Abstract
Introduction
Hepatitis C virus (HCV) is the second largest contributor to liver disease in the UK, with injecting drug use as the main risk factor among the estimated 200 000 people currently infected. Despite effective prevention interventions, chronic HCV prevalence remains around 40% among people who inject drugs (PWID). New direct-acting antiviral (DAA) HCV therapies combine high cure rates (>90%) and short treatment duration (8 to 12 weeks). Theoretical mathematical modelling evidence suggests HCV treatment scale-up can prevent transmission and substantially reduce HCV prevalence/incidence among PWID. Our primary aim is to generate empirical evidence on the effectiveness of HCV ‘Treatment as Prevention’ (TasP) in PWID.
Methods and analysis
We plan to establish a natural experiment with Tayside, Scotland, as a single intervention site where HCV care pathways are being expanded (including specialist drug treatment clinics, needle and syringe programmes (NSPs), pharmacies and prison) and HCV treatment for PWID is being rapidly scaled-up. Other sites in Scotland and England will act as potential controls. Over 2 years from 2017/2018, at least 500 PWID will be treated in Tayside, which simulation studies project will reduce chronic HCV prevalence among PWID by 62% (from 26% to 10%) and HCV incidence will fall by approximately 2/3 (from 4.2 per 100 person-years (p100py) to 1.4 p100py). Treatment response and re-infection rates will be monitored. We will conduct focus groups and interviews with service providers and patients that accept and decline treatment to identify barriers and facilitators in implementing TasP. We will conduct longitudinal interviews with up to 40 PWID to assess whether successful HCV treatment alters their perspectives on and engagement with drug treatment and recovery. Trained peer researchers will be involved in data collection and dissemination. The primary outcome – chronic HCV prevalence in PWID – is measured using information from the Needle Exchange Surveillance Initiative survey in Scotland and the Unlinked Anonymous Monitoring Programme in England, conducted at least four times before and three times during and after the intervention. We will adapt Bayesian synthetic control methods (specifically the Causal Impact Method) to generate the cumulative impact of the intervention on chronic HCV prevalence and incidence. We will use a dynamic HCV transmission and economic model to evaluate the cost-effectiveness of the HCV TasP intervention, and to estimate the contribution of the scale-up in HCV treatment to observe changes in HCV prevalence. Through the qualitative data we will systematically explore key mechanisms of TasP real world implementation from provider and patient perspectives to develop a manual for scaling up HCV treatment in other settings. We will compare qualitative accounts of drug treatment and recovery with a ‘virtual cohort’ of PWID linking information on HCV treatment with Scottish Drug treatment databases to test whether DAA treatment improves drug treatment outcomes.
Ethics and dissemination
Extending HCV community care pathways is covered by ethics (ERADICATE C, ISRCTN27564683, Super DOT C Trial clinicaltrials.gov: NCT02706223). Ethical approval for extra data collection from patients including health utilities and qualitative interviews has been granted (REC ref: 18/ES/0128) and ISCRCTN registration has been completed (ISRCTN72038467). Our findings will have direct National Health Service and patient relevance; informing prioritisation given to early HCV treatment for PWID. We will present findings to practitioners and policymakers, and support design of an evaluation of HCV TasP in England.
Keywords: Hepatology, INFECTIOUS DISEASES, Epidemiology, PUBLIC HEALTH, Public health, Infection control
Strengths and limitations of this study.
Our control sites in the rest of Scotland and England were not randomised so there will be confounding and uncertainty in the intervention effect estimates.
Hepatitis C virus treatment and prevention strategy in UK (and Europe) is evolving - motivated both by WHO ‘elimination targets’ and falling drug prices – which may contaminate our controls.
However, our statistical models suggest that we should have sufficient power to detect an intervention effect and can model changes over time.
We will develop dynamic transmission and economic models that can estimate cost-effectiveness including the prevention benefit of this intervention.
We are conducting multiple nested qualitative studies and training and using peer researchers.
Introduction and background
Infection with hepatitis C virus (HCV) is a progressive disease that over 20 to 40 years can lead to liver cancer and premature death. HCV is the second largest contributor to liver disease in the UK and one of the few causes that is curable.1 In the UK it is estimated that approximately 200 000 people are infected with HCV, over 85% of whom are people who inject or have injected drugs (PWID).2–5 Chronic HCV prevalence and incidence among PWID remains high in UK at 20% to 50% and 5 to 15 per 100 person-years, respectively.4 6–18 Prevention of HCV transmission among PWID is critical to long-term prevention of HCV related liver disease.19
We have reviewed the effectiveness of traditional primary prevention against HCV – opioid substitution treatment (OST) and needle and syringe programmes (NSPs).12 20–22 Ongoing exposure to OST and high-coverage NSPs can reduce the risk of HCV transmission by 50% to 80%.12 22 In Scotland HCV incidence among PWID decreased from approximately 14 to 6 per 100 person-years from 2008/2009 to 2011/2012 coinciding with the launch of the Scottish HCV strategy and action plan which incorporated scale-up of harm reduction interventions and HCV treatment.10 23 We estimated that 60% of this decline could be attributed to the scale-up of OST and NSP during the action plan and that 1400 HCV infections were averted by 2015.24 However, there was no appreciable reduction in overall anti-HCV prevalence over this short period, and there is some suggestion that incidence has increased recently to ~10 per 100 person-years (http://www.hps.scot.nhs.uk/resourcedocument.aspx?id=5863). HCV transmission models suggest that primary prevention through NSP and OST alone is insufficient to achieve substantial reductions (of the order of 40% or more within 10 years) in HCV prevalence among PWID in the UK.25 26
Prevention of hepatitis C disease and HCV transmission is now possible because highly effective, tolerable, short-course interferon-free direct-acting antiviral therapies (DAAs) are available for all HCV genotypes with cure rates – defined as sustained virological response (SVR) - exceeding 90%.27–29 We, and others, hypothesise that HCV treatment scale-up for PWID, and resulting HCV Treatment as Prevention (TasP) could enhance other primary interventions and reduce HCV incidence and chronic prevalence to negligible levels (ie, towards elimination as a major public health concern).30–35 TasP refers to the concept whereby future transmission is reduced by treating affected individuals36 37: in HIV TasP antiretroviral treatment reduces transmission because individuals have undetectable infection38 in HCV TasP people are cured so reducing opportunities for future transmission. WHO targets for HCV elimination, adopted by UK and other countries, aim to reduce HCV incidence by 80% and associated mortality by 65% by 2030.39–44
Clinical guidelines in Europe and USA changed from recommending prioritising HCV treatment to people with moderate-to-severe liver disease towards removing any restrictions and recommending that people at risk of transmission irrespective of fibrosis stage are offered treatment.45–49 Cost-effectiveness models that incorporate the population prevention benefit suggest early treatment should be prioritised to PWID over other patient groups (unless chronic HCV prevalence and transmission is very high).50 There is direct evidence that SVR following HCV treatment reduces liver disease progression and mortality risk,51–53 but in two recent reviews we found no empirical evidence that HCV treatment scale-up has reduced chronic HCV prevalence and incidence in PWID populations.36 37 In part this is because in most settings HCV treatment rates in PWID are too low and any changes generally too small to be detected, as we show in two studies of seven sites in UK7 and an extension to 11 sites in Europe.54 Until very recently in the UK, the annual number of HCV DAA treatments was restricted - as drug costs could be expensive (>£10 000 per patient). There is the opportunity now to test whether scaling up HCV treatment will reduce chronic HCV prevalence and transmission among PWID.44
In a pilot study (‘Eradicate C’) in Tayside we showed that we can increase HCV case-finding and engage and successfully treat PWID in the community.55 Combining further studies on extending community HCV treatment pathways in Tayside and additional treatments provided by National Health Service (NHS) Tayside and Scottish Government we can establish an immediate natural experiment (with Tayside as the intervention site and other sites in Scotland and England as controls) to test and generate UK empirical evidence on the and potential impact and cost-effectiveness of HCV TasP in PWID. The UK is one of few countries worldwide to have an established nationwide surveillance system monitoring HCV infection among PWID.9 12 17 22 56–60 This is undertaken through a series of cross-sectional voluntary anonymous surveys of PWID recruited at harm reduction services, referred to as the Unlinked Anonymous Monitoring Programme (UAM) in England and Wales and the Needle Exchange Surveillance Initiative (NESI) in Scotland.61 62 In addition, the UK has established sentinel laboratory surveillance of HCV testing and national monitoring of HCV treatment.8 63–65 The data collected in both UAM and NESI will be used to assess out outcome.
Alongside a natural experiment in Tayside, we will collect information to assess the treatment facilitators and barriers. Historically it has proven very hard to engage PWID in HCV treatment.66–69 Some barriers to engagement, such as poor efficacy or fear of interferon treatment side-effects, may be ameliorated by DAA therapy. However, other barriers such as mistrust of health services, stigma and competing priorities faced by PWID may persist. In addition, providers may be reticent to refer or provide HCV treatment to PWID due to concerns about adherence, reinfection and perceptions of treatment ‘worth’.70 71 It is expected that co-locating HCV treatment within existing services will reduce many system and provider level barriers to PWID accessing care.66–68 72–77 However, this has not been tested in the context of community wide scale-up of interventions across multiple potential pathways. It is critical, therefore, that we understand how HCV TasP is embedded within the existing service landscape and incorporated into providers’ professional roles.
Finally it has been hypothesised that successful HCV treatment in PWID may positively impact on understandings of self and identity and improve treatment of drug use disorders.71 72 78–80 Accounts of ‘transformative’ outcomes extending beyond viral clearance alone include reference to reductions in drug and alcohol use, uptake of safer injecting practices, improved social relationships, enhanced sense of responsibility and self-worth. Hints of such collateral or indirect benefits are also found in quantitative studies reporting low re-infection rates and reductions in risky injecting behaviours among treated PWID.81 82 We aim to test this hypothesis in our qualitative follow-up study and compare the findings to quantitative data generated from a virtual cohort.
Methods and analysis
Study design
Our intention is to create and conduct a mixed methods study, including qualitative studies and economic evaluation, of a natural experiment of HCV TasP among PWID. We also will develop methods for evaluating HCV TasP.
Methods
Scaling-up HCV treatment
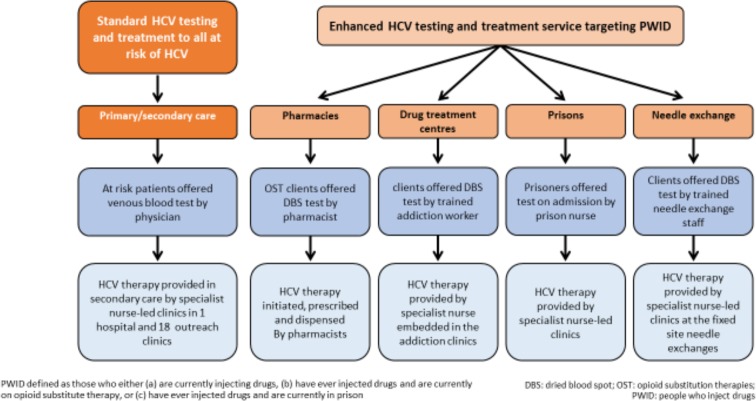
The intervention comprises the scale-up of HCV treatment in PWID which has started early in Tayside. By combining support from Scottish Government, National Health Board Tayside (NHS Tayside) and industry (MSD, Gilead, BMS) we can deliver rapid intensive scale-up of HCV treatments for PWID (comprising an extra 400 HCV treatments, a 3.5-fold increase from treatments for PWID prior to April 2017, see sample size below). We have developed multiple integrated community HCV care pathways, including novel care pathways in pharmacies, a low threshold NSP, drug treatment services and prisons (see figure 1). Our diagnostic pathways make extensive use of dried blood spot (DBS) testing for diagnosis of HCV antibody and chronic HCV with subsequent conventional laboratory testing in preparation for treatment (viral load, liver function and FIB-4 fibrosis score).83–85
Figure 1.
Overview of HCV testing and treatment pathways for the PWID population in NHS Tayside. DBS, dried blood spot; HCV, hepatitis C virus; NHS, National Health Service; OST, opioid substitution treatment; PWID, people who inject drugs.
Study population
Our intervention is delivered and measured at the population level – which we have created by combining several individual studies and treatment pathways as shown in figure 1 (see ethics section below for the individual studies). We gained ethical approval from East of Scotland Research Ethics Service REC 1 (ref: 18/ES/0128) to ask patients for permission to be recruited into the qualitative study (below) and extended clinical and behavioural drug history and data on health utilities (EQ5D-5L) at onset of treatment, during treatment and after the end of treatment.
Community HCV specialist nurses (3.5 full-time equivalent (FTE)) coordinate and deliver case-finding and treatment across the pathways in Tayside (figure 1).
The region of Tayside co-localises to NHS Tayside which is the provider of healthcare to a geographical area of 2903 sq mi (7519 km2) including the cities of Dundee and Perth and the counties of Angus and Perth & Kinross, situated in the east of Scotland with a population of 416 000. It is a mixture of urban and rural environments with some of the most affluent and most deprived areas in Scotland. It is therefore a representative microcosm of many areas in the UK.
HCV treatment
Apart from expansion of community HCV care pathways, no new clinical procedures will be investigated and all PWID with chronic HCV will be offered oral DAA HCV treatment compliant with the Scottish clinical guidelines (https://www.hps.scot.nhs.uk/resourcedocument.aspx?id=6621).
As per local standard of care, participants will be offered appropriate harm reduction advice.
Standard care for patients is to test for SVR at 12 weeks after end of treatment with patients being recommended for annual follow-up if at risk of re-infection. Specialist nurses concentrate on building a good relationship with the participant to ensure that they do return for follow-up appointments. Health Protection Scotland collates national public health surveillance data on the number, characteristics and response of patients initiated onto HCV therapy, through Clinical Databases installed in 17 specialist HCV treatment centres, across Scotland.41 86 A similar system also is available in England.
HCV surveillance and intervention outcome (chronic HCV in PWID)
The outcome is chronic HCV prevalence (HCV viraemia as measured by HCV PCR) among PWID in the community (not just in the patients who undergo HCV treatment). Prevalence will be monitored using the NESI and UAM surveys, as detailed below. During 2017 to 2022, three waves of data collection for NESI (n=7500) and five to six for UAM (n=17 000 in England) will measure this outcome.
In our pre-intervention period from 2010/2011 to 2016 there have been four NESI surveys in Scotland (n=10 000 participants in total) and six UAM surveys in England (n=16 000 in total), which have involved the collection of DBS linked to questionnaire data. Participants are recruited at sentinel sites by a team of trained interviewers in Scotland (at over 100 NSP sites) and by agency staff in over 60 low-threshold drug agencies across England.58 61 Participants complete a short questionnaire, with common questions across UAM and NESI, on demographics, injecting behaviour and service utilisation, and importantly (in relation to quantifying the intervention effect) both survey approaches have remained consistent over time.
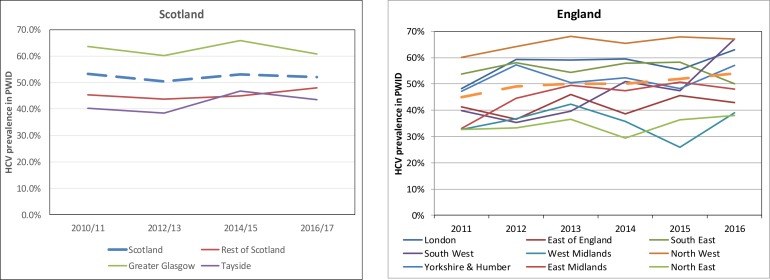
The DBS samples collected in NESI and UAM have all been tested for HCV antibody, using the same methods (where sensitivity and specificity of the assay on DBS are close to 100%),83 84 and illustrate that antibody prevalence (ever infection) has remained relatively stable among PWID during this time (figure 2). PCR positivity among antibody positive samples is used to determine chronic infection.
Figure 2.
Trends in HCV antibody prevalence among PWID in Scotland and England 2010/2011 to 2016. HCV, hepatitisC virus; PWID, people who inject drugs.
All NESI and UAM samples will be tested for HCV antibody and RNA PCR to assess the impact of HCV therapy scale-up – which is critical as trends in chronic infection and antibody status will diverge as more people are cured. In addition, we will undertake RNA PCR testing of all historical samples that were HCV antibody positive shown in figure 2 so that we can measure chronic HCV prevalence among PWID pre-intervention, as well as post-intervention, for analysis (below)
Data on HCV PCR positivity among antibody negative samples identify recent infections and is used to estimate HCV incidence – which has fluctuated between 5 to 10 infections per 100 person-years across the UK during the last 5 years.61 We will also estimate HCV incidence from our transmission dynamic models.24 54
Sample size, power and estimating intervention effect
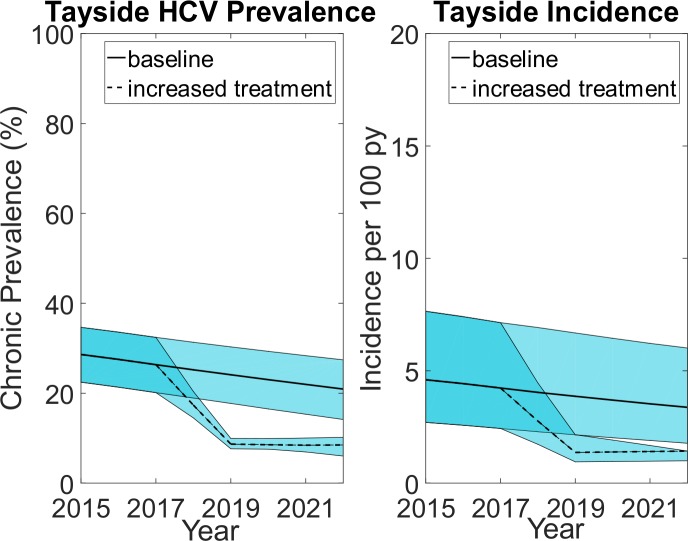
We updated estimates of the prevalence of PWID in Tayside5 which suggest there are 2760 (95% Credible Interval, CrI 2360 to 3170) PWID either currently injecting and/or in OST. NESI data suggest that approximately 30% have chronic HCV and over 75% of PWID with chronic HCV have been diagnosed. Prior to 2017 approximately 66 PWID were treated annually. From April 2017 we plan to treat at least 500 PWID in Tayside over 2 years (as a result of expanded community care pathways shown in figure 1 and extra HCV treatments provided by NHS, Scottish Government and Industry funding). Adapting a transmission dynamic model that has been used in Tayside,87 we hypothesise that within 2 years chronic HCV prevalence among PWID will reduce by approximately 62% from 26% (95% CrI 20 to 32) to at least 10% and chronic HCV incidence will fall by approximately 2/3 s from 4.2 (95% CrI 2.4 to 7.1) per 100 person-years (p100py) to 1.4 (95%CrI 1.0 to 1.4) p100py (as shown in figure 3). Modelling also suggests that maintaining these reductions after 2019 will require less than 40 treatments per year.
Figure 3.
Projected chronic HCV prevalence and incidence among PWID in Tayside with and without the intervention. Blue shaded area denotes the 95% credibility intervals of the model projections with and without the intervention. HCV, hepatitisC virus; PWID, people who inject drugs; py, person-years.
We will adapt the Causal Impact synthetic control Model (CIM) as proposed by Brodersen and colleagues.88 89
We have performed simulation studies to test power and evaluate the utility of the CIM assuming information on chronic HCV prevalence among PWID (shown in figure 4). Provided trends in the chronic HCV prevalence in the pre-intervention period are relatively stable (which is the case) there will be sufficient power to detect the projected reduction in chronic prevalence. For example, in figure 4d we see that for a prevalence reduction of 40% by year 2 to 3 the credible intervals of the estimated cumulative effect (cumulative drop in prevalence) exclude zero, correctly identifying evidence of a successful intervention. Whereas a cumulative reduction of <20% is unlikely to be detected.
Figure 4.
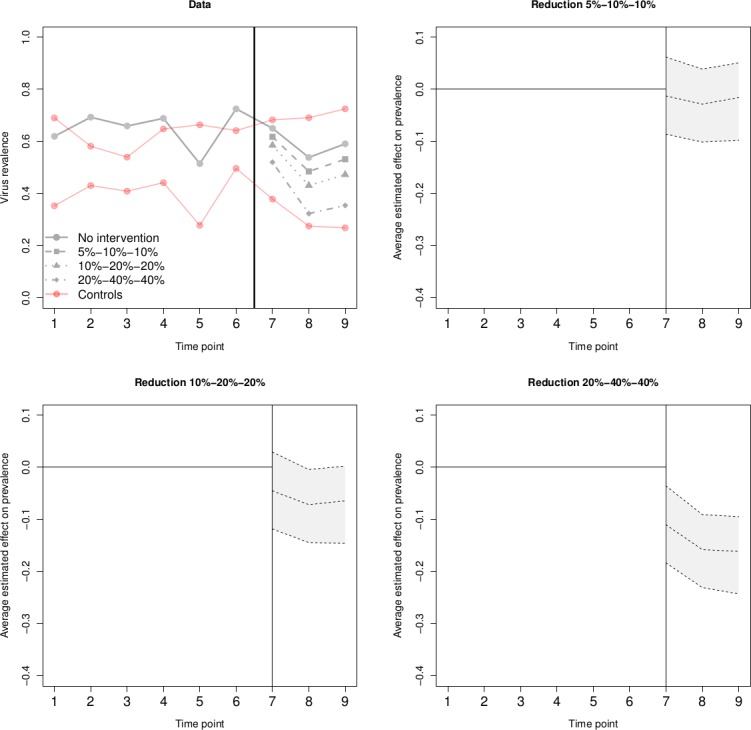
Causal impact synthetic control method (CIM) simulation and estimated intervention effects and 95% credible intervals for a range of assumed effects. Footnote: Illustration of CIM. First subplot shows a single data set, where solid lines represent the simulated prevalence in the absence of the intervention, and the dashed lines represent the outcome of treated site in the post-intervention period under different intervention magnitude scenarios. For each one of the three scenarios, we calculate the estimated average intervention effect along with credible intervals. These are shown in subplots 2 to 4. We see that as the effect increases, the intervals tend to move away for zero. However, the intervention effect only becomes significant in scenario 3, where zero is not included in any of the post-intervention time points.
Qualitative studies
Understanding the barriers and facilitators to scaling-up community-based HCV treatment
The qualitative study design has two distinct arms focusing on the intervention providers, and the intervention recipients.
Intervention providers
A purposive sample of 30 intervention providers, comprising nursing leads and key individuals from collaborating organisations will be approached directly by the lead hepatitis nurse. Seven focus groups will be convened according to professional role and locality:
HCV healthcare specialists (nurses and physicians).
Community pharmacists.
Prison staff (both healthcare and security).
‘Drug workers’ (from OST and NSP services).
Each focus group will consist of a maximum of six individuals and ideally comprise multi-agency mixed groups. Individual interviews by telephone will be offered for those hesitant to join a group (estimate 10 interviews). Topic guides informed by previous work in this area66 68 76 90 will facilitate group discussion.
Intervention recipients – cross-sectional and longitudinal
The intervention recipient arm of the study will comprise both cross-sectional and longitudinal elements. A cross-sectional approach will be employed to recruit 6 to 10 participants who do not take up the offer of treatment. These individuals will be recruited through the treatment pathways or through our peer-researcher networks. The longitudinal element will follow a cohort of up to 40 individuals recruited following their course of HCV treatment. These individuals will be purposively sampled from the existing services in which HCV TasP has been embedded (ie, pharmacy, prison and drug service), and then followed-up at 1 year post-treatment (with 70% expected to be followed-up).91 We aim to recruit women as well as men, younger and older people; those treated previously and first time; those injecting and not injecting at treatment onset. Follow-up interviews will explore collateral effects of HCV TasP including outcomes pertaining to drug use and injecting practices (secondary outcome below).
Participants will be recruited by hepatitis nurses or other clinical staff in Tayside and the face-to-face semi-structured interview will be conducted by peer-researchers, trained and guided by experienced qualitative researchers. Dr Magdalena Harris explains the importance of the use of peer researchers within the context of EPIToPe: https://www.youtube.com/watch?v=9ZZo3fKOXlg.92 93 The Scottish Drugs Forum (SDF) works with a group of Tayside peer-researchers with lived experience of injecting. Peer-researchers will receive study-orientated training and be provided with ongoing support to co-produce data and contribute to study outputs. A £20 shopping voucher will be offered to all interviewees except those in prison (Scottish prison service ethics did not permit thank you vouchers to prison participants).
Qualitative data analysis
Interviews and focus-groups will be audio-recorded using encrypted digital voice recorders, transcribed verbatim and anonymised. Nvivo v.10 software will be used to code and manage qualitative data. First level analysis will be deductive, guided by the research questions, and peer researchers will be consulted for input and feedback during the analytical process.94 A constant comparison method will be used to develop the thematic analysis and will reflect diverging and converging narratives, for example, across groups of intervention recipients at different time points in the treatment pathway, or between groups of intervention providers.94 The findings will be contextualised in the relevant theoretical perspectives which may include the diffusion of preventive innovations (staff) or social norms and values that might underpin health behaviour (recipients).95 96 We will assess TasP both from the providers’ perspective and from patients’ perspective including those who refuse treatment.
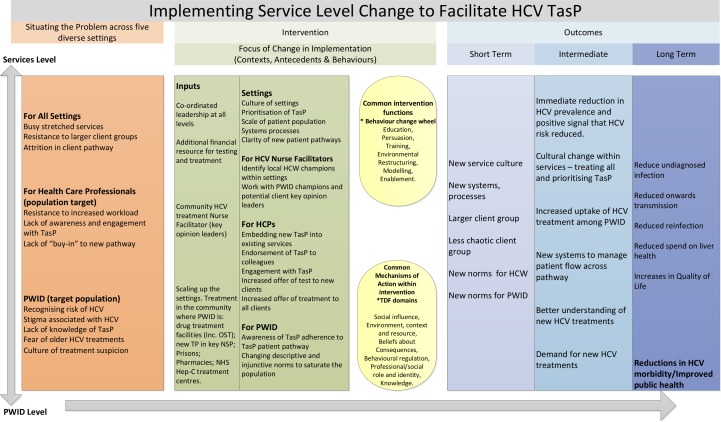
We will use the findings iteratively to update the HCV TasP logic model shown in figure 5. Our qualitative data will be used to generate a manual of an optimal intervention for other sites in UK. In previous examples, such as (https://www.youtube.com/channel/UCBV8smLmkOQVT9D0OR-md1g/videos) we have used the Behaviour Change Wheel96 as the framework to retrospectively analyse the success and failure of implementation within Tayside and then prospectively to formulate the optimal implementation intervention.
Figure 5.
Preliminary logic model HCV treatment as prevention (EPIToPe). HCPs, healthcare providers; HCV, hepatitis C virus; HCWs, healthcare workers; Hep-C, hepatitis C; NHS, National Health Service; NSP, needle and syringe programmes; TasP, Treatment as Prevention; PWID, people who inject drugs.
Mixed method study on drug use outcomes: OST retention, drug overdose, recovery and social transformation
Health Protection Scotland (HPS) link data on diagnostic HCV tests in the four largest Scottish NHS boards (including Tayside)8 and all persons undergoing HCV treatment in the Scottish HCV Clinical database97 which are also linked with other databases (including deaths, hospitalisations and drug treatment)8 42 98–100 and from 2018 Scotland's Prescribing Information System which holds data on OST and NHS prison health database (Prison Vision).101–105 PWID attending drug services who were HCV diagnosed, compared with those who were not, are at increased risk of drug-related and other cause-specific morbidity/mortality.106 107 Thus, we will create a virtual cohort of chronic HCV infected PWID (estimated to involve at least 600 individuals from Tayside and 3000 from elsewhere) and through linkage identify those who have been treated and attained SVR with those who have not. We will assess and compare the following outcomes: retention in drug treatment (determined through linkage to drug treatment and prescribing databases), drug-related and alcohol-related morbidity/mortality (through linkage to all hospital admission and mortality databases) and other markers of relapse (through linkage to prisons database).
Economic and impact evaluation
Infectious disease models can test the extent to which observed changes in disease transmission can be attributed to specific interventions,108–112 and assess cost-effectiveness of interventions that avert secondary infections, that is, have a population prevention benefit.50 85 113–117 We will update and adapt a transmission model of HCV among PWID in Scotland and Tayside to model the impact of the HCV treatment intervention based on historical trends and new observations collected as part of this programme.39 87 We will stratify the PWID population into current (injected in the previous year) and temporarily ceased (in OST and not injected in the previous year); as well as by duration of injecting (<3 years, 3 to 9 years, 10+years since onset), prevention intervention exposure (OST and/or high coverage NSP), and intervention settings for testing and treatment. We will use Approximate Bayesian Computation to calibrate the model to pre-intervention trends in chronic HCV prevalence and incidence among PWID in Tayside. The model will simulate the impact of observed rates of HCV treatment and cure rates for the intervention period, also incorporating any changes in the coverage of OST and NSP and injecting risk behaviours.
We will test consistency between the model impact projections and observed changes in HCV chronic prevalence and incidence from Tayside to disentangle the impact of HCV TasP from other interventions (OST/NSP) or epidemiological changes, and predict the impact of the TasP on number of HCV infections averted. If they are not consistent then alternative evidence-based hypotheses will be tested for why the model projects a different impact and the best fitting models will then be used to project the impact of the intervention. This will be assessed compared with two alternative counterfactuals where treatment rates are either at pre-scale-up levels in Tayside or at the average level achieved in other UK sites over the scale-up period. The impact of any changes in OST and NSP coverage will also be assessed to determine the contribution of those changes on observed effects. Impact will be assessed in terms of the relative decrease in prevalence and incidence, as well as the number and per cent of infections averted in the intervention model projections compared with each counterfactual over different time frames. These model projections can also be taken forward to evaluate the possible impact of the intervention over next 5 or 10 years.
We will evaluate the cost-effectiveness of the intervention (HCV treatment scale-up) compared with status quo (expected rate of HCV case-finding and treatment among PWID in the rest of the UK) from a healthcare provider (NHS) perspective, with the cost-effectiveness of the different settings where case-finding occurs also being assessed. The cost-effectiveness model will be based on the same dynamic impact model, adapted to include HCV disease progression stages and tracking of health outcomes among PWID after cessation of injecting.50 The economic evaluation will incorporate both individual benefits of HCV treatment (on disease progression) as well as population benefits (on HCV transmission). We will calculate the total number of infections and deaths over a 50 year time horizon for the intervention and counterfactual scenario and estimate the costs and quality-adjusted life years (QALYs) based on the number of individuals in each disease stage per year in the model. We will discount all future costs and QALYs at 3.5% (The National Institute for Health and Care Excellence (NICE) guidelines https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781). Probabilistic sensitivity analyses will be used to estimate the parametric uncertainty in the impact and cost projections. Cost-effectiveness results will be expressed in terms of incremental cost-effectiveness ratios and net monetary benefits estimated using NICE thresholds (£20 000 and £30 000 per QALY). We will plot cost-effectiveness acceptability curves to determine the probability of the intervention being cost-effective compared with different willingness-to-pay thresholds. Analyses of covariance methods will be used to summarise the proportion of the variability in the incremental costs and QALYs explained by uncertainty in different input parameters. Univariate sensitivity analyses will consider the effect of changes in important parameters such as time horizon, treatment cost and discount rate.
We focus on the incremental or additional resource costs associated with the intervention in Tayside. These costs, in part based on our earlier work for other studies, will include such things as the nurse time spent on intervention related activities (training other staff to offer HCV testing and treatment referral) as well as additional HCV testing and treatment costs, any additional OST costs due to HCV testing or treatment, and other staff time at the NSP, drug treatment centres and prisons involved with the intervention. Most of the incremental costs can be defined as variable (driven by extra nurse time and HCV testing/treatment costs). NHS HCV care costs and health utilities will be attached to each disease stage, based primarily on previous syntheses and models, which assume that PWID have a lower quality of life (QoL) than non-PWID of a similar age, gender and liver disease stage.118–120 Additional data using the EQ-5D-5L tool during this study will generate new health utility data on the QoL among PWID before and after DAA treatment.
Patient and public involvement
Patient and public involvement (PPI) was led by the Hepatitis C Trust and supported by qualitative research assessing barriers and facilitators to HCV treatment access (led by Dr Magdalena Harris). The SDF were also actively involved in the development of EPIToPe. The input from PPI groups has influenced the design of care pathways and has ensured that peer research is an essential element of the qualitative strand of EPIToPe.
A pilot National Institute for Health Research (NIHR) funded study in England (HEPCAT) responding to NICE Guidance on Hepatitis Case Finding was co-designed with Hepatitis C trust. It showed that Hepatitis C Facilitators and peer-support networks can increase the uptake of HCV case-finding and HCV treatment readiness in addiction services. This pilot study and our studies in Dundee/Tayside will influence how HCV treatment can be scaled up in England and our proposed evaluation HCV treatment as prevention.
Peer researchers will be trained to conduct the longitudinal study with PWID treated for HCV and will be involved and contribute to the analysis of the findings. Peer researchers and SDF will be members of the project management group and steering committee.
Dissemination events will be held in Dundee to discuss and present the findings from the qualitative studies with patient groups and services. These will be facilitated by SDF to support active contribution from our peer researchers. The study findings will be summarised and promoted through SDF website, social media platforms and through their sector-wide conferences in Scotland. Hepatitis Scotland, who are hosted within SDF, together with patient and public groups in England will take an active role in the wider national and international dissemination of the research, it’s translation into patient meaningful materials and its integration into a national policy context. The research will also be promoted via Hepatitis C Trust and Public Health England.
Discussion
Strengths and limitations of this study
Several limitations arise from the ‘natural experiment’ design as our intervention and controls were not randomised. In the UK and many other countries there is no longer sufficient equipoise in clinicians and policymakers – given WHO and national strategies on HCV ‘elimination’ – to mount an randomised controlled trial of HCV TasP. As a result, there will be confounding and additional uncertainty in the measurement of the intervention effect. However, we consider that a natural experiment and use of synthetic control methods to be a more robust design than simple before and after studies. Our preliminary simulation work also suggests that we should have sufficient power to detect the large intervention effect that is planned.
We know also that HCV treatment and prevention strategy in UK (and Europe) is evolving – motivated both by WHO ‘elimination targets’ and falling drug prices – and our control sites in Scotland and England may increase HCV treatment rates earlier than expected. This will complicate the analyses a little and potentially dilute the intervention effect. We are confident that we can adapt the synthetic control methods to take account of changes over time – and that because Tayside has started so early in scaling up HCV treatment that we will have time to detect a difference in the outcome.
The lack of randomised controls means that we have to generate the counterfactual of ‘no HCV treatment scale-up’ through our HCV transmission model so that we can subsequently estimate cost-effectiveness of the intervention in Tayside. This is not ideal but has become standard practice in economic models of novel HCV treatment interventions – and we are involved with the modelling of HCV treatment pathways through homeless centres, prison, Accident and Emergency (A&E), pharmacies, specialist drug clinics and NSPs (P Vickerman personal communication and for example55 85 121). We know also, however, that the benefit in terms of additional Quality of Life Years and averted HCV infections accrues and occurs over a prolonged period.50 It is more critical for any economic evaluation of HCV interventions in PWID that a dynamic model is used so that the prevention benefit (in terms of HCV infections averted) is correctly accounted for.
We are using peer researchers in the qualitative arm of patients’ perspectives on the intervention and on the impact of HCV treatment on addiction outcomes. This is novel but adds additional challenges to obtaining NHS passports and ensuring data quality across the interviews and interviewees. We are also intending to support peers in analysis and interpretation of the findings which we believe has not been done before. We have trained the interviewers and will be monitoring their performance of the interviewers to ensure consistent study quality – and will replace peers with our qualitative researcher if required.
Future study: natural experiment of TasP in England
In England HCV treatment is delivered through 22 operational delivery networks (ODNs). NHS England’s HCV strategy (2016 to 2019) prioritised 10 000 patients per year in line with the declared priorities of the network which could (and in many cases did) include people who use drugs at risk of transmission.44 In October 2018 it is anticipated that a new procurement deal will substantially increase the number of patients who can access DAAs and this will enable ‘trace and treat’ options to be introduced. We will use the first part of EPIToPe including the manual generated by the qualitative study, enhancements to historical and ongoing surveillance of chronic HCV in PWID, infectious disease models and methodological developments of causal impact model, to co-design with ODN leads a natural experiment of HCV TasP in England.
Supplementary Material
Acknowledgments
We would like to thank all those involved in PPI from both SDF and the Hepatitis C Trust.
Footnotes
Contributors: All authors contributed to editing of the manuscript. MH and SJH are co-PIs of EPIToPe and prepared first draft of the manuscript. JD leads intervention scale-up in Tayside in collaboration with Tayside CTU LJZB, PD, SKI, AR and AE. LE leads qualitative component of EPIToPe in collaboration with Scottish Drug Forum DL, EH and AM and support from MH, GV and DW on qualitative research and training of peer support workers, and PF on behavioural science. DDA leads synthetic control estimation and multiple parameter evidence synthesis in collaboration with PS, RH, AP, and NM. PV leads dynamic impact and economic modelling in collaboration with NM, ZW, HF with health economics led by W Hollingworth (WH), with GM, as part of Bristol Randomised Trial Collaboration (BRTC) with advice on trial design from JH, CM and AL. GF is leading design of evaluation in England based on EPIToPe with support from BRTC and KD. SH is leading on outcome measurement in Scotland with Health Protection Scotland DG, AMA, and in collaboration with LG from ISD, RNG, HI, NEP and AY. SM and SI are leading on outcome measurement in England with RG, HH, EH, VH, SM, MR, RS, SK. JM is the Programme Manager.
Funding: ‘This study is funded by the National Institute for Health Research (NIHR) Programme Grants for Applied Research programme (Grant Reference Number RP-PG-0616-20008). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care ’. In addition, we acknowledge support from NIHR Health Protection Research Unit in Evaluation, and the Bristol Randomised Trials Collaboration (BRTC), a UKCRC Registered Clinical Trials Unit in receipt of NIHR Clinical Trials Units (CTU) support funding. The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. NM is supported by the National Institute for Drug Abuse [grant number R01 DA037773] and the University of California San Diego Center for AIDS Research (CFAR), a National Institute of Health (NIH) funded program [grant number P30 AI036214].
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384:1953–97. 10.1016/S0140-6736(14)61838-9 [DOI] [PubMed] [Google Scholar]
- 2. De Angelis D, Sweeting M, Ades AE, et al. An evidence synthesis approach to estimating hepatitis C prevalence in England and Wales. Stat Methods Med Res 2009;18:361–79. 10.1177/0962280208094691 [DOI] [PubMed] [Google Scholar]
- 3. Harris RJ, Ramsay M, Hope VD, et al. Hepatitis C prevalence in England remains low and varies by ethnicity: an updated evidence synthesis. Eur J Public Health 2012;22:187–92. 10.1093/eurpub/ckr083 [DOI] [PubMed] [Google Scholar]
- 4. Hutchinson SJ, Roy KM, Wadd S, et al. Hepatitis C virus infection in Scotland: epidemiological review and public health challenges. Scott Med J 2006;51:8–15. 10.1258/RSMSMJ.51.2.8 [DOI] [PubMed] [Google Scholar]
- 5. Prevost TC, Presanis AM, Taylor A, et al. Estimating the number of people with hepatitis C virus who have ever injected drugs and have yet to be diagnosed: an evidence synthesis approach for Scotland. Addiction 2015;110:1287–300. 10.1111/add.12948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hutchinson SJ, McIntyre PG, Molyneaux P, et al. Prevalence of hepatitis C among injectors in Scotland 1989–2000: declining trends among young injectors halt in the late 1990s. Epidemiol Infect 2002;128:473–7. 10.1017/S0950268802006945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin NK, Foster GR, Vilar J, et al. Hcv treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. J Viral Hepat 2015;22:399–408. 10.1111/jvh.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McLeod A, Weir A, Aitken C, et al. Rise in testing and diagnosis associated with Scotland's action plan on hepatitis C and introduction of dried blood spot testing. J Epidemiol Community Health 2014;68:1182–8. 10.1136/jech-2014-204451 [DOI] [PubMed] [Google Scholar]
- 9. Palmateer NE, Hutchinson SJ, Innes H, et al. Review and meta-analysis of the association between self-reported sharing of needles/syringes and hepatitis C virus prevalence and incidence among people who inject drugs in Europe. International Journal of Drug Policy 2013;24:85–100. 10.1016/j.drugpo.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 10. Palmateer NE, Taylor A, Goldberg DJ, et al. Rapid decline in HCV incidence among people who inject drugs associated with national scale-up in coverage of a combination of harm reduction interventions. PLoS One 2014;9:e104515 10.1371/journal.pone.0104515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roy KM, Hutchinson SJ, Wadd S, et al. Hepatitis C virus infection among injecting drug users in Scotland: a review of prevalence and incidence data and the methods used to generate them. Epidemiol Infect 2007;135:433–42. 10.1017/S0950268806007035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turner KME, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 2011;106:1978–88. 10.1111/j.1360-0443.2011.03515.x [DOI] [PubMed] [Google Scholar]
- 13. Harris RJ, Hope VD, Morongiu A, et al. Spatial mapping of hepatitis C prevalence in recent injecting drug users in contact with services. Epidemiol Infect 2011:1–10. [DOI] [PubMed] [Google Scholar]
- 14. Hickman M, Hope V, Brady T, et al. Hepatitis C virus (HCV) prevalence, and injecting risk behaviour in multiple sites in England in 2004. J Viral Hepat 2007;14:645–52. 10.1111/j.1365-2893.2007.00855.x [DOI] [PubMed] [Google Scholar]
- 15. Hope VD, Hickman M, Ngui SL, et al. Measuring the incidence, prevalence and genetic relatedness of hepatitis C infections among a community recruited sample of injecting drug users, using dried blood spots. J Viral Hepat 2011;18:262–70. 10.1111/j.1365-2893.2010.01297.x [DOI] [PubMed] [Google Scholar]
- 16. Sutton AJ, Gay NJ, Edmunds WJ, et al. Modelling the force of infection for hepatitis B and hepatitis C in injecting drug users in England and Wales. BMC Infect Dis 2006;6 10.1186/1471-2334-6-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sweeting MJ, Hope VD, Hickman M, et al. Hepatitis C infection among injecting drug users in England and Wales (1992-2006): there and back again? Am J Epidemiol 2009;170:352–60. 10.1093/aje/kwp141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiessing L, Ferri M, Grady B, et al. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One 2014;9:e103345 10.1371/journal.pone.0103345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hickman MM, editor N. Hepatitis C among drug users in Europe: epidemiology, treatment and prevention. Lisbon: EMCDDA, 2016. [Google Scholar]
- 20. Van Den Berg C, Smit C, Van Brussel G, et al. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam cohort studies among drug users. Addiction 2007;102:1454–62. 10.1111/j.1360-0443.2007.01912.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. Journal of Infectious Diseases 2011;204:74–83. 10.1093/infdis/jir196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Platt L, Reed J, Minozzi S, et al. Effectiveness of needle/syringe programmes and opiate substitution therapy in preventing HCV transmission among people who inject drugs. Cochrane Database Syst Rev 2016 2016;(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutchinson SJ, Dillon JF, Fox R, et al. Expansion of HCV treatment access to people who have injected drugs through effective translation of research into public health policy: Scotland's experience. Int J Drug Policy 2015. [DOI] [PubMed] [Google Scholar]
- 24. Fraser H, Mukandavire C, Martin NK, et al. Modelling the impact of a national scale-up of interventions on hepatitis C virus transmission among people who inject drugs in Scotland. Addiction 2018;113:2118–31. 10.1111/add.14267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Vos AS, van der Helm JJ, Matser A, et al. Decline in incidence of HIV and hepatitis C virus infection among injecting drug users in Amsterdam; evidence for harm reduction? Addiction 2013;108:1070–81. 10.1111/add.12125 [DOI] [PubMed] [Google Scholar]
- 26. Vickerman P, Martin N, Turner K, et al. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? model projections for different epidemic settings. Addiction 2012;107:1984–95. 10.1111/j.1360-0443.2012.03932.x [DOI] [PubMed] [Google Scholar]
- 27. Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of "perfectovir". Clinical Infectious Diseases 2015;60:1829–36. 10.1093/cid/civ197 [DOI] [PubMed] [Google Scholar]
- 28. Gogela NA, Lin MV, Wisocky JL, et al. Enhancing our understanding of current therapies for hepatitis C virus (HCV). Curr HIV/AIDS Rep 2015;12:68–78. 10.1007/s11904-014-0243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker DR, Pedrosa MC, Manthena SR, et al. Early view of the effectiveness of new direct-acting antiviral (DAA) regimens in patients with hepatitis C virus (HCV). Adv Ther 2015;32:1117–27. 10.1007/s12325-015-0258-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hellard M, Doyle JS, Sacks-Davis R, et al. Eradication of hepatitis C infection: the importance of targeting people who inject drugs. Hepatology 2014;59:366–9. 10.1002/hep.26623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hagan LM, Wolpe PR, Schinazi RF. Treatment as prevention and cure towards global eradication of hepatitis C virus. Trends Microbiol 2013;21:625–33. 10.1016/j.tim.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grebely J, Matthews GV, Lloyd AR, et al. Elimination of hepatitis C virus infection among people who inject drugs through treatment as prevention: feasibility and future requirements. Clinical Infectious Diseases 2013;57:1014–20. 10.1093/cid/cit377 [DOI] [PubMed] [Google Scholar]
- 33. Bruggmann P. Treatment as prevention: the breaking of taboos is required in the fight against hepatitis C among people who inject drugs. Hepatology 2013;58:1523–5. 10.1002/hep.26539 [DOI] [PubMed] [Google Scholar]
- 34. Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013;58:1598–609. 10.1002/hep.26431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wedemeyer H, Duberg AS, Buti M, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat 2014;21(Suppl. 1):60–89. 10.1111/jvh.12249 [DOI] [PubMed] [Google Scholar]
- 36. Martin NK, Vickerman P, Dore GJ, et al. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Current opinion in HIV and AIDS 2015;10:374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hickman M, De Angelis D, Vickerman P, et al. Hcv treatment as prevention in people who inject Drugs–testing the evidence. Current opinion in infectious diseases 2015;28:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hayes R, Ayles H, Beyers N, et al. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment – a study protocol for a cluster randomised trial. Trials 2014;15:57 10.1186/1745-6215-15-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fraser H, Zibbell J, Hoerger T, et al. Scaling-up HCV prevention and treatment interventions in rural United States-model projections for tackling an increasing epidemic. Addiction 2018;113:173–82. 10.1111/add.13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Organization WH Global health sector strategy on viral hepatitis 2016-2021: towards ending viral hepatitis. Geneva: WHO, 2016. [Google Scholar]
- 41. Innes HA, McDonald SA, Dillon JF, et al. Toward a more complete understanding of the association between a hepatitis C sustained viral response and cause-specific outcomes. Hepatology 2015;62:355–64. 10.1002/hep.27766 [DOI] [PubMed] [Google Scholar]
- 42. Innes HA, Hutchinson SJ, Allen S, et al. Excess liver-related morbidity of chronic hepatitis C patients, who achieve a sustained viral response, and are discharged from care. Hepatology 2011;54:1547–58. 10.1002/hep.24561 [DOI] [PubMed] [Google Scholar]
- 43. Innes H, Goldberg D, Dillon J, et al. Strategies for the treatment of hepatitis C in an era of interferon-free therapies: what public health outcomes do we value most? Gut 2015;64:1800–9. 10.1136/gutjnl-2014-308166 [DOI] [PubMed] [Google Scholar]
- 44. Harris RJ, Martin NK, Rand E, et al. New treatments for hepatitis C virus (HCV): scope for preventing liver disease and HCV transmission in England. J Viral Hepat 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2014. J Hepatol 2014;61:373–95. 10.1016/j.jhep.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 46. European Association for Study of Liver EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015;63:199–236. 10.1016/j.jhep.2015.03.025 [DOI] [PubMed] [Google Scholar]
- 47. Panel A. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015. [DOI] [PubMed] [Google Scholar]
- 48. Grebely J, Robaeys G, Bruggmann P, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. International Journal of Drug Policy 2015;26:1028–38. 10.1016/j.drugpo.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. European Association for the Study of the Liver Electronic address eee. EASL recommendations on treatment of hepatitis C 2016. J Hepatol 2016. [DOI] [PubMed] [Google Scholar]
- 50. Martin NK, Vickerman P, Dore GJ, et al. Prioritization of HCV treatment in the direct-acting antiviral era: an economic evaluation. J Hepatol 2016;65:17–25. 10.1016/j.jhep.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aleman S, Rahbin N, Weiland O, et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis 2013;57:230–6. 10.1093/cid/cit234 [DOI] [PubMed] [Google Scholar]
- 52. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308:2584–93. 10.1001/jama.2012.144878 [DOI] [PubMed] [Google Scholar]
- 53. Innes H, Hutchinson SJ, Obel N, et al. Liver mortality attributable to chronic hepatitis C virus infection in Denmark and Scotland-Using spontaneous resolvers as the benchmark comparator. Hepatology 2016;63:1506–16. 10.1002/hep.28458 [DOI] [PubMed] [Google Scholar]
- 54. Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol 2018;68:402–11. 10.1016/j.jhep.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schulkind J, Stephens B, Ahmad F, et al. High response and re-infection rates among people who inject drugs treated for hepatitis C in a community needle and syringe programme. J Viral Hepat 2019;26:519–28. 10.1111/jvh.13035 [DOI] [PubMed] [Google Scholar]
- 56. MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012;345:e5945 10.1136/bmj.e5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. MacArthur GJ, van VE, Palmateer N, et al. Interventions to prevent HIV and hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. IntJ Drug Policy 2013. [DOI] [PubMed] [Google Scholar]
- 58. Palmateer N, Hutchinson S, McAllister G, et al. Risk of transmission associated with sharing drug injecting paraphernalia: analysis of recent hepatitis C virus (HCV) infection using cross-sectional survey data. J Viral Hepat 2014;21:25–32. 10.1111/jvh.12117 [DOI] [PubMed] [Google Scholar]
- 59. Palmateer N, Kimber J, Hickman M, et al. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction 2010;105:844–59. 10.1111/j.1360-0443.2009.02888.x [DOI] [PubMed] [Google Scholar]
- 60. Presanis AM, Gill ON, Chadborn TR, et al. Insights into the rise in HIV infections, 2001 to 2008: a Bayesian synthesis of prevalence evidence. AIDS 2010;24:2849–58. 10.1097/QAD.0b013e32834021ed [DOI] [PubMed] [Google Scholar]
- 61. Harris HE. Hepatitis C in the UK 2016 report: working towards its elimination as a major public health threat. London: Public Health England, 2016. [Google Scholar]
- 62. Health Protection Scotland UotWoS, Glasgow Caledonian University and the West of Scotland Specialist Virology Centre The needle exchange surveillance initiative: prevalence of blood-borne viruses and injecting risk behaviours among people who inject drugs attending injecting equipment provision services in Scotland, 2008-09 to 2015-16. Scotland HP: Glasgow, 2017. [Google Scholar]
- 63. Brant LJ, Hurrelle M, Balogun MA, et al. Sentinel laboratory surveillance of hepatitis C antibody testing in England: understanding the epidemiology of HCV infection. Epidemiol Infect 2007;135:417–26. 10.1017/S0950268806006832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brant LJ, Hurrelle M, Balogun MA, et al. Where are people being tested for anti-HCV in England? results from sentinel laboratory surveillance. J Viral Hepat 2008;15:729–39. 10.1111/j.1365-2893.2008.01000.x [DOI] [PubMed] [Google Scholar]
- 65. Lattimore S, Irving W, Collins S, et al. Using surveillance data to determine treatment rates and outcomes for patients with chronic hepatitis C virus infection. Hepatology 2014;59:1343–50. 10.1002/hep.26926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Treloar C, Newland J, Rance J, et al. Uptake and delivery of hepatitis C treatment in opiate substitution treatment: perceptions of clients and health professionals. J Viral Hepat 2010;17:839–44. 10.1111/j.1365-2893.2009.01250.x [DOI] [PubMed] [Google Scholar]
- 67. Treloar C, Rance J, Dore GJ, et al. Barriers and facilitators for assessment and treatment of hepatitis C virus infection in the opioid substitution treatment setting: insights from the ethos study. J Viral Hepat 2014;21:560–7. 10.1111/jvh.12183 [DOI] [PubMed] [Google Scholar]
- 68. Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J 2013;10:7 10.1186/1477-7517-10-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Swan D, Long J, Carr O, et al. Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration. AIDS Patient Care STDS 2010;24:753–62. 10.1089/apc.2010.0142 [DOI] [PubMed] [Google Scholar]
- 70. Rhodes T, Harris M, Martin A. Negotiating access to medical treatment and the making of patient citizenship: the case of hepatitis C treatment. Sociol Health Illn 2013;35:1023–44. 10.1111/1467-9566.12018 [DOI] [PubMed] [Google Scholar]
- 71. Harris M, Albers E, Swan T. The promise of treatment as prevention for hepatitis C: meeting the needs of people who inject drugs? International Journal of Drug Policy 2015;26:963–9. 10.1016/j.drugpo.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 72. Rance J, Treloar C, Group ES, ETHOS Study Group . 'Not just methadone Tracy': transformations in service-user identity following the introduction of hepatitis C treatment into Australian opiate substitution settings. Addiction 2014;109:452–9. 10.1111/add.12392 [DOI] [PubMed] [Google Scholar]
- 73. Treloar C, Rance J, Backmund M. Understanding barriers to hepatitis C virus care and stigmatization from a social perspective. Clin Infect Dis 2013;57(suppl_2):S51–S55. 10.1093/cid/cit263 [DOI] [PubMed] [Google Scholar]
- 74. Treloar C, Rance J, Bryant J, et al. Harm reduction workers and the challenge of engaging couples who inject drugs in hepatitis C prevention. Drug Alcohol Depend 2016;168:170–5. 10.1016/j.drugalcdep.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 75. Treloar C, Rance J, Bryant J, et al. Understanding decisions made about hepatitis C treatment by couples who inject drugs. J Viral Hepat 2016;23:89–95. 10.1111/jvh.12451 [DOI] [PubMed] [Google Scholar]
- 76. Jack K, Islip N, Linsley P, et al. Prison officers' views about hepatitis C testing and treatment: a qualitative enquiry. J Clin Nurs 2016. [DOI] [PubMed] [Google Scholar]
- 77. Rich ZC, Chu C, Mao J, et al. Facilitators of HCV treatment adherence among people who inject drugs: a systematic qualitative review and implications for scale up of direct acting antivirals. BMC Public Health 2016;16:994 10.1186/s12889-016-3671-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Clark JA, Gifford AL. Resolute efforts to cure hepatitis C: Understanding patients’ reasons for completing antiviral treatment. Health 2015;19:473–89. 10.1177/1363459314555237 [DOI] [PubMed] [Google Scholar]
- 79. Batchelder AW, Peyser D, Nahvi S, et al. “Hepatitis C treatment turned me around:” Psychological and behavioral transformation related to hepatitis C treatment. Drug Alcohol Depend 2015;153:66–71. 10.1016/j.drugalcdep.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Harris M. Managing expense and expectation in a treatment revolution: Problematizing prioritisation through an exploration of hepatitis C treatment ‘benefit’. International Journal of Drug Policy 2017;47:161–8. 10.1016/j.drugpo.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 81. Aspinall EJ, Hutchinson SJ, Janjua NZ, et al. Trends in mortality after diagnosis of hepatitis C virus infection: an international comparison and implications for monitoring the population impact of treatment. J Hepatol 2015;62:269–77. 10.1016/j.jhep.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 82. Aspinall EJ, Corson S, Doyle JS, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis 2013;57(suppl_2):S80–S89. 10.1093/cid/cit306 [DOI] [PubMed] [Google Scholar]
- 83. Judd A, Parry J, Hickman M, et al. Evaluation of a modified commercial assay in detecting antibody to hepatitis C virus in oral fluids and dried blood spots. J Med Virol 2003;71:49–55. 10.1002/jmv.10463 [DOI] [PubMed] [Google Scholar]
- 84. Bennett S, Gunson RN, McAllister GE, et al. Detection of hepatitis C virus RNA in dried blood spots. Journal of Clinical Virology 2012;54:106–9. 10.1016/j.jcv.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 85. Martin NK, Vickerman P, Brew IF, et al. Is increased hepatitis C virus case-finding combined with current or 8-week to 12-week direct-acting antiviral therapy cost-effective in UK prisons? A prevention benefit analysis. Hepatology 2016;63:1796–808. 10.1002/hep.28497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McDonald SA, Innes HA, Hayes PC, et al. What is the impact of a country-wide scale-up in antiviral therapy on the characteristics and sustained viral response rates of patients treated for hepatitis C? J Hepatol 2015;62:262–8. 10.1016/j.jhep.2014.08.046 [DOI] [PubMed] [Google Scholar]
- 87. Ward Z, Platt L, Sweeney S, et al. Impact of current and scaled-up levels of hepatitis C prevention and treatment interventions for people who inject drugs in three UK settings-what is required to achieve the who's HCV elimination targets? Addiction 2018;113:1727–38. 10.1111/add.14217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brodersen KH, Gallusser F, Koehler J, et al. Inferring causal impact using Bayesian structural time-series models. Ann Appl Stat 2015;9:247–74. 10.1214/14-AOAS788 [DOI] [Google Scholar]
- 89. Scott SL, Varian HR. Predicting the present with Bayesian structural time series. IJMMNO 2014;5 10.1504/IJMMNO.2014.059942 [DOI] [Google Scholar]
- 90. Rance J, Newland J, Hopwood M, et al. The politics of place(ment): Problematising the provision of hepatitis C treatment within opiate substitution clinics. Soc Sci Med 2012;74:245–53. 10.1016/j.socscimed.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 91. Lankenau SE, Sanders B, Hathazi D, et al. Recruiting and retaining mobile young injection drug users in a longitudinal study. Subst Use Misuse 2010;45:684–99. 10.3109/10826081003594914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stewart KE, Wright PB, Sims D, et al. The “Translators”: Engaging Former Drug Users as Key Research Staff to Design and Implement a Risk Reduction Program for Rural Cocaine Users. Subst Use Misuse 2012;47:547–54. 10.3109/10826084.2011.644379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Harris M. "Three in the Room": Embodiment, Disclosure, and Vulnerability in Qualitative Research. Qual Health Res 2015;25:1689–99. 10.1177/1049732314566324 [DOI] [PubMed] [Google Scholar]
- 94. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 95. Rogers EM. Diffusion of preventive innovations. Addict Behav 2002;27:989–93. 10.1016/S0306-4603(02)00300-3 [DOI] [PubMed] [Google Scholar]
- 96. Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implementation Sci 2012;7 10.1186/1748-5908-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Innes HA, Hutchinson SJ, Allen S, et al. Ranking predictors of a sustained viral response for patients with chronic hepatitis C treated with pegylated interferon and ribavirin in Scotland. Eur J Gastroenterol Hepatol 2012;24:646–55. 10.1097/MEG.0b013e32835201a4 [DOI] [PubMed] [Google Scholar]
- 98. McDonald SA, Hutchinson SJ, Bird SM, et al. Excess morbidity in the hepatitis C-diagnosed population in Scotland, 1991-2006. Epidemiol Infect 2011;139:344–53. 10.1017/S0950268810001421 [DOI] [PubMed] [Google Scholar]
- 99. McDonald SA, Hutchinson SJ, Bird SM, et al. A record-linkage study of the development of hepatocellular carcinoma in persons with hepatitis C infection in Scotland. Br J Cancer 2008;99:805–10. 10.1038/sj.bjc.6604563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. McDonald SA, Hutchinson SJ, Bird SM, et al. A population-based record linkage study of mortality in hepatitis C-diagnosed persons with or without HIV coinfection in Scotland. Stat Methods Med Res 2009;18:271–83. 10.1177/0962280208094690 [DOI] [PubMed] [Google Scholar]
- 101. Gao L, Dimitropoulou P, Robertson JR, et al. Risk-factors for methadone-specific deaths in Scotland’s methadone-prescription clients between 2009 and 2013*. Drug Alcohol Depend 2016;167:214–23. 10.1016/j.drugalcdep.2016.08.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bird SM, Fischbacher CM, Graham L, et al. Impact of opioid substitution therapy for Scotland's prisoners on drug-related deaths soon after prisoner release. Addiction 2015;110:1617–24. 10.1111/add.12969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bird SM, Hutchinson SJ. Male drugs-related deaths in the fortnight after release from prison: Scotland, 1996-99. Addiction 2003;98:185–90. 10.1046/j.1360-0443.2003.00264.x [DOI] [PubMed] [Google Scholar]
- 104. Alvarez-Madrazo S, McTaggart S, Nangle C, et al. Data resource profile: the Scottish national prescribing information system (PIs). Int J Epidemiol 2016;45:714–5. 10.1093/ije/dyw060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Graham L, Fischbacher CM, Stockton D, et al. Understanding extreme mortality among prisoners: a national cohort study in Scotland using data linkage. Eur J Public Health 2015;25:879–85. 10.1093/eurpub/cku252 [DOI] [PubMed] [Google Scholar]
- 106. Merrall ELC, Bird SM, Hutchinson SJ. Mortality of those who attended drug services in Scotland 1996–2006: record-linkage study. International Journal of Drug Policy 2012;23:24–32. 10.1016/j.drugpo.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. McDonald SA, Hutchinson SJ, Bird SM, et al. Hospitalisation for an alcohol-related cause among injecting drug users in Scotland: increased risk following diagnosis with hepatitis C infection. International Journal of Drug Policy 2011;22:63–9. 10.1016/j.drugpo.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 108. de Vos AS, Prins M, Kretzschmar MEE. Hepatitis C virus treatment as prevention among injecting drug users: who should we cure first? Addiction 2015;110:975–83. 10.1111/add.12842 [DOI] [PubMed] [Google Scholar]
- 109. Boily M-C, Lowndes CM, Vickerman P, et al. Evaluating large-scale HIV prevention interventions: study design for an integrated mathematical modelling approach. Sex Transm Infect 2007;83:582–9. 10.1136/sti.2007.027516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Boily M-C, Pickles M, Lowndes CM, et al. Positive impact of a large-scale HIV prevention programme among female sex workers and clients in South India. AIDS 2013;27:1449–60. 10.1097/QAD.0b013e32835fba81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hallett TB, Gregson S, Mugurungi O, et al. Assessing evidence for behaviour change affecting the course of HIV epidemics: a new mathematical modelling approach and application to data from Zimbabwe. Epidemics 2009;1:108–17. 10.1016/j.epidem.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 112. Pickles M, Boily M-C, Vickerman P, et al. Assessment of the population-level effectiveness of the Avahan HIV-prevention programme in South India: a preplanned, causal-pathway-based modelling analysis. The Lancet Global Health 2013;1:e289–99. 10.1016/S2214-109X(13)70083-4 [DOI] [PubMed] [Google Scholar]
- 113. Martin NK, Hickman M, Miners A, et al. Cost-Effectiveness of HCV case-finding for people who inject drugs via dried blood spot testing in specialist addiction services and prisons. BMJ Open 2013;3:e003153 10.1136/bmjopen-2013-003153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Martin NK, Miners A, Vickerman P. Assessing the cost-effectiveness of interventions aimed at promoting and offering hepatitis C testing to injecting drug users: an economic modelling report. London, 2012. [Google Scholar]
- 115. Martin NK, Pitcher AB, Vickerman P, et al. Optimal control of hepatitis C antiviral treatment programme delivery for prevention amongst a population of injecting drug users. PLoS One 2011;6:e22309 10.1371/journal.pone.0022309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Martin NK, Vickerman P, Miners A, et al. Cost-Effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology 2012;55:49–57. 10.1002/hep.24656 [DOI] [PubMed] [Google Scholar]
- 117. Martin NK, Vickerman P, Miners A, et al. How cost-effective is hepatitis C virus treatment for people who inject drugs? J Gastroenterol Hepatol 2013;28:590–2. 10.1111/jgh.12113 [DOI] [PubMed] [Google Scholar]
- 118. Kimber J, Copeland L, Hickman M, et al. Survival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatment. BMJ 2010;341:c3172 10.1136/bmj.c3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. McDonald SA, Hutchinson SJ, Palmateer NE, et al. Decrease in health-related quality of life associated with awareness of hepatitis C virus infection among people who inject drugs in Scotland. J Hepatol 2013;58:460–6. 10.1016/j.jhep.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 120. Wright M, Grieve R, Roberts J, et al. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess 2006;10:1–113. 10.3310/hta10210 [DOI] [PubMed] [Google Scholar]
- 121. Harrison GI, Murray K, Gore R, et al. The hepatitis C awareness through to treatment (HepCATT) study: improving the cascade of care for hepatitis C virus‐infected people who inject drugs in England. Addiction 2019;114:1113–22. 10.1111/add.14569 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.