ABSTRACT
Cyclophosphamide (CPM), an agent widely used in breast cancer therapy, has strong gonadotoxic effects. Female reproductive potential after therapy relies on ovulated oocytes deriving from primordial follicles surviving CPM toxic insult. In this study, we investigated in the mouse model whether pre-conceptional maternal exposure to CPM has epigenetic effects on offspring oocytes and if they are inherited. Adult female mice mated following CPM exposure, generated an offspring (F1) with delayed growth, normal fertility and altered methylation of three imprinted genes (H19, Igf2r and Peg3) in their oocytes. These alterations were present in oocytes generated by F2 mice. Pre-conceptional maternal exposure to fertoprotective agents AS101 and crocetin prior to CPM was not able to fully counteract alterations in offspring oocyte imprinting. For the first time, current study evidences that pre-conceptional CPM maternal exposure can affect the competence of offspring’s oocytes and warns on possible long-term effects on the health of next generations.
KEYWORDS: Cyclophosphamide, epigenetics, imprinting, oocyte, fertility, AS101, crocetin
Introduction
The remarkable advances in oncology practice in the last two decades have significantly improved the prognosis for patients highlighting the need of increasing the quality of life of cancer survivors by reducing harmful effects of cancer therapies on healthy organs [1]. Preservation of fertility has become one of the major quality of life issues for patients undergoing chemotherapy at reproductive age [2,3]. The ovary contains oocytes within immature (primordial) follicles that are fixed in number at birth. During reproductive life, the follicles, known as the ovarian reserve, start growing leading to cyclic production of mature oocytes and a gradual and irreversible decline in reproductive potential, until menopause, occurs [4]. Fertility loss associated with chemo- and radiotherapy is due to accelerated depletion of ovarian reserve resulting from direct toxic effects to the primordial follicle oocytes [5,6]. Since the effectiveness of protective pharmacological treatments in clinical settings is still under investigation, fertility preservation programs rely on germ cells or embryo cryopreservation before therapy [3].
Among the most ovotoxic drugs is cyclophosphamide (CPM), an alkylating agent widely used in allogeneic bone marrow transplantation and breast cancer therapy [7]. CPM alters the ovarian reserve in a manner that is dose-, duration- and age-dependent, being mutagenic, teratogenic and embryolethal. In addition, genetic risks to the growing oocytes exist. Indeed, animal studies reported that conceptions early after CPM exposure and thus attributable to follicles exposed to CPM during growth or at a mature stage result in a high rate of pregnancy failure and high malformation rate [8]. Nevertheless, it is important to consider that reproductive potential in cancer patients after the early post-treatment period relies on ovulated oocytes deriving from primordial follicles surviving the CPM toxic insult [9]. Although there is no evidence of mutations with effects on embryo viability and development, potential effects of maternal exposure to CPM on the health of offspring conceived during the ‘safe’ period following CPM exposure have never been investigated. In this study, we focused on offspring reproductive health and investigated in the mouse model whether pre-conceptional exposure to CPM has adverse effects on oocyte competence and fertility potential of female offspring. In mammals, a crucial factor for oocyte competence and embryo development is the correct establishment of the epigenetic information of imprinted genes (erasure/re-establishment) during oogenesis [10,11]. Imprinted genes are erased when primordial germ cells arrive at genital ridges and re-established during gametogenesis. The methylation of maternally imprinted genes including IGF2R and PEG3 is completed in the fully grown oocyte, whereas paternally imprinted genes including H19, remain unmethylated [12]. To accomplish our objective, female mice were administered a non-sterilizing dose of CPM and mated with unexposed males following 12 weeks from CPM exposure. Oocyte competence of female F1 mice was evaluated in terms of correct methylation status of Igf2r, Peg3 and H19, and their fertility was assessed throughout natural mating. We also investigated whether alterations found in F1 female mice were present in F2 (transgenerational effect). Finally, the hypothesis that maternal treatments with protective effects on ovarian reserve could counteract alterations found in F1 and F2 female mice was explored (Figure 1).
Figure 1.
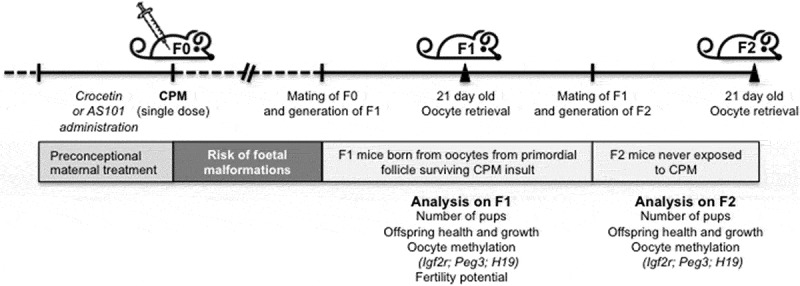
Experimental design.
Schematic representation of the experimental design. F0 female mice aged 4–8 weeks were administered a non-sterilizing dose of CPM with or without concurrently administration of fertoprotective agents crocetin and AS101 and mated with unexposed males following 12 weeks from CPM exposure. Oocytes were retrieved from F1 and F2 female mice aged 21 d. Methylation analysis was run on oocytes from F1 and F2 and compared with age-matched control mice.
Results
Analysis of F1 characteristics at birth, weaning and adult life
Twelve-weeks after CPM single dose, mice of all experimental groups were able to conceive after mating, got pregnant and gave birth to F1 pups. The litter obtained from all groups was similar in terms of number and health of pups. Indeed, no malformations or intrauterine growth retardation (IUGR) were observed (Table 1). At weaning F1 mice from CPM group presented a reduction of weight of about 30% in comparison to F1 mice born from control group. This weight reduction was recovered when F1 reached adult life at 2 months of age. Interestingly, maternal administration of crocetin or AS101 prior to CPM prevented the growth retardation observed in F1 mice born from mice receiving CPM alone 12 weeks prior to conceivement. No differences were observed between male and female characteristics.
Table 1.
F1 mice characteristics at birth, weaning and mating.
| Experimental group | Number of pups | P | Weight at birth (g) | P | Weight at weaning (g) | P | Weight at mating (g) | P |
|---|---|---|---|---|---|---|---|---|
| CTRL-F1 | 11.44 ± 0.96 | - | 1.83 ± 0.09 | - | 10.88 ± 0.79 | - | 20.28 ± 0.52 | - |
| CPM-F1 | 10.78 ± 0.95 | n.s. | 1.80 ± 0.10 | n.s. | 6.42 ± 0.58 | P < 0.001 | 19.28 ± 0.45 | n.s. |
| CRO+CPM-F1 | 10.55 ± 0.83 | n.s. | 1.75 ± 0.06 | n.s. | 9.06 ± 0.92 | n.s. | 20.21 ± 0.35 | n.s. |
| AS101+ CPM-F1 | 11.89 ± 01.28 | n.s. | 1.76 ± 0.06 | n.s. | 11.11 ± 0.58 | n.s. | 20.47 ± 0.48 | n.s. |
Values are means ±SEM.
n.s., not statistically significant; P < 0.001; One Way ANOVA
Peg3, Igf2r and H19 methylation in germinal vesicles (GV) oocytes from generation F1
Oocytes retrieved from CPM-treated F1 generation showed methylation alteration for the three analyzed genes. Peg3 and Igf2r methylation resulted reduced by about 20% and 60%, respectively (Figure 2(a,b)). H19 methylation was increased by about 25% (Figure 2(c)). The co-treatment with crocetin was able to partially rescue methylation levels in Peg3 and Igf2r (Figure 2(a,b)) whereas H19 resulted in methylated of an additive 20%. AS101 compound did not show any effect on Peg3 methylation which remained low as for CPM-treated oocytes whereas was able to recover the methylation status of Igf2r reaching 90% of total methylation. Similarly, AS101 completely recovered the methylation status for H19, until 7% (Figure 2(c)).
Figure 2.
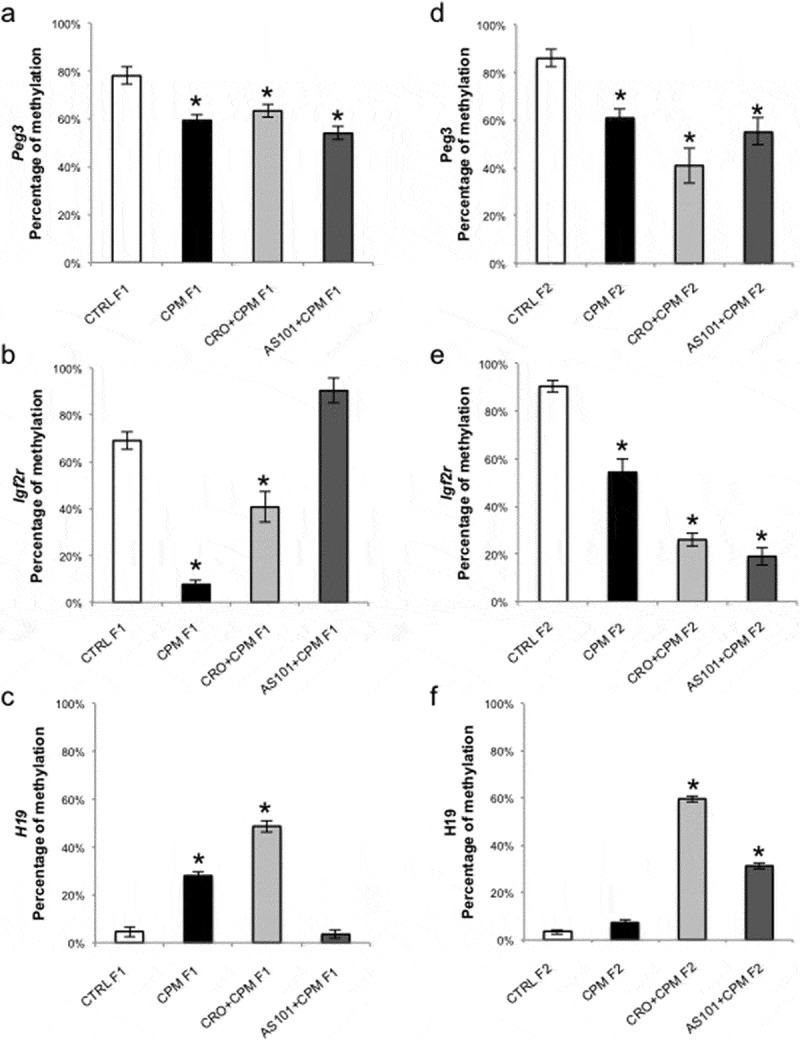
Peg3, Igf2r and H19 methylation in GV oocytes from F1 and F2 mice.
a) Methylation levels for Peg3 in F1 oocytes from controls, CPM-treated, CPM+crocetin-treated and CPM+AS101-treated mice. b) Methylation levels for Igf2r in F1 oocytes from controls, CPM-treated, CPM+crocetin-treated and CPM+AS101-treated mice. c) Methylation levels for H19 in F1 oocytes from controls, CPM-treated, CPM+crocetin-treated and CPM+AS101-treated mice. d) Methylation levels for Peg3 in F2 oocytes from controls, CPM-treated, CPM+crocetin-treated and CPM+AS101-treated mice. e) Methylation levels for Igf2r in F2 oocytes from controls, CPM-treated, CPM+crocetin-treated and CPM+AS101-treated mice. f) Methylation levels for‘ H19 in F2 oocytes from controls, CPM-treated, CPM+crocetin-treated and CPM+AS101-treated mice. All experiments were run in triplicate, ANOVA test was used to assess significance among the analysed groups considering p < 0.05 significant.
Analysis of female F1 fertility and F2 characteristics at birth and weaning
F1 mice of all experimental groups were able to conceive after mating, got pregnant and gave birth to F2 offspring with similar litter size. All F2 pups were healthy and no malformations were observed (Table 2). Similar weight at birth and weaning were observed in F2 mice born from all experimental groups.
Table 2.
F2 mice characteristics at birth and weaning.
| Experimental group | Number of pups | P | Weight at birth (g) | P | Weight at weaning (g) | P |
|---|---|---|---|---|---|---|
| CTRL-F2 | 14.33 ± 0.60 | - | 1.79 ± 0.06 | - | 11.24 ± 0.49 | - |
| CPM-F2 | 14.89 ± 0.58 | n.s. | 1.71 ± 0.04 | n.s. | 10.57 ± 0.36 | n.s. |
| CRO+CPM-F2 | 13.33 ± 0.65 | n.s. | 1.71 ± 0.03 | n.s. | 11.00 ± 0.44 | n.s. |
| AS101+ CPM-F2 | 14.00 ± 0.85 | n.s. | 1.74 ± 0.05 | n.s. | 10.88 ± 0.45 | n.s. |
Values are means ±SEM.
n.s., not statistically significant; One Way ANOVA
PEG3, IGF2R and H19 methylation in oocytes from generation F2
When analyzing oocytes retrieved from CPM-treated F2 generation, methylation modifications were found for Peg3 and Igf2r but not for H19, compared to F2 controls (Figure 2(d,e,f)). Peg3 and Igf2r had lower methylation of about 30%, showing methylation levels comparable to those in F1 generation (Figure 2). A difference in DNA methylation between control oocytes of F1 and F2 was observed for Igf2r, respectively, about 70% and 90% (Figure 2). This difference was considered biologically not significant since Igf2r methylation has been reported in MII oocytes ranging from about 60% to 100% [13]. Crocetin in F2 oocytes induced a deeper methylation reduction for Peg3 and Igf2r (Figure 2(d,e)), whereas H19 resulted methylated of more than 50% compared to controls and CPM-treated F2 (Figure 2(f)). The F2 oocytes from the group treated with CPM and AS101 showed a partial recovery of proper methylation of Peg3 and H19, whereas a further slight reduction for Igf2r compared to F1-matched oocytes was observed (Figure 2(d,e,f)).
Discussion
Although the risk of infertility associated with CPM administration is well known and its molecular basis is under continuous investigation, potential effects of pre-conceptional maternal exposure to this anticancer drug on the health of offspring have been poorly investigated [7,8]. Moreover, research has paid attention to the litter conceived early after the end of therapy and provided recommendation of conceiving at least 12 weeks following the last CPM administration in mice [8]. In this study, we focused on offspring reproductive health and investigated in the mouse model whether pre-conceptional exposure to CPM has adverse effects on oocyte competence and fertility potential of female offspring conceived during the ‘safe’ period following CPM exposure in mice.
We previously reported that CPM-treated mice shows a significant reduction of primordial and growing follicles, which is prevented by the administration of fertoprotective agents AS101 and crocetin, most likely due to modulation of antioxidant signalling response [14]. The detrimental effects of CPM reducing mature oocytes competence as well as the protective effects of crocetin and AS101 have been recently described in vitro [15].
In this study, for the first time, we have shown that the pre-conceptional exposure to CPM of female mice negatively affects the health of the offspring. In particular, we observed that adult female mice that received a non-sterilizing dose of CPM 12 weeks prior to mating with untreated males resulted in offspring F1 with postnatal growth retardation at the time of weaning. This effect may represent an evidence of toxicity produced by CPM in gametes, but also of alterations in the preconception period at the level of all maternal organs and tissues that contribute to a correct prenatal and postnatal development.
By focusing on germ cells and reproductive functions of the offspring, we found that CPM-F1 female mice produced oocytes with altered methylation of three imprinted genes. In particular, the methylation of the maternally imprinted genes Igf2r and Peg3 was reduced compared to the control, while the methylation of the paternal H19 imprinted gene was increased. However, CPM-F1 female mice were fertile and produced a CPM-F2 offspring similar to the control in terms of number, absence of malformations and growth rate. In mouse oocytes de novo methylation begins at 7–10 d post-natal when oocytes have a diameter of 45 microns and is completed during the growth phase when oocytes reach a diameter of 70–80 microns [12]. Hence, as demonstrated in cattle, DNA methylation of imprinted genes is closely related to oocyte size which, in turn, is associated with its level of competence [16,17]. Despite the close association between growth and oocyte competence, the functional role of oocyte methylation is unclear. In fact, the oocyte can resume and complete the meiosis and be fertilized even in the absence of DNA methylation as demonstrated by studies with oocytes null for DNMT3A and DNMT3L [18–20].
The epigenetic alterations found in CPM-F1 oocytes could be the consequence of perturbations in the ovarian microenvironment with direct and indirect effects on the numerous signals that regulate methylation during folliculogenesis. The alterations of CPM-F1 weight at weaning, when mice should have reached the reproductive maturity to start cycling, could be taken as evidence of metabolic alterations with possible influence on the ovarian microenvironment and the gametes development via redox state. Oxidative stress can induce metabolic changes that stimulate glutathione synthesis and the recycling of homocysteine, a molecule that interferes with the methylation process [21]. Foetal, birth and weaning weight are an indicator of foetal wellness and could be predictors of adulthood health outcomes such as cardiovascular disease, type 2 diabetes, and obesity [22]. Weight regulation is also known to depend on proper methylation of imprinted genes [23]. The mechanisms through which altered methylation of imprinted regions affect foetal growth and weight are complex since these genes participate in an intricate gene network called imprinted gene network (IGN) [24]. The genes of the IGN show a co-regulation pattern [25] and H19, in particular, seems to be one of the major trans-regulator of the IGN [26] regulating the transcription and translation of the IGF2 cluster.
A crucial goal of this work was to investigate whether the methylation aberrations were trans-generationally inherited by the F2 generation, which had not been directly exposed to CPM. The oocytes produced by CPM-F2 mice showed a methylation pattern of the maternally imprinted genes similar to CPM-F1, suggesting the presence of a trans-generational effect. The modifications of the ovarian microenvironment, previously hypothesized, could have induced stable modifications in the machinery that determine the correct methylation of the imprinted genes, which is still difficult to understand [27]. Some regions evade the genome-wide DNA demethylation occurring in the mammalian germline and pre-implantation embryos, to induce possible trans-generational effects based on the ancestor’s environment [28].
Another important aspect of this study is the observation that the pre-conceptional administration of fertoprotective agents prior to treatment with CPM counteracts the growth retardation observed in CPM-F1 mice. Thus, AS101 and crocetin, known for their anti-inflammatory and antioxidant activity, may mitigate the toxicity produced by CPM on all maternal organs and tissues that contribute to a correct prenatal and postnatal development. In addition, we demonstrate that both fertoprotective agents are able to partially rescue methylation alteration induced by CPM on Peg3 and Igf2r. H19 proper methylation status was reached only in F1 oocytes from mice treated with CPM+AS101. Noteworthy, F2 oocytes from the CPM co-treatment with crocetin or AS101 groups showed methylation alterations even more defective than those observed in oocytes from the only CPM-treated group. Although crocetin and AS101 play as both antioxidant and anti-inflammatory agents, they are known to have a different ability to modulate ovarian signalling pathways in response to CPM [14]. Therefore, the differences seen in the present work may be ascribed to different pathways activated by the two fertoprotective agents.
These data suggest that fertoprotective agents may induce inheritable effects with consequences on the health of the offspring that require further analyses in the context of oncofertility projects.
Conclusions
CPM pre-conceptional administration may have effects on the health of offspring conceived during the ‘safe’ period following treatment by causing alterations of methylation in F1 germline, which are trans-generational inherited by F2, which has not been directly exposed to CPM. Alterations of imprinting in cells of the somatic line of F1 that have not been the subject of the present study may be also hypothesized and proposed as a matter in future studies.
Supplementation with fertoprotective agents is proposed to reduce the risk of potentially harmful effects, but their use may sometimes be detrimental and induces epigenetic long-lasting effects. Even if the molecular mechanisms that drive the germline epigenetic modulation and inheritance linked to pre-conceptional exogenous chemicals exposure are still to be elucidated, this study is the first step towards an answer for oncofertility programs. Finally, current study warns on possible long-term effects on the health of next generations.
Material and methods
Cyclophosphamide, crocetin, and AS101 preparation
Cyclophosphamide (CPM) was obtained from Baxter, Rome, Italy. A solution of CPM at a concentration of 25 mg/mL in PBS (pH 7.4) was freshly prepared. Crocetin isolation was performed by crocetin esters and purified by an internal method of the Verdù Cantò Saffron Spain Company (Novelda, Alicante, Spain) as previously described [14]. AS101 was obtained from Tocris Biosciences, Bristol, UK. A solution of AS101 at a concentration of 150 mg/mL in PBS (pH 7.4) was freshly prepared.
Animals
CD1 mice were maintained in a temperature-controlled environment under a 12 h light/dark cycle (7.00–19.00) and free access to feed and water ad libitum. All the experiments were carried out in conformity with national and international laws and policies (European Economic Community Council Directive 86/609, OJ 358, 1 December 2012, 1987; Italian Legislative Decree 116/92, Gazzetta Ufficiale della Repubblica Italiana n. 40, 18 February 1992; National Institutes of Health Guide for the Care and Use of Laboratory Animals, NIH publication no. 85–23, 1985). The project was approved by the Italian Ministry of Health and the internal Committee of the University of L’Aquila. Mice were sacrificed by an inhalant overdose of carbon dioxide (CO2, 10–30%), followed by cervical dislocation. All efforts were made to minimize suffering.
Pre-conceptional treatments of female mice
A total of 32 CD-1 female mice aged 4 to 8 weeks (Charles River Italia s.r.l., Calco, Italy) were used in the present study. All the experiments were carried out in accordance with the guidelines for the care and use of laboratory animals approved by the Animal Care Committee of the University of L’Aquila. Mice treatments were performed as previously described [14]. Briefly mice received vehicle, crocetin (CRO + CPM, daily administration, 100 mg/kg by gastric gavage) or AS101 (AS101+ CPM, 10 μg per mouse, by intraperitoneal injections on alternate days) for 15 d; then, mice were subjected to a single dose of CPM (100 mg/kg).
Mating protocols
In order to avoid the risk of CPM-related foetal malformations [8], 12 weeks after CPM, female mice from each experimental group were mated with untreated proven fertile males (two females to one male) for 1 week. Then, the females were separated for the duration of pregnancy (21 d) until 3 weeks after the birth of the litter. The mean number of pups (F1) per mouse was counted in all experimental groups and weight were determined at birth, weaning (day 21) and at 2 months of age. At this age F1 females were mated and F2 was generated. The females were separated for the duration of pregnancy (21 d) until 3 weeks after the birth of the litter. The mean number of pups (F2) per mouse was counted in all experimental groups and weight was determined at birth, weaning and at 2 months of age.
Oocyte collection
At the age of 21 d, 10–15 F1 and F2 female mice of each experimental group were sacrificed for oocyte retrieval. Briefly, ovaries were excised and GV oocytes were obtained by puncturing large antral follicles in M2 medium (Sigma). Pools of at least 200 healthy GV oocytes were collected and processed for DNA extraction.
Oocyte DNA extraction and bisulfite pyrosequencing
DNA was extracted from oocyte pools for each condition by using the NucleoSpin Tissue kit (Macherey-Nagel, Düren, Germany), following the manufacturer’s instructions. DNA quantity and quality were assessed by Qubit 2.0 (ThermoFisher Scientific, Waltham MA, USA).
500 ng of DNA was bisulfite-converted using the EpiJET Bisulfite Conversion Kit (ThermoFisher Scientific, Waltham, MA, USA). PCR amplification of H19, Peg3 and Igf2r promoters was performed as previously reported [29,30]. PCR mix included KAPA Hi-Fi Uracil Mix (Kapa Biosystem), 0.3 µM of each primer, 3 µL of converted-DNA and nuclease-free water to a final volume of 50 µL. Pyrosequencing reaction was run on a PyroMark Q96ID (Qiagen) and CpGs methylation analysis was conducted by the PyroMark CpG software (Qiagen).
A triplicate was generated for each PCR using bisulfite-converted DNA from three different conversion reactions. The methylation for each amplicon was calculated as the median of methylation status of each analyzed CpG. Differences in methylation pattern across samples and controls were calculated by ANOVA test, considering significant p-Values <0.05.
Author contributions
Conceptualization: V.G., C.T.; Experiments: G.D.E., M.D.A., M.P., S.F., G.R.; Data analysis: G.D.E., M.D.A.; Writing – Editing: V.G., C.T., G.D.E., M.D.A.; Review: G.P.A., L.S.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Siegel RL, Miller KD, Jemal A.. Cancer statistics. 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Logan S, Perz J, Ussher JM, et al. Systematic review of fertility-related psychological distress in cancer patients: informing on an improved model of care. Psychooncology. 2019;28:22–30. [DOI] [PubMed] [Google Scholar]
- [3].Anazodo A, Laws P, Logan S, et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update. 2019;25:159–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tatone C, Amicarelli F, Carbone MC, et al. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14:131–142. [DOI] [PubMed] [Google Scholar]
- [5].Roness H, Kashi O, Meirow D. Prevention of chemotherapy-induced ovarian damage. Fertil Steril. 2016;105:20–29. [DOI] [PubMed] [Google Scholar]
- [6].Bedoschi GM, Navarro PA, Oktay KH. Novel insights into the pathophysiology of chemotherapy-induced damage to the ovary. Panminerva Med. 2019;61:68–75. [DOI] [PubMed] [Google Scholar]
- [7].Leroy C, Rigot JM, Leroy M, et al. Immunosuppressive drugs and fertility. Orphanet J Rare Dis. 2015;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Meirow D, Epstein M, Lewis H, et al. Administration of cyclophosphamide at different stages of follicular maturation in mice: effects on reproductive performance and fetal malformations. Hum Reprod. 2001;16:632–637. [DOI] [PubMed] [Google Scholar]
- [9].Arnon J, Meirow D, Lewis-Roness H, et al. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum Reprod Update. 2001;7:394–403. [DOI] [PubMed] [Google Scholar]
- [10].Lucifero D, Mertineit C, Clarke HJ, et al. Methylation dynamics of imprinted genes in mouse germ cells. Genomics. 2002;79:530–538. [DOI] [PubMed] [Google Scholar]
- [11].Hanna CW, Demond H, Kelsey G. Epigenetic regulation in development: is the mouse a good model for the human? Hum Reprod Update. 2018;24:556–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Song Z, Min L, Pan Q, et al. Maternal imprinting during mouse oocyte growth in vivo and in vitro. Biochem Biophys Res Commun. 2009;387:800–805. [DOI] [PubMed] [Google Scholar]
- [13].Mendonça Ados S, Guimarães AL, Da Silva NM, et al. Characterization of the IGF2 imprinted gene methylation status in bovine oocytes during folliculogenesis. PLoS One. 2015;10:e0142072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Di Emidio G, Rossi G, Bonomo I, et al. The natural carotenoid crocetin and the synthetic tellurium compound AS101 protect the ovary against cyclophosphamide by modulating SIRT1 and mitochondrial markers. Oxid Med Cell Longev. 2017;2017:8928604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jeelani R, Khan SN, Shaeib F, et al. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic Biol Med. 2017;110:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Okae H, Chiba H, Hiura H, et al. Genome-wide analysis of DNA methylation dynamics during early human development. PLoS Genet. 2014;10:e1004868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O’Doherty AM, O’Shea LC, Fair T. Bovine DNA methylation imprints are established in an oocyte size-specific manner, which are coordinated with the expression of the DNMT3 family proteins. Biol Reprod. 2012;86:67. [DOI] [PubMed] [Google Scholar]
- [18].Bourc’his D, Xu GL, Lin CS, et al. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294:2536–2539. [DOI] [PubMed] [Google Scholar]
- [19].Kaneda M, Okano M, Hata K, et al. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429:900–903. [DOI] [PubMed] [Google Scholar]
- [20].Hata K, Okano M, Lei H, et al. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 2002;129:1983–1993. [DOI] [PubMed] [Google Scholar]
- [21].Menezo YJ, Silvestris E, Dale B, et al. Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reprod Biomed Online. 2016;33:668–683. [DOI] [PubMed] [Google Scholar]
- [22].Küpers LK, Monnereau C, Sharp GC, et al. Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight. Nat Commun. 2019;10:1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hoyo C, Daltveit AK, Iversen E, et al. Erythrocyte folate concentrations, CpG methylation at genomically imprinted domains, and birth weight in a multiethnic newborn cohort. Epigenetics. 2014;9:1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fortier AL, McGraw S, Lopes FL, et al. Modulation of imprinted gene expression following superovulation. Mol Cell Endocrinol. 2014;388:51–57. [DOI] [PubMed] [Google Scholar]
- [25].Fauque P, Ripoche MA, Tost J, et al. Modulation of imprinted gene network in placenta results in normal development of in vitro manipulated mouse embryos. Hum Mol Genet. 2010;19:1779–1790. [DOI] [PubMed] [Google Scholar]
- [26].Gabory A, Ripoche MA, Le Digarcher A, et al. H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. Development. 2009;136:3413–3421. [DOI] [PubMed] [Google Scholar]
- [27].van Otterdijk SD, Michels KB. Transgenerational epigenetic inheritance in mammals: how good is the evidence? Faseb J. 2016;30:2457–2465. [DOI] [PubMed] [Google Scholar]
- [28].Leroux S, Gourichon D, Leterrier C, et al. Embryonic environment and transgenerational effects in quail. 9114088. 2017;49:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stouder C, Paoloni-Giacobino A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction. 2010;139:373–379. [DOI] [PubMed] [Google Scholar]
- [30].Faulk C, Barks A, Liu K, et al. Early-life lead exposure results in dose- and sex-specific effects on weight and epigenetic gene regulation in weanling mice. Epigenomics. 2013;5:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]


