Abstract
Hyperbranched polyamidoamines (h-PAMAM) were prepared using a one-pot reaction to have similar molecular weight to third generation PAMAM (G3-PAMAM) dendrimers, and then functionalized with N-diazeniumdiolate nitric oxide (NO) donors. A wide range of NO storage capacities (~1-2.50 μmol mg−1) and NO-release kinetics (t1/2 ~30-80 min) were achieved by changing the extent of propylene oxide (PO) modification. The therapeutic potential of these materials was evaluated by studying their antibacterial activities and toxicity against common dental pathogens and human gingival fibroblast cells, respectively. Our results indicate that the combination of NO release and PO modification is necessary to yield h-PAMAM materials with efficient bactericidal action without eliciting unwarranted cytotoxicity. Of importance, NO-releasing PO-modified h-PAMAM polymers exhibited comparable biological properties (i.e., antibacterial action and cytotoxicity) to defect-free G3-PAMAM dendrimers, but at a substantially lower synthetic burden.
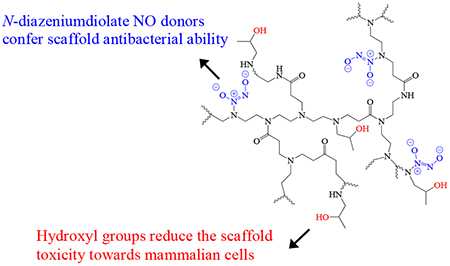
Graphical Abstract
Introduction
Oral disease is among the most prevalent health problems faced by humans.1 Gram-positive cariogenic (e.g., Streptococcus mutans, Actinomyces viscosus) and Gram-negative periodontal (e.g., Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans) bacteria represent the main aggravators associated with the evolution and progression of dental caries and periodontal disease, respectively. Unfortunately, current treatments to combat these pathogens come with undesirable side effects.2–3 For example, the systemic use of antibiotics may result in gastrointestinal disturbance and foster bacterial resistance.4 Chlorhexidine, a common oral antiseptic, can alter taste, stain teeth and tongue, and irritate buccal mucosa.5–7 As such, the development of oral therapeutics remains a vibrant research area. 8–11
Nitric oxide (NO), an endogenously produced free radical, is a potent antibacterial agent that acts on bacteria via nitrosative and oxidative stress.12–15 Our group and others have developed macromolecular NO delivery scaffolds (e.g., silica, gold, and polymeric nanoparticles) as antibacterial agents.15–21 Among these materials, dendrimers, a family of globular macromolecules with perfectly branched architecture and well-defined molecular weight, were particularly attractive due to a high density of exterior functional groups available for further modification and NO loading.22 The NO-releasing dendrimers were characterized by large NO payloads and antibacterial activities against a wide range of pathogenic bacteria, including Pseudomonas aureginosa and Staphylococcus aureus.23–25 More recently, we reported the efficacy of NO-releasing generation 1 (G1)-polyamidoamine (PAMAM) dendrimers against periodontal pathogens (i.e., P. gingivalis and A. actinomycetemcomitans).26 These dendrimers exhibited poor biocidal action against cariogenic bacteria (i.e., S. mutans and S. sanguinis). The PAMAM dendrimers were subsequently modified with long alkyl chains to facilitate antibacterial activity against the cariogenic bacteria by both membrane disruption and NO-related stress.27 The use of these dual-action dendrimers also resulted in toxicity to human gingival fibroblast cells.27–28
Higher generation NO-releasing PAMAM (e.g., G3 and G4) dendrimers were shown to exhibit greater antibacterial efficacy compared to their lower generation (e.g., G1) counterparts against P. aeruginosa and S. aureus, as a result of improved NO delivery afforded by the increased terminal group densities.24–25, 28–29 In this respect, higher generation NO-releasing PAMAM dendrimers may facilitate enhanced antibacterial activity against both cariogenic and periodontal pathogens without compromising the viability of mammalian cells. Unfortunately, the synthesis of higher generation PAMAM dendrimers is both time- and labor-intensive due to multistep purification, thus limiting scale-up and potential clinical trials.30–31
Hyperbranched polymers, an important subclass of dendritic polymers, are readily prepared in bulk via one-pot reactions with minimal purification.31–36 Compared to their structurally defect-free dendrimer counterparts, hyperbranched polymers are irregular in structure, but still retain a high density of exterior functional groups. Prior work has demonstrated that hyperbranched polyamidoamine (h-PAMAM) can be synthesized with similar unit structure and molecular weight to G3-PAMAM dendrimers.37–40 Wang et al. reported the synthesis of phenylalanine-modified h-PAMAM as a novel gene delivery vehicle with low toxicity towards mammalian cells.41 Zhang et al. evaluated the antibacterial properties of h-PAMAM against S. aureus and E. coli.42 Labena et al. demonstrated that h-PAMAM possessed broad spectrum antimicrobial and anti-biofilm action against a number of bacteria, yeast and fungal strains.43 While these studies indicate the benefits of using h-PAMAM polymers for biomedical applications, the antibacterial performance of this new NO-release material remains unknown.
Herein, we report the synthesis and characterization of N-diazeniumdiolate NO donor-modified h-PAMAM polymers. The NO-release properties (i.e., payloads and release kinetics) of h-PAMAM were evaluated as a function of chemical modifications. The potential of this scaffold as an oral therapeutic was assessed in terms of antibacterial activity to common dental pathogens and toxicity to human gingival fibroblast cells. Lastly, the properties of h-PAMAM derivatives were compared to G3-PAMAM counterparts with respect to therapeutic potential.
RESULTS AND DISCUSSION
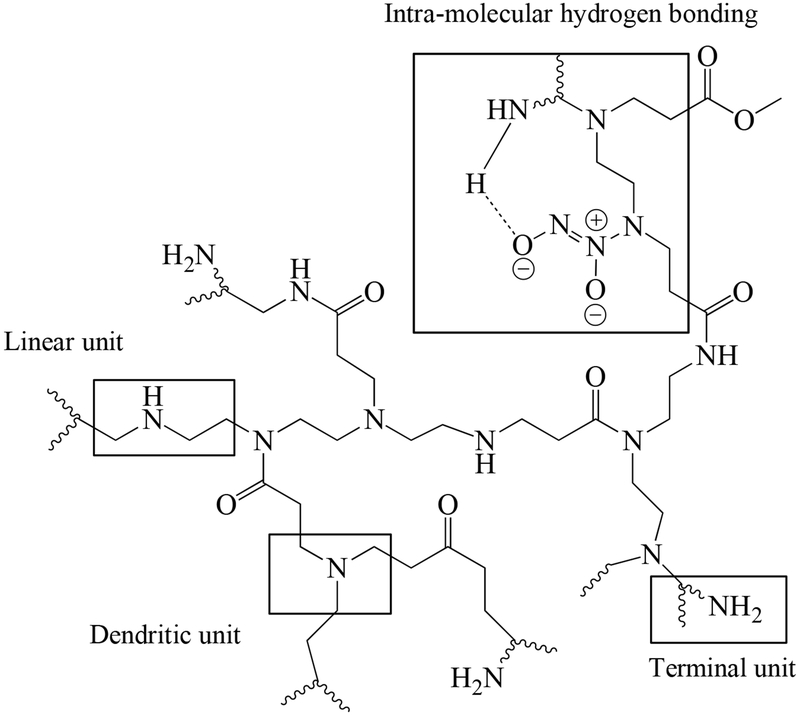
Hyperbranched polyamidoamine (h-PAMAM) polymer was synthesized by the polymerization of diethylenetriamine (DETA) and methyl acrylate (MA).38–39, 43 Size exclusion chromatography with a multi-angle light scattering (SEC-MALS) detector indicated that the weight-average molecular weight (MW) of h-PAMAM polymer was 6.39 × 103 g mol−1 with PDI of 1.89. Third generation PAMAM (G3-PAMAM; theoretical molecular weight of 6909 g mol−1) dendrimer was also prepared to investigate the influence of architecture on the properties of PAMAM scaffolds independent of molecular weight. The measured MW of G3-PAMAM dendrimer was 7.19 × 103 g mol−1 with PDI of 1.04. Relative to G3-PAMAM dendrimer (i.e., 32 primary amines per scaffold), the h-PAMAM polymer contained fewer primary amine groups (~8 primary amines per scaffold). This result was somewhat expected based on the structural differences between the two materials. Indeed, h-PAMAM polymer is composed of dendritic, linear, and terminal units (Scheme 1). The linear units induce structural defects, decreasing the number of exterior primary amines while increasing secondary amines along the polymer backbone. In contrast, structurally perfect G3-PAMAM dendrimer consists only of dendritic and terminal units.
Scheme 1.
The structure of N-diazeniumdiolate NO donor-modified h-PAMAM.
Secondary amine functionalities are necessary for forming stable N-diazeniumdiolate nitric oxide (NO) donors.22 In contrast to PAMAM dendrimer that requires a subsequent reaction to produce secondary amines prior to NO loading, h-PAMAM polymer is capable of direct reaction with NO gas to form N-diazeniumdiolates because of the secondary amines present on the linear units along the polymer backbone. To enhance NO payloads, every primary amine of h-PAMAM polymer (~8 primary amines per scaffold) was modified with one molar equivalent of propylene oxide (PO) via a ring opening reaction, yielding h-PAMAM-PO-1. For the comparative study, h-PAMAM polymer and G3-PAMAM dendrimer were modified with one molar equivalent of PO with respect to the primary amines of G3-PAMAM dendrimer (32 primary amine per scaffold), yielding h-PAMAM-PO-2 and G3-PAMAM-PO, respectively. In this manner, the impact of exterior modification on NO release properties (e.g., payloads and release kinetics) of h-PAMAM polymer could also be studied. The PO modification was verified using 1H NMR spectroscopy. As shown in Figure S1, the appearance of a distinct peak at 3.82 ppm was assigned to the protons adjacent to the hydroxyl group of the product (R2CHOH).39, 41
Nitric oxide loading and release.
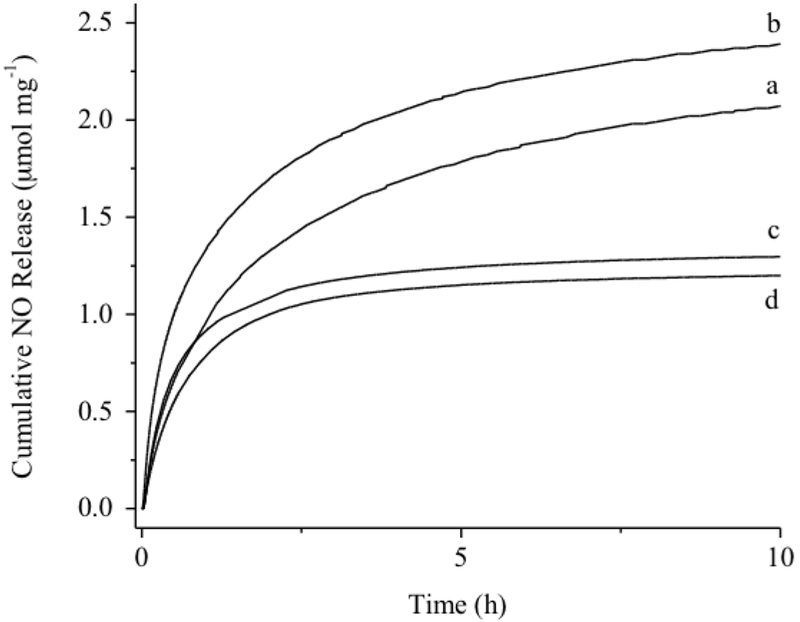
The reaction of the PAMAM scaffolds with NO gas under high pressures (10 atm) and high pH (basic conditions) yielded NO-releasing PAMAM, designated h-PAMAM/NO, h-PAMAM-PO-1/NO, h-PAMAM-PO-2/NO, and G3-PAMAM-PO/NO. Formation of N-diazeniumdiolate NO donor was verified by the appearance of a ~250 nm characteristic UV-vis peak (Figure S4).44 FT-IR spectra also indicated O-N-N-O deformation (1350-1370 cm−1) and N-N stretching (1230-1250 cm−1) vibrations.18, 44–46 Real-time NO release data was measured in PBS (10 mM, pH 7.4, 37 °C). As shown in Table 1, h-PAMAM-PO-1/NO stored the largest amount of NO (~2.50 μmol mg−1), followed by h-PAMAM/NO (~2.16 μmol mg−1). This result was anticipated given the greater number of secondary amine content of h-PAMAM-PO-1 via the PO modification. Indeed, approximately half of h-PAMAM’s primary amines were converted to secondary amines upon formation of h-PAMAM-PO-1. Additional PO modification of the h-PAMAM (i.e., h-PAMAM-PO-2) began to consume secondary amines along the polymer backbone, as evidenced in 1H NMR spectra. The greater PO conversion efficiencies (11 and 49% for h-PAMAM-PO-1 and h-PAMAM-PO-2, respectively) actually resulted in lower NO totals (~1.33 μmol mg−1) for h-PAMAM-PO-2/NO. The extent of PO modification also influenced NO-release kinetics, with faster NO release correlating with the extent of PO modification (Figure 1). For example, h-PAMAM/NO (0% PO modification) exhibited the most extended NO release (t1/2 ~80 min), while h-PAMAM-PO-2/NO (49% PO modification) released NO most rapidly (t1/2 ~30 min). As N-diazeniumdiolate anions are stabilized by neighboring cationic amines through intramolecular hydrogen bonding (Scheme 1),46–47 the PO modification of these amines likely diminished such stabilization, resulting in faster NO release (i.e., lower half-lives).
Table 1.
NO-release properties of N-diazeniumdiolate NO donor-modified PAMAM polymers in physiological buffer (PBS; 10 mM, pH 7.4, 37 °C).a
| PAMAM polymer | t[NO]b (μmol mg−1) | t[NO]2hc (μmol mg−1) | t1/2d (min) |
|---|---|---|---|
| h-PAMAM/NO | 2.16 ± 0.24 | 1.30 ± 0.24 | 78 ± 5 |
| h-PAMAM-PO-1/NO | 2.50 ± 0.39 | 1.62 ± 0.21 | 57 ± 5 |
| h-PAMAM-PO-2/NO | 1.33 ± 0.17 | 1.08 ± 0.17 | 31 ± 7 |
| G3-PAMAM-PO/NO | 1.10 ± 0.21 | 0.96 ± 0.17 | 30 ± 6 |
n ≥ 3 separate syntheses;
Total NO storage per milligram scaffold;
NO released amount for the initial 2h;
Half-life of NO release.
Figure 1.
Cumulative NO release from (a) h-PAMAM/NO; (b) h-PAMAM-PO-1/NO; (c) h-PAMAM-PO-2/NO; (d) G3-PAMAM-PO/NO in PBS (10 mM, pH 7.4, 37 °C).
The NO-release properties of h-PAMAM-PO-2/NO and G3-PAMAM/NO were nearly identical (Figure 1). In addition, the 2 h NO-release totals, corresponding to the dose of NO delivered during a 2 h bactericidal assay, were also comparable, allowing for a direct examination of PAMAM polymer structure (i.e., dendrimer vs. hyperbranched) on bactericidal action (Table 1).
Planktonic bactericidal assays.
The bactericidal activity of PAMAM scaffolds was evaluated against Gram-negative periodontal pathogens (P. gingivalis and A. actinomycetemcomitans) and Gram-positive cariogenic bacteria (S. mutans and A. viscosus). Assays were carried out over a 2 h period under static conditions. The minimum bactericidal concentration (MBC, mg mL−1), corresponding to a 3-log reduction in bacterial viability, was used to determine and compare antibacterial efficacy of the materials against the bacteria. The bactericidal NO dose was derived by multiplying the corresponding MBC values with the amount of NO delivered over the 2 h exposure time (i.e., t[NO]2h × 30 g mol−1 ) as measured in PBS by the NOA.
Polyamines have been shown to be bactericidal previously, stemming from electrostatic interactions between positively charged protonated amino groups and the negatively charged bacterial membrane.37, 48–49 This association can cause replacement of essential metal cations, thereby disrupting bacterial membrane.24, 43, 50 The h-PAMAM scaffold was hypothesized to have inherent bactericidal properties as a result of the high density of primary and secondary amines. As shown in Table 2, the Gram-negative pathogens tested were more susceptible to h-PAMAM treatment than the Gram-positive pathogens, as evidenced by the lower MBCs. This behavior is attributed to the thicker peptidoglycan layers of Gram-positive bacteria that may restrict scaffold association and mitigate membrane degradation.24–25, 29, 51 The antibacterial action of h-PAMAM was then assessed as a function of PO modification. Diminished antibacterial action was observed for h-PAMAM-PO-1 relative to h-PAMAM, likely the result of partial (~50%) exterior primary amines conversion to less potent secondary amines via the PO modification.52 It is also possible that the nonionic hydroxyl groups from PO shield cationic amines, thus inhibiting their interaction with bacterial membranes.53 Extensive PO modification of h-PAMAM as in the case of h-PAMAM-PO-2 further mitigated the antibacterial action based on the greater consumption of cationic amines and/or hydroxyl group shielding effect. In this study, the observed decrease in antibacterial action was most pronounced for the Gram-positive bacteria. For instance, the use of h-PAMAM-PO-2 did not elicit antibacterial action (as defined by a minimum 3-log viability reduction) against S. mutans even at 16 mg mL−1. Calabretta et al. similarly reported that modifying PAMAM dendrimer with neutral functional groups like polyethylene glycol (MW= 685 g mol−1) reduced the eradication potency against Gram-positive S. aureus, with little effect (i.e., potency loss) towards Gram-negative P. aeruginosa.49
Table 2.
Minimum bactericidal concentration (MBC2h) and bactericidal NO dose (μg mL−1) required to achieve 3-log reduction in bacterial viability for planktonic dental pathogens after 2 h exposure in PBS (10 mM, pH 7.4, 37 °C)a
| Periodontal pathogens (Gram-negative) | Cariogenic bacteria (Gram-positive) | |||||||
|---|---|---|---|---|---|---|---|---|
| Scaffold | A. ab | P. gingivalis | A. viscosus | S. mutans | ||||
| MBC2h (mg mL−1) | NO dose (μg mL−1) | MBC2h (mg mL−1) | NO dose (μg mL−1) | MBC2h (mg mL−1) | NO dose (μg mL−1) | MBC2h (mg mL−1) | NO dose (μg mL−1) | |
| G3-PAMAM | 0.10 | 0.50 | 1 | 8 | ||||
| h-PAMAM | 0.10 | 0.10 | 0.50 | 4 | ||||
| h-PAMAM/NO | 0.50 | 20 | 1 | 39 | 1 | 39 | 4 | 156 |
| h-PAMAM-PO-1 | 0.25 | 0.25 | 1 | 8 | ||||
| h-PAMAM-PO-1/NO | 0.50 | 24 | 0.50 | 24 | 1 | 49 | 4 | 194 |
| h-PAMAM-PO-2 | 0.50 | 2 | 4 | >16 | ||||
| h-PAMAM-PO-2/NO | 0.25 | 8 | 1 | 32 | 1 | 32 | 4 | 130 |
| G3-PAMAM-PO | 0.50 | 4 | 4 | >16 | ||||
| G3-PAMAM-PO/NO | 0.25 | 7 | 1 | 29 | 1 | 29 | 4 | 115 |
n ≥ 3 replicates.
A. a is the abbreviation for A. actinomycetemcomitans.
Nitric oxide release benefited the bactericidal action of h-PAMAM-PO-2 (Table 2). For example, the concentration of h-PAMAM-PO-2/NO required to eradicate S. mutans was < 25% of that for h-PAMAM-PO-2.12–14 However, both h-PAMAM/NO and h-PAMAM-PO-1/NO exhibited lower antibacterial action compared to controls. The charge (negative) of the N-diazeniumdiolate functionalities likely diminished the ability to associate with bacteria,24, 28 lessening the amine-directed contact killing observed for h-PAMAM and h-PAMAM-PO-1. This hypothesis is also supported by the extended NO-release rates of h-PAMAM/NO and h-PAMAM-PO-1/NO (t1/2 ~60-80 min) relative to h-PAMAM-PO-2/NO (t1/2 ~30 min). The increased NO release half-lives indicate a prolonged effect of the negatively charged N-diazeniumdiolates on h-PAMAM/NO and h-PAMAM-PO-1/NO over the entire bactericidal assay. Similar doses of NO-releasing scaffolds were required to eradicate the dental pathogens regardless of the degree of PO modification, suggesting a similar NO delivery efficacy. Consistent with previous reports, the Gram-positive bacteria evaluated here were slightly more resistant to NO treatment relative to the Gram-negative bacteria.24–25
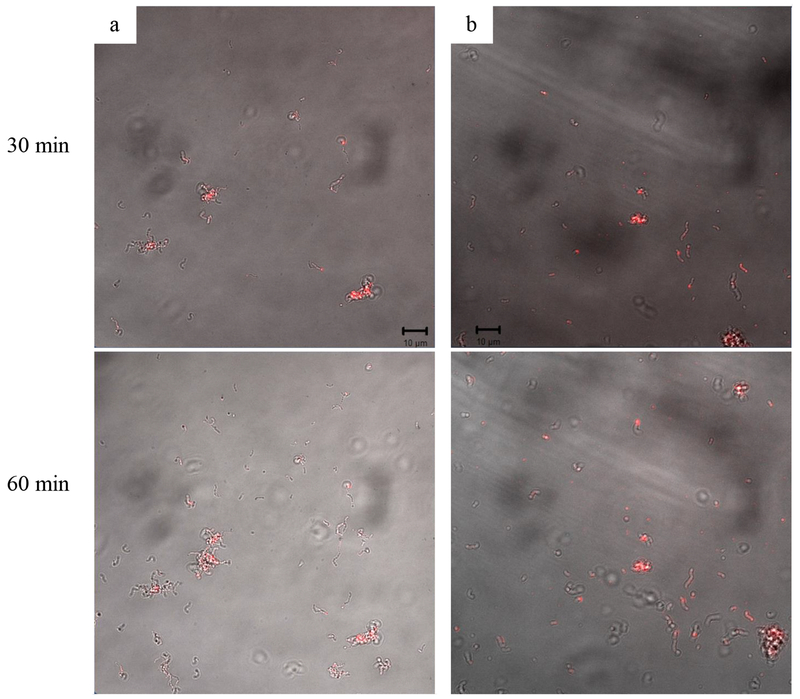
The antibacterial activities of the h-PAMAM and G3-PAMAM systems were compared to elucidate the effect of PAMAM structure on bactericidal properties. Despite a larger MBC required for unmodified G3-PAMAM dendrimers relative to h-PAMAM polymers, comparable antibacterial activities were achieved upon modification with PO for both NO-releasing and control scaffolds (Table 2). Given the identical NO-release payloads and kinetics between h-PAMAM-PO-2/NO and G3-PAMAM-PO/NO, this equivalent antibacterial activity suggests comparable polymer-bacterial association. Rhodamine B isothiocyanate (RITC)-modified h-PAMAM-PO-2 and G3-PAMAM-PO materials were prepared to facilitate visualization of the macromolecular scaffold with bacteria (S. mutans) via confocal fluorescence microscopy.27 Nearly identical fluorescence signal accumulation was observed for each time point (Figure 2), confirming the pivotal killing mechanism. Of note, prior work has pointed to the benefits of using dendrimers (e.g., G1-PAMAM-PO) as NO-release agents over other macromolecular scaffolds (e.g., silica) because of enhanced association and bactericidal efficacy.24, 26 The studies herein confirm the equivalent behavior between h-PAMAM-PO-2 and G3-PAMAM-PO, despite the imperfect structure characteristics of the hyperbranched polymer.
Figure 2.
Overlay of confocal fluorescence and bright-field images of RITC-modified PAMAM scaffolds (100 μg mL−1) association with S. mutans cells (a) h-PAMAM-PO-2; (b) G3-PAMAM-PO.
Of note, the MBC values determined for both NO-releasing hyperbranched PAMAM and G3-PAMAM were lower than that of previously reported smaller PAMAM dendrimer macromolecules (i.e., G1-PAMAM-PO/NO), especially for cariogenic bacteria (S. mutans).26, 28 This result confirms the importance of PAMAM dendrimer size in killing dental pathogens, consistent with data for other planktonic pathogens.24–26, 29 Overall, h-PAMAM-PO-2/NO is equivalent to G3-PAMAM-PO/NO with respect to bactericidal efficacy, but accessible at much lower synthetic cost.
In Vitro Cytotoxicity.
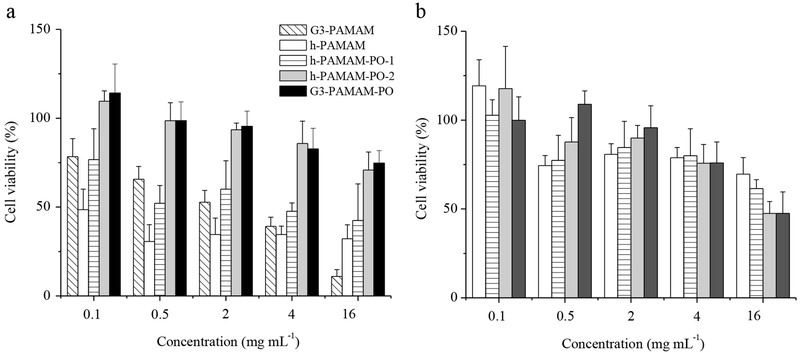
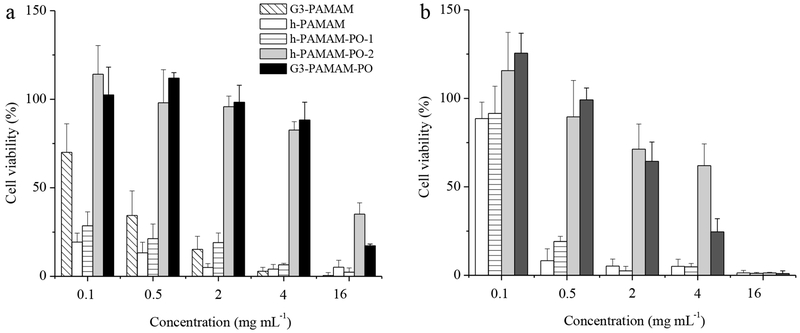
The toxicity of the PAMAM scaffolds to human gingival fibroblasts (HGF-1) was evaluated after 2 and 24 h exposure times. The 2 h incubation period was selected to correspond with the exposure time for the bactericidal assays. As shown in Figure 3, h-PAMAM proved to be the most toxic to HGF-1, likely due to the cationic amine disruption of cell membranes. The toxicity of h-PAMAM was lessened as might be expected from the reduced amine content and shielding of non-ionic hydroxyl groups. The addition of NO mitigated the toxicity of h-PAMAM and h-PAMAM-PO-1 further (Figure 3b), an effect of negatively charged N-diazeniumdiolate functional groups that restrained HGF-1 interaction.24
Figure 3.
Human gingival fibroblast (HGF-1) viability (%) following 2 h exposure to (a) control and (b) NO-releasing PAMAM scaffolds.
At 24 h incubation periods (Figure 4), h-PAMAM and h-PAMAM-PO-1 exhibited considerable HGF-1 toxicity even at low concentration (i.e., ≤ 30% viability at 0.1 mg mL−1) relative to h-PAMAM-PO-2 (i.e., ≥80% viability at 4 mg mL−1). These results suggest that partial PO modification of exterior primary amines (i.e., h-PAMAM-PO-1) is insufficient to mitigate macromolecular toxicity over extended periods. The addition of NO release to the scaffolds via NO donor modification (i.e., h-PAMAM, h-PAMAM-PO-1, and h-PAMAM-PO-2) resulted in a decreased viability of HGF-1 cells at high concentrations (> 0.5 mg mL−1) relative to controls, indicating that a high dose of NO is toxic to mammalian cells.28 In contrast, NO-releasing h-PAMAM polymers at low concentrations (0.1 mg mL−1) exhibited less toxicity versus controls, consistent with previous observations that low level of NO is tolerable and may even promote cell proliferation.27, 54–55 Even though the h-PAMAM and h-PAMAM-PO-1 materials exhibited antibacterial efficacy, the cytotoxicity data may limit their potential utility. The h-PAMAM-PO-2/NO polymer elicited minimal toxicity to HGF-1 cells at 4 mg mL−1 (the highest effective bactericidal concentration), representing an advantage of h-PAMAM-PO-2/NO over previously reported NO-releasing macromolecular scaffolds (silica nanoparticles and G1-PAMAM dendrimers).26–27
Figure 4.
Human gingival fibroblast (HGF-1) viability (%) following 24 h exposure to (a) control and (b) NO-releasing PAMAM scaffolds.
Lastly, the cytotoxicity was compared between h-PAMAM polymers and G3-PAMAM dendrimers using HGF-1 cells. Despite a lower observed toxicity for unmodified G3-PAMAM dendrimers relative to h-PAMAM polymers at concentrations <2 mg mL−1, significant toxicity was still observed for G3-PAMAM dendrimers even at 0.5 mg mL−1. Upon modification with PO, comparable toxicity was achieved for h-PAMAM polymers and G3-PAMAM dendrimers (i.e., h-PAMAM-PO-2 and G3-PAMAM-PO systems, respectively). Collectively, these data illustrate the potential of h-PAMAM-PO-2/NO as a safe antibacterial agent for oral antibacterial therapies.
CONCLUSIONS
The combination of NO release ability with propylene oxide (PO) modification on a hyperbranched PAMAM scaffold (h-PAMAM-PO-2/NO) enabled the efficient eradication of select oral pathogens with minimal toxicity to human gingival fibroblasts. Despite structural defects, the antibacterial activity of h-PAMAM-PO-2/NO was comparable to structurally perfect G3-PAMAM-PO/NO. In this regard, h-PAMAM-PO-2/NO shows promise as potentially scalable therapeutic. We are currently evaluating the antibacterial action of h-PAMAM-PO-2/NO against clinically relevant ex vivo multi-species dental biofilms. Future studies must evaluate the effects of NO release on the oral microbiome, to fully elucidate the potential therapeutic utility of NO-releasing hyperbranched polymers for oral care applications.
EXPERIMENTAL SECTION
Materials and Methods
Ethylene diamine (EDA), diethylene triamine (DETA), methyl acrylate (MA), propylene oxide (PO), 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS), and Dulbecco’s phosphate-buffered saline (DPBS) for cell culture were purchased from Sigma-Aldrich (St. Loius, MO). Streptococcus mutans (ATCC #25715), Actinomyces viscosus (ATCC #15987), and Aggregatibacter actinomycetemcomitans (ATCC #43717) were purchased from the American Type Culture Collection (Manassas, VA, USA). Porphyromonas gingivalis strain A7436 was provided by the UNC School of Dentistry, Chapel Hill, NC. CDC anaerobe 5 vol % sheep blood agar, brain heart infusion (BHI) broth and agar, and GasPak™ EZ campy container system sachets were purchased from Becton, Dickinson, and Company (Franklin Lakes, NJ). Wilkins-Chalgren (W-C) broth was purchased from Acumeida Neogen Corporation (Lansing, MI). Human gingival fibroblast cell line and FibroLife fibroblast serum-free media were purchased from Lifeline Cell Technology LLC (Frederick, MD). Pure nitric oxide (99.5%), argon, nitrogen, and nitric oxide calibration (25.87 ppm in nitrogen) were purchased from Airgas (Durham, NC). Common laboratory salts and solvents were purchased from Fisher Scientific (Pittsburgh, PA). Water was purified using a Millipore Milli-Q UV Gradient A10 System (Bethlehem, PA) to a final resistivity of 18.2 MΩ cm and total organic content of ≤10 ppb. Third generation polyamidoamine (G3-PAMAM) dendrimer was prepared by repeated alkylation and amidation reactions using MA/EDA monomer from an EDA core.28–29
Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a 400 MHz Bruker spectrometer. Carbon nuclear magnetic resonance (13C NMR) spectra were collected on a 600 MHz Bruker instrument. Size exclusion chromatography with multi-angle light scattering (SEC-MALS) was used to determine the molecular weight and polydispersity of polymer. The eluent (PBS, 0.01% azide, pH 7.4) was passed through a miniDawn TREOS multi-angle light scattering detector (Wyatt Technology; Santa Barbara, CA) coupled to a Waters 2414 refractive index detector (Waters Chromatography; Milford, MA).
Synthesis of hyperbranched polyamdioamine (h-PAMAM).
The synthesis of h-PAMAM was adapted from previous reports.37–39 Briefly, DETA (6.8 mL; 0.06 mol) and MA (6.8 mL; 0.072 mol) were mixed in methanol (10 mL) and stirred for 2 d. The reaction mixture was then heated to 60 °C for 1 h, 100 °C for 2 h, 120 °C for 2 h, and 140 °C for 2 h under a rotary evaporator to finish polymerization and remove unreacted monomer. The product (i.e., h-PAMAM) was a yellow oil. The h-PAMAM was re-dissolved in methanol at 100 mg mL−1 and stored in the freezer until future use. The h-PAMAM was characterized using 1H NMR, 13C NMR, and FTIR with peaks as follows. 1H NMR: (400 MHz, CD3OD, δ): 2.22-2.90 (COCH2, NHCH2, and NH2CH2), 3.15-3.58 (CONCH2), 3.60 (CH3O). 13C (600 MHz, CD3OD, δ): 30-60 (CH2 and CH3), 170-175 (C=O). FTIR (cm−1): 3308 (NH2), 2957 (CH2), 2848 (CH2), 1647 (C=O), and 1556 (NH).
Ninhydrin assay.
The free primary amine content of h-PAMAM was determined using ninhydrin assay.56–59 Briefly, the 2 wt% ninhydrin stock solution was prepared freshly before use by dissolving 0.2 g of ninhydrin in a mixture of 7.5 mL of DMSO and 2.5 mL of a 0.2 M sodium acetate buffer (pH 5.4). For the experiment, 2 mg of h-PAMAM was dissolved in 1 mL of sodium acetate buffer and mixed with 0.5 mL of ninhydrin stock solution. This solution was heated to 100 °C for 5 min, cooled to room temperature, and diluted 10-fold in ethanol. The absorbance was measured at 570 nm using a UV-vis Lambda 40 spectrophotometer (PerkinElmer; Waltham, MA) and compared to G3-PAMAM standard solutions prepared identically. Diethyl amine was also examined by the ninhydrin assay to investigate the potential influence of secondary amine functionalities. The results indicated that the iminium salts, product from the reaction between secondary amines and ninhydrin reagent, had negligible absorbance at 570 nm, suggesting minimal effects from the secondary amines of h-PAMAM.
Synthesis of N-diazeniumdiolate nitric oxide donor-modified PAMAM scaffolds.
Secondary amine-modified PAMAM scaffolds (including h-PAMAM and G3-PAMAM) were synthesized as previously described.24, 29In attempt to reach maximum secondary amines, 300 mg of h-PAMAM was reacted with 24 μL of propylene oxide (PO) (i.e., 1 equivalent amount with respect to the molar amount of primary amines of h-PAMAM), yielding h-PAMAM-PO-1. To achieve comparable NO-release properties, 300 mg of h-PAMAM or G3-PAMAM was reacted with 97 μL of PO (i.e., 1 equivalent amount with respect to the molar amount of primary amines of G3-PAMAM), yielding h-PAMAM-PO-2 or G3-PAMAM-PO. The reagents were mixed in 6 mL of methanol and stirred for 3 days. Unreacted PO and solvent were removed under reduced pressure. 1H NMR data of the resulting PO-modified PAMAM scaffolds consisted of the following peaks: h-PAMAM-PO-1 or h-PAMAM-PO-2 (400 MHz, D2O, δ): 1.05 (NHCH2CH(OH)CH3), 2.22-2.90 (COCH2, NHCH2, and NH2CH2), 3.15-3.58 (CONHCH2), 3.60 (CH3O), and 3.82 (NHCH2CH(OH)CH3). G3-PAMAM-PO consisted of the following peaks (400 MHz, D2O, δ): 1.05 (NHCH2CH(OH)CH3), 2.37 (CH2N(CH2CH2CO)2), 2.38-2.78 (NCH2, NHCH2), 3.08−3.28 (CONHCH2CH2), and 3.82 (NHCH2CH(OH)CH3). According to the 1H NMR data, the conversion efficiencies for h-PAMAM-PO-1, h-PAMAM-PO-2, and G3-PAMAM-PO were estimated to be 11, 49, and 63%, respectively.28, 41
The scaffolds containing secondary amines were exposed to high pressure of gaseous NO under basic conditions, yielding N-diazeniumdiolate NO donors.22, 25, 28–29 Specifically, 50 mg of scaffold (h-PAMAM, h-PAMAM-PO-1, h-PAMAM-PO-2, or G3-PAMAM-PO) was combined with 50 μL of NaOMe (5.4 M in MeOH; approximately 1.2 equivalent molar amount relative to primary amines of G3-PAMAM) in 1 mL of anhydrous methanol. The solutions were placed in a Parr hydrogenation reactor and stirred continuously. The reactor was purged with argon 6 times to remove oxygen, and then pressurized to 10 atm with NO gas for 3 d to yield NO donor-modified systems (i.e., h-PAMAM/NO, h-PAMAM-PO-1/NO, h-PAMAM-PO-2/NO or G3-PAMAM-PO/NO). The reactor was then purged with argon to remove unreacted NO, and solvent removed under reduced pressure. The NO-releasing materials were re-dissolved in anhydrous MeOH as 50 mg mL−1 and stored at −20 °C until later use.
Characterization of nitric oxide release.
Nitric oxide-releasing PAMAM scaffolds (1 mg) in 20 μL of MeOH were added to deoxygenated 10 mM phosphate buffered saline (30 mL, pH 7.4) at 37 °C. Nitrogen was bubbled through this solution at a flow rate of 70 mL min−1 to carry the liberated NO to a Sievers chemiluminescence nitric oxide analyzer (Boulder, CO). Additional nitrogen flow was provided into the flask to match the collection rate of the instrument (200 mL min−1). Real-time NO release profiles were recorded until the observed NO levels decreased to below 10 ppb mg−1 scaffold.
Planktonic bactericidal assays.
Planktonic bacteria (i.e., P. gingivalis, A. actinomycetemcomitans, S. mutans, and A. viscosus) were stored in 15 vol% glycerol PBS at −80 °C. To perform the bactericidal assay, this frozen stock was incubated in BHI broth (W-C anaerobic broth for P. gingivalis) at 37 °C overnight. A 500 μL aliquot of this solution was added into fresh broth and incubated at 37 °C until the bacterial concentration reached 1×108 colony forming units per milliliter (CFU mL−1), as determined by optical density (OD, 600 nm). P. gingivalis was cultured anaerobically in an atmosphere of 5 vol% CO2, 10 vol% H2, and 85 vol% N2. A. actinomycetemcomitans and A. viscosus were cultured under a microaerophilic environment (6 −16 vol% O2 and 2-10 vol% CO2) in a GasPak EZ Campy Container System (Becton, Dickinson and Company; Franklin Lakes, NJ). S. mutans was cultured aerobically. Prior to the 2h planktonic bactericidal assay, bacteria were diluted to 106 CFU mL−1 in 1 vol% BHI-supplemented PBS (W-C anaerobic broth for P. gingivalis). The NO-releasing or corresponding control materials were then introduced at 37 °C. Of note, the addition of broth had negligible effect on the NO-release totals (i.e., NO-release totals were ≤5% lower in 1 vol% broth-supplemented PBS). At 2h, the bacteria suspensions were diluted 10- and 100-fold, and spiral plated using BHI agar (CDC Anaerobic agar for P. gingivalis). To quantify the antibacterial capacities of materials against planktonic bacteria, the minimum bactericidal concentration (i.e., the minimum concentration of materials required to achieve a 3-log reduction in viability after 2 hours) was determined by counting the colonies formed on the agar plate. Of note, the detection limit for the plate counting method was 2.5×103 CFU mL−1.60
Confocal Microscopy.
Rhodamine B isothiocyanate (RITC)-labeled h-PAMAM-PO-2 and G3-PAMAM-PO were synthesized following prior reports.25, 27–28 S. mutans was cultured as described above and diluted to 107 CFU mL−1 with PBS. This bacterial solution (3 mL) was incubated in a glass bottom confocal dish for 30 min at 37 °C. A Zeiss 510 Meta inverted laser scanning confocal microscope (Carl Zeiss; Thornwood, NY) with a 543 nm HeNe excitation laser (1.0 mW, 25.0% intensity) and BP 560−615 nm filter was used to obtain fluorescence images of the RITC-modified PAMAM-PO. Both bright field and fluorescence images were collected using a N.A.1.2 C-apochromat water immersion lens with a 40× objective. RITC-labeled PAMAM-PO was added to the bacteria solution to achieve a final concentration of 100 μg mL−1. Images were collected every 10 min to visualize the association of PAMAM-PO with the bacteria.24
In Vitro Cytotoxicity.
Human gingival fibroblasts (HGF-1) were grown in FibroLife fibroblast serum-free media, and incubated in 5 vol % CO2 under humidified conditions at 37 °C. The cells were trypsinized at 80% confluency and seeded into tissue culture-treated polystyrene 96-well plates at a density of ~104 cells/well. The plates were incubated for an additional 24 h at 37 °C. The supernatant was aspirated and replaced with 100 μL of fresh growth medium containing various concentrations of PAMAM scaffolds. After two incubation time points (i.e., 2 and 24 h) at 37 °C, the supernatant was aspirated and the cells were washed with DPBS. A 100 μL solution of media/MTS/PMS (105/20/1, v/v/v) solution was added to each well, and incubated for 3 h at 37 °C. The absorbance of the colored supernatant was quantified at 490 nm using a Thermoscientific Multiskan EX plate reader (Waltham, MA). Measurements for untreated cells (control) and media/MTS/PMS mixture (blank) were also collected. Results were expressed as percentage of relative cell viability as follows:
| (Eq. 1) |
A killing curve was constructed for NO-releasing and control PAMAM scaffolds by plotting % cell viability versus concentration (mg mL−1).
Supplementary Material
ACKNOWLEGDMENTS
We thank Professor David B. Hill and Professor Mehmet Kesimer of the Marsico Lung Institute at University of North Carolina at Chapel Hill for providing access to SEC-MALS instrument. We would also thank Robert Currin at the Hooker Imaging Core of North Carolina at Chapel Hill for confocal microscopy assistance. Lastly, we acknowledge Dr. Brittany V. Worley for the preparation of G3-PAMAM dendrimers.
Funding
Financial support was provided by the National Institutes of Health (DE025207). A portion of this work was performed using the FTIR instrument in the UNC EFRC Instrumentation Facility established by the UNC EFRC Center for Solar Fuels, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award DE-SC0001011.
Footnotes
Supporting Information
NMR spectra, FT-IR spectra, and UV-vis spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare the following competing financial interest(s): Mark H. Schoenfisch is a co-founder and maintains a financial interest in Novan, Inc. and Novoclem Therapeutics, Inc. Both companies are commercializing macromolecular nitric oxide storage and release vehicles as therapeutics.
REFERENCES
- (1).Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C (2005) The global burden of oral diseases and risks to oral health. Bull. World Health Organ 83, 661–669. [PMC free article] [PubMed] [Google Scholar]
- (2).Slots J, Rams TE (1990) Antibiotics in periodontal therapy: advantages and disadvantages. J. Clin. Periodontol 17, 479–493. [DOI] [PubMed] [Google Scholar]
- (3).Jones CG (1997) Chlorhexidine: is it still the gold standard? Periodontology 2000 15, 55–62. [DOI] [PubMed] [Google Scholar]
- (4).Radvar M, Pourtaghi N, Kinane D (1996) Comparison of 3 periodontal local antibiotic therapies in persistent periodontal pockets. J. Periodontol 67, 860–865. [DOI] [PubMed] [Google Scholar]
- (5).Van Strydonck D, Timmerman M, Van Der Velden U, Van Der Weijden G (2005) Plaque inhibition of two commercially available chlorhexidine mouthrinses. J. Clin. Periodontol 32, 305–309. [DOI] [PubMed] [Google Scholar]
- (6).Charbonneau D, Snider A (1997) Reduced chlorhexidine tooth stain coverage by sequential administration of monoperoxyphthalic acid in the beagle dog. J. Dent. Res 76, 1596–1601. [DOI] [PubMed] [Google Scholar]
- (7).Imfeld T (1999) Chewing gum—facts and fiction: a review of gum-chewing and oral health. Crit. Rev. Oral. Biol. Med 10, 405–419. [DOI] [PubMed] [Google Scholar]
- (8).Paster BJ, Olsen I, Aas JA, Dewhirst FE (2006) The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontology 2000 42, 80–87. [DOI] [PubMed] [Google Scholar]
- (9).Silva B. R. d., Freitas V. A. A. d., Nascimento-Neto LG, Carneiro VA, Arruda FVS, Aguiar A. S. W. d., Cavada BS, Teixeira EH (2012) Antimicrobial peptide control of pathogenic microorganisms of the oral cavity: A review of the literature. Peptides 36, 315–321. [DOI] [PubMed] [Google Scholar]
- (10).Paula A, Koo H (2017) Nanosized building blocks for customizing novel antibiofilm approaches. J. Dent. Res 96, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Gao L, Koo H (2017) Do catalytic nanoparticles offer an improved therapeutic strategy to combat dental biofilms? Nanomed. Nanotech. Biol. Med 12, 275–279. [DOI] [PubMed] [Google Scholar]
- (12).Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, Cebula TA, Koch WH, Andrews AW, Allen JS et al. (1991) DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science, 1001–1003. [DOI] [PubMed] [Google Scholar]
- (13).Jones ML, Ganopolsky JG, Labbé A, Wahl C, Prakash S (2010) Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol 88, 401–407. [DOI] [PubMed] [Google Scholar]
- (14).Carpenter AW, Schoenfisch MH (2012) Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev 41, 3742–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, Schoenfisch MH (2008) Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. ACS nano 2, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wo Y, Brisbois EJ, Bartlett RH, Meyerhoff ME (2016) Recent advances in thromboresistant and antimicrobial polymers for biomedical applications: just say yes to nitric oxide (NO). Biomater. Sci 4, 1161–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Duong HTT, Adnan NNM, Barraud N, Basuki JS, Kutty SK, Jung K, Kumar N, Davis TP, Boyer C (2014) Functional gold nanoparticles for the storage and controlled release of nitric oxide: applications in biofilm dispersal and intracellular delivery. J. Mater. Chem. B 2, 5003–5011. [DOI] [PubMed] [Google Scholar]
- (18).Park D, Kim J, Lee YM, Park J, Kim WJ (2016) Polydopamine Hollow Nanoparticle Functionalized with N‐diazeniumdiolates as a Nitric Oxide Delivery Carrier for Antibacterial Therapy. Adv. Healthcare Mater 5, 2019–2024. [DOI] [PubMed] [Google Scholar]
- (19).Nguyen T-K, Selvanayagam R, Ho KK, Chen R, Kutty SK, Rice SA, Kumar N, Barraud N, Duong HT, Boyer C (2016) Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem. Sci 7, 1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Duong HTT, Jung K, Kutty SK, Agustina S, Adnan NNM, Basuki JS, Kumar N, Davis TP, Barraud N, Boyer C (2014) Nanoparticle (Star Polymer) Delivery of Nitric Oxide Effectively Negates Pseudomonas aeruginosa Biofilm Formation. Biomacromolecules 15, 2583–2589. [DOI] [PubMed] [Google Scholar]
- (21).Park J, Kim J, Singha K, Han D-K, Park H, Kim WJ (2013) Nitric oxide integrated polyethylenimine-based tri-block copolymer for efficient antibacterial activity. Biomaterials 34, 8766–8775. [DOI] [PubMed] [Google Scholar]
- (22).Stasko NA, Schoenfisch MH (2006) Dendrimers as a scaffold for nitric oxide release. J. Am. Chem. Soc 128, 8265–8271. [DOI] [PubMed] [Google Scholar]
- (23).Lu Y, Sun B, Li C, Schoenfisch MH (2011) Structurally Diverse Nitric Oxide-Releasing Poly(propylene imine) Dendrimers. Chem. Mater 23, 4227–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Sun B, Slomberg DL, Chudasama SL, Lu Y, Schoenfisch MH (2012) Nitric oxide-releasing dendrimers as antibacterial agents. Biomacromolecules 13, 3343–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Worley BV, Slomberg DL, Schoenfisch MH (2014) Nitric oxide-releasing quaternary ammonium-modified poly (amidoamine) dendrimers as dual action antibacterial agents. Bioconjugate Chem. 25, 918–927. [DOI] [PubMed] [Google Scholar]
- (26).Backlund CJ, Sergesketter AR, Offenbacher S, Schoenfisch MH (2014) Antibacterial efficacy of exogenous nitric oxide on periodontal pathogens. J. Dent. Res 93, 1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Backlund CJ, Worley BV, Schoenfisch MH (2016) Anti-biofilm action of nitric oxide-releasing alkyl-modified poly(amidoamine) dendrimers against Streptococcus mutans. Acta biomaterialia 29, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Worley BV, Schilly KM, Schoenfisch MH (2015) Anti-biofilm efficacy of dual-action nitric oxide-releasing alkyl chain modified poly (amidoamine) dendrimers. Mol. Pharm 12, 1573–1583. [DOI] [PubMed] [Google Scholar]
- (29).Lu Y, Slomberg DL, Shah A, Schoenfisch MH (2013) Nitric oxide-releasing amphiphilic poly (amidoamine)(PAMAM) dendrimers as antibacterial agents. Biomacromolecules 14, 3589–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Tomalia D, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1985) A New Class of Polymers: Starburst-Dendritic. Polym. J 17, 117–132. [Google Scholar]
- (31).Gao C, Yan D (2004) Hyperbranched polymers: from synthesis to applications. Prog. Polym. Sci 29, 183–275. [Google Scholar]
- (32).Caminade A-M, Yan D, Smith DK (2015) Dendrimers and hyperbranched polymers. Chem. Soc. Rev 44, 3870–3873. [DOI] [PubMed] [Google Scholar]
- (33).Wang D, Zhao T, Zhu X, Yan D, Wang W (2015) Bioapplications of hyperbranched polymers. Chem. Soc. Rev 44, 4023–4071. [DOI] [PubMed] [Google Scholar]
- (34).Jin H, Huang W, Zhu X, Zhou Y, Yan D (2012) Biocompatible or biodegradable hyperbranched polymers: from self-assembly to cytomimetic applications. Chem. Soc. Rev 41, 5986–5997. [DOI] [PubMed] [Google Scholar]
- (35).Zheng Y, Li S, Weng Z, Gao C (2015) Hyperbranched polymers: advances from synthesis to applications. Chem. Soc. Rev 44, 4091–4130. [DOI] [PubMed] [Google Scholar]
- (36).Yang L, Lu Y, Soto RJ, Shah A, Ahonen MJR, Schoenfisch MH (2016) S-Nitrosothiol-modified hyperbranched polyesters. Polym. Chem 7, 7161–7169. [PMC free article] [PubMed] [Google Scholar]
- (37).Zhang F, Chen Y, Lin H, Lu Y (2007) Synthesis of an amino‐terminated hyperbranched polymer and its application in reactive dyeing on cotton as a salt‐free dyeing auxiliary. Color. Technol 123, 351–357. [Google Scholar]
- (38).Cao L, Yang W, Wang C, Fu S (2007) Synthesis and striking fluorescence properties of hyperbranched poly (amido amine). J. Macromol. Sci. Pure Appl. Chem 44, 417–424. [Google Scholar]
- (39).Liu C, Gao C, Yan D (2006) Synergistic supramolecular encapsulation of amphiphilic hyperbranched polymer to dyes. Macromolecules 39, 8102–8111. [Google Scholar]
- (40).Zhang D, Chen L, Zang C, Chen Y, Lin H (2013) Antibacterial cotton fabric grafted with silver nanoparticles and its excellent laundering durability. Carbohydr. Polym 92, 2088–2094. [DOI] [PubMed] [Google Scholar]
- (41).Wang X, He Y, Wu J, Gao C, Xu Y (2009) Synthesis and evaluation of phenylalanine-modified hyperbranched poly (amido amine) s as promising gene carriers. Biomacromolecules 11, 245–251. [DOI] [PubMed] [Google Scholar]
- (42).Zhang F, Zhang D, Chen Y, Lin H (2009) The antimicrobial activity of the cotton fabric grafted with an amino-terminated hyperbranched polymer. Cellulose 16, 281–288. [Google Scholar]
- (43).Labena A, Kabel K, Farag R (2016) One-pot synthesize of dendritic hyperbranched PAMAM and assessment as a broad spectrum antimicrobial agent and anti-biofilm. Mater. Sci. Eng C 58, 1150–1159. [DOI] [PubMed] [Google Scholar]
- (44).Keefer LK, Flippen-Anderson JL, George C, Shanklin AP, Dunams TM, Christodoulou D, Saavedra JE, Sagan ES, Bohle DS (2001) Chemistry of the Diazeniumdiolates I. Structural and Spectral Characteristics of the [N (O) NO]− Functional Group. Nitric Oxide 5, 377–394. [DOI] [PubMed] [Google Scholar]
- (45).Liu T, Zhang W, Song T, Yang X, Li C (2015) Hollow double-layered polymer microspheres with pH and thermo-responsive properties as nitric oxide-releasing reservoirs. Polym. Chem 6, 3305–3314. [Google Scholar]
- (46).Zhou Z, Annich GM, Wu Y, Meyerhoff ME (2006) Water-soluble poly (ethylenimine)-based nitric oxide donors: preparation, characterization, and potential application in hemodialysis. Biomacromolecules 7, 2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Shin JH, Schoenfisch MH (2007) Inorganic/organic hybrid silica nanoparticles as a nitric oxide delivery scaffold. Chem. Mater 20, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Lenoir S, Pagnoulle C, Galleni M, Compère P, Jérôme R, Detrembleur C (2006) Polyolefin matrixes with permanent antibacterial activity: preparation, antibacterial activity, and action mode of the active species. Biomacromolecules 7, 2291–2296. [DOI] [PubMed] [Google Scholar]
- (49).Calabretta MK, Kumar A, McDermott AM, Cai C (2007) Antibacterial activities of poly (amidoamine) dendrimers terminated with amino and poly (ethylene glycol) groups. Biomacromolecules 8, 1807–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Gibney KA, Sovadinova I, Lopez AI, Urban M, Ridgway Z, Caputo GA, Kuroda K (2012) Poly (ethylene imine) s as antimicrobial agents with selective activity. Macromol. Biosci 12, 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Kawahara K, Tsuruda K, Morishita M, Uchida M (2000) Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent. Mater 16, 452–455. [DOI] [PubMed] [Google Scholar]
- (52).Boas U, Heegaard PM (2004) Dendrimers in drug research. Chem. Soc. Rev 33, 43–63. [DOI] [PubMed] [Google Scholar]
- (53).Xu F, Chai M, Li W, Ping Y, Tang G, Yang W, Ma J, Liu F (2010) Well-defined poly (2-hydroxyl-3-(2-hydroxyethylamino) propyl methacrylate) vectors with low toxicity and high gene transfection efficiency. Biomacromolecules 11, 1437–1442. [DOI] [PubMed] [Google Scholar]
- (54).Carpenter AW, Worley BV, Slomberg DL, Schoenfisch MH (2012) Dual action antimicrobials: nitric oxide release from quaternary ammonium-functionalized silica nanoparticles. Biomacromolecules 13, 3334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Nichols SP, Storm WL, Koh A, Schoenfisch MH (2012) Local delivery of nitric oxide: Targeted delivery of therapeutics to bone and connective tissues. Adv. Drug Deliv. Rev 64, 1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Kailasan A, Yuan Q, Yang H (2010) Synthesis and characterization of thermoresponsive polyamidoamine–polyethylene glycol–poly (d, l-lactide) core–shell nanoparticles. Acta Biomater. 6, 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Stasko NA, Johnson CB, Schoenfisch MH, Johnson TA, Holmuhamedov EL (2007) Cytotoxicity of polypropylenimine dendrimer conjugates on cultured endothelial cells. Biomacromolecules 8, 3853–3859. [DOI] [PubMed] [Google Scholar]
- (58).Luo D, Haverstick K, Belcheva N, Han E, Saltzman WM (2002) Poly (ethylene glycol)-conjugated PAMAM dendrimer for biocompatible, high-efficiency DNA delivery. Macromolecules 35, 3456–3462. [Google Scholar]
- (59).Sun Y-X, Yang B, Chen S, Lei Q, Feng J, Qiu X-F, Dong N-G, Zhuo R-X, Zhang X-Z (2013) Oligoamines grafted hyperbranched polyether as high efficient and serum-tolerant gene vectors. Colloids Surf. B 111, 732–740. [DOI] [PubMed] [Google Scholar]
- (60).Breed RS, Dotterrer W (1916) The number of colonies allowable on satisfactory agar plates. J. Bacteriol 1, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.