Abstract
A complex chain of intracellular signaling events, critically important in motor control, is activated by the stimulation of D1-like dopamine (DA) receptors in striatal neurons. At corticostriatal synapses on medium spiny neurons, we provide evidence that the D1-like receptor-dependent activation of DA and cyclic adenosine 3′,5′ monophosphate-regulated phosphoprotein 32 kDa is a crucial step for the induction of both long-term depression (LTD) and long-term potentiation (LTP), two opposing forms of synaptic plasticity. In addition, formation of LTD and LTP requires the activation of protein kinase G and protein kinase A, respectively, in striatal projection neurons. These kinases appear to be stimulated by the activation of D1-like receptors in distinct neuronal populations.
Keywords: basal ganglia, brain slices, dopamine, intracellular recordings, nitric oxide synthase-positive interneurons, phosphatases, protein kinase C, protein kinase G
Dopamine (DA)-containing fibers arising from midbrain neurons and glutamatergic inputs originating from the cortex extensively interact in the neostriatum to ensure correct information processing through the basal ganglia. Degeneration of DAergic terminals causes both an altered striatal glutamatergic neurotransmission and the motor and cognitive deficits observed in Parkinson's disease (Calabresi et al., 1997a). At the cellular level, both nigral DAergic inputs and cortical glutamatergic terminals converge on the same striatal neuronal subtype, the spiny projection neuron, which represents >90% of the striatal cell population and is the only cell type projecting out of the striatum. Medium spiny neurons contain both D1-like (D1, D5) and D2-like (D2, D3, D4) DA receptors (Gerfen, 1992; Sibley and Monsma, 1992; Surmeier et al., 1996) and also express both NMDA and non-NMDA classes of ionotropic glutamate receptors (Calabresi et al., 1996; Gotz et al., 1997). In addition, DA receptors are located in other critical sites in the striatum, such as interneurons, which also express glutamate receptors (Le Moine et al., 1991; Kawaguchi et al., 1995). This evidence suggests a complex action of DA on striatal physiological activity.
DA receptor activation has been found to represent a critical factor in the formation of two alternative forms of neuroplasticity at corticostriatal synapses, long-term depression (LTD) (Calabresi et al., 1992a; Choi and Lovinger, 1997) and long-term potentiation (LTP) (Centonze et al., 1999). Corticostriatal LTD, in fact, is prevented by unilateral nigral lesions, by D1- or D2-like DA receptor antagonists (Calabresi et al., 1992a), and by genetic disruption of D2 receptors (Calabresi et al., 1997b). Similarly, DAergic denervation of the striatum blocks LTP (Centonze et al., 1999). Striatal LTD and LTP are elicited in vitro by repetitive activation of corticostriatal glutamatergic terminals, in the presence and absence, respectively, of external magnesium in the bathing solution. Nevertheless, it should be noted that some authors have observed striatal LTP even in physiological concentrations of magnesium, when the experimental condition allows a certain activation of NMDA glutamate receptors (Wickens et al., 1996; Charpier and Deniau, 1997;Charpier et al., 1999; Dos Santos Villar and Walsh, 1999). Accordingly, whereas LTD is expressed in the presence of NMDA receptor antagonists, LTP is fully prevented by these pharmacological agents, indicating that corticostriatal LTP, but not LTD, is dependent on NMDA glutamate receptor activation (Calabresi et al., 1992b). These enduring changes in the efficacy of corticostriatal transmission may profoundly affect the pattern of firing of striatal spiny neurons, because, in the intact brain, the activity of striatal neurons mainly depends on the release of glutamate from cortical terminals (Stern et al., 1998).
In the striatum, D1- and D2-like receptors trigger opposite effects on intracellular levels of cAMP, stimulating and inhibiting adenylyl cyclase activity, respectively. These effects modulate cAMP-dependent protein kinase A (PKA) activity. A major substrate for PKA in spiny neurons is the DA and cyclic adenosine 3′,5′ monophosphate-regulated phosphoprotein 32 kDa (DARPP-32). This small protein is expressed in very high concentrations in virtually all spiny neurons and acts, in its phosphorylated but not dephosphorylated form, as a potent inhibitor of protein phophatase-1 (PP-1). PP-1, in turn, regulates the phosphorylation state and activity of many physiological effectors, including NMDA and AMPA glutamate receptors (Greengard et al., 1999), that are involved, respectively, in corticostriatal LTP and LTD (Calabresi et al., 1992a,b, 1996). The functional activity of these receptors is also modulated by the direct action of PKA (Roche et al., 1996; Tingley et al., 1997), as well as that of other kinases, such as protein kinase C (PKC) (Roche et al., 1996; Tingley et al., 1997;Carvalho et al., 1999). In addition, DARPP-32 also represents an excellent substrate for protein kinase G (PKG) (Hemmings et al., 1984a,b; Tsou et al., 1993), the activity of which is stimulated by cytosolic cGMP.
The aim of the present study was to address how the concomitant activation of ionotropic glutamate receptors and D1-like DA receptors initiates a cascade of biochemical events leading to the formation of opposing forms of corticostriatal plasticity, LTD and LTP.
MATERIALS AND METHODS
Electrophysiological experiments. Male wild-type (WT) and DARPP-32 knock-out (KO) (Fienberg et al., 1998) mice (2–3 months) were used for the electrophysiological experiments. The preparation and maintenance of coronal corticostriatal slices have been described previously (Calabresi et al., 1990, 1997b). Briefly, coronal slices (200–300 μm) were prepared from tissue blocks using a vibratome. The slices included the neostriatum and the neocortex. A single slice was transferred to a recording chamber (0.5 ml) and submerged in a continuously flowing Krebs' solution (32–33°C, 2–3 ml/min) gassed with a 95% O2 and 5% CO2 mixture and containing 10 μm bicuculline to avoid contamination of the corticostriatal EPSPs with depolarizing GABAA-mediated potentials. The composition of the solution was (in mm): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 11 glucose, and 25 NaHCO3. For intracellular recordings, sharp electrodes were used; they were filled with 2m KCl (30–60 MΩ). An Axoclamp 2A amplifier (Axon Instruments, Foster City, CA) was used for recordings either in current-clamp or in voltage-clamp mode. In single-electrode voltage-clamp mode, the switching frequency was 3 kHz. The headstage signal was continuously monitored on a separate oscilloscope. Traces were displayed on an oscilloscope and stored on a digital system. For synaptic stimulation, bipolar electrodes were used. The stimulating electrode was located either in the cortical areas close to the recording electrode or in the white matter between the cortex and the striatum. As the conditioning tetanus, we used three trains (3 sec duration, 100 Hz frequency, at 20 sec intervals). The duration of each individual pulse was 0.01–0.03 msec. During tetanic stimulation, the intensity was increased to levels producing an action potential on the EPSP (approximately twice the test intensity). Quantitative data on post-tetanic modifications are expressed as percentage of the controls, the latter representing the mean of responses recorded during a stable period (15–30 min) before tetanic stimulation or drug application. Each data point in the graphs in figures was obtained from at least five single neurons. Student's t test and χ2 analysis (for paired and unpaired observations) were used to compare the means, and ANOVA was used when multiple comparisons were made against a single control group. Drugs were applied by dissolving them to the desired final concentration in saline and by switching the perfusion from control saline to drug-containing saline. The following drugs were used:d(-)-2-amino-5-phosphonopentanoic acid (APV), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), SCH 23390,S-nitroso-N-acetylpenicillamine (SNAP), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), 7-nitroindazole monosodium salt (7-NINA) (from Tocris Cookson, Bristol, UK), calphostin C, nifedipine, phorbol 12,13-diacetate (PDAc) (Sigma-RBI, St. Louis, Mo), bicuculline (Sigma), H89 (Calbiochem, La Jolla, CA), zaprinast (Rhône-Poulenc Rorer, Dagenham, UK), okadaic acid, and calyculin A (from Alexis, Läufelfingen, Switzerland).
Biochemical experiments. Male C57BL/6 mice (5–6 weeks old) were decapitated. Their brains were removed rapidly and placed in ice-cold, oxygenated Krebs' solution. Coronal slices (300 μm) were prepared using a vibratome. For high-frequency stimulation (HFS) experiments, each slice was placed in the recording chamber and perfused with Krebs' solution containing bicuculline (10 μm). The HFS of the corticostriatal pathway was delivered as indicated above. For experiments using SNAP and zaprinast, striata were dissected from the slices in ice-cold Krebs' solution. Each slice was placed in a polypropylene incubation tube with 2 ml of fresh Krebs' solution and incubated at 30°C under constant oxygenation with 95% O2 and 5% CO2. After a 30 min incubation, the buffer was replaced with fresh Krebs' solution containing bicuculline (10 μm). After another 30 min, the slices were treated with SNAP (100 μm) or zaprinast (15 μm), plus bicuculline or with bicuculline only, for 5, 10, or 20 min. After the drug or control treatment, the slices were frozen rapidly on dry ice and stored at −80°C until assayed. Frozen tissue samples were sonicated in 1% SDS and boiled for 10 min. Aliquots (5 μl) of the homogenate were used for protein determination according to the bicinchoninic acid protein assay method (Pierce, Rockford, IL). Equal amounts of protein (25 μg; containing equal amounts of DARPP-32) from each sample were loaded onto 10% polyacrylamide gels. The proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (0.2 μm) (Schleicher & Schuell, Keene, NH) by the method of Towbin et al. (1979). Phospho[Thr34]-DARPP-32 was detected using a monoclonal antibody (mAb-23; 1:750 dilution) (Snyder et al., 1992). Phospho[Thr75]-DARPP-32 was detected using purified rabbit antiserum (1:10,000 dilution) (Bibb et al., 1999), and total DARPP-32 was detected using a mAb (1:10,000 dilution) (Hemmings et al., 1984a). Antibody binding was revealed using goat anti-mouse horseradish peroxidase-linked IgG (diluted 1:7500) for phospho[Thr34]-DARPP-32 and nonphosphorylated DARPP-32, a goat anti-rabbit horseradish peroxidase-linked IgG (diluted 1:6000) for phospho[Thr75]-DARPP-32, and the enhanced chemiluminescence immunoblotting detection system (Amersham Pharmacia Biotech, Arlington Heights, IL). Chemiluminescence was detected by autoradiography. Quantification of the phospho-DARPP-32 bands was done by densitometry, using NIH Image (version 1.52) software. Data were analyzed by one-way ANOVA followed by Dunnett's test for paired comparisons. A statistical difference was defined asp < 0.05.
RESULTS
Intrinsic and synaptic properties of striatal neurons in WT and DARPP-32 KO mice
Conventional intracellular sharp microelectrode recordings were performed in corticostriatal slices obtained from WT and DARPP-32 KO mice. The intrinsic membrane properties of striatal neurons, such as the resting membrane potential (RMP), input resistance, and current–voltage relationship, did not significantly differ in neurons recorded from the two groups of animals (WT: RMP = −85 ± 5 mV, input resistance = 42 ± 10 MΩ, n = 50; DARPP-32 KO: RMP = −84 ± 4, input resistance = 40 ± 13, n = 21; p > 0.05) and closely resembled the electrical activity described previously for rat and mouse spiny neurons (Fig.1A,B) (Calabresi et al., 1990, 1997b; Jiang and North, 1991). Also the pharmacology of cortically evoked EPSPs was similar in WT mice (n = 5) and DARPP-32 KO mice (n = 5). In the presence of a physiological concentration of external magnesium (1.2 mm), the AMPA glutamate receptor antagonist CNQX (10 μm) fully suppressed the EPSPs in both groups of animals. In contrast, in both groups, removal of external magnesium, a procedure that removes the voltage-dependent block of NMDA receptors, was necessary to uncover an NMDA component of the EPSP that could be blocked by APV (50 μm) (Fig.1C).
Fig. 1.
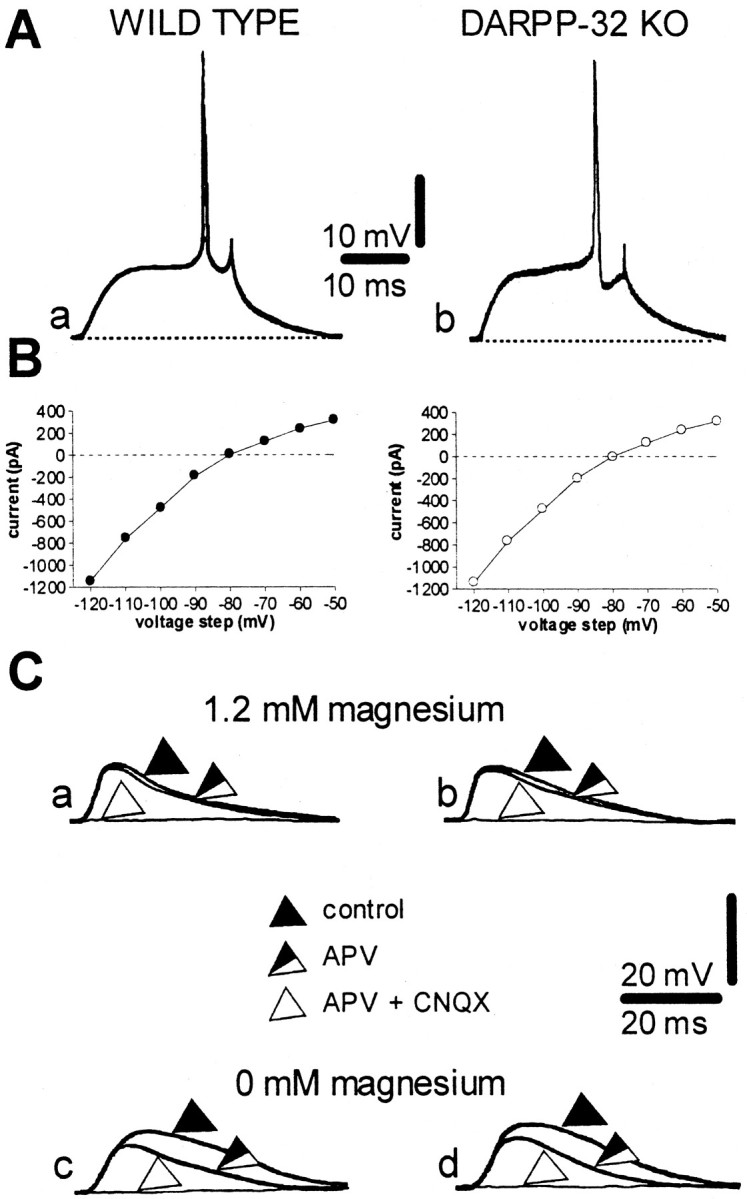
Intrinsic and synaptic properties of striatal spiny neurons recorded from WT and DARPP-32 KO mice. A, Action potential discharge was induced by depolarizing current steps (+0.9 nA) both in WT (a, RMP = −86 mV) and in DARPP-32 KO (b, RMP = −85 mV) neurons.B, Current–voltage plots obtained during single-microelectrode voltage-clamp experiments from WT (left) and DARPP-32 KO (right) neurons. The membrane potential of both neurons was held at −85 mV.C, In 1.2 mm magnesium, the blockade of glutamate NMDA receptors by 30 μm APV did not affect the EPSP recorded from striatal neurons from either WT (a) or DARPP-32 KO (b) mice. By contrast, in both experimental groups, the EPSP was fully suppressed by coadministration of 30 μm APV plus 10 μm CNQX. The RMP of both neurons was −86 mV. In magnesium-free medium, an APV-sensitive component was present in striatal neurons in slices from both WT (c) and DARPP-32 KO (d) mice. The RMP of both neurons was −86 mV.
Effects of high-frequency stimulation in WT and DARPP-32 KO mice
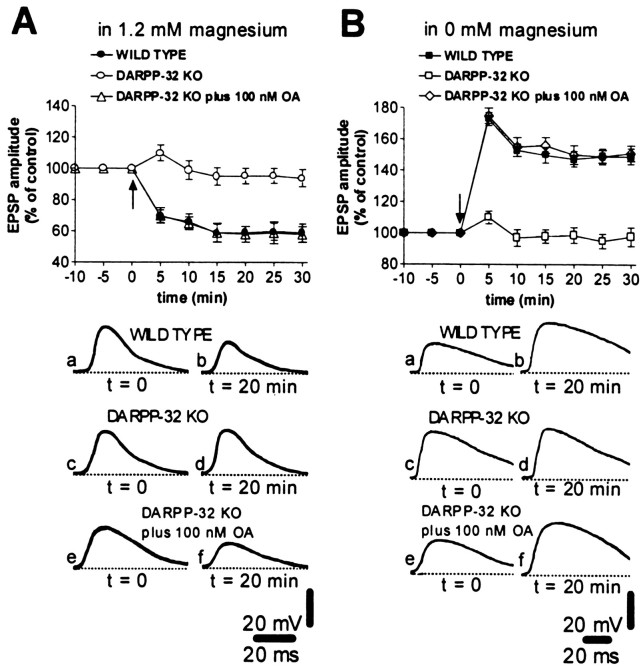
As previously described in rats (Calabresi et al., 1992a,b, 1996) and mice (Calabresi et al., 1997b), HFS (three trains, 100 Hz frequency, 3 sec duration, 20 sec interval) of corticostriatal fibers was able to produce long-term changes in the amplitude of EPSPs in WT mice. In particular, a stable LTD was recorded in 1.2 mmexternal magnesium (n = 12; Fig.2A), whereas LTP was revealed after the removal of this ion from the bathing solution (n = 11; Fig. 2B). In contrast, both forms of synaptic plasticity were absent in DARPP-32 KO mice (n = 9 in 1.2 mm magnesium;n = 10 in magnesium-free medium; p < 0.01 for both experimental conditions), indicating that changes triggered by the stimulation of the DARPP-32/PP-1 signaling cascade are critically involved in the formation of corticostriatal synaptic plasticity (Fig. 2A,B). To test directly whether the absence of both LTD and LTP in DARPP-32 KO mice was because of an inability to inhibit PP-1 activity, we examined the effect of PP-1 inhibitors in DARPP-32 KO mice using the same experimental paradigm. In DARPP-32 KO mice, bath application of okadaic acid (100 nm), an inhibitor of PP-1, restored both forms of synaptic plasticity (n = 4 for each experimental condition, Fig. 2A,B). Similarly, another rather selective inhibitor of this enzyme, calyculin A (100 nm), restored a normal LTP (n = 4; p > 0.05) and LTD (n = 4;p > 0.05) These two inhibitors of PP-1 by themselves had no effect on EPSP amplitude (p > 0.05).
Fig. 2.
Role of DARPP-32 and PP-1 in the expression of HFS-induced corticostriatal LTD and LTP. The graphs summarize the results from intracellular experiments performed in the presence of physiological concentrations of magnesium (A) and in the absence of magnesium (B) from WT slices (filled circles and squares), DARPP-32 KO slices in control medium (open circles andsquares), and DARPP-32 KO slices bathed in medium containing okadaic acid (OA) (open triangles and diamonds). Okadaic acid was applied 10 min before HFS was delivered. The arrowsindicate when HFS was delivered. The bottom part of the figure shows EPSPs recorded in six neurons from WT slices (a, b), DARPP-32 KO slices perfused in control medium (c, d), and DARPP-32 KO slices bathed in okadaic acid (e, f) immediately before (a, c, e) and 20 min after (b, d, f) HFS. RMPs were (in mV): −86 (A, WT and KO; B, WT), −84 (B, KO), and −82 (A, B, KO plus okadaic acid).
Role of D1-like DA receptors and PKA in corticostriatal LTD and LTP
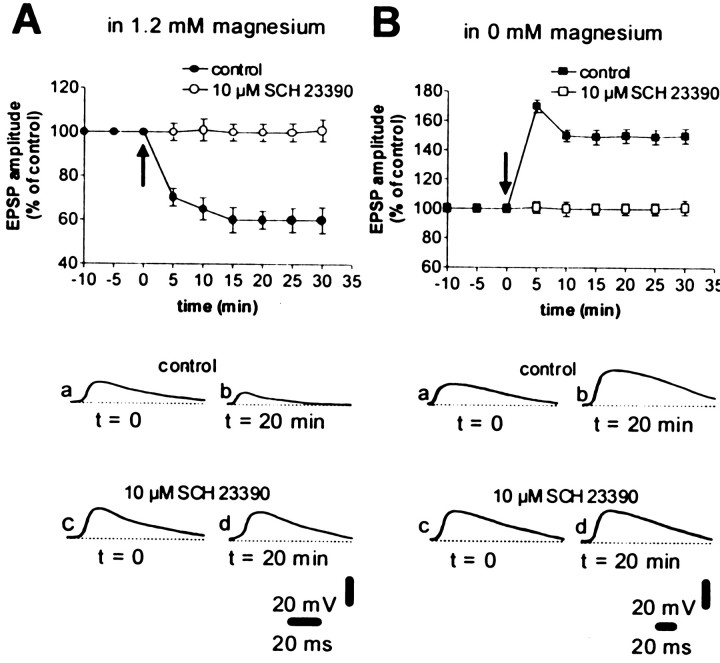
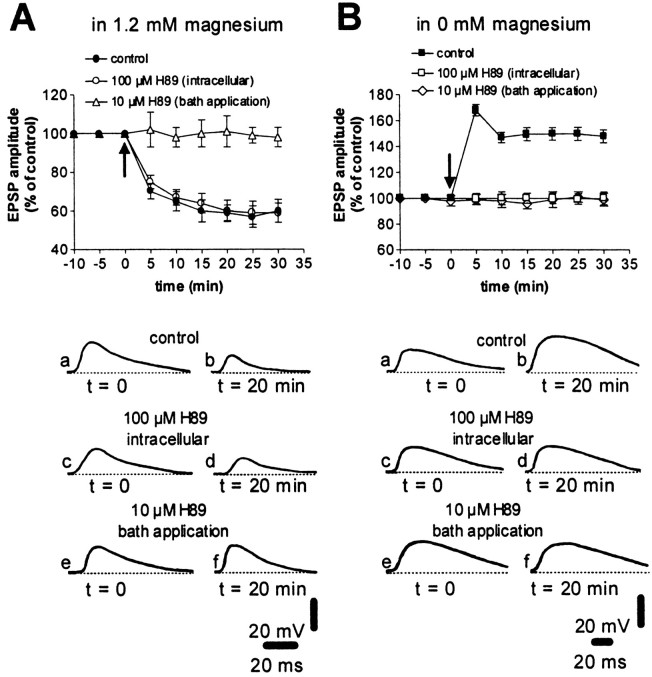
In accordance with the idea that D1-like DA receptor stimulation is the main factor required for DARPP-32 activation, we tested whether the pharmacological blockade of this class of DA receptors was equally effective in blocking both forms of synaptic plasticity. Consistently, the D1-like DA receptor antagonist SCH 23990 (10 μm) prevented both LTD, as reported earlier in rats (Calabresi et al., 1992) (n = 6, p > 0.05), and LTP (n = 11, p > 0.05) (Fig.3) in WT mice. Activation of the D1-like receptor stimulates PKA, leading to the phosphorylation of DARPP-32. Therefore, we tested the effect of the selective PKA inhibitor H89 on LTD and LTP. Surprisingly, in WT mice the intracellular injection of H89 (100 μm) fully blocked LTP (n = 5; p > 0.05) but failed to prevent HFS-induced LTD (n = 6; p < 0.01) (Fig. 4). In contrast, when H89 (10 μm, n = 4 for each experimental condition) was added to the perfusing solution, both LTD and LTP were prevented (Fig. 4; p > 0.05 for both conditions). These findings confirm the requirement of the D1-like receptor/PKA pathway for corticostriatal LTD and LTP induction. However, these data suggest different cellular loci for the action of PKA in the development of LTP and LTD. Thus, for LTP but not for LTD, this pathway is activated postsynaptically on spiny neurons. For LTD, it might be activated in striatal neuronal subpopulations other than the recorded spiny neurons.
Fig. 3.
The D1 DA receptor antagonist SCH 23390 blocks both corticostriatal LTD and LTP in WT mice. The graphs summarize the results obtained from intracellular experiments performed in WT mice in the presence of a physiological concentration of magnesium (A) and in the absence of magnesium (B) in control medium (filled circles and squares) and in the presence of 10 μm SCH 23390 (applied 10 min before HFS was delivered,open circles and squares). Thearrows indicate when HFS was delivered. Thebottom part of the figure shows EPSPs recorded in four neurons in control condition (a, b) and in the presence of 10 μm SCH 23390 (c, d), immediately before (a, c) and 20 min after (b, d) HFS. RMPs were (in mV): −82 (A, control), −85 (A, SCH 23390), −87 (B, control) and −84 (B, SCH 23390).
Fig. 4.
Role of PKA in the expression of HFS-induced corticostriatal LTP and LTD. The graphs summarize the results obtained in WT mice from intracellular experiments performed in the presence of physiological concentrations of magnesium (A) and in the absence of magnesium (B) in control condition (filled circles andsquares), after intracellular injection of 100 μm H89, a PKA inhibitor (open circles andsquares), and after bath application of 10 μm H89 (10 min before HFS was delivered, open triangles and diamonds). Thearrows indicate when HFS was delivered. Thebottom part of the figure shows EPSPs recorded in six neurons in control condition (a, b), after intracellular injection of 100 μm H89 (c, d), and in the presence of bath-applied H89 (10 μm) (e, f) immediately before (a, c, e) and 20 min after (b, d, e) HFS. RMPs were (in mV): −83 (A, control, intracellular, and bath-applied H89), −84 (B, bath-applied H89), and −88 (B, control and intracellular H89).
Role of nitric oxide/cGMP pathway in corticostriatal LTD recorded from WT and DARPP-32 KO mice
The sustained activation of glutamate receptors occurring during HFS of corticostriatal fibers triggers the massive release of endogenous DA from nigrostriatal terminals and also stimulates other intrastriatal neuronal subtypes, such as nitric oxide synthase (NOS)-positive and cholinergic striatal interneurons (Calabresi et al., 1999a,b). Nitric oxide (NO) and acetylcholine are critical transmitters involved in striatal synaptic plasticity, by promoting through a feed-forward mechanism the stimulation of different signaling pathways in spiny projection neurons. In particular, the stimulation of the NO/cGMP/PKG pathway, and of postsynaptic muscarinic M1 receptors leading to PKC activation, is required for LTD (Calabresi et al., 1999a) and LTP (Calabresi et al., 1999b), respectively. It is possible, therefore, that the absence of both striatal LTD and LTP in DARPP-32 KO mice might depend not only on interference with the D1 DA receptor-initiated intracellular signaling systems but also with these additional signal transduction mechanisms. For instance, activation of the NO/cGMP/PKG pathway should lead to increased phosphorylation of DARPP-32 (Greengard et al., 1999), and so one could predict an effect of the DARPP-32 KO on this pathway. Similarly, the absence of PP-1 inhibition in DARPP-32 KO mice, by causing an elevated phosphatase activity, might counteract the action of PKC on NMDA receptors.
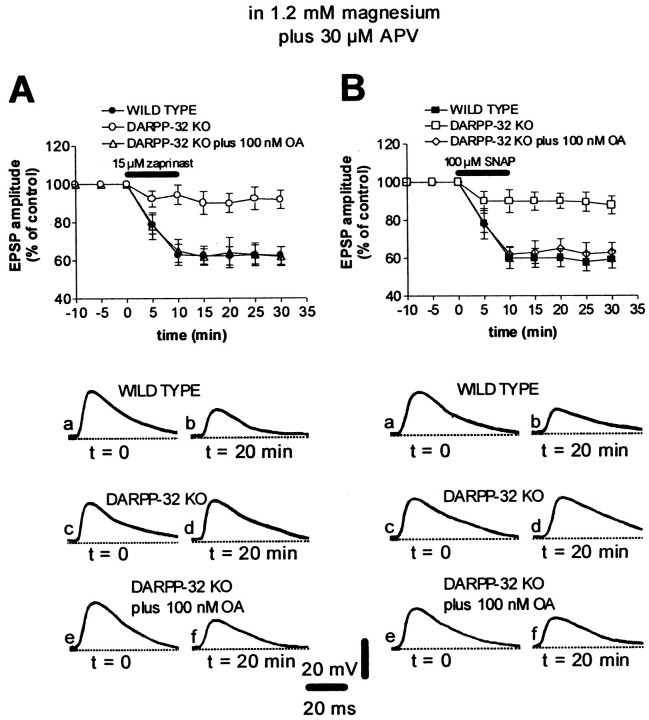
To address these issues, several types of experiments have been performed. It has been reported that the intracellular blockade of PKG fully prevents HFS-induced LTD, suggesting a key role for this kinase in this phenomenon (Calabresi et al., 1999a). Conversely, pharmacological stimulation of the NO/cGMP/PKG pathway is able to induce corticostriatal LTD. To test whether stimulation of DARPP-32 was required for the induction of this pharmacological LTD, we studied the effect of both the NO donor SNAP (100 μm) and the selective cGMP–phosphodiesterase inhibitor zaprinast (15 μm) in DARPP-32 KO mice. Incubation of the slices with SNAP (WT, n = 5; DARPP-32 KO, n = 7) or zaprinast (WT, n = 6; DARPP-32 KO, n = 7) induced corticostriatal LTD in WT animals (p< 0.01 for both experimental conditions) but not in DARPP-32 KO animals (p > 0.05 for both conditions) (Fig.5), suggesting that DARPP-32 represents a critical effector in this pharmacological LTD as in HFS-induced LTD. When applied intracellularly, zaprinast (100 μm) depressed EPSP amplitude in WT mice (n = 5; p < 0.01) but not in DARPP-32 KO mice (n = 5; p > 0.05), further suggesting a role for postsynaptic cGMP elevation in LTD. Furthermore, as seen for HFS-induced LTD and LTP (Fig. 2), bath application of okadaic acid (100 nm, n = 4 andp > 0.05 for each experimental condition) restored zaprinast- and SNAP-induced LTD in DARPP-32 KO mice (Fig. 5).
Fig. 5.
Stimulation of the NO/cGMP pathway induces AMPA receptor-mediated corticostriatal LTD in WT but not in DARPP-32 KO mice. A, In the presence of 1.2 mm magnesium plus 30 μm APV, the cGMP phosphodiesterase inhibitor zaprinast induced LTD of AMPA-mediated EPSPs in WT mice (filled circles) but not in DARPP-32 KO mice (open circles). In these mutant animals, the pharmacological LTD was restored after the application (10 min before zaprinast application) of the PP-1 inhibitor okadaic acid (100 nm, open triangles). The bottom part of the figure shows EPSPs recorded in neurons from WT (a, b) and DARPP-32 KO (c–f) mice immediately before (a, c, e) and 20 min after (b, d, f) the application of zaprinast. The RMP of neurons was −86 mV. B, In the presence of 1.2 mmmagnesium plus 30 μm APV, the NO donor SNAP induced LTD of AMPA-mediated EPSPs in WT (filled squares) but not DARPP-32 KO (open squares) mice. In these mutant animals the SNAP-induced LTD was restored after the bath application (10 min before SNAP application) of 100 nm okadaic acid (open diamonds). The bottom part of the figure shows EPSPs recorded in neurons from WT (a, b) and DARPP-32 KO (c–f) mice immediately before (a, c, e) and 20 min after (b, d, f) the application of SNAP). RMPs were (in mV): −85 (WT) and −84 (DARPP-32 KO).
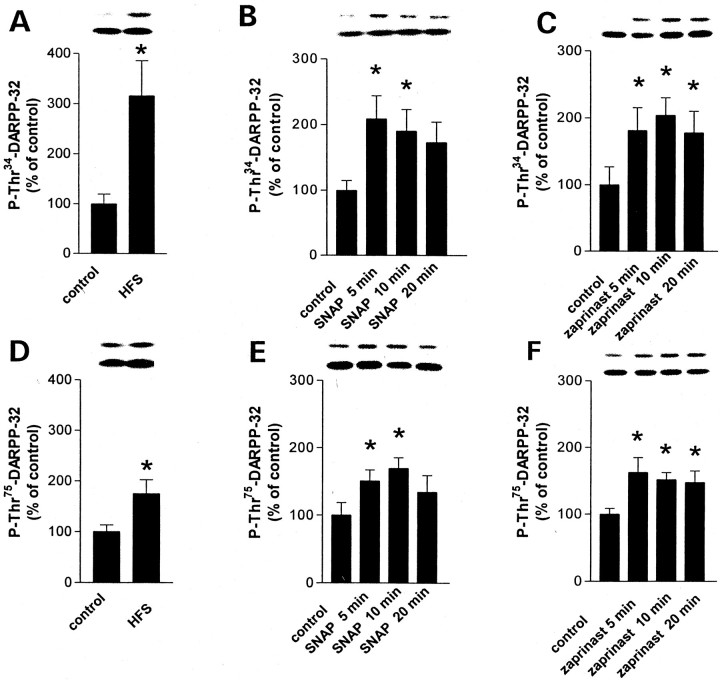
High-frequency stimulation, SNAP, and zaprinast increase Thr34 and Thr75 phosphorylation of striatal DARPP-32
The electrophysiological data presented above indicate that both HFS of corticostriatal fibers and pharmacological stimulation of the NO/cGMP pathway by SNAP or zaprinast induce striatal synaptic plasticity by stimulating DARPP-32 activity. To further address this idea, we measured the effects of HFS, SNAP, and zaprinast on the phosphorylation state of DARPP-32 at its Thr34 residue, a measure of its ability to serve as an inhibitor of PP-1. In addition, because it has been shown recently that DARPP-32 acts as an inhibitor of PKA when phosphorylated at Thr75 (Bibb et al., 1999), we evaluated the ability of HFS, SNAP, and zaprinast to modulate this additional phosphorylation site. As shown in Figure 6, HFS of corticostriatal fibers, 100 μm SNAP, and 15 μm zaprinast were each able to increase significantly both Thr34 and Thr75 phosphorylation of DARPP-32 in corticostriatal slices, further confirming that the different induction protocols used to elicit striatal synaptic plasticity also modulate the physiological activity of DARPP-32.
Fig. 6.
Effect of HFS (A, D), SNAP (B, E), and zaprinast (C, F) on the level of phospho[Thr34]-DARPP-32 (A–C) and phospho[Thr75]-DARPP-32 (D–F) in striatal slices. The amount of phospho-DARPP-32 was normalized to the level of total DARPP-32 in each sample and is expressed as a percentage of that measured in control slices. Data represent means ± SEM for four experiments performed in duplicate or triplicate (n = 9–12). *p < 0.05 versus the control group; ANOVA was followed by Dunnett's test. The top lanes above each graph illustrate phospho-DARPP-32. The bottom lanesillustrate the corresponding total DARPP-32.
Role of D1-like/PKA pathway in pharmacological LTD
To evaluate the possible involvement of the activation of the D1-like DA receptor and of PKA in the formation of SNAP- and zaprinast-induced LTD, we examined the effect of the PKA inhibitor H89 and of the D1-like DA receptor antagonist SCH 23390 on pharmacological LTD in WT mice. In experiments using intracellular H89 (n = 6), the LTD induced by SNAP was not significantly different from the control LTD (−41% ± 3 at 30 min after HFS, p > 0.05). Similar results were obtained with SCH 23390 (n = 6, −40% ± 4 at 30 min after HFS,p > 0.05). H89 and SCH 23390 also failed to significantly affect zaprinast-induced corticostriatal LTD (n = 6 for each condition, p > 0.05). It has been demonstrated that D1-like receptors stimulate striatal NOS-positive interneurons and increase cGMP level (Altar et al., 1990; Morris et al., 1997). Those results, taken together with the evidence that D1 receptor activation is required for HFS-induced LTD, but not for pharmacological LTD, indicate that the D1 receptor lies upstream of NO activation of guanylyl cyclase in HFS-induced LTD.
Absence of role of NO/cGMP pathway in corticostriatal LTP
We subsequently evaluated whether stimulation of the NO/cGMP/PKG/DARPP-32 pathway was involved in corticostriatal LTP. In magnesium-free external solution plus 10 μm CNQX, a purely NMDA-mediated EPSP was obtained after the stimulation of corticostriatal terminals in both WT and DARPP-32 KO mice. In this condition, application of zaprinast (15 μm) (WT,n = 5; DARPP-32 KO, n = 7) or SNAP (100 μm) (WT, n = 5; DARPP-32 KO,n = 5) to increase intracellular cGMP levels failed to produce significant changes in the amplitude and duration of the EPSPs, either in WT animals or in DARPP-32 KO mice (p> 0.05 in both groups of animals).
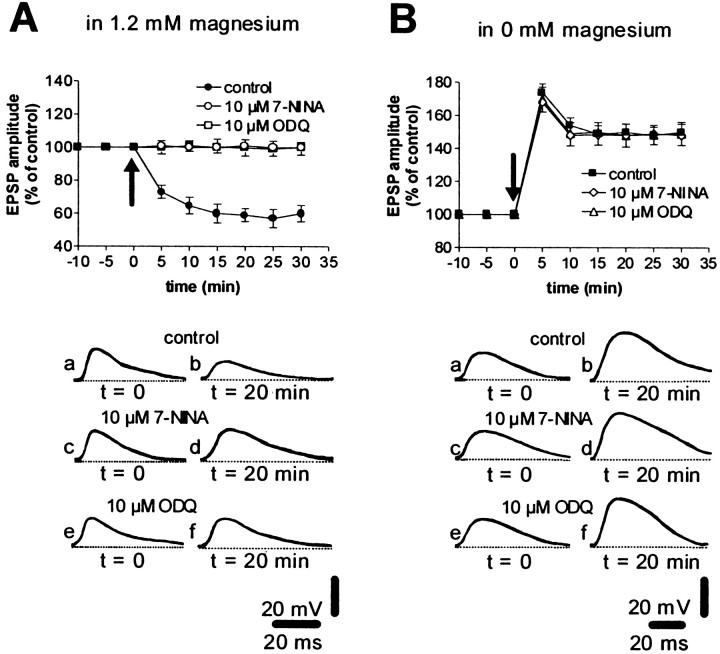
We also studied the effects on HFS-induced LTP of the NOS inhibitor 7-NINA and of ODQ, a potent and selective inhibitor of soluble NO-sensitive guanylyl cyclase. In previous studies, both agents have been found to be capable of fully blocking corticostriatal LTD in the rat (Calabresi et al., 1999a), and do so in WT mice as well (Fig.7A). In contrast, incubation of corticostriatal slices from WT mice with 7-NINA (n = 5) or ODQ (n = 5) failed to prevent HFS-induced LTP (Fig. 7B), supporting the idea that the stimulation of the NO/cGMP/PKG pathway is not involved in the cellular changes necessary for the formation of this form of synaptic plasticity.
Fig. 7.
Inhibition of the NO/cGMP pathway blocks corticostriatal LTD but not LTP in WT mice. The graphs summarize the results obtained in WT mice in 1.2 mm magnesium (A) and in the absence of magnesium (B) in control condition (filled circles and squares), in the presence of the neuronal NOS inhibitor 7-NINA (10 μm, open circles and diamonds), and in the presence of the soluble guanylyl cyclase inhibitor ODQ (10 μm,open squares and triangles). 7-NINA and ODQ were applied 10 min before HFS was delivered. Thearrows indicate when HFS was delivered. Thebottom part of the figure shows EPSPs recorded from six neurons in control condition (a, b), in the presence of 10 μm 7-NINA (c, d) and in the presence of 10 μm ODQ (e, f) immediately before (a, c, e) and 20 min after (b, d, f) HFS of the corticostriatal pathway. RMPs were (in mV): −86 (A, control), −85 (A, 7-NINA), −80 (A, ODQ; B, 7-NINA), −81 (B, control), and −88 (B, ODQ).
Role of PKC in corticostriatal LTP
In rat corticostriatal slices, PDAc, an activator of PKC, increases the membrane depolarization produced by exogenous NMDA application (Pisani et al., 1997). This PKC-dependent enhancement of NMDA currents represents a possible mechanism underlying corticostriatal LTP. Conversely, M1 muscarinic receptor antagonists prevent LTP by blocking PKC stimulation (Calabresi et al., 1999b,2000). To further define the role of PKC in corticostriatal LTP and to study the possible interaction with the D1/cAMP/PKA/DARPP-32 pathway, we first tested whether pharmacological inhibition of PKC activity by calphostin C (0.3 μm) altered the expression of LTP in WT mice. As shown in Figure8A, this PKC inhibitor fully prevented the expression of this form of synaptic plasticity (n = 5; p > 0.05). One possible explanation for the absence of LTP in DARPP-32 KO mice is that, in these animals, the lack of this physiological inhibitor of PP-1 decreases the phosphorylation state at the PKC site of the NMDA receptor. According to this hypothesis, phosphorylation by PKC and inhibition of dephosphorylation by the D1/cAMP/PKA/DARPP-32/PP-1 pathway, both of which are important for LTP, might synergistically interact to increase the phosphorylation state of the NMDA receptor at this PKC site. To test this possibility, we measured the effects produced by PDAc (0.1–10 μm) on NMDA-mediated EPSPs recorded from WT and DARPP-32 KO mice. Stimulation of PKC activity by PDAc produced a dose-dependent increase in the amplitude of the NMDA-mediated corticostriatal EPSPs in both groups of mice (Fig.8B) (WT, n = 15; DARPP-32 KO,n = 15). In DARPP-32 KO mice, however, we observed a shift to the right of the dose–response curve for PDAc, suggesting that the phosphorylation state of the NMDA receptor is critically dependent both on PKC-mediated phosphorylation and PP-1-mediated dephosphorylation. Stimulation of PKC activity did not result in significant changes in AMPA-mediated synaptic potentials in either WT (n = 5) or DARPP-32 KO (n = 5) mice (Fig. 8C), suggesting that PKC-dependent enhancement of glutamate ionotropic-mediated transmission in the striatum is specific for NMDA receptors.
Fig. 8.
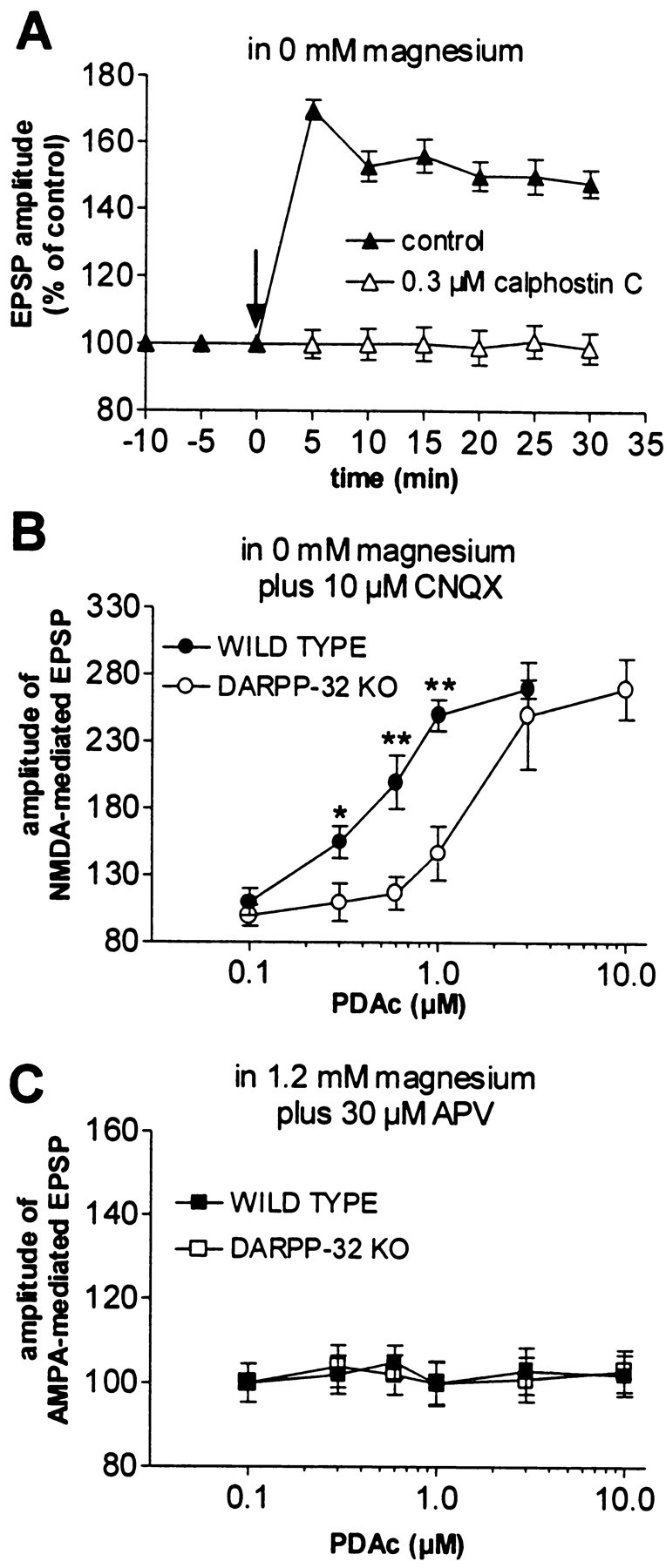
PKC activity is required for striatal LTP.A, Blockade of PKC function by the selective inhibitor calphostin C (0.3 μm) prevented LTP in WT mice. Thearrow indicates when HFS was delivered.B, In magnesium-free solution plus 10 μmCNQX, the stimulation of PKC activity by PDAc (applied for 10 min) produces a dose-dependent enhancement of the cortically evoked NMDA-mediated EPSPs in both WT (filled circles) and DARPP-32 KO (open circles) mice. Note that disruption of the DARPP-32 gene significantly reduced the PDAc-induced enhancement of NMDA-mediated EPSPs (*p < 0.05; **p < 0.01). C, In both WT (filled squares) and DARPP-32 KO (open squares) mice, the AMPA component of corticostriatal EPSPs, recorded in the presence of a physiological concentration of external magnesium plus 30 μm APV, was not modified by PDAc.
Effects of nifedipine on corticostriatal LTP
An elevation of intracellular calcium is a critical event leading to LTP (Schiller et al., 1998). This elevation may be caused simply by the activation of NMDA receptors. However, because it has been shown that activation of the D1/cAMP/PKA/DARPP-32/PP-1 pathway enhances L-type calcium currents (Surmeier et al., 1995), it also seemed possible that these channels might contribute to LTP induction. Therefore, we tested the pharmacological effects of nifedipine, a blocker of L-type calcium channels, on the expression of this form of synaptic plasticity. Nifedipine (10 μm, 10 min,n = 5, p > 0.05) did not alter the amplitude or duration of HFS-induced corticostriatal LTP (data not shown), ruling out the possibility that the control of LTP by the D1/cAMP/PKA/DARPP-32/PP-1 pathway is exerted through the modulation of L-type calcium channels.
DISCUSSION
This study provides the first evidence that activation of DARPP-32, and the resultant inhibition of PP-1 activity, is of critical importance for the expression of two opposing forms of brain synaptic plasticity, striatal LTD and LTP. Both forms of plasticity are also critically linked to the activation of DA receptors, supporting the idea that in the striatum, a close interplay among DA receptors, DARPP-32 activity, and glutamatergic transmission might underlie the functional role of this structure in motor control and cognitive activities. Moreover, alterations of these transduction pathways might play a role in pathophysiological conditions involving striatal DAergic mechanisms, such as Parkinson's disease, Huntington's disease, and schizophrenia.
Stimulation of D1/PKA/DARPP-32/PP-1 pathway is required for the induction of both striatal LTD and LTP
In agreement with a previous report (Fienberg et al., 1998), knocking out the DARPP-32 gene does not result in gross changes in the measured intrinsic membrane properties of recorded striatal spiny neurons. Similarly, the physiological and pharmacological properties of the glutamatergic EPSP evoked by a single cortical stimulation were not altered in DARPP-32 KO mice. In contrast, both LTP and LTD were normally expressed in WT mice but fully abolished in mutants. These findings are particularly interesting because these forms of plasticity involve different ionotropic glutamate receptors: activation of NMDA glutamate receptors is required for the expression of striatal LTP but not for LTD. In addition, DAergic denervation (Calabresi et al., 1992a; Centonze et al., 1999) or blockade of D1-like DA receptors abolishes both forms of synaptic plasticity. Although D1 receptor activation and DARPP-32 phosphorylation are involved in both forms of synaptic plasticity, our data also provide evidence that LTD and LTP require activation of distinct intermediate signaling steps in spiny neurons. The demonstration that HFS, SNAP, and zaprinast each increases the phosphorylation state of DARPP-32 in corticostriatal slices further indicates that under our experimental conditions, the various induction protocols of striatal synaptic plasticity require DARPP-32 activation to be effective.
In WT mice, inhibition of PKA by intracellular application of H89 prevents the induction of LTP but not of LTD. Conversely, LTD, but not LTP, is blocked by inhibitors of the NO/cGMP cascade and is mimicked by the pharmacological stimulation of this signaling pathway.
The lack of effect of postsynaptic application of the PKA inhibitor H89 on LTD induction is an unexpected finding. In fact, previously described electrophysiological effects mediated by D1-like receptor activation involved PKA stimulation (Surmeier et al., 1995). However, it is important to note that in isolated striatal neurons, stimulation of PKA activity via D1-like receptor activation causes potentiation of AMPA currents rather than their inhibition (Yan et al., 1999). Interestingly, we found that bath application of the PKA inhibitor H89 was able to block HFS-induced LTD. Thus, it can be postulated that D1 receptors required for LTD expression are not located on spiny neurons but on other neuronal subtypes such as NOS-positive interneurons (Kawaguchi et al., 1995). In accordance with this interpretation, evidence has been presented that activation of D1 receptors augments striatal NO production (Morris et al., 1997). Thus, D1-mediated increased NO levels might result in the activation of the cGMP/DARPP-32 cascade (Hemmings et al., 1984a,b; Tsou et al., 1993).
Various kinases are involved in the expression of corticostriatal synaptic plasticity
The evidence that inhibition of either PKA or PKC is able to prevent HFS-induced LTP and LTD suggests that the simultaneous activation of these kinases is required to induce these forms of synaptic plasticity. Moreover, both forms of synaptic plasticity may require both an increase in kinase activity and a decrease in phosphatase activity, resulting in a synergistic increase in the state of phosphorylation of residues on key substrate proteins such as AMPA and NMDA receptors. This concept would explain for example why in DARPP-32 KO mice relative to WT mice there is a reduced response to various agonists such as DA, cocaine (Fienberg et al., 1998), and phorbol esters (Fig. 7) at low but not at high concentrations of these agonists. Thus, at high concentrations of these agonists, the stimulation of kinase activity might be so great that the simultaneous inhibition of phosphatase activity provided by DARPP-32 phosphorylation would be unnecessary. The ability of zaprinast and SNAP to induce pharmacological LTD may result from a synergistic action of activated PKG, in which both an increased PKG-mediated direct phosphorylation of substrate protein residues as well as a PKG/DARPP-32-mediated inhibition of dephosphorylation of these substrate proteins occur. It is also possible that zaprinast and SNAP induce a sufficiently strong inhibition of PP-1 to make the stimulation of phosphorylation by PKA or PKC activity unnecessary.
The observation that the L-type calcium channel blocker nifedipine does not affect HFS-induced LTP indicates that these channels, although modulated by the DA-initiated intracellular pathway (Surmeier et al., 1995), are not involved in LTP expression. It would be interesting to test the effect of blockers of calcium channels other than L-type on synaptic plasticity. Unfortunately, most of these drugs affect synaptic transmission, precluding the possibility of testing their effects on striatal plasticity. However, PKA and PKC might, by phosphorylating the NMDA–receptor complex at two distinct sites, synergistically augment the intracellular calcium concentration required for LTP. It has been shown, in fact, that the NR1 subunit of NMDA-type glutamate receptors is phosphorylated at Ser897 by PKA and at Ser890 by PKC (Tingley et al., 1997; Greengard et al., 1999). Alternatively, PKA might increase the state of phosphorylation of the NMDA receptor at the PKC-dependent site by phosphorylating DARPP-32, thereby inhibiting PP-1-mediated dephosphorylation of NMDA receptors at this site. In support of this possibility, the phosphorylation of the PKC site is attenuated in neurons from DARPP-32 KO mice in response to DA (Snyder et al., 1998). In good agreement with these experimental findings, we found that PDAc-mediated enhancement of the NMDA component of corticostriatal EPSPs is significantly attenuated in DARPP-32 KO mice. It is conceivable, therefore, that the simultaneous phosphorylation of the NMDA–receptor complex at Ser897 and Ser890 by PKA and PKC, respectively, coupled to the inhibition of PP-1-mediated dephosphorylation, may synergistically interact to ensure LTP generation at corticostriatal synapses.
Concluding remarks
DARPP-32 KO mice show a reduced behavioral response to drugs of abuse such as cocaine and d-amphetamine. Moreover, neuroleptic-induced catalepsy is severely attenuated in these animals (Fienberg et al., 1998). These findings indicate that DARPP-32 controls some mechanisms underlying reward-motivated behavior and DA-related motor control. We suggest that the DARPP-32-mediated control of DA-related behavioral activities may require long-term changes of corticostriatal excitatory synaptic transmission, such as LTD and LTP.
The lack of gross behavioral alterations found in DARPP-32 KO mice does not exclude the possibility that more subtle but equally important changes might occur in these mice even in the absence of a pharmacological challenge; these alterations might reflect the absence of corticostriatal synaptic plasticity in these mice.
Footnotes
This work was supported by a Biomed grant (P.C.), by a Telethon grant (P.C.), by Consiglio Nazionale delle Ricerche grants (P.C. and G.B.), by National Institutes of Health Grants MH 40899 and DA 10044 (P.G.), by a fellowship from the Swedish Society for Medical Research (K.C.), by a travel fellowship from the Swedish Medical Research Council (K.C.), and by a fellowship from Stiftelsen för Internationalisering av högre utbilding och forskning (P.S.).
Correspondence should be addressed to Dr. Paolo Calabresi, Clinica Neurologica, Dipartimento di Neuroscienze, Università di Roma “Tor Vergata,” Via di Tor Vergata 135, 00133 Rome, Italy. E-mail:calabre@uniroma2.it.
REFERENCES
- 1.Altar CA, Boyar WC, Kim HS. Discriminatory roles for D1 and D2 dopamine receptor subtypes in the in vivo control of neostriatal cyclic GMP. Eur J Pharmacol. 1990;181:17–21. doi: 10.1016/0014-2999(90)90240-7. [DOI] [PubMed] [Google Scholar]
- 2.Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 3.Calabresi P, Mercuri NB, Bernardi G. Synaptic and intrinsic control of membrane excitability of neostriatal neurons. II. An in vitro analysis. J Neurophysiol. 1990;63:663–675. doi: 10.1152/jn.1990.63.4.663. [DOI] [PubMed] [Google Scholar]
- 4.Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci. 1992a;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent block of NMDA receptor channels. Eur J Neurosci. 1992b;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 6.Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunction of the basal ganglia. Trends Neurosci. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi P, De Murtas M, Bernardi G. The neostriatum beyond the motor function: experimental and clinical evidence. Neuroscience. 1997a;78:39–60. doi: 10.1016/s0306-4522(96)00556-8. [DOI] [PubMed] [Google Scholar]
- 8.Calabresi P, Saiardi A, Pisani A, Baik H-H, Centonze D, Mercuri NB, Bernardi G, Borrelli E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci. 1997b;17:4536–4544. doi: 10.1523/JNEUROSCI.17-12-04536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabresi P, Gubellini P, Centonze D, Sancesario G, Morello M, Giorgi M, Pisani A, Bernardi G. A critical role of the nitric oxide/cGMP pathway in corticostriatal long-term depression. J Neurosci. 1999a;19:2489–2499. doi: 10.1523/JNEUROSCI.19-07-02489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabresi P, Centonze D, Gubellini P, Bernardi G. Activation of M1-like muscarinic receptors is required for the induction of corticostriatal LTP. Neuropharmacology. 1999b;38:323–326. doi: 10.1016/s0028-3908(98)00199-3. [DOI] [PubMed] [Google Scholar]
- 11.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho AL, Kameyama K, Huganir RL. Characterization of phosphorylation sites on the glutamate receptor 4 subunit of the AMPA receptors. J Neurosci. 1999;19:4748–4754. doi: 10.1523/JNEUROSCI.19-12-04748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centonze D, Gubellini P, Picconi B, Giacomini P, Calabresi P, Bernardi G. Unilateral dopamine denervation blocks corticostriatal LTP. J Neurophysiol. 1999;82:3575–3579. doi: 10.1152/jn.1999.82.6.3575. [DOI] [PubMed] [Google Scholar]
- 14.Charpier S, Deniau JM. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci USA. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charpier S, Mahon S, Deniau JM. In vivo induction of striatal long-term potentiation by low-frequency stimulation of the cerebral cortex. Neuroscience. 1999;91:1209–1222. doi: 10.1016/s0306-4522(98)00719-2. [DOI] [PubMed] [Google Scholar]
- 16.Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci USA. 1997;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dos Santos Villar F, Walsh JP. Modulation of long-term synaptic plasticity at excitatory striatal synapses. Neuroscience. 1999;90:1031–1041. doi: 10.1016/s0306-4522(98)00504-1. [DOI] [PubMed] [Google Scholar]
- 18.Fienberg AA, Hiroi N, Mermelstein PG, Song W-J, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J-A, Nestler EJ, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 19.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 20.Gotz T, Kraushaar U, Geiger J, Lubke J, Berger T, Jonas P. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J Neurosci. 1997;17:204–215. doi: 10.1523/JNEUROSCI.17-01-00204.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 22.Hemmings HC, Jr, Nairn AC, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated neuronal phosphoprotein. II. Comparison of the kinetics of phosphorylation of DARPP-32 and phosphatase inhibitor I. J Biol Chem. 1984a;259:14491–14497. [PubMed] [Google Scholar]
- 23.Hemmings HC, Jr, Greengard P, Tung HYL, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984b;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Z-C, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol (Lond) 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 26.Le Moine C, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing D1 dopamine receptor gene. Proc Natl Acad Sci USA. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris BJ, Simpson CS, Mundell S, Maceachern K, Johnston HM, Nolan AM. Dynamic changes in NADPH-diaphorase staining reflect activity of nitric oxide synthase: evidence for a dopaminergic regulation of striatal nitric oxide release. Neuropharmacology. 1997;36:1589–1599. doi: 10.1016/s0028-3908(97)00159-7. [DOI] [PubMed] [Google Scholar]
- 28.Pisani A, Calabresi P, Centonze D, Bernardi G. Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Br J Pharmacol. 1997;120:1007–1014. doi: 10.1038/sj.bjp.0700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 30.Schiller J, Schiller Y, Clapham DE. NMDA receptors amplify calcium into dendritic spines during associative pre- and post-synaptic activation. Nat Neurosci. 1998;1:114–118. doi: 10.1038/363. [DOI] [PubMed] [Google Scholar]
- 31.Sibley DR, Monsma FJ., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13:61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- 32.Snyder GL, Girault J-A, Chen J, Czernik AJ, Kebabian JW, Nathanson JA, Greengard P. Phosphorylation of DARPP-32 and inhibitor-1 in rat choroids plexus: regulation by factors other than dopamine. J Neurosci. 1992;12:3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stern EA, Jaeger D, Wilson CJ. Membrane potential synchrony of simultaneously recorded striatal spiny neurons in vivo. Nature. 1998;394:475–478. doi: 10.1038/28848. [DOI] [PubMed] [Google Scholar]
- 35.Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 36.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of the protein kinase A and protein kinase C phosphorylation of the N-methyl-d-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 38.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsou K, Snyder GL, Greengard P. Nitric oxide/cGMP pathway stimulates phosphorylation of DARPP-32, a dopamine- and cGMP-regulated phosphoprotein, in the substantia nigra. Proc Natl Acad Sci USA. 1993;90:3462–3465. doi: 10.1073/pnas.90.8.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wickens JR, Begg AJ, Arbuthnott GW. Dopamine reverses the depression of rat corticostriatal synapses which normally follows high-frequency stimulation of cortex in vitro. Neuroscience. 1996;70:1–5. doi: 10.1016/0306-4522(95)00436-m. [DOI] [PubMed] [Google Scholar]
- 41.Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]