Abstract
LC3-associated phagocytosis (LAP) exists at the crossroads of the two evolutionary pathways of phagocytosis and autophagy. When a phagocyte engulfs an extracellular particle that engages receptor signaling, components of the autophagy machinery and Rubicon are recruited to the cargo-containing phagosome or LAPosome. Formation of the LAPosome is critical for both cargo clearance as well as mediating the proper signaling cascade. Globally, LAP functions as an immunosuppressive mechanism, as LAP deficiency often results in hyperinflammation. As defects in the autophagy machinery have been long associated with aberrant immune responses and autoimmune disorders, it is vital that we now revisit these associations with forms of non-canonical autophagy, like LAP, in mind.
Introduction
“A long time ago in a galaxy far, far away” – opening crawl
Within a cell, the natural consequence of time and function is the accumulation of damaged or unnecessary components, and in order to maintain homeostasis, cells employ degradative pathways to rid their environment of this waste, often recycling these components into basic building blocks for future use. One of these critical degradative pathways is macroautophagy (herein autophagy), a catabolic and cannibalistic process, classically triggered during times of nutrient deprivation or other stress, by which cells sequester portions of their own cytoplasm within a double membraned structure (autophagosome) which ultimately fuses with the lysosome to facilitate degradation and recycling of components for use by the nutrient-limited cell [1]. From the Greek for self-eating, the term autophagy was first coined by Christien du Nuve in 1963, and the groundbreaking work by Yoshinori Ohsumi delineating the molecular mechanisms that govern autophagy were honored with the 2016 Nobel Prize in medicine [2]. Autophagy is largely controlled by the ATG (AuTophaGy) family of proteins, and progress in six phases: inactivation of mTOR and pre-initiation complex activation, phagophore nucleation, vesicle curvature and elongation, autophagosome closure/formation, lysosome fusion, and component degradation [3]. The process of autophagy is highly conserved across all eukaryotic cells, suggesting that this mechanism is critical enough for cellular quality control and survival to withstand the rigors of evolutionary selection.
Considering autophagy’s importance across a wide swath of organisms, it is unsurprising that this machinery also participates in non-canonical functions outside the realm of non-specific scavenging during starvation. Evidence for non-canonical roles of autophagic machinery is strengthened by the diverse biological processes it is involved in and the differential roles that each component plays in development and disease pathology. Oftentimes, the deletion of even a single autophagy gene results in embryonic or perinatal lethality, though the difference in timing of lethality highlights key differences among autophagy molecules [4]. One such example of this dichotomy is Beclin1, wherein homozygotic deletion results in embryonic lethality after approximately E7.5 and heterozygotic deletion results in spontaneous tumor development [5]. Deletion of more downstream autophagy genes, such as Atg5 or Atg7, do not impede development, yet deficient pups die around P1–2. Moreover, animals heterozygous for Atg5 or Atg7, or other autophagy genes, have not been reported to be susceptible to spontaneous tumor development [4]. Thus, it is apparent that the requirement for genes such as Beclin1 supersede the requirement for more downstream autophagy genes, potentially placing Beclin1 (and others) in non-canonical roles.
The autophagy machinery can also be targeted specifically to damaged organelles (such as damaged mitochondria via mitophagy), protein aggregates (via aggrephagy), and intracellular pathogens (via xenophagy) [6]. In addition to these intracellular substrates, we now appreciate that this crucial autophagy machinery is also recruited to phagosomal membranes containing extracellular cargo that engage receptor signaling during its phagocytosis, in a recently defined pathway called LC3-associated phagocytosis or LAP [7–10]. During LAP, the power of the autophagic process is harnessed during phagocytosis to rapidly and efficiently process intraphagosomal contents and mediate the subsequent immune response. In this review, we will examine the unique molecular mechanisms that underlie LAP, the substrates that trigger this pathway, its role in shaping immunity, and its biological relevance in pathology.
Molecular differences between LAP and autophagy
“These aren’t the droids you’re looking for.” – Obi-Wan Kenobi
While canonical autophagy and LAP share many ATG components, LAP is a molecular distinct pathway in multiple ways. Under homeostatic conditions, autophagy is held in an inactive state by mTORC1, which phosphorylates ULK1 at Serine757 of the pre-initiation complex. Under starvation conditions, however, AMPK is activated and its kinase activity both inhibits mTORC1 (thus releasing ULK1 from inhibition) and activates ULK1 via phosphorylation at Serine317 and Serine777 [11]. LAP, on the other hand, does not require the pre-initiation complex [8,10,12], nor is LAP inhibited by starvation or mTOR inhibition with rapamycin [7]. The most upstream complex known to be required for LAP is the Class III PI3K complex, composed of the core components VPS34 (the Class III PI3 kinase), Beclin 1, and VPS15 [10]. Autophagy also requires either ATG14 or UVRAG in association with the Class III PI3K complex, and deletion of either results in autophagic defects, though recent evidence suggests that UVRAG is more important for endosomal maturation [13]. LAP, however, functions independently of ATG14 and exclusively requires UVRAG [10].
LAP also requires the activity of Rubicon (RUN domain protein as Beclin-1 interacting and cysteine-rich containing). Originally identified as a negative regulator of autophagy associated with the UVRAG-containing Class III PI3K complex, it has now been clearly demonstrated that LAP is a Rubicon-dependent process, whereas autophagy is not [9,10]. Thus, Rubicon deficiency provides the opportunity to study the mechanisms and roles of LAP without the confounding factor of concurrent autophagy deficiency. Rubicon’s function in LAP is two-fold. First, Rubicon is required for localization and stabilization of the Class III PI3K complex at the LAP-engaged phagosome, a structured termed the LAPosome. This association allows for efficient and localized generation of the phosphatidyl inositol (PI[3]P), which serves as a recruitment signal for downstream autophagic [10].
Second, Rubicon is required for the assembly and function of NADPH oxidase 2 (NOX2), the primary NADPH oxidase in phagocytic cells. Upon TLR or FcR stimulation, the cytosolic NOX2 components, p40phox, p47phox, p67phox, and Rac1, assemble at the phagosome with membrane bound p22phox and gp91phox (or NOX2). This complete complex is now capable of generating reactive oxygen species (ROS) to destroy invading pathogens and mediate signal transduction [14]. Rubicon binds to and stabilizes p22phox, as well as facilitating the production of PI(3)P, which binds and activates p40phox [10,14]. Both of these functions are required for optimal ROS production, and the absence of ROS results in defective LAP [10].
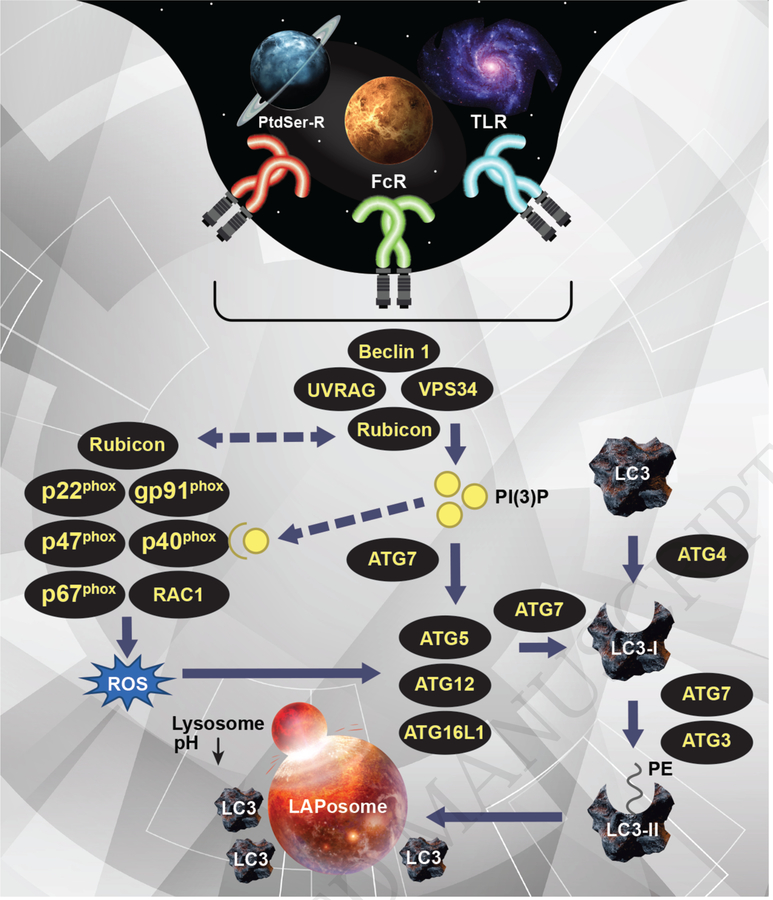
Both PI(3)P and ROS are uniquely required for recruitment or activation of the downstream ubiquitin-like conjugation systems, the ATG5-ATG12 conjugation system and the LC3-PE conjugation system [3,10]. These conjugation systems are required for and function similarly in both autophagy and LAP ATG7, an E1-like enzyme, activates ATG12, which is then transferred to the E2 enzyme, ATG10. ATG12 is then conjugated to ATG5, allowing for further association with ATG16L1 to form the multimeric ATG15-ATG12-ATG16L1 complex. This ATG5/12/16L1 complex also exhibits E3 enzyme activity and is critical for the generation of the lipidated and autophagosomal membrane bound form of LC3 (LC3-II). Cytosolic LC3 is cleaved by ATG4 and conjugated to phosphatidylethanolamine (PE) via the activity of ATG7 and the E2-like enzyme ATG3 [3]. LC3-II association with the LAPosome is required for fusion to the lysosomal and thus successful processing of the engulfed cargo (Figure 1) [10].
Figure 1: The molecular mechanisms of LC3-associated phagocytosis (LAP).
Upon engagement of TLRs, FcRs, or PtdSer-Rs, the Class III PI3 K complex, comprised of Beclin 1, VPS34, UVRAG, and Rubicon, is recruited to the cargo-containing phagosome or LAPosome. This complex is required for the sustained and localized production of PI(3)P at the LAPosome. Rubicon also interacts with and stabilizes the NOX2 complex (via interaction with p22phox) for the production of ROS. Furthermore, PI(3)P binds and activates p40phox of the NOX2 complex for efficient ROS production. Both ROS and PI(3)P are required for the recruitment of the downstream LAP machinery (such as ATG5, ATG12, ATG16L, and ATG7) and successful LC3-II decoration of the LAPosome, which is required for fusion to the lysosome and maturation of LAPosome.
LAP-engaging stimuli and the immune response
“How you get so big eating food of this kind?” - Yoda
LAP is triggered by receptor engagement of extracellular particles during phagocytosis (Figure 2). Pathogens represent a critical threat to homeostasis. Phagocytes surveil the body to identify, contain, and alert in the event of foreign pathogen invasion. Equipped with pathogen recognition receptors (PRRs), such as Toll like receptors (TLRs), these phagocytic cells are designed to seek out and destroy non-self entities [15]. Engagement of TLR1/2/4 is capable of initiating LAP in response to fungi (zymosan, Aspergillus fumigatus) or bacteria (Legionella dumoffii, Mycobacterium tuberculosis) [7,10,16–19]. In the absence of LAP, control of these pathogens in compromised and production of pro-inflammatory molecules, such as IL-6, and IL-1β is significantly increased [10,18].
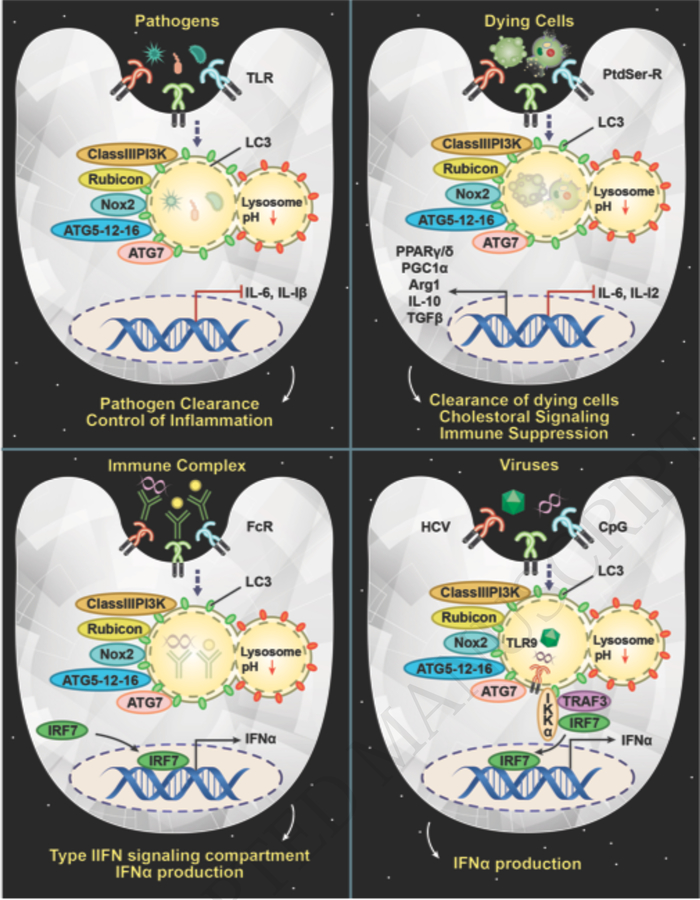
Figure 2: LAP as an immunological rheostat in response to multiple stimuli.
A) Engulfment of pathogens, such as A. fumigatus, L. dumoffii, and M. tuberculosis, that engage TLRs can trigger LAP and is required for suppression of inflammation and pathogen clearance. B) Uptake of immune complexes via FcRs can trigger LAP, which is required to establish the IRF7 signaling compartment for production of IFNα in pDC. C) Efferocytosis triggers LAP, which is required for an immunotolerant response. LAP-deficient cells have a hyperinflammatory response to dying cells and contribute to autoimmunity. D) Viral stimulation or CpG DNA stimulation triggers the formation of the LAPosome, wherein LC3 directly interacts with IKKα via LIR domains to promote further association with TRAF3 and IRF7 for IFNα production.
In addition to TLRs, engagement of Fc receptors (FcRs) can also trigger LAP. Immune complexes (IC) are autoantibodies bounds to self-antigens, such as DNA, and are common in patients with autoimmune disorders, such as systemic lupus erythematosus (SLE) [20]. Engagement of immune complexes with FcγR on plasmacytoid dendritic cells (pDCs) results in robust production of type I interferons, such as IFNα, which is preceded by and requires LC3 translocation to the IC-containing phagosome in an ATG5- and ATG7-dependent, but ULK1-, FIP200-, and ATG13-independent manner [12,13]. LAP deficiency results in a failure of the phagosome to mature into a late-endolysosomal phenotype, and subsequently a failure to form the signaling compartment required to activate interferon regulatory factor 7 (IRF7). Thus, LAP-deficient pDCs fail to produce IFNα in response to DNA-ICs, indicating that inhibition of LAP in pDC could lead to possible therapeutic options for SLE patients [12]. Recent work demonstrates that LAP also participates in TLR9 signaling, independently of FcγR engagement. Upon stimulation with the synthetic TLR9 agonist, CpG-ODN, both LC3 and the kinase IKKα were recruited to TLR9-containing endosomes in a LAP-dependent manner. Strikingly, IKKA contains three putative LC3-interacting regions (LIRs) that are required for further association with TRAF3 and IRF7 for IFNα production [21].
Phagocytes also employee a variety of receptors that are capable of distinguishing living cells from dying cells that require clearance. Programmed cell death, most commonly apoptosis, is required for proper development, cellular homeostasis, and immunity, yet the responsibility of silently and efficiently removing these cellular corpses falls to the cells of the phagocytic system. The sheer power of the phagocytic system is highlighted by the fact that is rare to observe uncleared dead cells under normal physiological conditions, despite constant turnover of cells [22]. During the lifespan of an organism, this need for dying cells clearance, a process termed efferocytosis, is both normal and reoccurring and must occur in an immunological silent manner, so as to not inappropriately alert the immune system [23]. Uncleared apoptotic cells or cells that have undergone necrotic forms of cell death (like necrosis, necroptosis, or pyroptosis, reviewed [24]) can release potentially immunogenic intracellular contents, such as nucleic acids.
Efferocytosis generally occurs via 4 main steps. The first step is the recruitment and priming of phagocytes by “find-me” signals (such as CX3CL1, ATP, UTP, Lysophosphatidylcholine [LPC], sphingosine-1-phosphate [S1P]) released by dying cells. These signals are sensed by cognate receptors on phagocytes and mediate their migration to sites of cell death and prepare the phagocyte for engulfment. The second step is recognition of dying cells by “eat-me” signals, which allow for the discernment of dead cells from viable cells. The most well-characterized “eat-me” signal is phosphatidylserine (PtdSer), a lipid maintained on the inner leaflet of the plasma membrane in healthy cells, but actively flipped via caspase-dependent activity to the outer leaflet during apoptosis [22]. Necrotic cells also inadvertently “expose” PtdSer via plasma membrane rupture. Phagocytes recognize exposed PtdSer via membrane receptors, such as T cell immunoglobulin mucin receptor 4 (TIM4), brain-specific angiogenesis inhibitor 1 (BAI1), and stabilin-2, or bridging molecules, such as milk fat globule-EGF factor 8 (MFG-E8) and Gas6, that mediate engulfment by linking to surface receptors such as integrin αvβ3, αvβ5, or Tryo3-Axl-Mer (or TAM) receptors [25].
Engagement of these receptors results in the third step, cytoskeletal rearrangements that facilitate the engulfment of the cellular corpse. The final step is the digestion, degradation, and processing of the cellular corpse. Fusion with lysosomes provides acidic proteases and nucleases to process engulfed dying cells into their basic cellular components. How phagocytes handle the metabolic stress of essentially doubling its content of cellular components is one mechanism by which they mediate an immunologically quiescent response to apoptotic cells. Efferocytosis results in the activation of peroxisome proliferator-activated receptor γ/δ (PPARγ/δ) and liver x receptor (LXR) families, both important regulators of cellular lipid homeostasis and immunotolerance [26]. Moreover, the expression of pro anti-inflammatory cytokines, such as such as tumor necrosis factor (TNF), IL-1, and IL-12 are actively suppressed [27].
Studies have demonstrated that engagement of PtdSer by dying cells results in the rapid recruitment of LC3 to the dying cell containing phagosome (REF) in a Rubicon-dependent manner [8,28]. In the absence of LAP, dying cells are not degraded and persist within phagosomes in the phagocyte. Subsequently, LAP-deficient macrophages fail to initiate an anti-inflammatory immune response, and instead produce significant amounts of pro-inflammatory molecules, such as IL-6 and IL-12 [8]. The molecular mechanisms that underlie LAP’s promotion of the immunotolerant response during efferocytosis remains to be explored, though we now appreciate the pathophysiological role of LAP in mediating immunosuppression in a variety of circumstances.
LAP and disease pathology
“Now, witness the firepower of this fully armed and operational battle station.” – Emperor Palpatine
Recent studies have identified LAP as a critical component of host defense. Mice deficient for components of the LAP pathway, such as Rubicon, fail to effectively control Aspergillus fumigatus infection in vivo and display increased tissue and serum levels of pro-inflammatory cytokines, such as IL-6 and IL-1β [10]. Mechanistically, it was demonstrated that A. fumigatus melanin sequesters calcium within the phagosome to inhibit calmodulin signaling and subsequently LAP, which implicates calcium signaling in the LAP pathway and fungal disease pathogenesis [29]. The intracellular vacuolar pathogen, Legionella dumoffii, is specifically targeted by LAP in a Dot/Icm T4SS-dependent manner [16]. LAP has also been implicated in the control of Mycobacterium tuberculosis via the activity of LRRK2, a gene commonly associated with Parkinson’s disease. LRRK2 kinase activity inhibits phagosome maturation via the recruitment of Rubicon to the Tb-containing phagosome in macrophages. Moreover, inhibition of LRRK2 kinase activity resulted in enhanced mycobacterial control independently of autophagy, suggesting that Rubicon can play critical roles outside of the autophagic realm [19]. M. tuberculosis employs an active strategy to avoid LAP via CpsA, an exported virulence factor found widely in Gram-positive bacilli, interferes with the recruitment of NOX2 to the mycobacterial phagosome [17]. Viral pathogens have also been demonstrated to engage LAP components. Hepatitis C virus (HCV) induces Rubicon and UVRAG expression, with Rubicon promoting HCV replication, and UVRAG suppressing HCV replication [30]. Rubicon can also act as a negative regulator for virus-triggered type I IFN, and as a result, Rubicon-deficient cells were able to more efficiently control influenza or VSV infection in vitro [31]. In addition, it was demonstrated that Rubicon can interact with IRF3, thus preventing IRF3 dimerization and and subsequent type I IFN production [32]. Thus, evasion of LAP is an evolutionarily important function for pathogens, highlighting LAP’s critical role in host defense.
Phagocytes outside of the immune system also undergo LAP. The uptake and processing of photoreceptor outer segments (POS) by the retinal pigment epithelium (RPE) is fundamental to vision. Indeed, RPE phagocytosis of POS triggers LAP, and mice with LAP-deficient RPE cells that showed evidence of disrupted lysosomal processing. These mice also exhibited decreased retinoid levels and subsequently decreased photoreceptor responses to light stimuli, demonstrating that LAP is required for homeostatic maintenance in the eye [33,34]. Efferocytosis of apoptotic germ cells by Sertoli cells also triggers LAP, which is vital for germ cell development and differentiation [35]. Other studies have demonstrated that Rubicon was also up-regulated in livers of mice fed a high-fat diet (HFD), and hepatocyte-specific Rubicon knockout mice were significantly protected against liver steatosis and injury associated with HFD [36]
Defects in the clearance of dying cells have long been associated with autoimmune disorders, as have defects in autophagic machinery [25]. The generation of the Rubicon deficient mouse model has allowed researchers to examine the effect of LAP on pathology without the confounding factor of concurrent loss of autophagy. LAP deficient animals develop spontaneous lupus-like syndrome with age, with increased serum levels of pro-inflammatory cytokines, increased circulating autoantibodies, increased autoantibody deposition in the glomeruli of the kidney, and increased evidence of kidney damage and dysfunction. Broadly, aged Rubicon-deficient mice contain elevated titers of autoantibodies to over 95 autoantigens associated with SLE. Importantly, the onset of this autoinflammatory phenotype could be linked to defects in efferocytosis, as chronic administration of dying cells hastened and exacerbated pathology [9]. Interestingly, while LAP is required in pDCs for type I IFN production in response to ICs, aged Rubicon-deficient mice still display an enhanced IFN signature, characteristic of SLE. It is worth noting, however, that mice with myeloid-specific LAP deficiency, such as LysM-Cre+ Becn1flox/flox mice, displayed a more pronounced IFN signature, indicating that LAP can play different roles in different cell types [9]. While LAP may be critical for IFNα production by pDC, its role in phagocytic cells, such as macrophages or conventional DCs, seems to be primarily that of suppressing inflammation. As different autoimmune pathologies involve both different cell types at different levels, it will be of great interest to explore how LAP functions in different scenarios [37].
Conclusions
“I am altering the deal, pray I don’t alter it any further.” – Darth Vader
The participation of the autophagic machinery in processes outside the purview of its canonical role in survival and stress response highlights its importance in the homeostasis and host defense of an organism. The autophagic machinery is capable of multi-tasking, contributing to starvation response, organelle quality control, and expediated clearance and processing of extracellular threats. However, we must now re-examine the pathways and pathologies that we have long associated with so-called autophagy defects and determine whether the perpetrator is canonical or non-canonical autophagy. Further understanding of how these non-canonical autophagic pathways, like LAP, function can lead to develop of targeted therapies that, for example, modulate LAP but do not disrupt the quality control aspect of autophagy. Genome wide association studies (GWAS) that can correlate LAP dysfunction with disease can help guide this avenue of research. Together, we can harness the Force of LAP.
Highlights.
LC3-associated phagocytosis (LAP) is a form of non-canonical autophagy that requires the activity of Rubicon.
LAP is triggered by the engulfment of pathogens, immune complexes, and dying cells via engagement of TLRs, FcRs, and PtdSer-Rs, respectively.
LAP is an important mediator of immunosuppression, and defect in LAP often result in exacerbated inflammation and autoimmune disorders.
Acknowledgments
This was work was supported by NIH Intramural Research Program 1ZIAES10328601.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang Z, Klionsky DJ: Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 2010, 22:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dikic I, Elazar Z: Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol 2018, 19:349–364. [DOI] [PubMed] [Google Scholar]
- 3.Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, et al. : Molecular definitions of autophagy and related processes. EMBO J 2017, 36:1811–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizushima N, Levine B: Autophagy in mammalian development and differentiation. Nat Cell Biol 2010, 12:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yue Z, Jin S, Yang C, Levine AJ, Heintz N: Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A 2003, 100:15077–15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stolz A, Ernst A, Dikic I: Cargo recognition and trafficking in selective autophagy. Nat Cell Biol 2014, 16:495–501. [DOI] [PubMed] [Google Scholar]
- 7.Sanjuan MA, Milasta S, Green DR: Toll-like receptor signaling in the lysosomal pathways. Immunol Rev 2009, 227:203–220. [DOI] [PubMed] [Google Scholar]
- 8.Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR: Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proceedings of the National Academy of Sciences of the United States of America 2011, 108:17396–17401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• 9.Martinez J, Cunha LD, Park S, Yang M, Lu Q, Orchard R, Li QZ, Yan M, Janke L, Guy C, et al. : Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 2016, 533:115–119.This study demonstrated for the first time that LAP deficiency in vivo results in autoinflammation in a manner independent of canonical autophagy.
- 10.Martinez J, Malireddi RK, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan JL, Tan H, Peng J, et al. : Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol 2015, 17:893–906. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Kim J, Kundu M, Viollet B, Guan KL: AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature cell biology 2011, 13:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, et al. : Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity 2012, 37:986–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itakura E, Kishi C, Inoue K, Mizushima N: Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 2008, 19:5360–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang CS, Lee JS, Rodgers M, Min CK, Lee JY, Kim HJ, Lee KH, Kim CJ, Oh B, Zandi E, et al. : Autophagy protein Rubicon mediates phagocytic NADPH oxidase activation in response to microbial infection or TLR stimulation. Cell Host Microbe 2012, 11:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow J, Franz KM, Kagan JC: PRRs are watching you: Localization of innate sensing and signaling regulators. Virology 2015, 479–480:104–109. [DOI] [PMC free article] [PubMed]
- • 16.Hubber A, Kubori T, Coban C, Matsuzawa T, Ogawa M, Kawabata T, Yoshimori T, Nagai H: Bacterial secretion system skews the fate of Legionella-containing vacuoles towards LC3-associated phagocytosis. Sci Rep 2017, 7:44795.In this manuscript, the authors describe mechanisms utililzed by bacteria to trigger specifically LAP.
- • 17.Koster S, Upadhyay S, Chandra P, Papavinasasundaram K, Yang G, Hassan A, Grigsby SJ, Mittal E, Park HS, Jones V, et al. : Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc Natl Acad Sci U S A 2017, 114:E8711–E8720.This study describes pathogen evasion mechanisms used by Mycobacterium tubercolosis to avoid LAP.
- 18.Yang CS, Rodgers M, Min CK, Lee JS, Kingeter L, Lee JY, Jong A, Kramnik I, Lin X, Jung JU: The autophagy regulator Rubicon is a feedback inhibitor of CARD9-mediated host innate immunity. Cell Host Microbe 2012, 11:277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 19.Hartlova A, Herbst S, Peltier J, Rodgers A, Bilkei-Gorzo O, Fearns A, Dill BD, Lee H, Flynn R, Cowley SA, et al. : LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages. EMBO J 2018.Here the author describe the role of LRRK2 in negatively regulating Rubicon and phagosome maturation during M. tuberculosis infection.
- 20.Liu Z, Davidson A: Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med 2012, 18:871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• 21.Hayashi K, Taura M, Iwasaki A: The interaction between IKKalpha and LC3 promotes type I interferon production through the TLR9-containing LAPosome. Sci Signal 2018, 11.This study demonstrates for the first time that IKKα contains LIR domain to faciltate interaction with LC3 on the LAPosome, which is required for downstream signaling.
- 22.Green DR, Oguin TH, Martinez J: The clearance of dying cells: table for two. Cell Death Differ 2016, 23:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart SP, Dransfield I, Rossi AG: Phagocytosis of apoptotic cells. Methods 2008, 44:280–285. [DOI] [PubMed] [Google Scholar]
- 24.Kearney CJ, Martin SJ: An Inflammatory Perspective on Necroptosis. Mol Cell 2017, 65:965–973. [DOI] [PubMed] [Google Scholar]
- 25.Martinez J: Prix Fixe: Efferocytosis as a Four-Course Meal. Curr Top Microbiol Immunol 2017, 403:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott MR, Koster KM, Murphy PS: Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses. J Immunol 2017, 198:1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Elkon KB, Ma X: Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 2004, 21:643–653. [DOI] [PubMed] [Google Scholar]
- 28.Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M: Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol 2011, 13:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• 29.Kyrmizi I, Ferreira H, Carvalho A, Figueroa JAL, Zarmpas P, Cunha C, Akoumianaki T, Stylianou K, Deepe GS Jr., Samonis G, et al. : Calcium sequestration by fungal melanin inhibits calcium-calmodulin signalling to prevent LC3-associated phagocytosis. Nat Microbiol 2018.This study places calcium-calmodulin signaling in the LAP pathway and describes the pathogen evasion mechanism of calcium sequestration by fungal pathogens.
- 30.Wang L, Tian Y, Ou JH: HCV induces the expression of Rubicon and UVRAG to temporally regulate the maturation of autophagosomes and viral replication. PLoS Pathog 2015, 11:e1004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan Y, Cao W, Han T, Ren S, Feng J, Chen T, Wang J, Broering R, Lu M, Zhu Y: Inducible Rubicon facilitates viral replication by antagonizing interferon production. Cell Mol Immunol 2017, 14:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JH, Kim TH, Lee HC, Nikapitiya C, Uddin MB, Park ME, Pathinayake P, Lee ES, Chathuranga K, Herath TUB, et al. : Rubicon Modulates Antiviral Type I Interferon (IFN) Signaling by Targeting IFN Regulatory Factor 3 Dimerization. J Virol 2017, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JY, Zhao H, Martinez J, Doggett TA, Kolesnikov AV, Tang PH, Ablonczy Z, Chan CC, Zhou Z, Green DR, et al. : Noncanonical autophagy promotes the visual cycle. Cell 2013, 154:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muniz-Feliciano L, Doggett TA, Zhou Z, Ferguson TA: RUBCN/rubicon and EGFR regulate lysosomal degradative processes in the retinal pigment epithelium (RPE) of the eye. Autophagy 2017, 13:2072–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 35.Panneerdoss S, Viswanadhapalli S, Abdelfattah N, Onyeagucha BC, Timilsina S, Mohammad TA, Chen Y, Drake M, Vuori K, Kumar TR, et al. : Cross-talk between miR-471–5p and autophagy component proteins regulates LC3-associated phagocytosis (LAP) of apoptotic germ cells. Nat Commun 2017, 8:598.In this manuscript, the authors demonstrate that LAP is a critical regulator of clearance of dying germ cells and is modulated by miR471–5p.
- 36.Tanaka S, Hikita H, Tatsumi T, Sakamori R, Nozaki Y, Sakane S, Shiode Y, Nakabori T, Saito Y, Hiramatsu N, et al. : Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 2016, 64:1994–2014. [DOI] [PubMed] [Google Scholar]
- 37.Ronnblom L, Eloranta ML: The interferon signature in autoimmune diseases. Curr Opin Rheumatol 2013, 25:248–253. [DOI] [PubMed] [Google Scholar]