Abstract
Introduction:
Freezing of gait is an episodic inability to move the feet forward despite the intention to walk. It is a common cause of falls and subsequent morbidity and mortality in Parkinson’s disease. Virtual reality paradigms provide an opportunity to safely evaluate freezing of gait, in order to better understand the underlying pathophysiology. This article focuses on the methodology, threshold used to define freezing of gait, results, limitations of studies using virtual reality paradigms, and proposes future directions of research. Summarizing these articles improves our understanding of freezing of gait in Parkinson’s disease, and critical evaluation provides an opportunity for future studies to improve upon these efforts.
Methods:
We performed a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines, of studies using VR paradigms to elucidate the underlying pathophysiology of PD-FOG.
Results:
This review initially identified 57 articles, but after exclusion of duplicates, abstracts, and studies not focused on the underlying pathophysiology of this disorder, 12 peer-reviewed articles using virtual reality paradigms to evaluate freezing of gait in Parkinson’s disease were found.
Conclusion:
Virtual reality paradigms are able to reproduce freezing of gait. Studies using MRI compatible virtual reality to evaluate freezing of gait found dysfunctional connectivity between cortical and subcortical structures during episodes. However, several important limitations of these studies should caution our interpretation of these results. Future studies which improve the design and methodology are needed to ultimately identify the cause and subsequent treatments for freezing of gait in Parkinson’s disease.
Keywords: Parkinson’s disease, virtual reality, freezing of gait
Introduction
Freezing of gait (FOG) is a disabling phenomenon characterized by a periodic absence or reduction of forward progression of the feet despite the intention to walk [1]. FOG is one of the most common causes of falls and subsequent morbidity and mortality in Parkinson’s Disease (PD) [2]. Approximately 50% of PD patients experience FOG (PD-FOG), but the pathophysiology behind this phenomenon remains unclear.
Assessment of PD-FOG remains challenging and can be dangerous in the clinical setting due to risk of falls [3]. Multiple triggers of PD-FOG have been defined, and tests implementing these triggers may help identify and quantify FOG. These include starting to walk, passing through narrow spaces like doorways, turning, dual-tasking, and reaching one’s intended destination [4]. Virtual reality (VR) paradigms offer the potential for safe and objective PD-FOG evaluation without exhaustive and potentially dangerous tests requiring long examination periods. Although these paradigms may not fully represent postural adjustment and balance during gait initiation, previous studies have shown that they can elicit FOG in PD participants by including known triggers. Additionally, VR paradigms enable the assessment of FOG during neuroimaging, which has helped elucidate the underlying mechanisms of PD-FOG. It is important to understand how advances in the implementation of VR paradigms have increased our knowledge of PD-FOG, as a model for use in other disease states as well. This systematic review discusses the methodology, results, and limitations of studies using VR paradigms to evaluate PD-FOG, in order to summarize efforts to date. We provide a critical review and propose future directions.
Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [5]. The electronic databases PubMed, Web of Science, and EMBASE were searched using the terms “Parkinson disease”, “freezing” and “virtual reality”.
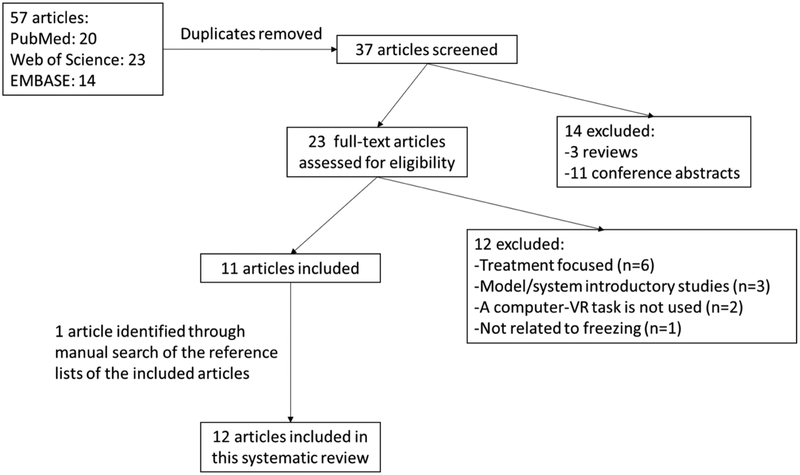
The searches were limited to original studies in English published before June, 2018. Reviews and conference abstracts were excluded. Titles and abstracts were screened for relevancy, then the full texts were reviewed for possible inclusion. Only studies using VR paradigms to elucidate the underlying pathophysiology of PD-FOG were selected. Reference lists of selected studies were manually scanned to identify additional relevant studies which were left out during the initial searches. The selection process is illustrated in Figure 1.
Figure 1.
Flowchart of the selection process for the included studies in this systematic literature review
Results
Following the PRISMA guidelines, 57 articles in total were identified from three databases. After removing the duplicates, 37 articles were screened for inclusion. Exclusion of reviews and conference abstracts identified 23 articles to be assessed for eligibility. Out of these 23 articles, 12 were excluded as they were not primarily focused on investigation of the pathophysiology of PD-FOG using a VR paradigm. After searching reference lists of the 11 included articles, one additional article was found, which was not included through the database search. Overall, 12 articles utilizing VR paradigms to evaluate PD-FOG were included in this systematic review. The methodology of these studies is summarized in detail in Table 1.
Table 1.
Methodology of the VR studies in PD-FOG
| Author, year |
Participants (age and gender data if stated in the article) |
Country where study was conducted |
Hoehn and Yahr stage (range) (mean/standard deviation if range is not stated) |
UPDRS- Part III score |
FOG categorization before the VR paradigm |
Medications | Dopaminergic state during VR paradigm |
VR paradigm methodology ** |
FOG definition in the VR paradigm |
|---|---|---|---|---|---|---|---|---|---|
| Naismith and Lewis, 2010 [10] | 12 PD • Age 65.6/4.7 |
Australia | 1-4 | 37.8 | Not done | Three not medicated, 4 on levodopa monotherapy (1 had bilateral STN DBS), 2 on levodopa with entacapone, 2 on dopamine agonist monotherapy, 1 on levodopa and dopamine agonist therapy | ON | First person perspective of a three-dimensional virtual environment with hands used to control stepping movements. Environment constructed using asoftware development kit by ID Software (Mesquite, TX, USA) | Responses falling 2 SD outside of the mean step latency |
| Shine et al., 2011 [11] | 1 PD-FOG • 61 year-old male |
Australia | Not stated | OFF: 33.0 ON: 23.0 |
FOG-Q | Levodopa, catechol-O-methyltransferase inhibitor, dopamine agonist | OFF and ON (14 days apart) | The task from Naismith and Lewis, 2010 with foot pedals. | Footsteps falling 1.5 SD outside the mean step latency |
| Shine et al., 2013 [12] | 24 PD-FOG • Age 69.5/7.3 14 PD-nFOG • Age 64.0/8.2 |
Australia | PD-FOG: 2.5–3 PD-nFOG: 1-2 |
PD-FOG: 39.8 PD-nFOG: 23.5 |
FOG-Q-item 3, Timed Up-and-Go | Two not medicated, 6 on dopamine agonist monotherapy, 30 on levodopa: 14 on additional entacapone, 20 on additional dopamine agonist, 2 on apomorphine infusion, 1 on apomorphine infusion and amantadine, 6 s/p DBS | OFF | The task from Shine et al., 2011 | Epochs greater than twice the modal footstep latency |
| Shine et al., 2013 [13] | 14PD-FOG • Age 63.2/7.0 15 PD-nFOG • Age 63.4/8.3 |
Australia | PD-FOG: 2.2/0.3 PD-nFOG: 1.9/0.5 |
PD-FOG: 31.9 PD-nFOG: 29.1 |
FOG-Q- item 3, MDS-UPDRS III-item 14, Timed Up-and-Go | Not stated | OFF | The task from Shine et al., 2011 | Epochs greater than twice the modal footstep latency |
| Shine et al., 2013 [14] | 18 PD-FOG • Age 66.8/8.2 |
Australia | 2-4 | 39.2 | FOG-Q-item 3, Timed Up-and-Go | Not stated | OFF | The task from Shine et al., 2011 | Epochs greater than twice the modal footstep latency |
| Shine et al., 2013 [15] | 10 PD-FOG • Age 67.1/6.4 10 PD-nFOG • Age 66.3/6.2 |
Australia | PD-FOG: 2.3/0.9 PD-nFOG: 2.7/0.6 |
PD-FOG: OFF 32.5 ON 26.2 PD-nFOG: OFF 23.9 ON 19.7 |
FOG-Q-item 3, Timed Up-and-Go | Not stated | OFF and ON (average of four weeks apart) | The task from Shine et al., 2011 | Epochs greater than twice the modal footstep latency |
| Matar et al., 2013 [16] | 36 PD-FOG • Age 66.2/9.6 37 PD-nFOG • Age 63.2/8.8 18 HC • Age 69.3/7.6 |
Australia | 1-4 | PD-FOG: 29.4 PD-nFOG: 21.0 |
FOG-Q-item3 | Not stated | ON | The task from Shine et al., 2011 | Not defined |
| Gilat et al., 2013 [17] | 17 PD-FOG • Age 66.1/6.5 11 PD-nFOG • Age 63.4/8.1 |
Australia | PD-FOG: 1.9/0.4 PD-nFOG: 1.8/0.6 |
PD-FOG: OFF 32.4 ON 24.2 PD-nFOG: OFF 22.2 ON 12.5 |
FOG-Q-item 3, clinical observation | Not stated | OFF and ON (average of nine weeks apart) | The task from Shine et al., 2011 with only wide doorways as environmental cue, and only direct “Walk” and “Stop” cues | Epochs greater than twice the modal footstep latency |
| Matar et al., 2014 [18] | 27 PD-FOG • Age 65.8/1.8 14 PD-nFOG • Age 62.6/7.9 |
Australia | PD-FOG: 2.3/0.9 (mean/standard error) PD-nFOG: 1.8/0.1 (mean/standard error) |
PD-FOG: 34.7 PD-nFOG: 24.1 |
FOG-Q-item 3, standardized gait assessment incorporating rapid turns | Not stated | OFF and ON (at least two weeks apart) | The task from Shine et al., 2011 with only direct “Walk” and “Stop” cues | Not defined |
| Gilat et al., 2015 [19] | 17 PD-FOG • Age 67.4/6.2 10 PD-nFOG • Age 64.8/4.1 |
Australia | PD-FOG: 2-3, PD-nFOG: 2-2.5 |
PD-FOG: 37.2 PD-nFOG: 30.1 |
FOG-Q-item 3 | Not stated | OFF | Task from Shine et al., 2011 including 90° turns without any environmental cues and with only direct “Walk” and “Stop”cues | Epochs greater than twice the modal footstep latency |
| Georgiades et al., 2016 [20] | 31 PD-FOG • Age 66.2/6.5 • 24 male/7 female 23 PD-nFOG • Age 64.7/6.8 • 18 male/5 male 15 HC • Age 64.8/5.1 • 6 male/9 female |
USA | PD-FOG: 2.2/0.4 PD-nFOG: 2.0/0.5 |
*PD-FOG: 25.8 PD-nFOG: 22.8 |
FOG-Q-item 3, MDS-UPDRS III-item 10 and 11 | Not stated | OFF | The task from Shine et al., 2011 | Epochs greater than twice the modal footstep latency |
| Ehgoetz Martens et al., 2018 [6] | 41 PD-FOG • Age 67.5/6.4 • 32 male/9 female 20 included in the fMRI analysis • Age 66.5/5.3 • 17 male/3 female |
Australia | Not stated | *All participant s: 33.9 Participants included in the fMRI analysis: 34.2 | FOG-Q-item 3, clinical observation | Not stated | OFF | The task from Shine et al., 2011 | Epochs greater than twice the modal footstep latency |
PD: Parkinson’s disease patients, PD-FOG: Parkinson’s disease patients with freezing of gait, PD-nFOG: Parkinson’s disease patients without freezing of gait, HC: healthy controls, fMRI: functional magnetic resonance imaging, UPDRS: Unified Parkinson’s Disease Rating Scale, FOG: freezing of gait, MDS-UPDRS: Movement Disorders- Unified Parkinson’s Disease Rating Scale, VR: virtual reality, FOG-Q: Freezing of gait questionnaire, NFOG-Q: New freezing of gait questionnaire, SD: standard deviation. Age is reported as mean/standard deviation. UPDRS scores are reported as mean.
MDS-UPDRS part III was used
All studies used the environment constructed by a software development kit by ID Software (Mesquite, TX, USA) for the VR paradigm.
Participants and initial categorization:
All 12 studies included participants who met criteria for the diagnosis of idiopathic PD. Aside from the inaugural VR study of PD-FOG by Naismith and Lewis [6], the other eleven studies preliminarily categorized PD participants as either PD-FOG or PD-nFOG (PD without evidence of freezing of gait). Of these 12 studies, only two included healthy controls (HC) as a baseline comparison [7,8].
Preliminary categorization of PD-FOG or PD-nFOG prior to performance in the VR paradigm varied among the twelve studies. Two studies categorized participants based solely on a subjective self-report measure, the Freezing of Gait Questionnaire (FOG-Q). Three studies by Shine et al initially categorized participants using the FOG-Q, and then confirmed FOG using the Timed-Up-and-Go (‘TUG’, which was modified to include turns). Matar et al [9] initially categorized participants using the FOG-Q, and confirmed FOG via gait assessment incorporating turns [9]. Ehgoetz Martens et al [10] initially categorized participants using the FOG-Q, and confirmed FOG in the clinical setting with observation by a movement disorders specialist. Georgiades et al [8] categorized participants using the FOG-Q and the Movement Disorders Society Unified Parkinson’s Disease Rating Scale motor exam (MDS-UPDRS part III), which includes a component for brief assessment of freezing of gait. Shine et al [11] categorized participants using the FOG-Q, modified TUG, and the MDS-UPDRS part III component to assess for FOG.
Dopaminergic state while performing the VR paradigm:
Dopaminergic state while performing the VR paradigm varied significantly between studies. PD participants in two studies performed the VR paradigm in the “on” state only [6,7]. Six studies evaluated PD participants with the VR paradigm in the “off” state only [8,10-14]. Four studies evaluated PD participants in both the “off” and “on” state, but these evaluations were performed a minimum of two weeks apart [9,15-17]. All twelve studies included PD participants taking dopaminergic medication. However, only four of these studies disclosed in detail which dopaminergic medications participants were taking [5,11,12,14]. The other eight studies included only the dopaminergic dose equivalence scores of the participants [7-11,14,16,17].
In the first study by Naismith and Lewis, participants were evaluated on their regular dopaminergic medication: four on levodopa monotherapy (one of whom also had bilateral deep brain stimulation), two were taking levodopa and entacapone, two were taking only dopamine agonists, and one was taking levodopa and a dopamine agonist. Three participants were not on dopaminergic medication [6]. In the case study by Shine et al, the participant evaluated during “on” and “off” was taking levodopa, a catechol-O-methyltransferase inhibitor, and a dopamine agonist [15]. In the next study by Shine et al, participants were evaluated while “off”, but dopaminergic medication regimens between groups were not standardized. Two participants were not taking dopaminergic medication, six were taking dopamine agonists, 30 were taking levodopa (14/30 were also taking entacapone, and 20/30 were also taking a dopamine agonist). Two patients also had apomorphine infusion, one had apomorphine infusion in addition to amantadine, and six had deep brain stimulation which was switched off an hour before testing. Three of the patients in another study by Shine et al were taking dopamine agonists [12].
VR paradigm methodology:
Naismith and Lewis [6] were the first to introduce a VR paradigm to evaluate PD-FOG. A first person representation of a virtual environment consisting of corridors with narrow and wide automated sliding doorways was created. Participants, sitting in front of the computer screen, were asked to respond with left and right hands corresponding to left and right stepping movements during the task. In the training phase, “STOP” and “WALK” signals appeared on the screen to cue the patient to stop or resume walking. In the experiment, a modified Stroop color word task with the words “RED”, “BLUE” and “GREEN” presented in congruent or incongruent colors was used for stop and walk cues. Congruent color-word pairs were used to cue walking, and incongruent color-word pairs were used as a stop cue. Following this study, Shine et al used the same paradigm by including foot pedals for the participants to respond by feet instead of hands to simulate walking [15]. All other studies in this review included the same paradigm, with only three studies [9,14,17] making minor modifications based on the aim of the study.
Definition of FOG in the VR paradigm:
There was consistency between studies regarding the definition of FOG in the VR paradigm. The first two studies used the mean step latency as a baseline measurement of normal walking cadence. Mean step latency is the average time between footsteps recorded at all time points throughout the VR paradigm, except when influenced by an external cue. Subsequent studies modified this by using modal footstep latency as the baseline gait measurement. Modal footstep latency also records time between footsteps, but in contrast to mean step latency it excludes footstep latency during freezing episodes, as well as those influenced by external cues. Therefore, modal footstep latency is a more accurate baseline measure of normal walking cadence. 10 of the 12 studies calculated the modal footstep latency (and standard deviation), and any responses greater than 2 standard deviations of this mean were considered a FOG episode.
Findings:
Findings of the studies included in this review are given in Table 2.
Table 2.
Result of the VR studies in PD-FOG
| Author, year | Aim/Hypothesis | Results | Was the aim achieved/ hypothesis confirmed? |
|---|---|---|---|
| Naismith and Lewis, 2010 [10] | To validate a VR paradigm modeling FOG without requiring the individual to walk | Inaugural study which designed the standard VR paradigm used to elicit FOG in PD participants. Worse performance on the VR paradigm correlated with higher scores on FOG-Q (p<0.05). | + |
| Shine et al., 2011 [11] | To determine the neural correlates of FOG | Case study using fMRI compatible VR which identified a distinct pattern of brain activation and deactivation underlying freezing behavior (p<0.001; corrected with a false detection rate of p<0.05). | + |
| Shine et al., 2013 [12] | To determine whether the VR measures for FOG can differentiate PD-FOG and PD-nFOG, and whether these measures correlate with FOG elicited during Timed Up-and-Go | VR induced more motor arrests in PD-FOG compared to PD-nFOG (p<0.01). The number and duration of FOG episodes during the VR paradigm positively correlated with clinically observed FOG duration during the Timed Up-and-Go (p<0.05). | + |
| Shine et al., 2013 [13] | To investigate whether increased cognitive load was associated with different activation patterns in PD-FOG and PD-nFOG | PD-FOG are unable to properly recruit specific cortical and subcortical regions within the Cognitive Control Network while performing simultaneous motor and cognitive functions (corrected with a false detection rate of p<0.05) | + |
| Shine et al., 2013 [14] | To determine neural correlates of FOG during “off” | FOG is associated with increased basal ganglia inhibitory output, leading to a decrease in thalamic and brainstem information processing (p<0.05 with family wise error correction for multiple comparisons). | + |
| Shine et al., 2013 [15] | To determine whether FOG during the VR task was associated with impaired functional connectivity between the networks likely to be involved in FOG | During FOG, PD-FOG demonstrated decreased functional connectivity between the basal ganglia and the Cognitive Control Network in each hemisphere (alpha controlled by astrict Bonferroni; p=0.01, α/5 comparisons for network activity and p=0.005, α/10 comparisons for network connectivity). | + |
| Matar et al., 2013 [16] | Greater conflict resolution and environmental stimuli requiring increased processing will be associated with delayed motor outflow in PD-FOG compared to PD-nFOG and HC. | PD-FOG had increased response latency when exposed to stimuli with higher levels of conflict resolution or when increased levels of visuospatial processing were required, compared to PD-nFOG and HC (p<0.05). | + |
| Gilat et al., 2013 [17] | VR task will reproduce the key features of gait variability in PD. | The characteristic features of gait disturbance observed in PD-FOG can also be demonstrated with a VR paradigm: stride-time variability was greater in PD-FOG vs PD-nFOG, and was partially ameliorated by dopaminergic therapy (p<0.05 one-tailed). | + |
| Matar et al., 2014 [18] | Footstep latency within the VR task will be increased during the “off” state compared to the “on” state in PD-FOG. | Motor delays when processing environmentally salient cues (wide and narrow doorways, and opening of a sliding door) improved with levodopa in PD-FOG but not PD-nFOG (p<0.05). | + |
| Gilat et al., 2015 [19] | To investigate the neural correlates of turning deficits in PD and PD-FOG | Activation of inferior frontal regions and deactivation of premotor and superior parietal cortices; increased functional connectivity between subcortical regions while turning in PD-FOG (p<0.05 with false detection rate correction in the network-based statistics software). | + |
| Georgiades et al., 2016 [20] | PD-FOG will experience footstep initiation and cessation deficits during the VR task compared to PD-nFOG and HC. | PD-FOG had more deficits in motor initiation and stopping performance, including stop failure, compared to PD-FOG and HC (p<0.05). | + |
| Ehgoetz Martens et al., 2018 [6] | During episodes of FOG, fMRI will demonstrate be abnormal functional connectivity within and between cortico-striatal circuits. Network correlates of FOG will be associated with individual differences in the cognitive, motor and limbic features of FOG | Functional connectivity during FOG is correlated with particular cognitive, motor and limbic features, which may indicate subyptes of PD-FOG. During FOG, there was an overall loss of synchrony between the cortex and the striatum, as well as a loss of segregation and specificity between examined cortico-striatal pathways (p<0.05, connectivity strength either stronger or weaker than the 97.5th (or 2.5th) percentile of the null distribution, Cohen’s d ≥0.08) | + |
FOG: freezing of gait, PD-FOG: Parkinson’s disease patients with freezing of gait, PD-nFOG: Parkinson’s disease patients without freezing of gait, VR: virtual reality, HC: healthy controls, FOG-Q: Freezing of gait questionnaire, +: achieved/confirmed.
Studies without neuroimaging:
Six of the 12 studies reviewed evaluated PD-FOG using a VR paradigm, without neuroimaging [6-9,13,17]. Naismith and Lewis [6] initially demonstrated that a VR paradigm was capable of inducing motor arrests in PD. The number of motor arrests recorded using the VR paradigm was significantly correlated with self-reported severity on the FOG-Q. In addition, performance correlated with recorded measures of disease severity, quality of life, and the hypothesized cognitive deficits in PD-FOG (deficiencies in set-shifting and concurrent motor processing).
In 2013, Shine et al [13] evaluated whether performance on a VR paradigm gait task correlated with actual episodes of FOG in patients with PD. This study demonstrated a VR task can accurately model freezing behavior in PD, and that severity of freezing during the VR task correlated with severity of freezing during the TUG assessment. Importantly, the VR task could be done while seated, which minimizes gravity and the influence of neural postural control. The study concluded by hypothesizing that freezing in PD is due to dysfunctional integration of cortical and subcortical information processing within neural networks underlying executive function.
Matar et al [7] utilized a VR paradigm (without neuroimaging) to investigate motor outflow in PD-FOG vs. PD-nFOG in settings of conflict resolution or environmental FOG triggers. The study further demonstrated the utility of VR paradigms in the evaluation of PD gait disorders – specifically the use of the maximum footstep latency to identify PD-FOG. PD-FOG had delayed response latencies which were exacerbated by the Go-No Go task and by environmental features requiring increased processing, implying an association with impaired regulation of automatic behavior.
Gilat et al [17] evaluated the feasibility of using the VR paradigm to reproduce key features of gait variability in PD-FOG. Step time variability (STV) was the primary outcome measure between groups. As the five steps preceding a freeze have been previously characterized by increased STV compared to five steps unrelated to freezing [18], STV of the five steps preceding a freezing episode was analyzed. Additionally, the footstep latencies of the three steps immediately prior to a freezing episode were measured. All participants displayed greater STV in the “off” compared to “on” state. PD-FOG had greater step time variability compared to PD-nFOG in the “off” state. The five steps leading up to a freezing episode in the VR paradigm showed a significant increase in STV, but the final three steps preceding the freeze were not characterized by a progressive shortening of latency. The study concluded a VR paradigm can reproduce the key features of stride time variability allowing a model of FOG for further studies.
Matar et al [9] compared motor performance of PD-FOG and PD-nFOG participants on a VR paradigm in the “off” and “on” states to explore the role of dopamine in doorway-provoked freezing. While “off”, PD-FOG had prolonged scaled maximum footstep latency in response to doorways. This was not observed in PD-nFOG. The study concluded that doorway provoked freezing behavior is dopamine dependent and recommended future neuroimaging studies evaluating this with the VR paradigm.
Georgiades et al [8] used a VR paradigm to examine deficits in voluntary footstep initiation and inhibition in PD-FOG. The study defined FOG as a measure of start hesitation. Specifically, as a delay in footstep initiation greater than twice the duration of a subject’s modal footstep initiation latency (most frequent duration between the presentation of a “WALK” cue and the following first step). The study further demonstrated the detrimental effects of cognitive load on voluntary step initiation and inhibition in PD-FOG. The results suggest PD-FOG have marked impairments in motor initiation and inhibition compared to PD and HC.
Overall, these studies found PD-FOG was associated with longer start hesitation, longer duration and higher number of FOG episodes, increased STV and maximum step latency, and more frequent stop failures compared to PD-nFOG [7,8,13,14,16,17] and HC [7,8]. No differences in these measures was observed between PD-nFOG and HC[7,8]. Going through narrow doorways and automated doors [7], as well as turning [14] induced a greater maximum footstep latency. Indirect cues increased the stop signal reaction time in PD-FOG compared to PD-nFOG and HC [8]. Levodopa improved the performance in PD-FOG, as shown by reduced step time variability [16], maximum step latency [9], and lower number of FOG episodes with shorter duration while “on” [15,17].
Studies with neuroimaging:
Six of the twelve studies incorporated neuroimaging while using a VR paradigm to assess PD-FOG [10-12,14-16]. Shine et al [15] initially performed a case study to investigate the utility of using a VR paradigm to elucidate the underlying mechanisms of PD-FOG with functional magnetic resonance imaging (fMRI). The results showed distinct BOLD patterns during “walking”, “dual-task walking” and during episodes of freezing. The study showed feasibility of using a VR paradigm while performing fMRI to evaluate the underlying neural correlates of PD-FOG.
In 2013, Shine et al [11] used an fMRI compatible VR paradigm to evaluate whether neural activity in response to an increased cognitive burden while walking differed in PD patients with and without FOG. This study demonstrated a VR paradigm can elicit activation patterns associated with cognitive processing in PD. PD-FOG showed reduced activation in a number of regions within the Cognitive Control Network (CCN) while attempting to process an increased cognitive load during a VR gait task.
A separate 2013 study by Shine et al [12] utilized the previously validated VR paradigm during fMRI [11] to investigate the neural correlates of freezing of gait in Parkinson’s disease. This study showed that freezing is associated with increased basal ganglia inhibitory output, leading to decreased thalamic processing and inhibition of the mesencephalic locomotor region. The study demonstrated VR and fMRI have the potential to elucidate the neural correlates underlying freezing.
Shine et al [16] then evaluated task based functional connectivity analysis to test the hypothesis that PD-FOG is secondary to dysfunctional connectivity between frontoparietal cortical regions and subcortical structures. Participants were evaluated in both the “off” on “on” states. High cognitive load in the “on” state increased recruitment of the motor network and CCN, with increased connectivity between the motor network and left CCN. But PD-FOG participants could not sustain this increase in the motor or basal ganglia networks. High cognitive load in the “off” state was coupled with increased activity in the motor network and the right CCN, and a relative decrease in activity within the motor network, basal ganglia, and ventral attention network in PD-FOG. The study found PD-FOG is associated with paroxysmal functional decoupling between widespread neural networks, including the basal ganglia network and bilateral CCN. It concluded PD-FOG is due to impaired communication between cortical and subcortical structures in the presence of an increased cognitive load and low levels of dopamine.
Gilat et al [14] used an fMRI compatible task based VR paradigm to evaluate the neural correlates underlying turning in PD-FOG compared to PD-nFOG. During turns, PD-FOG appear to have overactivity within the cortical and subcortical ‘stopping network’ (inferior frontal regions and subthalamic nucleus), with impaired pre-supplementary motor area input and failure to activate premotor and superior parietal cortices. The study therefore concluded while turning, PD-FOG have an increased propensity towards stopping with reduced sensorimotor integration, resulting in FOG.
Most recently, Ehgoetz Martens et al [10] used a task based VR paradigm to evaluate functional connectivity across cognitive, motor, and limbic domains in PD-FOG. In the cognitive network during freezing, there was decreased functional connectivity between the CCN and striatum, and increased connectivity within the CCN. There was also increased connectivity between the cerebellum, caudate, and CCN. In the motor network during freezing episodes, there was decreased connectivity within the cortical motor network and within the putamen. The motor network rearranged functional connectivity to other structures by decoupling from the putamen and limbic structures and coupling with the dorsal caudate nucleus. In the limbic network during freezing, there was increased coupling of cortical and subcortical limbic structures with the ventral striatum and CCN. There was decoupling between cortical and subcortical limbic structures and within the subcortical limbic network. The findings support the hypothesis that freezing is associated with impairments within and between cognitive, motor and affective domains.
Discussion
This review identified twelve studies using VR paradigms to evaluate PD-FOG, to date. There have been significant advances in our ability to detect, quantify, and understand PD-FOG with the advent of these studies. Initial studies focused on development and validation of the VR paradigm. Naismith and Lewis [6] and Shine et al [15] demonstrated a VR paradigm was capable of inducing motor arrests in PD participants, and that the number of motor arrests recorded using the VR paradigm significantly correlated with self-reported severity of PD-FOG. Future studies expanded on these efforts to increase our understanding of the underlying pathophysiology of PD-FOG, using functional neuroimaging. These studies found that during episodes of PD-FOG, there is dysfunctional connectivity between cortical and subcortical structures. The most recent study by Ehgoetz Martens et al [10] eloquently expanded on this foundation and proposed that PD-FOG is associated with impairments within and between cognitive, motor, and affective domains.
The VR paradigm used to evaluate PD-FOG in these studies was initially designed by Naismith and Lewis, 2010 [6]. The VR paradigm provides a first person perspective while navigating a course with some of the common triggers known to induce PD-FOG, including narrow doorways and initiation of gait. In 2011, Shine et al [15] incorporated the use of foot-pedals, rather than using hand-pedals, to navigate the VR paradigm. Freezing episodes for most studies consisted of determining the baseline time taken between footsteps (alternating depression of footpedals) with normal walking. After this modal footstep latency was calculated, freezing episodes were generally considered to be greater than two times the modal footstep latency between steps. Although logical, the definition of freezing used in these studies is arbitrary, and these measurements have not been used in the clinical setting to define a freezing episode.
There are several other important limitations of studies to date. First and perhaps most importantly, initial identification and categorization of those with PD-FOG appears flawed. Several studies identified PD-FOG solely by use of self-report measures, such as the FOG-Q. However in 2011, Shine et al evaluated the utility of the FOG-Q and found that scores on this questionnaire did not correlate with the frequency or duration of freezing episodes during objective assessment [19]. Other studies included in this review added clinical assessments to categorize PD-FOG, such as the TUG, MDS-UPDRS III, or clinical observation of freezing episodes by participants. Although the TUG is a validated measure of mobility and can be used to predict falls in PD [20,21], it has not been validated as an objective measure to assess PD-FOG. Additionally, assessment of PD-FOG by MDS-UPDRS III is performed by watching a patient walk ten meters in a straight line, turning, and then returning. PD-FOG is an episodic phenomenon which is frequently not captured during this brief clinical assessment, which does not include triggers such as walking through a narrow space or dual-tasking. Therefore, the MDS-UPDRS III is likely a specific, but not sensitive measure of PD-FOG. Indeed, a recent review by Barthel et al of measures used to assess PD-FOG concluded that a “unique methodological tool that encompasses the entire complexity of FOG is lacking” [3]. It is possible our current assumption of the underlying pathophysiology of PD-FOG is based on improper characterization of participants.
Studies of the prevalence of PD-FOG show that nearly 60% of these episodes occur while “off” levodopa and 36-38% of episodes occur while “on” levodopa [22,23]. Accordingly, there are levodopa and non-levodopa responsive subtypes of PD-FOG [24]. It is therefore important to know which subtype participants are, and which dopaminergic state PD participants are in when they perform the VR paradigm to assess PD-FOG. Additionally, the dopaminergic state impacts the fMRI findings of each PD participant. The reviewed studies did not identify PD-FOG levodopa subtypes and employed inconsistent methodology used to investigate the effects of levodopa on freezing behavior and fMRI findings in PD-FOG. The majority of studies evaluated participants only in the ‘off’ state. Four of the twelve studies evaluated participants in both the practically defined ‘off’ and ‘on’ levodopa states, and the minimum time period between assessments was two weeks apart. Given that PD-FOG is an episodic phenomenon, assessments in the ‘off’ and ‘on’ state should be performed consecutively to avoid within-subject variability and introduction of potential confounds. This is especially true for studies using fMRI, as this modality is sensitive to changes in brain metabolism and chemistry which can differ on a daily basis, adding a further layer of complexity to evaluation of fMRI changes induced by levodopa in PD-FOG [25].
Future directions:
Future studies of PD-FOG utilizing VR paradigms and neuroimaging would likely benefit from the following modifications and additions. As mentioned above, identification and quantification of PD-FOG is difficult in the clinical setting. For this reason, Ziegler et al developed a freezing assessment course designed to quantify freezing severity with a measure called the “FOG Score” [26]. This course includes the five most common triggers of PD-FOG: initiation of gait, turns, walking through a narrow doorway, dual-tasking (simple and complex), and cessation of gait. The course could improve the ability to initially identify and categorize PD-FOG, which would improve classification and subsequent reliability of results. Additionally, the course can be filmed and designed as a VR paradigm, which would augment the paradigm by Naismith and Lewis as it includes more triggers of PD-FOG. Validation of the FOG assessment course as a VR paradigm could be done simply by having participants traverse the course in reality and in VR, and comparing the results to ensure there is concordance in the calculated FOG score in both settings.
Importantly, therapeutic modalities (i.e. pharmacologic or visual and auditory cues) known to improve PD-FOG should be introduced to see how functional connectivity changes while traversing the VR paradigm. If levodopa is used, the VR paradigm should be traversed in the ‘off’ and ‘on’ state in the same day to avoid introduction of potential confounds in fMRI analysis. Other therapeutic modalities such as amantadine (which has level C evidence in the treatment of PD-FOG) and their effects on functional connectivity in PD-FOG while traversing a VR paradigm have not been evaluated to date either. PD-FOG improves with the projection of a laser line onto the floor, cueing one to step over the line in order to break the freezing episode. This has not been introduced into VR paradigms to date either [27]. Ultimately, evaluation of the effects of current therapeutic modalities on functional connectivity in a VR paradigm could allow for translation and development of newer and more effective treatments for PD-FOG.
Neuroimaging in the reviewed studies has focused on functional connectivity, but further elucidation of the underlying pathophysiology of PD-FOG would benefit from multimodal imaging of participants. Structural connectivity (i.e. diffusion tensor imaging) has shown degeneration of tracts between the pedunculopontine nucleus and the cerebellum, thalamus, and multiple regions of the frontal cortex, for example [1]. Voxel based morphometry, a measure of cortical atrophy, has also been used in previous studies and found reduced grey matter in several key areas presumed to be associated with PD-FOG [28,29]. Integration of functional connectivity analysis while performing the VR paradigm with baseline analysis of participants’ structural connectivity will provide a more comprehensive picture of brain networks.
Conclusions
There have been significant advances in our ability to evaluate and understand PD-FOG using VR paradigms. Our understanding of the underlying pathophysiology has increased significantly by incorporating evaluation of functional connectivity during freezing episodes. However, current classification of PD-FOG is suboptimal, and we can likely improve on previous VR paradigms by using the validated FOG Score assessment course. Proper evaluation of the effect of known treatments for PD-FOG, and using multimodal neuroimaging will allow for development of improved therapeutic modalities for this disabling phenomenon.
Virtual reality paradigms can elicit freezing of gait.
Freezing episodes are associated with connectivity disruptions in brain networks.
Virtual reality can help elucidate freezing of gait pathophysiology.
Acknowledgements
This publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 5P20GM109025.
Declaration of interest:
Dr. Bluett has received speaking and consulting fees from Abbvie, and speaking fees from Teva pharmaceutical. His research is supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 5P20GM109025.
Dr. Bayram does not have any interests to declare.
Dr. Litvan was a member of the Abbvie advisory board, consultant for Toyama Pharmaceuticals and member of the Biotie/Parkinson Study Group Medical Advisory Board. Her research is supported by the National Institutes of Health grants: 5P50 AG005131-31, 5T35HL007491, and 1U54NS092089-01; Parkinson Study Group, Michael J Fox Foundation, International Parkinson and Movement Disorder Society, Parkinson Foundation, AVID Pharmaceuticals, C2N Diagnostics/Abbvie and Bristol-Myers Squibb/Biogen. She receives her salary from the University of California San Diego and as Chief Editor of Frontiers in Neurology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB, Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait, Brain. 136 (2013) 2405–2418. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Okuma Y, Freezing of gait and falls in Parkinson’s disease, J. Parkinsons. Dis 4 (2014) 255–260. doi: 10.3233/JPD-130282. [DOI] [PubMed] [Google Scholar]
- [3].Barthel C, Mallia E, Debû B, Bloem BR, Ferraye MU, The Practicalities of Assessing Freezing of Gait, J. Parkinsons. Dis 6 (2016) 667–674. doi: 10.3233/JPD-160927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Plotnik M, Giladi N, Hausdorff JM, Bilateral coordination of gait and Parkinson’s disease: The effects of dual tasking, J. Neurol. Neurosurg. Psychiatry 80 (2009) 347–350. doi: 10.1136/jnnp.2008.157362. [DOI] [PubMed] [Google Scholar]
- [5].Moher D, Liberati A, Tetzlaff J, Altman DG, Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statements, PLOS Med. 6 (2009) e1000097. doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Naismith SL, Lewis SJG, A novel paradigm for modelling freezing of gait in Parkinson’s disease, J. Clin. Neurosci 17 (2010) 984–987. doi: 10.1016/j.jocn.2009.12.006. [DOI] [PubMed] [Google Scholar]
- [7].Matar E, Shine JM, Naismith SL, Lewis SJG, Using virtual reality to explore the role of conflict resolution and environmental salience in Freezing of Gait in Parkinson’s disease, Park. Relat. Disord 19 (2013) 937–942. doi: 10.1016/j.parkreldis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- [8].Georgiades MJ, Gilat M, Ehgoetz Martens KA, Walton CC, Bissett PG, Shine JM, Lewis SJG, Investigating motor initiation and inhibition deficits in patients with Parkinson’s disease and freezing of gait using a virtual reality paradigm, Neuroscience. 337 (2016) 153–162. doi: 10.1016/j.neuroscience.2016.09.019. [DOI] [PubMed] [Google Scholar]
- [9].Matar E, Shine JM, Naismith SL, Lewis SJG, Virtual reality walking and dopamine: Opening new doorways to understanding freezing of gait in Parkinson’s disease, J. Neurol. Sci 344 (2014) 182–185. doi: 10.1016/j.jns.2014.06.054. [DOI] [PubMed] [Google Scholar]
- [10].Ehgoetz Martens KA, Hall JM, Georgiades MJ, Gilat M, Walton CC, Matar E, Lewis SJG, Shine JM, The functional network signature of heterogeneity in freezing of gait, Brain. (2018). doi: 10.1093/brain/awy019. [DOI] [PubMed] [Google Scholar]
- [11].Shine JM, Matar E, Ward PB, Bolitho SJ, Pearson M, Naismith SL, Lewis SJG, Differential Neural Activation Patterns in Patients with Parkinson’s Disease and Freezing of Gait in Response to Concurrent Cognitive and Motor Load, PLoS One. 8 (2013). doi: 10.1371/journal.pone.0052602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shine JM, Matar E, Ward PB, Bolitho SJ, Gilat M, Pearson M, Naismith SL, Lewis SJG, Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease, Brain. 136 (2013) 1204–1215. doi: 10.1093/brain/awt049. [DOI] [PubMed] [Google Scholar]
- [13].Shine JM, Matar E, Bolitho SJ, Dilda V, Morris TR, Naismith SL, Moore ST, Lewis SJG, Modeling freezing of gait in Parkinson’s disease with a virtual reality paradigm, Gait Posture. 38 (2013) 104–108. doi: 10.1016/j.gaitpost.2012.10.026. [DOI] [PubMed] [Google Scholar]
- [14].Gilat M, Shine JM, Walton CC, O’Callaghan C, Hall JM, Lewis SJG, Brain activation underlying turning in Parkinson’s disease patients with and without freezing of gait: A virtual reality fMRI study, Parkinsons. Dis 1 (2015). doi: 10.1038/npjparkd.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shine JM, Ward PB, Naismith SL, Pearson M, Lewis SJG, Utilising functional MRI (fMRI) to explore the freezing phenomenon in Parkinson’s disease, J. Clin. Neurosci 18 (2011) 807–810. doi: 10.1016/j.jocn.2011.02.003. [DOI] [PubMed] [Google Scholar]
- [16].Shine JM, Matar E, Ward PB, Frank MJ, Moustafa AA, Pearson M, Naismith SL, Lewis SJG, Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia, Brain. 136 (2013) 3671–3681. doi: 10.1093/brain/awt272. [DOI] [PubMed] [Google Scholar]
- [17].Gilat M, Shine JM, Bolitho SJ, Matar E, Kamsma YPT, Naismith SL, Lewis SJG, Variability of Stepping during a Virtual Reality Paradigm in Parkinson’s Disease Patients with and without Freezing of Gait, PLoS One. 8 (2013) 1–6. doi: 10.1371/journal.pone.0066718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moreau C, Defebvre L, Bleuse S, Blatt JL, Duhamel A, Bloem BR, Destée A, Krystkowiak P, Externally provoked freezing of gait in open runways in advanced Parkinson’s disease results from motor and mental collapse, J. Neural Transm 115 (2008) 1431–1436. doi: 10.1007/s00702-008-0099-3. [DOI] [PubMed] [Google Scholar]
- [19].Shine JM, Moore ST, Bolitho SJ, Morris TR, Dilda V, Naismith SL, Lewis SJG, Assessing the utility of Freezing of Gait Questionnaires in Parkinson’s Disease, Park. Relat. Disord 18 (2012) 25–29. doi: 10.1016/j.parkreldis.2011.08.002. [DOI] [PubMed] [Google Scholar]
- [20].Morris S, Morris ME, Iansek R, Reliability of measurements obtained with the Timed “Up, & Go” Test in people with Parkinson disease, Phys. Ther 81 (2001) 810–818. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- [21].Nocera JR, Stegemöller EL, Malaty IA, Okun MS, Marsiske M, Hass CJ, Using the timed up & go test in a clinical setting to predict falling in parkinson’s disease, Arch. Phys. Med. Rehabil 94 (2013) 1300–1305. doi: 10.1016/j.apmr.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Amboni M, Stocchi F, Abbruzzese G, Morgante L, Onofrj M, Ruggieri S, Tinazzi M, Zappia M, Attar M, Colombo D, Simoni L, Ori A, Barone P, Antonini A, Prevalence and associated features of self-reported freezing of gait in Parkinson disease: The DEEP FOG study, Park. Relat. Disord 21 (2015) 644–649. doi: 10.1016/j.parkreldis.2015.03.028. [DOI] [PubMed] [Google Scholar]
- [23].Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A, Meissner WG, Schelosky L, Tison F, Rascol O, Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease, JAMA Neurol. 71 (2014) 884–890. doi: 10.1001/jamaneurol.2014.753. [DOI] [PubMed] [Google Scholar]
- [24].Bloem BR, Hausdorff JM, Visser JE, Giladi N, Falls and freezing of Gait in Parkinson’s disease: A review of two interconnected, episodic phenomena, Mov. Disord 19 (2004) 871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- [25].Skup M, Longitudinal fMRI analysis: A review of methods., Stat. Interface 3 (2010) 235–252. doi: 10.1016/j.bbi.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ziegler K, Schroeteler F, Ceballos-Baumann AO, Fietzek UM, A new rating instrument to assess festination and freezing gait in Parkinsonian patients, Mov. Disord 25 (2010) 1012–1018. doi: 10.1002/mds.22993. [DOI] [PubMed] [Google Scholar]
- [27].Egerton CJ, McCandless P, Evans B, Janssen J, Richards JD, Laserlight visual cueing device for freezing of gait in Parkinsons disease: A case study of the biomechanics involved, Physiother. Theory Pract 31 (2015) 518–526. doi: 10.3109/09593985.2015.1037874. [DOI] [PubMed] [Google Scholar]
- [28].Kostic VS, Agosta F, Pievani M, Stefanova E, Jecmenica-Lukic M, Scarale A, Spica V, Filippi M, Pattern of brain tissue loss associated with freezing of gait in Parkinson disease, Neurology. 78 (2012) 409–416. doi: 10.1212/WNL.0b013e318245d23c. [DOI] [PubMed] [Google Scholar]
- [29].Tessitore A, Amboni M, Cirillo G, Corbo D, Picillo M, Russo A, Vitale C, Santangelo G, Erro R, Cirillo M, Esposito F, Barone P, Tedeschi G, Regional gray matter atrophy in patients with Parkinson disease and freezing of gait, Am. J. Neuroradiol 33 (2012) 1804–1809. doi: 10.3174/ajnr.A3066. [DOI] [PMC free article] [PubMed] [Google Scholar]