Abstract
Introduction:
The advent of checkpoint blockade immunotherapy has revolutionized cancer treatment, but clinical response to immunotherapies is highly heterogeneous among individual patients and between cancer types. This represents a challenge to oncologists when choosing specific immunotherapies for personalized medicine. Thus, biomarkers that can predict tumor responsiveness to immunotherapies before and during treatment are invaluable.
Areas covered:
We review the latest advances in ‘liquid biopsy’ biomarkers for noninvasive prediction and in-treatment monitoring of tumor response to immunotherapy, focusing primarily on melanoma and non-small cell lung cancer. We concentrate on high-quality studies published within the last five years on checkpoint blockade immunotherapies, and highlight significant breakthroughs, identify key areas for improvement, and provide recommendations for how these diagnostic tools can be translated into clinical practice.
Expert opinion:
The first biomarkers proposed to predict tumor response to immunotherapy were based on PD1/PDL1 expression, but their predictive value is limited to specific cancers or patient populations. Recent advances in single-cell molecular profiling of circulating tumor cells and host cells using next-generation sequencing has dramatically expanded the pool of potentially useful predictive biomarkers. As immunotherapy moves toward personalized medicine, a composite panel of both genomic and proteomic biomarkers will have enormous utility in therapeutic decision-making.
Keywords: Checkpoint blockade immunotherapy, liquid biopsy, noninvasive diagnostics, circulating tumor cells, personalized medicine
1. Introduction and organization of review
The explosion of research into checkpoint blockade immunotherapies (CBI) is owed to their resounding clinical success in dramatically increasing survival rates across multiple cancer types, most notably metastatic melanoma which historically has had an extremely poor prognosis. In recognition of this achievement, James P. Allison and Tasuku Honjo jointly received the 2018 Nobel Prize in Physiology or Medicine for their fundamental contributions to the discovery of CBI. The list of cancers with FDA-approved indications for CBI now include metastatic melanoma, non-small cell lung cancers, renal cell carcinoma, head and neck squamous cell carcinoma, and bladder cancer, with a multitude of other tumor-therapy combinations under investigation in ongoing clinical trials.
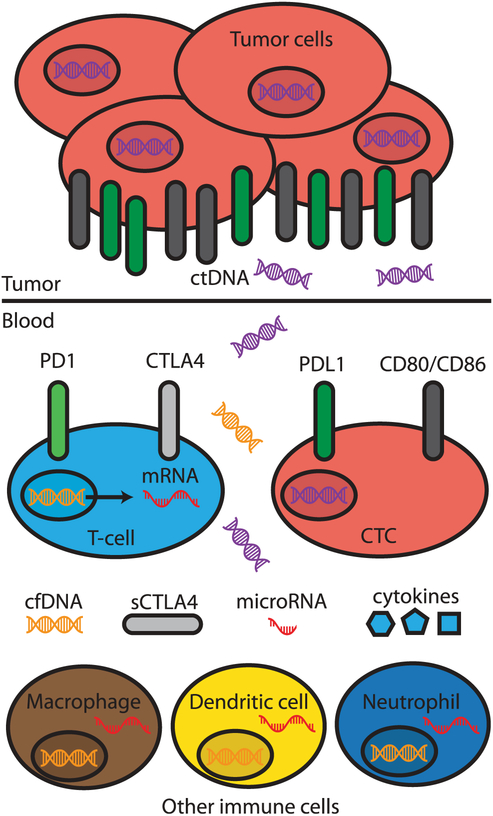
Despite the success of CBI, several barriers still exist in extending clinical benefit to a greater number of patients. Although checkpoint immunotherapies work well for patients that do achieve clinical responses, a subset of patients do not respond or respond poorly to the same treatment. At present, it is not well understood how and why this occurs. Recent work has implicated dynamic changes in both host immunology as well as heterogeneities in tumor genetics and microenvironment. In addition, there is no consensus as to which objective metrics best enable prediction of clinical response. Identification of such metrics would enable oncologists to choose specific therapies before initiation, and potentially adapt and modify therapeutic strategies as they are monitored throughout therapy. Biomarkers reflecting tumor immune microenvironment and tumor cell-intrinsic features, obtained directly from tumor samples, have been studied as potential markers of response to CBI. Examples include intratumor PDL1 expression, density of tumor-infiltrating lymphocyte (TIL), tumor mutational burden (TMB) [1], tumor transcriptomics [2,3], and tumor mismatch-repair (MMR) deficiency [4], which have been shown to predict treatment effects of CBI [5]. However, these biomarkers require invasive sampling and are not practical from a risk-benefit standpoint for monitoring tumor response during treatment. Circulating ‘liquid biopsy’ biomarkers have recently shown promise as metrics predictive of tumor immunotherapy response, because they can be non-invasively obtained from patients and trended over time (Figure 1) [6]. In this review, we begin by providing a brief overview of the FDA-approved checkpoint blockade immunotherapies, their mechanisms of actions, and the basic immunology of checkpoint inhibitors. We then explore and synthesize findings from studies published in the last five years identifying potential biomarkers predictive of clinical response for different cancer types and immunotherapies. We outline the major classes of potential biomarkers, highlight significant breakthroughs, identify key areas for improvement, and provide expert recommendations for how these diagnostic tools can be translated into clinical practice. Identification of promising biomarkers can potentially expedite the implementation of personalized medicine in cancer immunotherapy.
Figure 1.
Overview of potential circulating ‘liquid biopsy’ biomarkers predictive of treatment response to checkpoint blockade immunotherapy. Genomic, transcriptomic, proteomic, and immunologic biomarkers are depicted.
2. Overview of immunotherapies: drug classes and mechanisms of action
Checkpoint inhibitor immunotherapies are monoclonal antibodies directed at disrupting either the cytotoxic T lymphocyte-associated antigen 4 (CTLA4) pathway or the programmed cell death protein 1 pathway (PD1/PDL1). At present, the seven FDA-approved cancer immunotherapies are ipilimumab (CTLA4), nivolumab (PD1), pembrolizumab (PD1), atezolizumab (PDL1), avelumab (PDL1), durvalumab (PDL1), and cemiplimab (PD1) [7]. Ipilimumab is the only FDA-approved CTLA4-based therapy and was the first immunotherapy to market, while the most recently approved therapy was cemiplimab.
During normal T cell-mediated immune responses, T lymphocytes patrol the body for signs of infection, disease, or cancer. Before initiating a response, they first probe the target for cell surface markers, which may reveal its identity as healthy or unhealthy. Recognition of peptide antigens on unhealthy cells or on antigen-presenting cells (APCs) via the T cell receptor (TCR) leads to T cell activation and proliferation. T cells also normally express PD1 and CTLA4 on their surface, which are inhibitory receptors that prevent T cell activation when bound by ligands PDL1 or CD80/CD86, respectively [8,9]. Tumors may escape this type of surveillance by aberrantly expressing PDL1 or CD80/CD86, which activate PD1 or CTLA4, inducing inhibition of T cell activation and proliferation. The detailed immunology of CBI is reviewed thoroughly elsewhere [8].
The newest class of immunotherapies, chimeric antigen receptor (CAR) T cells, do not rely on checkpoint inhibition. Instead, they induce anti-tumor immunity by customizing the patient’s T cells to recognize specific cell-surface markers on the target cancer. The process involves isolating the patient’s own T cells, genetically engineering them to express CARs specific their tumor, and then injecting them back into the patient [10]. In this review, we will focus primarily on studies of cancer patients treated with checkpoint blockade immunotherapies.
3. Circulating markers predictive of response to immunotherapy
The majority of candidate biomarkers being vetted for prediction of treatment response can be categorized into genomic and proteomic markers. Examples of genomic studies include whole-exome sequencing circulating tumor cells [11], profiling of naked cell-free DNA (cfDNA) or circulating tumor DNA (ctDNA) [12], and RNA transcriptomic signatures of host immune cells [13]. Proteomic markers include either soluble proteins present in circulation, such as host cytokines and chemokines [14], or cell-surface markers on circulating tumor or immune cells such as PD1/PDL1 and TCRs [9]. Another class of soluble biomarkers that can predict response to immunotherapy are exosomes and extracellular vesicles [15,16]. Several studies have also proposed ‘immune biomarkers’ that measure the magnitude of the immune response generated with CBI, which are a direct reflection of host immunology and have been associated with response to treatment [17].
PDL1 expression was the first proposed proteomic biomarker for prediction of treatment response to CBI, since a number of studies noticed that patients undergoing CBI with tumors overexpressing PDL1 measured via immunohistochemistry had improved clinical outcomes. However, a large number of patients who had low levels of PDL1 also exhibited robust responses, which complicates the use of PD1/PDL1 as an exclusive biomarker [18]. While PDL1 expression has been associated with more favorable response rates to PD1/PDL1 agents, PDL1 is not a static biomarker capable of binary discrimination of responsiveness [19]. Furthermore, a number of clinical trials have measured PDL1 in patients, but the assessment methodology of PDL1 is heterogenous, making it different to compare reproducibility across trials [20]. Other proteomic and cell-based biomarkers are related to host immunology. For example, the neutrophil to lymphocyte ratio (NLR) has been found to be prognostic of survival in many solid tumor types. Interestingly, a recent meta-analysis showed that an NLR > 4 was associated with increased overall survival. Other studies have identified the absolute lymphocyte count (ALC) to be positively correlated with survival, and serum lactate dehydrogenase (LDH) to be correlated with a negative prognosis in patients with melanoma following ipilimumab therapy [21]. Other immunologic markers include the TCR, the inducible costimulatory (ICOS) molecule, and serum autoantibodies.. Additional potential biomarkers positively correlated with active response include mutated tumor antigens, cytokine signature indicative of CD8 activation, PDL1 expression, whereas those associated with no or limited response include high levels of immunosuppression [22].
3.1. Melanoma
Since the first FDA approval of ipilimumab, the community has searched for biomarkers predictive of response in melanoma, especially in specific genotypes (e.g. BRAF, NF1, NRAS) and subtypes of melanoma (e.g. desmoplastic, acral lentiginous melanoma). Emerging metrics to track responses to cancer immunotherapy in melanoma can be categorized broadly into genomic markers, proteomic markers, and immunologic markers [23]. The most studied markers include PDL1, ctDNA, transcriptomic signatures, and host immunologic markers, including absolute counts and ratios of immune cell subtypes, cytokines, chemokines, and other soluble proteins [24].
In a study of 49 melanoma patients, the molecular signature of microfluidically enriched circulating tumor cells (CTCs) were analyzed using a quantitative 19-gene digital RNA signature (CTC score) [25]. Patients with high quantities of CTCs had a significantly higher risk of relapse, whereas those with decreasing or stable numbers had longer overall survival, and a decrease in CTC score within 7 weeks of CBI correlated with an increase in progression-free survival (hazard ratio (HR), 0.17; P = 0.008) and overall survival (HR, 0.12; P = 0.04) [13]. Tumor-specific mutations in ctDNA such as BRAF and NRAS mutations for melanoma patients have been proposed for monitoring of immunotherapy response [26]. In a review of seven cases, a recent study showed that comprehensive BRAF/NRAS ctDNA monitoring during anti-PD1 can be used during anti-PD1 treatment to monitor clinical benefit [27], which agrees from findings in another study of 229 patients [28]. In a study of 35 patients with combined CTLA4 and PD1 blockade therapy, 710 tumor-associated genes were studied from repeated liquid biopsies before and during treatment. TMB obtained from ctDNA was higher in responders than non-responders with a cutoff of TMB > 23.1. Furthermore, a decrease in over 50% of TMB after the first 3 weeks of treatment led to increased overall survival [29].
Another emerging class of biomarkers are microRNAs (miRNAs), which are released dynamically from dying tumor cells. One of the first miRNAs shown to predict CBI responses was circulating miRNA-21 [30]. Since then, other studies have surveyed larger panels of microRNAs in circulating blood. Interestingly, in one study, several tumor-derived microRNAs were found to induce myeloid suppressor cells and predict immunotherapy resistance in melanoma and poor survival (miR-146a, miR-155, miR-125b, miR-100, let-7e, miR-125a, miR-146b, miR-99b) [31]. Circulating tumor DNA is another emerging biomarker to monitor treatment response during CBI in melanoma [32,33]. In a study of 86 patients, ctDNA was collected and correlated with stage and outcome, and found to be an accurate predictor of tumor response. Conversely, elevated ctDNA after therapy correlated with a poor prognosis [34]. Another study extended this work by using droplet digital PCR (ddPCR) to study ctDNA post-therapy as opposed to pre-therapy or during therapy. ddPCR data showed that ctDNA levels fell upon treatment response and rose with detectable disease progression, and was superior to LDH as a blood-based marker [35].
Host immune cell-derived biomarkers such as serum immunoregulatory proteins also have emerged. A great deal of work has been done to identify signatures of strong immune host responses, which are thought to correlate with increased probability of therapy response. Soluble CTLA4 (sCTLA4) was explored as a possible biomarker for identifying a subset of patients that respond to ipilimumab therapy. In 113 patients, high sCTLA4 serum levels predicted favorable clinical outcomes with ipilimumab treatment [36]. In contrast, high baseline levels of soluble CD25 (sCD25) are associated with a poor prognosis and treatment resistance with CTLA4 blockade [37]. Another study of 194 patients showed that low levels of soluble NKG2D ligands MICB, ULBP1, and ULBP2 were associated with clinical outcomes in CBI [38]. LDH was one of the first biomarkers to make it into clinical guidelines as an independent predictor of survival in melanoma [39]. In one study of 209 patients, a baseline signature of low LDH, absolute monocyte counts (AMC), and myeloid-derived suppressor cells (MDSCs) as well as high absolute eosinophil counts (AEC), regulatory T-cells (Tregs), and relative lymphocyte counts (RLC) are associated with favorable outcome following ipilimumab [40]. In a cognate study, LDH levels significantly increased over baseline (10–40%) was associated with significantly shorter survival times [41]. Similarly, elevated levels of IL-15, TIM-3, and NK cell subsets predict responsiveness to anti-CTLA4 treatment in melanoma [42]. Circulating IL-17, TGF-β1, and IL-10 are predictors of response in ipilimumab neoadjuvant therapy of melanoma. In 35 patients, IL-17 was associated with toxicity/side effects, while TGF-β1 and IL-10 led to improvement in therapeutic clinical outcome with respect to progress-free survival [43]. In 273 patients receiving CTLA4 blockade, elevated levels of chemokine CXCL11 and soluble MHC class I polypeptide-related chain A (sMICA) were linked to poor overall survival [44].
In a separate study looking at cell-surface markers on host immune cells, the authors leveraged advances in high-through mass cytometry to conduct high-dimensional single-cell analysis utilizing a machine learning-based pipeline for characterization of diverse immune cell subsets in the peripheral blood of patients with stage IV melanoma before and after 12 weeks of anti-PD1 immunotherapy. They found that the strongest predictor of progression-free and overall survival was the presence of CD14+ CD16− HLA-DRhi monocytes in response to CBI [45]. Similarly, another trial found that increases in ALC and circulating CD4 and CD8 T cells are linked to increased positive clinical outcomes with ipilimumab treatment [46]. An increase in total circulating lymphocytes [47] and decreased NLR [48] were also associated with higher survival. Similarly, elevated levels of CD16-expressing monocytes at baseline were associated with higher response rates [49]. Conversely, in another study of 720 patients with advanced melanoma, those with both absolute neutrophil counts (ANC) ≥ 7500 and NLR ≥ 3 had a significantly increased risk of death due to decreased response [50]. An increased diversity of TCRs and T cell repertoire are both associated with a favorable response to CTLA4 blockade [51,52]. In concordance with these findings, both PDL1 expression on peripheral circulating T cells and the presence of CD137 on circulating CD8 T cells was associated with better prognosis with respect to overall and progression-free survival [53]. Gene expression profiles in host immune cells were also explored as potential markers. Expression of genes involved in cytolytic activity and proliferation of NK cells and T cells were related to positive responses in both anti-CTLA4 and anti-PD1 therapies [54]. In a subset of patients with melanomas expressing the NY-ESO-1 tumor antigen, the presence of anti-NY-ESO-1 antibodies along with corresponding CD8 T cells experienced more frequency clinical benefit with ipilimumab [55].
A newer class of biomarker is represented by exosomes and extracellular vesicles, which are released from tumors into the circulation. A study of patients with metastatic melanoma showed that exosomes released from melanomas carry PDL1 on their surface, and that the increase in levels of circulating exosomal PDL1 correlates with tumor response to anti-PD1 therapy, and also tracks with IFN-γ stimulation [56]. Suppression of exosomal PDL1 was found to induce systemic anti-tumor immunity and promotes T cell activity in the draining tumor lymph node, suggesting that exosomal PDL1 could be a therapeutic target in metastatic melanoma [57]. Interestingly, measurement of mRNA levels of exosomal PDL1 by PCR rather than direct measurement of PDL1 proteins within circulating exosomes is sufficiently predictive [58].
3.2. Non-small cell lung cancer (NSCLC)
Currently, PDL1 is the only biomarker used in clinical practice to select patients most likely to benefit from CBI in NSCLC. Conflicting findings have been associated with PDL1 as a biomarker in NSCLC. In the largest study, 2102 patients who underwent nivolumab therapy were studied. For those with PDL1 expression of <1% via immunohistochemistry (IHC), nivolumab showed a trend for improved survival compared with docetaxel. Although PDL1 expression is related to greater response, PDL1 negative patients had also some benefit [59]. In another study of 914 patients, pooled analysis showed that patients with PDL1 positive tumors had a significantly higher overall response rate, compared to patients with PDL1 negative tumors (OR: 2.44; 95% CIs: 1.61–3.68). They suggested an IHC cutoff point of 1% for positivity as a predictive biomarker for the selection of patients to treat with immune-checkpoint inhibitors [60]. CTC counts were one of the early markers proposed for therapy responses in primary lung cancer [61]. In a study of 24 stage 4 NSCLC patients treated with nivolumab, CTCs were analyzed for PDL1 expression. Interestingly, patients with PDL1 negative CTCs all obtained a clinical benefit, while patients with PDL1 positive CTCs all experienced progressive disease [62]. In another study using an engineered microfluidic system to isolate and concentrate NSCLC CTCs, PDL1 expression on CTCs alone was not predictive of progression-free survival [63]. These findings have triggered research into identification of other biomarkers that are more reliable for predicting response to CBI. Within the last several years, genomic and host immunology-based markers borrowed from the melanoma literature have been proposed and studied, such as TMB, tumor microenvironment, and immune cell-related biomarkers.
A key theme is that the genomic landscape of lung cancers shape responses to anti-PD1 therapy. Recent data show that TMB obtained from peripheral sampling of ctDNA in the blood is one likely candidate ready to enter clinical practice to aid treatment selection [64]. TMB from tumor samples, as measured by next-generation sequencing (NGS) [65], whole-exome sequencing (WES) or a cancer gene panel (CGP) [66], is known to be associated with immunotherapy responses. However, whether TMB estimated by ctDNA in circulating blood (bTMB) is associated with clinical outcomes of immunotherapy remains to be explored. In a groundbreaking study, a CGP named NCC-GP150 was designed and virtually validated using a large-scale patient database based on blood samples. In 50 patients, they found that the bTMB estimated by NCC-GP150 distinguished between patients that would or would not benefit from CBI, and validating the bTMB as a useful prognostic biomarker [11]. In a study of 136 patients with NSCLC, a higher ctDNA TMB was significantly correlated with poor clinical outcomes with CBI [67], which is interestingly in contrast with a separate study of TMB in direct tumor samples, which showed a higher mutational burden in ctDNA was associated with improved overall survival and progress-free survival [68]. This suggests that ctDNA may reflect a different genomic signature than the tumor DNA. In a later study, blood-based TMB predicted clinical benefit in NSCLC to atezolizumab, which was validated with a retrospective analysis of two large randomized trials [69]. Recently, a plasma immune-related miRNA-signature classifier (MSC) that is typically used in screening patients for lung cancer was applied to determine whether it was also predictive of response to CBI in NSCLC [70]. They found that the MSC test was associated with overall and progression-free survival, and that the MSC and PDL1 combination panel of biomarkers were able to risk-stratify patients into three groups. Furthermore, the MSC risk level remained low in patients until tumor progression was measured, suggesting that it can be used to track recurrence. Another distinct study proposed that ctDNA levels could enable early assessment of immunotherapy efficacy. A drop in ctDNA level is an early marker of therapeutic efficacy and predicts prolonged survival in patients treated with immune-checkpoint inhibitors for NSCLC [71]. Very early response of circulating tumor-derived DNA in plasma predicts efficacy of nivolumab treatment in patients with non-small cell lung cancer. Fourteen patients who were treated with nivolumab. Levels of ctDNA measured, basal and serial ctDNA analysis revealed that a decrease in allelic frequency (AF) of ctDNA showed high-level correspondence with a good durable response at the 2-week mark [72]. Overall, ctDNA represents an extremely promising biomarker in NSCLC.
Similar to metastatic melanoma, immunologic host-related biomarkers are now being explored as potential predictors of response to CBI. Examples of these immunologic host-related biomarkers reflective of immunotherapy response in NSCLC include ALC [73], ANC [73], NLR [74], platelet to lymphocyte ratio (PLR) [75], AEC [73], and CD8 T-cell density [76]. These markers have demonstrated prognostic value in small studies. In a recent study, NLR is correlated with survival in patients treated with PD1/PDL1 blockade, where low NLR Is associated with better outcomes. NSCLC patients undergoing PD1/PDL1 blockade showed that reduction in NLR during treatment is correlated with treatment response using computed tomography imaging as an endpoint, with progressive disease corresponding to an increase in the NLR [77]. In a study of 70 treatment naïve NSCLC patients, decreased survival was associated with elevated levels PD1+, PD1+ CD3+, PDL1+ CD3+, PDL1+ CD3+ CD8+, PDL2+ CD3 +, PDL2+ CD3+ CD4+, or PDL2+ CD3+ CD8+ PBMCs. Interestingly, the cytokines IL-2 and TNF-α were strongly associated with the expression of PDL1 on T cells in responding patients [78].
4. Other cancers and cancer invariant ‘universal biomarkers’
Compared to metastatic melanoma and NSCLC, fewer studies have been conducted on identifying novel biomarkers for urothelial carcinoma [4], head and neck cancers [79], colorectal cancer [80], and breast cancer [81]. Here, we summarize the results of a subset of these studies. Potential biomarkers that have been identified in genitourinary malignancies include mutational burden, PDL1, cytokine panels, and autoimmune responses like vitiligo, colitis, and thyroiditis [82]. In urothelial carcinoma, a recent study identified alterations in DNA damage and repair (DDR) genes and mutational load as correlated with improved clinical outcomes after PD1/PDL1 blockade. Sixty patients with urothelial cancer enrolled in prospective trials of anti-PD1/PDL1 antibodies met inclusion criteria. DDR alterations are independently associated with response to PD1/PDL1 blockade in patients with metastatic urothelial carcinoma [4]. In head and neck cancers, anti-PD1 agents have become the standard of care for platinum-refractory recurrent/metastatic head and neck squamous cell carcinoma (HNSCC). A recent study showed that a combination of PDL1 expression and circulating CD8 T cells have positive predictive value [83]. In 113 patients with HNSCC, detection of CTCs overexpressing PDL1 was found to have prognostic value in HNSCC. Overexpression of PDL1 at end of treatment had poor survival compared to those without, and the abscess of PDL1 overexpression at end of treatment was associated with complete response [79]. In 25 patients with muscle invasive and metastatic bladder cancers, PDL1 was characterized on CTCs, which could potentially guide treatment selection [84]. Historically, response to CBI in colorectal carcinoma has been poor but a small subset of patients do respond to CBI. In 50 patients with metastatic colorectal carcinoma, a panel of six CTC markers were measured (GAPDH, VIL1, CLU, TIMP1, LOXL3, and ZEB2) and correlated with overall and progress-free survival. Reduction in these six CTC markers corresponded to a doubling in both outcomes, compared to those with high CTC markers. Interestingly, treatment-refractory patients could be identified using the same panel that were misidentified as responders via computed tomography imaging [80]. Circulating levels of PDL1 present on exosomes, but not freely circulating PDL1, released from head and neck cancers were associated with disease progression, and blockade of PDL1 exosome signaling correlated with a robust immune response [85]. Exosomes may also represent early biomarkers for ovarian cancer [86].
Most studies thus far have assessed biomarkers predictive of response in specific cancer types, but recent work has expanded the idea of cancer invariant ‘universal biomarkers’ that are indicative of pan-tumor responses to CBI. In a key study, other proteomic markers such as CTLA4 expression and the absence of the cytokine fractalkine (CX3CL1) were also associated with strong immune responses [87] across multiple cancer types. Other cytokines or chemokines prognostic of positive response included increase levels of IFN-γ and IL-18, and decreased levels of IL-6. Transient increases in CD8+ HLA-DR+ Ki-67+ lymphocytes were associated with CBI response in bladder cancer and other cancers [87,88]. In advanced solid tumor patients, CTCs were analyzed for PDL1 expression. PDL1 positive CTC and PDL1 high CTC correlate with disease outcome (P < 0.001, P = 0.002, and P = 0.007, respectively), and an abundance of PDL1 CTCs at baseline before treatment were predictive of progression-free survival [89]. Using next-generation sequencing from plasma/serum–derived cfDNA, another study quantified chromosomal instability across multiple cancer types. They identified that cfDNA could be used as a real-time surrogate for disease progression, as well as an early indicator of response to immunotherapy [12]. In NSCLC, uveal melanoma, or colorectal cancer patients treated with nivolumab or pembrolizumab monotherapy, changes in ctDNA levels during therapy could be a promising tool for very accurate monitoring of treatment efficacy [90]. They found that patients with undetectable ctDNA at 8 weeks responded well to therapy, and predicted higher PFS and overall survival [91].
5. Conclusions
In this review, we began by briefly outlining the FDA-approved checkpoint blockade immunotherapies and their mechanisms of actions. We summarized key findings from primary studies published in the last five years identifying potential metrics predictive of clinical response, with a focus on biomarkers for metastatic melanoma, NSCLC, and ‘cancer invariant’ biomarkers. We outline the major classes of potential biomarkers, which can be divided broadly into genomic signatures and proteomic signatures. Although many candidate biomarkers have been described to date, only three assays are FDA-approved (one as a companion and two as a complementary diagnostic [92]) to identify patients who are more likely to benefit from anti-PD1 /PDL1 therapies [93]. We discussed advancements in biomarker identification and validation utilizing multimodal approaches such as deep sequencing, transcriptomics, and machine learning. A number of studies identified combinations of genomic and proteomic biomarkers that were not necessarily predictive of immune response by themselves but were strongly correlated with survival when considered in combination [94]. As a result, multiplexed detecting methods and biomarker panels may provide new strategies for addressing the question of predicting therapy response. Several studies have identified circulating tumor DNA [95] and tumor mutational burden as prognostic, in addition to some oncogene mutations. Circulating proteomic markers like cytokines/chemokines and the numbers of or ratios of specific tumor-tropic immune cells, such as neutrophils, CD4 T cells, and CD8 T cells are of high predictive value as well. As current evidence of those potential predictors, a consensus and standardization is required to apply these biomarkers broadly to larger patient populations [96]. The most promising biomarker strategies beyond PD1/PDL1 encompass genomic analysis of circulating tumor DNA and cell-free DNA (microsatellite instability, specific tumor mutations, DNA damage), the tumor mutational landscape, and proteomic and transcriptomic signatures of host immunology [97]. Future development of predictive biomarkers for CBI must integrate multiple approaches to characterize host immunology and tumor immunology [98].
The highest quality evidence at the present moment is available for metastatic melanoma and non-small cell lung cancer. Further studies will need to be conducted for other cancer types, including urothelial carcinoma, colorectal carcinoma, and renal cell carcinoma. Although we focused on biomarkers predictive of tumor response to checkpoint inhibitor immunotherapies, other immunotherapy modalities are being readily explored in both basic research and clinical trials. One example is CAR T cell immunotherapy, which has been recently PDA approved for lymphoma. At present, there are no studies identifying circulating biomarkers predictive of tumor response to CAR T cell immunotherapy, and more work will need to be done to identify such biomarkers. In the broader class of non-invasive biomarkers, several recent studies have identified multimodal-targeted imaging-based biomarkers for tumor response, termed ‘radiomics’ [99,100]. For example, positron emission tomography (PET) with the development of new tracers specific for various cancers can enable another non-invasive and quantitative strategy to monitor treatment response [6].
6. Expert opinion
Selecting an optimal panel of biomarkers that are predictive of response to tumor immunotherapy is confounded by numerous factors, including but not limited to patient-to-patient heterogeneity, tumor genetic heterogeneity, sensitivity and specificity of diagnostic tests, costs, and regulatory considerations [93]. The first biomarkers proposed to predict treatment response to CBI were based on PD1 and PDL1 expression on tissue sections, but their predictive value seems to be limited when evaluated in a vacuum, since some patients with PDL1 negative tumors retain robust immune responses, while in other cancers, it does not correlate with treatment response at all. Dissecting how and why this occurs from an immunologic standpoint is currently under investigation. At present, there is no consensus or standardization of approaches for identifying and validating potential biomarkers. Right now, the main barriers to clinical adoption are two-fold: selecting and validating biomarkers for specific patient populations using a standardized procedure [93,101] and translating novel findings from individual smaller studies toward broad applicability in larger patient populations. Some work has been done to standardize approaches to identifying biomarkers. Recently, the Society for Immunotherapy of Cancer convened the Immune Biomarkers Task Force, consisting of a multidisciplinary panel of experts to make recommendations [102]. Addressing these problems will require both advancement of our basic science knowledge of how CBI works, specifically how administration affects both host and tumor genetics and immunology, as well as clinical testing of potential markers with real-world patient data.
Common limitations of some present studies include typical statistical limitations such as deficiencies in sample size and power, which can be easily rectified. A very important feature of well-validated biomarkers that most likely will be implemented in the clinic is that they must possess a strong negative predictive value, which do not limit patients with falsely negative results from receiving benefit from CBI [23]. This should be kept in mind during the selection of biomarkers. Other considerations for biomarker development include their use an adjunct to guide selection of medications with unfavorable risk–benefit balance, especially those with severe side effects. Identifying ‘hidden responders’ in a haystack of mostly non-responders may uncover new biomarkers that are indicative of response. Conversely, those that are not necessarily predictive of response can still identify patients that can respond to therapy, as evidenced by PD1/PDL1 [103]. More importantly, proper clinical trial design and implementation of biomarker monitoring before and during treatment will be central to collecting high-quality data patient data [104].
The studies discussed in this review outline not only potential new biomarkers for prediction of response to CBI but also illuminate new tools and technologies for selecting optimal biomarkers from a pool of candidates. Lessons learned from other major fields, such as computer science and biomedical engineering can be applied effectively to oncology. For example, engineered microfluidic devices can assist in capturing circulating tumor cells for molecular characterization [63], while machine learning approaches can help identify immunological signatures predictive of responses [45]. Due to increased interest in exosomes and extracellular vesicles containing PDL1 as circulating biomarkers, state of the art methods aimed at isolating and purifying exosomes from varying bodily fluids has been developed [105]. Due to the fast-growing nature of the field, we believe that changes can be realistically implemented into clinical and research practice. However, this will require a multidisciplinary approach, involving collaborations between surgeons, oncologists, immunologists, bioinformaticians, computer scientists, and regulatory bodies across multiple institutions. We believe that technical and technological limitations lie primarily in the novel application of existing technologies, rather than the lack of developed technology, and that the CBI field will benefit immensely from cross-disciplinary assimilation of ideas.
We anticipate that in the next 5–10 years, integration of genomic and proteomic methods in concert with advancements in artificial intelligence and next-generation sequencing will enable cancer immunotherapy to transition toward personalized medicine [21,106,107]. With numerous ongoing clinical trials testing new and existing CBIs, there will be a wealth of data moving forward that can be efficiently mined and analyzed using bioinformatics [108]. We envision that efficient selection and validation of biomarkers to predict tumor response to CBI will require cross-correlations between an individual’s genetic background, tumor micro-environment, and immunological signatures. Our hope is that a number of biomarker panels will become FDA approved for screening patients. We also anticipate that new combination drug regimens [109] as well as new modalities such as CAR T-cell therapy [10] and cancer vaccines [110] will prove useful in further improving overall survival and progression-free survival, and that new biomarkers will need to be identified to track treatment responses for these therapies.
Article highlights.
Circulating ‘liquid biopsy’ biomarkers are promising non-invasive metrics for the prediction and tracking of treatment response to checkpoint blockade immunotherapy, and the highest quality evidence is available for metastatic melanoma and non-small cell lung cancer.
Tumor PDL1 expression alone does not adequately capture the complexity of the host immunology and tumor microenvironment, and its predictive value seems to be limited to specific cancers or patient populations.
Circulating tumor DNA, blood tumor mutational burden, transcriptomic signatures, circulating tumor cells, and host immunological markers are the most promising next-generation ‘liquid biopsy’ biomarkers with potential for translation into clinical practice.
Selection and validation of biomarkers to predict tumor response to checkpoint blockade immunotherapies require cross-correlations between an individual’s genetic background, tumor microenvironment, and immunological signatures.
Integration of genomic and proteomic methods in concert with advancements in artificial intelligence and next-generation sequencing will enable cancer immunotherapy to transition toward personalized medicine.
Acknowledgments
Declaration of interest
E.Y.L. acknowledges support from the UCLA-Caltech Medical Scientist Training Program (T32GM008042), the Dermatology Scientist Training Program (T32AR071307) at UCLA, and an Early Career Research Grant from the National Psoriasis Foundation. R.P.K. acknowledges support from the OHSU Physician-Scientist Award, the Department of Defense (W81XWH-17-1-0098 and W81XWH-17-1-0514), Cancer Research Institute, LUNGevity, and Melanoma Research Alliance. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Reviewers Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auslander N, Zhang G, Lee JS, et al. Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat Med. 2018;24(10):1545–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang P, Gu S, Pan D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. 2018;36(17):1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quandt D, Zucht HD, Amann A, et al. Implementing liquid biopsies into clinical decision making for cancer immunotherapy. Oncotarget. 2017;8(29):48507–48520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. [DOI] [PubMed] [Google Scholar]
- 8.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive review on the basic immunology of checkpoint blockade immunotherapies.
- 9.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.June CH, O’Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Duan J, Cai S, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 2019;5(5):696. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A high impact study demonstrating the utility of non-invasively measuring tumor mutational burden from circulating tumor DNA as a biomarker for CBI in NSCLC.
- 12.Weiss GJ, Beck J, Braun DP, et al. Tumor Cell-Free DNA Copy Number Instability Predicts Therapeutic Response to Immunotherapy. Clin Cancer Res. 2017;23(17):5074–5081. [DOI] [PubMed] [Google Scholar]
- 13.Hong X, Sullivan RJ, Kalinich M, et al. Molecular signatures of circulating melanoma cells for monitoring early response to immune checkpoint therapy. Proc Natl Acad Sci U S A. 2018;115 (10):2467–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Outlines a novel transcriptome-based molecular signature derived from melanoma circulating tumor cells that predicted improvement in progress-free and overall survival after seven weeks of therapy with CBI.
- 14.Zhang M, Yang J, Hua W, et al. Monitoring checkpoint inhibitors: predictive biomarkers in immunotherapy. Front Med. 2019;13 (1):32–44. [DOI] [PubMed] [Google Scholar]
- 15.Syn NL, Wang L, Chow EK-H, et al. Exosomes in cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol. 2017;35(7):665–676. [DOI] [PubMed] [Google Scholar]
- 16.Tucci M, Passarelli A, Mannavola F, et al. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. Oncoimmunology. 2018;7(2):e1387706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother. 2011;60 (3):433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. [DOI] [PubMed] [Google Scholar]
- 19.Friedman CF, Postow MA. Emerging tissue and blood-based biomarkers that may predict response to immune checkpoint inhibition. Curr Oncol Rep. 2016;18(4):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teixidó C, Karachaliou N, González-Cao M, et al. Assays for predicting and monitoring responses to lung cancer immunotherapy. Cancer Biol Med. 2015;12(2):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Music M, Prassas I, Diamandis EP. Optimizing cancer immunotherapy: is it time for personalized predictive biomarkers? Crit Rev Clin Lab Sci. 2018;55(7):466–479. [DOI] [PubMed] [Google Scholar]
- 22.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4–328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Axelrod ML, Johnson DB, Balko JM. Emerging biomarkers for cancer immunotherapy in melanoma. Semin Cancer Biol. 2018;52(Pt 2):207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manson G, Norwood J, Marabelle A, et al. Biomarkers associated with checkpoint inhibitors. Ann Oncol. 2016;27(7):1199–1206. [DOI] [PubMed] [Google Scholar]
- 25.Pachmann K, Willecke-Hochmuth R, Schneider K, et al. Circulating epithelial tumor cells as a prognostic tool for malignant melanoma. Melanoma Res. 2018;28(1):37–43. [DOI] [PubMed] [Google Scholar]; • Study outlining the utility of measured levels of circulating tumor cells as a prognostic indicator for melanoma.
- 26.Calapre L, Warburton L, Millward M, et al. Circulating tumour DNA (ctDNA) as a liquid biopsy for melanoma. Cancer Lett. 2017;404:62–69. [DOI] [PubMed] [Google Scholar]
- 27.Seremet T, Planken S, Schreuer M, et al. Illustrative cases for monitoring by quantitative analysis of BRAF/NRAS ctDNA mutations in liquid biopsies of metastatic melanoma patients who gained clinical benefits from anti-PD1 antibody therapy. Melanoma Res. 2018;28(1):65–70. [DOI] [PubMed] [Google Scholar]
- 28.Johnson DB, Lovly CM, Flavin M, et al. Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol Res. 2015;3(3):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forschner A, Battke F, Hadaschik D, et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma - results of a prospective biomarker study. J Immunother Cancer. 2019;7(1):180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Recent article highlighting biomarkers for combined dual CBI in melanoma based on tumor mutational burden estimated from ctDNA.
- 30.Wang Z, Han J, Cui Y, et al. Circulating microRNA-21 as noninvasive predictive biomarker for response in cancer immunotherapy. Med Hypotheses. 2013;81(1):41–43. [DOI] [PubMed] [Google Scholar]
- 31.Huber V, Vallacchi V, Fleming V, et al. Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J Clin Invest. 2018;128(12):5505–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Study identifying microRNAs from myeloid-derived suppressor cells as a novel class of circulating biomarkers for melanoma.
- 32.Ashida A, Sakaizawa K, Uhara H, et al. Circulating tumour DNA for monitoring treatment response to anti-PD-1 immunotherapy in melanoma patients. Acta Derm Venereol. 2017;97(10):1212–1218. [DOI] [PubMed] [Google Scholar]
- 33.Khagi Y, Goodman AM, Daniels GA, et al. Hypermutated circulating tumor DNA: correlation with response to checkpoint inhibitor– based immunotherapy. Clin Cancer Res. 2017;23(19):5729–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JH, Long GV, Boyd S, et al. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann Oncol. 2017;28(5):1130–1136. [DOI] [PubMed] [Google Scholar]
- 35.Tsao SC-H, Weiss J, Hudson C, et al. Monitoring response to therapy in melanoma by quantifying circulating tumour DNA with droplet digital PCR for BRAF and NRAS mutations. Sci Rep. 2015;5(1):11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pistillo MP, Fontana V, Morabito A, et al. Soluble CTLA-4 as a favorable predictive biomarker in metastatic melanoma patients treated with ipilimumab: an Italian melanoma intergroup study. Cancer Immunol Immunother. 2019;68(1):97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hannani D, Vétizou M, Enot D, et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015;25 (2):208–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maccalli C, Giannarelli D, Chiarucci C, et al. Soluble NKG2D ligands are biomarkers associated with the clinical outcome to immune checkpoint blockade therapy of metastatic melanoma patients. Oncoimmunology. 2017;6(7):e1323618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogan SA, Levesque MP, Cheng PF. Melanoma immunotherapy: next-generation biomarkers. Front Oncol. 2018;8:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martens A, Wistuba-Hamprecht K, Geukes FM, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22(12):2908–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]; • High quality study identifying a series of immunologic biomarkers predictive of response to CTLA4 blockade in melanoma.
- 41.Diem S, Kasenda B, Spain L, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114(3):256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tallerico R, Cristiani CM, Staaf E, et al. IL-15, TIM-3 and NK cells subsets predict responsiveness to anti-CTLA-4 treatment in melanoma patients. Oncoimmunology. 2017;6(2):e1261242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer. 2015;3(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koguchi Y, Hoen HM, Bambina SA, et al. Serum immunoregulatory proteins as predictors of overall survival of metastatic melanoma patients treated with ipilimumab. Cancer Res. 2015;75(23):5084–5092. [DOI] [PubMed] [Google Scholar]
- 45.Krieg C, Nowicka M, Guglietta S, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24(2):144–153. [DOI] [PubMed] [Google Scholar]; •• Study characterizing the novel use of high-dimensional single-cell mass cytometry to identify a subpopulation of host mono-cytes predictive of progression-free and overall survival.
- 46.Martens A, Wistuba-Hamprecht K, Yuan J, et al. Increases in absolute lymphocytes and circulating CD4+ and CD8+ T cells are associated with positive clinical outcome of melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22(19):4848–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelderman S, Heemskerk B, van Tinteren H, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63 (5):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Giacomo AM, Calabrò L, Danielli R, et al. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother. 2013;62(6):1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romano E, Kusio-Kobialka M, Foukas PG, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112(19):6140–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27(4):732–738. [DOI] [PubMed] [Google Scholar]
- 51.Kvistborg P, Philips D, Kelderman S, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6(254):254ra128–254ra128. [DOI] [PubMed] [Google Scholar]
- 52.Cha E, Klinger M, Hou Y, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6(238):238ra70–238ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacquelot N, Roberti MP, Enot DP, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun. 2017;8(1):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das R, Verma R, Sznol M, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194(3):950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108(40):16723–16728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Study identifying PDL1-containing exosomes released from tumors as a circulating biomarker associated with CBI response.
- 57.Poggio M, Hu T, Pai C-C, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177 (2):414–427.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Del Re M, Marconcini R, Pasquini G, et al. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. Br J Cancer. 2018;118(6):820–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aguiar PN, Santoro IL, Tadokoro H, et al. A pooled analysis of nivolumab for the treatment of advanced non-small-cell lung cancer and the role of PD-L1 as a predictive biomarker. Immunotherapy. 2016;8(9):1011–1019. [DOI] [PubMed] [Google Scholar]
- 60.Passiglia F, Bronte G, Bazan V, et al. PD-L1 expression as predictive biomarker in patients with NSCLC: a pooled analysis. Oncotarget. 2016;7(15):19738–19747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res. 2009;15(22):6980–6986. [DOI] [PubMed] [Google Scholar]
- 62.Nicolazzo C, Raimondi C, Mancini M, et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor nivolumab. Sci Rep. 2016;6 (1):31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dhar M, Wong J, Che J, et al. Evaluation of PD-L1 expression on vortex-isolated circulating tumor cells in metastatic lung cancer. Sci Rep. 2018;8(1):2592. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Characterization of an engineered microfluidic-based platform for the isolation and measurement of PDL1 expression on circulating tumor cells in NSCLC.
- 64.Prelaj A, Tay R, Ferrara R, et al. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer. 2019;106:144–159. [DOI] [PubMed] [Google Scholar]
- 65.Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti–programmed cell death (PD)-1 and anti–programmed death-ligand 1 (PD-L1) blockade in patients with non– small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campesato LF, Barroso-Sousa R, Jimenez L, et al. Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget. 2015;6(33):34221–34227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chae YK, Davis AA, Agte S, et al. Clinical implications of circulating tumor DNA tumor mutational burden (ctDNA TMB) in non-small cell lung cancer. Oncologist. 2019;24(6):820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24 (9):1441–1448. [DOI] [PubMed] [Google Scholar]; •• Report of a new blood-based assay to estimate TMB from ctDNA in NSCLC to identify patients that would benefit from atezolizumab.
- 70.Boeri M, Milione M, Proto C, et al. Circulating miRNAs and PD-L1 tumor expression are associated with survival in advanced NSCLC patients treated with immunotherapy: a prospective study. Clin Cancer Res. 2019;25(7):2166–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldberg SB, Narayan A, Kole AJ, et al. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin Cancer Res. 2018;24(8):1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iijima Y, Hirotsu Y, Amemiya K, et al. Very early response of circulating tumour-derived DNA in plasma predicts efficacy of nivolumab treatment in patients with non-small cell lung cancer. Eur J Cancer. 2017;86:349–357. [DOI] [PubMed] [Google Scholar]
- 73.Tanizaki J, Haratani K, Hayashi H, et al. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol. 2018;13(1):97–105. [DOI] [PubMed] [Google Scholar]; • Identification of immunologic biomarkers such as ANC, AEC, and ALC associated with progression free and overall survival in NSCLC treated with nivolumab.
- 74.Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. [DOI] [PubMed] [Google Scholar]
- 75.Suh KJ, Kim SH, Kim YJ, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67(3):459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Evans M, O’Sullivan B, Smith M, et al. Predictive markers for anti-PD −1/PD-L1 therapy in non-small cell lung cancer—where are we? Transl Lung Cancer Res. 2018;7(6):682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang H, Jayaprakash KT, Aslam S, et al. Neutrophil-to-lymphocyte ratio (NLR) trends and treatment response to programmed death/ligand 1 (PD-1/PD-L1) inhibitors in non-small cell lung cancer (NSCLC). Lung Cancer. 2019;127:S41–S42. [Google Scholar]
- 78.Arrieta O, Montes-Servín E, Hernandez-Martinez J-M, et al. Expression of PD-1/PD-L1 and PD-L2 in peripheral T-cells from non-small cell lung cancer patients. Oncotarget. 2017;8 (60):101994–102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strati A, Koutsodontis G, Papaxoinis G, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol. 2017;28 (8):1923–1933. [DOI] [PubMed] [Google Scholar]
- 80.Barbazán J, Muinelo-Romay L, Vieito M, et al. A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer. Int J Cancer. 2014;135(11):2633–2643. [DOI] [PubMed] [Google Scholar]; • The authors report a panel of six circulating biomarkers derived from circulating tumor cells in colorectal cancer that predict immunotherapy response.
- 81.Mazel M, Jacot W, Pantel K, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 2015;9(9):1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slovin SF. Biomarkers for immunotherapy in genitourinary malignancies. Uro Oncol. 2016;34(4):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oliva M, Spreafico A, Taberna M, et al. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann Oncol. 2019;30(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anantharaman A, Friedlander T, Lu D, et al. Programmed death-ligand 1 (PD-L1) characterization of circulating tumor cells (CTCs) in muscle invasive and metastatic bladder cancer patients. BMC Cancer. 2016;16(1):744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Theodoraki M-N, Yerneni SS, Hoffmann TK, et al. Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang MKS, Wong AST. Exosomes: emerging biomarkers and targets for ovarian cancer. Cancer Lett. 2015;367(1):26–33. [DOI] [PubMed] [Google Scholar]
- 87.Herbst RS, Soria J-C, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• High impact study identifying several circulating cytokine and cell-surface immune cell markers predicting success of PDL1 blockade across multiple cancer types.
- 88.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. [DOI] [PubMed] [Google Scholar]
- 89.Yue C, Jiang Y, Li P, et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients under-going PD-1 blockade therapy. Oncoimmunology. 2018;7(7): e1438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goldberg SB, Patel AA. Monitoring immunotherapy outcomes with circulating tumor DNA. Immunotherapy. 2018;10(12):1023–1025. [DOI] [PubMed] [Google Scholar]
- 91.Cabel L, Riva F, Servois V, et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol. 2017;28(8):1996–2001. [DOI] [PubMed] [Google Scholar]
- 92.Ma W, Gilligan BM, Yuan J, et al. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dobbin KK, Cesano A, Alvarez J, et al. Validation of biomarkers to predict response to immunotherapy in cancer: volume II — clinical validation and regulatory considerations. J Immunother Cancer. 2016;4(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tray N, Weber JS, Adams S. Predictive biomarkers for checkpoint immunotherapy: current status and challenges for clinical application. Cancer Immunol Res. 2018;6(10):1122–1128. [DOI] [PubMed] [Google Scholar]
- 95.Cabel L, Proudhon C, Romano E, et al. Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat Rev Clin Oncol. 2018;15(10):639–650. [DOI] [PubMed] [Google Scholar]
- 96.Teng F, Meng X, Kong L, et al. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett. 2018;414:166–173. [DOI] [PubMed] [Google Scholar]
- 97.Meng X, Huang Z, Teng F, et al. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41 (10):868–876. [DOI] [PubMed] [Google Scholar]
- 98.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17(12): E542–E551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19(9):1180–1191. [DOI] [PubMed] [Google Scholar]
- 100.Trebeschi S, Drago SG, Birkbak NJ, et al. Predicting response to cancer immunotherapy using non-invasive radiomic biomarkers. Ann Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Masucci GV, Cesano A, Hawtin R, et al. Validation of biomarkers to predict response to immunotherapy in cancer: volume I - pre-analytical and analytical validation. J Immunother Cancer. 2016;4(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Perspective article outlining strategies to validate new potential biomarkers for prediction of response to CBI.
- 102.Gnjatic S, Bronte V, Brunet LR, et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J Immunother Cancer. 2017;5(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hegde PS, Karanikas V, The where ES. The when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22(8):1865–1874. [DOI] [PubMed] [Google Scholar]
- 105.Inamdar S, Nitiyanandan R, Rege K. Emerging applications of exosomes in cancer therapeutics and diagnostics. Bioeng Transl Med. 2017;2(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan J, Hegde PS, Clynes R, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oellerich M, Schütz E, Beck J, et al. Using circulating cell-free DNA to monitor personalized cancer therapy. Crit Rev Clin Lab Sci. 2017;54(3):205–218. [DOI] [PubMed] [Google Scholar]
- 108.Roszik J, Haydu LE, Hess KR, et al. Novel algorithmic approach predicts tumor mutation load and correlates with immunotherapy clinical outcomes using a defined gene mutation set. BMC Med. 2016;14(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kitahara M, Hazama S, Tsunedomi R, et al. Prediction of the efficacy of immunotherapy by measuring the integrity of cell-free DNA in plasma in colorectal cancer. Cancer Sci. 2016;107(12):1825–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]