Abstract
The stoned locus of Drosophila melanogaster encodes two novel proteins, stonedA (STNA) and stonedB (STNB), both of which are expressed in the nervous system. Flies with defects at the stoned locus have abnormal behavior and altered synaptic transmission. Genetic interactions, in particular with the shibire (dynamin) mutation, indicated a presynaptic function for stoned and suggested an involvement in vesicle cycling. Immunological studies revealed colocalization of the stoned proteins at the neuromuscular junction with the integral synaptic vesicle protein synaptotagmin (SYT). We show here that stoned interacts genetically with synaptotagmin to produce a lethal phenotype. The STNB protein is found by co-immunoprecipitation to be associated with synaptic vesicles, and glutathione S-transferase pull-downs demonstrate an in vitro interaction between the μ2-homology domain of STNB and the C2B domain of the SYTI isoform. The STNA protein is also found in association with vesicles, and it too exhibits an in vitro association with SYTI. However, we find that the bulk of STNA is in a nonmembranous fraction. By using the shibire mutant to block endocytosis, STNB is shown to be present on some synaptic vesicles before exocytosis. However, STNB is not associated with all synaptic vesicles. We hypothesize that STNB specifies a subset of synaptic vesicles with a role in the synaptic vesicle cycle that is yet to be determined.
Keywords: Drosophila melanogaster, protein interactions, stoned, synaptotagmin, synaptic vesicles
The proteins encoded by thestoned locus of Drosophila, stonedA (STNA) and stonedB (STNB), are critical for normal synaptic function. Viable mutant alleles of stoned, stoned-temperature-sensitive(stnts) (Grigliatti et al., 1973) and stonedC(stnC) (Homyk and Sheppard, 1977), are behaviorally abnormal, whereas lethal alleles (Miklos et al., 1987) demonstrate that stoned products are essential. Mosaic and Northern analysis suggested neural-specific expression (Petrovich et al., 1993; Andrews et al., 1996), and both STNA (Stimson et al., 1998) and STNB proteins (Fergestad et al., 1999) have been localized to presynaptic terminals at larval neuromuscular junctions.
The functions of the stoned proteins are unknown, but a combination of viable mutations at both the stoned and shibireloci is synthetically lethal (Petrovich et al., 1993). Theshibire gene encodes dynamin (Chen et al., 1991; van der Bliek and Meyerowitz, 1991), a GTPase with a pivotal role in endocytosis and vesicle recycling (Masur et al., 1990; Takei et al., 1995; Vallee and Okamoto, 1995). STNB has amino acid sequence homology to μ-adaptin proteins (Andrews et al., 1996). The μ2-adaptin (Thurieau et al., 1988) is a subunit of AP2, a clathrin adaptor-protein-complex (for review, see Robinson, 1994; Kirchhausen, 1999). STNA contains signature sequences, DPF, (Stimson et al., 1998) that can bind the appendage domain of α-adaptin (Owen et al., 1999), also a subunit of AP2 and required for synaptic vesicle endocytosis (Gonzalez-Gaiten and Jäckle, 1997). In addition, the lethal alleles of stoned show alterations in the size and morphology of synaptic vesicles (Fergestad et al., 1999), a phenotype similar to that observed in Drosophila with defects in the clathrin adaptor-protein AP180 (Zhang et al., 1998). All of these observations imply that stoned proteins function in endocytosis.
In contrast to the above, electrophysiological studies ofstoned mutants, involving either the adult visual system (Kelly, 1983; Homyk and Pye, 1989) or the embryonic and larval neuromuscular junctions (Stimson et al., 1998; Fergestad et al., 1999), all show defects in synaptic transmission. Furthermore, bothstnc andstnts mutations increase the frequency of miniature-endplate potentials (Stimson et al., 1998). These alterations are consistent with changes in exocytosis and neurotransmitter release.
Hypomorphic mutations at the Drosophila melanogaster sytI locus result in behavioral, electrophysiological, and morphological phenotypes similar to those seen in stnmutants (DiAntonio et al., 1993; Littleton et al., 1993a, 1994; Reist et al., 1998). Synaptotagmin I (SYTI) is a synaptic vesicle protein with a proposed role in both exocytotic and endocytotic functions in both mammals (for review, see Zhang et al., 1994; Südhof and Rizo, 1996) and invertebrates (DiAntonio et al., 1993; Littleton et al., 1993a, 1994; Nonet et al., 1993; Jorgensen et al., 1995).
In this paper we report a synthetically lethal interaction in flies with mutations at both the sytI and stn loci, that STNB interacts with synaptic vesicles in vivo via SYTI, and that this interaction may be maintained throughout the vesicle cycle. In addition, in vitro, studies show that domains of both STNA and STNB bind the C2B domain of the SYTI protein.
MATERIALS AND METHODS
Drosophila strains and crosses. TheDrosophila strains that we used—[white stonedts1(wstnts1), white stonedts2(wstnts2),shibirets1(shits1),shibirets3(shits3),Suppressor of stoned (Su(stn)), andOregon-R]—were as described previously (Lindsley and Zimm, 1992; Petrovich et al., 1993). The synaptotagmin mutantsw/w;sytAD4/CyO;+/+ and w/w;sytD27/Gla,Bc;P[elav-syt I]/+, both strains homozygous/hemizygous for the w mutation, were supplied by T. L. Schwarz (Stanford University Medical Center, Stanford, CA) and were as published (DiAntonio et al., 1993; DiAntonio and Schwarz, 1994).
Crosses to produce flies hemizygous for each of the viablestoned alleles, stnts andstnC, doubly heterozygous for thesyt null mutations, sytD27 and sytAD4, and heterozygous for the syt+minigene, were as outlined in Figure 1. Control flies were genetically identical for the syt alleles but wild-type at thestoned locus.
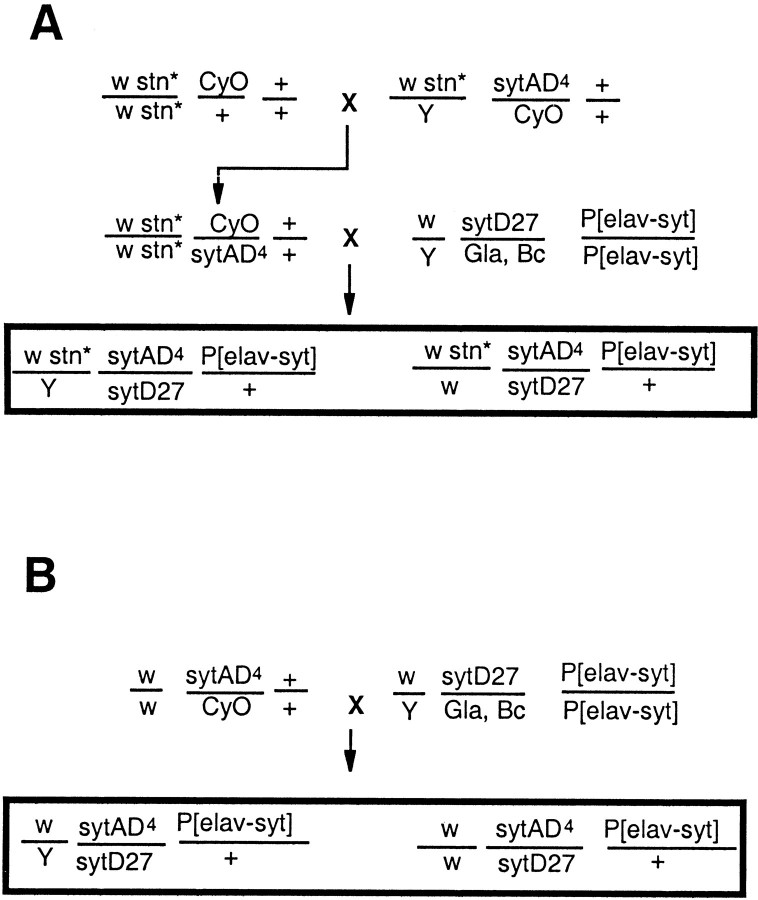
Fig. 1.
Generation of thestoned–synaptotagmin double mutants. The diagram shows the crossing strategy used to generate flies hemizygous for stn and with the sytI hypomorphic combination, that is, doubly heterozygous for sytI null mutations sytAD4 and sytD27 but heterozygous for the sytI+ transgene on chromosome 3. (For simplicity, we have not shown the early crosses introducing the stn alleles into the strain carrying thesytAD4 mutation, and the genotypes shown are only those of offspring used in later crosses and of the double mutants from the crosses.) A, The experimental crosses. Three separate crosses were established. The crosses differed only in thestn mutation present on chromosome 1, that is,stn* indicates stnts1,stnts2, orstnC. B, The control cross. This generated flies identical in genotype to the experimental crosses except for being wild type at the stnlocus.
Antibodies. Polyclonal antisera to an MBP/STNA (residues 20–349) fusion was raised in rabbits as described previously (Andrews et al., 1996) and used at a dilution of 1:20,000 on Western blots, or affinity-purified for immunoprecipitations. Rabbit anti-stonedB antisera were from two sources. Antiserum raised to an MBP/STNB (residues 1025–1261) fusion protein was used at a dilution of 1:1000 on Western blots or affinity-purified as described previously (Andrews et al., 1996). Rabbit anti-stonedB antisera raised against two combined peptides (residues 88–104 and 1244–1261) was affinity-purified against a glutathione S-transferase (GST)/STNB (residues 643–1261) fusion protein. The purified STNB antibodies were used in separate immunoprecipitation experiments. Experiments using both purified STNB antibodies gave identical results. Rabbit anti-synaptotagmin I (DSYT-2) antibody was a gift to M.R. from Troy Littleton and Hugo Bellen (Littleton et al., 1993b) (Baylor College of Medicine, Waco, TX). The DSYTI antisera was used at a dilution of 1:2000, or when affinity-purified against an MBP/SYTI (cytoplasmic domain) fusion, at a dilution of 1:5000. Rabbit anti-synaptobrevin (SYB) raised against a peptide, CMADAAPAGDAPPNA, from the cytoplasmic domain was a gift from J. Roos and R. B. Kelly (University of California Medical School, San Francisco, CA). It was used at a dilution of 1:2000 on Western blots. Mouse anti-cysteine string protein (CSP) antibody (mAb49) was kindly provided by Konrad Zinsmaier (University of Pennsylvania School of Medicine, Philadelphia, PA) and was used at a dilution of 1:200. The mouse anti-syntaxin (SYX) antibody (mAb 8C3) was a gift to M.R. from Seymour Benzer (California Institute of Technology, Pasadena, CA) and was used at a dilution of 1:500.
Fractionation of head homogenates. For the differential centrifugation, heads (0.1 gm) from Oregon-R flies were homogenized, Teflon on glass (20 strokes at 400 rpm), in 1 ml of buffer A in the absence of Ca2+ [10 mmHEPES, pH 7.4, 1 mm EGTA, 0.1 mmMgCl2, 1 mm phenylmethylsulfonyl fluoride (PMSF)] or in buffer A plus Ca2+[10 mm HEPES, pH 7.4, 1 mmCaCl2, 0.1 mmMgCl2, 1 mm PMSF]. Homogenates were then centrifuged at 1000 × g to produce the P1 pellet fraction. The supernatant (S1) was centrifuged at 25,000 ×g and 4°C for 40 min to produce the P2 pellet fraction. The resulting S2 supernatant was then subjected to centrifugation at 4°C at an average of 125,000 × g for 1 hr in a TL-100.3 rotor (Beckman TL-100 Ultracentrifuge) to obtain the P3 pellet and the final supernatant, S3. For organelle immunoprecipitation the P3 pellet was then resuspended in 1 ml of the homogenization buffer.
To generate material for the glycerol gradients, heads were homogenized in buffer A at a tissue-to-buffer ratio of 1:2, and the resulting homogenate was centrifuged at 1000 × g for 10 min. The S1 supernatant (150 μl) was then loaded onto the surface of a 5–25% glycerol gradient (1.7 ml) over a 150 μl 50% sucrose pad. The gradients were spun at 50,000 rpm using a TLS-55 rotor in a Beckman TL100 tabletop ultracentrifuge for 30 min. Fifteen 133 μl fractions were collected from each gradient. SDS sample buffer, without reducing agents, was added to each fraction, and the samples were boiled immediately and stored at −20°C. The absence of reducing agent allows the STNB protein to run at its expected molecular weight of 138 kDa (Andrews et al., 1996).
Co-immunoprecipitations. Anti-STNA and anti-STNB antibodies raised against MBP-STNA and MBP-STNB fusion proteins were affinity-purified (Andrews et al., 1996). Tosyl-activated magnetic beads (Dynal, Dynabeads M-500 Subcellular) were prepared per manufacturer's instructions using Protein-A as the linker protein (120 μg/6 × 107 beads). The Protein A-linked beads were incubated overnight at 4°C with either affinity-purified anti-STNB antibodies, affinity-purified anti-STNA antibodies, nonspecific rabbit IgG (preimmune serum), or no antibodies. The IgGs were covalently linked to the Protein A beads using dimethylpimelimidate as described (Harlow and Lane, 1988). The beads were mixed with the P3 (vesicle enriched) fraction overnight at 4°C, and then washed with 0.1% bovine serum albumin in PBS. Bound material was eluted by boiling in reducing SDS sample buffer, electrophoresed on SDS PAGE, and Western-blotted. The blots were prepared and probed as previously described (Andrews et al., 1996).
Scanning electron microscopy. Dynabeads (2 × 107 beads per sample) prepared for the co-immunopreciptation experiments were incubated with 25 μl of P3 fraction (equivalent to proteins from 25 μg wild-type fly heads). Beads (105) from each treatment were then washed in PBS and prepared for scanning electron microscopy by standard techniques. The beads were fixed in 2.5% glutaraldehyde, washed in PBS, attached to polyethyleneimine-coated coverslips, coated with OsO4, and subjected to critical point drying. Silver-coated, mounted samples were examined with a Phillips XL30 FEG Field Emission Scanning Electron Microscope (Phillips, Eindhoven, The Netherlands).
Fusion constructs. Fusion constructs were obtained by ligating restriction fragments from cDNA clones, and PCR fragments, in-frame, into pMalc2 (New England Biolabs) and pGEX-4T-1 (Pharmacia Biotech) expression vectors.
The in-frame restriction sites used at the 5′ end of ORF 2 constructs were the SpeI site at 4529 bp and an introducedEcoRI site at 5249 bp. The SmaI site at 5399 bp, the SalI site at 5927 bp, and the EcoRV site at 6782 bp were used as 3′ insert/vector ligation sites (the numbering of bases is from the corrected sequence GenBank accession no. U54982, with base 1 the A of the ATG initiating translation codon). A pGEX-4T-1 clone containing the SpeI–EcoRV fragment was produced by sequential subcloning of this fragment into Bluescript SK+ (Stratagene, La Jolla, CA), pMal, then via Bluescript SK+, into the pGEX vector (see Fig. 5A, Construct 1). Other subclones were generated similarly to Construct 1.
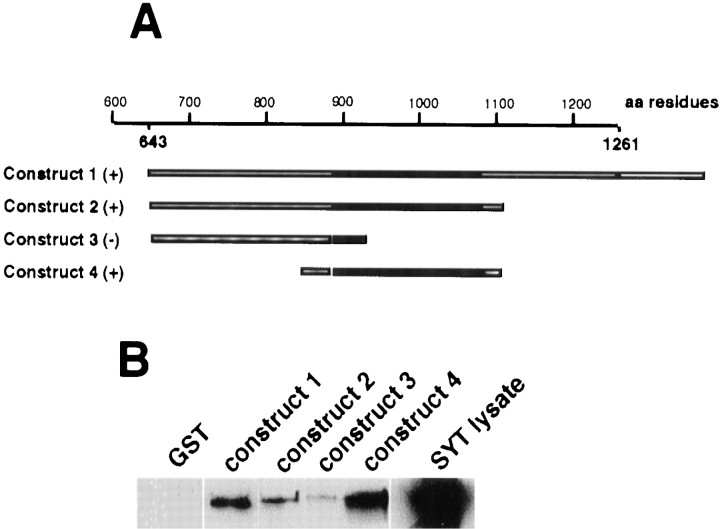
Fig. 5.
Molecular analysis of SYT binding to domains of STNB. A, A diagram indicating the STNB μ2-like domain regions that were subcloned into the pGEX expression vector. Theblack regions represent the (discontinuous) μ2-like domain (Andrews et al., 1996), the hatched regionrepresents coding region, and the unfilled regionrepresents the 3′ untranslated sequence. B, Western blots probed with affinity-purified anti-SYT antibody. Lane 1 is a negative control with GST bound to the resin, showing that GST does not bind SYT. The other lanes are as labeled, with the final lane being a sample of the Escherichia coli lysate expressing the ∼39 kDa 6x-His SYT.
DNA encoding the N-terminal 306 amino acids of STNA was obtained from both wild-type and stnts2genomic DNA by PCR using commercially obtained oligonucleotides and standard techniques. The sequence of the 5′ oligonucleotide was 5′-CCGGATCCCTTAAGCTACCAAAAGGCC-3′, incorporating an in-frame BamHI site at the initiating methionine codon. The sequence of the 3′ oligonucleotide was 5′-GCTCGGCGTTCGAAGTGG-3′. The PCR fragments were ligated into the pGEM-T Easy vector (Promega, Madison, WI) and sequenced to identify any introduced errors. Clones with no errors were subcloned into pGEX4-T-1 using the introducedBamHI site and an EcoRI site present in the pGEM-T Easy vector polylinker. These clones represent constructs 1 and 3 of STNA in Figure 6. The 966 bp XhoI fragment (bases 79–1045) was subcloned in frame into the XhoI site of pGEX-4T-1 to produce GST/Xho-p33 (see Fig. 6, Construct 2).
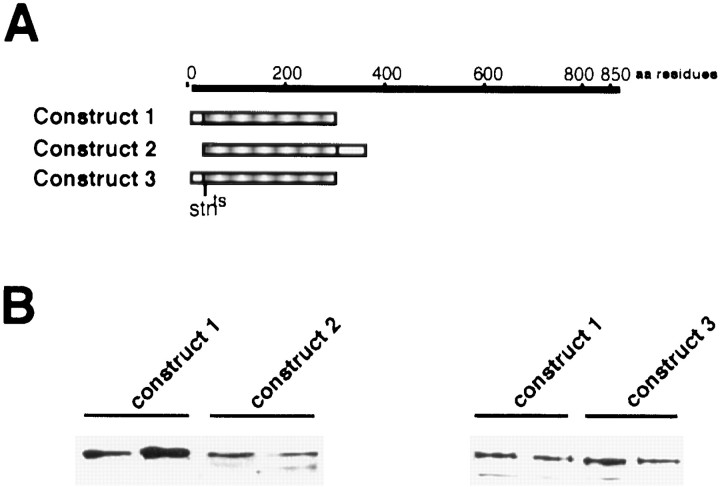
Fig. 6.
The N-terminal domain of STNA binds synaptotagmin.A, A diagram showing the STNA regions that were subcloned into the pGEX expression vector. The linerepresents the complete STNA protein. The hatched boxrepresents the domain common to the fusion constructs that bound SYT. The position of the stnts mutation is also shown. B, Western blots of protein eluates probed with SYT antibodies. The first blot shows the SYT bound to approximately equal quantities of fusion proteins fromConstructs 1 and 2. The second blot is a separate but equivalent experiment comparing the ability of the wild-type (Construct 1) and thestnts mutant fusion protein (Construct 3) to bind SYT.
The cytoplasmic domain of synaptotagmin-I, 760–1780 bp in pET-15b, was used as a complete construct or cloned as separate C2A and C2B domains into the pMal vector. An EcoRI site was introduced at 408 bp into the coding sequence using the oligonucleotide 5′-cataGTGTGCGGGAATTCCTGAAGAAGCG-3′, and an XhoI site was generated immediately 3′ to the translation stop codon using the oligonucleotide 5′-gcagctcgagcTACTTCATGTTCTTCAG-3′ (lower case bases are homologous to the pET vector sequence of the cDNA clone). The 1020 bp sytI fragment produced was subcloned into pGEM-T Easy. The resulting EcoRI–XhoI fragment was then subcloned into pMalc2 to produce the MBP/SYT fusion protein. Separate constructs for each of the two C2 domains of the synaptotagmin-I cDNA, including some adjacent sequence, were obtained from this construct. DNA from the pGEM-T Easy clone was digested with EcoRI andEcoRV to obtain the fragment encoding the C2A domain. This fragment was subcloned into Bluescript and then into pMalc2 usingEcoRI and HindIII restriction sites. The SYTC2A construct that was generated contains amino acid residues 137–327, (21,978 Da) of the SYT protein. The SYTC2B construct was produced by introducing an EcoRI site at 857 bp using the oligonucleotide 5′-CTGGTCAGCGTTGAATTCGAGGGC-3′, and a C-terminalXhoI site using the 3′ oligonucleotide was used to produce the 1020 bp fragment. The 474 bp sytI fragment produced was subcloned into pGEM-T Easy and then into pMalc2 using theEcoRI and SalI sites in the pMal vector. The SYTC2B construct contains residues 317–473, (17,702 Da) of the SYT protein and so extends beyond the sequence encoding the C2B domain. The fusion proteins were bound to amylose resin, and the SYT domains were cleaved from the MBP in 50 μl digests using FactorXa (New England Biolabs) according to the manufacturer's instructions. After digestion, EGTA was added to the samples to inactivate Ca2+ in the digestion buffer, and the isolated SYTI domains were identified on SDS-PAGE gels and by Western blotting. Equivalent amounts of the SYT C2A and SYT C2B fragment did not give equivalent signals on Western blots, and so the amounts of the two fragments used in binding to STN fusion proteins were adjusted to give approximately equivalent signals.
The C2B domain of SYTIV was generated by PCR fromDrosophila genomic DNA using a 5′ oligonucleotide that introduced an EcoRI site at 1114 bp (Accession No.AF181098), 5′-GCCACGGGAATTCAAGATTCGAGCC-3′, and a 3′oligonucleotide that introduced a HindIII site at 1581bp 5′-GCGCCCAGAAGCTTCCTGCC-3′. The PCR product was subcloned into pGEMT and sequenced to confirm that there were no Taq-induced errors. After the 467 bp fragment was subcloned into the pMal vector using the introduced restriction sites, SYTIV C2B/MBP was prepared and digested with FactorXa as described above for the SYTI constructs.
SYT binding experiments. GST fusion proteins were incubated with glutathione-Sepharose4B resin at 4°C overnight on a rocking platform. The resin was then washed with 10,000-fold volumes of PBS, or when performing studies involving Ca2+, it was washed with Tris-buffered saline (TBS). Bound protein was eluted from 20 μl of resin and assayed by the Bradford method (Bio-Rad) to determine protein eluted per microliters of resin. The protein-bound resin was incubated with a crude cell extract of the SYT fusion and washed with 10,000-fold volumes of buffer (or buffer containing Ca2+). For Ca2+ studies, the resin was washed with an additional 2000-fold volumes of TBS to remove Ca2+, before elution. Protein eluted from resin was separated by SDS-PAGE, Western-blotted, and probed with affinity-purified SYTI polyclonal antibodies.
Sequencing. Sequencing was performed by the Sanger method (Sanger et al., 1977). Fragments generated by PCR using commercially obtained oligonucleotides (Bresatec) were subcloned into the pGEM-T and pGEM-T Easy vectors (Promega). Wild-type cDNA and genomic clones, and genomic DNA and cDNA derived from total RNA, were used as controls to identify strain polymorphisms and when sequence obtained from the mutant differed from the published sequence. Some errors in the published sequence were identified and have been corrected in the data base (GenBank accession no. U54982).
RESULTS
Genetic interactions of stonedand synaptotagmin
The aim of these experiments was to determine whether either of the viable stn alleles, when in combination with mutations at the synaptotagmin I locus, enhanced or suppressed thesyt I phenotype. ThestnC andstnts alleles are homozygous viable as adult flies and were isolated in the same genetic background, and both possess the same insertional polymorphism as the original Oregon-R strain (Andrews et al., 1996). Flies heterozygous for twosyt I null mutations, sytAD4 andsytD27, were supplied by the Schwarz laboratory. The P-element-mediated transposition of a syt I minigene to the third chromosome provides 10% of wild-type SYTI protein levels and allows flies with lethal null mutations on both chromosomes at thesytI locus to survive to produce fertile adults (DiAntonio et al., 1993; DiAntonio and Schwarz, 1994). Double mutant combinations were constructed (Fig. 1), and the viability and behavior of the resulting flies were investigated.
The total number of flies analyzed in each cross, the proportion of male to female offspring, and the proportion of doubly mutant offspring are shown in Table 1. Hemizygous mutantstn males eclosed later than females heterozygous for the same mutant stn allele, and the male/female ratios, ∼60%, are as expected from previous viability data relating to stnmutants (Petrovich et al., 1993). Control males carrying only thesyt hypomorphic combination (+/Y; sytD27/sytAD4; P[elav-syt I]/+), eclosed in approximately expected proportions (20.6%) as shown in Table 1. In contrast, the combination of thesyt hypomorph with thestnts alleles significantly reduced the viability of these hemizygousstnts males. Only 9 doubly mutant males of thestnts1/Y; sytD27/sytAD4; P[elav-syt I]/+ genotype (2.3% of total males) and 13 (5%) equivalent males carrying thestnts2 allele eclosed. That the reduction in numbers of the double mutants carrying thestnts mutations was not caused by genetic background effects is demonstrated by thestnC data. Double mutants with the stnC allele (stnC/Y; sytD27/sytAD4; P[elav-syt I]/+) eclosed in the expected numbers, as did female flies heterozygous for eitherstnts orstnC (Table 1).
Table 1.
Relative viabilities of stn/syt double mutants
| Sex-chromosome genotype | sytAD4/sytD27 P[elav syt+] (as % of total) | n | Male/female ratio |
|---|---|---|---|
| stnts1/Y | 2.3* | 399 | |
| stnts1/+ | 21.5 | 731 | 0.55 |
| stnts2/Y | 5.8* | 223 | |
| stnts2/+ | 23.8 | 416 | 0.54 |
| stnC/Y | 16.9 | 633 | |
| stnC/+ | 24.9 | 884 | 0.72 |
| +/Y | 20.6 | 247 | |
| +/+ | 18.2 | 248 | 0.99 |
+ indicates the presence of a wild-type stoned allele, and Y represents a Y chromosome. n = total number of flies of each sex resulting from the crosses (see Fig. 1).
denotes progeny values that are significantly different from expected values. (*p < 0.001 using contingency χ2, actual numbers, not percentages, were used for calculation.)
Heterozygous females (stnts1/+; sytAD4/sytD27; P[elav-syt I]/+) showed abnormal behavior including a spread leg, “spider-like” appearance, and a slightly uncoordinated gait that was not seen in control flies. The few survivingstnts/Y; sytAD4/sytD27; P[elav-syt I]/+ males had an extreme behavioral phenotype and were very sedentary and severely uncoordinated. These males all died within 48 hr of eclosion. It was not possible to discriminate between the surviving stnts1 orstnts2 combinations (see later data on the stnts1 andstnts2 mutations). Control males, +/Y: sytAD4/sytD27; P[elav-syt I]/+, although uncoordinated [see also DiAntonio et al. (1993)], appeared well coordinated by comparison withstnts1/Y; sytAD4/sytD27; P[elav-syt I]/+ flies. A video showingstnts, the syt I hypomorph, and the doubly mutant males can be seen onhttp://www.genetics.unimelb.edu.au/Kelly/Kelmov.html.
The stnC/Y; sytAD4/sytD27; P[elav-syt I]/+ double mutants were behaviorally indistinguishable from syt hypomorphic controls (+/Y; sytAD4/sytD27; P[elav-syt I]/+).
These data clearly indicate a genetic interaction between thestnts mutations and syt I. Because synaptotagmins are an integral component of synaptic vesicle membranes, this data also suggests an interaction between thestoned protein(s) and synaptic vesicles.
Association of STNB with synaptic vesicle membranes
Hydrophobicity analysis of both the STNA and STNB proteins suggests that they should be soluble proteins. Wild-type fly head extracts, homogenized in both the presence and absence of calcium, were subjected to differential centrifugation to produce P1 (1000 ×g), P2 (25,000 × g), and P3 (125,000 × g) pellets and a final supernatant fraction, S3 (see Materials and Methods). Western blots prepared from these fractions were probed with anti-STNA, anti-STNB, and anti-SYTI antibodies. The anti-SYTI antibodies were raised against the recombinant cytoplasmic region of the SYTI protein and recognize a number of isoforms of synaptotagmin (54–69 kDa), as described previously (Littleton et al., 1993b). Neither the STNA nor STNB proteins could be visualized in the supernatant fraction. The STNB protein co-sedimented with the synaptic vesicle protein marker SYT, primarily in the P2 and P3 fractions. STNA, on the other hand, preferentially partitioned into the P1 fraction, although some STNA was found in both the P2 and P3 fractions (Fig.2A). This indicates that both stoned proteins preferentially partition into either membrane fractions or fractions containing large protein complexes. The association of STNA with the P1 fraction was investigated further. Solublization of STNA from P1 was not achieved with Triton X-100, deoxycholate, or high NaCl concentrations; however, the chaotropic agent KI (1 m) effectively solubilized all of the STNA protein from the P1 fraction (data not shown). This indicates that STNA in the P1 fraction is not associated with heavy membranes but is more likely to be associated with a large protein complex.
Fig. 2.
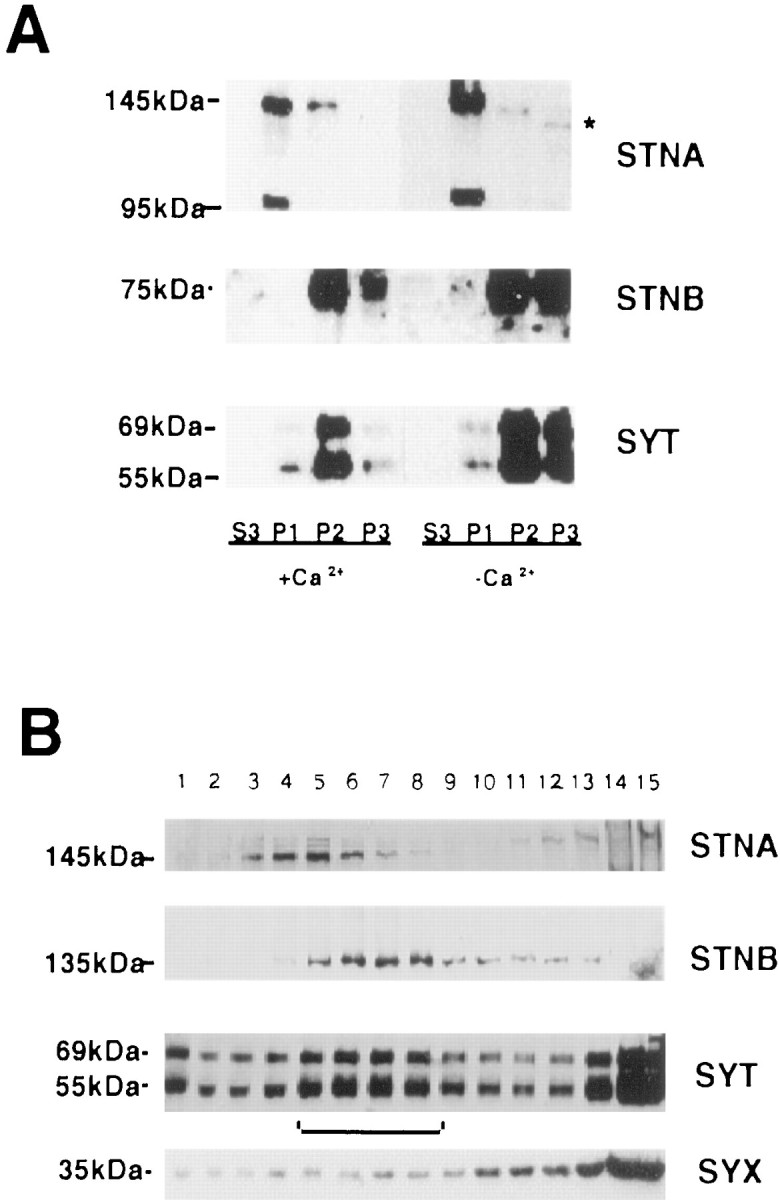
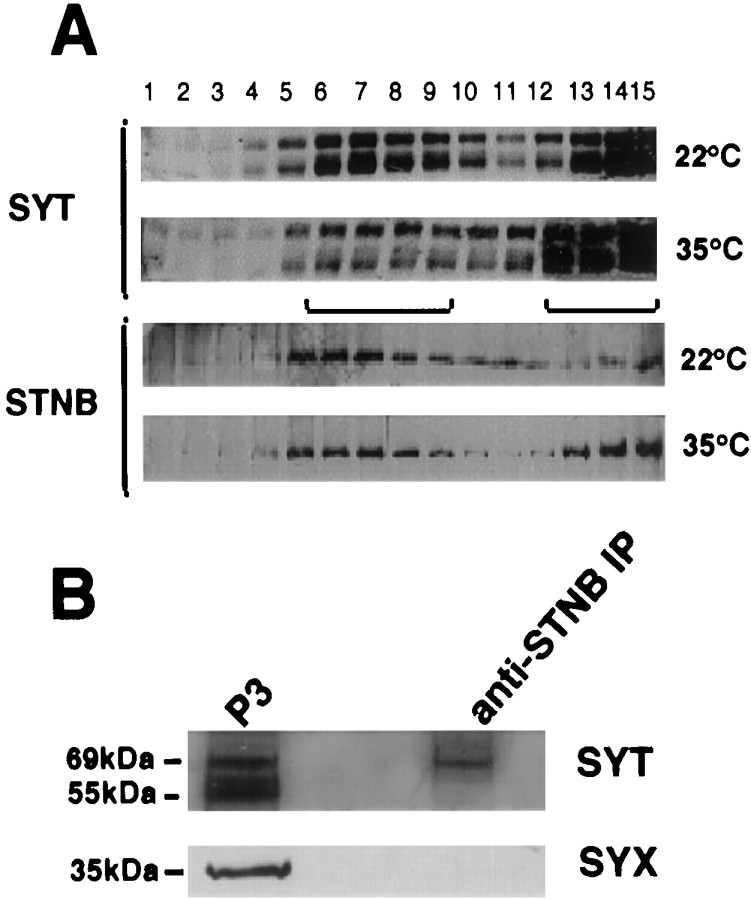
Distribution of STNA, STNB, and SYT proteins in head extracts. A, Heads were homogenized either in the presence or absence of Ca2+ and fractionated to produce a 1000 × g pellet (P1), a 25,000 × g pellet (P2), a 125,000 × g pellet (P3), and a final supernatant (S3). The protein content of each fraction was determined, and equal amounts of protein were electrophoresed. The resulting Western blots were probed with anti-STNA, anti-STNB, and anti-SYT antibodies. The figure shows that the bulk of the twostoned proteins partition into different fractions with STNA predominating in P1 fraction. The asteriskindicates that the STNA protein shows altered mobility in the P3 fraction. The distribution of the STNB protein parallels that of SYT. Under reducing conditions, the C-terminal fragment of STNB migrates with an apparent molecular weight of 75 kDa (Andrews et al., 1996).B, The numbered lanes are fractions from glycerol gradients of the S1 from wild-type flies. Fraction 1 represents the top of the gradient, and 15 represents the bottom of the gradient. Equal volumes from these fractions were then applied to nonreducing SDS-PAGE, Western-blotted, and probed with the antisera as indicated.
As expected, the SYT isoforms were associated with both the P2 and P3 fractions, plasma membrane, and vesicle-enriched fractions, respectively. Also observed was a coincidental shift of SYT and STNB from the P3 to the P2 fraction when homogenization was performed in the presence of Ca2+. The supernatant fraction from the P1 centrifugation (S1) was applied to a glycerol gradient and centrifuged to separate membrane components. A peak of SYT (Fig.2B, fractions 5-8), corresponding to the synaptic vesicle fraction, was observed as previously described (van de Goor et al., 1995). STNB protein co-sedimented with the SYT peak, whereas STNA, although entering the gradient, peaked in fractions 3–6 (Fig. 2B). These two results, the coincident redistribution of STNB and SYT in the presence of Ca2+ and their co-sedimentation in glycerol gradients, are consistent with an association of the STNB protein with synaptic vesicles. The plasma membrane marker syntaxin was also present in the gradients, probably indicating fragmentation of plasma membrane during homogenization, although its distribution did not mirror that of synaptotagmin/STNB or STNA.
To determine whether the STNB present in the P3 fraction is associated with synaptic vesicles, anti-STNB antibodies were attached to Protein A-coated magnetic beads, and incubated with a P3 fraction prepared fromDrosophila head homogenates in the absence of calcium. These beads were then analyzed for the presence of STNB and the synaptic vesicle protein markers SYT, CSP, and SYB as well as the plasma membrane marker SYX. The results indicate that a major proportion of the STNB protein in the P3 fraction is immunoprecipitated (Fig.3A). The STNB antibodies coprecipitate all three synaptic vesicle markers (SYT, CSP, and SYB), but not SYX (Fig. 3A). Although multiple species of SYT can be identified in P3 fractions, as was previously described for head homogenates (Littleton et al., 1993b), only the 69 kDa SYTI isoform is present in these precipitates.
Fig. 3.
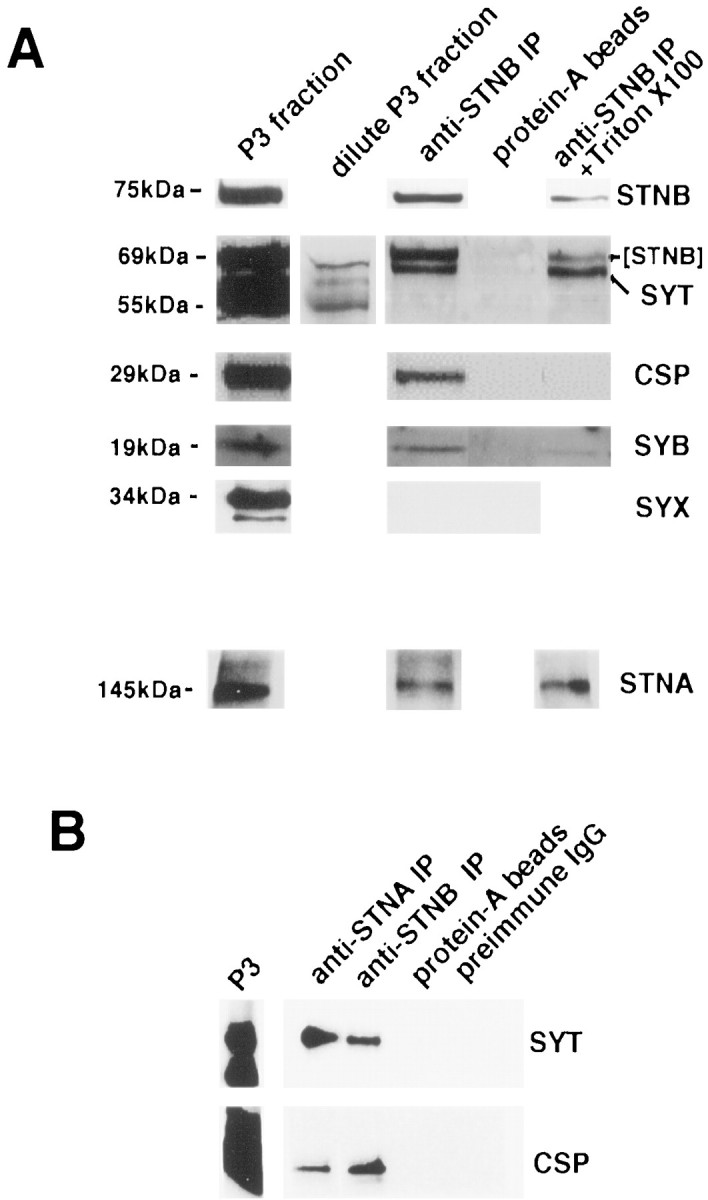
Immunoprecipitation studies using anti-STN antibodies. A, Western blots of protein eluates from immunoprecipitation experiments using the beads as shown inA. Blots were probed with anti-STNB, anti-SYT, anti-CSP, anti-SYB, anti-SYX, and anti-STNA antibodies. The lanesshow the P3 fraction, the bead-associated fraction, the fraction associated with Protein A-only beads, and the bead-associated fraction remaining after washing with Triton X-100. The material shown in all lanes was derived from the same P3 fraction. The additional blot shown in the STNB + SYT panel contains a reduced amount of the original P3 fraction probed with anti-SYT to show the relative positions of the various SYT isoforms. The SYT panel is the blot from the top panel (STNB alone) reprobed with anti-SYT antibodies showing the presence of both the 69 kDa SYTI and the signal remaining from STNB. B, Immunoprecipitation of vesicles by anti-STNA antibodies. Western blot of protein eluates from beads coated with Protein A bound to anti-STNA, anti-STNB, Protein A alone, or nonspecific antibodies (preimmune IgG) as well as a P3 fraction. These blots were probed with anti-SYT and anti-CSP. All gels used in these assays were run under reducing conditions.
On the basis of its deduced amino acid sequence, STNB does not contain any putative transmembrane segments and is unlikely to be an integral membrane protein. What then is the molecular nature of the STNB/vesicle association? To address this question, fractions of the STNB immunoprecipitations were washed extensively with 1% Triton X-100. The presence of the detergent entirely removed CSP from the precipitates and considerably reduced the amount of SYB present. However, Triton X-100 had no effect on the amount of SYTI bound to the beads (Fig.3A). This result suggests that STNB is not associating with the lipid components of the vesicle membrane and that the interaction may be via SYTI.
There was relatively little STNA seen in the P3 fraction on the Western blots (Fig. 2A). However, we did observe STNA protein on the glycerol gradients (Fig. 2B), and although there was no coincidence of the peak fractions, there was overlap between STNA and the synaptic vesicle peak. The immunoprecipitations were therefore further probed for the presence of STNA. The STNA protein was found to be associated with the immunoprecipitates and to be insensitive to the Triton X-100 washes (Fig. 3A). When immunoprecipitations were performed using anti-STNA antibodies attached to beads, again SYTI and CSP were coprecipitated (Fig. 3B). Therefore, both STNB and STNA can be found associated with synaptic vesicles in the P3 fraction.
Where does STNB act in the vesicle cycle?
The structure of STNB, containing as it does a μ2 homology domain (Andrews et al., 1996), along with the interaction ofstnts with shibire(Petrovich et al., 1993) and the alteration in the size of vesicles in stoned lethal mutants (Fergestad et al., 1999), all suggest that STNB may play a role in the synaptic vesicle endocytotic pathway (Zhang et al., 1998). Because STNB can be found associated with free synaptic vesicles in the P3 fraction, it might be presumed that these vesicles have recently been endocytosed. We investigated this possibility by looking at the distribution of STNB in extracts from heat-treatedshits1 flies. If STNB becomes associated with synaptic vesicles only as they are budding from the plasma membrane, then in heat-treated shibire flies, where endocytosis is blocked at the budding stage, STNB should no longer colocalize with free synaptic vesicles. If recruitment of STNB to budding vesicles occurs after dynamin activity, then STNB should be in the supernatant fraction (the top of the gradient). If recruitment of STNB occurs before dynamin action, then in heat- treatedshibire flies, STNB should be associated with the plasma membrane fraction (bottom of the gradient). To block endocytosis but not deplete all synapses of synaptic vesicles, the shibireflies were heat-treated in the dark. Figure4A shows glycerol gradients prepared using S1 extracts fromshits1 flies kept at 22°C (control) compared with similar flies treated, in the dark, for 15 min at 35°C. Contrary to expectation, the behavior of STNB mimics that of SYTI. As reported previously (van de Goor et al., 1995), there is a heat treatment-dependent shift in the SYT isoforms from the synaptic vesicle fraction (fractions 5–8) to the bottom of the gradient (fractions 12–15), although less than in previous studies, as the flies were dark-adapted. There is also a partial shift of STNB to the bottom of the gradient, but some STNB, like SYTI, remained associated with the free synaptic vesicle fraction (Fig. 4A). There is no indication of a shift of STNB to the soluble fractions (top of the gradient). The distribution of STNA on the gradients is unaffected by the heat treatment (data not shown).
Fig. 4.
The effects of heat-treatingshits1 flies on the distribution of STNA and STNB. A, Glycerol gradients were loaded with equal quantities of S1 material from heat-treated and untreatedshits1 flies, and fractions 1–15 were collected as for Figure 2B. Western blots were probed with anti-SYT, anti-STNA, and anti-STNB antibodies. A peak of free synaptic vesicles was observed in fractions 6–9, most of which shifts to the bottom of the gradient in extracts from heat-treated flies. A similar shift is observed with both STNB and SYT.B, A P3 fraction from heat-treatedshits1 flies (left-hand lane) was applied to Protein A/anti-STNB-coated beads, and the eluants were Western-blotted and probed with anti-SYT and anti-SYX antibodies (right-hand lane), showing that anti-STNB continues to immunoisolate synaptic vesicles but not plasma membrane.
To check that STNB in the vesicle fractions of heat-treatedshits1 extracts remains bound to synaptic vesicles, P3 fractions from control and heat-treatedshibire flies were immunoprecipitated with anti-STNB antibodies. Figure 4B shows that anti-STNB antibodies co-immunoprecipitate SYTI from P3 fractions of heat-treatedshits1 flies. These immunoprecipitates still do not have syntaxin associated with them and so are unlikely to have arisen from fragmented plasma membrane. These results suggest that STNB-associated vesicles do not represent only those recently endocytosed.
Molecular interactions between STNB and SYTI
The continued association of SYTI with immunoprecipitated STNB even after Triton X-100 treatment suggested a direct interaction between STNB and SYTI. It appeared likely that this interaction would be via the μ2-like domain of STNB. To investigate this, a 621 residue protein containing the μ2-like region, residues 883–1089, and adjacent sequence up to residue 1261 (Fig.5A, Construct 1), was expressed in the pGEX expression vector to produce a GST fusion. The resin-bound GST/μ2-like fusion protein was incubated overnight with a crude extract of bacterial cells expressing the cytoplasmic portion of Drosophila SYTI as a 6x-His (pET) fusion protein of 39 kDa. When proteins eluted from the resin were Western-blotted and the blot was exposed to anti-SYTI antibody, the expected 39 kDa SYTI fusion protein was identified (Fig.5B). The GST protein itself was unable to bind SYTI in this assay (Fig. 5B).
A series of fusion constructs with different regions of the μ2-like domain of STNB (Fig. 5A) were similarly assayed. In repeated experiments, fusion proteins terminating at amino acid residue 930 (Fig. 5A,B, Construct 3) were unable to bind SYTI. However, SYTI bound to all fusion proteins containing amino acid residues 847–1108 of the STNB protein. Because residues 883–1138 of STNB constitute the μ2-like domain (Andrews et al., 1996), this domain is sufficient for SYTI binding in vitro. It is possible, however, that residues outside the μ2-like domain influence the strength of this interaction in vivo.
The effect of Ca2+ on binding of SYTI to the GST/STNB (Fig. 5A, Construct 1) was assayed by performing the overnight incubations and washes in the presence or absence of added Ca2+ up to 250 μm. SYTI bound to the fusion in both the presence and absence of Ca2+, indicating that the interaction of the STNB μ2-like domain with SYTI, like that of AP2 (Zhang et al., 1994), is independent of Ca2+ (data not shown).
The stnts mutation
Because the stnts alleles are known to interact with other mutant neurological loci, includingsytI, the nature of thesestnts mutations was important. Genomic DNA and cDNAs obtained fromstnts2 mutant flies were sequenced. The only sequence difference found between wild type andstnts2 was an A to T transversion present 104 bp into the coding sequence of ORF 1, resulting in a change from lysine (K) to methionine (M) at residue 35. The A to T substitution was found to be present instnts1,stnts2, andSu(stn);stnts2 flies but not in stnC mutants,shits mutants, or Oregon-R wild-type controls. Because bothstnts alleles andstnC andshits3 were isolated from the same Oregon-R strain, the absence of the mutation instnC andshits3 shows that this is not a polymorphism. These data also imply that there is only a singlestnts mutation,stnts1 being identical tostnts2.
Molecular interactions between STNA and SYTI
We have shown (Fig. 3) that STNA can be found associated with synaptic vesicles immunoabsorbed by the STNB antibodies. To determine whether the STNA protein might be associated directly with synaptotagmin, SYTI binding to STNA fusion proteins was analyzed as described previously for the STNB constructs. Three constructs were used. The first was a fusion protein that included the first 290 residues of STNA (GST/5′STNA), the second included residues 26–350 (GST/STNA Xho-p33), which removes most of the N-terminal region that would be missing if the methionine in thestnts mutant acted as a novel translation initiation site, and the third was identical to GST/5′STNA but contained the sequence encoding the K to M substitution found in the stnts flies (Fig.6A). The results (Fig. 6B) indicate that residues 26–290 of the amino terminal region of STNA can bind SYTI. This region includes the sequence altered in thestnts mutation. Binding of SYTI to the GST/5′STNA construct containing thestnts mutation showed that this mutant protein also bound SYTI (Fig. 6B). The affinity of STNA for SYTI appears less than that seen with the STNB constructs; however, the binding of STNA to SYTI in vitrowas observed consistently.
Domain specificity of stoned–synaptotagmin interactions
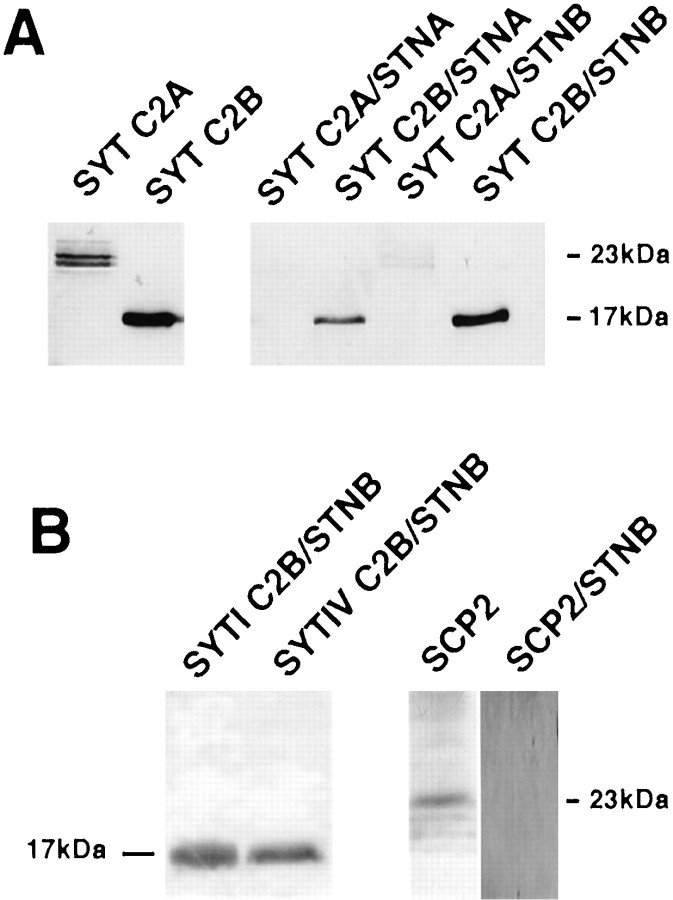
There are two C2 domains, C2A and C2B, in the SYTI monomer, and the two domains have been found to interact with different intracellular components (Davletov and Südhof, 1993; Fukuda et al., 1995; Li et al., 1995; Sugita et al., 1996). Two SYTI protein constructs were produced, each containing one of the C2 domains, and the ability of these protein constructs to bind to both the STNA/GST and STNB/GST fusions was investigated. The protein containing the cytoplasmic sequences including the C2A domain (residues 137–327) but excluding the C2B domain failed to bind to either STNA or STNB. However, the protein containing only the C2B domain (residues 317–473) was found to bind to both STNA and STNB (Fig.7A).
Fig. 7.
Stoned proteins bind to the C2B domain of synaptotagmin. A, The SYTC2A domain, or SYTC2B domain, were cleaved from maltose-binding-protein fusions (see Materials and Methods) and are shown in lanes 1 and 2. The SYTC2B binds to the STNA and STNB fusions (lanes 4 and6) but SYTC2A does not (lanes 3and 5). B, The SYTC2B domains of both SYTI and SYTIV are capable of binding to the μ2-homology domain of STNB. The cleaved SYTI and SYTIV pMal fusion proteins both produce 17 kDa fragments. As a control for the specificity of this interaction, the Drosophila SCP2 (Kelly et al., 1997) protein fused in pMAL and cleaved from the fusion protein to produce a 23 kDa protein was used in the binding assay. No SCP2 protein was found associated with the beads.
The immunoprecipitation studies indicated that only the 69 kDa SYTI isoform was coprecipitated with STNB. However, Littleton et al. (1999)identified genes for other synaptotagmin species inDrosophila, including a SYTIV homolog of 55 kDa that was shown to be associated with synaptic vesicles. The C2B domain (residues 325–374) of SYTIV was expressed as a pMAL fusion protein and shown to cross-react with the polyclonal anti-SYTI antibodies. We asked, therefore, whether STNB could interact with SYTIV in vitro. The SYTIV fusions were then assayed for their ability to bind to STNB as described above for the SYTI C2 domains. The SYTIV C2B domain was shown to interact with the μ2-homology region of STNB (Fig.7B).
DISCUSSION
The results presented here clearly show that there is both anin vivo and an in vitro interaction between the products of the stoned gene and the integral synaptic vesicle protein synaptotagmin I. We hypothesized that a genetic interaction would be observed between the viable stn alleles and the syt hypomorphic combination of genes, and our data show a synergistic lethality between thestnts mutation andsytI. Individually, both thestnC mutation and thesyt hypomorphic mutations remove the transient responses of the ERG (Homyk and Pye, 1989; DiAntonio and Schwarz, 1994), increase the frequency of miniature end plate potentials, and decrease the amplitude of the excitatory junction current (EJC) at the larval neuromuscular junction (Littleton et al., 1993a; DiAntonio and Schwarz, 1994; Stimson et al., 1998). Given the phenotypic similarities between these mutants, we anticipated a phenotypic enhancement in thestnC/Y; sytD27/sytAD4; P[elav-syt]/+ doubly mutant fly. However, we found no genetic interaction between these mutations. In contrast, thestnts mutant, which shows an increase in the off-transient amplitude (Kelly 1983) and no effect on the larval neuromuscular EJC (Stimson et al., 1998), shows a lethal interaction with sytI as was previously observed forshibire and dunce. The shibireproduct, dynamin, is essential for vesicle endocytosis and linksstoned to the endocytotic branch of the vesicle cycle. Thedunce locus encodes a cAMP phosphodiesterase, and mutation at this locus results in a chronic increase in cAMP levels (Byers et al., 1981). The calcium dependence of neurotransmitter release is altered in dunce mutants (Zhong and Wu, 1991), suggesting an indirect effect of cAMP on exocytosis. A retrograde effect of cAMP on quantal output from larval presynaptic terminals has also been observed (Davis et al., 1998). Therefore the interaction betweenstoned and dunce suggests a possible effect ofstoned on exocytosis. Because synaptotagmin has been implicated in both exocytosis and endocytosis, the stn/sytIgenetic interaction does not discriminate between the endocytotic or exocytotic branches of vesicle cycling; however, the data strengthens the argument for a role for stoned in these processes.
Investigation of possible protein interactions have led to the conclusion that STNB and SYTI interact directly in vivo. Both SYTI and STNB are localized to nerve terminals (Fergestad et al., 1999), although the co-sedimentation properties and the colocalization on glycerol gradients of STNB and SYT reported here are the first indications that the proteins might interact. Western blot analysis of the proteins attached to the anti-STNB antibody-coated beads indicates the presence of several integral synaptic vesicle proteins and clearly identifies the vesicles as free synaptic vesicles. The absence of SYX from these immunoprecipitates suggests low or no contamination with fragmented plasma membrane. That the detergent solubilization of vesicle membranes removed CSP and SYB but not SYT from these precipitates strongly implied a direct binding of STNB to SYT in vivo. The ability of STNB to directly bind to SYT was confirmed by the STNB-GST pull-downs showing that the μ2-homology domain of STNB can bind to the C2B domain of SYTI independently of Ca2+.
We observe that anti-STNB antibodies precipitate synaptic vesicles, but only those containing the 69 kDa isoform of synaptotagmin. More than one band is recognized by affinity-purified anti-SYTI antibodies on the gradients and in the P3 fraction. Although we cannot exclude the possibility that proteolysis of SYTI causes at least some of the bands that are seen (Littleton et al., 1993b), we would expect that SYTIV would be identified by the anti-SYTI antibodies as a band at 55 kDa in P3 fractions. SYTIV is present in Drosophila head protein homogenates and is an integral synaptic vesicle protein (Littleton et al., 1999). Our results show that SYTIV is recognized by the antibody and can bind to STNB in vitro (Fig. 7B). It is possible that STNB and SYTIV are never present at the same synapses, although a previous report states that SYTI and SYTIV are present together, in at least some synapses (Littleton et al., 1999). It seems likely that STNB binds specifically to SYTI and not to SYTIV in vivo, but a physiological interaction between STNB and SYTIV cannot be excluded.
The behavior of STNB and SYT in heat-treatedshits1 flies indicates that STNB is not restricted to vesicles that have been recently endocytosed but may be constitutively associated with vesicles. There is movement of a high proportion of the 55 kDa band recognized by the SYTI antibodies to the plasma membrane fractions in heat-treatedshits1 flies [van de Goor et al. (1995), and Fig. 4, this report]. The 55 kDa band may be proteolyzed SYTI, SYTIV, or a combination of both proteins, but theshibire data suggest that vesicles containing this moiety are capable of exocytosis and, according to van de Goor et al. (1995), endocytosis. Our immunoprecipitation data show no association between STNB and vesicles containing anti-SYTI cross-reacting proteins with molecular weights lower than 69 kDa (Fig. 3). We conclude that the presence of STNB on synaptic vesicles is not essential for either exocytosis or endocytosis of all vesicles. The constitutive association of STNB, therefore, marks a specific pool of vesicles and suggests that they are somehow differentiated from the remainder of the vesicle pool. A number of structurally and physiologically differentiated pools have been proposed (Koenig and Ikeda, 1996; Kuromi and Kidokoro, 1998) that are involved in both clathrin-dependent and clathrin-independent recycling (for review, see Palfrey and Artalejo, 1998). Because both AP2 and STNB bind SYTI, it is possible that STNB competes with and inhibits AP2 and prevents clathrin-mediated cycling, perhaps resulting in fast vesicle cycling. The finding that α-adaptin mutant embryos lack vesicles in presynaptic terminals (Gonzalez-Gaiten and Jäckle, 1997) is evidence against this hypothesis. However, currently there is insufficient data to determine how a chronic loss of α-adaptin may affect vesicle biogenesis and recycling. An understanding of the number of vesicle pools, their physiological role, and the contribution to these pools made by STNB remains to be determined.
The distribution of the STNA protein differs markedly from that of STNB. The presence of STNA protein in the P1 fraction, along with its solubilization only in the presence of the highly chaotropic agent KI, suggests that most of this protein is involved in a dense multiprotein complex. It is tempting to speculate that STNA is associated with cytoskeletal elements, but this has yet to be shown. The presence of small amounts of STNA protein in the P3 fraction and the association of this STNA protein with STNB-containing vesicles implies an interaction between STNA and STNB. The observation that the P3 STNA has an altered mobility by comparison with the bulk of the protein may indicate that post-translational modification of STNA affects its interaction with the P1 protein complex or with synaptic vesicles, or both.
We have shown that the stntsmutation is located in the STNA protein. The amino terminal domain of STNA (residues 26–290) binds to the C2B domain of synaptotagminin vitro. The ORF containing STNA is highly unusual in that it contains no sequences encoding internal methionine residues (Andrews et al., 1996). The stntsmutation, on the other hand, introduces such an internal methionine codon early in the STNA ORF. Because STNA and STNB are products of a dicistronic mRNA (Andrews et al., 1996), which in eukaryotes is rare, we currently have no understanding of the effects of this novel methionine on translation initiation or the read-through mechanism translating the second ORF. However, preliminary data indicate no major quantitative alteration in the levels of either STNA or STNB instnts flies. We suggest that STNA has a specific role to play in the regulation of synaptic output, but it is unclear at present how and where this protein acts in the vesicle cycle.
The function of both the STNA and STNB proteins remains unknown; however, in this report we have shown that both of these proteins can associate with synaptic vesicles via SYTI and hypothesize that the association of STNB with SYTI defines a physiologically distinct pool of synaptic vesicles.
Footnotes
This research was supported by Grant 960117 from the National Health and Medical Research to L.E.K., by National Institutes of Health grants to M.R. (NS34889 and KO2-NS02001), and by a Human Frontiers Science Program grant to L.E.K. and M.R. (and four others), as well as by funds from the McKnight and Alfred P. Sloan Foundations (M.R.). We thank Tom Schwarz for the synaptotagmin mutants, Jack Roos and Reg Kelly for the antibodies to synaptotagmin, synaptobrevin, and syntaxin, and Konrad Zinsmaier for the CSP antibody. We are grateful to Quentin Lang for assistance with figures. Excellent technical assistance was provided by Jennifer Shirriffs.
Correspondence should be addressed to Dr. A. Marie Phillips, Department of Genetics, University of Melbourne, Parkville, Victoria, Australia 3052. E-mail: m.phillips@genetics.unimelb.edu.au.
REFERENCES
- 1.Andrews J, Smith M, Merakovsky J, Coulson M, Hannan F, Kelly LE. The stoned locus of Drosophila melanogaster produces a dicistronic transcript and encodes two distinct polypeptides. Genetics. 1996;143:1699–1711. doi: 10.1093/genetics/143.4.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byers D, Davis RL, Kiger JA., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981;289:79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- 3.Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- 4.Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- 5.Davletov BA, Südhof TC. A single C2 domain from synaptotagminI is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- 6.DiAntonio A, Schwarz TL. The effect on synaptic physiology of synaptotagmin mutations in Drosophila. Neuron. 1994;12:909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 7.DiAntonio A, Parfitt KD, Schwarz TL. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993;73:1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- 8.Fergestad T, Davis WS, Broadie K. The stoned proteins regulate synaptic vesicle recycling in the presynaptic terminal. J Neurosci. 1999;19:5847–5860. doi: 10.1523/JNEUROSCI.19-14-05847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda M, Moreira JE, Lewis FM, Sugimori M, Niinobe M, Mikoshiba K, Llinas R. Role of the C2B domain of synaptotagmin in vesicle release and recycling as determined by specific antibody injection into the squid giant synapse preterminal. Proc Natl Acad Sci USA. 1995;92:1533–1539. doi: 10.1073/pnas.92.23.10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Gaitan M, Jäckle H. Role of Drosophila α-adaptin in presynaptic vesicle recycling. Cell. 1997;88:767–776. doi: 10.1016/s0092-8674(00)81923-6. [DOI] [PubMed] [Google Scholar]
- 11.Grigliatti TA, Hall L, Rosenbluth R, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol Gen Genet. 1973;120:107–114. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- 12.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1988. Immunoaffinity purification. pp. 522–523. [Google Scholar]
- 13.Homyk T, Jr, Pye Q. Some mutations affecting neural or muscular tissues alter the physiological components of the electroretinogram in Drosophila. J Neurogenet. 1989;5:37–48. doi: 10.3109/01677068909167263. [DOI] [PubMed] [Google Scholar]
- 14.Homyk T, Jr, Sheppard DE. Behavioral mutants of Drosophila melanogaster. I. Isolation and mapping of mutations which decrease flight ability. Genetics. 1977;87:95–104. doi: 10.1093/genetics/87.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgensen EM, Hartwieg E, Schuske K, Nonet ML, Jin Y, Horvitz HR. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature. 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- 16.Kelly LE. An altered electroretinogram transient associated with an unusual jump response in a mutant of Drosophila. Cell Mol Neurobiol. 1983;3:143–149. doi: 10.1007/BF00735278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly LE, Phillips AM, Delbridge M, Stewart R. Identification of a gene family from Drosophila melanogaster encoding proteins with homology to invertebrate sarcoplasmic calcium-binding proteins. Insect Biochem Mol Biol. 1997;27:783–792. doi: 10.1016/s0965-1748(97)00062-3. [DOI] [PubMed] [Google Scholar]
- 18.Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- 19.Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Ullrich B, Zhang JZ, Anderson RGW, Brose N, Südhof TC. Ca2+-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 22.Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. Academic; San Diego: 1992. [Google Scholar]
- 23.Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca2+-activated neurotransmitter release. Cell. 1993a;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- 24.Littleton JT, Bellen HJ, Perin MS. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993b;118:1077–1088. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]
- 25.Littleton JT, Stern M, Perin M, Bellen HJ. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc Natl Acad Sci USA. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Littleton JT, Serano TL, Rubin GM, Ganetzky B, Chapman ER. Synaptic function modulated by changes in the ratio of synaptotagmin I and IV. Nature. 1999;400:757–760. doi: 10.1038/23462. [DOI] [PubMed] [Google Scholar]
- 27.Masur SK, Kim Y-T, Wu C-F. Reversible inhibition of endocytosis in cultured neurons from the Drosophila temperature-sensitive mutant shibrets1. J Neurogenet. 1990;6:191–206. doi: 10.3109/01677069009107110. [DOI] [PubMed] [Google Scholar]
- 28.Miklos GLG, Kelly LE, Coombe PE, Leeds C, Lefevre G. Localization of the genes Shaking-B, small optic lobes, sluggish-A, stoned and stress sensitive-C to a well-defined region on the X-chromosome of Drosophila melanogaster. J Neurogenet. 1987;4:1–19. doi: 10.3109/01677068709102329. [DOI] [PubMed] [Google Scholar]
- 29.Nonet ML, Grundahl K, Meyer BJ, Rand JB. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- 30.Owen DJ, Vallis Y, Noble MEM, Hunter JB, Dafforn TR, Evans PR, McMahon HT. A structural explanation for the binding of multiple ligands by the α-adaptin appendage domain. Cell. 1999;97:805–815. doi: 10.1016/s0092-8674(00)80791-6. [DOI] [PubMed] [Google Scholar]
- 31.Palfrey HC, Artalejo CR. Vesicle recycling revisited: rapid endocytosis may be the first step. Neuroscience. 1998;83:969–989. doi: 10.1016/s0306-4522(97)00453-3. [DOI] [PubMed] [Google Scholar]
- 32.Petrovich TZ, Merakovsky J, Kelly LE. A genetic analysis of the stoned locus and its interaction with dunce, shibire and Suppressor of stoned variants of Drosophila melanogaster. Genetics. 1993;133:955–965. doi: 10.1093/genetics/133.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reist NE, Buchanan J, Li J, DiAntonio A, Buxton EM, Schwarz TL. Morphologically docked synaptic vesicles are reduced in synaptotagmin mutants of Drosophila. J Neurosci. 1998;18:7662–7673. doi: 10.1523/JNEUROSCI.18-19-07662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 35.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain termination inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stimson DT, Estes PS, Smith M, Kelly LE, Ramaswami M. A product of the Drosophila stoned genetic locus regulates neurotransmitter release. J Neurosci. 1998;18:9638–9649. doi: 10.1523/JNEUROSCI.18-23-09638.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Südhof TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 38.Sugita S, Hata Y, Südhof TC. Distinct Ca2+-dependent properties of the first and second C2-domains of synaptotagmin 1. J Biol Chem. 1996;271:1262–1265. doi: 10.1074/jbc.271.3.1262. [DOI] [PubMed] [Google Scholar]
- 39.Takei K, McPherson PS, Schmid SL, De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-γS in nerve terminals. Nature. 1995;374:186–192. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- 40.Thurieau C, Brosius J, Burne C, Jolles P, Keen JH, Mattaliano RJ, Pingchang Chow E, Ramachandran KL, Kirchhausen T. Molecular cloning and complete amino acid sequence of AP50, an assembly protein associated with clathrin-coated vesicles. DNA. 1988;7:663–669. doi: 10.1089/dna.1988.7.663. [DOI] [PubMed] [Google Scholar]
- 41.Vallee RB, Okamoto PM. The regulation of endocytosis: identifying dynamin's binding partners. Trends Cell Biol. 1995;5:43–47. doi: 10.1016/s0962-8924(00)88937-0. [DOI] [PubMed] [Google Scholar]
- 42.van de Goor J, Ramaswami M, Kelly RB. Redistribution of synaptic vesicles and their proteins in temperature-sensitive shibirets1 mutant Drosophila. Proc Natl Acad Sci USA. 1995;92:5739–5743. doi: 10.1073/pnas.92.12.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- 44.Zhang B, Koh YH, Beckstead RB, Budnik V, Ganetzky B, Bellen HJ. Synaptic vesicles are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 45.Zhang JZ, Davletov BA, Südhof TC, Anderson RGW. Synaptotagmin 1 is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 46.Zhong Y, Wu C-F. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]