Abstract
Background:
Lapatinib and capecitabine cross the blood tumor-barrier in breast cancer brain metastasis but have modest clinical efficacy. Administration of high dose tyrosine kinase inhibitor (TKI) has been evaluated in brain metastases and primary brain tumors as a strategy to improve drug exposure in the central nervous system (CNS). We derived a rational drug scheduling of intermittent high dose lapatinib alternating with capecitabine-based on our preclinical data and Norton-Simon mathematical modeling. We tested this intermittent, sequential drug schedule in breast cancer patients with CNS metastasis.
Patients and methods:
We conducted a phase I trial using an accelerated dose escalation design in HER2-positive breast cancer patients with CNS metastasis. Lapatinib was given Day 1–3 and Day 15–17 with capecitabine on Day 8–14 and Day 22–28 on an every 28-day cycle. Lapatinib dose was escalated, and capecitabine given as a flat dose at 1500mg BID. Toxicity and efficacy were evaluated.
Results:
Eleven patients were enrolled: brain only (four patients,36%), leptomeningeal, (five patients,45%), and intramedullary spinal cord (two patients,18%). Grade 3 nausea and vomiting were dose-limiting toxicities. The maximum tolerated dose of lapatinib was 1500mg BID. Three patients remained on therapy for greater than six months.
Conclusions:
High dose lapatinib is tolerable when given intermittently and sequentially with capecitabine. Antitumor activity was noted in both CNS and non-CNS sites of disease. This novel administration regimen is feasible and efficacious in HER2-positive breast cancer patients with CNS metastasis and warrants further investigation.
Keywords: brain metastasis, leptomeningeal metastasis, intramedullary spinal cord metastasis, metastatic breast cancer, high dose lapatinib
Introduction:
Central nervous system (CNS) metastases are associated with poor overall survival in metastatic breast cancer patients (1–3). HER2-positive (HER2+) breast cancer patients have a higher incidence of CNS metastasis (2,4). The advent of anti-HER2 targeted therapy has changed the landscape for HER2+ metastatic breast cancers (MBC) with unprecedented improvement in overall survival (OS) associated with the introduction of newer HER2-targeted drugs such as pertuzumab and ado-trastuzumab-emtansine (5–7). However, management of CNS metastasis remains a clinical challenge in HER2+ MBC patients. For example, among HER2+ MBC patients treated with trastuzumab-emtansine (TDM-1), CNS was the first site of progression in 56% of patients with prior brain metastasis and 18% in those without prior brain metastasis (8). Isolated CNS progression while maintaining disease control in non-CNS sites is not uncommon in this patient population (9,10).
Due to the limited survival and unclear extent of blood-brain or blood-tumor barrier penetration, patients with CNS metastasis had, until recently, been historically excluded from clinical trials of systemic therapy (11). There are several trials conducted specifically in patients with brain metastasis, but there is a paucity of prospective trials for patients with other types of CNS involvement such as leptomeningeal or intramedullary spinal cord metastasis where the median survival is much shorter compared to that of brain metastasis patients (1,12). There is a critical unmet need for novel effective systemic treatment in such patients(13).
Lapatinib and capecitabine have shown preclinical and clinical antitumor activity as monotherapy and in combination for MBC and have been studied specifically in HER2 positive brain metastases (14–17). We previously demonstrated the brain metastasis uptake of capecitabine, its metabolites, and lapatinib in MBC patients undergoing clinically indicated craniotomy (18). The study showed detectable drug concentrations of both capecitabine and lapatinib in resected brain metastasis tissue. While the concentration of 5-FU (the active metabolite of capecitabine) approached the cytotoxic inhibitory concentration, lapatinib concentration was variable and less optimal. This finding was consistent with the modest clinical activity reported in the clinical trials for brain metastasis (lapatinib monotherapy 2.6% and the combination approximately 38%) (15,17). Considering that the combination response rate noted in the brain metastases trial is comparable to the median response rate of 28% noted for capecitabine as a single agent in pretreated metastatic breast cancer without brain metastases (19), insufficient lapatinib uptake is a potential barrier to lapatinib or lapatinib capecitabine combination efficacy in the brain metastases population (20).
Based on our preclinical study (online only Supplement A) and studies reported by others (21–26), we hypothesize that a higher dose of lapatinib would improve the efficacy and drug penetration in CNS metastases (18,23,24,27). However, given that both capecitabine and lapatinib are known to have overlapping gastrointestinal toxicities, high dose lapatinib given in a standard concurrent administration would be too toxic. Intermittent dosing of capecitabine in a 7 days on/7 days off schedule has previously been shown to improve tolerance based on Norton-Simon mathematical modeling (28,29).
We tested three intermittent dosing schedules against standard continuous dosing and found that there was no difference between the intermittent schedules (Supplement A). In addition, a higher tumor reduction was noted with intermittent high dose administration compared to lower continuous dosing. Concurrent administration of capecitabine and high dose lapatinib was not feasible due to prohibitive toxicity but sequential dosing appeared to be feasible. Based on these results, we derived a novel drug scheduling regimen that would allow administration of high dose “pulsatile” lapatinib with capecitabine using a xenograft model: high dose lapatinib (3 days on/11 days off) given sequentially with intermittent capecitabine (7 days on/7 days off) (27–29)1. We chose to dose lapatinib twice daily to increase drug exposure (30), We conducted a phase I trial to test our hypothesis that this novel administration regimen will allow high dose lapatinib administration in combination with capecitabine in HER2+ MBC patients with CNS metastases.
Material and Methods:
This was a prospective multi-center phase I dose-escalation study in HER2+ MBC with CNS metastasis using an accelerated titration design (Clinical trials.gov Identifier: ). The study sites included Memorial Sloan Kettering Cancer, New York, Queens Cancer Center of Queens Hospital, New York, and the University of Michigan. The study was conducted in accordance with the U.S. Common Rule. Appropriate approval was obtained from each institutional review board and in accordance with an assurance approved by the U.S. Department of Health and Human Services. Informed written consent was obtained from each subject. The primary objective of the study was to assess the toxicity and determine the maximum tolerated dose (MTD) of the intermittent high dose lapatinib alternating with capecitabine. Secondary and exploratory objectives included the clinical efficacy and feasibility of assessing circulating tumor cells (CTC) in cerebrospinal fluid (CSF) samples for patients with known or suspected LMD and compare CSF CTCs with blood CTCs.
The study included patients with HER2 +breast cancer as defined by immunohistochemistry with the score of 3+, or if 2+ with confirmatory fluorescence in situ hybridization ratio of ≥ 2.0. Radiologic evidence of new or progressive parenchymal brain metastasis (BM) or leptomeningeal disease (LMD) by magnetic resonance imaging of the brain or spine or CSF cytology evidence of new or persistent LMD were required. The study was later amended to include new or progressive intramedullary spinal cord metastases, as this diagnosis was considered as CNS metastases. Prior lapatinib at a standard FDA approved dose and capecitabine were allowed as long as capecitabine exposure was more than six months prior to the study enrollment. Patients must have received prior trastuzumab or chemotherapy for MBC unless the CNS was the only site of metastasis. Measurable disease was not required. Performance status between 0 – 2 on Eastern Cooperative Oncology Group (ECOG) scale was allowed. Patients must have had adequate organ function. Prior CNS-targeted therapies (including radiotherapy, surgery, or intrathecal therapy) were allowed.
Study design:
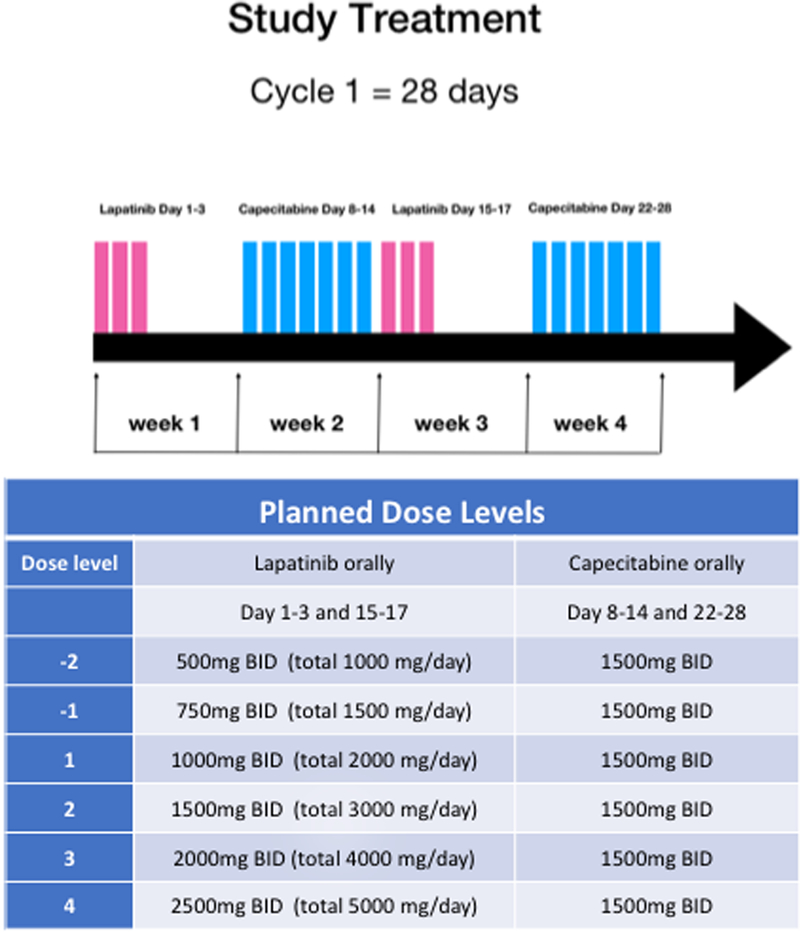
The study used an accelerated titration design for lapatinib dose escalation. No dose escalation was conducted for capecitabine. No intra-patient dose escalation was allowed. Lapatinib dose levels consisted of 1000mg twice a day (BID), 1500mg BID, 2000mg BID, and 2500mg BID. Lapatinib was given BID for 3 days followed by 11 days of rest based on the Norton-Simon modeling and our preclinical study(27). Patients received lapatinib on days 1–3 and days 15–17 alternating with intermittent capecitabine as shown in Figure 1. Capecitabine was administered as flat dosing of 1500 mg oral BID for 7 days followed by 7 days of rest according to the Norton-Simon modeling as previously published (28,29). Verbal and written diarrheal management instruction along with a prescription for loperamide was provided at the start of the study. However, no scheduled or required the prophylactic use of supportive medications, including anti-emetics was prescribed as a part of the study treatment. Patients were allowed to continue concurrent intravenous (IV) trastuzumab or endocrine therapy if they were previously treated with the same drug.
Figure 1:
Study treatment schema and planned drug dose levels: Study treatment schema showing lapatinib 3 days on/11 days off alternating with capecitabine 7 days on/7days off.
A cycle was defined as four weeks in duration, and the dose-limiting toxicity (DLT) was defined and assessed during cycle 1. Patients continued treatment until progression of the disease, unacceptable toxicity, or withdrawal from the study. Dose reductions were allowed for lapatinib (maximum of two dose reductions in 500mg per day increments) and capecitabine (maximum of two dose reductions by 500mg per day increments).
Study assessments:
Patients were assessed weekly during cycle 1 (for four weeks) and then every 4 weeks. Patient history, physical examination, laboratory tests were performed at each visit. A review of medication compliance was conducted with a pill diary weekly for the first cycle and at the start of each subsequent cycle.
Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0. DLT was defined as the following toxicities attributable to study treatment during cycle 1: any grade ≥3 non-hematologic toxicity (except alopecia, altered taste, nail changes), and any grade 3 nausea, vomiting, or dehydration (except those occurred in setting of inadequate compliance with supportive care measures lasting for less than 48 hours), grade 3 diarrhea not responsive to supportive care measures within 72 hours, grade 4 neutropenia lasting ≥5 days in duration or with fever > 38.5 and/or infection requiring antibiotic or antifungal therapy, grade 4 thrombocytopenia, and grade 4 anemia. Any unresolved drug-related toxicity requiring drug interruption for > 14 days in the first cycle and any drug-related toxicity leading to a dose modification of lapatinib in the first cycle were also considered as DLT.
All patients were required to undergo baseline radiologic assessment with computed tomography (CT) of chest/abdomen/pelvis and magnetic resonance imaging (MRI) of brain and spine with contrast. Imaging was performed every eight weeks for the first six months and subsequently every 12 weeks with a CT scan and MRI brain. MRI spine was required at baseline for all but follow up MRI spine was required only for patients with spinal cord metastasis or LMD. BM patients with measurable disease (defined as a lesion ≥ 1cm) were evaluated for CNS response with or without non-CNS response (depending on the site of measurable disease). For BM patients, CNS Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 and RECIST 1.1 criteria were used to evaluate target lesions. For LMD patients, responses were assessed by CSF cytology, radiology imaging, and clinical signs/symptoms. Complete response was defined by complete cytologic and radiologic response with stable or improved clinical function. Partial response was defined as either complete cytologic with stable imaging or complete radiologic response with stable cytology and at least stable clinical function. Progressive disease was defined either radiologic progression or worsening clinical signs/symptoms or new clinical signs/symptoms attributable to LMD and warrant a change in therapy per the treating investigator. Stable disease was defined as a response that did not meet complete, partial or progressive disease response criteria for LMD patients as described above.
Patients with LMD with no contraindication to CSF collection were approached for a CSF biomarker collection. For consenting LMD patients, CSF was collected for routine cytology, CSF CTC, and blood CTC at baseline, Cycle 2 day 1, Cycle 3 Day 1 then every eight weeks for the first six months and subsequently every 12 weeks. 3.5ml of CSF and 7.5ml of whole blood were collected in CellSave Tubes (Veridex LLC, Warren NJ, USA) and shipped to the central study laboratory (Dr. Martin Fleisher, Memorial Sloan Kettering, New York, USA) within 48 hours of collection. The CellSearch® (Menarini Silicon Biosystems) platform was used to enumerate CTCs in CSF and serum specimens. Results were reported as number of CTCs per 3 ml of CSF (CSF CTCs/3ml) and as number of CTCs per 7.5 ml of blood.
Statistical analysis:
MTD was determined using an accelerated titration design without intra-patient dose escalation. In the accelerated titration design, 1 patient is accrued per each sequential dose cohort until 1 DLT or 2 instances of moderate toxicity are observed. After such an observation, the study reverts to a traditional 3+3 design. Toxicity data were summarized with descriptive statistics. If a patient withdrew or discontinued the study drugs prior to day 29 due to reasons unrelated to adverse events, death, or progression of the disease, an additional patient was allowed to enroll and replace that patient. However, if a patient had any dose of study drug, that patient was included in the study analysis including toxicity and DLT assessments.
Initially, for those patients with measurable disease, response rate, progression-free survival and overall survival were considered as exploratory objectives. However, after the completion of enrollment, the study population consisted of various CNS metastasis types (BM, LMD, and spinal cord metastasis) with the differing historical clinical outcome. Therefore, we present the exploratory clinical outcomes in a graphical format.
Results:
Patients:
From February 2016 to June 2017, eleven patients were enrolled. The clinical characteristics of all patients are summarized in Table 1. One patient came off study due to non-compliance after three doses of lapatinib, and an additional patient was enrolled to fill that dose level per the protocol. There were four patients with parenchymal BM only, five patients with LMD (two of which had concurrent BM), and two patients with spinal cord metastasis with concurrent BM or BM and LMD. All but two had prior radiotherapy for CNS metastasis. Three patients (27%) had prior capecitabine, and two patients (18%) had prior lapatinib exposure. Of the five patients with LMD, three had prior intrathecal/intraventricular therapy that included IT trastuzumab, IT methotrexate, and/or IT topotecan.
Table 1.
Patient characteristics (N=ll)
| Characteristics | Number | Percentage | Median (range) | |
|---|---|---|---|---|
| Age (years) | 52 (34–75) | |||
| ECOGperfbnrance status | 0 | 2 | 18% | |
| 1 | 6 | 55% | ||
| 2 | 3 | 27% | ||
| ER/PR receptor status | ER+/PR+ | 3 | 27% | |
| ER+/PR− | 2 | 18% | ||
| ER−/PR− | 6 | 55% | ||
| CNS metastabis type | Parenchymal BM only | 4 | 36% | |
| Leptomeninges* | 5 | 45% | ||
| Spinal Cord* | 2 | 18% | ||
| Non–CNS metastasis status** | Yes | 6 | 55% | |
| No | 5 | 45% | ||
| Previous CNS targeted treatment | Surgery | 1 | 9% | |
| Radiation | 9 | 82% | ||
| Intrathecal therapy | 3 | 27% | ||
| Previous capecitabine exposure | Yes | 3 | 27% | |
| No | 8 | 73% | ||
| Previous lapatinib exposure | Yes | 2 | 18% | |
| No | 9 | 82% | ||
| Prior anti-HER2 therapy (other than lapatinib) | IV trasutuzmab | 11 | 100% | |
| IT trasutuzmab | 2 | 18% | ||
| IV pertuzumab | 8 | 73% | ||
| TDM–1 | 5 | 45% | ||
Patients may have concurrent parenchymal BM and/or leptomeningeal metastasis,
“No”= no evidence of disease on CT CAP
Abbreviations: N, sample size, ECOG, Easten Cooperative Oncology Group, ER, estrogen receptor, PR, progresterone receptor CNS, central nervous system, NED, no evidence of active disease, BM, brain metastasis, TDM-l,trasutuzmab-emtansine, CT CAP, computer tomography of chest/abdomen/pelvic
Determination of the MTD and toxicities:
During the accelerated titration portion, the dose was escalated up to 2000 mg BID, at which point the 3+3 portion was triggered. In total, one patient was treated at the 1000mg BID lapatinib dose level without any DLT. There were no DLTs seen in six patients treated at the 1500 mg BID lapatinib dose level. At the 2000 mg BID dose, there were four patients treated and two DLTs: one Grade 3 nausea and one Grade 3 vomiting despite institution of and compliance with the supportive measure as specified in the protocol. One patient underwent one dose reduction to 1500mg BID as allowed per the protocol and subsequently remains on study to date. Another patient treated at 2000 mg BID dose level came off study due to Grade 3 nausea and discontinued treatment without dose reduction during the first cycle. The MTD was determined to be 1500 mg BID lapatinib 3 days on 11 days off alternating with capecitabine 1500mg oral BID on 7 days on 7 days off.
All eleven patients (including the patient who came off study for non-compliance) were evaluable for toxicity. During the cycle 1, most common toxicities (>20%) regardless of attribution were Grade 1 and 2 diarrhea (45.5% and 27.3% respectively), Grade 1 nausea (36.4%), Grade 1 fatigue (27.3%), Grade 2 vomiting (27.3%), and Grade 1 palmar-plantar erythrodysesthesia (27.3%). There were three Grade 3 adverse events, which two were attributed to the study treatment (DLTs). The most common (> 20%) treatment-emergent toxicities, regardless of attribution, were Grade 2 vomiting, Grade1 and 2 diarrhea, Grade1 abdominal pain, Grade 1 and 2 fatigue, Grade 1 headache, Grade 1 and 2 nausea, and Grade1 palmar-plantar erythrodysesthesia. Commonly reported toxicities (>20%) regardless of attribution to study treatment by maximum grade of toxicity per patient are summarized in Table 2.
Table 2.
Adverse events regardless of relationship to study treatment (occurring in > 20% of treated patients, N=11)
| Maximum grade | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhea | (45%) | (27%) | (9%) | (0%) | (82%) | |||||
| Nausea | (27%) | (27%) | (9%) | (0%) | (64%) | |||||
| vomiting | (0%) | (55%) | (9%) | (0%) | (64%) | |||||
| Abdominal pain | (36%) | (9%) | (0%) | (0%) | (45%) | |||||
| Anorexia | (18%) | (18%) | (0%) | (0%) | (36%) | |||||
| Headache | (36%) | (0%) | (0%) | (0%) | (36%) | |||||
| PPE | (27%) | (9%) | (9%) | (0%) | (45%) | |||||
| Peripheral neuropathy | (18%) | (9%) | (0%) | (0%) | (27%) | |||||
| Fatigue | (36%) | (27%) | (0%) | (0%) | (64%) | |||||
| Generated muscle weakness | (0%) | (18%) | (9%) | (0%) | (27%) | |||||
| Back pain | (18%) | (9%) | (0%) | (0%) | (27%) | |||||
N, number of patients, PPE, Palmar-plantar erythrodysesthesia
Clinical activity:
All eleven patients had baseline radiologic assessments. Two patients came off study prior to the first follow–up radiologic assessment (for non-compliance and toxicity). Three patients with parenchymal BM completed at least 1 cycle of study treatment and had a follow up radiologic assessment. Of the two BM patients with measurable target lesion, 1 had unconfirmed stable disease, and other had confirmed stable disease. Of the four LMD patients who had completed at least 1 cycle of study treatment, one patient had a partial response and one had stable disease. One patient with multilevel intramedullary spinal cord metastases had significant, albeit transient improvement in sensory symptoms and motor symptoms resulting in improved functional status and ambulation.
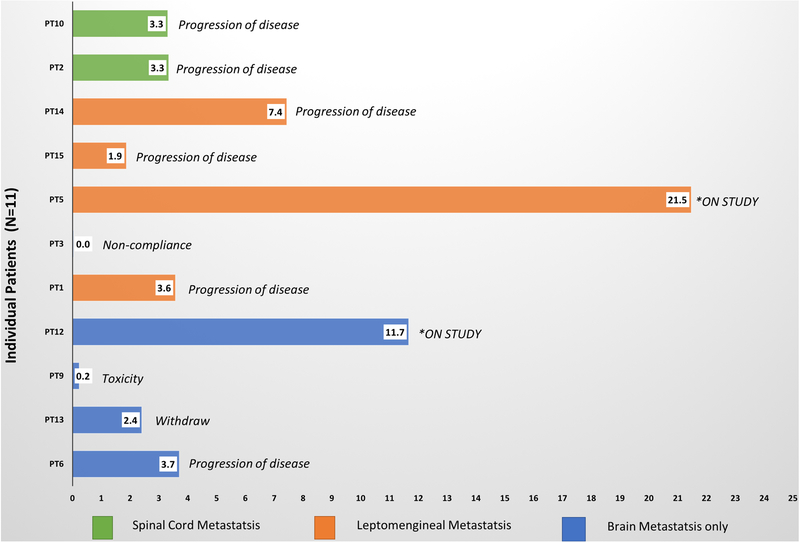
The duration of therapy and reason for treatment discontinuation for each patient grouped by the CNS site of disease is summarized in Figure 2. Five patients had CNS as the only site of detected disease (defined as no evidence of disease detect on non-CNS CT body imaging): PT 1, 3, 5, 12, and 13. Five patients continued intravenous trastuzumab as allowed by the protocol: Pt 5, 6, 9, 10, and 14.
Figure 2:
Treatment duration: The duration of on-study treatment per patient is shown for all eleven patients on the study. Sites of central nervous disease are indicated by color-coding. Two patients remain on the study (the duration is at the time of data-lock).
Three patients were on study treatment for at least six months. Two of three patient had LMD. One patient who came off study treatment after seven months had prior exposure to intrathecal therapy (including intrathecal trastuzumab) and RT but not to capecitabine and lapatinib exposure. The patient (PT5) who has completed 22 cycles of study treatment to date did not have capecitabine, lapatinib, or intrathecal treatment prior to the study enrollment and remains on study. Another patient (PT12) who remains for more than 12 months on the study treatment had a progressive BM after CNS RT and had previously discontinued capecitabine and due to toxicity before the study entry.
As of April 2018, two patients remain on study treatment. Nine patients are off protocol therapy for the following reasons: progression of disease (six patients), toxicity (one patient), non-compliance (one patient with LMD), voluntary withdrawal (one patient with BM). Of the six patients who came off study treatment for progression, CNS was the site of progression for five patients (83%). There have been four deaths, all of which were unrelated to the study treatment.
Exploratory circulating cell analysis:
Patients with LMD with or without spinal cord metastasis were approached for optional CSF and blood research collection for CTC analysis). Three patients (all with intraventricular reservoir) consented to the serial CSF and blood collection, and the data is summarized in Table 3. Two patients had LMD, and one patient had LMD and spinal cord metastasis. All had detectable CTCs per 3mls of CSF at baseline (range: 2 −328 CTCs/3ml). Two LMD patients had a baseline positive conventional CSF cytology which became negative after one cycle of therapy. Concurrently, the number of CTCs decreased and remained detectable while CSF cytology was negative. Subsequently, the number of detectable CTCs in CSF increased as patients experienced the progression of CNS disease noted radiologically and clinically. The CTCs in blood was discordant with the CTCs in CSF for the LMD patients (PT1) with no evidence of disease on non-CNS imaging, while the CTCs in CSF and blood similarly decreased for the LMD patients who had disease present in both CNS and non-CNS imaging (PT14). For LMD with spinal cord metastasis patient (PT10), the CTCs in CSF remained very low at baseline and subsequently, even when the disease progressed. Interestingly, this patient was taken off the study by the treating investigator for an increase in intramedullary metastases with expansile intamedullary edema.
Table 3.
CTC analysis of matched CSF and blood.
| Patient PT1 | Baseline | C2D1* | C3D1* | CNS Progression/off study | ||
| ctc csf | 328 | 76 | 117 | |||
| CTC Blood | 0 | Not done | 0 | |||
| CSF cytology | Positive | Negative | Positive | |||
| MRI brain/spine | lesions present | Not done | Stable | |||
| CTCAP | No lesions | Not done | No lesions | |||
| Patient PT14 | Baseline | C2D1 | C3D1 | C5D1 | C7D1 | CNC Progression/off study |
| CTC CSF | 22 | 13 | 13 | 2 | 9 | |
| CTC Blood | 14 | 0 | 0 | 0 | 1 | |
| CSF cytology | Atypical/Positive** | Negative | Atypical/Negative** | Negative | Atypical/Negative** | |
| MRI brain/spine | No lesions | Not done | Stable | Stable | Stable | |
| CT CAP | lesions present | Not done | Stable | Stable | Stable | |
| Patient PT10*** | Baseline | C2D1 | C3D1 | CNS Progression/off study | ||
| CTC CSF | 2 | 3 | 1 | |||
| CTC Blood | 0 | 1 | 0 | |||
| CSF cytology | Suspicious/Negative** | Negative | Negative | |||
| MRI brain/spine | Lesions present | Not done | Stable | |||
| CT CAP | Lesions present | Not done | Stable | |||
Abbreviations: CTC, circulating tumor cell, CSF, cerebrospinal fluid, MRI, magnetic resonance imaging, CT CAP, computer tomography
of chest/abdomen/pelvis.
Study specified assessments made prior to the start of cycle 2,3,5, and 7. Patients were taken off study for progression of disease
based on non-study scheduled clinical, cytologic, or radiologic assessments made during clinical follow up.
if the CSF cytology was atypical or suspicious, a repeat CSF study was conducted for a confirmatory testing.
PT10 had concurrent brain and spinal cord metastases.
Discussion:
We evaluated the safety and toxicity of high dose pulsatile lapatinib alternating with capecitabine in HER2+ breast cancer patients with CNS metastasis. The MTD regimen was defined as lapatinib 1500mg BID on Day 1–3 and Day 15–17 with capecitabine 1500mg BID on Day 8–14 and Day 22–28 on an every 28-day cycle. Lapatinib and capecitabine combination has shown clinical activity in HER2+ breast cancer patients in several phase II trials(14,17). However, the degree of clinical activity has been limited. The trial was motivated by our previous observation made in our ‘window-of-opportunity’ presurgical study where we reported that capecitabine and lapatinib do cross the blood-tumor barrier but variably and suboptimally for lapatinib(18), by mathematical modeling, and xenograft studies (Online supplement).
We aimed to improve the clinical efficacy of these drugs which are already FDA approved, and whose toxicities are well characterized. The drugs have overlapping gastrointestinal toxicities that often make the combination therapy difficult to administer in clinical practice. We tested a dosing schedule that was designed based on Norton-Simon mathematical modeling where we hypothesized that the pulsatile dosing and intermittent alternating scheduling of these drugs will improve tolerability and allow a higher dose of lapatinib to be administered(27). We then conducted a preclinical xenograft study, further informing the dosing schedule for this trial (Supplement A, online). In this trial, we translated and evaluated our preclinical finding that a high dose of lapatinib given 3 days on/11 days off alternating with capecitabine 7 days on/7 days off would be tolerable in breast cancer patients with CNS metastasis.
In the randomized phase II trial of capecitabine plus lapatinib or lapatinib plus topotecan in HER2-positive breast cancer BM, of the 13 patients randomized to lapatinib (given 1250mg orally daily) with capecitabine (2000mg/m2 BID on days 1–14 of a 21 day cycle), 23% experienced Grade 3 and 8% had Grade 4 diarrhea (17). Grade 3 palmar-plantar erythrodysesthesia (PPE) was reported in 15% of patients (17). While we noted less Grade 3 and 4 diarrhea and PPE, there was more Grade 2 vomiting, which may have been partly due to the pill burden, and/or a CNS emetogenic effect of pulsatile high-dose lapatinib. Most recently, neratinib, another HER2 targeted TKI was studied in combination with capecitabine for brain metastasis where Grade 3 diarrhea occurred in 29% of patients (31). Therefore, we believe the tolerability of our study regimen is at least comparable to other reported TKI/capecitabine combinations.
Unfortunately, as this was a phase I trial with the primary objective of assessing safety and toxicity, we did not have many patients with measurable disease in each CNS metastasis type to meaningfully examine response rate or progression-free survival. Moreover, historically there were no standard criteria to assess response in LMD or intramedullary spinal cord metastasis. We used a study-specific LMD response criteria, but no response criteria was developed for spinal cord metastasis, as this patient population was not included at the time of study conception. Only recently, there is a proposal to standardize LMD response criteria in trials (32). Despite having a mixed population, we noted that 3 patients remained on study therapy for more than six months with two remaining on study for more than a year.
We conducted an exploratory comparative analysis of CTCs in CSF and blood. For the three patients with LMD who enrolled, CTCs in CSF were detectable even when conventional cytology was negative. The trajectory of changes in CTC enumeration correlated with the clinical course during the study for two patients. We also noted discordance between the detection of CTCs in CSF and blood that was reflective of the discordance between the CNS and non-CNS disease extent. Although the number of patients was very small for the CTC analysis, the finding warrants further evaluation of CTC analysis in CSF as a diagnostic and disease monitoring tool for patients with LMD. It is notable that for the patients with both LMD and spinal cord metastasis, CTCs was not informative. Of note CSF was collected from an intraventricular reservoir for this patient, and it is unclear if the source of CSF collection may impact CTC detection in such case. The question remains if CTC analysis in CSF is useful only in LMD or also in other types of CNS metastasis.
Our accelerated titration phase I design was efficient, thus resulting in a modest sample size of study patients to robustly ascertain clinical efficacy as a secondary endpoint. It is worth noting that there were patients who remained on study therapy for more than six months. In contrast, the recently reported Phase II trial of intrathecal trastuzumab in HER2 positive LMD patients had a median progression-free survival of 2.4 months(33). Additionally, the Phase II trial of pertuzumab and high dose trastuzumab (PATRICIA) reported median treatment duration of 4.4months (range 1.2–8.3) among the 15 patients analyzed in the interim analysis(34). However, the clinical activity observed does not easily lend itself to comparison to historical controls or other trials, and as such, further phase II investigation is warranted.
We would proposed that the next step is to evaluate clinical efficacy of this study regimen in a phase II setting (separate cohorts of brain and LMD patients) randomized against a standard of care or physician’s choice (which may include various anti-HER2 TKIs or non-TKIs but reported to have CNS activity such as TDM-1) to provide a contemporary benchmark. Moreover, the extent of clinical benefit associated with the intermittent high dose approach (rather than the specific regimen tested in this study) can be further evaluated and explored using newer anti-HER2 TKIs such as neratinib and tucatinib (31,35). This may be more relevant for neratinib where diarrhea is a significant concern as mentioned above.
Our preclinical model showed a ceiling effect for lapatinib when given at a very high dose and did not allow sufficient evaluation of the additive value of capecitabine. In this study, we decided to optimize the combination regimen of lapatinib and capecitabine which is well-established with known CNS and non-CNS activity (36). A separate cohort of single agent lapatinib was considered during the conception of this study but ultimately not pursued partly due to prioritizing study efficiency and concern for tumor heterogeneity (unlike cell lines, clinical disease may consist of HER2-driven and non-HER2 driven cells) and a very modest activity of standard dose lapatinib single agent. However, a single agent high dose TKI is certainly a possible approach to explore to further mitigate toxicity and maximize clinical activity in the CNS as opposed to a combination with capecitabine especially for newer TKIs with more robust single agent activity.
Although the epidemiology for HER2 positive BM patients has evolved with a more extended median survival, we previously reported that approximately 36% of HER2 positive BM patients present with CNS as the only site of active disease (10). Moreover, Le Scodan et al. reported that 61% of trastuzumab-treated HER2 positive BM patients ultimately died despite well controlled or no evidence of non-CNS disease (37). Therefore, the treatment of CNS metastasis in HER2 positive patients remains an important clinical problem.
LMD and intramedullary spinal metastasis are still associated with severely limited survival of only a few months(1,12,38), and such patients have limited therapeutic options. Although there are more therapeutic trials that include BM patients, there is a paucity of prospective trials that allows patients with progressive LMD. Moreover, to the best of our knowledge, there are no published prospective trial data for patients with progressive intramedullary spinal metastasis. This trial was uniquely inclusive in this regard in an effort to offer trials to patients who are traditionally excluded from clinical trials(11).
In summary, the present study provides evidence that phase I evaluation of new regimens in patients with CNS metastases is feasible, and that optimized drug dosing and scheduling may mitigate toxicity while providing greater potential for efficacy in the CNS compartment. We are encouraged by the durable clinical activity observed in some of our patients. The study regimen and strategy warrants expanded evaluation in this population as supported by our data. Drug development and trial options for patients with CNS metastasis remain an area of urgent and unmet need.
Supplementary Material
Translational Relevance.
Treatment options for breast cancer patients with central nervous system (CNS) metastasis are limited. Lapatinib and capecitabine have demonstrated CNS activity in HER2-positive breast cancer with brain metastasis. However, the clinical efficacy is limited, dosing has been empiric and based on conventional labeled scheduling for each single agent, and the overlapping gastrointestinal toxicities from these drugs can be considerable. This study evaluated the safety and toxicities associated with intermittent high dose lapatinib 3 days on/11 days off alternating with intermittent fixed dose capecitabine 7days on/7days off in HER2-positive breast cancer patients with CNS metastasis. This study is the first prospective trial to study the combination of lapatinib and capecitabine in HER2-positive breast cancer patients with leptomeningeal and intramedullary spinal cord metastasis. This regimen provides a rationally designed, tolerable alternative to conventional drug dosing and warrants further evaluation of its clinical efficacy.
Financial support:
Novartis, the Breast Cancer Research Fund, the Judah Gubbay Memorial Fund, and Core Grant P30 CA008748
Conflict of interest/Disclosures:
ADS: research support (Novartis, Bayer) consulting (Genomic Health, Genentech, Puma, Novartis, Lilly, Eisai, Odonate, Nektar); speaker (Genomic Health, Genentech, Novartis, Lilly, Pfizer, Eisai, Celgene, AstraZeneca, Merck)
AM: research support (Novartis, Bayer, Genentech, Lilly, Merrimack)
LN: honoraria or consulting (Ionis Pharmacetuicals, Lockwood, Bristol-Myers Squibb, Oncolytic Biotech, Pfizer, BluPrint Oncology Concepts,Celgene, Lilly, Sermonix)
CVP: research support (Bayer); royalties (UpToDate)
BTL: consulting (Genentech, Mersana Therapeutics, Hengrui Therapeutics, Guardant Health, Biosceptre Australia).
Footnotes
ClinicalTrials.gov Identifier:
Norton-Simon Modelling is based on the Norton-Simon Hypothesis: If N is the tumor size at the time of therapy and N’ is the expected unperturbed growth rate at that size then after therapy the perturbed growth (or regression) rate can be expressed as (1-D) N’, with slower that unperturbed growth when 0 < D < 1 and tumor shrinkage when D > 1. Taking time t into account, a single dose of therapy results in a function D(t) ≥ 0, which (if non-zero) reaches a maximum at some time after therapy before thereafter decreasing, sometimes to zero. The maxima D’(T) = 0 occurs at the time T ≥ t of maximum therapy effect, which would be the ideal time to introduce a different growth-perturbing therapy. Xenograft experiments with lapatinib confirmed a T of approximately 9±1 days after three daily administrations. Prior published analyses of capecitabine confirmed a T of 9±1 days after seven daily administrations. Pulsatile use of these agents allows dose escalations, thereby increasing their efficacy since for both agents there is a monotonically rising dose-level-response relationship.
Reference:
- 1.Morikawa A, Jordan L, Rozner R, Patil S, Boire A, Pentsova E, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin breast cancer 2017;17(1):23–8 doi 10.1016/j.clbc.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol 2017;3(8):1069–77 doi 10.1001/jamaoncol.2017.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ording AG, Heide-Jorgensen U, Christiansen CF, Norgaard M, Acquavella J, Sorensen HT. Site of metastasis and breast cancer mortality: a Danish nationwide registry-based cohort study. Clin Exp Metastasis 2017;34(1):93–101 doi 10.1007/s10585-016-9824-8. [DOI] [PubMed] [Google Scholar]
- 4.Olson EM, Najita JS, Sohl J, Arnaout A, Burstein HJ, Winer EP, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast 2013. doi 10.1016/j.breast.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes J, Fumoleau P, Bianchi GV, Petrella TM, Gelmon K, Pivot X, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012;30(14):1594–600 doi 10.1200/JCO.2011.37.4207. [DOI] [PubMed] [Google Scholar]
- 6.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367(19):1783–91 doi 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372(8):724–34 doi 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okines A, Irfan T, Khabra K, Smith I, O’Brien M, Parton M, et al. Development and responses of brain metastases during treatment with trastuzumab emtansine (T-DM1) for HER2 positive advanced breast cancer: A single institution experience. Breast J 2017. doi 10.1111/tbj.12906. [DOI] [PubMed] [Google Scholar]
- 9.Burstein HJ, Lieberman G, Slamon DJ, Winer EP, Klein P. Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol 2005;16(11):1772–7 doi 10.1093/annonc/mdi371. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa A, Wang R, Patil S, Diab A, Yang J, Hudis CA, et al. Characteristics and prognostic factors for patients with HER2-overexpressing breast cancer and brain metastases in the era of HER2-targeted therapy: an argument for earlier detection. Clin Breast Cancer 2017. doi 10.1016/j.clbc.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Kim ES, Bruinooge SS, Roberts S, Ison G, Lin NU, Gore L, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol 2017;35(33):3737–44 doi 10.1200/JCO.2017.73.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payer S, Mende KC, Westphal M, Eicker SO. Intramedullary spinal cord metastases: an increasingly common diagnosis. Neurosurg Focus 2015;39(2):E15 doi 10.3171/2015.5.FOCUS15149. [DOI] [PubMed] [Google Scholar]
- 13.Lin NU, Prowell T, Tan AR, Kozak M, Rosen O, Amiri-Kordestani L, et al. Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol 2017;35(33):3760–73 doi 10.1200/JCO.2017.74.0761. [DOI] [PubMed] [Google Scholar]
- 14.Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 2013;14(1):64–71 doi 10.1016/s1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 15.Lin NU, Carey LA, Liu MC, Younger J, Come SE, Ewend M, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2008;26(12):1993–9 doi 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin NU, Dieras V, Paul D, Lossignol D, Christodoulou C, Stemmler HJ, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 2009;15(4):1452–9 doi 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 17.Lin NU, Eierman W, Greil R, Campone M, Kaufman B, Steplewski K, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol 2011;105(3):613–20 doi 10.1007/s11060-011-0629-y. [DOI] [PubMed] [Google Scholar]
- 18.Morikawa A, Peereboom DM, Thorsheim HR, Samala R, Balyan R, Murphy CG, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol 2015;17(2):289–95 doi 10.1093/neuonc/nou141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ershler WB. Capecitabine monotherapy: safe and effective treatment for metastatic breast cancer. Oncologist 2006;11(4):325–35 doi 10.1634/theoncologist.11-4-325. [DOI] [PubMed] [Google Scholar]
- 20.Petrelli F, Ghidini M, Lonati V, Tomasello G, Borgonovo K, Ghilardi M, et al. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: A systematic review and pooled analysis. Eur J Cancer 2017;84:141–8 doi 10.1016/j.ejca.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Chien AJ, Auerback, G., Rugo, H.S., Melisko, M; MUnster, P.N., Khanafshar, E., Ordovas, E., Petricoin, K., Koch, K.M., Moasser, M.M. A phase I dose-escalation study of 5-day intermittent oral lapatinib therapy with biomarker anlaysis in patients with HER-2-overexpressing breast cancer. J Clin Oncol 2014; 32(14): 1472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien AJ, Illi JA, Ko AH, Korn WM, Fong L, Chen LM, et al. A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound Paclitaxel for advanced solid malignancies. Clin Cancer Res 2009;15(17):5569–75 doi 10.1158/1078-0432.CCR-09-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol 2010;99(2):283–6 doi 10.1007/s11060-010-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grommes C, Oxnard GR, Kris MG, Miller VA, Pao W, Holodny AI, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 2011;13(12):1364–9 doi 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taskar KS, Rudraraju V, Mittapalli RK, Samala R, Thorsheim HR, Lockman J, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res 2012;29(3):770–81 doi 10.1007/s11095-011-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivanco I, Robins HI, Rohle D, Campos C, Grommes C, Nghiemphu PL, et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov 2012;2(5):458–71 doi 10.1158/2159-8290.CD-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morikawa A, De Stanchina E, Patil S, Chandarlapaty S, Li B, Norton L, et al. Optimization of intermittent high dose lapatinib administration with or without capecitabine: A rational approach to drug dosing and scheduling using Norton-Simon modeling. [abstract] In: Proceedings of the Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposium: 2015 Dec 8–12; San Antonio, TX: Philadelphia (PA): AACR; Cancer Res; 2016;76(4 Suppl):Abstract nr P4-14-24 [Google Scholar]
- 28.Gajria D, Gonzalez J, Feigin K, Patil S, Chen C, Theodoulou M, et al. Phase II trial of a novel capecitabine dosing schedule in combination with lapatinib for the treatment of patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat 2012;131(1):111–6 doi 10.1007/s10549-011-1749-y. [DOI] [PubMed] [Google Scholar]
- 29.Traina TA, Theodoulou M, Feigin K, Patil S, Tan KL, Edwards C, et al. Phase I study of a novel capecitabine schedule based on the Norton-Simon mathematical model in patients with metastatic breast cancer. J Clin Oncol 2008;26(11):1797–802 doi 10.1200/JCO.2007.13.8388. [DOI] [PubMed] [Google Scholar]
- 30.Burris HA 3rd, Taylor CW, Jones SF, Koch KM, Versola MJ, Arya N, et al. A phase I and pharmacokinetic study of oral lapatinib administered once or twice daily in patients with solid malignancies. Clin Cancer Res 2009;15(21):6702–8 doi 10.1158/1078-0432.CCR-09-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman RA, Gelman RS, Anders CK, Melisko ME, Parsons HA, Cropp AM, et al. TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J Clin Oncol 2019: 10.1200/JCO.18.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlain M, Junck L, Brandsma D, Soffietti R, Ruda R, Raizer J, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro-oncology 2017;19(4):484–92 doi 10.1093/neuonc/now183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumthekar P, Gradishar W, Lin N, Pentsova E, Groves M, Jeyapaln S, et al. Intrathecal (IT) trastuzumab (T) for the treatent of leptomeningeal metastases (LM) in patients (pts) with human epidermal growth factor receptor 2-positive (HER2+) cancer: a multicenter Phase ½ study. 2018. Neuro-Oncology. p vi58. [Google Scholar]
- 34.Lin NU, Stein A, Nicholas A, Fung A, Kumthekar P, Ibrahim NK, et al. Planned interim analysis of PATRICIA: An open-label, single-arm, phase II study of pertuzumab (P) with high-dose trastuzumab (H) for the treatment of central nervous system (CNS) progression post radiotherapy (RT) in patients (pts) with HER2-positive metastatic breast cancer (MBC). 2017. J Clin Oncol. p 2074-. [Google Scholar]
- 35.Murthy R, Borges VF, Conlin A, Chaves J, Chamberlain M, Gray T, et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol 2018;19(7):880–8 doi 10.1016/S1470-2045(18)30256-0. [DOI] [PubMed] [Google Scholar]
- 36.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355(26):2733–43 doi 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 37.Le Scodan R, Jouanneau L, Massard C, Gutierrez M, Kirova Y, Cherel P, et al. Brain metastases from breast cancer: prognostic significance of HER-2 overexpression, effect of trastuzumab and cause of death. BMC cancer 2011;11:395 doi 10.1186/1471-2407-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosmas C, Koumpou M, Nikolaou M, Katselis J, Soukouli G, Markoutsaki N, et al. Intramedullary spinal cord metastases in breast cancer: report of four cases and review of the literature. J Neurooncol 2005;71(1):67–72 doi 10.1007/s11060-004-9177-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.