ABSTRACT
Islet transplantation is efficacious to prevent severe hypoglycemia and glycemic liability of selected patients of type 1 diabetes. However, since calcineurin inhibitor (CNI) causes β-cell and nephrotoxicity, alternative drug(s) with similar potency and safety profile to CNI will be highly desirable. Here we tested whether JAK3 inhibitor, tofacitinib could be used instead of tacrolimus in CIT07 immunosuppression regimen in cynomolgus nonhuman primate (NHP) model. Five independent streptozotocin (STZ)-induced diabetic monkeys were transplanted with MHC-mismatched allogeneic islets and three animals were further re-transplanted upon insufficient glycemic control or early islet graft rejection. After islet transplantation, blood glucose levels were quickly stabilized and maximal islet graft survival as measured by serum C-peptide concentration was >330, 98, >134, 31, or 22 days, respectively, after transplantation (median survival day; 98 days). Cellular and humoral immune responses were efficiently suppressed by JAK3 inhibitor-based immunosuppression during the follow-up periods. Although intermittent increases of the genome copy number of cynomolgus cytomegalovirus (CMV) were detected by quantitative real-time PCR analyses, serious infections or posttransplant lymphoproliferative disease (PTLD) was not found in all animals. Taken together, we have shown that JAK3 inhibitor could be used in replacement of tacrolimus in a highly translatable NHP islet transplantation model and these results suggest that JAK3 inhibitor will be potentially incorporated in human allogeneic islet transplantation.
KEYWORDS: Islet transplantation, JAK3 inhibitor, Calcineurin inhibitor, NHP
Introduction
Pancreatic islet transplantation has been started as treatment option for type 1 diabetes in late 1970s from the experimental stage,1 and it is now well established in the clinics via a breakthrough result from the Edmonton group in 2000.2 Importantly, recent phase 3 clinical trial demonstrated the efficacy and safety of the standardized human pancreatic islet product in the patients with impaired awareness of hypoglycemia (IAH) and severe hypoglycemic events (SHEs) using Clinical Islet Transplantation (CIT07) immunosuppression regimen.3 In this regimen, the patients received induction and maintenance immunosuppression in an open-label fashion consisting of rabbit anti-thymocyte globulin (ATG; basiliximab instead of ATG for the second and third transplants, if applicable), etanercept, sirolimus, and low-dose tacrolimus.4 Calcineurin inhibitors such as tacrolimus is an essential component in the immunosuppression regimen. It can dephosphorylate nuclear factor of activated T cell, cytoplasmic (NFATc), a transcription factor, which is essential for interleukin-2 (IL-2) production and the subsequent T cell activation and differentiation.5 However, calcineurin inhibitor has been known to be very toxic to the pancreatic β-cells. Indeed, it has been demonstrated to cause β-cell death,6 inhibit β-cell proliferation and differentiation and impair insulin biosynthesis and secretion.7 Moreover, it is also nephrotoxic such that its dose has to be reduced or completely withdrawn in islet recipients.8 Therefore, it is important to develop an alternative drug, which is equally potent for preventing T cell activation and differentiation, and at the same timeless or non-toxic to β-cells or kidney epithelial cells.
Recently, significant attention has been paid to develop Janus kinase 3 (JAK3) inhibitor as a novel immunosuppressive drug in organ transplantation, because this class of inhibitor can be theoretically appealing for several reasons.9 First, the mutation in the gene encoding JAK3, a cytoplasmic tyrosine kinase resulted in a phenotype similar to X-linked severe-combined immunodeficiency disease (X-SCID), which is characterized by immune deficiency such as T−B+NK− cell phenotype. Second, JAK3 has a more restricted expression pattern than other JAKs such that it is expressed at high levels in immune cells. Third, JAK3 is tightly associated with the cytokine receptor subunit, common γ chain, which is important for signal transduction of many cytokines such as IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. Thus, JAK3 inhibitor would inhibit the function of T cells, B cells, NK cells, memory T cells through IL-2, IL-4, IL7, IL-15 signaling pathway, respectively. Consistent with this notion, Busque et al. recently reported that long-term tofacitinib was effective in preventing renal allograft acute rejection and preserving renal function in open-label, long-term extension (LTE) study.10 All these features led us to hypothesize that JAK3 inhibitor may replace tacrolimus in immunosuppressive regimen in allogeneic islet transplantation setting. To test this hypothesis, clinically available JAK3 inhibitor, tofacitinib, was used in replacement with tacrolimus in CIT07 immunosuppressive regimen in cynomolgus monkey allogeneic islet transplantation model.
Results
Overview
Our primary goal in this study was to test whether clinically approved JAK3 inhibitor tofacitinib could be used in replacement of tacrolimus in allogeneic islet transplantation, given that all the other drugs were kept similar in human islet transplantation CIT07 immunosuppression regimen. For this end, five independent diabetic cynomolgus monkeys were used as islet recipients and three of them were re-transplanted with another batches of the islets after the first islet graft had been rejected or when the function of transplanted islet was insufficient to maintain normoglycemia. Islets were harvested from either survival partial pancreatectomy or whole pancreatectomy following necropsy and then infused into a portal vein in the diabetic recipients (Table 1). The transplanted islet mass ranged from 3,900 to 12,500 islet equivalent (IEQ)/kg (median 8,950 IEQ/transplant) and subsequent follow-up period after islet transplantation was at least 3 weeks (median 89.5 days) (Table 2). The long term follow-up data were limited to only three animals except the fasting blood glucose level, because two animals (C242 and C244) accidently died due to iatrogenic reasons; C242 died from peritonitis by iatrogenic laceration of gastrostomy site and C244 died from brain infarct due to arterial catheterization. Both reasons of death were not directly related to islet transplantation and subsequent immunosuppression medication. The endpoint of follow-up from three long-term survivors was set to at least 6 months after islet transplantation, because this 6 month period was considered enough for evaluating islet graft function.11
Table 1.
Donor and recipient information and stimulation index of anti-donor mixed lymphocyte culture.
| Recipient |
Donor |
|||||||
|---|---|---|---|---|---|---|---|---|
| ID | Body Weight (kg) | Age (months) | Sex | ID (donor pancreas type*) |
Body Weight (kg) | Age (months) | Sex | Anti-donor MLC, Stimulation index (-fold increase) |
| C241 | 4.0 | 54 | M | C250 (P) | 4.2 | 62 | F | 14.5 |
| C241 (D90 Re-Tx) | 11Cm07 (W) | 7.2 | 97 | M | 5.3 | |||
| C242 | 4.0 | 48 | M | C17 (W) | 6.5 | 84 | M | 12.3 |
| C243 | 4.8 | 58 | M | C244 (P) | 4.2 | 61 | M | 12.2 |
| C243 (D70 Re-Tx) | C17 (W) | 6.5 | 84 | M | 21.6 | |||
| C244 | 4.2 | 61 | M | C242 (P) | 4.0 | 48 | M | 43.0 |
| C245 | 5.2 | 84 | M | C249 (W) | 3.9 | 64 | F | 12.0 |
| C245 (D83 Re-Tx) | 11Cm07 (W) | 7.2 | 97 | M | 66.0 | |||
*P and W indicate partial pancreatectomy and whole pancreatectomy, respectively.
Table 2.
Transplantation outcome of intrahepatic allogeneic islet transplantation in cynomolgus monkeys.
| Recipient | Infused allogenic islet mass (IEQ/kg) | Insulin independence day | Monkey C-peptide positive (>0.1 ng/ml) |
|---|---|---|---|
| C241 | 10,000 | 3 | 90 |
| C241 (D90 Re-Tx) | 7,900 | 6 | 98 |
| C242 | 12,500 | 22 | 22 |
| C243 | 11,000 | 3 | 41 |
| C243 (D70 Re-Tx) | 10,000 | 3 | >330 |
| C244 | 6,200 | 31 | 31 |
| C245 | 3,900 | 4 | 83 |
| C245 (D83 Re-Tx) | 7,400 | >134 | >134 |
Immunosuppression regimen was ATG, Humira, Sirolimus, Tofacitinib, and Anakinra except C243 (without Anakinra) in the first islet transplantation and Basiliximab, Humira, Sirolimus, Tofacitinib, and Anakinra in the second islet transplantation (Re-Tx;retransplantation), respectively.
Tofacitinib was effective in prolongation of islet graft survival
Diabetic status of all five islet recipients was confirmed by persistent hyperglycemia over 250 mg/dl and the need for exogenous insulin injection to control glycemia at least 2 weeks prior to islet transplantation. Upon islet transplantation, blood glucose levels were quickly stabilized (100 ~ 200 mg/dl) in all five animals with exogenous insulin being significantly reduced (10 ~ 20% of pre-transplant level) and one animal became insulin-free (C242) (Figure 1). Three animals (C241, C243, and C245) received the second islet infusion after the first islet had been rejected (C243) or when glycemic control was insufficient (C241 and C245). These animals maintained near-normal blood glucose levels with significantly reduced exogenous insulin (C241 and C243) or without insulin (C245) for >330, 98, and >134 days, respectively, after islet transplantation (Figure 1 and Table 2). In three animals where follow-up period was long enough to test the function of the transplanted islet (C243, C241, and C245), serial IVGTT was performed. As shown in Figure 2, glucose disposal capacity after a bolus of glucose load was significantly improved than in pre-transplant period. However, this capacity gradually deteriorated over time. Importantly, monkey C-peptide in serum was reproducibly detected during IVGTT, albeit the levels were clearly lower than that of non-diabetic condition.
Figure 1.
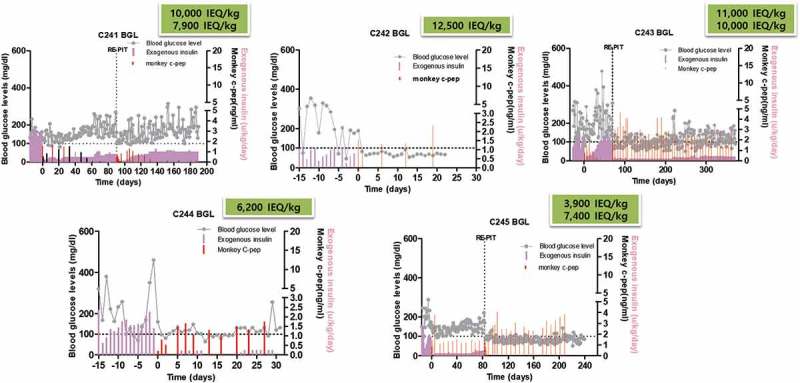
Blood glucose control by the transplanted islets. Fasting blood glucose levels and monkey C-peptide were measured in five monkeys that were intraportally transplanted with allogeneic islets. Maximal duration of islet graft survival as measured by C-peptide in serum was 98, 22, >330, 31, and >134 days after islet transplantation (median survival day; 98 days) in C241, C242, C243, C244, and C245, respectively. Absence of monkey C-peptide measured at pre-transplant period indicates that blood glucose control was entirely dependent on C-peptide from the transplanted islets. gray line: fasting blood glucose, red bar: monkey C-peptide, pink bar: exogenous insulin.
Figure 2.
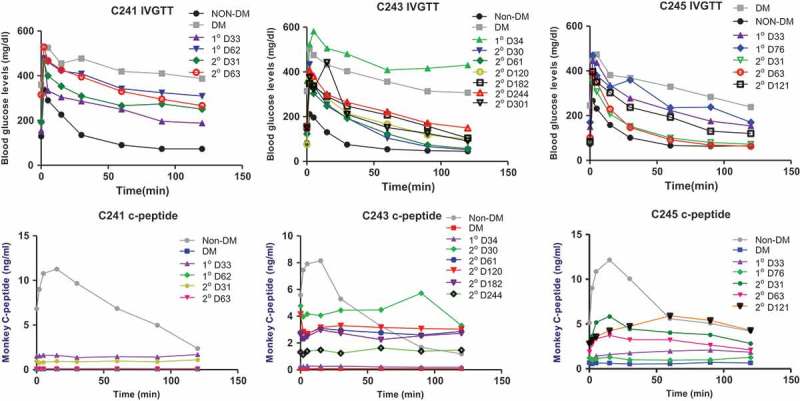
Intravenous glucose tolerance test and C-peptide response. Three animals were transplanted with allogeneic islets twice [first (1°) and second transplantation (2°)] and an IVGTT was performed at the indicated time-points. Glucose disposal capacity was significantly improved by islet transplantation, but this capacity slowly deteriorated over time. C-peptide was negative before transplantation (DM), but after transplantation C-peptide secretion in response to glucose load was observed in all monkeys.
Control of humoral and cellular immune responses
In addition to monitoring of blood glucose levels and glucose disposal capacity, humoral and cellular responses to allogeneic antigens were examined by flow cytometry and ELISPOT, respectively. Levels of plasma DSA remained constant and did not differ from pre-transplantation levels (Figure 3A) in three animals. Also, the number of IFN γ-secreting cells among PBMCs against donor antigen did not significantly increase in two animals but marginally increased in C241 (Figure 3B). These results suggested that tofacitinib-based immunosuppression was effective for controlling humoral and cellular immune responses to donor antigens. Because JAK3 signaling is important for hematopoiesis and NK cell development, the numbers of peripheral T, B, and NK cells were measured. As shown in Figure 4, these cells decreased shortly after ATG induction, and then repopulated to pre-transplantation levels around 30 days after transplantation. Interestingly, NK cells were present at relatively lower numbers than other cells.
Figure 3.
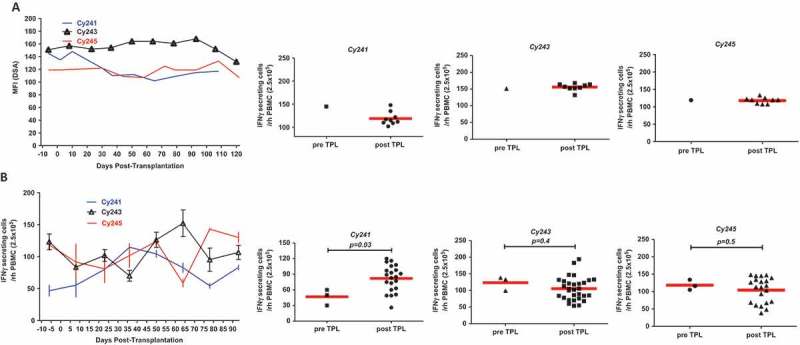
Donor-specific antibody (DSA) and cellular reactivity to donor antigen. (a) DSA was measured by mean fluorescence intensity (MFI), using donor-specific antigen (PBMC) and plasma samples taken at indicated time points. (b) Cellular reactivity toward donor antigen was measured by interferon-γ secreting PBMCs taken at indicated time points by ELISPOT. DSA and interferon-γ secreting cells were well controlled by immunosuppression compared to pre-transplant levels in most cases.
Figure 4.
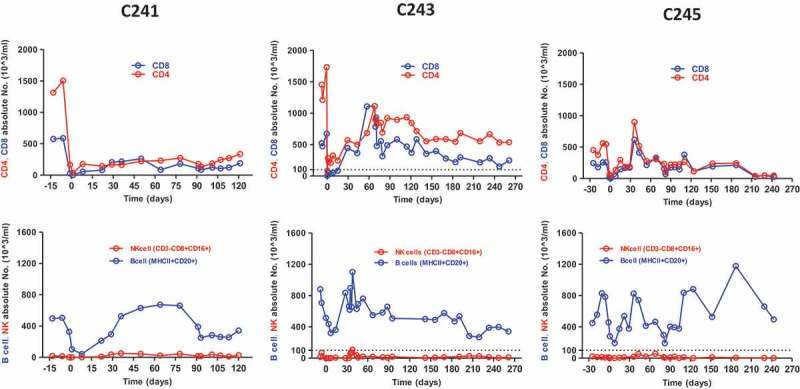
Changes of CD4+, CD8+ T cells, B cells, and NK cells. Absolute numbers of each cell type were regularly monitored in the peripheral blood. T and B cells were significantly depleted by ATG induction, repopulated and then restored around 30 days. Very small number of NK cells was detected throughout the follow-up periods.
General condition, adverse effects, and graft monitoring
All animals remained healthy without any noticeable serious infections or palpable lymph nodes. Most animals kept their body weight within ±10% of pre-transplantation levels and some recipients (C243 and C245) even gained the body weight (Figure 5A). When the copy number of cynomolgus cytomegalovirus was measured by real-time RT-PCR in serum during the follow-up periods, intermittent increases of the copy number were observed, but serious symptom was not detected (Figure 5B). Finally, to examine islet graft in the liver, liver biopsy or liver harvested upon necropsy was investigated by immunohistochemistry. As shown in Figure 6, the islet was observed either infiltrated mostly with CD3 T cells or relatively intact depending on liver retrieval time at which blood glucose levels rose to hyperglycemia or remained normal, respectively. Importantly, the possibility of endogenous β-cell regeneration after STZ-induced diabetes in NHPs could be excluded out from a recent paper.12 Taken together, these results demonstrated that JAK3 inhibitor-based immunosuppression is capable of prolonging allogeneic islet graft without serious infections or PTLD in the same context of human islet clinical transplantation except the replacement of tacrolimus with tofacitinib.
Figure 5.
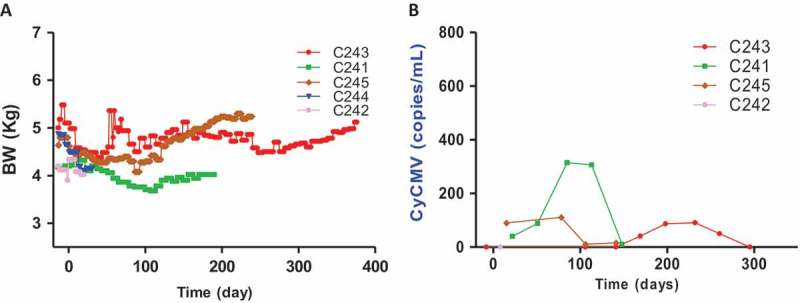
Body weight (BW) changes and monitoring of cynomolgus CMV in peripheral blood of monkeys after islet transplantation. (A) All monkeys lost ~20% of BW after STZ injection, but they maintained (C241 and C243) or gained (C245) BW after islet transplantation. (B) The copy numbers of cynomolgus CMV (CyCMV) DNA in plasma were determined by quantitative real-time PCR. Intermittent CyCMV viremia was observed in three monkeys, but illness and clinical signs associated with CyCMV reactivation were never observed.
Figure 6.
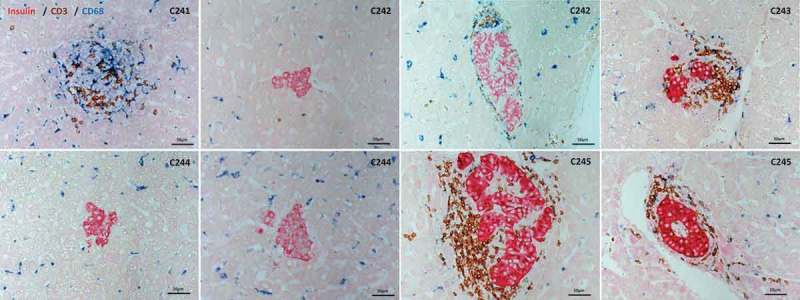
Immunohistochemical staining of liver samples. Liver was biopsied from C241, C243, and C245 at day post-transplantation (DPT) 70, 147, and 63, respectively. Liver samples were obtained at necropsy of C242 and C244 at DPT 22 and 31, respectively. Liver samples were analyzed by immunohistochemical staining using anti-insulin, CD3, CD68 antibodies with appropriate secondary antibodies to reveal β-cells, T cells, and macrophages, respectively. Islets from normoglycemic C242 and C244 were devoid of immune cell infiltration, whereas islets were infiltrated with T cells in varying degrees in the other three animals.
Discussion
In this study, we demonstrated that JAK3 inhibitor tofacitinib could be used in replacement of tacrolimus while all the other drugs were kept the same in human clinical islet transplantation protocol called CIT07, in a highly translatable NHP islet allogeneic transplantation model. The five diabetic islet recipients restored normoglycemia either without insulin or with significantly reduced amount of insulin after intraportal islet transplantation. Serial IVGTTs and immunohistochemical analyses on the liver samples showed sustained function of transplanted islets for up to >330 days in one animal (C243). Importantly, immune monitoring data showed that JAK3 inhibitor-based immunosuppression was able to control DSA and cellular reactivity toward donor antigens without any serious infections and PTLD.
Although islet transplantation has been clinically practiced for the last 3 decades, it is rather surprising to note the paucity of the study wherein the efficacy of clinically practiced immunosuppression regimen consisting of ATG, tacrolimus, and sirolimus was tested using NHP models. Only two studies could be retrieved from the literature; Hirshberg et al. reported that islet grafts were rejected at 0, 11, 40, and 41 days in four animals and insulin independence were achieved for 5, 30, 90, 210 days in other four animals with median survival being 40 days.13 Berman et al. showed that only three out of 20 animals were insulin-independent for 11, 20, and 46 days and other 17 animals were C-peptide-positive for variable periods from 11 to 515 days (median survival; 101.5 days).14 Although direct comparison of our results with those data was not entirely rational due to the differences in transplanted islet mass and NHP strain, it is notable that median survival of the islet grafts in our small set of experiment reached to 86.5 days based on JAK3 inhibitor-based immunosuppression. In addition to the efficacy of islet graft prolongation, the safety profile was also very excellent. Since JAK3 signaling pathway through the common γ chain is important for IL-7 and IL-15-mediated NK cell development and survival, potential viral infection or reactivation of latent virus would be problematic when JAK3 inhibitor was used. However, while the genome copy number of cytomegalovirus was intermittently increased in quantitative real-time PCR analyses, noticeable symptoms were never found. Mild inhibition of hematopoiesis was detected as evidenced by decreased hemoglobin levels, but this problem was easily overcome by dose adjustment of tofacitinib.
Limitation of our study was the small number of animals (n = 5) and the lack of control group whereby parallel comparison between tacrolimus- and tofacitinib-based immunosuppression regimen in islet transplantation would have been outstanding. Therefore, an additional study involving more animals including the control group is needed. Taken together, we have shown that JAK3 inhibitor could be used in replacement of tacrolimus in the setting of allogeneic islet transplantation without any serious infections and PTLD in highly translatable NHP model. These results suggest that incorporation of JAK3 inhibitor instead of tacrolimus deserves to be tested in human islet transplantation.
Materials and methods
Animals
A total of nine cynomolgus monkeys (Macaca fascicularis), 4–9 years of age, were used in this study. Five animals (C241~ C245) were islet recipients and other four independent animals (C17, C249, C250, and 11Cm07) were used as islet donors. C242 and C244 among the islet recipients were also used as islet donor after partial pancreatectomy (Table 1). After being imported from Vietnam, the quarantine process of 1 month concluded that all subjects were in good general condition. Then, the monkeys were acclimated to our animal facility at least for 6 months. The monkeys were maintained in single-housed cages and had daily access to food (2050 Teklad Global 20% Protein Primate Diet, Harlan Laboratories; fresh fruit, local market) and unlimited access to water. The room maintained 24 ± 4°C and a relative humidity of 50 ± 10%, with an artificial light-dark cycle of 12:12 (7:00 AM onset) and with 13–18 air changes per hour. All procedures that affected handling and care of animals were in compliance with the guidelines set forth in the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86–23, revised 2011), and approved by the Seoul National University Institutional Animal Care and Use Committee (IACUC no. 16–0192).
Donor pancreatectomy and islet isolation
Monkey pancreata were obtained from either survival partial pancreatectomy (70 ~ 75%) or whole pancreatectomy following necropsy. For partial pancreatectomy, the procedure was performed under general anesthesia through a long vertical midline incision. The splenocolic and splenorenal ligaments were divided so that the spleen, together with the tail of the pancreas, was mobilized. The common bile duct, the main, and the accessory pancreatic ducts were identified and ligated. The head of the pancreas was excised from the second portion of the duodenum by sharp dissection with the caput pancreatic and uncinated process unaffected. Whole pancreatectomy was similarly proceeded as above except the whole pancreas was removed en bloc. Cynomolgus monkey islet isolation was undertaken via minor modifications of the automated method for human islet isolation by using Liberase MTF (Roche Diagnostics Corp., Indianapolis, IN, USA) at a concentration of 0.47 mg/ml.15 Continuous Ficoll gradient and a COBE 2991 cell processor (Cobe Laboratories, Lakewood, CO) were used for purification of islets from the pancreatic digest. Samples of the final islet preparation (100–150 islets) were stained with dithizone, and the diameter of each islet was measured and converted to the mean islet equivalent (IEQ) according to Ricordi method.16 The purity of isolated islets (n = 6) was 90.1 ± 6.0% and fragmentation index was 1.7 ± 0.3.
Induction of diabetes in NHPs
Monkeys were made diabetic by injecting a single high dose (110 mg/kg) of streptozotocin (STZ) or partial pancreatectomy accompanied by low dose injection (60 mg/kg) of STZ as previously described.17,18 Butorphanol or metoclopramide was administered to prevent vomiting caused by STZ. Complete diabetes induction was confirmed by persistent hyperglycemia (>250 mg/dl), <1 ng/ml of fasting C-peptide levels and absence of C-peptide responses in intravenous glucose tolerance test (IVGTT) as previously reported.17
Islet transplantation into NHPs
All monkeys were fasted for 12 h before surgery. A laparotomy was performed, and the jejunal arch was exposed to infuse the islets. A 24- or 22-gauge catheter was inserted through the jejunal vein and advanced toward the portal vein. The monkey islets were infused under gravity pressure over 8–12 min. After infusion, the vessel was ligated with a 5–0 Prolene suture. Ketamine (50 μg/kg/min) and lidocaine (0.6 mg/kg/hr) were continuously infused for 3 days. Meloxicam (0.1 mg/kg, i.v. SID) was injected for 3 days and butorphanol (0.05 mg/kg, i.v.) was injected when necessary for postoperative analgesic regimen. When the first islet graft was fully rejected as evidenced by undetectable C-peptide and hyperglycemia, a second islet transplantation was performed similarly as above. After surgery, the tether system was applied for continuous fluid therapy and infusion of low-dose dextrose, if necessary. Valganciclovir (15 mg/kg/day) was administered orally from day 1 to day 28 after islet transplantation to prevent reactivation of cytomegalovirus.
Immunosuppressive therapy
Immunosuppressive regimen was basically same to that in human CIT07 protocol except that tacrolimus was replaced with tofacitinib, and an anti-inflammatory agent, anti-IL-1β blocker (anakinra; Kineret®, Amgen, San Diego, USA), was added. Immunosuppression was induced with ATG (Thymoglobulin®, Genzyme), sirolimus (Rapamune®, Wyeth), and tofacitinib (Pfizer, NY, USA) (Table 1). TNF-α neutralizing mAb, adalimumab (Humira®, Abbott Laboratories, Queensborough, UK), and anakinra was used as anti-inflammation therapy during peri-transplantation period. ATG (5 mg/kg) was infused i.v. on days −3 and −1 of the transplantation until total CD3 T cell count reached <500/μl. Sirolimus was orally administered daily to achieve stable trough levels (3 ~ 8 ng/ml). Adalimumab was administered subcutaneously 2 ~ 3 h before islet infusion at a dose of 5 mg/kg. Tofacitinib was orally administered twice a day (0.75 mg/kg, bid). The target trough level was sustained by adjusting dosages of sirolimus (0.08–0.3 mg/kg/day) after confirming serum concentration by liquid chromatography-tandem mass spectrometry. At the second islet transplantation, induction was performed with anti-CD25 antibody, basiliximab (Simulect®, Novartis Pharmaceuticals Corp. Basel, Switzerland), instead of ATG.
C-peptide measurement
For measuring serum C-peptide concentrations, blood samples were collected in a serum separating tube. Blood samples were centrifuged at 3,000 rpm for 20 min at 4°C, and the separated serum was stored frozen at −80°C until further use. Monkey C-peptide concentrations were determined by an immunoradiometric assay (Insulin IRMA Kit, Diasource, Belgium) according to the manufacturer’s instructions.
Immune monitoring
The absolute counts of the T cells, B cells, and NK cells were measured by a flow cytometer (BD FACSCantoTM II; BD Biosciences, San Jose, CA) using suitable fluorochrome-conjugated antibodies. Development of donor-specific antibody (DSA) was assessed by the incubation of donor peripheral blood mononuclear cells (PBMCs) with plasma obtained from the recipient after islet transplantation. After incubation with donor PBMCs, samples were washed extensively and were incubated with FITC-conjugated anti-monkey IgG. After further incubation, samples were washed again, and then the mean fluorescence intensity (MFI) of DSA was measured by flow cytometry. Normal monkey plasma was used as a negative control. The cell count and the MFI data were analyzed with FACSDiva software (BD Biosciences). ELISPOT analysis was performed by the method previously described.19 Briefly, the frequencies of interferon (IFN) γ-secreting donor-specific PBMCs of recipient NHPs were measured using an ELISPOT kit (Mabtech, Nacka Strand, Sweden). The resulting spots were enumerated by a computer-assisted ELISPOT Reader System (AID, Strassberg, Germany).
Anti-donor mixed leukocyte culture (MLC)
Allo-reactive donor–recipient pairs were chosen based on positive mixed leukocyte culture reactivity (Table 1). Recipient peripheral blood mononuclear cells (PBMCs) were used as responders against γ-irradiated (3,000 rad) donor PBMC in a one-way mixed leukocyte culture (MLC). Culture media consisted of RPMI medium 1640 supplemented with 10% normal bovine serum, antibiotics, Hepes, sodium pyruvate, and L-glutamine. Responder cells were labeled with CFSE and were harvested and analyzed on day 6 by CSFE dilution assay using FACS. Data were expressed as stimulation index: anti-donor CFSE dilution percent divided by that of the responder only (Table 1).
Biopsy, necropsy, and immunohistochemistry
When the study was ended, the recipient monkeys were humanely killed. The livers were fixed with 10% neutral buffered formalin and then embedded in paraffin. Embedded tissues were sectioned (4 μm) using a microtome. Liver biopsy and subsequent immunohistochemistry were conducted as described previously.20
Quantitation of cynomolgus cytomegalovirus (CyCMV)
The quantification of CyCMV was determined by real-time PCR using forward and reverse primers, 5ʹ-CCTTAACATGAGGCTTGGCATAC-3ʹ and 5ʹ-CAGCCTGAGGCAGCAGAGA-3ʹ, and probe 5ʹ FAM- CTCAGTCTGCTCTACCTG-3ʹ TAMRA (Bioneer Co.). Their sequences are located in the highly conserved CyCMV 156 region. PCR was performed with universal temperature which is recommended by Applied Biosystems as follows; 40 cycles of denaturation for 10 s at 95°C were followed by annealing and extension for 60 s at 60°C. The PRISM 7500 Sequence Detection System (Applied Biosystems) was used for the actual quantification of the signals.
Statistical analysis
The statistical software GRAPHPAD PRISM5 (GraphPad Software, La Jolla, CA) was used for Student’s t-test.
Funding Statement
This work was supported by a grant from the Korea Healthcare Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry for Health and Welfare, Republic of Korea (Grant No. HI13C0954). This work was partly supported by the National Research Foundation of Korea (Grant No. 2016R1D1A1B03932507, 2016R1D1A1B03930468, 2017R1D1A1B03030897 to J. M. Kim, J. S. Shin, and B. H. Min, respectively).
Abbreviations
- ATG
anti-thymoglobulin
- AST
arginine stimulation test
- JAK3
Janus Kinase 3
- IVGTT
Intravenous glucose tolerance test
- NHP
Nonhuman primate
- STZ
Streptozotocin
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Acknowledgments
The authors would like to thank S. K. Park, J. W. Choi, and M. S. Lee for isolating porcine islets; H. Y. Nam for conducting FACS and ELISPOT analyses; W. Y. Jung, M. S. Kim, G. E. Lee, J. E. Kim and S.E. Kwon for caring for all NHPs.
References
- 1.Ballinger WF, Lacy PE.. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72:175–186. [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV.. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 3.Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39:1230–1240. doi: 10.2337/dc15-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13:268–277. doi: 10.1038/nrendo.2016.178. [DOI] [PubMed] [Google Scholar]
- 5.Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol. 2013;191:5785–5791. doi: 10.4049/jimmunol.1390055. [DOI] [PubMed] [Google Scholar]
- 6.Bugliani M, Masini M, Liechti R, Marselli L, Xenarios I, Boggi U, Filipponi F, Masiello P, Marchetti P. The direct effects of tacrolimus and cyclosporin A on isolated human islets: A functional, survival and gene expression study. Islets. 2009;1:106–110. doi: 10.4161/isl.1.2.9142. [DOI] [PubMed] [Google Scholar]
- 7.Van Belle T, von Herrath M. Immunosuppression in islet transplantation. J Clin Invest. 2008;118:1625–1628. doi: 10.1172/JCI35639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senior PA, Zeman M, Paty BW, Ryan EA, Shapiro AM. Changes in renal function after clinical islet transplantation: four-year observational study. Am J Transplant. 2007;7:91–98. doi: 10.1111/j.1600-6143.2006.01573.x. [DOI] [PubMed] [Google Scholar]
- 9.Wojciechowski D, Vincenti F. Targeting JAK3 in kidney transplantation: current status and future options. Curr Opin Organ Transplant. 2011;16:614–619. doi: 10.1097/MOT.0b013e32834c23ce. [DOI] [PubMed] [Google Scholar]
- 10.Busque S, Vincenti FG, Tedesco Silva H, O’Connell PJ, Yoshida A, Friedewald JJ, Steinberg SM, Budde K, Broeders EN, Kim YS, et al. Efficacy and safety of a tofacitinib-based immunosuppressive regimen after kidney transplantation: results from a long-term extension trial. Transplant Direct. 2018;4:e380. doi: 10.1097/TXD.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Du X, He S, Yuan Y, Han P, Wang D, Chen Y, Liu J, Tian B, Yang G, et al. A preclinical evaluation of alternative site for islet allotransplantation. PLoS One. 2017;12:e0174505. doi: 10.1371/journal.pone.0174505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin JS, Kim JM, Min BH, Chung H, Park CG. Absence of spontaneous regeneration of endogenous pancreatic beta-cells after chemical-induced diabetes and no effect of GABA on alpha-to-beta cell transdifferentiation in rhesus monkeys. Biochem Biophys Res Commun. 2019;508:1056–1061. doi: 10.1016/j.bbrc.2018.12.062. [DOI] [PubMed] [Google Scholar]
- 13.Hirshberg B, Montgomery S, Wysoki MG, Xu H, Tadaki D, Lee J, Hines K, Gaglia J, Patterson N, Leconte J, et al. Pancreatic islet transplantation using the nonhuman primate (rhesus) model predicts that the portal vein is superior to the celiac artery as the islet infusion site. Diabetes. 2002;51:2135–2140. doi: 10.2337/diabetes.51.7.2135. [DOI] [PubMed] [Google Scholar]
- 14.Berman DM, O’Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P Jr., Pileggi A, Ricordi C, Kenyon NS. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009;9:91–104. doi: 10.1111/j.1600-6143.2008.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, Kirk AD, Harlan DM, Burkly LC, Ricordi C. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci U S A. 1999;96:8132–8137. doi: 10.1073/pnas.96.14.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, Lake SP, London NJ, Socci C, Alejandro R. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27:185–195. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Shin JS, Min BH, Kim HJ, Kim JS, Yoon IH, Jeong W-Y, Lee G-E, Kim M-S, Kim J-E, et al. Induction, management, and complications of streptozotocin-induced diabetes mellitus in rhesus monkeys. Xenotransplantation. 2016;23:472–478. doi: 10.1111/xen.12266. [DOI] [PubMed] [Google Scholar]
- 18.Jin X, Zeng L, He S, Chen Y, Tian B, Mai G, Yang G, Wei L, Zhang Y, Li H, et al. Comparison of single high-dose streptozotocin with partial pancreatectomy combined with low-dose streptozotocin for diabetes induction in rhesus monkeys. Exp Biol Med (Maywood). 2010;235:877–885. doi: 10.1258/ebm.2010.009361. [DOI] [PubMed] [Google Scholar]
- 19.Kim H-J, Yoon I-H, Min B-H, Kim Y-H, Shin J-S, Kim J-M, Kim J-S, Nam H-Y, Lee -W-W, Park C-G. Porcine antigen-specific IFN-gamma ELISpot as a potentially valuable tool for monitoring cellular immune responses in pig-to-non-human primate islet xenotransplantation. Xenotransplantation. 2016;23:310–319. doi: 10.1111/xen.12248. [DOI] [PubMed] [Google Scholar]
- 20.Shin JS, Kim JM, Kim JS, Min BH, Kim YH, Kim HJ, Jang JY, Yoon IH, Kang HJ, Kim J, et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015;15:2837–2850. doi: 10.1111/ajt.13345. [DOI] [PubMed] [Google Scholar]


