ABSTRACT
Severe adverse events (AEs) following post-exposure rabies vaccination had been occasionally described in previous studies. Once AEs occurred, immediate medical treatment and appropriate change of vaccine and vaccination schedule were of significance. It was also important and challengeable to determine the relationship among adverse reactions, vaccines residues and laboratory tests for patients, to choose a proper vaccine in resumed vaccination, to avoid the reoccurrence of AEs and to ensure adequate immune response. Here, we present steps about how to cope with AEs by giving an example with a two-year-old girl who was identified as category II exposure to rabies, suffered from anaphylaxis after first dose administration with human diploid rabies vaccine (HDCV) so vaccination was temporarily suspended. Dexamethasone was prescribed to her in anti-allergy therapy. Allergy tests indicated that the patient was not sensitive to allergens and heterologous proteins. Vaccine test report showed that residual kanamycin existed in that batch of vaccines. This reminded us to provide her antibiotic skin sensitivity test which found she was allergic to kanamycin. Thus, we could conclude it was the cause of AEs. Then, 0.5 mL lyophilized Purified Vero Cell Rabies Vaccine (PVRV) without any residues was enrolled in the resumed vaccination. To ensure successful immunization, immunogenicity test was also provided which showed adequate immune response (RVNA ≥ 0.5 IU/mL) starting from day14. Besides, no further AEs occurred afterward. This study emphasized the importance of in-depth survey, analysis and implied the necessity to scientifically and properly choose the optimal vaccine for patients and appropriately provide treatments if AEs occurred.
Keywords: rabies vaccines, anaphylaxis, immune response, safety, vaccines residuals, cortisol hormone, vaccine selection, resumed vaccination
Introduction
Rabies is a deadly zoonotic disease that can cause severe symptoms on humans and 100% mortality if patients haven’t received timely and adequate PEP. It is responsible for an estimated 59,000 human deaths and over 3.7 million disability-adjusted life years (DALYs) lost every year1 so to achieve prompt PEP is essential for patients when they were bitten or scratched by suspected rabid or rabid animals. Most cases occur in Africa and Asia, in which China has the second largest population of human rabies death, only after India.2 Rabies deaths occur mainly in those who cannot access timely and effective PEP. Prompt PEP following severe exposures is 100% effective in preventing rabies.3 Slight adverse events (AEs) like pain, erythema, swelling occasionally occur after vaccination, but severe AEs like local and systemic allergic reactions, neurologic disorders are rarely seen while using modern rabies vaccine. A 25-year review indicated that most AEs were non-serious.4 In India, a 2-year surveillance reported five AEs related to rabies vaccine, account for 0.6% of all AEs associated to various vaccines.5 An immediate allergic reaction associated with polygeline in rabies vaccine occurred in Thailand, which was also rare.6 Once severe AEs occur, timely medication and resumed vaccination is urgent and significant in view of 100% fatality of rabies. As WHO position stated, there is no contraindication to PEP vaccination.7 In the subsequent immunization, it is critical to choose an optimal vaccine and administer it to patient with proper regimen, avoiding reoccurrence of AEs. Also, we need to consider if prescription like steroids will suppress immune response. In this study, we put forward approaches coping with severe adverse events by taking a case as example who experienced serious allergic reaction due to presence of potential allergen in rabies vaccine and concurrent treatments, that will be good use for reference to our future work.
Post-exposure prophylaxis and the onset of adverse events
In the clinical courses of PEP, sensitivity to components of vaccines varies among individuals and various adverse events usually occur. It is significant to carefully review the potential risk factors, properly cope with AEs once they occur and offer patients different treatments and new PEP according to their conditions. The case below is a good example about how to appropriately respond to AEs. A two-year-old girl was hospitalized in the emergency department at 23:00 P.M. on September 21st, 2017 for suffering from acute anaphylaxis. Her body temperature was 39.4°C, accompanied with the complaint of continuous 3-hours palpitation and 1-hour excessive sweating. She was scratched by a dog 13 hours ago. Scratches (5–10 mm) without bleeding appeared on the back of her left hand immediately. Her parents sent her to the clinic of Wuhan Center of Disease Control and Prevention and reported that the dog was suspected rabid with hyperexcitation and salivation. There in the clinic, her level of exposure was defined as category II exposure according to World Health Organization Recommendations.8 Immediate PEP was offered to her, which included thorough wound cleaning, human diploid cell rabies vaccine (HDCV) (1.0 mL/dose) vaccination with Essen regimen (day-0,3,7,14,28) at PM12:10. No abnormal reactions were seen in the following 30-minute observation. However, at 16:00 on the same day, the patient suffered a swelling (15 × 20 mm) on the injection site, low-grade fever (37.6°C), listlessness, following the occurrence of flushing and wheal rash on her neck and shoulders at 20:00, which rapidly spread to both arms and back at 22:00. No history of food or drug allergy, birth defects, congenital diseases, hereditary diseases or cardiopathy were reported by her parents. Given her state, the doctor prescribed intravenous infusion (40 drops per minute) with 5 mg dexamethasone. After that, her symptoms relieved. Her temperature gradually decreased to 38.2°C. On the next day, intravenous infusion was continuously provided with 3 mg dexamethasone. Flushing and wheal rash gradually faded and her temperature went down to 36.8°C (Figure 1).
Figure 1.
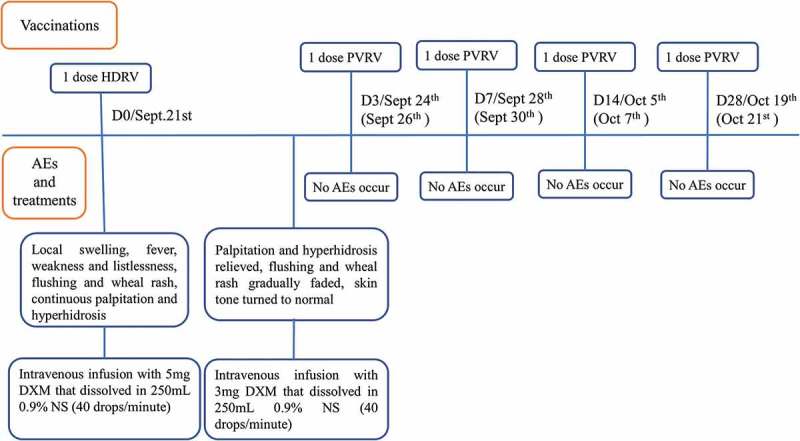
Vaccinations, AEs and treatments on this patient.
Notes. Dates (Sept 24th, Sept 28th, Oct 5th, Oct 19th) followed forward slashes were the dates that rabies vaccines should have been administered on the patient. Nevertheless, due to the anaphylaxis, PEP needed to delay and the regimen resumed since Sept 26th. Dates (Sept 26th, Sept 30th, Oct 7th, Oct 21th) in brackets were the exact dates of following vaccinations. DXM is abbreviation of dexamethasone and NS is normal saline.
Considering the subsequent risk of rabies from the suspected rabid or rabid dog, PEP was essential for the patient. The exact cause of anaphylaxis needed to be figured out first. Bovine serum is one component in HDCV so bovine serum albumin (BSA) was highly likely to be the risk because high sensitivity to BSA was a possible reason of anaphylaxis.9 To determine whether it was or not, allergy testing was performed with UniCAP systems (Pharmacia Biotech, Uppsala, Sweden) by Tongji Hospital, Tongji Medical College of Huazhong University of Science & Technology for her. The test results found that in this patient, no hypersensitivity reactions to allergen exposure from routine life, including pollen assemblage, house dust, cat, dog, cockroach, penicillium notatum, branch spore, aspergillus fumigatus, alternaria, albumen, milk, egg, peanut, soybean, seafoods, freshwater fish, beef, mutton and other heterologous proteins like bovine serum albumin.
After that, we referred to test report of lot release on vaccines, and finally speculate a possible reason of the AEs described above. The advanced bioreactor used in production technique of vaccines was totally hermetic so contamination was impossible to happen. The test report also indicated no endotoxin existed. The whole producing process was free of any antibiotics. Nevertheless, cell culture used in vaccine manufacturing has experienced a thawing procedure from −80°C to ambient temperature, during which contaminations usually occur. To avoid analogous contamination, antibiotics were usually used in cell matrix. We speculated that the cause of AEs was a certain kind of antibiotic. The test report also showed that there did exist kanamycin residue. The antibiotics skin sensitivity test indicated that the patient was allergic to kanamycin. Until now, it was clear that the cause of AEs was the residual kanamycin in that batch of vaccines.
New vaccination schedule, safety and immunogenicity results
Survival from clinical rabies is extremely rare; it has been well documented in 15 cases, albeit with severe sequelae in most of those cases10 so this patient needed another rabies vaccine allow for the risk of 100% mortality. It is necessary to prescribe a proper vaccine among a variety of rabies vaccines in China pharmaceutical market (Table 1). To date, human rabies vaccines in China were in four kinds of cell culture and strains. Vaccines containing residue of antibiotics were not allowed to use on patient who is allergic to certain antibiotics. A previous study11 found that children aged under 5 years occurred less AEs when they were immunized with small-dose rabies vaccines, so that study stated that dosage was a factor to affect the safety of vaccines. With the same level of potency, vaccines in small dosage would contain fewer residues. Although a study concluded that gelatin was not likely to result in allergic reactions to Sri Lankan children,9 in this case, we tended to avoid potential risks from gelatin and heterologous protein. Vaccines produced in roller bottles led to relatively low yields12 while vaccines produced in bioreactors were safer due to the reduction of residual cell protein, cell DNA and BSA.11 With the development of technique, advanced microcarrier bioreactor culture technique could generate larger yields and reduced the risk of contamination during the entire completely closed pipe production processes.12 Thus, a lyophilized PVRV (0.5 mL/dose) produced in microcarrier reactor without any residues like gelatin, antibiotics and serum protein was enrolled. This vaccine used PM strain, similar to PV strain in HDCV that could avoid adverse reaction resulted from difference between strains. Also, PM strain and PV strain were both certificated by WHO.7
Table 1.
Human rabies vaccine marketed in China.
| Cell matrix | Dose (mL) | Form | Strain | Cultivation | Purification | Potency (IU/mL) | Excipient | Preservatives | Regimen |
|---|---|---|---|---|---|---|---|---|---|
| Vero | 0.5 | liquid | PV2061 from USA ATCC | microcarrier bioreactor | 4-tandemed-columns chromatography | 4.5 | - | thiomersal | Zagreb/Essen |
| Vero | 0.5 | lyophilized | 4.5 | dextran | - | ||||
| Vero | 1.0 | lyophilized | aG strain | fixed-bed bioreactor | chromatography | 4.3 | saccharose | gentamycin | Essen |
| Vero | 0.5 | lyophilized | aG strain | fixed-bed bioreactor | chromatography | 4 | lactose/dextran/gelatin | gentamycin | Essen |
| Vero | 1.0 | lyophilized | aG strain | roller bottle | chromatography | 4 | Dextran/gelatin | Essen | |
| Vero | 1.0 | liquid | aG strain | bioreactor | chromatography | 4 | - | thiomersal | Essen |
| HDRV | 1.0 | lyophilized | PM strain | bioreactor | chromatography | 4 | maltose | kanamycin | Essen |
| PHKC | 1.0 | liquid | aG strain | roller bottle | chromatography | 4 | - | - | Essen |
| PHKC | 1.0 | liquid | aG strain | roller bottle | chromatography | 4 | - | - | Essen |
| CEF | 1.0 | lyophilized | Flury LEP strain | cell plant | gradient centrifugation | 4 | dextran | gentamycin | Zagreb/Essen |
After determined that no residual antibiotics left according test report of that batch of vaccines, it was administered to the patient. The initial PEP was resumed rather than restarted, that was in accordance with the latest WHO position.7 A change in the route of administration or in vaccine product during a PEP course was acceptable if such a change was unavoidable. No AEs were found following the later resumed vaccination via cautious observation within 30 minutes and active telephone inquiries within 24 and 48 hours. Pharmacopoeia of the People’s Republic of China13 had suggested that administration of PVRV should not provide simultaneously with the prescription of corticosteroids. However, this patient was in complex conditions so both prescription and immunization were needed.
To ensure adequate immunization, before the third dose of PVRV, we tested rabies virus neutralizing antibody (RVNA) titer with the same method described in our previous study.14 A review of literature stated that despite vaccines and immunization schedules, on day 14, all patients obtained protective level of RVNA15 and some of them obtained on day7, so immunogenicity test conducted on D7, D14 and D42 in this case. Although the second dose was injected 5 days after the first dose, it was regarded as 3 d in Essen regimen. Serum RVNA titer test on Sept 30th (7 d) reflected immune response of previous two doses vaccinations. RVNA titer on 7d was 0.37 IU/mL, increased to 0.61 IU/mL on 14 d, which reflected immune response of previous three doses vaccinations, and reached to 9.36 IU/mL on 42 d that reflected immune response of the entire vaccination regimen. RVNA titer higher than 0.5 IU/mL indicated adequate immune response, proposed by WHO.8 Although compared with the reference,16 RVNA titers were lower in this case, possibly due to the immune suppression caused by dexamethasone.17 Successful protection has been built up on Oct.7th (4th dose, 14 d) (Figure 2).
Figure 2.
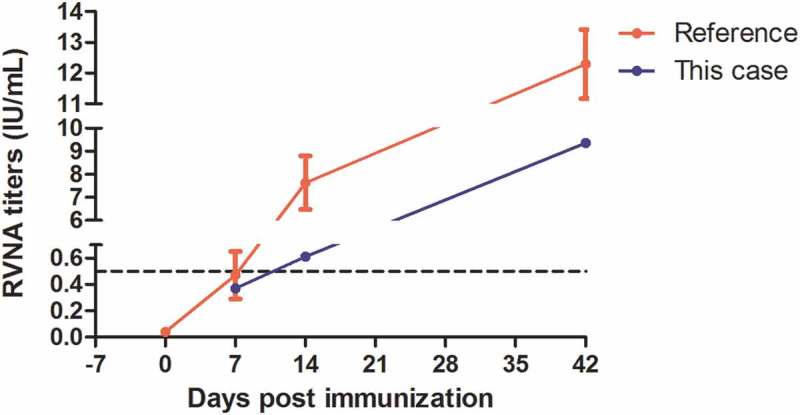
RVNA titers of this case and reference data from a previous study16 which used the same kind of vaccine with Essen regimen. The black dotted line (RVNA = 0.5 IU/mL) indicated minimum protective level of sero-conversion. Day 7 was actually 9 days post the first dose because of two-days delay on anaphylaxis treatment. It was the third dose in regimen and reflected the immune response of previous two doses, so in this figure, it was marked as day 7. It was comparative to the reference data. As for day 14, it reflected the immune response of previous three doses, though it was 16 days post the first dose, it was the fourth dose (Day 14) in regimen.
Discussion
Slight side effects like fever, weakness, headache, nausea, local pain and swelling usually occurred in patients following rabies vaccination, among whom patients aged younger than 15 years old were more often suffered from fever, vomiting and cough.18 Mostly, these symptoms were transient and could relieved naturally. In China, a multi-center study documented that in Essen route, adverse reactions were the highest reported in first/second injection (8.9%), progressively lower in third (8.5%), fourth (5.9%), fifth (2.3%) injection and most of them were non-serious.16 Severe allergic reactions related to vaccines were rarely reported and it was of difficulty and urgency to figure out the specific reason that cause AEs. In a previous study.19 a child suffered from AEs was allergic to eggs and milk while the first vaccine applied to him after dog bite was stabilized by gelatin and components from cattle, so it finally concluded that the vaccine containing heterologous protein was the culprit to him. Occurrence of Henoch Schönlein purpura (HSP) in an adult patient post rabies vaccination was also rare and serious.20 Vaccines contained additional components like potential allergen (egg, milk, yeasts), additives (stabilizers, gelatin, dextran), preservatives (thimerosal, phenoxyethanol, antibiotics), possibly lead to allergic reactions in children vaccinated.21,22 Consisted of various components, produced from diverse cell culture with different techniques, rabies vaccines can possibly bring risks to individuals because of their varieties of sensitivity to components. Therefore, various kinds of vaccines needed to be prepared in clinics of CDC. Once severe adverse events following immunization happen, medication and another optimal vaccine should be offered to patients to treat adverse reactions and avoid potential risk of rabies. As stated above, Pharmacopoeia of the People’s Republic of China suggested that PVRV should not apply on patients with steroids, but in this case, dexamethasone was necessary to treat allergic reaction. Meanwhile, in view of the almost invariably fatal outcome of rabies, there was no contraindication to PEP vaccination, so subsequent immunization was crucial.
In 2006, China Food and Drug Administration (CFDA) launched regulation stipulated that all biological products including vaccines must be certificated before accessing to clinics and each batch of vaccines would accompanied with test report, which was also called lot release certificate. Test reports could not only prove quality and validity of vaccines but also determine associations between reactions and components & residues of vaccines once adverse events occurred. Otherwise, they could be references to choose another vaccine when AEs occurred.
When specifying the exact cause to AEs, both individual conditions and constituents of vaccines ought to be considered. This case had no allergy history reported by her parents and allergy test proved it. Thus, components of vaccine were suspected. It was necessary for clinicians and other health staff to ascertain whether there were antibiotics or other allergens left in vaccines by referring to test reports. After determining the cause, another vaccine would be enrolled but which one? Regimen, dose, residues, purification way, inactivation way and stabilizers (Table 1) should be considered when change vaccination regimen for the patient. Based on our previous study,23 Essen regimen was better than Zagreb regimen for children younger than 5 years old. And with similar potency, small dosage was relatively safer for children, particularly for young children.11 Finally, 0.5 mL lyophilized PVRV without any residues was selected. This patient was allergic to kanamycin and it was unrealistic to perform all kinds of antibiotics skin sensitivity tests for her, so to avoid risks, vaccine without residues like gentamycin was appropriate. Careful monitoring was still needed to make sure no further AEs occurred. And immunogenicity test was required to ensure adequate immune response while using steroids. Though RVNA titers in this patient was lower compared with the reference data, it was also protective since day14. Dexamethasone prescription during rabies vaccination may affect the immunization response because of immune suppression. Besides, children < 5 years old may have significantly lower antibody levels at day7, possibly due to immature immune system.14
In this case, identifying the cause of AEs and offering prompt medication was challengeable but it could remind clinicians of several points in the future clinical works. To date, reports of allergic reactions resulted from antibiotics in vaccines are limited.24 It is essential to gain more information by asking patients about their physical conditions, especially their allergy histories and offer allergy tests. Then as there are various kinds of vaccines in China, clinicians need to compare their characteristics according to Table 1 and choose the most proper one for patients. Table 1 could possibly be a brief handbook for health staff working on rabies prevention in China. After all, if AEs still occur unfortunately and corticosteroids are administered, which may affect the efficacy of vaccination, serum RVNA titer tests will be significant to ensure adequate immune response. In this case, cortisol hormone indeed suppressed immunization (Figure 2) but luckily, she finally obtained protective level of sero-conversion. However, it remained unknown that whether protective level of RVNA titers can obtain or not when patients need continuous prescription and higher dosage of corticosteroid, so more cases and further studies are imperative. To sum up, this study highlights the importance of clinical detailed inquiries, scientifically choosing suitable vaccine and immune response monitoring.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, et al. Estimating the global burden of endemic canine rabies. Plos Neglect Trop D. 2015;9(4):e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao X, Tang Q, Rayner S, Guo Z, Li H, Lang S, Yin C, Han N, Fang W, Adams J, et al. Molecular phylodynamic analysis indicates lineage displacement occurred in Chinese rabies epidemics between 1949 to 2010. Plos Neglect Trop D. 2013;7(7):e2294. doi: 10.1371/journal.pntd.0002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilde H. Failures of post-exposure rabies prophylaxis. Vaccine. 2007;25(44):7605–7609. doi: 10.1016/j.vaccine.2007.08.054. [DOI] [PubMed] [Google Scholar]
- 4.Moro PL, Woo EJ, Paul W, Lewis P, Petersen BW, Cano M. Post-marketing surveillance of human rabies diploid cell vaccine (Imovax) in the Vaccine Adverse Event Reporting System (VAERS) in the United States, 1990‒2015. Plos Neglect Trop D. 2016;10(7):e0004846. doi: 10.1371/journal.pntd.0004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh AK, Wagner AL, Joshi J, Carlson BF, Aneja S, Boulton ML. Application of the revised WHO causality assessment protocol for adverse events following immunization in India. Vaccine. 2017;35(33):4197–4202. doi: 10.1016/j.vaccine.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Beckham JM, Noroski L, McNeil JC. Immediate hypersensitivity reaction related torabiespost-exposure-prophylaxis in thailand with subsequent rabies vaccine change to avoid polygeline vaccine excipient with successful challenge and treatment tolerance in the United States. Open Forum Infect Dis. 2017;4(Suppl 1):S124–S125. doi: 10.1093/ofid/ofx163.164. [DOI] [Google Scholar]
- 7.World Health Organization WHO expert consultation on rabies: third report. WHO press; 2018.
- 8.World Health Organization WHO expert consultation on rabies. Second report. World Health Organ Tech Rep. 2013;982(982):1–139.. [PubMed] [Google Scholar]
- 9.de Silva R, Dasanayake WMDK, Wickramasinhe GD, Karunatilake C, Weerasinghe N, Gunasekera P, Malavige GN. Sensitization to bovine serum albumin as a possible cause of allergic reactions to vaccines. Vaccine. 2017;35(11):1494–1500. doi: 10.1016/j.vaccine.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Jackson AC. Human rabies: a 2016 update. Curr Infect Dis Rep. 2016;18(11):38. doi: 10.1007/s11908-016-0540-y. [DOI] [PubMed] [Google Scholar]
- 11.Peng J, Lu S, Zhu Z, Zhang M, Hu Q, Fang Y. Safety comparison of four types of rabies vaccines in patients with WHO category II animal exposure. Medicine. 2016;95(47):e5049. doi: 10.1097/MD.0000000000004864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu P, Huang Y, Zhang Y, Tang Q, Liang G. Production and evaluation of a chromatographically purified Vero cell rabies vaccine (PVRV) in China using microcarrier technology. Hum Vacc Immunother. 2014;8(9):1230–1235. doi: 10.4161/hv.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinese Pharmacopoeia Commission Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science and Technology Press; 2010. [Google Scholar]
- 14.Fang Y, Chen L, Liu M, Zhu Z, Zhu Z, Hu Q. Comparison of safety and immunogenicity of PVRV and PCECV immunized in patients with WHO Category II animal exposure: A study based on different age groups. Plos Neglect Trop D. 2014;8(12):e3412. doi: 10.1371/journal.pntd.0003412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett S, Levis R, Meltzer MI, Schaffner W, et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine. 2009;27(51):7141–7148. doi: 10.1016/j.vaccine.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Shi N, Zhang Y, Zheng H, Zhu Z, Wang D, Li S, Li Y, Yang L, Zhang J, Bai Y, et al. Immunogenicity, safety and antibody persistence of a purified vero cell cultured rabies vaccine (Speeda) administered by the Zagreb regimen or Essen regimen in post-exposure subjects. Hum Vacc Immunother. 2017;13(6):1338–1345. doi: 10.1080/21645515.2017.1279770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng J, Chen L, Zhu Z, Zhu Z, Hu Q, Fang Y. Effect of Corticosteroids on RVNA production of a patient with acute disseminated encephalomyelitis following rabies vaccination as well as administration of HRIG. Hum Vacc Immunother. 2014;10(12):3622–3626. doi: 10.4161/21645515.2014.979621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sari T, Tulek N, Bulut C, Oral B, Tuncer Ertem G. Adverse events following rabies post-exposure prophylaxis: A comparative study of two different schedules and two vaccines. Travel Med Infect Di. 2014;12(6,Part A):659–666. doi: 10.1016/j.tmaid.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Fang Y, Liu M, Chen L, Zhu Z, Zhu Z, Hu Q. Rabies post-exposure prophylaxis for a child with severe allergic reaction to rabies vaccine. Hum Vacc Immunother. 2016;12(7):1802–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Z, Zheng Y, Lu S, Hu Q, Fang Y. Rabies post-exposure prophylaxis for a male with severe Henoch Schönlein purpura following rabies vaccination. Hum Vacc Immunother. 2018:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franceschini F, Bottau P, Caimmi S, Crisafulli G, Lucia L, Peroni D, Saretta F, Vernich M, Povesi Dascola C, Caffarelli C. Vaccination in children with allergy to non active vaccine components. Clin Transl Med. 2015;4:1. doi: 10.1186/s40169-014-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreskin SC, Halsey NA, Kelso JM, Wood RA, Hummell DS, Edwards KM, Caubet J, Engler RJM, Gold MS, Ponvert C, et al. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9:1. doi: 10.1186/s40413-016-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Q, Liu M, Zhu Z, Zhu Z, Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vacc Immunother. 2014;10(6):1645–1649. doi: 10.4161/hv.28420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hee CE. Vaccine allergies. Clin Exp Vaccine Res. 2014;3(1):50–57. doi: 10.7774/cevr.2014.3.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]


