annurev.ps.41.020190.003013. [DOI] [PubMed] [Google Scholar]
- 6.Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9:743–751. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coles SK, Iles JF, Nicolopoulos-Stournaras S. The mesencephalic center controlling locomotion in the rat. Neuroscience. 1989;28:149–157. doi: 10.1016/0306-4522(89)90239-x. [DOI] [PubMed] [Google Scholar]
- 8.Darracq L, Gervasoni D, Souliere F, Lin JS, Fort P, Chouvet G, Luppi PH. Effect of strychnine on rat locus coeruleus neurons during sleep and wakefulness. NeuroReport. 1996;8:351–355. doi: 10.1097/00001756-199612200-00069. [DOI] [PubMed] [Google Scholar]
- 9.D'Ascanio P, Bettini E, Pompeiano O. Tonic inhibitory influences of locus coeruleus on the response gain of limb extensors to sinusoidal labyrinth and neck stimulations. Arch Ital Biol. 1985;123:69–100. [PubMed] [Google Scholar]
- 10.D'Ascanio P, Horn E, Pompeiano O, Stampacchia G. Injections of beta-adrenergic substances in the locus coeruleus affect the gain of vestibulospinal reflexes in decerebrate cats. Arch Ital Biol. 1989;127:187–218. [PubMed] [Google Scholar]
- 11.Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung SJ, Barnes CD. Evidence of facilitatory coeruleospinal action in lumbar motoneurons of cats. Brain Res. 1981;216:299–311. doi: 10.1016/0006-8993(81)90132-3. [DOI] [PubMed] [Google Scholar]
- 13.Fung SJ, Barnes CD. Membrane excitability changes in hindlimb motoneurons induced by stimulation of the locus coeruleus in cats. Brain Res. 1987;402:230–242. doi: 10.1016/0006-8993(87)90029-1. [DOI] [PubMed] [Google Scholar]
- 14.Fung SJ, Pompeiano O, Barnes CD. Suppression of the recurrent inhibitory pathway in lumbar cord segments during locus coeruleus stimulation in cats. Brain Res. 1987;402:351–354. doi: 10.1016/0006-8993(87)90043-6. [DOI] [PubMed] [Google Scholar]
- 15.Fung SJ, Pompeiano O, Barnes CD. Coeruleospinal influence on recurrent inhibition of spinal motonuclei innervating antagonistic hindleg muscles of the cat. Pflügers Arch. 1988;412:346–353. doi: 10.1007/BF01907550. [DOI] [PubMed] [Google Scholar]
- 16.Fung SJ, Manzoni D, Chan JY, Pompeiano O, Barnes CD. Locus coeruleus control of spinal motor output. Prog Brain Res. 1991;88:395–409. doi: 10.1016/s0079-6123(08)63825-x. [DOI] [PubMed] [Google Scholar]
- 17.Fung SJ, Chan JYH, Manzoni D, White SR, Lai YY, Strahlendorf HK, Zhuo H, Liu R-H, Reddy VK, Barnes CD. Cotransmitter-mediated locus coeruleus action on motoneurons. Brain Res Bull. 1994;35:423–432. doi: 10.1016/0361-9230(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Rill E. Connections of the mesencephalic locomotor region (MLR) 3. Intracellular recordings. Brain Res Bull. 1983;10:73–81. doi: 10.1016/0361-9230(83)90077-1. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Rill E, Skinner RD. Modulation of rhythmic function in the posterior midbrain. Neuroscience. 1988;27:639–654. doi: 10.1016/0306-4522(88)90295-3. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Rill E, Skinner RD, Fitzgerald JA. Activity in the mesencephalic locomotor region during locomotion. Exp Neurol. 1983;82:609–622. doi: 10.1016/0014-4886(83)90084-5. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Rill E, Skinner RD, Conrad C, Mosley D, Campbell C. Projections of the mesencephalic locomotor region in the rat. Brain Res Bull. 1986;17:33–40. doi: 10.1016/0361-9230(86)90158-9. [DOI] [PubMed] [Google Scholar]
- 22.Gervasoni D, Darracq L, Fort P, Souliere F, Chouvet G, Luppi PH. Electrophysiological evidence that noradrenergic neurons of the rat locus coeruleus are tonically inhibited by GABA during sleep. Eur J Neurosci. 1998;10:964–970. doi: 10.1046/j.1460-9568.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- 23.Guyenet PG. The coeruleospinal noradrenergic neurons: anatomical and electrophysiological studies in the rat. Brain Res. 1980;189:121–133. doi: 10.1016/0006-8993(80)90012-8. [DOI] [PubMed] [Google Scholar]
- 24.Hajnik T, Lai YY, Siegel JM. Atonia related regions in the rodent pons and medulla. J Neurophysiol. 2000;84:1942–1948. doi: 10.1152/jn.2000.84.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henley K, Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol Exp (Warsz) 1974;34:215–232. [PubMed] [Google Scholar]
- 26.Hisamitsu T, Wu C, Takeshige C. Comparison of motor cortex-induced flexor muscle activity inhibition by hard pressure on various parts of the body and by light pinch of abdomen of animals with gastro-duodenal ulcers. Acupunct Electrother Res. 1987;12:171–183. doi: 10.3727/036012987816358797. [DOI] [PubMed] [Google Scholar]
- 27.Hobson JA, McCarley RW, Wyzinski PV. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 28.Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotoninergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1179–1200. doi: 10.1016/0306-4522(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 29.Holstege JC, Kuypers HG. Brainstem projections to spinal motoneurons: an update. Neuroscience. 1987;23:809–821. doi: 10.1016/0306-4522(87)90160-6. [DOI] [PubMed] [Google Scholar]
- 30.Hoshino K, Pompeiano O. Selective discharge of pontine neurons during the postural atonia produced by an anticholinesterase in the decerebrate cat. Arch Ital Biol. 1976;114:244–247. [PubMed] [Google Scholar]
- 31.Iwakiri H, Oka T, Takakusaki K, Mori S. Stimulus effects of the medial pontine reticular formation and the mesencephalic locomotor region upon medullary reticulospinal neurons in acute decerebrate cats. Neurosci Res. 1995;23:47–53. [PubMed] [Google Scholar]
- 32.Jacobs BL. Single unit activity of locus coeruleus neurons in behaving animals. Prog Neurobiol. 1986;27:183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- 33.Jankowska E, Lund S, Lunberg A, Pompeiano O. Inhibitory effect evoked by through ventral reticulospinal pathways. Arch Ital Biol. 1968;106:124–140. [PubMed] [Google Scholar]
- 34.Jouvet M, Delorme F. Locus coeruleus et sommeil paradoxal. C R Seances Soc Biol Fil. 1965;159:895–899. [PubMed] [Google Scholar]
- 35.Juch PJ, Schaafsma A, van Willigen JD. Brainstem influences on biceps reflex activity and muscle tone in the anaesthetized rat. Neurosci Lett. 1992;140:37–41. doi: 10.1016/0304-3940(92)90676-x. [DOI] [PubMed] [Google Scholar]
- 36.Katayama Y, DeWitt DS, Becher DP, Hayes RL. Behavioral evidence for cholinoceptive pontine inhibitory area: descending control of spinal motor output and sensory input. Brain Res. 1984;296:241–262. doi: 10.1016/0006-8993(84)90062-3. [DOI] [PubMed] [Google Scholar]
- 37.Kawahara K, Nakazono Y, Kumagai S, Yamauchi Y, Miyamoto Y. Neuronal origin of parallel suppression of postural tone and respiration elicited by stimulation of midpontine dorsal tegmentum in the decerebrate cat. Brain Res. 1988;474:403–406. doi: 10.1016/0006-8993(88)90460-x. [DOI] [PubMed] [Google Scholar]
- 38.Kohyama J, Lai YY, Siegel JM. Inactivation of the pons blocks medullary-induced muscle tone suppression in the decerebrate cat. Sleep. 1998;21:695–699. doi: 10.1093/sleep/21.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai YY, Siegel JM. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai YY, Siegel JM. Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J Neurosci. 1990a;10:2727–2734. doi: 10.1523/JNEUROSCI.10-08-02727.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai YY, Siegel JM. Cardiovascular and muscle tone changes produced by microinjection of cholinergic and glutamatergic agonists in dorsolateral pons and medial medulla. Brain Res. 1990b;514:27–36. doi: 10.1016/0006-8993(90)90432-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai YY, Siegel JM. Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression. J Neurosci. 1991;11:2931–2937. doi: 10.1523/JNEUROSCI.11-09-02931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luppi PH, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Afferent projections to the rat locus coeruleus demonstrated by retrograde and anterograde tracing with cholera-toxin B subunit and Phaseolus vulgaris leucoagglutinin. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- 44.Magoun HW, Rhines R. An inhibitory mechanism in the bulbar reticular formation. J Neurophysiol. 1946;9:165–171. doi: 10.1152/jn.1946.9.3.165. [DOI] [PubMed] [Google Scholar]
- 45.Mileikovskii BYu. Influence of stimulation of movement-inhibiting areas of the pons on the activity of neurons of the medial region of the medulla oblongata. Neurosci Behav Physiol. 1994;24:423–428. doi: 10.1007/BF02359795. [DOI] [PubMed] [Google Scholar]
- 46.Mileikovskii BYu, Verevkina SV, Nozdrachev AD. Central neurophysiological mechanisms of the regulation of inhibition. Neurosci Behav Physiol. 1991;21:263–268. doi: 10.1007/BF01191667. [DOI] [PubMed] [Google Scholar]
- 47.Mileikovsky BY, Nozdrachev AD. Neuronal reactions in the dorsolateral pontine area during the immobility response in rats. Fiziol Zh Im I M Sechenova. 1997;83:80–93. [PubMed] [Google Scholar]
- 48.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Suppression of medullary reticulospinal and pontine neurons during stimulation of the pontine inhibitory area. Soc Neurosci Abstr. 1999;25:626. [Google Scholar]
- 49.Milner KL, Mogenson GJ. Electrical and chemical activation of the mesencephalic and subthalamic locomotor regions in freely moving rats. Brain Res. 1988;452:273–285. doi: 10.1016/0006-8993(88)90031-5. [DOI] [PubMed] [Google Scholar]
- 50.Morales FR, Engelhardt JK, Soja PJ, Pereda AE, Chase MH. Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J Neurophysiol. 1987;57:1118–1129. doi: 10.1152/jn.1987.57.4.1118. [DOI] [PubMed] [Google Scholar]
- 51.Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- 53.Pompeiano O, Hoshino K. Tonic inhibition of dorsal pontine neurons during the postural atonia produced by an anticholinesterase in the decerebrate cat. Arch Ital Biol. 1976;114:310–340. [PubMed] [Google Scholar]
- 54.Rampon C, Peyron C, Gervasoni D, Pow DV, Luppi PH, Fort P. Origins of the glycinergic inputs to the rat locus coeruleus and dorsal raphe nuclei: a study combining retrograde tracing with glycine immunohistochemistry. Eur J Neurosci. 1999;11:1058–1066. doi: 10.1046/j.1460-9568.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- 55.Sakai K, Sastre JP, Salvert D, Touret M, Tohyama M, Jouvet M. Tegmentoreticular projections with special references to the muscular atonia during paradoxical sleep in the cat: an HRP study. Brain Res. 1979;176:233–254. doi: 10.1016/0006-8993(79)90981-8. [DOI] [PubMed] [Google Scholar]
- 56.Schenck CH, Mahowald MW. Injurious sleep behavior disorders (parasomnias) affecting patients on intensive care units. Intensive Care Med. 1991;17:219–224. doi: 10.1007/BF01709881. [DOI] [PubMed] [Google Scholar]
- 57.Schenkel E, Siegel JM. REM sleep without atonia after lesions of the medial medulla. Neurosci Lett. 1989;98:159–165. doi: 10.1016/0304-3940(89)90503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shefner SA, Osmanovic SS. GABAA and GABAB receptors and the ionic mechanisms mediating their effects on locus coeruleus neurons. Prog Brain Res. 1991;88:187–195. doi: 10.1016/s0079-6123(08)63808-x. [DOI] [PubMed] [Google Scholar]
- 59.Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the mid-brain. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- 60.Siegel JM, Nienhuis R, Tomaszewsky KS. Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res. 1983;268:344–348. doi: 10.1016/0006-8993(83)90501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of catalepsy-related cells in medial medulla. Science. 1991;252:1315–1318. doi: 10.1126/science.1925546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siegel JM, Nienhuis R, Fahringer HM, Chiu C, Dement WC, Mignot E, Lufkin R. Activity of medial mesopontine units during cataplexy and sleep-waking states in the narcoleptic dog. J Neurosci. 1992;12:1640–1646. doi: 10.1523/JNEUROSCI.12-05-01640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984;323:385–389. doi: 10.1016/0006-8993(84)90319-6. [DOI] [PubMed] [Google Scholar]
- 64.Stampacchia G, Barnes CD, D'Ascanio P, Pompeiano O. Effects of microinjection of a cholinergic agonist into locus coeruleus on the gain of vestibulospinal reflex in decerebrate cats. Arch Ital Biol. 1987;125:107–138. [PubMed] [Google Scholar]
- 65.Takakusaki K, Ohta Y, Mori S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effect via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res. 1989;74:11–23. doi: 10.1007/BF00248276. [DOI] [PubMed] [Google Scholar]
- 66.Takakusaki K, Shimoda N, Matsuyama K, Mori S. Discharge properties of medullary reticulospinal neurons during postural changes induced by intrapontine injections of carbachol, atropine and serotonin, and their functional linkages to hindlimb motoneurons in cats. Exp Brain Res. 1994;99:361–374. doi: 10.1007/BF00228973. [DOI] [PubMed] [Google Scholar]
- 67.White SR, Neuman RS. Facilitation of spinal motoneuron excitability by 5-hydroxytryptamine and noradrenaline. Brain Res. 1980;188:119–127. doi: 10.1016/0006-8993(80)90561-2. [DOI] [PubMed] [Google Scholar]
- 68.White SR, Neuman RS. Pharmacological antagonism of facilitatory but not inhibitory effects of serotonin and norepinephrine on excitability of spinal motoneurons. Neuropharmacology. 1983;22:489–494. doi: 10.1016/0028-3908(83)90168-5. [DOI] [PubMed] [Google Scholar]
- 69.Wu M-F, Gulyani SA, Yau E, Mignot E, Phan B, Siegel JM. Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience. 1999;91:1389–1399. doi: 10.1016/s0306-4522(98)00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]