ABSTRACT
Inflammation and myocardial weakness, two major hallmarks of chronic viral myocarditis (VMC), often lead to dilated cardiomyopathy or chronic heart failure. It has been reported that indoleamine 2,3-dioxygenase-1 (IDO1) may play a pathogenic role in the progression of inflammatory diseases. Hence, the study is set out to investigate the potential role of IDO1 in chronic VMC by establishing a mouse model of VMC by intraperitoneally injected with coxsackievirus B3 (CVB3). After model establishment, the expression of IDO1 was determined by RT-qPCR and Western blot analysis. IDO1 was identified as an up-regulated gene in CVB3-induced VMC. Then, in order to elucidate the potential role of IDO1 in VMC, macrophages were isolated and treated with the overexpression plasmid of IDO1 or IDO1 inhibitor (1-MT). After that, these transfected macrophages were co-cultured with normal cardiomyocytes, followed by measurement of inflammatory factors and evaluation of cardiomyocyte injury. The overexpression of IDO1 was observed to significantly enhance the levels of interleukin (IL)-6, IL-1β and tumor necrosis factor-α (TNF-α), as well as lactate dehydrogenase (LDH) activity and malondialdehyde (MDA) content. By contrast, the treatment of 1-MT in macrophages reversed the promoting effects of IDO1 on cardiomyocyte injury. Co-culture experiment showed that overexpressed IDO1 impaired cardiomyocyte, which was alleviated upon treatment of 1-MT. Taken together, the key findings of the present study provide evidence that 1-MT-mediated IDO1 suppression could potentially reduce inflammatory response in macrophages and consequently ameliorate cardiomyocyte injury in mice with VMC.
KEYWORDS: Chronic viral myocarditis, IDO1, inflammatory response, cardiomyocyte injury, macrophages
Introduction
Myocarditis is widely considered to be a significant cause leading to heart failure and sudden cardiac death, which is in most cases due to cardiotropic viral infection besides other microbes such as protozoan, fungi and bacterial [1,2]. Viral myocarditis often occurs as a consequence of the myocardial infiltration with either leukocytes or eosinophils, which trying to recognize and clear the invasive viruses [3]. Macrophages, known as a type of leukocytes, are the main inflammatory infiltrates and exert a pathogenic effect on the progression of viral myocarditis [4]. Coxsackievirus B3 (CVB3) belongs to the genus Enterovirus, which are among the most important and common pathogens in human beings [5]. It has been suggested that CVB3 infection may result in the development of chronic viral myocarditis (VMC) that can trigger macrophages to gather around, and lead to myocardial injury and inflammation, thereby promoting dilated cardiomyopathy and chronic heart failure [6]. VMC has been reported to induce long-term morbidity and mortality due to the inflammation [7]. Despite advances in myocarditis and virus molecular detection by drug therapy and endomyocardial biopsy, the therapeutic approaches for VMC are limited yet [1,8]. It is speculated that control of the immune response caused by macrophages can alleviate VMC symptoms [9]. Interestingly, several genes in macrophages are effective against VMC; for example, melanoma differentiation-associated gene-5 (MDA5) has been suggested to be a potential therapeutic target for VMC owing to its ability to protect the heart against myocardial dysfunction and viral injury [10,11].
Indoleamine 2,3-dioxygenase (IDO) is a tryptophan catabolic enzyme that inhibits adaptive T-cell immunity; the disruption of IDO has been reported to be a promising tool that may prove to be an effective immunotherapeutic approach for cancer treatment [12]. The IDO family consists of two enzymes: IDO1 and IDO2 [13], both of which are primarily highly expressed in tumor cells, dendritic cells, macrophages and other antigen-presenting cells following microbial or viral infections [14,15]. IDO1 has been implicated in the process of the generation of tolerogenic kynurenines in human vulvovaginal candidiasis [16], which propagates immunosuppressive metabolites. IDO1 exhibits up-regulated levels in childhood acute myeloid leukemia characterized by immune dysfunction, with a negative correlation identified between its expression and disease prognosis [17]. Moreover, the activity of IDO1 promotes colitis-related tumorigenesis in mice via activating β-catenin signaling pathway, acting to subsequently accelerate cell proliferation [18]. A previous literature has indicated the pathogenic role played by IDO1 in the inflammatory processes and its influence in stimulating immune tolerance via decreasing tryptophan and inducing various toxic kynurenine metabolites [19]. Hence, the central objective of the present study was to investigate the mechanism by which 1-methyl-DL-tryptophan (1-MT)-mediated down-regulation of IDO1 in inflammatory response in macrophages and myocardial injury in a mouse VMC model.
Materials and methods
Ethics statement
The study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol of the study was approved by the Ethics Committee of China–Japan Union Hospital of Jilin University.
Establishment of VMC mice model
Eighty specific pathogen-free (SPF) BALB/c male mice (aging 4–6 weeks and weighing 14 ± 2 g), provided by the Department of Laboratory Animal Science of China–Japan Union Hospital of Jilin University, were recruited in this study. The mice were subsequently assigned into a normal group (normal mice) and a VMC group (mice established as VMC models) with 40 mice in each group. The establishment of VMC mice model was conducted according to the following [20]: the mice were injected intraperitoneally with 0.1 mL virus solution containing 1 × 102 TCID50VMC CVB3 [(Nancy strain, American Type Culture Collection (ATCC, USA))]. The normal mice were inoculated intraperitoneally with 0.1 mL Eagle culture solution (Wisent Corporation, Nanjing, China) without VMC. The day of virus inoculation was regarded as day 0. After virus inoculation, the mice in the two groups were subjected to the same controlled conditions, with the number of mice surviving the process in each group recorded every day. Successful VMC modeling was confirmed by electrocardiogram (ECG), myocardial enzymogram, hematoxylin-eosin (HE) staining, immunohistochemistry and scanning electron microscopy (SEM). After the VMC model had been confirmed to have been successfully established on the 14th day, some of the remaining mice in the VMC group were injected intraperitoneally with 1.0 mg/kg 1-MT (IDO1 inhibitor, Sigma-Aldrich Chemical Company, St Louis, MO, USA) for 7 consecutive days in order to construct a 1-MT model.
ECG
On the 3rd, 5th, 7th and 14th day, five mice in each group were randomly selected and anesthetized with 1% pentobarbital sodium (Sigma-Aldrich Chemical Company, St Louis, MO, USA) by intraperitoneal injection of 45 mg/kg dose. After the mice were observed to be quiet, they were placed in an upright position and recorded with a pre-opened electrocardiograph (Shanghai Yuyan Instruments Co., Ltd., Shanghai, China), with the standard lead II ECG recorded. The abnormal ECG indicated an accelerated or slowed heart rate, a heart rate of more than 100 per min before and after the experiment, arrhythmias, conduction block or ST changes.
Echocardiography
Five mice in each group were anesthetized by intraperitoneal injection of 20–35 mg/kg sodium pentobarbital. The heart rate (HR), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), interventricular septal thickness at end-systole (IVSs) and left ventricular posterior wall thickness (LVPW) were all subsequently measured and recorded using Philips 7500 Doppler ultrasonography. Left ventricular ejection fraction (LVEF) and left ventricular short axis shortening rate (LVFS) were then calculated. The examination was repeated three times.
Detection of myocardial enzymogram
The euthanized mice on the 14th day were collected after which their serum was isolated from the blood by centrifugation at 3000 r/min for 10 min. Myocardial enzymes consisting of aspartate aminotransferase (AST), creatine kinase (CK), lactate dehydrogenase (LDH) were determined in accordance with the instructions of the Animal-3000 biochemical analyzer (Gelite Technology Co., Ltd., Jinan, China).
HE staining
The mice were euthanized on the 14th day, after which their hearts were extracted under sterile conditions and subsequently fixed in 10% formalin (Bouin’s fixative). Subsequently, the cardiac tissues were dehydrated with gradient ethanol (50%, 70%, 80%, 90%, 95%, and 100%, respectively, 2 h/time), cleared twice with dimethylbenzene (1 h/time), embedded with paraffin, and finally cut into 5 μm sections. The tissue sections were then dried at 45°C, dehydrated using gradient ethanol, and cleared with dimethylbenzene. Thereafter, the tissue sections were stained with hematoxylin (H8070, Beijing Solarbio Science & Technology Co. Ltd., Beijing, China) for 5–15 min, differentiated by 0.5% hydrochloric-alcohol solution, and counterstained by eosin (PT001, Shanghai Bogoo Biotechnology Co. Ltd., Shanghai, China). After that, the sections were then dehydrated by gradient ethanol, cleared twice by dimethylbenzene (1 min/time), and mounted with neutral gum in ventilator cabinet. Finally, the morphological characteristics of cardiac tissues were observed under a light microscope (DMM-300D, Shanghai Caikon Optical Instrument Co. Ltd., Shanghai, China).
Immunohistochemistry (IHC)
After antigen retrieval in a citrate buffer for 10 min, the tissue sections were subsequently immersed in 3% H2O2-methanol solution for 20 min to block endogenous peroxidase activity. Then, the sections were sealed with 10% normal goat serum (36119ES03, YEASEN Biotechnology Co., Ltd., Shanghai, China) for 10 min at room temperature. The sections were then incubated with the primary polyclonal rabbit anti-mouse IDO1 (ab106134, 1: 50, Abcam Inc., Cambridge, MA, USA) overnight at 4°C and with biotin-labeled secondary goat anti-rabbit immunoglobulin G (IgG) (ab6721, 1: 1000, Abcam Inc., Cambridge, MA, USA) for 30 min at 37°C, respectively. Phosphate buffered saline (PBS) replaced primary antibody as the negative control (NC). The sections were subsequently incubated with streptomycin ovalbumin-peroxidase solution (Beijing Zhongshan Biotechnology Co., Ltd., Beijing, China) at 37°C for 20 min and developed using diaminobenzidine (DAB, P0203, Beyotime Institute of Biotechnology, Shanghai, China) for 5 min. Next, the sections were counterstained using hematoxylin for 3 min, differentiated in 1% hydrochloric acid for 5 s, turned blue, and sealed with neutral resin. Finally, five high-power fields from each section were randomly selected prior to a microscopic observation. The cells found to have more than 25% area of staining and brown or yellow-brown granules in cytoplasm or membrane were regarded as positive cells. The positive expression rate of IDO1 rate = the number of positive cells/total cells × 100%.
SEM observation of morphology of phagocyte
After euthanasia on the 14th day, mice were injected intraperitoneally with 5 mL Roswell Park Memorial Institute (RPMI) 1640 culture medium (Hyclone Laboratories, Logan, UT, USA). The peritoneal fluid was absorbed 5 min later. After centrifugation and removal of supernatant, the phagocytes were resuspended in RPMI 1640 culture medium containing 10% fetal bovine serum (FBS, Wisent St-Bruno, Quebec, Canada). Next, after the cell density was diluted to 1 × 106 cells/mL with complete culture solution, the cells were inoculated into a 37°C incubator using a culture plate with 5% CO2 for 2 h. After that, the cell metabolites as well as the few non-adherent cells were absorbed using PBS. The remaining cells were then further cultured in RPMI 1640 culture medium without 10% FBS for later use.
The phagocytes were fixed using 2.5% glutaraldehyde and stabilized at 4°C overnight. The coverslips inside were dehydrated in a stepwise manner with ethanol (30%, 50%, 70%, 80%, 90%, and 100%) and then sprayed with gold. The morphological characteristics of macrophages were observed by a Philips 505 SEM (Philips Electron Optics, The Netherlands) (8000 ×).
Enzyme-linked immunoadsorbant assay (ELISA)
The macrophages were inoculated into 96-well plates. After the cells were confirmed to have reached 70% confluence, the cell supernatant was collected. The known antigens were diluted to 1–10 ug/mL with a buffer coated with carbonate (pH 9.6), 0.1 mL of which was added to each well at 4°C overnight. On the next day, 0.1 mL diluted supernatant was added to each well for further incubation at 37°C for 1 h. Meanwhile, the blank, negative and positive wells were prepared, which was then incubated with diluted 0.1 mL enzyme-labeled secondary antibody at 37°C for 35–60 min. Each well was permitted to temporarily react with 0.1 mL tetramethylbenzidine dihydrochloride (TMB) substrate solution. After that, the optical density (OD) of each well was measured at a wavelength of 450 nm within 20 min. The concentrations of interleukin-6 (IL-6), IL-1β and tumor necrosis factor-α (TNF-α) in the supernatant were determined in accordance with the regression equation of the standard curve. All the aforementioned reagents were obtained from ELISA kit. ELISA kit for IL-6 was purchased from Shanghai Jingkang Bioengineering Co., Ltd. (Shanghai, China) and that for TNF-α and IL-1β was purchased from Shanghai Guang Rui Biological Technology Co., Ltd. (Shanghai, China). The experiment was repeated three times in an independent manner with the mean value obtained.
Cell treatment
Phagocytes in the normal group were classified into five groups: a vehicle group (normal macrophages), a NC group (macrophages transfected with negative control plasmid), an overexpression (oe)-IDO1 group (macrophages transfected with IDO1 overexpression plasmid), a 1-MT group (macrophages transfected with IDO1 inhibitor, Sigma-Aldrich Chemical Company, St Louis, MO, USA) and an oe-IDO1 + 1-MT group (macrophages co-transfected with IDO1 overexpression plasmid and IDO1 inhibitor). IDO1 overexpression plasmid was designed and synthesized by Shanghai Shengbo Biomedical Technology Co., Ltd. (Shanghai, China) based on the sequence number (accession NC_000074.6) in National Center for Biotechnology Information (NCBI) database. After treatment with 0.25% trypsin (Shanghai Chemical Reagent Company, Difeo, Detroit, MI, USA), the cells were resuspended in an RPMI1640 culture medium containing 10% FBS to reach a density of 1 × 105 cells/mL and inoculated in six-well culture plates. When the cells reached 70% confluency, the medium was replaced with serum-free RPMI 1640 culture. The cells were inoculated into six-well plates 24 h prior to transfection and then promptly transfected using the Lipofectamine 2000 (11668027, Invitrogen Corp., Carlsbad, CA, USA). In briefly, 100 µL serum-free Opti-MEM medium (31985070, Gibco, Grand Island, NY, USA) was used to dilute 6 µL Lipofectamine 2000 and an additional 100 µL serum-free Opti-MEM medium was taken to dilute 2 μg target plasmids. These were then mixed and permitted to settle at room temperature for 5 min. Next, two samples were mixed in an even manner and incubated at room temperature for 20 min. The original culture solution in six-well plates was discarded with each well subsequently added with 180 μL Opti-MEM. The transfected complex was added into the corresponding wells, followed by an 18-h period of incubation in a 37°C incubator with 5% CO2. After renewal of fresh complete medium, cells were transfected for 48 h and then collected for detection.
Evaluation of cardiomyocyte injury
The body surface of mice was disinfected using 75% ethanol, with their hearts subsequently extracted by thoracotomy and cut into tissue blocks. After digestion and centrifugation, the obtained suspension was filtered through a 200-mm mesh screen to collect the filtrate, followed by further centrifugation. After that, the precipitation was collected and resuspended in a 2 mL medium. All reagents used during this experiment were purchased from Wuhan PriCells Biomedical Technology Co., Ltd. (Wuhan, China).
Macrophages at the logarithmic growth stage were collected and then washed three times with Dulbecco’s phosphate-buffered saline (D-PBS, Shanghai Haoran Biotechnology Co., Ltd., Shanghai, China) following transfection. The macrophages were subsequently cultured with addition of 1 mL serum-free RPMI-1640 (Shanghai Beinuo Biological Technology Co., Ltd., Shanghai, China) for 1 day. Next, the culture solution was collected and centrifuged at 4°C at 1610 × g for 20 min to collect the supernatant, which used as the conditioned medium. The supernatant was then mixed with RPMI-1640 medium at a ratio of 1: 1, which was then added with freshly prepared cardiomyocyte suspension. This mixture was regarded as the co-culture system of conditioned medium and cardiomyocytes. In the next step, the macrophages were made into cell suspension and mixed with cardiomyocyte suspension, after which the mixture was seeded into six-well plates at a density of 1 × 106 cells/well for co-culture, namely the co-culture system of macrophages and cardiomyocytes. Finally, the injury of cardiomyocytes in the system was assessed using LDH and malondialdehyde (MDA) kits (Nanjing JianCheng Bioengineering Institute, Nanjing, China).
RNA isolation and quantitation
Total RNA was extracted by using the Trizol kit (15596-026, Invitrogen, Gaithersburg, MD, USA). Then, the purity and the concentration of RNA were quantified using a spectrophotometry (BioPhotometer D30, Eppendorf, Hamburg, Germany). Following this, the RNA was then reversely transcribed into cDNA by the Reverse Transcription Kit (K1621, Fermentas, Maryland, NY, USA). Next, the reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed using a SYBR Premix Ex Taq reagent kit (RR820A, Action-award Biological Technology Co., Ltd, Guangzhou, Guangdong, China) on an ABI7500 FAST real-time PCR system (Applied Biosystems, Foster City, CA, USA). β-actin was regarded as the internal reference, and the fold changes were calculated by relative quantification (2−ΔΔCt method). The primers used are depicted in Table 1, which were synthesized by Shanghai Genechem Co., Ltd (Shanghai, China).
Table 1.
Primer sequences for RT-qPCR.
| Gene | Sequence (5ʹ-3ʹ) |
|---|---|
| IDO1 | F: GCTGTTCCTTACTGCCAACT |
| R: AGCAAAGTGTCCCGTTCT | |
| β-actin | F: GACATGGAGAAGATCTGGCA |
| R: GGTCTTTACGGATGTCAACG |
RT-qPCR, reverse transcription quantitative polymerase chain reaction; IDO1, indoleamine 2,3-dioxygenase-1; F, forward; R, reverse.
Western blot analysis
Total protein was extracted by radioimmunoprecipitation assay (RIPA) lysis containing phenylmethanesulfonyl fluoride (PMSF) and then quantified using a bicinchoninic acid (BCA) protein assay kit. The proteins (10 μg) were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% separation gel and 5% spacer gel) and transferred onto nitrocellulose (NC) membranes. The membrane was blocked using Tris-buffered saline Tween-20 (TBST) buffer (pH 7.6) and 5% skim milk for 2 h at room temperature, and incubated with diluted primary rabbit anti-mouse IDO1 (1: 50, ab106134, Abcam Inc., Cambridge, MA, USA) overnight at 4°C, followed by incubation with diluted secondary goat anti-rabbit polyclonal antibody (1: 2000, ab205718, Abcam Inc., Cambridge, MA, USA) with 5% skim milk on the following day. Finally, the developer was added with the results visualized and assessed using the Bio-Rad image analysis system (MG8600, Beijing Thmorgan Biological Technology Co., Ltd., Beijing, China). β-actin was employed as the internal control with the relative protein levels expressed as the ratio of the gray values of target genes to that of internal control protein. The experiment was repeated three times independently.
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay
Cells in each group were cultured to a density of approximately 90% and subsequently constructed into a single cell suspension by trypsinization. After that, they were inoculated into 96-well culture plates at a density of 4 × 104 cells/mL, and incubated at 37°C under 5% CO2 overnight, with the medium renewed with 1% Dulbecco’s modified Eagles Medium (DMEM). After 24-h incubation, 5 mg/mL MTT solution (M1020, Beijing Solarbio Science & Technology Co. Ltd., Beijing, China) was added and incubated in a 37°C incubator for 4 h under conditions void of light. After the removal of supernatant, 200 μL dimethyl sulfoxide (DMSO, D5879-100ML, Sigma-Aldrich Chemical Company, St Louis, MO, USA) solution was added to each well to solubilize the purple crystals. Next, the OD values of each well were measured at 490 nm using a microplate reader and analyzed with the cell activity in the normal group set to 100%.
Statistical analysis
All experimental data were processed by SPSS 21.0 statistical software (IBM Corp., Armonk, NY, USA). Measurement data were expressed as mean ± standard deviation. Comparisons between two groups were performed by t-test, and comparisons among multiple groups were assessed using one-way analysis of variance (ANOVA). Enumeration data were expressed as percentage or rate, with comparisons conducted using a χ2 test. The normality of variance was evaluated using Kolmogorov–Smirnov test. The normally distributed data among multiple groups were evaluated using one-way ANOVA, followed by Tukey post-hoc test. Comparisons of data with skewed distribution were analyzed using a Kruskal–Wallis test, followed by Dunn’s post hoc test for multiple comparisons. A p < 0.05 value was considered to be indicative of statistical significance.
Results
CVB3-induced VMC mice show abnormal ECG
Mice in the normal group were observed to be active and have bright fur, free diet and movement, normal stools, with a survival rate of was 100% (40/40). On the 3rd day, the mice in the VMC group exhibited a marked decrease in activity, mental retardation, tortile and lackluster hair, slow response to stimulation, and reduced intake of food and water. On the 5th day, the VMC mice began to die with the rate of death peaking between the 7th-8th day, culminating in a survival rate of 85% (34/40).
The ECG (Figure 1, Table 2) results revealed no abnormal ECG types in the normal mice during the whole observation. However, the VMC mice were found to have atrioventricular block, abnormal ST changes and Q type of ECG on the 3rd day after virus inoculation. With the advance of time, two or more types of abnormal ECG were manifested in VMC mice, such as atrioventricular block with heart rate changes, ST changes with abnormal Q type, and abnormal ECG gradually decreased.
Figure 1.
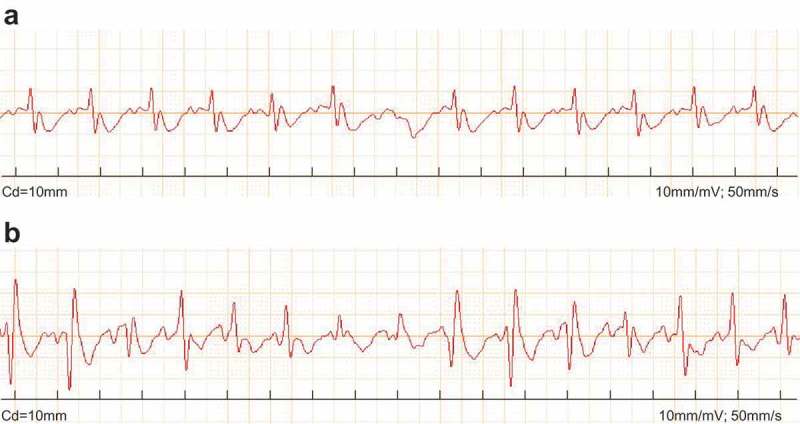
CVB3 leads to abnormal ECG in VMC mice. A, image of normal ECG; B, image of abnormal ECG; CVB3, coxsackievirus B3; ECG, electrocardiogram; VMC, viral myocarditis.
Table 2.
Abnormality detection in ECG of normal and VMC mice.
| The normal group |
The VMC group |
|||||
|---|---|---|---|---|---|---|
| Time | Case | Number of abnormal ECG | Percentage of abnormal ECG | Case | Number of abnormal ECG | Percentage of abnormal ECG |
| 3 | 5 | 0 | 0 | 5 | 1 | 20% |
| 5 | 5 | 0 | 0 | 5 | 1 | 20% |
| 7 | 5 | 0 | 0 | 5 | 2 | 40% |
| 14 | 5 | 0 | 0 | 5 | 2 | 40% |
| Total | 20 | 0 | 0 | 20 | 6 | 30% |
* p< 0.05 vs. normal mice without any treatment; statistical data were enumeration data and analyzed using a χ2 test; n = 20 in VMC and normal groups; the experiment was repeated 3 times independently. ECG, electrocardiogram; VMC, viral myocarditis.
The aforementioned findings revealed marked ECG abnormalities in the VMC mice following CVB3 treatment.
1-MT treatment contributes to the improvement of cardiac function
Our subsequent investigation revealed that HR, LVEDD, LVESD, IVSs and LVPW of the VMC mice were much higher than that of normal mice (p < 0.05). Compared with VMC mice, HR, LVEDD, LVESD, IVSs, and LVPW were lower in 1-MT mice (p < 0.05). Besides, LVEF and LVFS of VMC mice were lower than those of normal mice, and LVEF and LVFS of 1-MT-treated mice exhibited elevated levels when compared with VMC mice (Table 3), ultimately suggesting that 1-MT could improve cardiac function.
Table 3.
Cardiac function detection in normal, VMC and 1-MT mice.
| Normal | VMC | 1-MT | |
|---|---|---|---|
| HR (bpm) | 485.20 ± 27.57 | 613.9 ± 36.84* | 552.36 ± 37.52# |
| LVEDD (mm) | 2.25 ± 0.13 | 3.54 ± 0.18* | 2.71 ± 0.22# |
| LVESD (mm) | 1.38 ± 0.07 | 2.15 ± 0.13* | 1.74 ± 0.12# |
| IVSs (mm) | 1.18 ± 0.06 | 2.05 ± 0.14* | 1.57 ± 0.13# |
| LVPW (mm) | 0.71 ± 0.06 | 1.43 ± 0.15* | 0.92 ± 0.08# |
| LVEF (%) | 68.49 ± 9.32 | 33.21 ± 6.52* | 55.13 ± 8.26# |
| LVFS (%) | 39.68 ± 5.63 | 15.37 ± 2.12* | 25.35 ± 4.18# |
* p< 0.05 vs. normal mice without any treatment; # p< 0.05 vs. CVB3-induced VMC mice; statistical data were enumeration data and analyzed using one-way ANOVA; n = 40 in the normal group, n = 20 in the VMC group; n = 14 in the 1-MT group; the experiment was repeated 3 times independently; VMC, viral myocardit; HR, heart rate; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; IVSs, interventricular septal thickness at end-systole; LVPW, left ventricular posterior wall thickness; LVEF, left ventricular ejection fraction; LVFS, left ventricular short axis shortening rate.
1-MT treatment improves the impaired cardiomyocytes in CVB3-induced VMC mice
The morphological changes of myocardial tissues in the VMC mice were observed by HE staining. As depicted in Figure 2, cardiomyocytes in normal mice were observed to be uniformly arranged, with a compact intercellular space, with no vacuolar degeneration and monocyte infiltration detected. However, cardiomyocytes in VMC mice exhibited interstitial edema, fissure enlargement, turbidity and swelling, unclear transverse striation, and focal inflammatory infiltration. The cardiomyocytes in 1-MT treated VMC mice displayed a diminished degree of inflammatory infiltration, congestion, edema, and necrosis, indicating that the cardiomyocyte injury had been significantly ameliorated.
Figure 2.
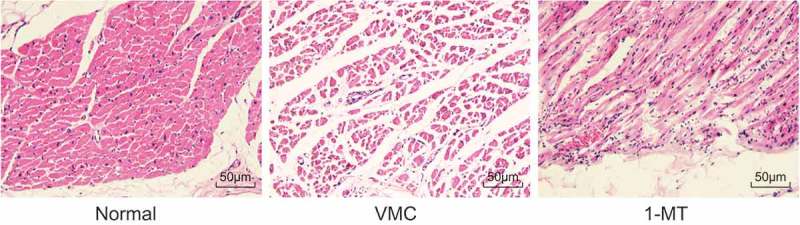
Observation of cardiomyocyte characteristics in normal, VMC and 1-MT treated VMC mice by HE staining (× 400). VMC, viral myocarditis; 1-MT was the inhibitor of IDO1.
The results obtained suggested that the severe cardiomyocyte injury in the VMC mice could be relieved by treatment with 1-MT.
1-MT treatment reduces the level of myocardial enzymogram in CVB3-induced VMC mice
The myocardial enzymogram was subsequently determined, the results (Figure 3) of which revealed that the activity of AST, CK, and LDH in VMC mice was higher than that in normal mice (p < 0.05). Compared with VMC mice, the activity of AST, CK, and LDH in 1-MT treated mice was lower (p < 0.05). The results obtained led us to conclude that the VMC mice were suffering from a metabolic disorder in myocardium and 1-MT treatment may normalize the level of myocardial enzymogram.
Figure 3.
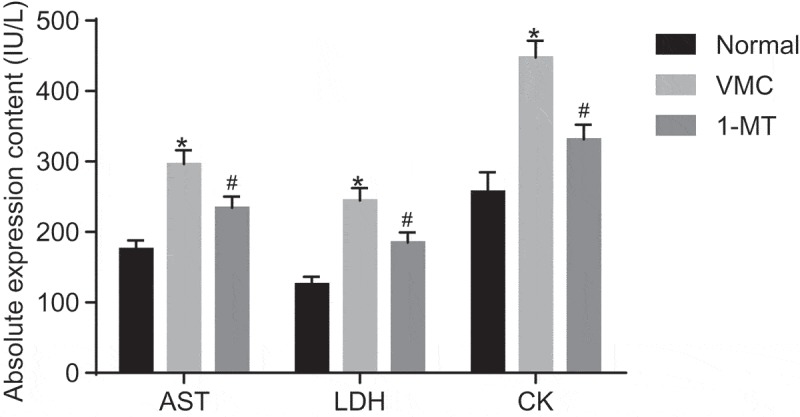
1-MT treatment reduces myocardial enzymogram in VMC mice. * p< 0.05 vs. normal mice without any treatment; # p< 0.05 vs. CVB3-induced VMC mice. The unit was IU/L; Statistical values were measurement data and analyzed using one-way ANOVA. n = 40 in the VMC group. n = 20 in the normal group. n = 14 in the 1-MT group. 1-MT was the inhibitor of IDO1. The experiment was repeated three times independently. VMC, viral myocarditis; CVB3, coxsackievirus B3; AST, aspartate aminotransferase; CK, creatine kinase; LDH, lactate dehydrogenase; ANOVA, analysis of variance.
1-MT treatment reduces the activation of macrophages in CVB3-induced VMC mice
The macrophage morphological characteristics were analyzed by means of SEM (Figure 4). The normal macrophages were observed to be generally spherical, small in size, with few protuberances on the surface and no pseudopods. However, compared with normal ones, macrophages in VMC mice displayed marked overactivation, which was highlighted by a significant increase in volume, irregular shape, a large area of clear protuberances on the surface, increased adherence area, and a large number of pseudopods on the cell surface. 1-MT treated VMC mice also exhibited activated macrophages, and the size was reduced and the number of pseudopods decreased significantly.
Figure 4.
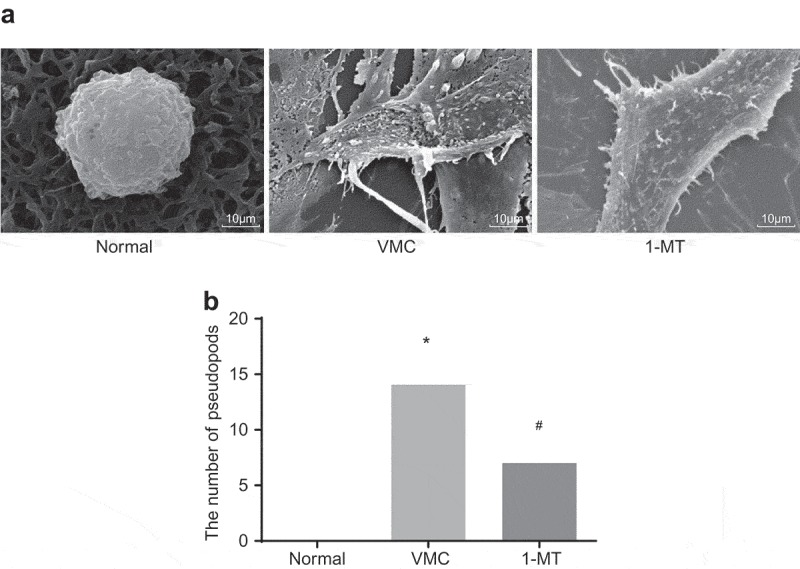
1-MT treatment impairs the activation of macrophages in VMC mice (× 8000). A, images of macrophages in abdominal cavity in normal, VMC and 1-MT treated VMC mice observed by SEM; B, the quantitative analysis of the number of pseudopods in normal, VMC and 1-MT treated VMC mice; * p< 0.05 vs. normal mice without any treatment; # p< 0.05 vs. CVB3-induced VMC mice. Statistical values were measurement data and analyzed using one-way ANOVA. n = 20 in the VMC group. n = 40 in the normal group. n = 14 in the 1-MT group. 1-MT was the inhibitor of IDO1. The experiment was repeated 3 times independently. VMC, viral myocarditis; SEM, scanning electron microscopy; CVB3, coxsackievirus B3; ANOVA, analysis of variance.
The results obtained demonstrated that CVB3 could activate macrophages in mice, and 1-MT could reduce the activation.
1-MT treatment diminishes the expression of IDO1 protein in CVB3-induced VMC mice
The positive expression rate of IDO1 protein was determined by means of IHC (Figure 5). The IDO1 protein was found to be mainly expressed in the cytoplasm and membrane of macrophages, and was represented by the brown and yellow granules. In the VMC mice, the brown and yellow granules were markedly increased and much darker than those in normal group, in which myocardial tissues were obviously infiltrated by macrophages. From a statistical point of view, the positive expression rate of IDO1 protein in VMC mice (71.28% ± 7.35%) was notably higher than that in normal mice (16.42% ± 2.75%) (p < 0.05). Compared with VMC mice, 1-MT treated VMC mice exhibited immune cell infiltration in the myocardial tissues, and the number and color of the yellow granules were distinctly diminished, with the expression of IDO1 found to be decreased (37.62% ± 4.26%) (p < 0.05).
Figure 5.
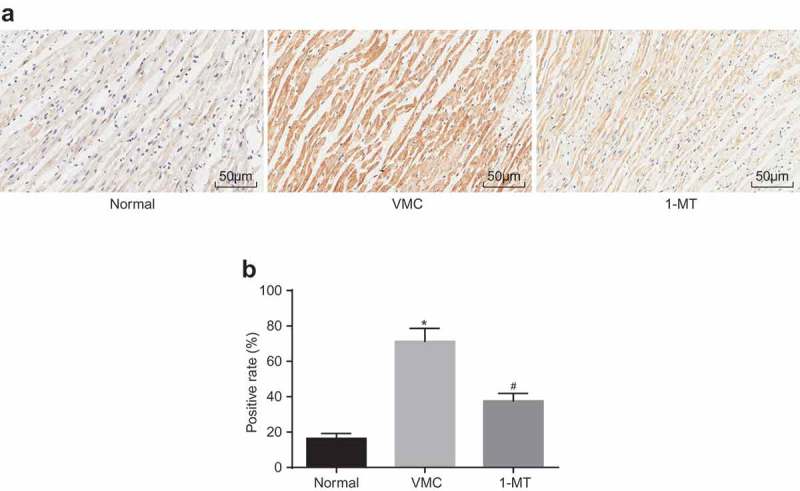
1-MT treatment decreases the up-regulation of IDO1 in VMC mice. A, IHC staining of IDO1 protein in myocardial tissues in normal, VMC and 1-MT treated VMC mice (× 200); B, the positive expression rate of IDO1 in normal, VMC and 1-MT treated VMC mice; * p< 0.05 vs. normal mice without any treatment; # p< 0.05 vs. CVB3-induced VMC mice. Statistical values were measurement data and analyzed using one-way ANOVA. n = 20 in the VMC group. n = 40 in the normal group. n = 14 in the 1-MT group. 1-MT was the inhibitor of IDO1. The experiment was repeated 3 times independently. VMC, viral myocarditis; IDO1, indoleamine 2,3-dioxygenase-1; IHC, Immunohistochemistry; CVB3, coxsackievirus B3; ANOVA, analysis of variance.
Based on the aforementioned results, we concluded that IDO1 was expressed at a high level in VMC mice and 1-MT could reduce the level of IDO1 and reduce macrophage infiltration.
IDO1 is highly expressed in macrophages in CVB3-induced VMC mice
RT-qPCR and Western blot analysis were employed to detect the expression of IDO1 in macrophages isolated from VMC mice, the results of which showed (Figure 6) that compared with VMC mice, the mRNA expression of IDO1 in macrophages in normal and 1-MT treated VMC mice decreased significantly (p < 0.05). The Western blot analysis results revealed that compared with VMC mice, the level of IDO1 protein in normal and 1-MT treated VMC mice was found to be significantly decreased (p < 0.05). The aforementioned results suggested that CVB3 infection could trigger an increase in the expression of IDO1 in macrophages, while 1-MT could attenuate the effect of CVB3 on IDO1.
Figure 6.
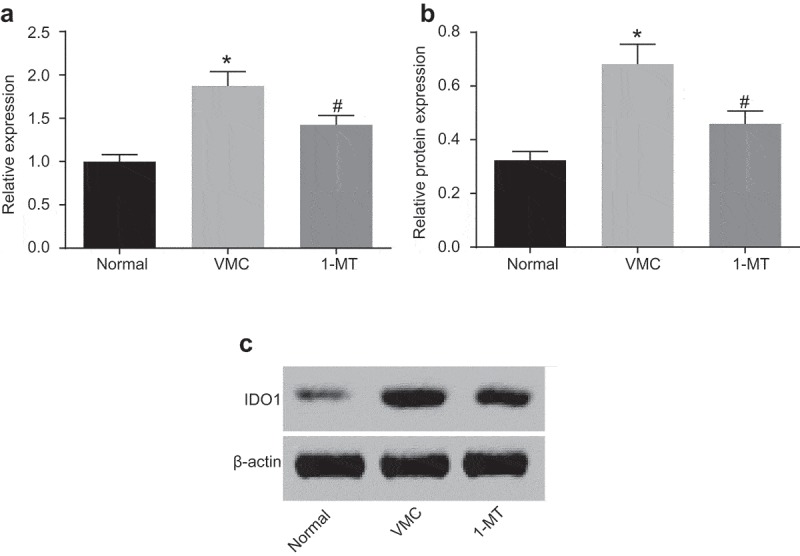
1-MT inhibits the expression of IDO1 in macrophages in VMC mice. A, the mRNA expression of IDO1 in macrophages in normal, VMC and 1-MT treated VMC mice; B and C, protein levels and bands of IDO1 in macrophages in normal, VMC and 1-MT treated VMC mice; * p< 0.05 vs. normal mice without any treatment; # p< 0.05 vs. CVB3-induced VMC mice. Statistical values were measurement data and analyzed using one-way ANOVA. n = 20 in the VMC group. n = 40 in the normal group. n = 14 in the 1-MT group. 1-MT was the inhibitor of IDO1. The experiment was repeated 3 times independently. VMC, viral myocarditis; IDO1, indoleamine 2,3-dioxygenase-1; CVB3, coxsackievirus B3; ANOVA, analysis of variance.
1-MT treatment enhances the viability of macrophages in CVB3-induced VMC mice
The effects of 1-MT on the viability of peritoneal macrophages in VMC mice were determined by MTT (Figure 7). Compared with normal mice, the viability of peritoneal macrophages in VMC mice was significantly decreased. Whereas the viability of peritoneal macrophages in 1-MT treated VMC mice was increased when compared with that in VMC mice (p < 0.05). Hence, we concluded that that CVB3 could decrease the viability of macrophages, while 1-MT can increase the viability.
Figure 7.
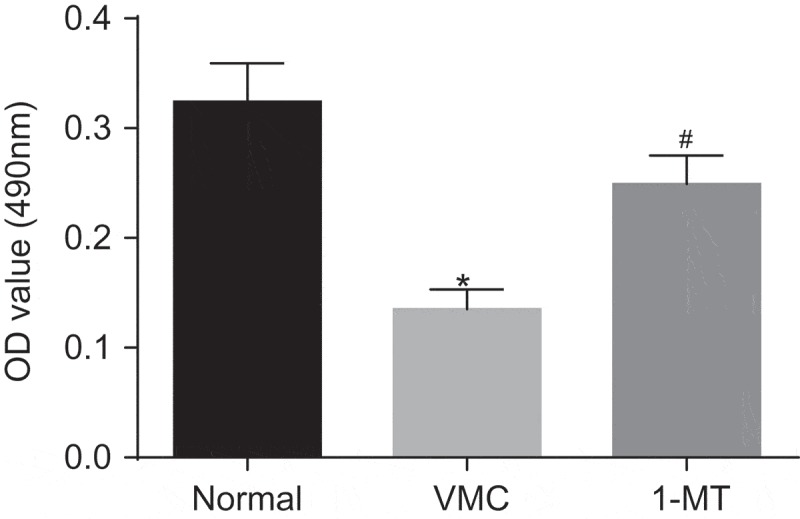
1-MT promotes the viability of macrophages in VMC mice. * p< 0.05 vs. normal mice without any treatment; # p< 0.05 vs. CVB3-induced VMC mice. Statistical values were measurement data and analyzed using one-way ANOVA. n = 20 in the VMC group. n = 40 in the normal group. n = 14 in the 1-MT group. 1-MT was the inhibitor of IDO1. The experiment was repeated 3 times independently. VMC, viral myocarditis; IDO1, indoleamine 2,3-dioxygenase-1; CVB3, coxsackievirus B3; OD, optical density; ANOVA, analysis of variance.
1-MT reduces the expression of IDO1 in macrophages
In order to elucidate the effects of 1-MT on IDO1, RT-qPCR and Western blot analysis were performed to examine the expression of IDO1 in macrophages treated with vehicle, NC, oe-IDO1, 1-MT, and oe-IDO1 + 1-MT. As illustrated in Figure 8, the mRNA and protein levels of IDO1 weren’t significantly different in the macrophages treated with vehicle, NC and oe-IDO1 + 1-MT (p> 0.05). In comparison with negative control groups, both the mRNA and protein levels of IDO1 in the cells treated with oe-IDO1 were significantly elevated (p< 0.05), and notable decreases in the cells transfected with 1-MT were identified (p< 0.05). Taken together, the results obtained confirmed the hypothesis that IDO1 expression in macrophages after transfection could be used for the subsequent experiments.
Figure 8.
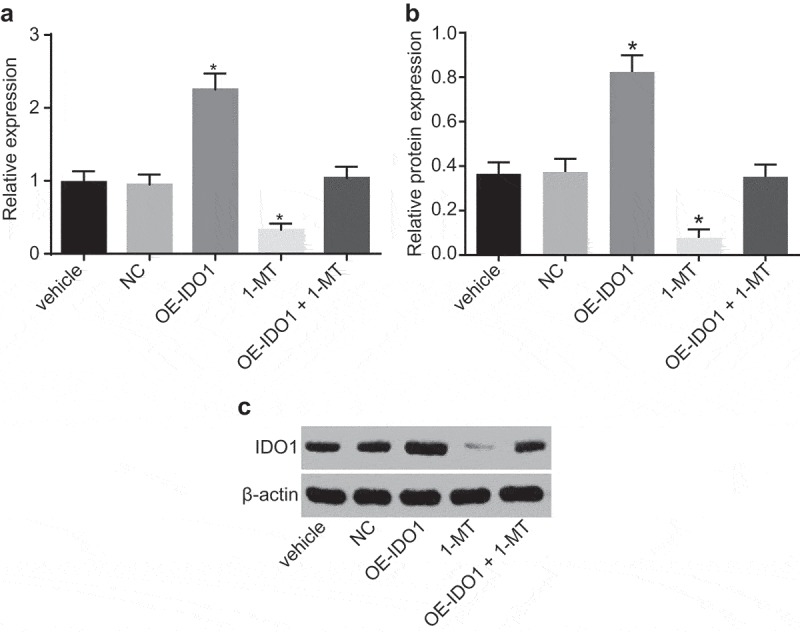
1-MT decreases IDO1 expression in macrophages. A, the mRNA expression of IDO1 in macrophages treated with vehicle, NC, oe-IDO1, 1-MT and oe-IDO1 + 1-MT; B and C, protein level and bands of IDO1 in macrophages treated with vehicle, NC, oe-IDO1, 1-MT and oe-IDO1 + 1-MT; * p< 0.05 vs. the vehicle group. Statistical values were measurement data and analyzed using one-way ANOVA. n = 3. The experiment was repeated 3 times independently. VMC, viral myocarditis; IDO1, indoleamine 2,3-dioxygenase-1; NC, negative control; oe, overexpression; 1-MT was the inhibitor of IDO1; ANOVA, analysis of variance.
Up-regulation of IDO1 over-activates macrophages
In order to identify the influence of IDO1 on macrophages, SEM was employed to analyze the morphological characteristics of macrophages in response to different treatment (Figure 9). The morphology of macrophages treated with vehicle, NC and oe-IDO1 + 1-MT was similar to that of the cells treated with 1-MT, presenting spherical shape, small size, few protuberances on the surface and no pseudopods. However, regarding the overexpression of IDO1, the macrophages displayed signs of over-activation, notably increased size, irregular shape, and pseudopods on the surface. The aforementioned results indicated that overexpressed IDO1 could over-activate macrophages, while down-regulated IDO1 could rescue the macrophages and return them to their normal status.
Figure 9.
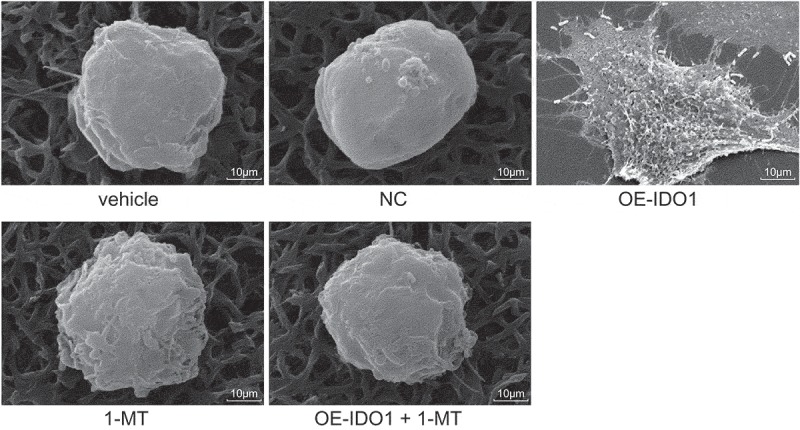
Observation of macrophages treated with vehicle, NC, oe-IDO1, 1-MT and oe-IDO1 + 1-MT by SEM (× 8000). IDO1, indoleamine 2,3-dioxygenase-1; NC, negative control; oe, overexpression; 1-MT was the inhibitor of IDO1; SEM, scanning electron microscopy.
Up-regulation of IDO1 in macrophages increases levels of inflammatory factors
ELISA was performed to investigate the possible effects associated with IDO1 on inflammatory factors (Figure 10). The levels of IL-6, IL-1β, and TNF-α in macrophages treated with vehicle, NC and oe-IDO1 + 1-MT were found with no significant difference (p> 0.05). Compared with macrophages treated with vehicle and NC, the levels of IL-6, IL-1β and TNF-α in response to overexpression of IDO1 were increased significantly (p < 0.05), highlighting a stimulated inflammatory response, while the levels of IL-6, IL-1β and TNF-α were markedly diminished following 1-MT treatment (p < 0.05), suggesting the inflammatory response was reversed by 1-MT. Hence, based on our results we concluded that up-regulated IDO1 expression could heighten the levels of inflammatory factors and enhance the inflammatory response. However, the reduction of IDO1 expression could diminish the inflammatory response.
Figure 10.
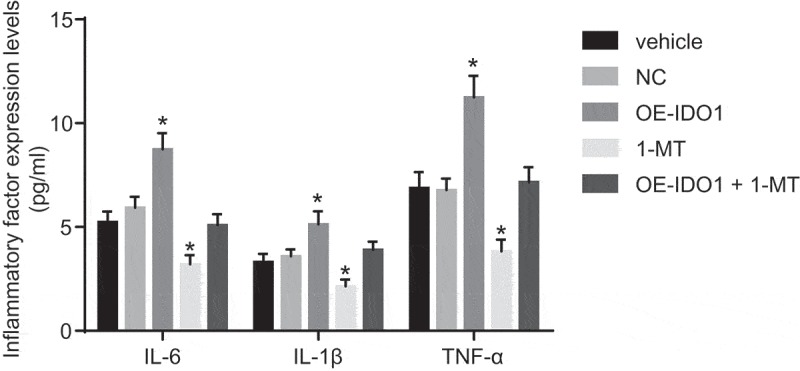
Up-regulation of IDO1 increases levels of inflammatory factors in macrophages (pg/mL). * p< 0.05 vs. the vehicle group. Statistical values were measurement data and analyzed using one-way ANOVA. n = 3. The experiment was repeated 3 times independently. IDO1, indoleamine 2,3-dioxygenase-1; NC, negative control; oe, overexpression; 1-MT was the inhibitor of IDO1; IL, interleukin; TNF-α, tumor necrosis factor-α; ANOVA, analysis of variance.
Up-regulation of IDO1 in macrophages impairs cardiomyocytes
In an attempt to detect the potential effects of over-expressed IDO1 in the conditioned medium on cardiomyocytes, LDH activity and MDA content in medium were evaluated. There were no notable changes in terms of LDH activity (U/L) and MDA content (nmol/mg) in the conditioned medium in response to vehicle and NC treatment (p > 0.05). Compared with vehicle and NC groups, LDH activity and MDA content in the conditioned medium in response to oe-IDO1 treatment were increased (p < 0.05), highlighting that cardiomyocytes were indeed injured, whereas the LDH activity and MDA content decreased in response to 1-MT treatment (p < 0.05), which was suggestive of cardiomyocytes injury alleviation (p < 0.05, Figure 11(A-B)).
Figure 11.
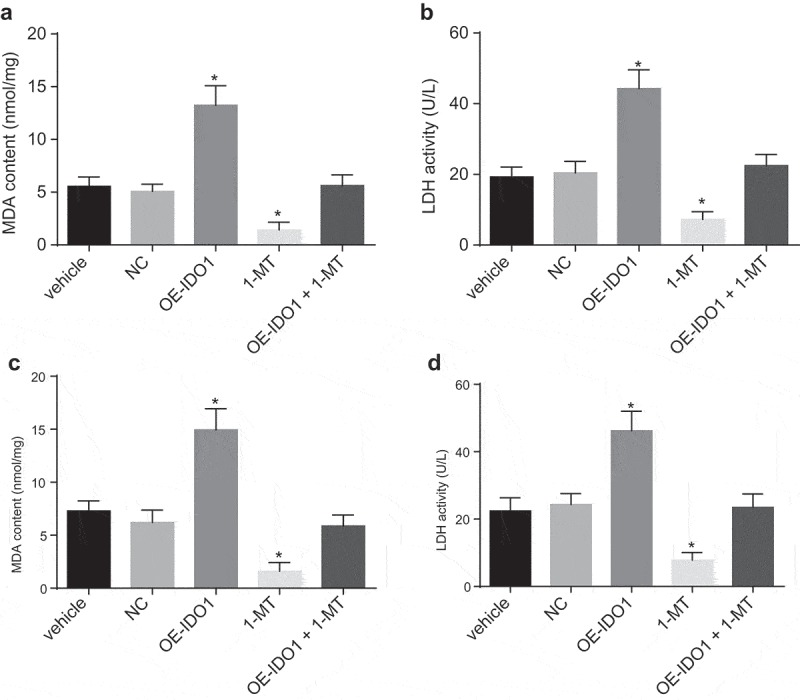
IDO1 up-regulation in macrophages causes cardiomyocytes injury. A and B, LDH activity and MDA content in the conditioned medium in response to the treatment of vehicle, NC, oe-IDO1, 1-MT and oe-IDO1 + 1-MT; C and D, LDH activity and MDA content in the co-culture system in response to the treatment of vehicle, NC, oe-IDO1, 1-MT and oe-IDO1 + 1-MT; * p< 0.05 vs. the vehicle group. Statistical values were measurement data and analyzed using one-way ANOVA. n = 3. The experiment was repeated 3 times independently. 1-MT was the inhibitor of IDO1; IDO1, indoleamine 2,3-dioxygenase-1; NC, negative control; oe, overexpression; LDH, lactate dehydrogenase; MDA, malondialdehyde; ANOVA, analysis of variance.
We subsequently set out to further investigate LDH activity (U/L) and MDA content (nmol/mg) in the co-culture system. In comparison to the vehicle and NC groups, LDH activity and MDA content in the co-culture system following oe-IDO1 treatment were increased (p < 0.05), which was indicative of cardiomyocytes injury, while LDH activity and MDA content decreased upon cultured with 1-MT-treated macrophages (p < 0.05), which was suggestive of cardiomyocytes injury alleviation (p < 0.05, Figure 11(C-D)).
Taken together, IDO1 up-regulation in normal macrophages could act to profoundly impair cardiomyocytes and 1-MT treatment and alleviate cardiomyocyte injury in response to up-regulated IDO1.
Discussion
Accompanied by severe myocardial inflammation, VMC is recognized as a chronic life-threatening disease and can initiate the loss of heart functions, remodeling process and the development of fibrosis in healthy adults [21,22]. Patients suffering from VMC usually exhibit severe myocardial injury, impaired left ventricular function, as well as an elevated inflammatory response, often resulting in a diminished survival rate [23]. Promisingly, therapeutic inhibition of IDO1 has been reported to be a feasible therapeutic method capable of treating immunodeficiency-associated abnormalities by diminishing cellular immunity [24]. Collectively, the findings of the study revealed that IDO1 was highly expressed in CVB3-induced VMC mouse models, and 1-MT-mediated down-regulation of IDO1 was found to prevent myocardial injury by inhibiting the inflammatory response in macrophages.
Based on our findings, IDO1 was found to be expressed at a high level in VMC mice induced by CVB3. Consistently, previous reports have indicated that IDO1 is up-regulated in non-Hodgkin lymphoma [25]. Up-regulation of IDO1 has been highlighted in the microglia in the cerebellum of mice with acute viral encephalitis [26]. IDO1 represents an interferon-γ-induced metabolic enzyme, capable of degrading tryptophan into kynurenine, which subsequently hampers effector T cells and stimulates regulatory T-cell (Treg) differentiation [17]. The overexpression of IDO1 has been demonstrated to play a contributory role in the inhibition of the immune system in cases of multiple myeloma, a plasma cell malignancy accompanied by immune dysfunction [27]. Cesario et al. revealed that inhibition of cyclooxygenase (COX)-2 by IDO1 overexpression engenders the chronic inflammation development [28]. Besides, IDO1 has been reported to exhibit up-regulated levels in 2,4,6-trinitrobenzene sulfate (TNBS)-induced colitis mice [29]. Therefore, IDO1 is a regulator to immune system and play a key role in inflammation occurrence in cardiomyocytes.
The up-regulation of IDO1 can over-activate macrophages in VMC mice. As major regulators of inflammation, macrophages with a high degree of plasticity are able to differentiate into M1 (classically activated) or M2 (alternatively activated) macrophages [4]. The current study demonstrated that the overexpression of IDO1 in macrophages could activate inflammatory response. Besides macrophages, similar inflammation responses have been observed in other cells. For instance, a recent study demonstrates that dysregulated expression of IDO1 in endometrial stromal cells contributes to inflammatory response, which subsequently stimulates the activation of immunosuppressive macrophages in endometriosis [30]. IDO has been shown to enhance the survival and invasiveness of endometrial stromal cells by activating the JNK signaling pathway [31]. In human colon cancer cells, blockade of IDO1 activity has been shown to reduce nuclear and activated b-catenin, transcription of its target genes (cyclin D1 and Axin2), and ultimately, proliferation [18]. Moreover, Epstein-Barr virus infections have been reported to induce IDO1 in macrophages through p38 and NF-κB pathways, and the inhibition of the two pathways abrogates TNF-α and IL-6 production and inhibits IDO production [32]. Consistently, ELISA analysis revealed that IDO1 increased the levels of inflammatory factors IL-6, IL-1β and TNF-α in macrophages. Also, after viral infection, patients with chronic myocarditis exhibit significantly increased levels of TNF-α, IL-6, and IL-1β in cardiac fibroblasts [30,33]. Thus, we arrived at the conclusion that IDO1 increased the levels of inflammation-related factors and then intensified inflammatory response.
Additionally, up-regulated IDO1 in macrophages leads to the impairment of cardiomyocytes, as evidenced by elevated LDH activity and MDA content. It has been demonstrated that LDH activity and MDA content are overexpressed in cardiomyopathy, and the decrease of which exerts antioxidant and cardioprotective effects [34]. LDH activity and MDA content are elevated following the gradual increase of clenbuterol dosage, which acts to generate myocardial injury and reduce the capacity of myocardial anti-oxidation [35], suggesting that LDH activity and MDA content are indicators of cardiomyocyte function. Previous reports have suggested that the up-regulation of IDO1 is associated with an increased in serum LDH level in non-Hodgkin lymphoma [25], while MDA content has been indicated to exhibit increases in mice with viral myocarditis [36]. Hence, treatment with overexpressed IDO1 enhances LDH activity and MDA content, and consequently triggers cardiomyocyte impairment.
1-MT is the common inhibitor of IDO1 expression found in numerous pathologies, such as chronic infection, allergy, autoimmunity, and cancer [37], which is a tryptophan mimetic chemical and hinders the transcription of the enzyme, thereby blocking its immunosuppressive effects [38]. Specifically, 1-MT can act to suppress the expression and activity IDO1 not only in macrophages but also in other professional antigen-presenting cells such as dendritic cells, which induce and coordinate immune responses [12,39]. 1-MT functions suppress metabolic processes including glucose uptake, mitochondrial function, and induce aerobic glycolysis and decrease immunosuppressive cytokine IL-10 production in human lymphocytes [40]. In addition, 1-MT-treated mice with acute viral myocarditis exhibited a greater rate of survival in addition to a suppressed viral load and myocardial injury (decreased CK and LDH activities) [14]. These findings demonstrated that 1-MT could act to restrain the stimulatory effects of IDO1 on myocardial injury by inhibiting the inflammatory response in VMC.
In conclusion, the key findings of the current study present evidence indicating that IDO1 gene silencing induced by 1-MT treatment reduced the inflammatory response of macrophages, which ultimately acts to alleviate myocardial injury in mice with VMC. Our study highlights the potential of IDO1 modulation as a potential strategy for treating VMC.
Acknowledgments
The authors thank the reviewers for their helpful comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Pollack A, Kontorovich AR, Fuster V, et al. Viral myocarditis–diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–680. [DOI] [PubMed] [Google Scholar]
- [2].Spitzbarth-Regrigny E, Petitcolin MA, Bueb JL, et al. Pertussis toxin-sensitive G(i)-proteins and intracellular calcium sensitivity of vasoconstriction in the intact rat tail artery. Br J Pharmacol. 2000;131:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huber SA. Viral myocarditis and dilated cardiomyopathy: etiology and pathogenesis. Curr Pharm Des. 2016;22:408–426. [DOI] [PubMed] [Google Scholar]
- [4].Zhang Y, Zhang M, Li X, et al. Silencing MicroRNA-155 attenuates cardiac injury and dysfunction in viral myocarditis via promotion of M2 phenotype polarization of macrophages. Sci Rep. 2016;6:22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lim BK, Ju ES, Lao DH, et al. Development of a enterovirus diagnostic assay system for diagnosis of viral myocarditis in humans. Microbiol Immunol. 2013;57:281–287. [DOI] [PubMed] [Google Scholar]
- [6].Helluy X, Sauter M, Ye YX, et al. In vivo T2* weighted MRI visualizes cardiac lesions in murine models of acute and chronic viral myocarditis. PLoS One. 2017;12:e0172084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakamura H, Kunitsugu I, Fukuda K, et al. Diverse stage-dependent effects of glucocorticoids in a murine model of viral myocarditis. J Cardiol. 2013;61:237–242. [DOI] [PubMed] [Google Scholar]
- [8].Zhang YY, Li JN, Xia HH, et al. Protective effects of losartan in mice with chronic viral myocarditis induced by coxsackievirus B3. Life Sci. 2013;92:1186–1194. [DOI] [PubMed] [Google Scholar]
- [9].Fairweather D, Cihakova D.. Alternatively activated macrophages in infection and autoimmunity. J Autoimmun. 2009;33:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fairweather D, Frisancho-Kiss S, Yusung SA, et al. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Philip J, Xu Z, Bowles NE, et al. Cardiac-specific overexpression of melanoma differentiation-associated gene-5 protects mice from lethal viral myocarditis. Circ Heart Fail. 2013;6:326–334. [DOI] [PubMed] [Google Scholar]
- [12].Sun T, Chen XH, Tang ZD, et al. Novel 1-alkyl-tryptophan derivatives downregulate IDO1 and IDO2 mRNA expression induced by interferon-gamma in dendritic cells. Mol Cell Biochem. 2010;342:29–34. [DOI] [PubMed] [Google Scholar]
- [13].Croitoru-Lamoury J, Lamoury FM, Caristo M, et al. Interferon-gamma regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase (IDO). PLoS One. 2011;6:e14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hoshi M, Matsumoto K, Ito H, et al. L-tryptophan-kynurenine pathway metabolites regulate type I IFNs of acute viral myocarditis in mice. J Immunol. 2012;188:3980–3987. [DOI] [PubMed] [Google Scholar]
- [15].Cheong JE, Ekkati A, Sun L. A patent review of IDO1 inhibitors for cancer. Expert Opin Ther Pat. 2018;28:317–330. [DOI] [PubMed] [Google Scholar]
- [16].De Luca A, Carvalho A, Cunha C, et al. IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis. PLoS Pathog. 2013;9:e1003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Folgiero V, Goffredo BM, Filippini P, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity in leukemia blasts correlates with poor outcome in childhood acute myeloid leukemia. Oncotarget. 2014;5:2052–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Thaker AI, Rao MS, Bishnupuri KS, et al. IDO1 metabolites activate beta-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology. 2013;145(416–425):e411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li F, Zhang R, Li S, et al. IDO1: An important immunotherapy target in cancer treatment. Int Immunopharmacol. 2017;47:70–77. [DOI] [PubMed] [Google Scholar]
- [20].Li-Sha G, Xing-Xing C, Lian-Pin W, et al. Right cervical vagotomy aggravates viral myocarditis in mice via the cholinergic anti-inflammatory pathway. Front Pharmacol. 2017;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gruhle S, Sauter M, Szalay G, et al. The prostacyclin agonist iloprost aggravates fibrosis and enhances viral replication in enteroviral myocarditis by modulation of ERK signaling and increase of iNOS expression. Basic Res Cardiol. 2012;107:287. [DOI] [PubMed] [Google Scholar]
- [22].Papageorgiou AP, Heymans S. Interactions between the extracellular matrix and inflammation during viral myocarditis. Immunobiology. 2012;217:503–510. [DOI] [PubMed] [Google Scholar]
- [23].Yue-Chun L, Guang-Yi C, Li-Sha G, et al. The protective effects of ivabradine in preventing progression from viral myocarditis to dilated cardiomyopathy. Front Pharmacol. 2016;7:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu X, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–3530. [DOI] [PubMed] [Google Scholar]
- [25].Liu XQ, Lu K, Feng LL, et al. Up-regulated expression of indoleamine 2,3-dioxygenase 1 in non-Hodgkin lymphoma correlates with increased regulatory T-cell infiltration. Leuk Lymphoma. 2014;55:405–414. [DOI] [PubMed] [Google Scholar]
- [26].Taguchi A, Niwa M, Hoshi M, et al. Indoleamine 2,3-dioxygenase 1 is upregulated in activated microglia in mice cerebellum during acute viral encephalitis. Neurosci Lett. 2014;564:120–125. [DOI] [PubMed] [Google Scholar]
- [27].Bonanno G, Mariotti A, Procoli A, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) activity correlates with immune system abnormalities in multiple myeloma. J Transl Med. 2012;10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cesario A, Rocca B, Rutella S. The interplay between indoleamine 2,3-dioxygenase 1 (IDO1) and cyclooxygenase (COX)-2 in chronic inflammation and cancer. Curr Med Chem. 2011;18:2263–2271. [DOI] [PubMed] [Google Scholar]
- [29].Takamatsu M, Hirata A, Ohtaki H, et al. IDO1 plays an immunosuppressive role in 2,4,6-trinitrobenzene sulfate-induced colitis in mice. J Immunol. 2013;191:3057–3064. [DOI] [PubMed] [Google Scholar]
- [30].Mei J, Chang KK, Sun HX. Immunosuppressive macrophages induced by IDO1 promote the growth of endometrial stromal cells in endometriosis. Mol Med Rep. 2017;15:2255–2260. [DOI] [PubMed] [Google Scholar]
- [31].Mei J, Li MQ, Ding D, et al. Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway. Int J Clin Exp Pathol. 2013;6:431–444. [PMC free article] [PubMed] [Google Scholar]
- [32].Liu WL, Lin YH, Xiao H, et al. Epstein-Barr virus infection induces indoleamine 2,3-dioxygenase expression in human monocyte-derived macrophages through p38/mitogen-activated protein kinase and NF-kappaB pathways: impairment in T cell functions. J Virol. 2014;88:6660–6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang JF, Meissner A, Malek S, et al. Propranolol ameliorates and epinephrine exacerbates progression of acute and chronic viral myocarditis. Am J Physiol Heart Circ Physiol. 2005;289:H1577–1583. [DOI] [PubMed] [Google Scholar]
- [34].Tang Y, Wang M, Le X, et al. Antioxidant and cardioprotective effects of Danshensu (3-(3, 4-dihydroxyphenyl)-2-hydroxy-propanoic acid from Salvia miltiorrhiza) on isoproterenol-induced myocardial hypertrophy in rats. Phytomedicine. 2011;18:1024–1030. [DOI] [PubMed] [Google Scholar]
- [35].Benge ZHL, Kedong B, et al. Effects of clenbuterol on leaves of LDH and antioxidant capacity of myocardium in mice.[J]. Chin J Anim Health Inspection. 2009;26:40–41. [Google Scholar]
- [36].Pei-Yong Z, Qin ZF, Xiao W. Influence of Yi Xin Drink to SOD and MDA activity in mice with viral myocarditis[J]. Tianjin J Traditional Chin Med. 2004;21:187. [Google Scholar]
- [37].Metz R, Smith C, DuHadaway JB, et al. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immunol. 2014;26:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Salvadori ML, Da Cunha Bianchi PK, Gebrim LH, et al. Effect of the association of 1-methyl-DL-tryptophan with paclitaxel on the expression of indoleamine 2,3-dioxygenase in cultured cancer cells from patients with breast cancer. Med Oncol. 2015;32:248. [DOI] [PubMed] [Google Scholar]
- [39].Adikari SB, Lian H, Link H, et al. Interferon-gamma-modified dendritic cells suppress B cell function and ameliorate the development of experimental autoimmune myasthenia gravis. Clin Exp Immunol. 2004;138:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Eleftheriadis T, Pissas G, Karioti A, et al. The indoleamine 2,3-dioxygenase inhibitor 1-methyl-tryptophan suppresses mitochondrial function, induces aerobic glycolysis and decreases interleukin-10 production in human lymphocytes. Immunol Invest. 2012;41:507–520. [DOI] [PubMed] [Google Scholar]


