ABSTRACT
Infection with the opportunistic fungal pathogen, Pneumocystis jirovecii causes life-threatening pneumonia in immunocompromised individuals. In addition to HIV-1 infected patients, individuals at risk of Pneumocystis infection include those receiving immunosuppressive therapies due to transplantation, cancer or autoimmune disease. Antibiotic treatment is not always successful, and it does not prevent obstructive lung disease after clearance of the pathogen. Therefore, it is essential to develop therapeutic alternatives that are more effective against PCP. We reported that Pneumocystis recombinant protein KEX1 induces protective immunity against the development of PCP in a non-human primate model of HIV-induced immunosuppression. In this study, we tested the immunogenicity KEX1 immunization of healthy rhesus macaques and the durability of these responses during drug-induced immunosuppression using tacrolimus (FK506) and methylprednisolone. We observed that vaccination with KEX1 prior to the start of the immunosuppressive regimen generated a robust and long-lasting antibody response that was maintained throughout the immunosuppressive treatment. Furthermore, boosting with KEX1 during immunosuppression induced recall of memory responses against recombinant KEX1. The durability of the anti-KEX1 response and the ability to induce a recall response during immunosuppressive therapy provide a proof-of-concept data supporting further investigation of the KEX1 as a prophylactic vaccine to prevent PCP in drug-induced immunosuppression. This approach provides fundamental knowledge for the elaboration of therapeutic and prophylactic alternatives for PCP in patients undergoing severe immunosuppressive therapy.
KEYWORDS: Pneumocystis, immunosuppression, tacrolimus, NHP, Kexin, vaccine
Introduction
Pneumocystis jirovecii (formerly Pneumocystis carinii) is a fungal opportunistic pathogen that can cause lethal pneumonia in immunocompromised patients. Pneumocystis Pneumonia (PCP) is the most prevalent opportunistic infection in individuals infected with HIV, with a mortality rate in patients with AIDS of 10–30%. Symptoms of PCP include progressive dyspnea, nonproductive cough and low-grade fever, which can progress to the development of a pneumothorax, and eventually permanent pulmonary obstruction and respiratory failure.1 In addition, there is an increased incidence of PCP in non-HIV infected individuals with impaired immunity, including patients undergoing transplantation, individuals treated for inflammatory disease (Crohn’s, rheumatoid arthritis, autoimmune and collagen vascular diseases), those undergoing cancer chemotherapy, and in individuals with congenital immune defects (e.g., severe-combined immunodeficiency, hyper-IgM syndrome).2,3 In these individuals, PCP has a higher mortality rate of 30–60%; it presents with acute fulminant pneumonia that rapidly progresses to acute respiratory failure, due to a larger inflammatory response and lower oxygenation in the lungs, compared to PCP in patients with AIDS.4,5 The number of individuals at risk of PCP continues to increase due to improved survival of these patient populations and widespread use of immunosuppressive therapeutics. Despite the fact that fungal diseases are an increasing clinical burden, there are no anti-fungal vaccines approved for clinical use. Therefore, there is an urgent need to find therapeutic and prophylactic alternatives that are safer and more effective for patients on immunosuppressive therapies.
Colonization by Pneumocystis is defined as the detection of Pneumocystis DNA in samples of Broncho Alveolar Lavage (BAL) fluid from asymptomatic individuals. Persistent colonization by Pneumocystis is a cofactor in the development of Chronic Obstructive Pulmonary Disease (COPD).6,7 Furthermore, colonization by Pneumocystis has been associated with an increased incidence of COPD among smokers, faster disease progression, and more severe disease. Although treatment with trimethoprim-sulfamethoxazole (TMP-SMX) or second line antibiotic treatments have been effective in reducing morbidity and mortality in some patient populations, there continues to be treatment-limiting adverse reactions and increased concerns of emerging drug resistance.8 Even though antibiotic treatment can be successful in clearing the infection from the lungs, colonization by Pneumocystis results in permanent obstructive changes in the tissue.9 Persistent colonization can also increase the risk of progression to PCP, it could play a role in transmission to susceptible individuals, and latent infection may cause inflammation that can be damaging to the lung.7
Previous studies have shown that natural exposure to Pneumocystis generates antibody responses to recombinant Kexin 1 (KEX1) in healthy adults and monkeys.10 Pneumocystis KEX1 is a protease that belongs to the family of fungal Kexin proteins and shares several characteristics with Kexin proteins form other fungal pathogens. Monoclonal antibodies against KEX1 can confer protection against PCP in susceptible mice and can recognize antigens from Pneumocystis species from different hosts, including ferrets, humans and rhesus macaques.11,12 We have also observed that low antibody titers to recombinant KEX1 are predictive of the development of PCP in HIV-1 infected individuals.13 We have shown that vaccination with Pneumocystis recombinant KEX1 elicits antibody responses that protect rhesus macaques from developing PCP during chronic SIV-induced immunosuppression.14 In this study, we have evaluated the immunogenicity of KEX1 in a novel Non-Human Primate (NHP) model of drug-induced immunosuppression.
Materials and methods
Study design
The study population comprised 14 adult female rhesus macaques (Macaca mulatta) of Chinese origin, which were purchased from vendors approved by the University of Georgia and housed in accordance with the Guide for the Care and Use of Laboratory Animals.15 Studies were approved by the Institutional Animal Care and Use Committee of the University of Georgia. Macaques were randomly assigned into one of the two groups for immunization with the 11 kDa KEX1 recombinant protein subunit vaccine. Blood and bronchoalveolar lavage (BAL) fluid samples were collected at baseline and every 2 and 4 weeks following immunization, respectively. Plasma samples and BAL supernatants were collected and stored at −80°C.
Immunization and immunosuppression
KEX1 expression was induced in Escherichia coli BL21 (DE3) containing the pET28b(+)-KEX1 plasmid; recombinant protein was purified by affinity chromatography.14 Eight animals were intramuscularly immunized with 100μg of recombinant KEX1 and aluminum hydroxide (Imject Alum, Thermo Scientific) mixed in a 1:1 ratio. Six animals were sham vaccinated with PBS. Animals were boosted with 50ug of KEX1 and Aluminum Hydroxide or PBS at 8 weeks after the first vaccination, and again at 10 weeks after the second vaccination. Five weeks after the third vaccination, all animals began the immunosuppressive regimen, consisting of 2mg/kg/day of tacrolimus (FK506) and methylprednisolone starting at 40mg/day, tapered over a period of two weeks, to a maintenance dose of 4mg/day. From weeks 18 to 22 after the start of the regimen, tacrolimus was eliminated from the regimen, and methylprednisolone was increased to 10mg/day. Levels of tacrolimus in the blood were monitored every week for the first month, and every two weeks thereafter for the duration of the study.
ELISA and ELISpot assays
Microtiter plates (Immunolon 4HBX; Thermo Fisher Scientific) were coated with purified KEX1 at 5 µg/ml in PBS. Heat-inactivated plasma samples were diluted 1:100 in blocking buffer (PBS with 5% nonfat milk) and 1:2 serial dilutions were made to determine endpoint titers. Goat anti-monkey immunoglobulin conjugated horseradish peroxidase (Nordic Immunology) was used for detection, and plates were developed with TMB. Normal (uninfected, Pc-negative determined by antibody titer) macaque plasma was used as a negative control, and sample from a monkey with PCP was used for positive control.10
Multiscreen HTS IP-filter plates with PVDF membrane plates (Millipore) were activated with ethanol following manufacturer’s instructions. Plates were then coated with either purified recombinant Kexin protein (KEX1; 5µg/ml), keyhole limpet hemocyanin (Pierce Imject mcKLH, 2.5 µg/ml; Thermo-Scientific), or affinity-purified anti-monkey IgG (5 µg/ml; Rockland) at 4°C overnight. For quantification of total IgG and KEX1-antibody secreting cells (ASCs), PBLs were isolated with red blood cell lysis buffer and 2 × 106 cells were stimulated with R848 at 500ng/ml, and IL-2 at 5ng/ml (Invivogen) for 5 days in complete culture media (RPMI containing 10% FBS, penicillin and streptomycin, non-essential amino acids, HEPES, Glutamax and B-mercaptoethanol).16 After stimulation, cells were harvested, washed and counted prior to plating. After blocking the plates with culture media, cells were seeded and incubated for 16 to 24 h at 37°C and 5% CO2. To detect KEX1-specific ASCs, 2 × 105 cells were seeded per well. For quantification of total IgG-secreting cells, serial dilutions starting at 5000 cells per well, were seeded. For detection, biotin-conjugated secondary antibody (Rockland) and streptavidin-horseradish peroxidase (BD Biosciences) were used. The plates were developed with AEC substrate (BD Biosciences). Plate images were acquired with an Immunospot CTL plate reader (CTL Technologies).
Pneumocystis challenge and diagnosis
Pneumocystis challenge of KEX1- and sham-immunized rhesus macaques was performed via natural airborne transmission by cohousing these animals with animals infected with Pneumocystis.10,14 During the administration of the immunosuppressive therapy, Pneumocystis infection status was evaluated at monthly intervals in BAL fluid samples. BAL fluid was examined by modified Giemsa stain and microscopic examination for the presence of Pneumocystis cystic and trophic forms. Pneumocystis infection was defined as detection of the Pneumocystis mitochondrial large subunit rRNA gene by PCR in BAL cell lysate.17,18 Colonization by Pneumocystis was defined as detection of Pneumocystis DNA by nested PCR on the first PCR product only. PCR for β-globin was performed as a control.10 The remaining fluid was filtered through a 40 µm pore-size cell strainer, 105 cells were removed and stained with modified Giemsa stain, and differential counts were performed manually.19 The remaining cells were stained and analyzed by flow cytometry and the supernatant fluid was collected and stored at −80°C.
Flow cytometry
Immunologic parameters were monitored following vaccination and in monthly intervals after the start of immunosuppression in blood and BAL. Blood samples were collected from all animals and leukocytes were isolated with red blood cell lysis buffer. Cells were stained in FACS buffer containing 20% FBS, 2% Human Serum, 2% Goat serum, 5mM EDTA, and 0.05% Azide. Cells were stained with CD3, clone SK1, Biolegend; CD4, clone L200, BD; CD8, clone SP34, Biolegend; CD20, clone 2H7, Biolegend. After staining, cells were fixed with paraformaldehyde and analyzed in the LSRII Flow cytometer (BD Bioscience). Data analysis was performed in FLowJo v10.
Results
We examined the antigenicity of the KEX1 vaccine candidate in rhesus macaques. One group of eight animals was vaccinated with recombinant KEX1 and Alum, and a second control group of six animals was sham vaccinated with PBS, according to the schedule shown in Figure 1(a). Anti-KEX1 antibody titers increased after each vaccination dose, with levels above 106 reciprocal endpoint titer (RET). Antibody levels were above 105 RET at the start of the immunosuppressive regimen, five weeks after the last KEX1-Alum boost (Figure 1(b)). In the sham-vaccinated group, antibody levels were undetectable or detected at very low levels, likely the result of natural exposure to Pneumocystis. These results corroborate our previous findings where we evaluated our KEX1 vaccine candidate in an SIV-induced immunosuppression model.14 We also observed that KEX1 memory B cell responses in the circulation can be detected up until 8 weeks after the third vaccination (Figure 1(c)). Therefore, our vaccination strategy with KEX1 was able to generate a robust antibody response and B cell memory, prior to the start of the immunosuppressive therapy.
Figure 1.
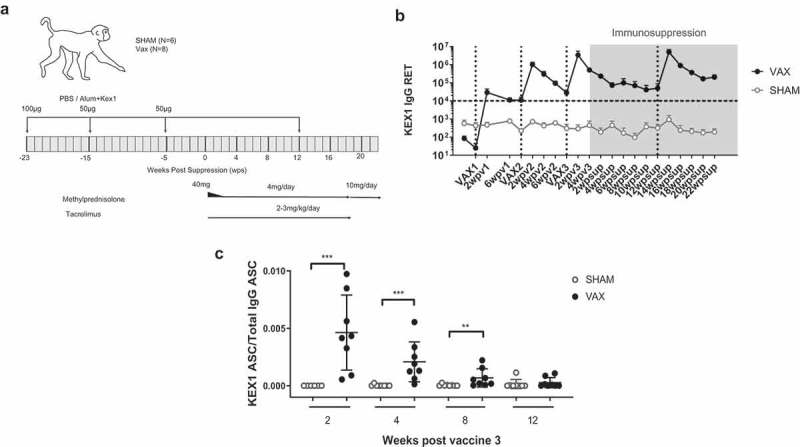
Vaccination with KEX1 induces a robust immune response. (a) Schematic representation of the vaccination strategy and treatment administration. (b) Mean reciprocal endpoint titer of anti-KEX1 IgG antibodies as determined by ELISA. Dotted lines on the X-axes indicate the times of vaccination. The period for administration of the immunosuppressive regimen is highlighted in grey. Dashed line indicates correlate of protection of 104 RET. (c) Ratio of KEX1 Antibody-secreting cells from total IgG-secreting cells, as determined by ELISpot. Mann–Whitney test was performed to evaluate the differences between the sham and vaccinated groups. *p < .05, **p < .01, ***p < .001.
In order to establish a drug-induced immunosuppression model in NHPs, we administered tacrolimus and methylprednisolone, two compounds commonly used to achieve immunosuppression in transplant patients. Tacrolimus is a calcineurin inhibitor, which has been shown to be successful in preventing experimental transplant rejection in cynomolgus macaques at a dose of 2 mg/kg/day.20 We administered tacrolimus at 2 mg/kg/day, combined with methylprednisolone, starting at 40 mg/day and tapered over to 4 mg/day maintenance dose. Upon the start of the immunosuppressive therapy, levels of tacrolimus in the blood were monitored every 2 weeks and were maintained above 5 ng/ml (Figure 2(a)). In cases where levels were below 5 ng/ml for 4 consecutive weeks, the dose was increased to 3mg/kg/day. Anti-KEX1 antibody titers were maintained during immunosuppression and did not drop below the previously established correlate of protection of 104 RET (Figure 1(b)).14 We also monitored the number of CD3+ lymphocytes, CD4+ and CD8+ T cells and B cells in the circulation. We observed that immunosuppressive therapy with tacrolimus and methylprednisolone did not affect the number of T and B cells in the circulation (Figure 2(b-i)).
Figure 2.
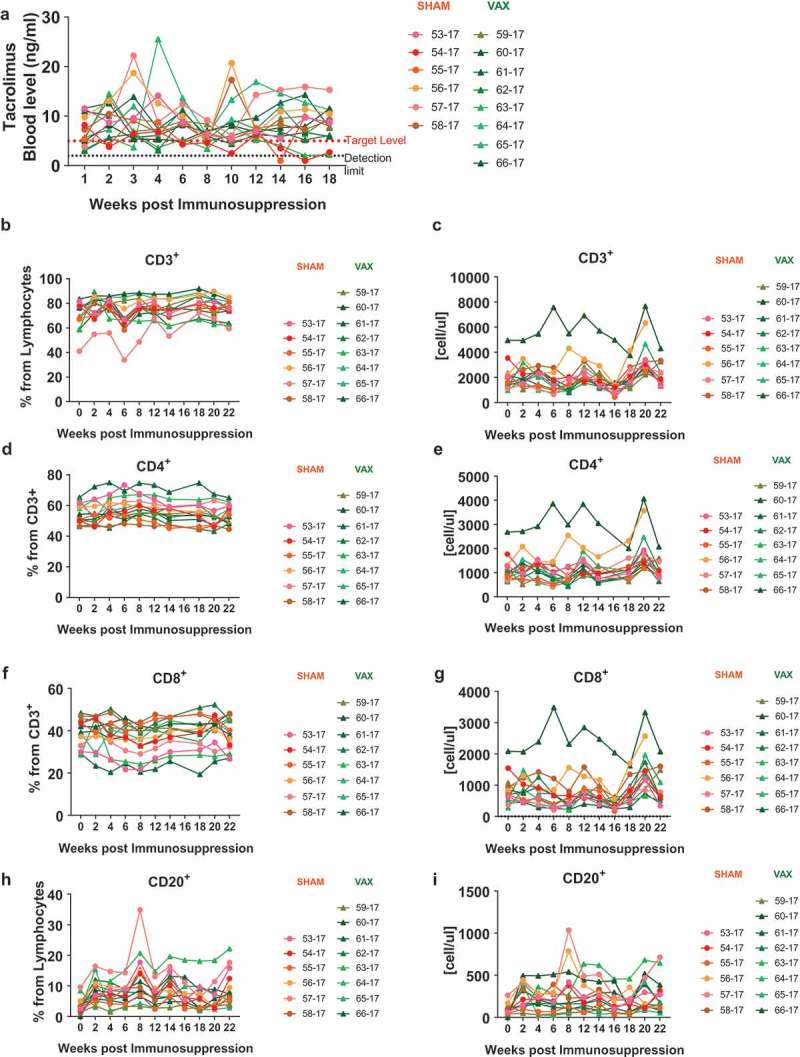
Monitoring during immunosuppression. (a) Tacrolimus levels were monitored every two weeks during treatment. Dotted line indicates the level of detection of 2 ng/ml, the dashed line indicates the target level of 5 ng/ml. (b-i) Changes in immune cell populations in the blood were monitored by flow cytometry. Frequency and absolute number of (b-c) CD3+ Lymphocytes, (d-e) CD4+ T cells, (f-g) CD8+ T cells and (h-i) B cells.
During the course of the immunosuppressive therapy, the Pneumocystis infection status was monitored by PCR in BAL fluid. Although none of the animals developed PCP, we detected colonization by Pneumocystis throughout the duration of the drug regimen. Detection of Pneumocystis DNA in the BAL fluid of healthy individuals and animals is observed, however, it is transient and asymptomatic.21 We have previously observed that Pneumocystis is detected in the BAL fluid only in a few cases during early stages of SIV infection in rhesus macaques, and colonization only becomes persistent during chronic viral-induced immunosuppression, prior to the development of PCP.10 Therefore, it is important to establish if animals became persistently colonized by Pneumocystis during the immunosuppressive regimen, since colonization plays an important role in the development of permanent obstructive changes in the lungs and it likely precedes the development of PCP.7,21 In this study, we observed that the incidence of Pneumocystis colonization expressed as the percentage of animals that became colonized at each time point, increased during the course of the drug regimen (Figure 3), indicating that the long-term immunosuppressive treatment resulted in persistent colonization by Pneumocystis in the lungs. In addition, fewer animals in the vaccinated group became colonized by Pneumocystis, although this is not significantly different from the sham group. This could indicate that antibodies against KEX1 in the mucosal tissue could play a role in interfering with Pneumocystis colonization of lung of animals at risk, in addition to preventing the development of PCP. However, these results do not constitute definite evidence of the effect of vaccination on preventing colonization by Pneumocystis.
Figure 3.
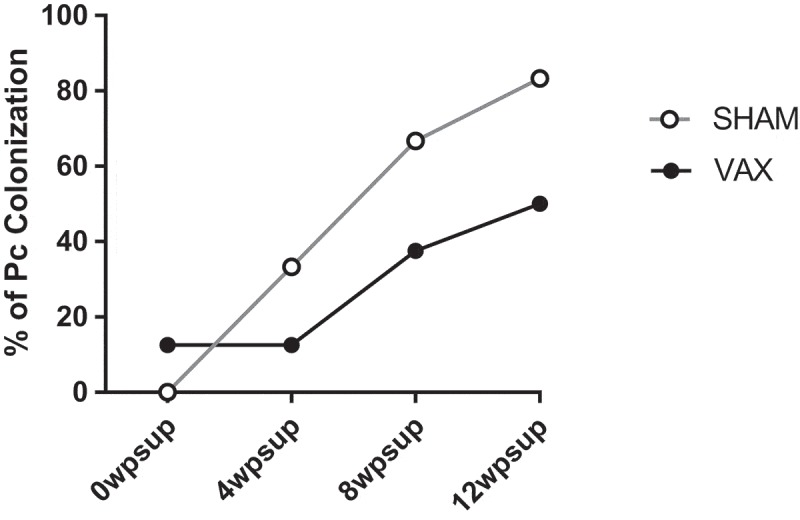
Colonization by Pneumocystis. Percentage of animals colonized with Pneumocystis was monitored by nested PCR in BAL samples, at monthly intervals. The difference between the sham and vaccinated groups was evaluated by repeated measures ANOVA and was found not to be significantly different.
We further examined whether the anti-KEX1 response generated by vaccination prior to the start of therapy could be recalled during the immunosuppressive state. We boosted the KEX1 vaccinated animals at 12 weeks after the start of the immunosuppressive regimen (Figure 1(a)). In Figure 1(b), we have highlighted in gray, the period of time when the immunosuppressive regimen was administered, and at 12 weeks post-suppression, noted by the dotted vertical line, we have indicated the time point at which the animals were boosted. We observed that the antibody titers increased in response to vaccination, achieving similar levels to those obtained after the second boost, prior to the start of immunosuppression (Figure 1(b)). This indicates that the memory response created by vaccination against KEX1 is robust, long-lasting and it can be recalled even when the immune system has been impaired by the use of immunosuppressive drugs.
Discussion
In this study, we have observed that vaccination with recombinant KEX1 is able to induce a robust and long-lasting antibody response that can be maintained during drug-induced immunosuppression. In addition, the memory responses generated by vaccination can also be recalled during the administration of the immunosuppressive regimen. We have established a model for drug-induced immunosuppression in NHPs, by administering a combined regimen of tacrolimus and methylprednisolone. We have shown that tacrolimus levels in the blood are comparable to those reported in other studies to be adequate to prevent transplant rejection.20 Furthermore, we have described that treatment with tacrolimus and methylprednisolone does not affect the number of circulating T and B cells. This is a highly relevant model for evaluating therapeutic or prophylactic treatments in patients with an impaired immune system due to the administration of immunosuppressive drugs, which are at risk of acquiring Pneumocystis infection and developing PCP. Furthermore, this model allows for the evaluation of changes in populations of immune cells, since it closely resembles the human immune system.
Our results indicate that the immunosuppressive regimen resulted in the impairment of the immune system, which led to persistent colonization by Pneumocystis in all animals. This indicates that this model of drug-induced immunosuppression may resemble early stages of the development of PCP in immunocompromised patients, where persistent colonization by Pneumocystis may occur prior to the development of PCP. The immunosuppressive regimen we administered in this study is not as aggressive as other treatments used in other animal models of transplantation, or heavy immunosuppressive regimens given to patients undergoing transplantation or cancer therapies,22–25 which may explain why the animals did not develop PCP. The knowledge we have gained from this study will allow us to evaluate other treatment regimens using this NHP model, which may cause severe immunosuppression, and where the animals will be at a higher risk of developing PCP.
In previous studies, we have shown that our vaccine candidate KEX1 is able to prevent the development of PCP during chronic SIV-induced immunosuppression.14 In this study, we observe that vaccination with KEX1 slightly reduced the number of animals that became persistently colonized by Pneumocystis in the context of drug-induced immunosuppression. This could be attributed to the role of anti-KEX1 antibodies in the mucosa of the lung. We have previously shown that vaccination with KEX1 induces antibody responses in the BAL fluid, which could play an important role in impairing the ability of infectious trophic forms of Pneumocystis to adhere to the epithelial cells and colonize the lung. However, the difference in the number of colonized animals between the vaccinated and sham groups is not significantly different, and thus we are unable to establish whether KEX1 vaccination can affect colonization by Pneumocystis.
There are limitations to this study. There is currently a wide variety of immunosuppressive drugs that are used in numerous combination therapies. The small size of our study allowed us to evaluate only one immunosuppressive regimen. Additionally, the length of the study did not allow for full evaluation of susceptibility of the treated animals to Pneumocystis infection.
Furthermore, we have shown that the memory response elicited by vaccination with KEX1 can be recalled during chronic immunosuppression with tacrolimus and methylprednisolone. This is an important finding, since it demonstrates that vaccination prior to the start of an immunosuppressive regimen can elicit protective antibody responses that can be recalled during treatment, and may be able to protect patients when they are most susceptible to developing PCP. Future efficacy studies will determine if vaccination can be protective in the context of a drug-induced immunosuppressive state and if this response can still be recalled when more severe regimens are used. Vaccination with KEX1 could be an important alternative for patients with an impaired immune system that not only confers protection but could also circumvent the side effects of life-long antibiotic treatments, which are not completely effective and do not prevent reinfection and pulmonary obstruction.
Funding Statement
This work was supported by the Georgia Research Alliance and the University of Georgia Research Foundation
Acknowledgments
We thank Dr. Steve Harvey, Michael Bennett Johnston, Tamara Boyles and Ellen Griggs for veterinary support. We also thank Brenda Noble and Lauren Lacefield for laboratory technical support.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Thomas CF Jr., Limper AH.. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–98. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 2.Fillatre P, Decaux O, Jouneau S, Revest M, Gacouin A, Robert-Gangneux F, Fresnel A, Guiguen C, Le Tulzo Y, Jego P, et al. Incidence of Pneumocystis jiroveci pneumonia among groups at risk in HIV-negative patients. Am J Med. 2014;127:1242e11–7. doi: 10.1016/j.amjmed.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Roblot F, Godet C, Le Moal G, Garo B, Faouzi Souala M, Dary M, De Gentile L, Gandji JA, Guimard Y, Lacroix C, et al. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis. 2002;21:523–31. doi: 10.1007/s10096-002-0758-5. [DOI] [PubMed] [Google Scholar]
- 4.Festic E, Gajic O, Limper AH, Aksamit TR. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest. 2005;128:573–79. doi: 10.1378/chest.128.2.573. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA. 2009;301:2578–85. doi: 10.1001/jama.2009.880. [DOI] [PubMed] [Google Scholar]
- 6.Morris A, Netravali M, Kling HM, Shipley T, Ross T, Sciurba FC, Norris KA. Relationship of pneumocystis antibody response to severity of chronic obstructive pulmonary disease. Clin Infect Dis. 2008;47:e64–8. doi: 10.1086/591701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris A, Sciurba FC, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, Huang L, Norris KA. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med. 2004;170:408–13. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 8.Khodavaisy S, Mortaz E, Mohammadi F, Aliyali M, Fakhim H, Badali H. Pneumocystis jirovecii colonization in Chronic Obstructive Pulmonary Disease (COPD). Curr Med Mycol. 2015;1:42–48. doi: 10.18869/acadpub.cmm.1.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kling HM, Shipley TW, Guyach S, Tarantelli R, Morris A, Norris KA. Trimethoprim-sulfamethoxazole treatment does not reverse obstructive pulmonary changes in pneumocystis-colonized nonhuman primates with SHIV infection. J Acquir Immune Defic Syndr. 2014;65:381–89. doi: 10.1097/QAI.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kling HM, Shipley TW, Patil S, Morris A, Norris KA. Pneumocystis colonization in immunocompetent and simian immunodeficiency virus-infected cynomolgus macaques. J Infect Dis. 2009;199:89–96. doi: 10.1086/595297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gigliotti F, Haidaris CG, Wright TW, Harmsen AG. Passive intranasal monoclonal antibody prophylaxis against murine Pneumocystis carinii pneumonia. Infect Immun. 2002;70:1069–74. doi: 10.1128/iai.70.3.1069-1074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee LH, Gigliotti F, Wright TW, Simpson-Haidaris PJ, Weinberg GA, Haidaris CG. Molecular characterization of KEX1, a kexin-like protease in mouse Pneumocystis carinii. Gene. 2000;242:141–50. [DOI] [PubMed] [Google Scholar]
- 13.Gingo MR, Lucht L, Daly KR, Djawe K, Palella FJ, Abraham AG, Bream JH, Witt MD, Kingsley LA, Norris KA, et al. Serologic responses to pneumocystis proteins in HIV patients with and without Pneumocystis jirovecii pneumonia. J Acquir Immune Defic Syndr. 2011;57:190–96. doi: 10.1097/QAI.0b013e3182167516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kling HM, Norris KA. Vaccine-induced immunogenicity and protection against Pneumocystis pneumonia in a nonhuman primate model of HIV and Pneumocystis coinfection. J Infect Dis. 2016;213:1586–95. doi: 10.1093/infdis/jiw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): The National Academies Press; 2011. [Google Scholar]
- 16.Walsh PN, Friedrich DP, Williams JA, Smith RJ, Stewart TL, Carter DK, Liao HX, McElrath MJ, Frahm N, Network NHVT . Optimization and qualification of a memory B-cell ELISpot for the detection of vaccine-induced memory responses in HIV vaccine trials. J Immunol Methods. 2013;394:84–93. doi: 10.1016/j.jim.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Board KF, Patil S, Lebedeva I, Capuano S 3rd, Trichel AM, Murphey-Corb M, Rajakumar PA, Flynn JL, Haidaris CG, Norris KA. Experimental Pneumocystis carinii pneumonia in simian immunodeficiency virus-infected rhesus macaques. J Infect Dis. 2003;187:576–88. doi: 10.1086/373997. [DOI] [PubMed] [Google Scholar]
- 18.Patil SP, Board KF, Lebedeva IP, Norris KA. Immune responses to Pneumocystis colonization and infection in a simian model of AIDS. J Eukaryot Microbiol. 2003;50(661–2). doi: 10.1111/j.1550-7408.2003.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 19.Croix DA, Board K, Capuano S 3rd, Murphey-Corb M, Haidaris CG, Flynn JL, Reinhart T, Norris KA. Alterations in T lymphocyte profiles of bronchoalveolar lavage fluid from SIV- and Pneumocystis carinii-coinfected rhesus macaques. AIDS Res Hum Retroviruses. 2002;18:391–401. doi: 10.1089/088922202753519179. [DOI] [PubMed] [Google Scholar]
- 20.Kinugasa F, Nagatomi I, Ishikawa H, Nakanishi T, Maeda M, Hirose J, Fukahori H, Ooshima S, Noto T, Higashi Y, et al. Efficacy of oral treatment with tacrolimus in the renal transplant model in cynomolgus monkeys. J Pharmacol Sci. 2008;108:529–34. [DOI] [PubMed] [Google Scholar]
- 21.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012;25:297–317. doi: 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes Transplant Work G KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(3):S1–155. [DOI] [PubMed] [Google Scholar]
- 23.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, Anderson D, Cowan S, Price K, Naemura J, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–53. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 24.Meyer C, Walker J, Dewane J, Engelmann F, Laub W, Pillai S, Thomas CR Jr., Messaoudi I. Impact of irradiation and immunosuppressive agents on immune system homeostasis in rhesus macaques. Clin Exp Immunol. 2015;181:491–510. doi: 10.1111/cei.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page EK, Page AJ, Kwun J, Gibby AC, Leopardi F, Jenkins JB, Strobert EA, Song M, Hennigar RA, Iwakoshi N, et al. Enhanced de novo alloantibody and antibody-mediated injury in rhesus macaques. Am J Transplant. 2012;12:2395–405. doi: 10.1111/j.1600-6143.2012.04074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


