ABSTRACT
The intestinal microbiota has been demonstrated to influence host metabolism, and has been proposed to affect the development of obesity and type 2 diabetes (T2D), possibly through short-chain fatty acids (SCFAs) produced by fermentation of dietary fiber. There are some indications that SCFAs inhibit glucose-stimulated insulin secretion (GSIS) in rodents, but research on this subject is sparse. However, it has been reported that receptors for SCFAs, free fatty acid receptor 2 (FFAR2) and FFAR3 are expressed not only on gut endocrine cells secreting GLP-1 and PYY, but also on pancreatic islet cells. We hypothesized that SCFAs might influence the endocrine secretion from pancreatic islets similar to their effects on the enteroendocrine cells. We studied this using isolated perfused mouse pancreas which responded adequately to changes in glucose and to infusions of arginine. None of the SCFAs, acetate, propionate and butyrate, influenced glucagon secretion, whereas they had weak inhibitory effects on somatostatin and insulin secretion. Infusions of two specific agonists of FFAR2 and FFAR3, CFMB and Compound 4, respectively, did not influence the pancreatic secretion of insulin and glucagon, whereas both induced strong increases in the secretion of somatostatin. In conclusion, the small effects of acetate, propionate and butyrate we observed here may not be physiologically relevant, but the effects of CFMB and Compound 4 on somatostatin secretion suggest that it may be possible to manipulate pancreatic secretion pharmacologically with agonists of the FFAR2 and 3 receptors, a finding which deserves further investigation.
KEYWORDS: Pancreas perfusions, metabolism, short-chain fatty acid, type 2 diabetes, FFAR2, FFAR3
Introduction
The intestinal microbiota has been shown to influence host metabolism and has been suggested to play a previously underestimated role in metabolic disorders such as obesity and type 2 diabetes (T2D).1 One possible mechanism by which microbes are thought to influence host metabolism involves the production of short-chain fatty acids (SCFAs).2,3 The SCFAs have a chain length of 1–6 carbon atoms and they are produced by the intestinal flora in the distal small intestine and colon by fermentation of dietary fiber and malabsorbed carbohydrates.4 The most abundant fermentation products (≥95%) are acetate (C2), propionate (C3) and butyrate (C4).5 Acetate is primarily used for host synthesis of lipids and cholesterol,6 propionate is mainly absorbed by the liver where it may serve as a substrate in gluconeogenesis,5-7 and butyrate primarily serves as fuel for colonic enterocytes.8 Whereas the total luminal concentration of SCFAs in the mammalian colon is about 100 mM under physiological conditions, their concentration in peripheral blood is as low as 100–150 µM for acetate, 4–5 µM for propionate, and 1–3 µM for butyrate9 due to colonic and hepatic extraction.
In addition to their roles as metabolic substrates, SCFAs may also function as signaling molecules. In 2003, the free fatty acid receptors, FFAR2 (GPR43) and FFAR3 (GPR41) were deorphanized and acetate, propionate, and butyrate were identified as the predominant ligands for these receptors.10-12 FFAR2 signals through coupling to either Gαq/11, which leads to increased intracellular calcium levels via activation of phospholipase C (PLC) β, or Gαi/0, which leads to decreased cAMP production,13 whereas FFAR3 only couples to Gαi/0.14 The expression of FFAR2 and FFAR3 in various cell types in the intestinal tract is well established, and includes expression by the enteroendocrine L-cells.15-17 Several studies have indicated that SCFAs are capable of increasing the secretion of either GLP-1,15,18,19 PYY20,21 or both22,23 from L-cells, and that these effects involve binding to either FFAR2 or both FFAR2 and FFAR3.15,19,22,23 Through the secretion of GLP-1 and PYY, SCFAs have been proposed to indirectly affect host metabolism by increasing satiety and decreasing gastric emptying and gut motility.24 Furthermore, glucose stimulated insulin secretion (GSIS) from pancreatic β-cells may be affected via the stimulated secretion of GLP-1.25
However, recent studies have demonstrated expression of FFAR2 and FFAR3 also on pancreatic islet cells indicating that SCFAs may also directly influence islet function. In particular, the expression of FFAR2 and FFAR3 on β-cells seems well documented13,26-31 and expression on α-cells has also been suggested15 The role of FFAR2 and FFAR3 for pancreatic islet cell secretion remains uncertain as reports are few and results conflicting.
In 1981, it was shown in a perfused rat pancreas preparation that acetate (1 mM) had no effect on β- or α-cell secretion by itself, but that it greatly modified the response of these cells to glucose and arginine. Thus, acetate inhibited GSIS as well as arginine-induced insulin secretion. Concomitantly, glucagon secretion induced by arginine was greatly stimulated by acetate.32 Similar effects on insulin were recently demonstrated in isolated mouse islets, but no effect of acetate on glucagon secretion was detected.29 The finding that acetate inhibits GSIS conflicts with earlier studies that found the opposite effect; namely that acetate should in fact augment GSIS.33,34 Research into the direct effects of the other SCFAs, propionate and butyrate, in the pancreas of non-ruminant animals are sparse. The only two available studies on this have shown opposite effects of propionate and butyrate on insulin secretion. In 1968 it was reported that butyrate strongly enhances insulin secretion at low glucose (2.5 mM) in isolated rat islets,35 whereas a study from 2007 employing the same model found propionate to inhibit insulin secretion at all glucose concentrations between 0 and 16.7 mM.36
Given the increased interest in FFAR2 and FFAR3 as potential drug targets for treatment of metabolic disorders such as obesity and T2D, we investigated the effects of the SCFAs, acetate, propionate and butyrate, using the isolated perfused mouse pancreas model. Experiments were also carried out with the synthetic agonists CFMB, selective for FFAR2,37 and Compound 4, selective for FFAR3.38
Results
Mouse pancreas perfusions with the endogenous FFAR2 and FFAR3 ligands, acetate, propionate and butyrate
The isolated perfused mouse pancreases were stimulated with either acetate (10 mM) (Figure 1(A)) propionate (1 or 10 mM) (Figure 1(B,C)) or butyrate (10 mM) (Figure 1(D)) at both low (3.5 mM) and high (15 mM) glucose levels. The concentrations were chosen based on dose response experiments performed earlier on within the physiological micromolar range (unpublished data) where no response was observed, thus the doses was increased to milimolar range. One minute effluent samples were analyzed for glucagon, insulin, and somatostatin concentrations. In all experiments, the 10 mM L-arginine control elicited a moderate to strong secretory response from all three hormones measured. Furthermore, in all experiments, the change in glucose concentration from 3.5 mM to 15 mM resulted in increases in insulin and somatostatin levels and a concomitant, marked decrease in glucagon levels. Together, this shows that the pancreases were alive and responding adequately to vascular stimuli in all experiments.
Figure 1.
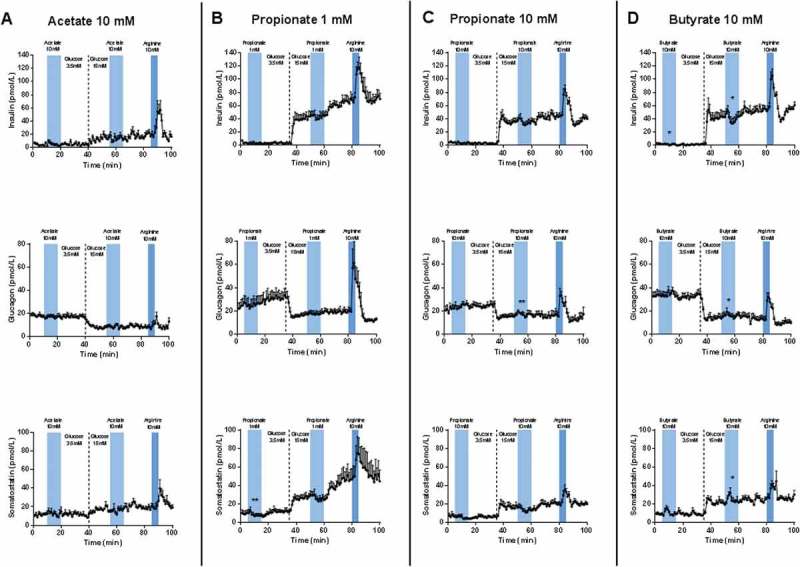
Pancreatic output of insulin, glucagon, and somatostatin in response to 10 mM Acetate, 1 and 10 mM propionate and 10 mM butyrate. Pancreases from 8–10 weeks old female and male C57BL/6 mice were perfused at a basal glucose concentration of 3.5 mM which was increased to 15 mM glucose after 35 min. Stimulations with test substances were carried out once at low and once at high glucose concentration. At the end of each protocol, the pancreases were stimulated with 10 mM L-arginine as a positive control. (A) Stimulation with 10 mM Acetate (n = 5–6). (B) 1 mM propionate (n = 6). (C) Stimulation with 10 mM propionate (n = 6). (D) Stimulation with 10 mM butyrate (n = 5). All results are plotted as mean ± SEM. Statistically significant changes in secretion are indicated by *(p < 0.05).
Insulin
When 10 mM of acetate was infused there was no effect on insulin levels whether at low or high glucose concentrations (Figure 1A top panel). Infusion of 1 mM of propionate did not show any significant response of insulin, neither at low nor high glucose concentrations, although a small numerical decrease was seen at high glucose (Figure 1B top panel). When the propionate concentration was increased to 10 mM, the picture was the same, although the tendency toward a decreased insulin secretion during the propionate infusion at high glucose seemed more pronounced (Figure 1C, top panel). For 10 mM butyrate, there was a significant suppression of insulin secretion at high glucose (p = 0.0299), but nothing at low glucose, which is consistent with the propionate data (Figure 1D, top panel).
Glucagon
Acetate did not affect the glucagon secretion at either low or high glucose concentration (Figure 1A middle panel). Neither 1 nor 10 mM propionate influenced glucagon secretion whether at low or high glucose (Figure 1B,C, middle panel). A similar result was obtained with infusion of 10 mM butyrate: no change in glucagon secretion at neither low nor high glucose (Figure 1D, middle panel).
Somatostatin
Acetate did not affect somatostatin secretion at any glucose concentrations (Figure 1A, bottom panel). Likewise, 1 mM propionate did not significantly affect somatostatin secretion, although a small insignificant decrease was observed at both low and high glucose (Figure 1B, bottom panel). When propionate concentration was increased to 10 mM, the same pattern as for 1 mM was observed, (Figure 1C, bottom panel). When 10 mM of butyrate was infused, a small, but significant increase in somatostatin secretion was observed at low glucose (p = 0.0091), and a similar (but not significant) small peak was observed at high glucose (Figure 1D, bottom panel). These peaks in somatostatin secretion were very short-lasting and had returned to baseline levels several minutes before the butyrate-infusion was terminated.
Mouse pancreas perfusions with the selective FFAR2 and FFAR3 agonists, CFMB and compound 4, respectively
In this series of experiments, the isolated perfused mouse pancreases were first stimulated with either 1 µM specific FFAR2-agonist, CFMB, (Figure 2(A)) or 1 µM specific FFAR3-agonist, Compound 4, (Figure 2(B)) at both low (3.5 mM) and high (15 mM) glucose levels. These concentrations were chosen based on potency studies.39,40 One minute effluent samples were collected from the experiments and analyzed for glucagon, insulin, and somatostatin. All pancreatic responses to the L-arginine control and the increase in glucose concentration followed the expected patterns.
Figure 2.
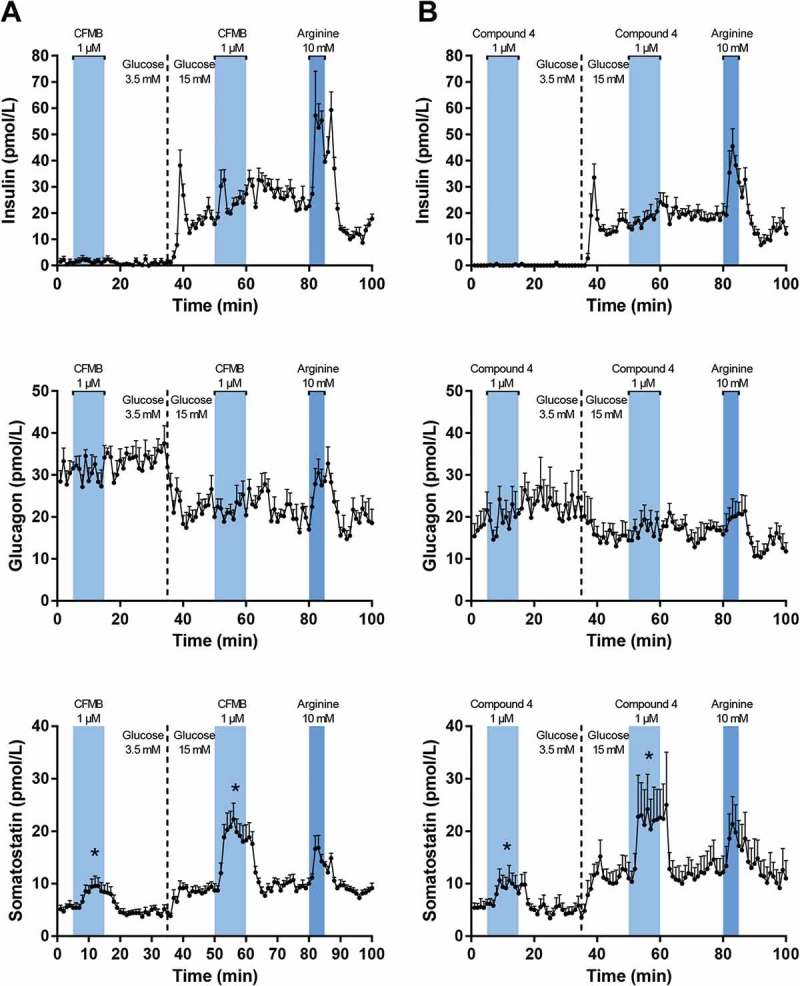
Pancreatic output of insulin, glucagon, and somatostatin in response to 1 µM specific FFAR2-agonist, CFMB, and specific FFAR3-agonist, Compound 4. Pancreases from 8–10 weeks old female C57BL/6 mice were perfused at a basal glucose concentration of 3.5 mM which was increased to 15 mM glucose after 35 min. Stimulations with test substances were carried out once at low and once at high glucose concentration. 80–85 minutes into each protocol, the pancreases were stimulated with 10 mM L-arginine as a positive control. (A) Stimulation with 1 µM CFMB (n = 7). (B) Stimulation with 1 µM Compound 4 (n = 5). All results are plotted as mean + SEM. Statistically significant changes in secretion are indicated by *(p < 0.05).
Insulin and glucagon
Infusion of 1 µM of CFMB or Compound 4 had no effect on the pancreatic secretion of insulin, regardless of glucose level (Figure 2A,B, top panels) and neither compound influenced glucagon secretion regardless of glucose level (Figure 2A,B, middle panels).
Somatostatin
1 µM of CFMB induced significant increases in somatostatin secretion at both low and high glucose levels (p = 0.0027 and p < 0.0001, respectively) (Figure 2A, bottom panel). Likewise, very strong somatostatin responses were also recorded for Compound 4 at both glucose levels (p = 0.0007 and p = 0.0002, respectively) (Figure 2B, bottom panel).
Materials and methods
Test substances
Acetic acid, Propionic acid and butyric acid were purchased from Sigma-Aldrich, Germany (cat# 695092, cat# 81910 and B103500, respectively). They were diluted in water and solutions were adjusted to pH 7.4 with 10 M NaOH. A selective FFAR2 agonist, CFMB ((S)-2-(4-chlorophenyl)-3,3-dimethyl-N-(5-phenylthiazol-2-yl)butamide), was purchased from Calbiochem, Germany (cat# 371725), and a selective FFAR3 agonist, Compound 4 (N-(2,5-dichlorophenyl)-4-(furan-2-yl)-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxamide) was synthesized as described elsewhere.37 CFMB (1 µM) and Compound 4 (1 µM) were both dissolved in dimethyl sulfoxide (DMSO) and diluted in perfusion buffer (see below) to a final concentration of 1% DMSO. The positive control, L-arginine, was purchased from Sigma-Aldrich, Germany (cat# A6969) and dissolved in perfusion buffer immediately prior to infusion.
Animals
Female and male C57BL/6 mice (8–10 weeks, 18–22 g for females and 26–27 g for males) bred in our own animal facility with free access to standard rodent chow and water were used for the experiments. They were housed 2–8 mice per cage under a 12 h light/dark cycle. All animal studies were carried out in accordance with local and international guidelines and with permission from the Danish Veterinary and Food Administration.
The isolated perfused mouse pancreas
Non-fasted mice were anesthetized by i.p. injection with Ketamine/Xylazine (0.1 mL/20 g) Ketamine 90 mg/kg (Ketaminol Vet.; MSD Animal Health, Madison, NJ, USA), Xylazine 10 mg/kg (Rompun Vet.; Bayer Animal Health, Leverkusen, Germany) and the abdominal cavity was exposed. The pancreas was isolated in situ from the circulation by ligating and removing the surrounding organs; first the entire intestine except the proximal fragment of the duodenum which is attached to the pancreas, then the spleen, and the stomach. Left and right renal arteries and veins were ligated. Cannulas were inserted into the abdominal aorta and the portal vein for access to (via the celiac and the superior mesenteric arteries) and drainage from the pancreatic vasculature, respectively, and the pancreas was immediately perfused with pre-heated (39°C) perfusion buffer (Krebs-Ringer bicarbonate buffer containing 0.1% BSA, 5% dextran T-70, 3.5 mM glucose, 5 mM of each pyruvate, fumarate, and glutamate, and 5 mL/L Vamin (14 g N/I mixture of amino acids, Fresenius Kabi AB, Uppsala, Sweden), pH adjusted to 7.4 with 5 M HCl). The perfusion buffer was oxygenated with a 95% O2/5% CO2 mixture. Immediately after cannulation, the mice were killed by perforation of the diaphragm, and a small incision was made in the distal end of the attached duodenum for drainage of exocrine secretions from the pancreas. The perfusate flow rate was kept constant at 1.5 mL/min, and one minute effluent samples were collected from the venous cannula using a fraction collector (Frac-920, GE Healthcare, Denmark) and stored at −20°C until analysis.
Experimental protocol
The surgical procedure was followed by a 30 min equilibration period in which the organ was perfused with the buffer alone in order to stabilize hormone levels. A glucose concentration of 15 mM was achieved by addition of glucose directly into the perfusion buffer, while the test substances and L-arginine control were infused via a sidearm syringe.
Hormone analyses
One minute effluent samples collected from the venous cannula were analyzed for glucagon, insulin, and somatostatin by in-house radioimmunoassays (RIA). Glucagon concentrations were measured by means of the C-terminally directed antiserum 4305, which only detects glucagon of pancreatic origin.41 Somatostatin was measured using a rabbit antiserum raised against synthetic cyclic somatostatin (1758), recognizing both somatostatin-14 and somatostatin-28.42 Insulin concentrations were determined using guinea-pig antiserum raised against porcine insulin (2006–3), which cross-reacts strongly with both human, rat, and mouse insulin.43 For glucagon and somatostatin, sensitivity was below 1 pmol/L, for insulin 3 pmol/L. Data were plotted using GraphPad Prism software, version 6.0 (La Jolla, CA, USA).
Statistical analysis
Distribution was tested using the Kolmogorov-Smirnov normality test. Differences between 5 minutes of mean baseline levels and 5 minutes mean response/peak levels both at low and high glucose were assessed statistically by a paired t-test. Statistical significance was accepted at p < 0.05. Data analysis was carried out using GraphPad Prism software, version 6.0 (La Jolla, CA, USA).
Discussion
SCFAs are produced from indigestible dietary fiber and malabsorbed carbohydrates by fermenting bacteria in the distal ileum and colon. SCFAs serve as fuel for enterocytes in the colon but may also influence host metabolism. We set out to explore the proposed impact of SCFAs on regulation of pancreatic endocrine secretions in mice using the in situ perfused mouse pancreas model. We believe this model has several advantages compared to islet- and cell-based models including e.g. preserved organ architecture with maintained microvasculature and islet organization, ensuring adequate respiration and metabolism for all cells and preserved paracrine relationships. Furthermore, this model offers the exclusive opportunity, compared to in vivo studies, to detect pancreatic hormone output before extraction occurs in the liver and before the secreted products are diluted into the systemic circulation or broken down by enzymatic mechanisms.
In our experiments, acetate did not seem to affect GSIS, whereas propionate seems to dose-dependently, but weakly, decrease GSIS. Although this tendency did not reach statistical significance, it is in line with a previous study that also found propionate (0.5 mM) to inhibit GSIS in perifused rat islets.36 However, that study also found propionate to inhibit insulin secretion at low glucose levels, which we were not able to detect in our setup. In our experiments, butyrate was also able to significantly suppress insulin secretion, but only at high glucose. This, however, contrast to earlier findings that butyrate (5 mM) strongly enhances insulin secretion from rat islets but at low glucose (2.5 mM).35 Should the three SCFAs propionate, butyrate, and acetate be expected to exert similar or differential effects on the pancreas? Not easy to tell, and current research is still much too limited to define a clear pattern. The discrepancies can be seen in regard to acetate where some groups have found acetate to potentiate GSIS,33,34 which has also been noted in mouse islets in a recent study by Priyadarshini et al.,30 whereas another group showed that deletion of FFAR2, the preferred ligand of which is acetate, leads to increased insulin levels and improved glucose tolerance in mice.30
Our experiments clearly demonstrate that neither acetate, propionate nor butyrate significantly impacted glucagon secretion in our experimental model. However, propionate (both 1 and 10 µM) seemed to decrease somatostatin secretion at both low and high glucose levels, although the effect was weak and not statistically significant. Interestingly, butyrate (10 mM) seemed to have the opposite effect, namely by shortly increasing the secretion of somatostatin at both low and high glucose, although again this effect was weak and significant only at low glucose. Lastly, no effect was observed when administrating acetate on somatostatin levels. The results suggest that propionate and butyrate may actually have differential effect on the same cell type. Furthermore, it is possible that FFAR2, which couples to both Gαq/11 and Gαi/0, can switch between these signal transducers under different conditions, a phenomenon that has been described for other types of GPCRs.44 Adding even further to the complexity, the SCFAs have been proposed to initiate signaling cascades that are independent of the FFAR2 and FFAR3 receptors.45
Altogether, the magnitudes of responses to the SCFAs acetate, propionate and butyrate in our perfused mouse pancreas model are quite modest. Over the years, the methods for determining SCFAs in plasma have improved, but it remains possible that the true values could be slightly higher than previously reported. Nevertheless, the concentrations used in our study (1 mM and 10 mM) are undoubtedly supraphysiologic.
The synthetic, specific FFAR2 agonist, CFMB (30 µM), has been shown to acutely elevate intracellular calcium levels in primary L-cells15 pointing toward a stimulatory role of this compound through a Gαq/11-pathway. However, another group found only a small, statistically insignificant increase in GLP-1 secretion from murine colonic crypt cultures in response to 10 or 30 µM CFMB.18 Interestingly, these inconsistencies observed in enteroendocrine cells also apply in pancreatic islet cells. McNelis et al.13 were recently able to demonstrate a stimulatory role of CFMB in islet cells by showing that 1 µM CFMB augments GSIS in islets from both mice and humans as well as in the immortal β-cell line, MIN6. Priyadarshini et al.30 also tested CFMB on isolated mouse islets, but, conversely, they observed a slight, although not significant, inhibition of GSIS. In our perfused mouse pancreas model, 1 µM CFMB did not induce any convincing changes in the secretion of either insulin or glucagon neither at low (3.5 mM) or high (15 mM) glucose levels. However, the infusion of CFMB clearly increased somatostatin secretion at both glucose levels. Likewise, the infusion of 1 µM of the FFAR3 specific agonist, Compound 4, did also not produce convincing changes in insulin or glucagon secretion, but somatostatin secretion was greatly increased by Compound 4 at both low and high glucose levels. The surprisingly strong effect of both CFMB and Compound 4 selectively on somatostatin secretion is puzzling because somatostatin normally suppresses the secretion of insulin and glucagon,46,47 but in the present experiments, no concomitant suppressions of the other pancreatic hormones was demonstrated.
Conclusion
We have shown here that the SCFAs, acetate, propionate, and butyrate, had no physiologically relevant effects in the perfused mouse pancreas. Furthermore, the selective agonists, CFBM and Compound 4 (selective for FFAR2 and FFAR3, respectively), neither influenced the pancreatic secretions of glucagon and insulin, whereas clear peaks in somatostatin secretion were recorded. We believe that the perfused mouse pancreas model with the preserved spatial organization of islet cells and microvasculature is superior to immortalized cell lines and isolated islet cell models. Based on this, we conclude that SCFAs probably exert their positive effects on metabolism and energy homeostasis mainly by acting on other tissues than the endocrine pancreas. Furthermore, the surprising results from the experiments with CFMB and Compound 4 suggest that potential pancreatic actions via stimulated somatostatin secretion must be taken into account when considering these agents for therapeutic purposes.
Funding Statement
The work presented here was partially supported by a grant from the NNF Center for Basic Metabolic Research.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Sonnenburg JL, Backhed F.. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakraborti CK. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol. 2015;6(4):110–119. doi: 10.4291/wjgp.v6.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartstra AV, Bouter KE, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159–165. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62(1):67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 5.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaut M. Gut microbiota and energy balance: role in obesity. Proc Nutr Soc. 2015;74(3):227–234. doi: 10.1017/S0029665114001700. [DOI] [PubMed] [Google Scholar]
- 7.Den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, Müller M, Groen AK, Hooiveld GJ, Bakker BM, et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol. 2013;305(12):G900–G910. doi: 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- 8.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaji I, Karaki S, Kuwahara A. Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release. Digestion. 2014;89(1):31–36. doi: 10.1159/000356211. [DOI] [PubMed] [Google Scholar]
- 10.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 11.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun. 2003;303:1047–1052. [DOI] [PubMed] [Google Scholar]
- 13.McNelis JC, Lee YS, Mayoral R, van der Kant R, Johnson AM, Wollam J, Olefsky JM. GPR43 potentiates β-cell function in obesity. Diabetes. 2015;64(9):3203–3217. doi: 10.2337/db14-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirasawa A, Hara T, Katsuma S, Adachi T, Tsujimoto G. Free fatty acid receptors and drug discovery. Biol Pharm Bull. 2008;31:1847–1851. [DOI] [PubMed] [Google Scholar]
- 15.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59 Suppl 2:251–262. [PubMed] [Google Scholar]
- 17.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324(3):353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 18.Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288(35):25088–25097. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nohr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 20.Cherbut C, Ferrier L, Roze C, Anini Y, Blottiere H, Lecannu G, Galmiche JP. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. 1998;275(6 Pt 1):G1415–G1422. doi: 10.1152/ajpgi.1998.275.6.G1415. [DOI] [PubMed] [Google Scholar]
- 21.Anini Y, Fu-Cheng X, Cuber JC, Kervran A, Chariot J, Roz C. Comparison of the postprandial release of peptide YY and proglucagon-derived peptides in the rat. Pflugers Arch. 1999;438:299–306. [DOI] [PubMed] [Google Scholar]
- 22.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond). 2015;39(3):424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin HV, Frassetto A, Kowalik EJ Jr., Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Holst JJ, Vilsboll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol. 2009;297(1–2):127–136. doi: 10.1016/j.mce.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng YW, Scanlan TS, Hebrok M, Coughlin SR. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest. 2007;117(12):4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kebede MA, Alquier T, Latour MG, Poitout V. Lipid receptors and islet function: therapeutic implications? Diabetes Obes Metab. 2009;11(Suppl 4):10–20. doi: 10.1111/j.1463-1326.2009.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Layden BT, Durai V, Newman MV, Marinelarena AM, Ahn CW, Feng G, Lin S, Zhang X, Kaufman DB, Jafari N, et al. Regulation of pancreatic islet gene expression in mouse islets by pregnancy. J Endocrinol. 2010;207(3):265–279. doi: 10.1677/JOE-10-0298. [DOI] [PubMed] [Google Scholar]
- 29.Tang C, Ahmed K, Gille A, Lu S, Grone HJ, Tunaru S, Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med. 2015;21(2):173–177. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- 30.Priyadarshini M, Villa SR, Fuller M, Wicksteed B, Mackay CR, Alquier T, Poitout V, Mancebo H, Mirmira RG, Gilchrist A, et al. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Mol Endocrinol. 2015;29(7):1055–1066. doi: 10.1210/me.2015-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priyadarshini M, Layden BT. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets. 2015;7(2):e1045182. doi: 10.1080/19382014.2015.1045182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiengo A, Valerio A, Molinari M, Meneghel A, Lapolla A. Effect of ethanol, acetaldehyde, and acetate on insulin and glucagon secretion in the perfused rat pancreas. Diabetes. 1981;30:705–709. [DOI] [PubMed] [Google Scholar]
- 33.Shah JH, Wongsurawat N, Aran PP. Effect of ethanol on stimulus-induced insulin secretion and glucose tolerance. A study of mechanisms. Diabetes. 1977;26:271–277. [DOI] [PubMed] [Google Scholar]
- 34.Patel DG, Singh SP. Effect of ethanol and its metabolites on glucose mediated insulin release from isolated islets of rats. Metabolism. 1979;28:85–89. [DOI] [PubMed] [Google Scholar]
- 35.Montague W, Taylor KW. Regulation of insulin secretion by short chain fatty acids. Nature. 1968;217(5131):853. doi: 10.1038/217853a0. [DOI] [PubMed] [Google Scholar]
- 36.Ximenes HM, Hirata AE, Rocha MS, Curi R, Carpinelli AR. Propionate inhibits glucose-induced insulin secretion in isolated rat pancreatic islets. Cell Biochem Funct. 2007;25(2):173–178. doi: 10.1002/cbf.1297. [DOI] [PubMed] [Google Scholar]
- 37.Lee T, Schwandner R, Swaminath G, Weiszmann J, Cardozo M, Greenberg J, Jaeckel P, Ge H, Wang Y, Jiao X, et al. Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol Pharmacol. 2008;74(6):1599–1609. doi: 10.1124/mol.108.049536. [DOI] [PubMed] [Google Scholar]
- 38.Leonard JN, Chu ZL, Bruce MA, Boatman DP. Gpr41 and modulators thereof for the treatment of insulin-related disorders. 2006. http://www.google.com/patents/WO2006052566A2.
- 39.Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nøhr MK, et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mole Metab. 2013;2(4):376–392. doi: 10.1016/j.molmet.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–G65. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- 41.Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87(2):415–423. doi: 10.1172/JCI115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baldissera FG, Nielsen OV, Holst JJ. The intestinal mucosa preferentially releases somatostatin-28 in pigs. Regul Pept. 1985;11:251–262. [DOI] [PubMed] [Google Scholar]
- 43.Brand CL, Jorgensen PN, Knigge U, Warberg J, Svendsen I, Kristensen JS, Holst JJ. Role of glucagon in maintenance of euglycemia in fed and fasted rats. Am J Physiol. 1995;269(3 Pt 1):E469–E477. doi: 10.1152/ajpendo.1995.269.3.E469. [DOI] [PubMed] [Google Scholar]
- 44.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390(6655):88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 45.Den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud D-J, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 46.Johnson DG, Ensinck JW, Koerker D, Palmer J, Goodner CJ. Inhibition of glucagon and insulin secretion by somatostatin in the rat pancreas perfused in situ. Endocrinology. 1975;96(2):370–374. doi: 10.1210/endo-96-2-370. [DOI] [PubMed] [Google Scholar]
- 47.Gerich JE, Lovinger R, Grodsky GM. Inhibition by somatostatin of glucagon and insulin release from the perfused rat pancreas in response to arginine, isoproterenol and theophylline: evidence for a preferential effect on glucagon secretion. Endocrinology. 1975;96(3):749–754. doi: 10.1210/endo-96-3-749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Leonard JN, Chu ZL, Bruce MA, Boatman DP. Gpr41 and modulators thereof for the treatment of insulin-related disorders. 2006. http://www.google.com/patents/WO2006052566A2.


