Abstract
No vaccines are currently licensed to prevent Crimean-Congo hemorrhagic fever virus (CCHFV) infection, which can cause mild self-limiting clinical signs or severe, often fatal hemorrhagic fever disease. Here we continued investigations into the utility of a single-dose virus replicon particle (VRP) vaccine regimen by assessing protection against Turkey or Oman strains of CCHFV. We found that all mice were completely protected from disease, supporting broad applicability of this platform for CCHFV prevention.
Keywords: Crimean-Congo hemorrhagic fever, virus, hemorrhagic fever, IFNAR−/− mice, disease, vaccine, heterologous protection, VRP, replicon particle
Crimean-Congo hemorrhagic fever (CCHF) is caused by the tick-borne Crimean-Congo hemorrhagic fever virus (CCHFV; order Bunyavirales; family Nairoviridae). Disease can vary from mild, non-specific febrile illness to severe hemorrhagic clinical signs. Despite a wide endemic range, including areas of Europe, the Middle East, Asia, and Africa, along with well-documented person-to-person transmission, no approved therapeutics or vaccines exist for treatment or prevention of CCHF (Bente et al., 2013). Other mammals are largely refractory to disease, but serve a critical role in maintenance and transmission of the virus in nature. With the exception of a recent study in cynomolgus macaques (Haddock et al., 2018), lethal disease models of CCHF are restricted to STAT-1−/− and interferon α/β-receptor knockout (IFNAR−/−) mice, deficient in essential innate immune pathways (Bente et al., 2010; Bereczky et al., 2010; Zivcec et al., 2013).
A concern for vaccine development is the high genetic diversity amongst CCHFV strains, especially between strains from different geographic regions (Carroll et al., 2010). However, all CCHFV vaccine studies to date have only investigated homologous challenge. We recently reported a replicon particle vaccine that completely protected mice from lethal challenge with CCHFV-IbAr10200 following single-dose vaccination (Scholte et al., 2019). The RNA genome of CCHFV is tri-segmented: small (S), medium (M), and large (L) segments encode the viral nucleoprotein (NP), glycoprotein precursor (GPC; proteolytically processed into Gn and Gc), and RNA polymerase, respectively. The virus replicon particle (VRP) vaccine contains the complete S and L genome segments of the IbAr10200 strain, but lacks the M segment, restricting the VRP to a single round of replication. To optimize cell entry, VRPs are generated and amplified by co-transfecting a plasmid encoding the codon-optimized GPC of the Oman-98 strain (Scholte et al., 2019; Zivcec et al., 2015; and supplementary methods). Here, to further assess the putative application of this VRP vaccine, we investigated protective efficacy against genetically diverse CCHFV strains.
First, to investigate disease progression after infection with genetically diverse strains representing two additional CCHFV clades, female B6.129S2-Ifnar1tm1Agt/Mmjax mice (MMRRC 032045-JAX; 7–8 weeks of age) were inoculated subcutaneously (SC) in the inter-scapular region with a target dose of 1 × 102 50% tissue culture infective dose (TCID50) of the Nigerian tick isolate (recombinant CCHFV-IbAr10200, Africa-3 clade), or with one of four low-passage clinical isolate strains representing either the Europe-1 clade (CCHFV-Turkey) or the Asia-1 clade (CCHFV-Oman-97, -Oman-98, or -UAE) (n = 5 each, Fig 1A). Mice were housed in a climate-controlled laboratory with a 12 h day/night cycle; provided sterilized commercially available mouse chow and water ad libitum; and group-housed on autoclaved corn cob bedding (Bed-o’Cobs® ¼”, Anderson Lab Bedding) with cotton nestlets in an isolator-caging system (Thoren Caging, Inc., Hazleton, PA, USA) with a HEPA-filtered inlet and exhaust air supply. Mice were humanely euthanized with isoflurane vapor at the indicated time points, or when clinical illness scores based on piloerection, behavior (i.e. reluctance to leave nest), activity level, neurological signs (i.e. ataxia, tremors, paresis/paralysis), dehydration, dyspnea, and/or weight loss (>20% from baseline at −1 dpi) indicated that the animal was in distress or in the terminal stages of disease.
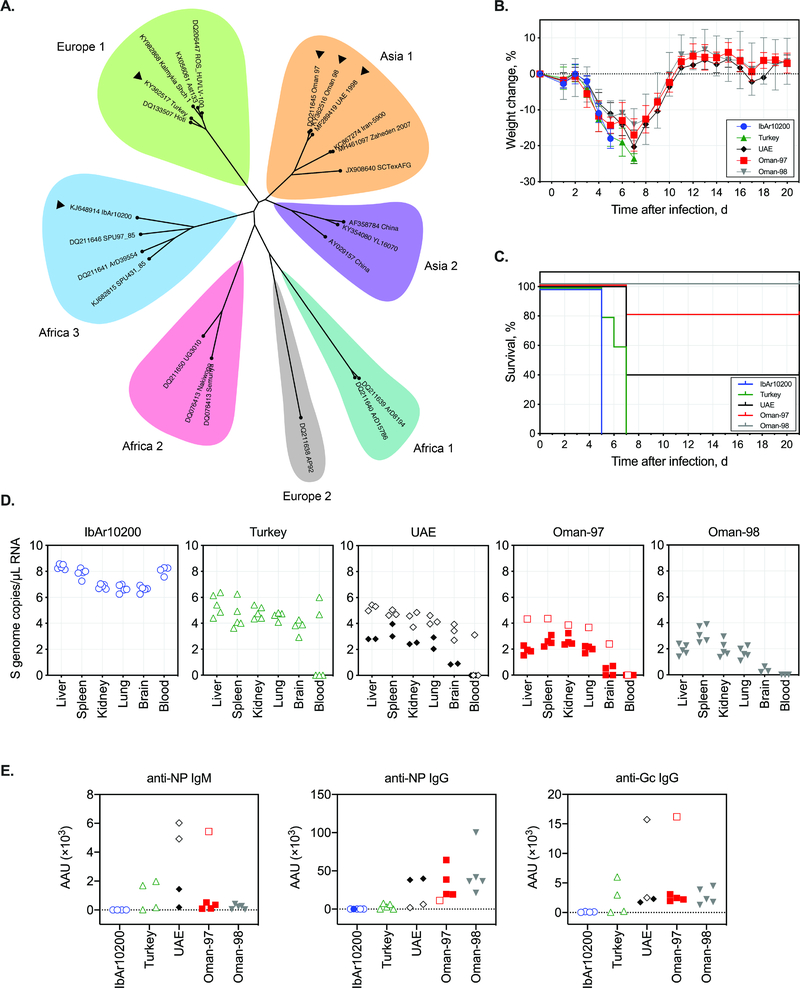
Fig 1. Comparing infection with diverse CCHFV strains in IFNAR−/− mice.
(A) Phylogenetic designation by clade of CCHFV (listed by GenBank no. and strain name) based on full-length S-genome segment (for full details of tree construction see supplementary methods). Strains used for in vivo comparison in mice are indicated by black arrowheads. (B) Weight change and (C) survival in mice inoculated SC with a target dose of 1 × 102 TCID50 of indicated CCHFV strains. (D) RT-PCR analyses of viral RNA (S segment) in blood and tissues, and (E) antibody activity units (AAU) of anti-NP IgM or IgG, and anti-Gc IgG in plasma collected when mice reached end-point criteria (open symbols) or at completion of the study (closed symbols; 21 dpi). Time post-infection of sampling, outcome, and corresponding antibody levels for individual mice is provided in Supplementary Table 1.
Following inoculation, all mice exhibited clinical signs beginning ~3 days post infection (dpi; Fig. 1B, C; Supplementary table 1). In addition to pronounced and progressive weight loss, we observed decreased activity and, in mice reaching end-stage disease, severe hypoactivity and moribundity. Clinical signs were most severe in mice infected with IbAr10200. Despite significant weight loss (up to 20% from baseline at −1 dpi), disease onset was less acute and clinical signs less pronounced in mice infected with CCHFV-Turkey, -Oman-97, -Oman-98, or UAE than in those infected with IbAr10200, even in animals reaching end-point criteria.
RNA was extracted from blood and homogenized tissue samples using the MagMAX-96 Total RNA Isolation Kit (Thermo-Fisher Scientific) on a 96-well ABI MagMAX extraction platform with a DNaseI treatment step according to manufacturer’s instructions. RNA was quantitated using a one-step real-time RT-PCR targeting a strain-specific NP gene sequence (Supplementary Table 2), and was standardized to 18S with a SuperScript III Platinum One-Step qRT-PCR Kit (Thermo-Fisher Scientific) according to manufacturer’s instructions. Relative viral S genome copy numbers were calculated using standards prepared from in vitro-transcribed S genomic RNA and expressed per μL of eluted RNA. As in previous reports, viral RNA was widely distributed in all mice, with levels highest in animals that succumbed early and lowest in convalescent animals (Fig 1D).
To better understand the kinetics and magnitude of antibody responses to CCHFV infection in mice, serological analyses were conducted on plasma obtained at the time of euthanasia (Fig 1E; Supplementary Table 1). Plasma was separated from whole blood collected in lithium heparin tubes by centrifuging 3 min at 8000 rpm. Samples were inactivated using gamma irradiation (5 million rads from a 60Co source). CCHFV NP IgG and IgM were detected using commercial ELISA kits [Alpha Diagnostics International, AE-320400–1 and AE-320410–1 (AP92 strain sequence)]. CCHFV Gc IgG levels were determined using an in-house ELISA assay using purified CCHFV-Oman Gc ectodomain bound to nickel-coated 96-well plates (1 μg per well). OD450 values were obtained for a 3-fold dilution series of plasma (1:100, 1:300, 1:900, 1:2700, 1:8100, 1:24300). Antibody activity units (AAU) for all assays were determined according to Alpha Diagnostics International’s recommended protocol. In brief, power trendlines (y = cxb) were fitted to the OD450 values, and AAUs were calculated for each assay based on an OD450 value determined from negative control animals (no VRP pre-bleed plasma, n = 8): anti-NP IgM = 0.35 OD450; anti-NP IgG = 0.15 OD450; anti-Gc IgG = 0.60 OD450.
As expected, antibody responses varied based on time after infection and disease outcome. In fatal human CCHF cases, detectable IgM or IgG antibodies are typically not produced (Bente et al., 2013), while robust development of IgM and IgG is considered a positive prognostic indicator (Shepherd et al., 1989). Similarly, no or low antibody reactivity was detected in animals that first succumbed to disease (IbAr10200-infected mice at 5 dpi). However, in mice that succumbed at 7 dpi, more robust reactivity was detected for all antibodies assessed. In almost all survivors, IgG against both NP and glycoprotein (Gc) were detected, but not IgM, when sampled at study completion (21 dpi).
Based on these data supporting use of IFNAR−/− mice infected with various CCHFV strains as alternative disease models, CCHFV-Turkey and -Oman-97 were subsequently used for challenge studies in IFNAR−/− mice vaccinated with a single dose of the VRP vaccine. Female B6.129S2-Ifnar1tm1Agt/Mmjax mice (MMRRC 032045-JAX; 6 weeks of age), housed as above, were vaccinated SC in the inter-scapular region with DMEM (mock, n = 3, each strain) or a target dose of 1 × 105 TCID50 of CCHFV-VRP (n = 3 for IbAr10200, or n = 6 for Turkey or Oman); back-titer dose: 2.15 × 105 TCID50) (Fig 2A). Consistent with our previous report (Scholte et al., 2019) and the safety profile we observed in suckling mice inoculated intracranially with VRP (Supplementary Fig 1), no clinical signs were observed in VRP-vaccinated IFNAR−/− mice during the post-vaccination period. Samples were collected 24 days post vaccination to assess antibody levels in plasma prior to challenge (Fig 2D; Supplementary Table 3). All vaccinated animals had detectable levels of anti-NP IgG, a subset had evidence of low-level anti-Gc IgG activity, and only a few had minimal anti-NP IgM antibody activity at the time of sampling.
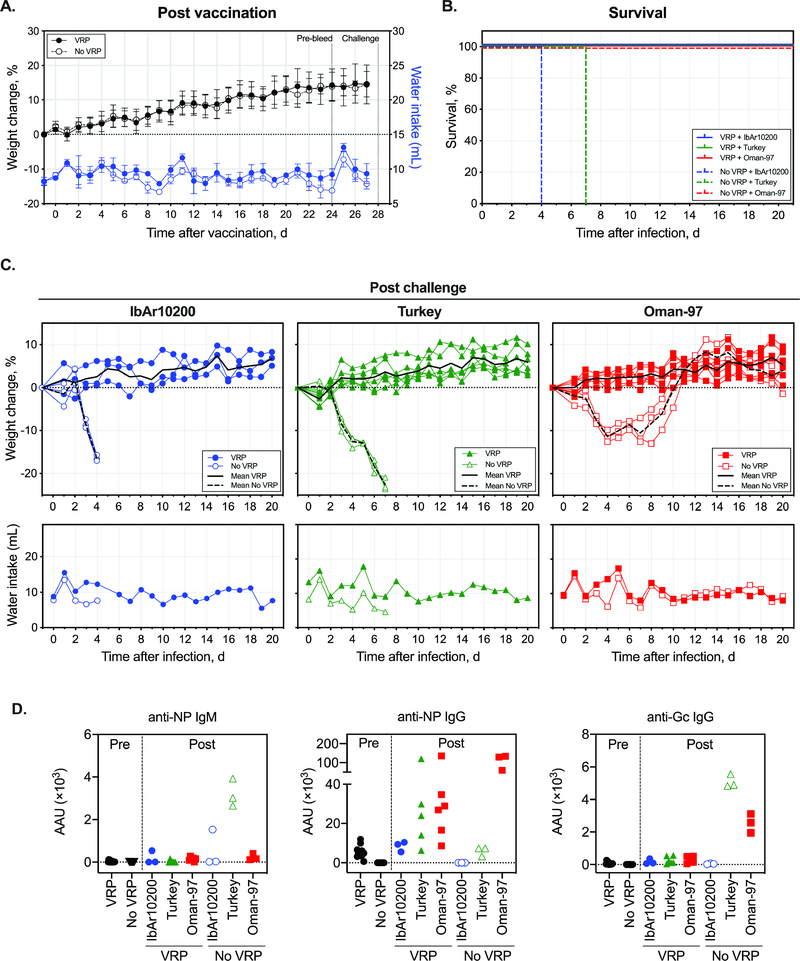
Fig 2. Clinical outcome of heterologous CCHFV challenge following VRP vaccination.
(A) Weight change in mice following VRP vaccination (1 × 105 TCID50). (B) Survival in mice challenged SC with a target dose of 100 TCID50 of indicated CCHFV strains 28 days after VRP vaccination. (C) Post-challenge weight change and water intake. (D) AAU of anti-NP IgM or IgG, and anti-Gc IgG in plasma collected when mice reached end-point criteria (open symbols) or at completion of the study (closed symbols; 21 days post challenge). Time post-infection of sampling, outcome, and corresponding antibody levels for individual mice is provided in Supplementary Table 3.
At 28 days post vaccination, groups of mice (n = 6 for recombinant CCHFV-IbAr10200, or n = 9 for CCHFV-Turkey or CCHFV-Oman-97) were challenged with a target dose of 1 × 102 TCID50 of indicated CCHFV strain (back-titer dose: 3.73 × 102 TCID50). Mice were humanely euthanized as above, with weight loss end-point criteria extended to >25% (from baseline at −1 dpi). Post-challenge, all unvaccinated mice developed clinical signs (weight loss, decreased water consumption, hunched posture, hypoactivity) and reached end-point criteria (CCHFV-IbAr10200 or -Turkey) or recovered from disease by ~12 dpi (CCHFV-Oman-97) (Fig 2B–C, Supplementary Fig 2). In contrast, all vaccinated mice were protected from both disease and death, demonstrating heterologous protection from CCHF disease in single-dose VRP-vaccinated IFNAR−/− mice. An increase in plasma anti-NP and anti-Gc IgG activity was observed in almost all VRP-vaccinated mice post challenge. In unvaccinated mice, both antibody and viral RNA levels were comparable to those detected in strain comparison studies (Fig 2D, Supplementary Fig 3).
A variety of CCHFV vaccine candidates has been screened in immunodeficient mouse models. The majority of these use a prime/boost vaccination approach. Prior to our report (Scholte et al., 2019), the only reported vaccine with a single dose regimen was a human adenovirus 5-vectored vaccine expressing the NP protein, which conferred 33% protection (Zivcec et al., 2018). Recently, use of a single dose of VSV-based vaccine was shown to provide protection from lethal outcome, but did not protect all mice from clinical disease (Rodriguez et al., 2019). While a number of vaccines based on viral NP antigen alone have demonstrated efficacy (reviewed in (S. D. Dowall et al., 2016)), to date, none of the CCHFV vaccine platforms expressing NP alone (modified Vaccinia virus Ankara (MVA) (S. D. Dowall et al., 2016) or human adenovirus 5 (Zivcec et al., 2018)) conferred complete protection against lethal disease. In addition to our VRP platform, four vaccines strategies have been reported to confer complete protection against lethal CCHFV in IFNAR−/− mice: (1) an MVA-based vaccine vector expressing the full-length glycoproteins (MVA-GPC) (Buttigieg et al., 2014); (2) plasmid DNA vaccination (NP, Gn, and Gc) (Hinkula et al., 2017); (3) adenovirus 5 expressing NP (Aligholipour Farzani et al., 2019); and; (4) a bovine herpes type vector expressing NP (Aligholipour Farzani et al., 2019). Notably, all these vaccines were administered with one or more booster doses prior to challenge.
Immune correlates of protection for CCHF are not yet fully characterized. Both antibody and T-cell responses have been indicated as required for protection; in follow-up studies using the MVA-GPC vaccine, transfer of both T-cells and antibodies was apparently required for protection in mice (Stuart D. Dowall et al., 2016). In contrast, vaccine studies indicate that the production of neutralizing antibodies does not correspond to efficacy (Hinkula et al., 2017; Kortekaas et al., 2015). In this study, we cannot correlate protection to a Gc-specific antibody response as Gc-antibodies were not detected in all vaccinated mice. This may represent an absence of response, or may be due to limitations of our assay. However, NP-specific antibodies were detected in all vaccinated mice, an antigen actively produced by the VRP, as opposed to Gc, which is only present on the VRP surface. While this correlates with results from other vaccine studies demonstrating that NP antigen alone can elicit protective responses, protective antibodies may also target other non-Gc GPC-derived antigens not captured in our analyses (e.g., Gn, GP38). Given the high efficacy of the VRP platform, now demonstrated to also protect against additional diverse CCHFV strains, future studies should continue to examine protective efficacy under varying vaccination regiments and focus on detailed investigations into immune correlates of protection elicited by the VRP vaccine. These will help guide informed design of efficacious CCHFV vaccines and support the optimization and refinement of the existing VRP platform.
Supplementary Material
Highlights.
A variety of CCHFV strains cause disease in mice, and can be used to assess therapeutic effect on disease and/or lethality.
Kinetics of viral RNA, and antibody detection vary by disease course and outcome, and in turn, challenge strain.
Single-dose of VRP vaccine confers protection against disease for 3 genetically diverse strains of CCHFV.
Acknowledgements
We thank Pierre Rollin for initial strain isolation studies; Tatyana Klimova for assistance with editing the manuscript; and members of the Centers for Disease Control and Prevention’s Comparative Medicine Branch for assistance in providing care for the animals.
Financial support. This work was partially supported by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC (S.R.W.), by a CDC foundation project funded by NIAID grant R01AI109008 (E.B.), and by CDC Emerging Infectious Disease Research Core Funds.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts.
Declaration of interests statement. Provisional US Patent Application No. 62/780,098
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aligholipour Farzani T, Földes K, Hanifehnezhad A, Yener Ilce B, Bilge Dagalp S, Amirzadeh Khiabani N, Ergünay K, Alkan F, Karaoglu T, Bodur H, Ozkul A, 2019. Bovine Herpesvirus Type 4 (BoHV-4) Vector Delivering Nucleocapsid Protein of Crimean-Congo Hemorrhagic Fever Virus Induces Comparable Protective Immunity against Lethal Challenge in IFNα/β/γR−/− Mice Models. Viruses 11, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente DA, Alimonti JB, Shieh W, Camus G, Ströher U, Zaki S, Jones SM, Stroher U, Wung-Shu S, 2010. Pathogenesis and Immune Response of Crimean-Congo Hemorrhagic Fever Virus in a STAT-1 Knockout Mouse Model. J. Virol 84, 11089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M, 2013. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 100, 159–89. [DOI] [PubMed] [Google Scholar]
- Bereczky S, Lindegren G, Karlberg H, Akerström S, Klingström J, Mirazimi A, 2010. Crimean-Congo hemorrhagic fever virus infection is lethal for adult type I interferon receptor-knockout mice. J. Gen. Virol 91, 1473–7. [DOI] [PubMed] [Google Scholar]
- Bergeron É, Zivcec M, Chakrabarti AK, Nichol ST, Albariño CG, Spiropoulou CF, 2015. Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin. PLoS Pathog. 11, e1004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttigieg KR, Dowall SD, Findlay-Wilson S, Miloszewska A, Rayner E, Hewson R, Carroll MW, 2014. A novel vaccine against Crimean-Congo Haemorrhagic Fever protects 100% of animals against lethal challenge in a mouse model. PLoS One 9, e91516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SA, Bird BH, Rollin PE, Nichol ST, 2010. Ancient common ancestry of Crimean-Congo hemorrhagic fever virus. Mol. Phylogenet. Evol 55, 1103–10. [DOI] [PubMed] [Google Scholar]
- Dowall SD, Buttigieg KR, Findlay-Wilson SJD, Rayner E, Pearson G, Miloszewska A, Graham VA, Carroll MW, Hewson R, 2016. A Crimean-Congo hemorrhagic fever (CCHF) viral vaccine expressing nucleoprotein is immunogenic but fails to confer protection against lethal disease. Hum. Vaccines Immunother. 12, 519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowall Stuart D., Graham VA, Rayner E, Hunter L, Watson R, Taylor I, Rule A, Carroll MW, 2016. Protective effects of a modified Vaccinia Ankara-based vaccine candidate against Crimean-Congo Haemorrhagic Fever virus require both cellular and humoral responses. PLoS One 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock E, Feldmann F, Hawman DW, Zivcec M, Hanley PW, Saturday G, Scott DP, Thomas T, Korva M, Avšič-Županc T, Safronetz D, Feldmann H, 2018. A cynomolgus macaque model for Crimean-Congo haemorrhagic fever. Nat. Microbiol. 3, 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkula J, Devignot S, Åkerström S, Karlberg H, Wattrang E, Bereczky S, Mousavi-Jazi M, Risinger C, Lindegren G, Vernersson C, Paweska J, van Vuren PJ, Blixt O, Brun A, Weber F, Mirazimi A, 2017. Immunization with DNA Plasmids Coding for Crimean-Congo Hemorrhagic Fever Virus Capsid and Envelope Proteins and/or Virus-Like Particles Induces Protection and Survival in Challenged Mice. J. Virol 91, JVI.02076–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas J, Vloet RPM, McAuley AJ, Shen X, Bosch BJ, de Vries L, Moormann RJM, Bente DA, 2015. Crimean-Congo Hemorrhagic Fever Virus Subunit Vaccines Induce High Levels of Neutralizing Antibodies But No Protection in STAT1 Knockout Mice. Vector-Borne Zoonotic Dis. 15, 759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H, 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27, 493–497. [Google Scholar]
- Rodriguez SE, Cross RW, Fenton KA, Bente DA, Mire CE, Geisbert TW, 2019. Vesicular Stomatitis Virus-Based Vaccine Protects Mice against Crimean-Congo Hemorrhagic Fever. Sci. Rep 9, 7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte FEM, Spengler JR, Welch SR, Harmon JR, Coleman-McCray JD, Freitas BT, Kainulainen MH, Pegan SD, Nichol ST, Bergeron É, Spiropoulou CF, 2019. Single-dose replicon particle vaccine provides complete protection against Crimean-Congo hemorrhagic fever virus in mice. Emerg. Microbes Infect 8, 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte FEM, Zivcec M, Dzimianski JV, Deaton MK, Spengler JR, Welch SR, Nichol ST, Pegan SD, Spiropoulou CF, Bergeron É, 2017. Crimean-Congo Hemorrhagic Fever Virus Suppresses Innate Immune Responses via a Ubiquitin and ISG15 Specific Protease. Cell Rep. 20, 2396–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ, Swanepoel R, Leman PA, 1989. Antibody Response in Crimean-Congo Hemorrhagic Fever. Rev. Infect. Dis 11, S801–6. [DOI] [PubMed] [Google Scholar]
- Zivcec M, Metcalfe MG, Albariño CG, Guerrero LW, Pegan SD, Spiropoulou CF, Bergeron É, 2015. Assessment of Inhibitors of Pathogenic Crimean-Congo Hemorrhagic Fever Virus Strains Using Virus-Like Particles. PLoS Negl. Trop. Dis 9, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivcec M, Safronetz D, Scott D, Robertson S, Ebihara H, Feldmann H, 2013. Lethal Crimean-Congo hemorrhagic fever virus infection in interferon α/β receptor knockout mice is associated with high viral loads, pro-inflammatory responses and coagulopathy. J. Infect. Dis 207, 1909–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivcec M, Safronetz D, Scott DP, Robertson S, Feldmann H, 2018. Nucleocapsid protein-based vaccine provides protection in mice against lethal Crimean-Congo hemorrhagic fever virus challenge. PLoS Negl. Trop. Dis 12, e0006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.