Abstract
Purpose
The Upper Gastrointestinal Cancer Registry (UGICR) was developed to monitor and improve the quality of care provided to patients with upper gastrointestinal cancers in Australia.
Participants
It supports four cancer modules: pancreatic, oesophagogastric, biliary and primary liver cancer. The pancreatic cancer (PC) module was the first module to be implemented, with others being established in a staged approach. Individuals are recruited to the registry if they are aged 18 years or older, have received care for their cancer at a participating public/private hospital or private clinic in Australia and do not opt out of participation.
Findings to date
The UGICR is governed by a multidisciplinary steering committee that provides clinical governance and oversees clinical working parties. The role of the working parties is to develop quality indicators based on best practice for each registry module, develop the minimum datasets and provide guidance in analysing and reporting of results. Data are captured from existing data sources (population-based cancer incidence registries, pathology databases and hospital-coded data) and manually from clinical records. Data collectors directly enter information into a secure web-based Research Electronic Data Capture (REDCap) data collection platform. The PC module began with a pilot phase, and subsequently, we used a formal modified Delphi consensus process to establish a core set of quality indicators for PC. The second module developed was the oesophagogastric cancer (OGC) module. Results of the 1 year pilot phases for PC and OGC modules are included in this cohort profile.
Future plans
The UGICR will provide regular reports of risk-adjusted, benchmarked performance on a range of quality indicators that will highlight variations in care and clinical outcomes at a health service level. The registry has also been developed with the view to collect patient-reported outcomes (PROs), which will further add to our understanding of the care of patients with these cancers.
Keywords: pancreatic cancer, oesophageal cancer, gastric cancer, liver cancer, biliary cancer, upper gastrointestinal cancers, clinical registry, quality improvement, quality of care, database, population health
Strengths and limitations of this study.
The Upper Gastrointestinal Cancer Registry is the first clinical quality registry (CQR) in Australia, designed to capture information on upper gastrointestinal (UGI) cancers with the aim to improve practice by monitoring and providing benchmarked reports to participating sites.
We describe the development of a CQR for UGI cancers, including the establishment of governance, recruitment framework, clinical quality indicators, minimum data set, data access policy and reporting structure.
This registry was developed as per the Australian Commission on Quality and Safety in Health Care’s (ACSQHC) Framework for Australian CQRs and follows ACSQHC’s Australian Operating Principles for CQRs and can be used as a model for researchers developing CQRs.
The time-consuming and labour-intensive site governance approval process in Australia is a major limitation for rollout of the registry.
Introduction
The five most common upper gastrointestinal (UGI) cancers in Australia are pancreas, oesophagus, stomach, liver (hepatocellular carcinoma) and biliary cancers; the combined incidence is approximately 10 000, and there are around 7500 deaths annually.1 The 5-year relative survival rates of UGI cancers are among the worst of all tumour types: 9.8% in pancreas; 18.5% in liver; 20.1% in biliary; 22% in oesophagus; and 30.3% in stomach.1 The dismal prognosis of these cancers can be largely attributed to their presentation at an advanced disease stage. Additionally, older age is a risk factor for mortality from these tumours, and significant cardiac and respiratory comorbidities may limit treatment options. As a result, only 15% of pancreas, 43% of liver, 20% of oesophagus and 50% of stomach cancers are potentially resectable at diagnosis.2 3
Resection, with radical lymph node dissection where appropriate, remains the principal potentially curative therapy for all localised UGI cancers. Disease management is almost invariably multimodal and may include chemotherapy and radiotherapy as neoadjuvant, adjuvant or palliative therapy and the provision of optimal supportive care.4–8
The aggressive nature of these cancers and the complexity of treatment often decrease health-related quality of life.9 Advances in surgical techniques and perioperative care have resulted in operative mortality falling to less than 5% in major centres.10 However, surgery remains a morbid procedure with postoperative complications resulting in prolonged hospital admission, adversely impacting on overall quality of life and the ability to undergo any adjuvant therapies.11 In those surviving 1–2 years following curative treatment, health-related quality of life generally recovers to baseline. However, there are still major challenges faced by survivors. For those having palliative or supportive therapy only, quality of life frequently deteriorates throughout the disease trajectory.9
Local or distant cancer recurrence occurs frequently following resection for all UGI cancers. A third of patients diagnosed with stomach12 and half of all patients diagnosed with oesophageal13 cancer develop recurrent disease within 2 years. In pancreatic cancer (PC), where only 10%–15% of tumours are considered resectable, the local recurrence rate ranges from 10% to 40% and distant recurrence is as high as 88%.14
There is evidence that variability exists in the management and outcomes of UGI cancers. For example, not all patients are presented to a multidisciplinary team meeting15; there are disparities in the utilisation of surgical resection and associated disease-specific survival based on where patients live16; there is wide variation in histopathological assessment of margins and the proportion that have clear margins14; the duration of surgery, postoperative complication rates and their management differ between public and private hospitals17 18; administration of adjuvant chemotherapy or radiotherapy is variable, often due to morbidity associated with postoperative complications19; and the 30-day postoperative mortality is lower in hospitals performing more resections each year.20 21 Patients with UGI cancers have significant unmet needs pertaining to quality of life, finance, relationships and family or caregiver distress; these are often exacerbated by a lack of understanding of the health system.22 23 In PC, over 50% of participants (n=136) in an Australian-based study reported moderate to high unmet physical or psychological needs.24
Measuring quality of care with clinical quality registries (CQRs)
To identify, understand and reduce unwarranted clinical variation and ensure that all patients receive optimal care, it is important to collect high-quality disease-specific data. CQRs support continuous improvements in patient outcomes by monitoring quality of care and providing risk-adjusted feedback to the relevant clinical community. These data describe patterns of treatment in order to identify variation and can provide a framework for research.25 Successful implementation of CQRs has been achieved in a range of disciplines include trauma, cardiac, transplant and bariatric surgery,26 joint replacement27 and cancer care (eg, prostate).28
The Australian Commission on Safety and Quality in Health Care (ACSQHC) supports the development of CQRs in Australia through the provision of the national framework for CQRs.29 The framework details the necessary principles, guidelines and standards for best practice design, build, operation and security of CQRs. A recent evaluation of the cost-effectiveness of CQRs determined that when funded sufficiently with robust operating procedures, CQRs provide a substantial return on investment.30 In prioritising the development of CQRs in Australia, the ACSQHC ranked the development of registries for high-burden cancers only behind those monitoring ischaemic heart disease and musculoskeletal disorders.31 PC is ranked fourth as a high-burden cancer in terms of its impact on disability-adjusted life years behind lung, bowel and breast cancer.32 It was predicted to be the third leading cause of cancer deaths in the USA in 2018 and by 2030 is predicted to be the second most common cause of cancer associated mortality.2
Although a number of generic population-based cancer registries exist, there are no CQRs specific to the five aforementioned UGI cancers. Disease-specific registries33 34 and audit databases35 provide much needed evidence about the management of patients with these cancers. However, little prospective data have been published from multi-institution databases and/or registries regarding the quality of UGI cancer care across the disease trajectory.
Rationale for the Upper Gastrointestinal Cancer Registry (UGICR)
Improvements in cancer outcomes for patients with UGI cancer will understandably come through establishment of models of care that are informed by close attention to clinical and patient-reported quality measures and standardisation of treatment that comply with agreed best practice. Given the lack of Australian population-level data regarding patient outcomes from UGI cancers, it was considered that a registry established to monitor treatment and outcomes of patients with cancers arising in the oesophagus, stomach, pancreas, liver and biliary system will improve management of these diseases. Furthermore, while detailed guidelines exist for each of these cancers, gaps remain regarding optimal care and management of these patient groups.4–8 36
The UGICR is a CQR established with the aims to:
Assess patterns of care and identify variations in clinical and patient reported outcomes.
Benchmark performance and provide feedback to service providers using a targeted quality improvement approach to drive improvements in current practice.
Provide confidence to public, clinician and wider stakeholders on the delivery of high-quality service.
Advance knowledge of best treatment protocols by facilitating future clinical, health service, psychosocial and biomedical research.
Cohort description
Overview
The UGICR is a multicentre, population-based, non-interventional prospective cohort study.
It was established in 2015 in Victoria and has since expanded to the state of New South Wales, Australia.
Governance
The UGICR is governed by a Steering Committee and, currently, two clinical working parties with the responsibility of each outlined in figure 1. The Steering Committee performs in accordance with the Australian Framework for CQRs.29
Figure 1.
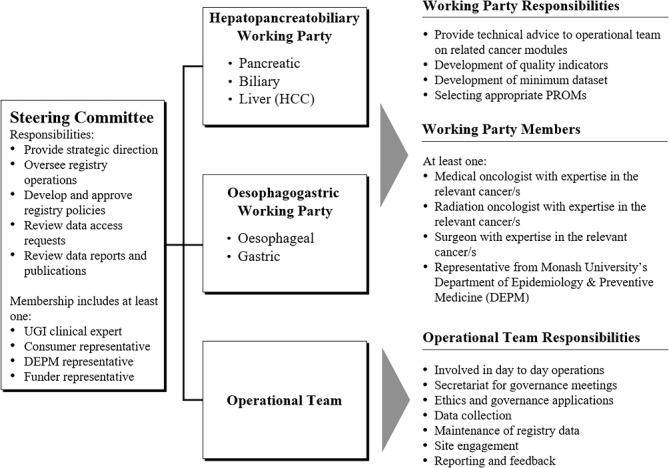
UGICR governance structure. HCC, hepatocellular carcinoma; PROMs, patient reported outcome measures; UGI, upper gastrointestinal.
A central research team provides operational oversights. A principal investigator at each participating hospital is responsible for ensuring that research activities undertaken at their site are conducted in accordance with the human research ethics committee (HREC) approval, the research protocol, site registry agreements and related policy documentation. At each site, patients are identified for recruitment and data collection occurs.
Registry design
The UGICR has a multimodular design with pancreatic, oesophagogastric (OG), liver and biliary cancer modules. Data are entered into Research Electronic Data Capture (REDCap), a secure web-based application, hosted and managed by Helix (Monash University).37 The registry was developed in REDCap, and all data are held securely on a Monash University server that has been accredited under the information security standard ISO27001.38
Participant recruitment and consent
The full recruitment schema is outlined in figure 2. Eligible patients are identified within each jurisdiction through state-based cancer registries or by individual health services. Eligibility criteria are listed in table 1. The UGICR uses an opt-out approach to minimise selection bias.39
Figure 2.
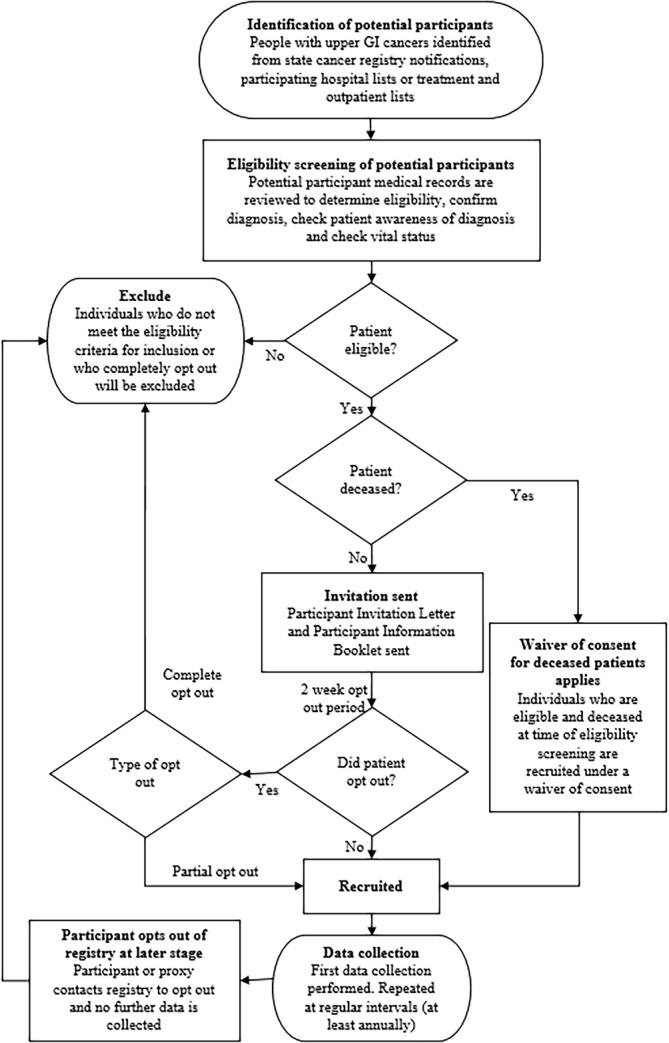
Registry recruitment schema. GI, gastrointestinal.
Table 1.
Eligibility criteria
| All modules | |
| Inclusion |
|
| Module specific | |||
| Modules | Tumour sites | Tumour cell types | |
| Pancreatic | Inclusion | Pancreas. Periampullary region
Distal bile duct. |
Ductal adenocarcinoma. Cholangiocarcinoma. Acinar cell carcinoma. Acinar cell cystadenocarcinoma. IPMN (invasive). Pancreatoblastoma. Serous cystadenocarcinoma. |
| Exclusion | Non-distal bile duct | Neuroendocrine neoplasms. Premalignant lesions. Mesenchymal tumours. Solid pseudopapilliary carcinoma. IPMN (non-invasive). |
|
| Oesophagogastric | Inclusion | Oesophagus (lower two-thirds). Gastro-oesophageal junction. Stomach. |
Carcinoma
|
| Exclusion | Upper third of oesophagus. | Neuroendocrine neoplasms. Lymphomas. Mesenchymal tumours. |
|
| Biliary | Inclusion | Perihilar (hilar) bile duct. Intrahepatic bile duct. Gall bladder. |
Carcinoma. Cholangiocarcinoma. Adenosquamous carcinoma. Squamous cell carcinoma. Cholangiosarcoma. |
| Exclusion | Distal bile duct. | Neuroendocrine neoplasms. Mesenchymal tumours. |
|
| Liver* | Inclusion | Liver. | Hepatocellular carcinoma. |
| Exclusion | Intrahepatic bile duct. | Cholangiocarcinoma. Mesenchymal tumours. Germ cell tumours. Lymphomas. |
|
*Liver module eligibility criteria still to be finalised.
IPMN, intraductal papillary mucinous neoplasm.
Eligible participants are mailed an introductory letter explaining the study and an information booklet outlining details of the registry, its purpose, possible outcomes of the research and the opt-out process. Participants are given 2 weeks to opt out of the registry before their participation is assumed, after which we commence collection of clinical and personal data covering diagnosis to end-of-life care. Patients can withdraw their consent from participation in the registry at any point by telephoning or emailing the UGICR office, as outlined in the participant information booklet. A waiver of consent applies where patients deemed eligible require an interpreter, have significant cognitive impairment or where there is evidence that the patient is deceased.
Findings to date
Data set
The first module developed was the PC module, which began with a pilot phase of approximately 1 year, during which we collected data for a provisional set of quality indicators in three Victorian sites from 2016 to 2017. The second module developed using a similar pilot phase was the OG module. Subsequently, we used a formal modified Delphi consensus process to establish a core set of quality indicators for PC. This process involved 19 PC care experts from three states in Australia. A detailed description of the methods of the modified Delphi process and the selected indicators has been published separately.40 In addition, a review was undertaken of the Australian Optimal Care Pathways (OCP) for PC41 and OGC42 to ensure that indicators are aligned with the seven themes described in the OCP (prevention and early detection; presentation, initial investigations and referral; diagnosis, staging and treatment planning; treatment; care after initial treatment and recovery; managing recurrent, residual or metastatic disease; and end-of-life care). An outline of this process for PC is provided in table 2. There are currently no clinical quality indicators in the UGICR that measure care for the prevention and early detection of PC. However, the UGICR is participating in a collaborative project, Symptom-UGI: Upper Gastrointestinal Cancer Symptom Study, to map the patient pathways from onset of symptoms to cancer diagnosis. Details of this study can be found within the UGICR website (https://ugicr.org.au/associated-studies/).
Table 2.
PC Optimal Care Pathway (OCP) mapped to modified Delphi quality indicators
| PC OCP | OCP elements | Mapped quality indicators from modified Delphi consensus40 |
| Step 1: Prevention and early detection | 1.1 Prevention. 1.2 Risk factors. 1.3 Early detection. |
Nil |
| Step 2: Presentation, initial investigations and referral | 2.1 Signs and symptoms. 2.2 Assessments by general practitioner or medical practitioner. 2.3 Referral. |
|
| 2.4, 3.5, 4.6, 5.4, 6.6 and 7.3 Support and communication |
Nil | |
| Step 3: Diagnosis, assessment and treatment planning | 3.1 Diagnostic workup. 3.2 Staging. 3.3 Treatment planning. |
|
| 3.4, 4.4, 5.3, 6.5 and 7.2 Research and clinical trials |
|
|
| 3.1 and 3.2 Timeframe |
|
|
| Step 4: Treatment | 4.1 Treatment intent | Nil |
| 4.2.1 Surgery (curative) |
|
|
| 4.2.1 Chemotherapy or chemoradiation. |
|
|
| 4.2.2 and 4.3 Treatment of unresectable PC/palliative care. |
|
|
| 4.5 Complementary or alternative therapies. | Nil | |
| Step 5: Care after initial treatment and recovery | 5.1 Survivorship. 5.2 Post-treatment care planning. |
|
| Step 6: Managing recurrent, residual and metastatic disease | 6.1 Signs and symptoms of recurrent, residual or metastatic disease. | |
| Step 7: End-of-life-care | 6.4 Palliative care. 7.1 Multidisciplinary palliative care. |
|
Some elements in each step of the pathway are overlapping. Elements 6.2 and 6.3 readdress steps 3 and 4. Please note: the purpose of this document is to provide a broad overview of the areas within the OCP that the developed PC quality indicators measure. Only the key indicators that map to the elements are listed.
ASA, American Society of Anesthesiologists (performance status); ECOG, Eastern Cooperative Oncology Group (performance status); MDT, Multidisciplinary Team.
The minimum data set was established to enable quality indicators to be calculated. Data items and definitions were aligned with national specifications where appropriate, and a comprehensive data dictionary was developed for each module. The core data items are outlined in table 3.
Table 3.
UGICR minimum dataset*
|
Participant details
Title First name Middle name(s) Surname Recruiting hospital Medical record number Date of birth Sex Medicare number Department of Veteran Affairs number Country of birth Preferred language Interpreter required Indigenous status Contact details
Next of kin and contact details General practitioner details Deceased status Date of death Cause of death |
Diagnosis and staging (prior to antitumour treatment) Diagnosis date Date mass first seen on imaging Diagnostic imaging tests completed† Pathology testing prior to anti-tumour treatment
Primary site of tumour Tumour morphology Clinical disease stage (TNM) Resectability of tumour at diagnosis CA 19–9 measured Discussion at a multidisciplinary team meeting Date earliest multidisciplinary team meeting discussion Diagnosing hospital Surgery Date of operation Type of resection Surgical approach Reason resection surgery abandoned Date of return to theatre Readmitted to hospital within 90 days of surgery (excluding same day chemotherapy) Date of readmission Died in surgical admission Name of consultant surgeon Hospital where surgery was performed Resection pathology
|
Chemotherapy
Treatment intent (Neoadjuvant/adjuvant/curativetive/palliative)‡ Date chemotherapy commenced
Radiotherapy Treatment intent (Neoadjuvant/adjuvant/curativetive/palliative)‡
Restaging after neoadjuvant therapy Date neoadjuvant therapy completed Resectability of tumour Clinical disease (TNM) Other treatment and end-of-life care Referral to or contact with palliative care Date of referral to palliative care ≥2 ED presentations in the last 30 days prior to death ≥14 days in acute hospital during last 30 days of life Died within 30 days of dose of chemotherapy |
*More detailed, module specific data dictionaries have been developed.
†Varies between modules.
‡All related data items collected for first cycle of each type of treatment intent.
ED, Emergency Department; TNM (staging), Tumour, Node, Metastasis; UGICR, Upper Gastrointestinal Cancer Registry.
The OGC module has been developed by the OGC working party following a literature review, and a consensus method was used to agree on the quality indicator set. The registry has future plans to begin the collection of patient-reported outcomes (PROs) and patient-reported experiences (PREs) to provide valuable patient perspectives. As an initial step, a systematic review evaluating patient-reported outcome measures (PROMs) in PC has been undertaken by the UGICR team to define which PROMs are most appropriate for this group of patients.
Data collection
If the participant has not opted out of the registry, data collectors abstract diagnosis, surgical, pathology and treatment data directly from the participant’s electronic and/or hard copy medical records from participating sites or from clinician rooms. Data collection begins close to the time of recruitment with at least annual follow-up until end of life.
Results from the pilot studies from the PC and OGC modules
The results of the pilot phase for both PC and OGC modules are displayed in table 4. Of the 123 participants eligible for the PC module and 189 for the OGC module, 8 (6.5%) and 9 (4.8%) opted out of the registry, respectively. Clinical stage at diagnosis was not well documented in both the PC module (n=80, 70%) and OGC cancer module (n=82, 46%) and is an area for future quality improvement. Around 20% of the pancreatic cohort received surgery as first treatment, which is broadly representative of surgical treatment in patients with PC.43 Furthermore, 73 participants in the PC and 94 participants in the OGC module had documented reasons for no surgery. The pilot results for both modules identified areas for improving data completeness, definitions, items and structure of data collection forms. Following the pilot phase, the registry focused on improving these areas before expanding to other participating hospitals.
Table 4.
PC and OGC module data from pilot data collection
| Variable | PC module | OGC module |
| n (%) | n (%) | |
| Recruited | 115 | 180 |
| Recruited via invitation letter | 88 (76.5) | 120 (66.7) |
| Recruited via waiver of consent (deceased) | 27 (23.5) | 60 (33.3) |
| Sex | ||
| Male | 56 (48.7) | 132 (73.3) |
| Female | 59 (51.3) | 48 (26.7) |
| Age at diagnosis (years) | ||
| <50 | 6 (5.2) | 11 (6.1) |
| 50–59 | 14 (12.2) | 22 (12.2) |
| 60–69 | 30 (26.1) | 54 (30.0) |
| 70–79 | 38 (33.0) | 54 (30.0) |
| ≥80 | 22 (19.1) | 33 (18.3) |
| Missing | 5 (4.3) | 6 (3.3) |
| Resectability at diagnosis | ||
| Resectable | 25 (21.7) | 58 (32.2) |
| Borderline resectable | 3 (2.6) | 11 (6.1) |
| Unresectable | 67 (58.3) | 64 (35.6) |
| Locally advanced (LA) | 24 (20.9) | 6 (3.3) |
| Metastatic (Mets) | 43 (37.4) | 58 (32.2) |
| Not documented | 14 (12.2) | – |
| Unknown | – | 41 (22.8) |
| Missing | 6 (5.2) | 6 (3.3) |
| Clinical stage at diagnosis | ||
| I or II | 5 (4.3) | 33 (18.3) |
| III | – | 7 (3.9) |
| IV | 18 (15.7) | 50 (27.8) |
| Complete TNM* not documented | 80 (69.6) | 82 (45.6) |
| Missing | 12 (10.4) | 8 (4.4) |
| First treatment | ||
| Neoadjuvant therapy | 4 (3.5) | 60 (33.3) |
| Attempted or completed resection surgery | 27 (23.5) | 13 (7.2) |
| Curative intent ChemoTx and/or RT | – | 7 (3.9) |
| Palliative intent ChemoTx and/or RT | 37 (32.2) | 55 (30.6) |
| No treatment | 29 (25.2) | 23 (12.8) |
| Unknown | – | 16 (8.9) |
| Missing | 18 (15.7) | 6 (3.3) |
| Reasons for no surgery† | ||
| LA or Mets | 62 | 60 |
| Advanced age | 1 | 6 |
| Comorbidities | 7 | 9 |
| Patient declined | 1 | 12 |
| Patient died prior to surgery | 0 | 7 |
| Performance status | – | 4 |
| Other reason | 1 | – |
| Reason not documented | 4 | 3 |
| Participant data collection status | ||
| Complete | 51 (44.3) | 107 (59.4) |
| Incomplete | 64 (55.7) | 73 (40.6) |
| Data entry subform completeness | ||
| Demographics | 113 (98.2) | 180 (100.0) |
| Vital status and tumour recurrence | 58 (50.4) | 145 (80.6) |
| Diagnosis details | 97 (84.3) | 165 (91.7) |
| Biliary stents | 94 (81.7) | – |
| Surgery | 102 (88.7) | 168 (93.3) |
| Pathology of resection sample | 102 (88.7) | – |
| Neoadjuvant therapy | 104 (90.4) | – |
| Adjuvant therapy | 98 (85.2) | – |
| Therapy for locally advanced disease | 95 (82.6) | – |
| Therapy for metastatic disease | 77 (67.0) | – |
| Other treatment and trials | 80 (70.0) | – |
| Treatment summary | – | 167 (92.8) |
| Restaging after neoadjuvant therapy | – | 167 (92.8) |
| Chemotherapy details | – | 162 (90.0) |
| Radiotherapy details | – | 163 (90.6) |
| End-of-life details | – | 81 (45.0) |
*TNM system of classification of cancer.
†Reason for no surgery: participants may have more than one reason documented.
ChemoTX, chemotherapy; RT, radiotherapy.
Population coverage
Population coverage in Victoria is based on data from the Victorian Cancer Registry. The population coverage in the pilot phase was 19% for the PC module and 11% for the OGC module. Current coverage is 73% for PC and 55% for the OGC module. In New South Wales, data are currently only being collected on the PC module with an estimated population coverage of 55%.
Reporting
The registry will produce risk-adjusted benchmarked reports that will feed back deidentified data to participating sites on the associated quality indicators. To provide fair and meaningful benchmarked reports, we have undertaken a review of risk models to identify demographic and baseline clinical variables (focusing on those over which clinicians have no control, for example, age, sex and disease stage) that predict patient outcomes for the purposes of risk adjustment. The data from the registry will also permit validation of current predictive risk models and enable further refinement of these tools. Publicly available annual reports that provide an overview of quality of care and the registry’s activities will be published. A UGICR website (https://ugicr.org.au/) has been developed to provide information about the registry to patients, clinicians and other stakeholders. This will be updated to include results as they become available.
Strengths and limitations
The UGICR is Australia’s first UGI cancer CQR. The aims of the registry are to monitor quality of care, benchmark clinical and patient-reported outcomes against best practice and provide high-quality population-based data for clinical research. Registries such as the UGICR provide much needed real-world evidence outside the context of randomised control trials about disease epidemiology, treatment patterns, burden of illness, survival outcomes, clinical variation and treatment safety.44
In recent decades, there has been increasing integration of PROMs into cancer registries to collect outcomes such as overall quality of life, functional and psychosocial well-being, lifestyle behaviours and supportive care needs.45 Clinicians and patients may place different emphasis on symptom impacts and expectations from their treatment.46 The collection of PROMs is an important step in understanding patients’ experience of their symptoms and management and the impact of the disease and its treatment on their quality of life. The UGICR will determine and integrate the most relevant PROMs for each UGI cancer type following thorough examination of the literature.
Through the accumulation of significant and consistent data on UGI cancers, the registry will assess how clinical management compares with best practice and communicate this to clinicians through the PIs or relevant hospital departments. Furthermore, the UGICR provides a platform for longer term clinical follow-up, randomised clinical trials and substudies exploring treatment outcomes and linking outcomes to tumour tissue characteristics.
An important consideration is the maturity of each module before useful quality indicator reports can be provided to participating hospitals, as some UGI cancers have a relatively low incidence in comparison with other cancers.1 The working groups in collaboration with statisticians will determine an analysis plan for each indicator with due consideration to data completeness and risk adjustment methods.
Identified challenges
The UGICR has faced some key challenges affecting its establishment and implementation. The introduction of the National Mutual Acceptance (NMA) scheme has significantly streamlined the ethics process for all public hospitals in Australia, except in the Northern Territory, making the process to gain approval for CQRs more manageable. However, obtaining governance approval at each site continues to be both labour intensive and time consuming.47 48 Furthermore, separate HREC approval is frequently required to access data from private hospitals and clinics.
Funding is another challenge faced by CQRs. As with many healthcare initiatives, the financial burden can be a major impediment.25 Data from CQRs are held in positive regard by clinicians, health managers and government. However, further funding will be required to progress national rollout of the registry.
Other identified barriers include reluctance of some healthcare providers to supply source data, and poor interoperability between clinical information systems leading to duplication of data entry. Where data are of high quality, such as for diagnosis and procedure codes, administrative data is appropriate, but there are limited data for comorbidities and risk factors.49 While automation of data collection from existing data sources would be ideal, this is hampered by inconsistent documentation and a lack of standardisation.50
Collaboration
The UGICR aims to capture whole of population, real-world data that monitors and aspires to improve the quality of care provided to patients with UGI cancers. The registry is currently recruiting hospitals to increase population capture and selecting the most relevant instruments for measuring PROs and PREs for inclusion in each module. The biliary module is entering its pilot phase, and the liver module is to be developed. Monash University is the UGICR’s data custodian and is accountable for the privacy, security and integrity of patient information held within the registry. Participating sites can request a copy of their own patient-level data. Researchers may access registry data following a formal submission to the UGICR data custodian and approval by the UGICR Steering Committee. They are required to complete a request form detailing their research aims and methods, potential impact on healthcare, and provide evidence relevant HREC approval before deidentified data will be released. The registry will harness new opportunities for data linkage with technologies such as the electronic medical records and collaborate with existing data repositories (eg, biomedical) to evolve and fulfil its aim of providing quality evidence.
Supplementary Material
Acknowledgments
The authors first and foremost gratefully acknowledge and thank our participants. We would also like to thank our consumer representatives, Jan Gibson and David Attwood, for their ongoing support and contribution to the registry. The authors would also like to acknowledge the participating hospitals, site investigators and Victorian Cancer Registry for providing ongoing data to the Upper Gastrointestinal Cancer Registry (UGICR).
Footnotes
ADM and JFH contributed equally.
Contributors: ADM and JFH are joint first authors on this manuscript. SME, WAB, DGC, CHCP, JGK, LRL, TL, JJM, MN and JZ are part of the UGICR Steering Committee. SME, LI, WAB, DGC, CHCP, JGK, LRL, TL, MN, AA, PRB, PAC, JC, CD, PE, DG, AH, MWH, BPFK, NM, MM, REN, JP, IWTP, MS, JS, PPT and JZ are part of the working parties. RS and JFH developed the registry protocol in consultation with the UGICR Steering Committee and working parties. All authors reviewed and provided feedback on the drafts of the manuscript and approved the final version.
Funding: The authors gratefully acknowledge the Victorian Government, Pancare Foundation, Specialised Therapeutics Australia, Servier Australia, Eli Lilly Australia, and the Australian National Health and Medical Research Council for the Pancreatic Cancer Registry for Quality Improvement grant (grant number APP1125395). The Victorian Government and Pancare Foundation were involved in the design of the study through steering committee representation.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This project has received human research ethics committee (HREC) approval from the following HRECs: Monash Health (Ref: 15482A) under the National Mutual Acceptance scheme (HREC/15/MonH/134); Cancer Council Victoria (HREC 1611); Epworth HealthCare (EH2017-227), Aboriginal Health & Medical Research Council (1387/18) and is registered with Monash University (CF16/119-2016000051).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Australian Institute of Health and Welfare Cancer in Australia 2019. Canberra: AIHW, 2019. 205p. Cancer series no.119. [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67:7–30. Jan 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3. Rugge M, Fassan M, Graham DY, et al. Epidemiology of Gastric Cancer [internet. Switzerland: Springer, 2015: p23–34. [Google Scholar]
- 4. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5(suppl 5):v56–68. 10.1093/annonc/mdv295 [DOI] [PubMed] [Google Scholar]
- 5. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29(Suppl 4):iv238–55. 10.1093/annonc/mdy308 [DOI] [PubMed] [Google Scholar]
- 6. Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27(suppl 5):v50–7. 10.1093/annonc/mdw329 [DOI] [PubMed] [Google Scholar]
- 7. Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27(suppl 5):v38–49. 10.1093/annonc/mdw350 [DOI] [PubMed] [Google Scholar]
- 8. Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Annal Oncol 2016;27(suppl_5):v28–37. 10.1093/annonc/mdw324 [DOI] [PubMed] [Google Scholar]
- 9. Whistance RN, Blazeby JM. Systematic review: quality of life after treatment for upper gastrointestinal cancer. Curr Opin Support Palliat Care 2011;5:37–46. 10.1097/SPC.0b013e3283436ecb [DOI] [PubMed] [Google Scholar]
- 10. Davis SS, Babidge WJ, Kiermeier A, et al. Perioperative mortality following oesophagectomy and pancreaticoduodenectomy in Australia. World J Surg 2018;42:742–8. 10.1007/s00268-017-4204-3 [DOI] [PubMed] [Google Scholar]
- 11. Zhou J, Hiki N, Mine S, et al. Role of prealbumin as a powerful and simple index for predicting postoperative complications after gastric cancer surgery. Ann Surg Oncol 2017;24:510–7. 10.1245/s10434-016-5548-x [DOI] [PubMed] [Google Scholar]
- 12. Spolverato G, Ejaz A, Kim Y, et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi-institutional analysis. J Am Coll Surg 2014;219:664–75. 10.1016/j.jamcollsurg.2014.03.062 [DOI] [PubMed] [Google Scholar]
- 13. Knight WRC, Zylstra J, Van Hemelrijck M, et al. Patterns of recurrence in oesophageal cancer following oesophagectomy in the era of neoadjuvant chemotherapy. BJS Open 2017;1:182–90. 10.1002/bjs5.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chandrasegaram MD, Goldstein D, Simes J, et al. Meta-Analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg 2015;102:1459–72. 10.1002/bjs.9892 [DOI] [PubMed] [Google Scholar]
- 15. Brauer DG, Strand MS, Sanford DE, et al. Utility of a multidisciplinary tumor board in the management of pancreatic and upper gastrointestinal diseases: an observational study. HPB 2017;19:133–9. 10.1016/j.hpb.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shapiro M, Chen Q, Huang Q, et al. Associations of socioeconomic variables with resection, stage, and survival in patients with early-stage pancreatic cancer. JAMA Surg 2016;151:338–45. 10.1001/jamasurg.2015.4239 [DOI] [PubMed] [Google Scholar]
- 17. Chua TC, Mittal A, Nahm C, et al. Pancreatoduodenectomy in a public versus private teaching hospital is comparable with some minor variations. ANZ J Surg 2018;88:E526–E531. 10.1111/ans.14191 [DOI] [PubMed] [Google Scholar]
- 18. Busweiler LA, Henneman D, Dikken JL, et al. Failure-To-Rescue in patients undergoing surgery for esophageal or gastric cancer. European Journal of Surgical Oncology 2017;43:1962–9. 10.1016/j.ejso.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 19. Schouwenburg MG, Busweiler LAD, Beck N, et al. Hospital variation and the impact of postoperative complications on the use of perioperative chemo(radio)therapy in resectable gastric cancer. Results from the Dutch Upper GI Cancer Audit. Eur J Surg Oncol 2018;44:532–8. 10.1016/j.ejso.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 20. Dikken JL, van Sandick JW, Allum WH, et al. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br J Surg 2013;100:83–94. 10.1002/bjs.8966 [DOI] [PubMed] [Google Scholar]
- 21. Skipworth RJE, Parks RW, Stephens NA, et al. The relationship between hospital volume and post-operative mortality rates for upper gastrointestinal cancer resections: Scotland 1982-2003. Eur J Surg Oncol 2010;36:141–7. 10.1016/j.ejso.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 22. Otutaha B, Srinivasa S, Koea J. Patient information needs in upper gastrointestinal cancer: what patients and their families want to know. ANZ J Surg 2019;89:20–4. 10.1111/ans.14565 [DOI] [PubMed] [Google Scholar]
- 23. Shaw J, Harrison J, Young J, et al. Coping with newly diagnosed upper gastrointestinal cancer: a longitudinal qualitative study of family caregivers' role perception and supportive care needs. Support Care Cancer 2013;21:749–56. 10.1007/s00520-012-1575-8 [DOI] [PubMed] [Google Scholar]
- 24. Beesley VL, Janda M, Goldstein D, et al. A tsunami of unmet needs: pancreatic and ampullary cancer patients' supportive care needs and use of community and allied health services. Psychooncology 2016;25:150–7. 10.1002/pon.3887 [DOI] [PubMed] [Google Scholar]
- 25. Wilcox N, McNeil JJ. Clinical quality registries have the potential to drive improvements in the appropriateness of care. Med J Aust 2016;205:21–6. 10.5694/mja15.00921 [DOI] [PubMed] [Google Scholar]
- 26. Stey AM, Russell MM, Ko CY, et al. Clinical registries and quality measurement in surgery: a systematic review. Surgery 2015;157:381–95. 10.1016/j.surg.2014.08.097 [DOI] [PubMed] [Google Scholar]
- 27. Owen DH, Russell NC, Smith PN, et al. An estimation of the incidence of squeaking and revision surgery for squeaking in ceramic-on-ceramic total hip replacement: a meta-analysis and report from the Australian orthopaedic association national joint registry. Bone Joint J 2014;96-B:181–7. 10.1302/0301-620X.96B2.32784 [DOI] [PubMed] [Google Scholar]
- 28. Evans SM, Millar JL, Wood JM, et al. The prostate cancer registry: monitoring patterns and quality of care for men diagnosed with prostate cancer. BJU Int 2013;111:E158–E166. 10.1111/j.1464-410X.2012.11530.x [DOI] [PubMed] [Google Scholar]
- 29. Australian Commission on safety and quality in healthcare (ACSQHC). framework for Australian clinical quality registries. Australia 2014. [Google Scholar]
- 30. Ahern S, Evans S, Hopper I, et al. Towards a strategy for clinical quality registries in Australia. Aust Heal Rev 2018. [DOI] [PubMed] [Google Scholar]
- 31. Australian Commission on Safety and Quality in Healthcare Prioritised list of clinical domains for clinical quality registry development: final report. Sydney: ACSQHC, 2016. [Google Scholar]
- 32. Australian Institute of Health and Welfare Burden of cancer in Australia: Australian burden of disease study 2011. Canberra: AIHW, 2017: 145p. [Google Scholar]
- 33. Egawa S, Toma H, Ohigashi H, et al. Japan pancreatic cancer registry; 30th year anniversary: Japan pancreas Society. Pancreas 2012;41:985–92. 10.1097/MPA.0b013e318258055c [DOI] [PubMed] [Google Scholar]
- 34. de Steur WO, Henneman D, Allum WH, et al. Common data items in seven European oesophagogastric cancer surgery registries: towards a European upper Gi cancer audit (EURECCA upper Gi). Eur J Surg Oncol 2014;40:325–9. 10.1016/j.ejso.2013.11.021 [DOI] [PubMed] [Google Scholar]
- 35. Busweiler LAD, Jeremiasen M, Wijnhoven BPL, et al. International benchmarking in oesophageal and gastric cancer surgery. BJS Open 2019;3 10.1002/bjs5.50107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Te Riele W, van Tinteren H, van Sandick J. Centralization of upper gastrointestinal cancer care should be dictated by quality of care. Ann Surg Oncol 2018;25(Suppl 3):984–5. 10.1245/s10434-017-6221-8 [DOI] [PubMed] [Google Scholar]
- 37. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. International Standards Organisation ISO/IEC 27001 Information technology - Security techniques - Information security management systems - Requirements. ISO, 2005. [Google Scholar]
- 39. Tu JV, Willison DJ, Silver FL, et al. Impracticability of informed consent in the registry of the Canadian stroke network. N Engl J Med 2004;350:1414–21. 10.1056/NEJMsa031697 [DOI] [PubMed] [Google Scholar]
- 40. Maharaj AD, Ioannou L, Croagh D, et al. Monitoring quality of care for patients with pancreatic cancer: a modified Delphi consensus. HPB (Oxford), 2018. [DOI] [PubMed] [Google Scholar]
- 41. Cancer Council Australia Optimal care pathway for people with pancreatic cancer, 2016. Available: https://www.cancer.org.au/content/ocp/health/optimal-care-pathway-for-people-with-pancreatic-cancer-june-2016.pdf [Accessed cited 2018 Oct 20].
- 42. Cancer Council Australia Optimal care pathway for people with oesophagogastric cancer, 2016. Available: https://www.cancer.org.au/content/ocp/health/optimal-care-pathway-for-people-with-oesophagogastric-cancer-june-2016.pdf [Accessed 2018 Nov 18].
- 43. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 44. Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE special Task force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf 2017;26:1033–9. 10.1002/pds.4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ashley L, Jones H, Thomas J, et al. Integrating patient reported outcomes with clinical cancer registry data: a feasibility study of the electronic patient-reported outcomes from cancer survivors (ePOCS) system. J Med Internet Res 2013;15:e230–e. 10.2196/jmir.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chassany O, Shaheen NJ, Karlsson M, et al. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol 2012;47:1412–21. 10.3109/00365521.2012.712999 [DOI] [PubMed] [Google Scholar]
- 47. Clay-Williams R, Taylor N, Braithwaite J. Potential solutions to improve the governance of multicentre health services research. Med J Aust 2018;208:152–4. 10.5694/mja16.01268 [DOI] [PubMed] [Google Scholar]
- 48. Brown WA, Smith BR, Boglis M, et al. Streamlining ethics review for multisite quality and safety initiatives: national bariatric surgery registry experience. Med J Aust 2016;205:200–1. 10.5694/mja16.00027 [DOI] [PubMed] [Google Scholar]
- 49. Alexander M, Evans SM, Wolfe R, et al. Risks of using medical record and administrative data for prognostic models. Med J Aust 2017;207 10.5694/mja16.00919 [DOI] [PubMed] [Google Scholar]
- 50. Gliklich RE DN, Leavy MB. Registries for Evaluating Patient Outcomes: A User's Guide[internet]. 3rd Ed Rockville (MD): Agency for Healthcare Research and Quality (US), 2014. Apr. Available from https://www.ncbi.nlm.nih.gov/books/NBK208616/ [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


