ABSTRACT
Invasive meningococcal disease (IMD), a rapidly progressing and potentially fatal illness, disproportionately affects adolescents and young adults. While IMD is best prevented by vaccination, vaccine uptake in these groups is low. An evidence-based understanding of the safety and effectiveness of concomitant vaccination of meningococcal vaccines, including the newer MenB protein vaccines and the more established MenACWY conjugate vaccines, with other vaccines recommended for adolescents and young adults may help maximize vaccination opportunities. We identified 21 studies assessing concomitant administration of meningococcal vaccines with other vaccines in adolescents and adults. Although studies varied in methodology, concomitant administration generally did not affect immunogenicity of the meningococcal or coadministered vaccines. In some cases, reactogenicity increased following concomitant administration, but no definitive safety concerns were raised. In general, data suggest that meningococcal vaccines can be safely and effectively coadministered with other vaccines.
KEYWORDS: Invasive meningococcal disease, concomitant, vaccination, MenB-FHbp, adolescents, adults
Introduction
Invasive meningococcal disease (IMD) is characterized by sudden onset, rapid progression, and high case fatality rates (~10% or more) despite appropriate medical care.1-3 About 20% of surviving patients experience long-term sequelae, such as limb amputation or hearing loss.4 Most IMD is caused by Neisseria meningitidis serogroups A, B, C, W, Y, and X.5 In adolescents and young adults in particular, serogroup B causes the majority of IMD in the United States and Europe compared with the other individual serogroups.6,7 Vaccination remains the most effective method for preventing IMD.
In the United States, despite current recommendations, vaccination adherence is low among adolescents,8,9 which may be due in part to their relatively fewer preventive healthcare visits.10 Simultaneous administration of multiple vaccines has been shown to improve immunization rates11,12 by decreasing the number of healthcare provider visits and associated logistic challenges. As part of national immunization programs, meningococcal vaccines may be administered around the same time as other vaccines, including yearly influenza; human papilloma virus (HPV); tetanus, diphtheria, and/or acellular pertussis (Tdap); hepatitis; and varicella vaccines.13-22
Although concomitant administration is essential to improving vaccination rates, it is critical that this approach be guided by available safety and immunogenicity data. Immune interference, which is the relative enhancement or suppression of immune responses when vaccines are given at the same time as opposed to separately,23 is a key concern. Interference may be related either to the vaccine formulation or to vaccine antigens and the immune response they elicit.24-27 Studies evaluating safety and immunogenicity data for a given vaccine administered concomitantly with other vaccines thus address an important informational need. Such studies are usually required for licensure by regulatory bodies across the globe and provide important data to support policy decisions on incorporation of a vaccine into an established national immunization schedule.
Currently available meningococcal conjugate vaccines include quadrivalent vaccines targeting serogroups A, C, W, and Y (MenACWY vaccines) which contain capsular polysaccharide antigens conjugated to different carrier proteins such as diphtheria toxoid (D), tetanus toxoid (TT), or CRM197.28-30 Available monovalent vaccines contain capsular polysaccharide antigens from serogroups A or C conjugated to TT or CRM197.31-34 Two combination vaccines target Haemophilus influenzae type b (Hib) and N meningitidis: Hib and MenC polysaccharides conjugated to TT; and Hib, MenC, and MenY polysaccharides conjugated to TT.35,36
A number of countries have MenACWY vaccination recommendations for infants or toddlers.37-39 Recommendations in EU countries typically target infants or toddlers for MenC vaccination and adolescents for MenACWY vaccination.18 In the United States, the Advisory Committee on Immunization Practices (ACIP) recommends universal routine administration of a single dose of a MenACWY conjugate vaccine at age 11 to 12 years and a booster dose at age 16 years;19 individuals of all ages at increased risk of meningococcal disease, other than young infants, are also recommended to receive MenACWY vaccines.40 Effectiveness studies indicate that the widespread use of meningococcal conjugate vaccines has led to substantial decreases in meningococcal disease incidence.41,42
Among approved MenB vaccines, MenB-FHbp (Trumenba®, bivalent rLP2086; Pfizer Inc, Collegeville, PA, USA) contains 2 recombinant lipidated factor H binding proteins (FHbp), one from each subfamily, A and B.43 MenB-4C (Bexsero®, 4CMenB; GSK Vaccines, Srl., Siena, Italy) includes 3 meningococcal proteins: FHbp from subfamily B, Neisserial adhesin A (NadA), and Neisserial Heparin Binding Antigen (NHBA), in addition to outer membrane vesicles that contain the New Zealand outbreak strain PorA subtype P1.4.44 Age groups at which MenB immunization is recommended outside of the United States vary by country, and immunization may be publicly funded or simply recommended without public funding.18,45 In the United States, the ACIP recommends routine vaccination with either MenB vaccine for individuals aged 10 years and older at higher risk for meningococcal disease.46 The ACIP additionally recommends that MenB vaccination be considered for all individuals aged 16 through 23 years (16 through 18 years preferred).47,48
This review focuses on the immunogenicity and safety of concomitant vaccines administered with the recently licensed MenB vaccines in adolescents and young adults, the ages for which these vaccines are licensed in the United States.43,44 These findings are compared with available information regarding concomitant administration of previously licensed MenACWY and other non-MenB meningococcal vaccines.
Studies investigating concomitant administration of meningococcal vaccines with other vaccines were identified using the following search string in PubMed (https://www.ncbi.nlm.nih.gov/pubmed) and Ovid MEDLINE: meningococcal AND (concomitant OR simultaneous OR coadminis*) AND (vaccin* OR immuniz*). Additional studies were identified from the most recently available package inserts of meningococcal vaccines and from review articles identified by the Ovid MEDLINE search. Studies were excluded if they did not primarily assess concomitant administration of a meningococcal vaccine with another licensed vaccine or vaccines in adolescents and young adults (studies with subjects 10 through 25 years of age, with the study population described as “adolescents” or “young adults” or “boys/girls”) or adults (any studies including subjects >25 years of age). Studies were divided into these 2 age groups for analysis. Immunogenicity of administered vaccines as well as overall safety was evaluated. The findings are presented in 3 sections for each age group (adolescents/young adults and adults): concomitant administration of meningococcal protein with meningococcal conjugate vaccines, concomitant administration of meningococcal protein with non-meningococcal vaccines, and concomitant administration of meningococcal conjugate and non-meningococcal vaccines.
Results
Figure 1 depicts the flow of citations identified for inclusion in this review. Of the 23 articles collectively evaluated for inclusion, 2 presented results from the same study, with one focusing on immunogenicity and one on safety;49,50 data from both publications were used as needed. One study was excluded because the concomitant vaccines differed from subject to subject rather than being consistent across all subjects;51 2 arms of another study were excluded for the same reason.52 In total, 21 studies were included in this review.
Figure 1.
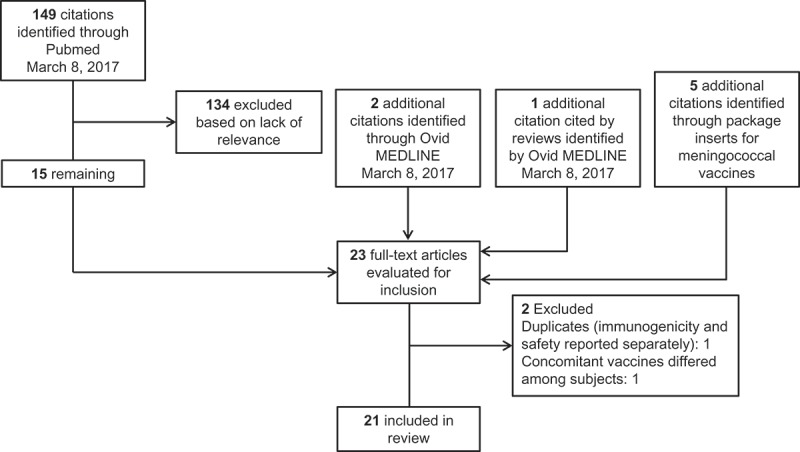
Selection of articles for inclusion.
Meningococcal vaccine immune responses are reported as titers from serum bactericidal assays using human (hSBA) or rabbit (rSBA) complement. Use of rSBA for serogroup A and C vaccines has been specified by the World Health Organization since 1976 on account of rabbit complement being easier to standardize and more easily available in large quantities as compared with hSBA.53,54 Postlicensure evaluation of MenC vaccine effectiveness has correlated with proportions of individuals with rSBA titers ≥1:8; rSBA titers ≥1:8 are therefore considered protective.55,56 For hSBA, titers ≥1:4 or ≥ 4-fold rises in titers from baseline levels are accepted as serologic correlates of protection based on original experiments performed in 1969 indicating that protection from IMD correlated with baseline hSBA titers ≥1:4.55,57,58 Of note, rabbit complement does not bind meningococcal FHbp, resulting in higher titers than those measured with hSBA.59,60 Licensure of MenACWY vaccines containing capsular polysaccharide antigens has generally relied on either or both hSBA and rSBA results.28-30 For MenB vaccines, including those containing protein antigens such as FHbp, humans are considered the only acceptable source of complement, and clinical studies for newer MenB vaccines have therefore used hSBA.43,44,61
Critical parameters such as subject ages, vaccines administered, and immune response assessments varied substantially across studies. No meta-analyses were undertaken on account of this heterogeneity.
Adolescent and young adult studies on concomitant administration
Table 1 summarizes the 12 studies assessing concomitant administration of meningococcal vaccines in adolescents and young adults (individuals 10–25 years of age).49,62-72 The following sections focus on potential changes to immune responses to vaccines when administered together with meningococcal vaccines. Most of the adolescent studies that compared immune responses to the meningococcal vaccine under individual and concomitant administration did not find any decreases in meningococcal immune responses under concomitant administration.62-65,67,70-72 Similarly, safety was generally comparable across groups in each of the adolescent studies when assessed.50,62-72 Exceptions to both of these general observations are specified where applicable.
Table 1.
Meningococcal concomitant vaccine study results in adolescents and young adults.
| Meningococcal vaccine class |
|||
|---|---|---|---|
| MenACWY (quadrivalent conjugate) | MenB | MenC (monovalent conjugate) | |
| Licensed vaccines included | MenACWY-CRM (Menveo) MenACWY-D (Menactra) MenACWY-TT (Nimenrix) |
MenB-FHbp (Trumenba) | MenC-TT (NeisVac-C) MenC-CRM (Meningitec, Menjugate) |
| Subject age range, y | 10–25 | 10–18 | 13–18 |
| Concomitant vaccine | Reduced immunogenicity of concomitant vaccinea | ||
| Tetanus/low-dose diphtheria (Aventis Pasteur) |
No68,b,c,d | – | No49,50,e,f,g |
| Tdap (Boostrix, Adacel) | Yes62,h,i,j Yes63,k,l No67,m,n No69,o,p N/A71,q,r,s N/A72,q,r |
No64,t,u | – |
| Tdap/IPV (Repevax) | – | No66,v,w,x | – |
| HPV2 (Cervarix) | No67,m,n | – | – |
| HPV4 (Gardasil) | No62,h,i,j No71,q,r,s N/A69,o,p |
Yes65,y,z | – |
| HPV9 (Gardasil9) | No72,q,r | – | – |
| MenACWY-D (Menactra) | – | No64,t,u | – |
| Hepatitis A/B (Twinrix) | No70 | ||
CRM = diphtheria toxin, CRM mutant; D = diphtheria toxoid; DT = diphtheria tetanus vaccine; FHA = filamentous hemagglutinin; GMC = geometric mean concentration; GMT = geometric mean titer; HPV = human papilloma virus; IPV = inactivated poliovirus vaccine; MenACWY = meningococcal serogroups A, C, W, Y; MenB = meningococcal serogroup B; MenB-FHbp = bivalent rLP2086 (Trumenba); PRN = pertactin; PT = pertussis toxoid; rSBA = serum bactericidal assay using rabbit complement; Td = tetanus/low-dose diphtheria vaccine; Tdap = tetanus, diphtheria, and acellular pertussis vaccine; TT = tetanus toxoid.
aNo = no decrease on immune responses of coadministered vaccine when administered concomitantly with a meningococcal vaccine; Yes = decrease detected; N/A = not applicable either because the concomitant vaccine was always administered together with meningococcal vaccine or because the immune responses to the concomitant vaccine were not assessed; footnotes indicate (1) important details regarding vaccination sequence, (2) any detected changes in meningococcal vaccine immunogenicity under concomitant administration (no footnote in cases of no change in meningococcal vaccine immunogenicity), (3) any findings regarding immune response dependence on vaccine schedule for studies in which no control group was included, and (4) notable findings regarding safety differences observed between groups.
bSubjects who received MenACWY-D and Td concomitantly had a higher anti-diphtheria immune response than subjects who received the vaccines sequentially.
cOverall rate of systemic adverse events was higher in the concomitant group.
drSBA titer increases for meningococcal serogroups C, W, and Y were higher when MenACWY-D was administered concomitantly with Td compared with sequential administration.
eAmong adolescents, systemic symptoms within 3 days were highest in the concomitant group.
fMeningococcal immune responses were not presented specifically for the adolescent age group. When 3.5–6 year and 13–18 year age groups were analyzed together, the immune response to MenC-TT was found to be slightly reduced with concomitant administration of DT/Td.
gHigher anti-tetanus responses were observed for concomitant administration with MenC-TT and higher anti-diphtheria responses were observed for concomitant administration with MenC-CRM.
hTdap and HPV4 administered together for concomitant administration with MenACWY; all 3 vaccines administered in sequence under 2 different schedules otherwise.
iNoninferiority not achieved for GMCs of FHA and PRN pertussis antigens under concomitant administration; otherwise, it was achieved for all antigens tested.
jLocal reactions and systemic events were slightly higher under concomitant vaccine administration.
kNoninferiority not achieved for proportions of subjects achieving ≥ 4-fold increases in antibody concentrations for PRN and PT pertussis antigens under concomitant administration; the concomitant group had a higher proportion of subjects achieving anti-diphtheria antibody titers ≥1.0 IU/mL and higher GMCs than the Tdap-alone group; immunogenicity otherwise was similar across groups for all antigens tested.
lProportions of subjects reporting local or systemic reactions were higher in the Tdap groups than the group receiving MenACWY alone.
mVarious sequential and concomitant administration schedules using different combinations of MenACWY, Tdap, and HPV2 were assessed, including concomitant administration with all 3 vaccines.
nMyalgia was reported by fewer participants receiving MenACWY followed by HPV2 than those receiving Tdap followed by HPV2 or the concomitant administration of all 3 vaccines.
oSubjects received concomitant MenACWY-CRM + Tdap + HPV or saline placebo + Tdap + HPV.
pRates of solicited systemic reactions were higher in the MenACWY-CRM + Tdap + HPV group than in the placebo + Tdap + HPV group.
qTdap and HPV were administered together for concomitant administration with MenACWY; Tdap + MenACWY were administered separately from HPV otherwise.
rHPV injection site swelling was more frequent under concomitant administration than separate administration.
sMenACWY + Tdap injection site bruising and pain were more frequent under concomitant administration than separate administration.
tTdap and MenACWY were administered concomitantly whenever given.
uLocal reactions and systemic events were generally similar across MenB-FHbp groups and higher than in the Tdap + MenACWY-alone group.
vLocal reactions, fever ≥38.0°C, headache (doses 2 and 3), and fatigue (doses 2 and 3) were reported by a higher proportion of participants receiving MenB-FHbp with Tdap/IPV than Tdap/IPV alone.
wAmong groups for which meningococcal immune responses were assessed, only 1 group was given MenB-FHbp.
xMeningococcal immune responses were not compared between different groups receiving the meningococcal vaccine.
yNoninferiority was not achieved for HPV-18 GMTs; otherwise, it was achieved for all antigens tested.
zLocal reactions and systemic events were generally similar and were higher in MenB-FHbp groups than the HPV4-alone group.
Meningococcal protein vaccines with meningococcal conjugate vaccines
One study examined concomitant administration of MenB-FHbp with Tdap and MenACWY-D compared to individual administration of either MenB-FHbp or Tdap and MenACWY-D vaccines; for this analysis, MenB-FHbp was considered to be the meningococcal vaccine and MenACWY-D and Tdap were considered to be the concomitant vaccines.64 Noninferiority criteria were met for all 6 Tdap antigens and all 4 MenACWY strains tested.
Safety assessments for this study indicated that local reactions and systemic events were more frequent when MenB-FHbp was administered, regardless of concomitant administration.64 Most local reactions and systemic events were mild or moderate in severity, and none of the infrequently reported serious adverse events (AEs) were considered vaccine-related.64
Meningococcal protein vaccines with non-meningococcal vaccines
Two studies evaluated concomitant administration of MenB-FHbp with non-meningococcal vaccines. In one study, concomitant administration of MenB-FHbp with Tdap and inactivated poliovirus vaccines (Tdap/IPV) was compared with individual administration of Tdap/IPV; noninferiority criteria were met for all 9 Tdap/IPV antigens under concomitant administration.66 An additional study assessed concomitant administration of MenB-FHbp with a 4-valent HPV vaccine (HPV4) compared with individual administration of either vaccine.65 Prespecified noninferiority criteria were that the lower limit of the 2-sided 95% CI of the geometric mean titer (GMT) ratio be >0.67 at 1 month after the third HPV vaccination; this was met for 3 HPV types (6, 11, and 16). For HPV-18, the lower bound of the 95% CI for the GMT ratio was 0.62. However, GMTs for HPV-18 and seroconversion rates for all 4 antigens (≥99%) were similar or even superior to those in the largest HPV4 vaccine licensure studies; importantly, the assays used to measure HPV immunogenicity were the same across studies.65,73-75 No studies evaluating concomitant administration of MenB-4C with any other vaccines administered to adolescents and/or young adults were identified by the literature search or in the US package insert.44
Similar to observations for the study evaluating concomitant administration of MenB-FHbp with MenACWY-D and Tdap,64 concomitant administration of MenB-FHbp with non-meningococcal vaccines was associated with increased reactogenicity; however, the frequencies of reactions were similar to those observed with individual administration of MenB-FHbp.65,66
Meningococcal conjugate vaccines with non-meningococcal vaccines
Concomitant administration studies of MenACWY vaccines in adolescents and young adults, which mostly considered concomitant administration with Tdap and/or HPV vaccines, generally showed no effect on immune responses; however, some individual antigen responses in concomitant vaccines did not achieve prespecified noninferiority criteria. Considering the effects of concomitant administration on Tdap antigens, one study in which MenACWY-CRM and Tdap were concomitantly administered with HPV4 demonstrated that noninferiority was achieved for GMTs or geometric mean concentrations (GMCs) of 3 of 5 Tdap antigens but not for filamentous hemagglutinin (FHA) and pertactin (PRN) pertussis antigens.62 In another MenACWY-CRM study that did not include HPV4, noninferiority was achieved for all Tdap antigens other than PRN and pertussis toxoid (PT).63 Importantly, noninferiority evaluations for pertussis differed between these 2 studies, which were respectively based on the ratio of GMCs and the proportions of subjects achieving a ≥ 4-fold increase in antibody titers over preimmunization baselines. A study that compared concomitant and sequential administration of MenACWY-D and tetanus/low-dose diphtheria (Td) vaccine found considerably greater immune responses to diphtheria in the concomitant group, while responses to tetanus were similar between groups.68 This study additionally found that rSBA titer increases for meningococcal serogroups C, W, and Y were higher when MenACWY-D was administered concomitantly with Td compared with sequential administration. Another study, which evaluated several schedules of various combinations of HPV4, Tdap, and MenACWY-D, found no significant differences in Tdap immunogenicity when Tdap was administered concomitantly with the MenACWY-D and HPV4 vaccines.67 Similarly, in a study that compared coadministration of MenACWY-CRM and Tdap/HPV4 versus a saline placebo and Tdap/HPV4, all non-inferiority criteria for immunologic responses against Tdap antigens were met.69
Several studies, some of which were discussed previously in the context of Tdap, assessed concomitant administration with MenACWY (either -CRM or -D) on the safety and immunogenicity of HPV vaccines, including HPV4,62,71 2-valent HPV (HPV2),67 or 9-valent HPV (HPV9)72 vaccines. Although these studies differed from one another in the comparator groups assessed, concomitant administration in all of the studies included administration of both vaccines simultaneously with Tdap. None of these studies, including the study discussed previously that did not demonstrate noninferiority for FHA and PRN, showed any effect of concomitant administration of MenACWY vaccines on HPV immune responses.
A single study evaluated concomitant MenACWY-TT and hepatitis A/B (HepA/B) vaccination. Results demonstrated that coadministration was immunologically noninferior to administration of either MenACWY-TT or HepA/B vaccine alone.70
One older study compared concomitant administration of any 1 of 3 different MenC vaccines with a Td booster in adolescents.49 This study found no evidence that concomitant MenC vaccine administration reduced antibody responses to tetanus or diphtheria toxoids; on the contrary, concomitant MenC-TT administration was associated with higher anti-tetanus responses, while concomitant MenC-CRM administration was associated with higher anti-diphtheria responses .49Additionally, in school-aged children and adolescents collectively, concomitant administration with MenC-TT was associated with slightly lower immunoglobulin G (IgG) GMCs or rSBA GMTs compared with administration before the tetanus/diphtheria vaccine.49
Similar to observations for the MenB-FHbp protein vaccine, one study evaluating concomitant administration of meningococcal conjugate vaccines with non-meningococcal vaccines found that local reactions and systemic events were reported more frequently when Tdap was administered, regardless of whether MenACWY-CRM was administered at the same time.63 Concomitant administration of MenACWY-D and Td was associated with an overall rate of systemic AEs that was marginally higher compared with Td alone, whereas similar frequencies of local reactions were observed between groups.68
Two different studies assessing concomitant administration of MenACWY-D with HPV vaccines found increased HPV vaccine injection site swelling with concomitant administration;71,72 one of these studies also found that bruising and pain were more frequent at the MenACWY-D and Tdap injection site with concomitant administration of HPV4.71 Another study examining multiple schedules of MenACWY-D, Tdap, and HPV2 vaccines found that myalgia was less frequent when subjects were vaccinated with MenACWY-D followed by HPV2 compared with those given Tdap followed by HPV2 or all 3 vaccines simultaneously.67 A similar study with HPV4 found that local reactions and systemic events were slightly more frequent with concomitant administration than when vaccines were administered at separate visits.62 When coadministration of MenACWY-CRM and Tdap/HPV4 was compared with coadministration of a saline placebo and Tdap/HPV4, the overall reported rate of solicited systemic reactions was higher in the MenACWY-CRM/Tdap/HPV4 group; however, there were no statistically significant differences between groups in the frequencies of each systemic reaction.69
The one study examining concomitant administration with MenC vaccines found that systemic events were reported more frequently when MenC vaccines were administered concomitantly with tetanus/low-dose diphtheria vaccines in adolescents.50
Adult studies on concomitant administration
Nine concomitant meningococcal vaccine studies performed in adults (individuals >25 years of age) are summarized in Table 2.52,68,76-82 All of these studies also included individuals ≤25 years of age; however, results were not reported by age subgroup, and all adult studies lacked the focus on adolescents and/or young adults that characterized the studies described previously. As for the adolescent and young adult studies, safety findings from adult studies were generally unremarkable;68,76-82 exceptions are noted in the following sections where applicable.
Table 2.
Meningococcal concomitant vaccine study results in adults.
| Meningococcal vaccine class |
||||
|---|---|---|---|---|
| MenACWY (quadrivalent conjugate) | MenB | MenC (monovalent polysaccharide) | Other multivalent vaccines | |
| Licensed vaccines | MenACWY-D (Menactra) MenACWY-CRM (Menveo) MenACWY-TT (Nimenrix) |
MenB-4C (Bexsero) | MenC (Meningovax C) | MenAC (Menomune A/C) |
| Subject age range, y | 17–64 | 23–55 | 24–50 | 24–60 |
| Concomitant vaccine | Reduced immunogenicity of concomitant vaccinea | |||
| Tdap (Boostrix/not specified) | No80,b,c,d,e N/A52,f,g |
– | – | – |
| Typhoid fever (Typhim Vi) | No76,h,i,j No68 |
– | – | – |
| Yellow fever (Stamaril) | No76,h,i,j | – | – | – |
| Hepatitis A/B (Twinrix, Havrix, Engerix-B) | No78,k | – | – | – |
| Japanese encephalitis (IXIARO) | No77,l,m,n | – | – | – |
| Rabies (Rabipur) | No77m,n,° | – | – | – |
| Hib (Merck, not specified) | – | – | No79,e,p | No79,e,p |
| PCV13 (Prevnar 13) | N/A80,b,c,d,e | – | – | – |
| PPSV14 (Pneumovax) | – | – | N/A79,e,p | N/A79,e,p |
| MenACWY-CRM (Menveo) | – | N/A82,q,r,s | – | |
| Seasonal influenza (Fluarix) | N/A81,t,u,v | – | – | |
AE = adverse event; CRM = diphtheria toxin, CRM mutant; D = diphtheria toxoid; GMC = geometric mean concentration; GMT = geometric mean titer; Hib = Haemophilus influenzae type b; IgG = immunoglobulin G; hSBA = serum bactericidal assay with human complement; JEV = Japanese encephalitis vaccine; MenACWY = meningococcal serogroups A, C, W, Y; MenB = meningococcal serogroup B; OPA = opsonophagocytic activity; PCEVC = rabies purified chick embryo cell-culture vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine; PPSV14 = 14-valent pneumococcal polysaccharide vaccine; rSBA = serum bactericidal assay with rabbit complement; Tdap = tetanus, diphtheria, and acellular pertussis vaccine; TF = typhoid fever; YF = yellow fever.
aNo = no decrease on immune responses of coadministered vaccine when administered comcomitantly with a meningococcal vaccine; Yes = decrease detected; N/A = not applicable either because the concomitant vaccine was always administered together with the meningococcal vaccine or because the immune responses to the concomitant vaccine were not assessed; footnotes indicate (1) important details regarding vaccination sequence, (2) any detected changes in meningococcal vaccine immunogenicity under concomitant administration (no footnote in cases of no change in meningococcal vaccine immunogenicity), (3) any findings regarding immune response dependence on vaccine schedule for studies in which no control group was included, and (4) notable findings regarding safety differences observed between groups.
bTdap administered either before, at the same time as, or after MenACWY + PCV13; all 3 vaccines were administered at separate sites when given concomitantly, with MenACWY and PCV13 given at separate sites of the right deltoid.
c≥ 4-fold rises in OPA titers and/or GMTs significantly lower for some PCV13 serotypes when PCV13 + MenACWY was administered after Tdap compared with before.
dLocal reactions were least frequent at the MenACWY and Tdap injection sites when given at different time points; systemic AEs were lowest when Tdap was given alone, followed by MenACWY + PCV13.
eMeningococcal immune responses were not assessed.
fSubjects receiving MenACWY + Tdap had higher IgG GMCs and rSBA GMTs for serogroups C and Y than those receiving MenACWY alone; Tdap immunogenicity was not evaluated.
gSafety was not evaluated.
hTF and YF vaccines were administered concomitantly whenever given.
iSerogroup W GMTs and seroresponse rates were significantly higher under concomitant administration.
jFewer AEs were reported by subjects receiving MenACWY than those in the TF + YF vaccine groups.
kHepatitis A/B booster vaccination depended on the previous vaccination history of a given subject.
lJEV was administered with PCECV for all groups.
mNoninferiority was not achieved for serogroup A GMTs under concomitant administration, but proportions of subjects with seroresponse or achieving hSBA titers ≥1:8 were similar across groups.
nSubjects receiving MenACWY alone reported fewer AEs than those receiving JEV and/or PCECV (with or without MenACWY), but AEs were collected for a shorter period in the MenACWY-alone group.
oPCECV was administered with JEV for concomitant administration with MenACWY, and either with or without JEV when MenACWY was not administered.
pHib and PPSV14 were administered together for concomitant administration with meningococcal vaccine; Hib was administered alone otherwise; PPSV14 immunogenicity was not evaluated.
qOnly coadministration of MenACWY-CRM and MenB-4C (separate sites) was evaluated; additional doses of MenB-4C were given later.
rPost-vaccination, 95%–100% and 90%–100% of subjects had protective SBA titers against A, C, W, Y and diverse MenB strains, respectively.
sSystemic reactions of nausea and headache were greatest after concomitant administration.
tSubjects were randomized to receive MenACWY-TT plus seasonal influenza vaccine, MenACWY-TT alone, or MPSV4 alone.
uThe MenACWY-TT plus seasonal influenza vaccine group met all pre-defined criteria for antibody responses against all influenza antigens evaluated.
vNon-inferiority criteria for the coadministration group compared with the MenACWY-TT-only group were met for meningococcal serogroups A, W, and Y but not for C.
Meningococcal protein vaccines with meningococcal conjugate vaccines
A small single-arm study assessed the coadministration of MenB-4C (3 doses given at months 0, 2, and 6) and MenACWY-CRM (1 dose given at month 0) in laboratory workers.82 Of note, MenB-4C is generally administered as 2 doses given ≥1 month apart.44 Although the first respective doses of MenB-4C and MenACWY-CRM were administered together, there was no comparator group included in this analysis and therefore immune interference was not systematically assessed. High rates of baseline immunity were observed, which increased following vaccination; the highest rSBA GMTs for groups A, C, and Y were measured at month 3 (ie, 3 months after ACWY-CRM administration and 1 month after MenB-4C dose 2).82
Safety findings from this study indicated higher rates of nausea and headache when MenACWY-CRM and MenB-4C were administered concomitantly than when MenB-4C was administered alone at subsequent vaccination visits.82
Meningococcal conjugate vaccines with non-meningococcal vaccines
Other adult studies involved concomitant administration of MenACWY vaccines with non-meningococcal vaccines, including 2 studies with Tdap. One study performed in prospective Hajj pilgrims assessed the administration of MenACWY-CRM and 13-valent pneumococcal conjugate vaccine (PCV13) before, concomitantly with, or after Tdap and found no effect on tetanus and diphtheria immunogenicity; however, responses to some PCV13 serotypes were lower when Tdap was administered before MenACWY-CRM and PCV13.80 Another study assessing concomitant administration of MenACWY-D and Tdap was performed in military recruits and did not evaluate Tdap immunogenicity.52 However, concomitant administration resulted in significantly higher meningococcal serogroup C and Y (the only serogroups tested) IgG GMCs and GMTs in rSBA compared with administration of MenACWY-D alone.
Immune responses to specific travel vaccines under concomitant administration with MenACWY-CRM were evaluated in several studies. Results generally demonstrated that there was no effect on immune responses of the concomitant vaccines, but immune responses to meningococcal vaccines were sometimes altered under concomitant administration. One study found that concomitant administration of MenACWY-CRM with Japanese encephalitis vaccine (JEV) and rabies purified chick embryo cell-culture vaccine (PCECV) had no effect on immune responses to JEV and PCECV. On the other hand, meningococcal GMTs of meningococcal serogroup A antibodies were lower with concomitant administration; however, proportions of subjects achieving human complement SBA titers ≥1:8 (above the accepted correlate of protection57) were similar across groups.77 A second study evaluating the concomitant use of MenACWY-D and typhoid fever (TF) vaccine found that immune responses to the vaccines administered concomitantly were comparable to those observed when either vaccine was given alone.68 A third study found that concomitant administration of MenACWY-CRM with TF and yellow fever (YF) vaccine did not affect immune responses to the TF and YF vaccines when compared to those receiving the TF and YF vaccines alone; interestingly, meningococcal serogroup W antibody GMTs and seroresponse rates were significantly higher under concomitant administration.76 Finally, a fourth study showed that concomitant administration of MenACWY-CRM with a HepA/B vaccine elicited noninferior immune responses to either HepA/B or MenACWY compared with individual administration of either vaccine.78
Two studies evaluated concomitant administration of meningococcal vaccines with routinely administered adult vaccines. One study evaluating the immunogenicity and safety of concomitant MenACWY-TT and the seasonal influenza vaccine administration found that all pre-defined immunogenicity criteria for influenza antigens were met in the concomitant group; the concomitant group also met non-inferiority criteria for meningococcal serogroups A, W, and Y but not for serogroup C.81 Additionally, a small study completed more than 30 years ago evaluated concomitant administration of either a MenC or MenAC polysaccharide vaccine with a Hib vaccine and a previously used 14-valent pneumococcal polysaccharide vaccine (PPSV14), and found no effect on Hib immunogenicity. Immune responses to PPSV14 or the meningococcal vaccines were not assessed.79
When MenACWY-CRM and PCV13 were administered separately from or together with Tdap, systemic AEs were lowest after Tdap vaccination, followed by MenACWY-CRM and PCV13, and highest with the concomitant administration of all 3 vaccines.80 Additionally, the study assessing concomitant administration of MenACWY-CRM with TF and YF vaccines found that AEs were more frequent under TF and YF administration, regardless of concomitant administration with MenACWY-CRM, compared with administration of MenACWY-CRM alone.76 Similarly, fewer subjects reported AEs when receiving MenACWY-CRM alone compared with those receiving JEV and/or PCECV (with or without MenACWY-CRM); however, of note, subjects in the former group were followed for a shorter period than those in the other groups.77
Discussion
We reviewed current knowledge regarding immune responses to vaccines concomitantly administered with meningococcal vaccines in the adolescent and adult populations. A literature search identified multiple published studies that evaluated the use of newer MenB protein vaccines and the more established MenACWY conjugate vaccines, as well as several other less frequently used meningococcal vaccines. For the MenB protein vaccines, among adolescents, the search uncovered concomitant use studies for the MenB-FHbp vaccine. Although outside the scope of this review, studies in infants have evaluated the concomitant use of MenB-4C with routinely administered pediatric vaccines, including diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated polio, and conjugate Hib and pneumococcal vaccines.83,84 In one study, although concomitant administration of MenB-4C with these vaccines compared with intercalated administration achieved prespecified noninferiority criteria, concomitant administration elicited notably lower percentages of subjects with hSBA titers ≥1:5.83 In a second, similarly designed study, immune responses to these vaccines were generally unaffected by concomitant administration with MenB-4C, with the exception of IPV, for which responses were somewhat lower; reactogenicity was notably increased when MenB-4C was given together with the other vaccines.84 For adolescents and young adults, the concomitant vaccines studied, notably Tdap and HPV, reflected the vaccination schedules prevalent for healthy individuals in this age group as a whole,13-19 whereas studies in adults reflected vaccines that may be recommended for individuals at elevated risk due to medical or circumstantial conditions.20
Immune responses to meningococcal vaccines generally remained similar to individual administration when administered concomitantly.62-65,67,68,70-72,78 A few studies noted either an increase52,68,76 or decrease49,77,81 when meningococcal vaccines were administered concomitantly with other vaccines when compared with individual administration. The remaining studies either did not assess meningococcal immune responses79,80 or did not compare these responses between different groups receiving the meningococcal vaccine.66,69,82
Studies involving both the MenB and MenACWY vaccines in adolescents and adults did not identify significant safety concerns. There were some reports of increased reactogenicity with concomitant administration compared with individual administration of each vaccine, but these did not appear to reflect an increase in severe or serious events or subsequent dosing.50,62,64–69,71,72,80,82 In 2 of the MenB-FHbp studies, local and/or systemic reactions under concomitant administration were noted to be similar to individual administration of MenB-FHbp.64,65
Most studies found that the administration of a meningococcal vaccine did not generally interfere with immune responses to the concomitant vaccine(s) under investigation, although study parameters varied and not all studies could be directly compared with one another. First, studies differed in how noninferiority was defined and whether or not it was formally assessed; this was true even when comparing 2 studies assessing the same concomitant vaccine. For example, Tdap responses for pertussis were assessed as the percentage of responders showing a 4-fold increase in pertussis antibody titers in one study with MenACWY-CRM63 and as GMCs of pertussis antibodies in another study involving MenB-FHbp and MenACWY-D.64 Second, noninferiority was generally defined as meeting prespecified criteria for all evaluated antigens of the concomitant vaccine studied. The number of antigens per vaccine tested varied across studies evaluating the same type of vaccine; for instance, in the aforementioned Tdap studies, one study evaluated 3 pertussis antigens,63 whereas the other evaluated 4 antigens64. Finally, comparing studies was also challenging because several multi-antigen vaccines were evaluated across broad age groups (10–25 years of age for adolescents and >25 years of age for adults), and vaccination schedules differed by study in terms of time points and sequence of vaccines administered.
Immune interference under concomitant administration may be associated with a number of different mechanisms. The study evaluating concomitant administration of different MenC vaccines with a Td booster in adolescents found that use of either a TT or CRM carrier protein led to higher tetanus or diphtheria responses, respectively, under concomitant administration,49 indicating potential enhancement of immune responses when the same toxoid is used in vaccines administered concomitantly.23 A similar hypothesis may apply to the enhanced responses to MenACWY-D observed in military recruits when the vaccine was administered concomitantly with Tdap.52 In cases where concomitant administration was associated with decreased immune responses, whether responses were lower could vary based on the method of measurement. For example, concomitant administration of MenACWY-CRM with JEV and PCECV was associated with lower serogroup A GMTs but not proportions of responders with hSBA titers ≥1:8.77 Furthermore, across studies, failure to meet noninferiority criteria generally occurred for a minority of the antigens evaluated (eg, only HPV-18 when MenB-FHbp was coadministered with HPV465). As such, understanding the mechanisms underlying these decreased responses may require further investigation.
From an implementation viewpoint, concomitant administration studies provide critical information for immunizers, in terms of clinical practice, and for vaccine technical committees, who evaluate the evidence and make recommendations to integrate new vaccines into national immunization schedules. In addition, data from concomitant vaccination studies help construct and support “immunization platforms,” which are primary care visits dedicated to the receipt of recommended age-appropriate vaccinations.85 These platforms catalyze discussions between parents, adolescents, and healthcare providers, help overcome vaccine hesitancy, and program the expectation of vaccination during the visit.
Immunization platforms are of particular significance for adolescents and young adults, as this subpopulation tends to have lower vaccination rates compared with infants.8,9,86 This may be due in part because individuals in this age group are generally healthy, have infrequent preventive care visits including fewer immunization-only visits, and because they are at an age where they are transitioning between care providers (ie, from pediatricians to adult care providers such as gynecologists and family practitioners).19,87 With the goal of improving adolescent vaccination rates in the United States, the Centers for Disease Control and Prevention established an 11- to 12-year-old immunization platform in 1996,88 and in 2017, a new 16-year-old immunization platform.19,89 Both age-based immunization platforms are now represented on the ACIP immunization schedule as shaded columns for 11 to 12 years and 16 years.19 At age 11 to 12 years, the ACIP recommends Tdap, HPV, and MenACWY vaccination, whereas at age 16 years, it recommends a booster dose of MenACWY and MenB vaccination, with the latter based on individual clinical decision-making in consultation with a provider.19 Catch-up immunizations should be evaluated at each interaction as well, such as a catch-up immunization for HPV at the 16-year-old platform visit. In this context, it is fitting that our literature search identified a number of concomitant vaccination studies conducted in adolescents and young adults who are at increased risk of IMD40 and for whom meningococcal vaccination is recommended in the United States and many European countries;18,19 we identified a number of additional studies in adults.
The ACIP recommended MenACWY vaccines in 200590 and MenB vaccines in 2015.47 Both MenB vaccines were recommended after the US Food and Drug Administration accelerated approval based on phase 2 data in the context of ongoing MenB disease outbreaks at 2 US colleges.46 At the time, the ACIP acknowledged the public health benefits of MenB vaccines but was awaiting more data on the breadth and persistence of protective coverage across diverse MenB disease causing strains;47 those data are now available and are published.48,91,92 The ACIP also evaluated the data from concomitant vaccination studies in adolescents and young adults.47 Importantly, MenB vaccines are compositionally distinct and therefore not interchangeable.19,20,43,44
There are some limitations of this review. Specifically, as detailed above, study parameters often differed, precluding direct comparison between studies; differences included whether and how noninferiority was evaluated, the number of antigens evaluated for a given vaccine, and differences in vaccination schedules and time points at which immune responses were assessed. Additionally, only published data or package inserts were included in this review, and it is possible that unpublished data or data on file from manufacturers may have been considered for developing recommendations for coadministration of particular vaccines.
Conclusions
Studies in adolescents and adults mostly showed that concomitant administration of meningococcal vaccines with various other vaccines did not negatively affect immune responses to either vaccine, although there were a few exceptions. Additionally, no overarching safety concerns were raised. Results from MenB-FHbp vaccine studies comparing concomitant with individual administration are similar to results from studies assessing concomitant administration with meningococcal capsular polysaccharide conjugate vaccines, supporting concomitant administration of MenB-FHbp with other vaccines recommended in adolescents and young adults. This information is particularly relevant for this age group, which has substantial gaps in vaccination coverage8,9 and for whom concomitant vaccine administration is a critical immunization strategy.11,12 Despite any potential vaccine interference, concomitant administration would nonetheless likely improve vaccine uptake in adolescents and young adults, populations that are hard to reach in vaccination campaigns. Additionally, as future vaccination recommendations may consider the use of a pentavalent MenABCWY vaccine,93 evaluating concomitant administration of MenB vaccines with MenACWY vaccines, as has been done for MenB-FHbp,64 will provide policymakers with added evidence of the safety and immunogenicity of a MenABCWY vaccine.
Funding Statement
This work was sponsored by Pfizer Inc.
Acknowledgments
Medical writing support was provided by Judith Kandel, PhD, at Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group company, and was funded by Pfizer Inc.
Disclosure of Potential Conflicts of Interest
All authors are employees of and may hold stock or stock options in Pfizer Inc.
References
- 1.Stein-Zamir C, Shoob H, Sokolov I, Kunbar A, Abramson N, Zimmerman D.. The clinical features and long-term sequelae of invasive meningococcal disease in children. Pediatr Infect Dis J. 2014;33(7):777–79. doi: 10.1097/INF.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 2.Baccarini C, Ternouth A, Wieffer H, Vyse A. The changing epidemiology of meningococcal disease in North America 1945-2010. Hum Vaccin Immunother. 2013;9(1):162–71. doi: 10.4161/hv.22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, Harnden A, Mant D, Levin M. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367(9508):397–403. doi: 10.1016/S0140-6736(06)67932-4. [DOI] [PubMed] [Google Scholar]
- 4.Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(suppl 2):B3–B9. doi: 10.1016/j.vaccine.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 5.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Enhanced meningococcal disease surveillance report, 2016. [accessed 2018 April2] https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf.
- 7.European Centre for Disease Prevention and Control Invasive meningococcal disease. [accessed 2017 November11] https://ecdc.europa.eu/sites/portal/files/documents/Invasive%20meningococcal%20disease%20AER.pdf.
- 8.Wong CA, Taylor JA, Wright JA, Opel DJ, Katzenellenbogen RA. Missed opportunities for adolescent vaccination, 2006-2011. J Adolesc Health. 2013;53(4):492–97. doi: 10.1016/j.jadohealth.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, Williams CL, Meyer SA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–82. doi: 10.15585/mmwr.mm6633a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams SH, Park MJ, Twietmeyer L, Brindis CD, Irwin CE Jr.. Association between adolescent preventive care and the role of the Affordable Care Act. JAMA Pediatr. 2017;172(1):43–48. doi: 10.1001/jamapediatrics.2017.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Center for Immunization and Respiratory Diseases General recommendations on immunization–recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1–64. [PubMed] [Google Scholar]
- 12.Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, Curtis RC, Markowitz L. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014–United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–24. [PMC free article] [PubMed] [Google Scholar]
- 13.European Centre for Disease Prevention and Control Recommended immunisations for diphtheria. [accessed 2017 November9] http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- 14.European Centre for Disease Prevention and Control Recommended immunisations for human papillomavirus infection. [accessed 2017 November9] http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- 15.European Centre for Disease Prevention and Control Recommended immunisations for tetanus. [accessed 2017 November9] http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- 16.European Centre for Disease Prevention and Control Recommended immunisations for pertussis. [accessed 2017 November9] http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- 17.European Centre for Disease Prevention and Control Recommended immunisations for influenza. [accessed 2017 November9] http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- 18.European Centre for Disease Prevention and Control Recommended immunisations for meningococcal disease. [accessed 2017 July20] http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- 19.Centers for Disease Control and Prevention Recommended immunization schedules for persons aged 0 through 18 years, United States, 2017. [accessed 2017 July18] https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf.
- 20.Centers for Disease Control and Prevention Recommended immunization schedule for adults aged 19 years or older, United States, 2017. [accessed 2017 June21] https://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf.
- 21.European Centre for Disease Prevention and Control Recommended immunisations for hepatitis B. [accessed 2019 March 6]. http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- 22.European Centre for Disease Prevention and Control Recommended immunisations for varicella. [accessed 2019 March 6]. http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- 23.Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: A review. Vaccine. 2010;28(34):5513–23. doi: 10.1016/j.vaccine.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Tejedor JC, Moro M, Ruiz-Contreras J, Castro J, Gomez-Campdera JA, Navarro ML, Merino JM, Martin-Ancel A, Roca J, Garcia-Del-Ri M, et al. Immunogenicity and reactogenicity of primary immunization with a novel combined Haemophilus influenzae Type b and Neisseria meningitidis Serogroup C-tetanus toxoid conjugate vaccine coadministered with a Diphtheria-tetanus-acellular Pertussis-hepatitis B-inactivated poliovirus vaccine at 2, 4 and 6 months. Pediatr Infect Dis J. 2007;26(1):1–7. doi: 10.1097/01.inf.0000247070.60063.09. [DOI] [PubMed] [Google Scholar]
- 25.Nolan T, Lambert S, Roberton D, Marshall H, Richmond P, Streeton C, Poolman J, Boutriau D. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine. 2007;25(51):8487–99. doi: 10.1016/j.vaccine.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Kitchin NR, Southern J, Morris R, Hemme F, Thomas S, Watson MW, Cartwright K, Miller E. Evaluation of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b vaccine given concurrently with meningococcal group C conjugate vaccine at 2, 3 and 4 months of age. Arch Dis Child. 2007;92(1):11–16. doi: 10.1136/adc.2005.076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Southern J, Crowley-Luke A, Borrow R, Andrews N, Miller E. Immunogenicity of one, two or three doses of a meningococcal C conjugate vaccine conjugated to tetanus toxoid, given as a three-dose primary vaccination course in UK infants at 2, 3 and 4 months of age with acellular pertussis-containing DTP/Hib vaccine. Vaccine. 2006;24(2):215–19. doi: 10.1016/j.vaccine.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 28.Menactra® (MCV4) Full Prescribing Information. Swiftwater (PA): Sanofi Pasteur Inc; 2014. [Google Scholar]
- 29.Menveo® (Meningococcal [Groups A, C, Y and W-135] Oligosaccharide Diphtheria CRM197 Conjugate Vaccine) Full Prescribing Information. Sovicille (Italy): Novartis; 2013. [Google Scholar]
- 30.Pfizer Canada Inc Nimenrix Product Monograph. Kirkland (QC, Canada): Pfizer Canada Inc; 2016. [Google Scholar]
- 31.MenAfriVac (meningococcal A conjugate vaccine) Full Prescribing Information. Pune (India): Serum Institute of India; 2015. [Google Scholar]
- 32.Menjugate 10 micrograms suspension for injection (meningococcal group C conjugate vaccine) Summary of Product Characteristics. Sovicille (Italy): Novartis Vaccines and Diagnostics S.r.l; 2016. [Google Scholar]
- 33.Meningitec (meningococcal serogroup c conjugate vaccine) Full Prescribing Information. West Ryde (NSW, Australia): Pfizer Australia Pty Ltd; 2011. [Google Scholar]
- 34.NeisVac-C (meningococcal C conjugate vaccine) Full Prescribing Information. New York (NY): Pfizer; 2015. [Google Scholar]
- 35.MENHIBRIX® (Hib-MenCY-TT) Full Prescribing Information. Rixensart (Belgium): GlaxoSmithKline; 2013. [Google Scholar]
- 36.Menitorix (Haemophilus type b and meningococcal group C conjugate vaccine) Summary of Product Characteristics. Middlesex (UK): SmithKline Beecham PLC; 2015. [Google Scholar]
- 37.Ministerio de Salud (Gobierno de Chile) Vaccination Calendar 2017. [accessed 2017 August29] http://vacunas.minsal.cl/calendario-de-vacunacion-2017/.
- 38.State of Kuwait Schedule of essential vaccination by age, State of Kuwait, 2008. [accessed 2017 August29] https://www.e.gov.kw/sites/kgoEnglish/Pages/CitizensResidents/Health/BasicVaccinationsInKuwait.aspx.
- 39.Kingdom of Saudia Arabia Ministry of Health Vaccination certificate. [accessed 2017 August29] http://www.moh.gov.sa/HealthAwareness/EducationalContent/HealthInstructions/Documents/جدول%20التطعيمات.pdf.
- 40.Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR–2):1–28. [PubMed] [Google Scholar]
- 41.Borrow R, Abad R, Trotter C, van der Klis FR, Vazquez JA. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine. 2013;31(41):4477–86. doi: 10.1016/j.vaccine.2013.07.083. [DOI] [PubMed] [Google Scholar]
- 42.Cohn AC, MacNeil JR, Harrison LH, Lynfield R, Reingold A, Schaffner W, Zell ER, Plikaytis B, Wang X, Messonnier NE, et al. Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine. Pediatrics. 2017;139(2):e20162193. doi: 10.1542/peds.2016-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trumenba® Meningococcal group B vaccine. Philadelphia (PA): Wyeth Pharmaceuticals Inc., a subsidiary of Pfizer Inc; 2016. [Google Scholar]
- 44.Bexsero® (Meningococcal group B vaccine) Full Prescribing Information. Cambridge (MA, USA): Novartis Vaccines and Diagnostics, Inc; 2015. [Google Scholar]
- 45.National Advisory Committee on Immunization Advice for the use of the multicomponent meningococcal serogroup B (4CMenB) vaccine. [accessed 2017 August29] http://publications.gc.ca/collections/collection_2014/aspc-phac/HP40-104-2014-eng.pdf.
- 46.Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR, Centers for Disease C . Control and Prevention (CDC). Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(22):608–12. [PMC free article] [PubMed] [Google Scholar]
- 47.MacNeil JR, Rubin L, Folaranmi T, Ortega-Sanchez IR, Patel M, Martin SW. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(41):1171–76. doi: 10.15585/mmwr.mm6441a3. [DOI] [PubMed] [Google Scholar]
- 48.Patton ME, Stephens D, Moore K, MacNeil JR. Updated recommendations for use of MenB-FHbp serogroup B meningococcal vaccine - Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(19):509–13. doi: 10.15585/mmwr.mm6619a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burrage M, Robinson A, Borrow R, Andrews N, Southern J, Findlow J, Martin S, Thornton C, Goldblatt D, Corbel M, et al. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect Immun. 2002;70(9):4946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Southern J, Gelb D, Andrews N, Waight PA, Morris R, Cartwright K, Miller E. Reactogenicity of meningococcal C conjugate vaccines when administered at the same time as, or a month prior to or after, tetanus and diphtheria booster vaccinations. Hum Vaccin. 2006;2:237–42. [DOI] [PubMed] [Google Scholar]
- 51.Tseng HF, Sy LS, Ackerson BK, Hechter RC, Tartof SY, Haag M, Slezak JM, Luo Y, Fischetti CA, Takhar HS, et al. Safety of quadrivalent meningococcal conjugate vaccine in 11- to 21-year-olds. Pediatrics. 2017;139:1. doi: 10.1542/peds.2016-2084. [DOI] [PubMed] [Google Scholar]
- 52.Broderick MP, Romero-Steiner S, Rajam G, Johnson SE, Milton A, Kim E, Choi LJ, Radin JM, Schmidt DS, Carlone GM, et al. Immune responses in U.S. military personnel who received meningococcal conjugate vaccine (MenACWY) concomitantly with other vaccines were higher than in personnel who received MenACWY alone. Clin Vaccine Immunol. 2016;23(8):672–80. doi: 10.1128/cvi.00267-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization WHO Expert Committee on biological standardization: Twenty-seventh report. [accessed 2018 August3] http://apps.who.int/iris/bitstream/handle/10665/37954/WHO_TRS_594.pdf?sequence=1&isAllowed=y.
- 54.Keiser P, Gill C. Defining efficacy in meningococcal vaccine trials. Future Sci. 2012;2:589–601. [Google Scholar]
- 55.Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine. 2005;23(17–18):2222–27. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 56.Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol. 2003;10(5):780–86. doi: 10.1128/CDLI.10.5.780-786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borrow R, Aaberge IS, Santos GF, Eudey TL, Oster P, Glennie A, Findlow J, Hoiby EA, Rosenqvist E, Balmer P, et al. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup b, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin Diagn Lab Immunol. 2005;12(8):970–76. doi: 10.1128/CDLI.12.8.970-976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McIntosh ED, Broker M, Wassil J, Welsch JA, Borrow R. Serum bactericidal antibody assays - the role of complement in infection and immunity. Vaccine. 2015;33(36):4414–21. doi: 10.1016/j.vaccine.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 60.Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27(suppl 2):B112–116. doi: 10.1016/j.vaccine.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 61.Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, Zollinger W, Robbins J, Aaberge I, Granoff DM, et al. Neisseria meningitidis group B correlates of protection and assay standardization–international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine. 2006;24(24):5093–107. [DOI] [PubMed] [Google Scholar]
- 62.Arguedas A, Soley C, Loaiza C, Rincon G, Guevara S, Perez A, Porras W, Alvarado O, Aguilar L, Abdelnour A, et al. Safety and immunogenicity of one dose of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine. 2010;28(18):3171–79. doi: 10.1016/j.vaccine.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 63.Gasparini R, Conversano M, Bona G, Gabutti G, Anemona A, Dull PM, Ceddia F. Randomized trial on the safety, tolerability, and immunogenicity of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis vaccine in adolescents and young adults. Clin Vaccine Immunol. 2010;17(4):537–44. doi: 10.1128/cvi.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muse D, Christensen S, Bhuyan P, Absalon J, Eiden JJ, Jones TR, York LJ, Jansen KU, O’Neill RE, Harris SL, et al. A phase 2, randomized, active-controlled, observer-blinded study to assess the immunogenicity, tolerability and safety of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with tetanus, diphtheria and acellular pertussis vaccine and serogroup A, C, Y and W-135 meningococcal conjugate vaccine in healthy US adolescents. Pediatr Infect Dis J. 2016;35(6):673–82. doi: 10.1097/INF.0000000000001124. [DOI] [PubMed] [Google Scholar]
- 65.Senders S, Bhuyan P, Jiang Q, Absalon J, Eiden J, Jones TR, York LJ, Jansen KU, O’Neill RE, Harris SL, et al. Immunogenicity, tolerability, and safety in adolescents of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with quadrivalent human papilloma virus vaccine. Pediatr Infect Dis J. 2016;35(5):548–54. doi: 10.1097/INF.0000000000001072. [DOI] [PubMed] [Google Scholar]
- 66.Vesikari T, Wysocki J, Beeslaar J, Eiden J, Jiang Q, Jansen KU, Jones TR, Harris SL, O’Neill RE, York LJ, et al. Immunogenicity, safety, and tolerability of bivalent rLP2086 meningococcal group B vaccine administered concomitantly with diphtheria, tetanus, and acellular pertussis and inactivated poliomyelitis vaccines to healthy adolescents. J Pediatric Infect Dis Soc. 2016;5(2):180–87. doi: 10.1093/jpids/piv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wheeler CM, Harvey BM, Pichichero ME, Simon MW, Combs SP, Blatter MM, Marshall GS, Catteau G, Dobbelaere K, Descamps D, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J. 2011;30(12):e225–234. doi: 10.1097/INF.0b013e31822d28df. [DOI] [PubMed] [Google Scholar]
- 68.Product Monograph: Menactra® [accessed 2018 June5] https://www.vaccineshoppecanada.com/document.cfm?file=menactra_e.pdf.
- 69.Gasparini R, Johnston W, Conversano M, Garscadden A, Alexanderian D, Giglioli N, Percell S, Han L, Smolenov I. Immunogenicity and safety of combined tetanus, reduced diphtheria, acellular pertussis vaccine when co-administered with quadrivalent meningococcal conjugate and human papillomavirus vaccines in healthy adolescents. J Vaccines Vaccin. 2014;5:231. doi: 10.4172/2157-7560.1000231. [DOI] [Google Scholar]
- 70.Ostergaard L, Silfverdal SA, Berglund J, Flodmark CE, West C, Bianco V, Baine Y, Miller JM. A tetravalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine is immunogenic and well-tolerated when co-administered with Twinrix® in subjects aged 11-17 years: an open, randomised, controlled trial. Vaccine. 2012;30(4):774–83. doi: 10.1016/j.vaccine.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 71.Reisinger KS, Block SL, Collins-Ogle M, Marchant C, Catlett M, Radley D, Sings HL, Haupt RM, Garner EI, Protocol 025 Investigators. Safety, tolerability, and immunogenicity of Gardasil given concomitantly with Menactra and Adacel. Pediatrics. 2010;125(6):1142–51. doi: 10.1542/peds.2009-2336. [DOI] [PubMed] [Google Scholar]
- 72.Schilling A, Parra MM, Gutierrez M, Restrepo J, Ucros S, Herrera T, Engel E, Huicho L, Shew M, Maansson R, et al. Coadministration of a 9-valent human papillomavirus vaccine with meningococcal and Tdap vaccines. Pediatrics. 2015;136(3):e563–572. doi: 10.1542/peds.2014-4199. [DOI] [PubMed] [Google Scholar]
- 73.Clinical review of Biologics License Application for human papillomavirus 6, 11, 16, 18 L1 virus like particle vaccine (S. cerevisiae) (STN 125126 GARDASIL), manufactured by Merck, Inc. [accessed 2018 May9] http://wayback.archive-it.org/7993/20170723091811/https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM111287.pdf.
- 74.US Food and Drug Administration Clinical review of biologics license application supplement STN# 125126/1297.0 – male indication for GARDASIL. [accessed 2017 October31] http://wayback.archive-it.org/7993/20170722072813/https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM190977.pdf.
- 75.Gardasil (human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant) Full prescribing information. Whitehouse Station (NJ): Merck & Co., Inc; 2014. [Google Scholar]
- 76.Alberer M, Burchard G, Jelinek T, Reisinger E, Beran J, Hlavata LC, Forleo-Neto E, Dagnew AF, Arora AK. Safety and immunogenicity of typhoid fever and yellow fever vaccines when administered concomitantly with quadrivalent meningococcal ACWY glycoconjugate vaccine in healthy adults. J Travel Med. 2015;22(1):48–56. doi: 10.1111/jtm.12164. [DOI] [PubMed] [Google Scholar]
- 77.Alberer M, Burchard G, Jelinek T, Reisinger E, Beran J, Meyer S, Forleo-Neto E, Gniel D, Dagnew AF, Arora AK. Co-administration of a meningococcal glycoconjugate ACWY vaccine with travel vaccines: a randomized, open-label, multi-center study. Travel Med Infect Dis. 2014;12(5):485–93. doi: 10.1016/j.tmaid.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 78.Alberer M, Burchard G, Jelinek T, Reisinger EC, Meyer S, Forleo-Neto E, Dagnew AF, Arora AK. Immunogenicity and safety of concomitant administration of a combined hepatitis A/B vaccine and a quadrivalent meningococcal conjugate vaccine in healthy adults. J Travel Med. 2015;22(2):105–14. doi: 10.1111/jtm.12180. [DOI] [PubMed] [Google Scholar]
- 79.Ambrosino DM, Siber GR. Simultaneous administration of vaccines for Haemophilus influenzae type b, pneumococci, and meningococci. J Infect Dis. 1986;154:893–96. [DOI] [PubMed] [Google Scholar]
- 80.Tashani M, Alfelali M, Barasheed O, Alqahtani AS, Heron L, Wong M, Rashid H, Booy R. Effect of Tdap when administered before, with or after the 13-valent pneumococcal conjugate vaccine (coadministered with the quadrivalent meningococcal conjugate vaccine) in adults: A randomised controlled trial. Vaccine. 2016;34(48):5929–37. doi: 10.1016/j.vaccine.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 81.Aplasca-De Los Reyes MR, Dimaano E, Macalalad N, Dbaibo G, Bianco V, Baine Y, Miller J. The investigational meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate vaccine (ACWY-TT) and the seasonal influenza virus vaccine are immunogenic and well-tolerated when co-administered in adults. Hum Vaccin Immunother. 2012;8(7):881–87. doi: 10.4161/hv.20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Findlow J, Bai X, Findlow H, Newton E, Kaczmarski E, Miller E, Borrow R. Safety and immunogenicity of a four-component meningococcal group B vaccine (4CMenB) and a quadrivalent meningococcal group ACWY conjugate vaccine administered concomitantly in healthy laboratory workers. Vaccine. 2015;33(29):3322–30. doi: 10.1016/j.vaccine.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 83.Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Becker B, et al. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307(6):573–82. doi: 10.1001/jama.2012.85. [DOI] [PubMed] [Google Scholar]
- 84.Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381(9869):825–35. doi: 10.1016/S0140-6736(12)61961-8. [DOI] [PubMed] [Google Scholar]
- 85.Society for Adolescent Health and Medicine Establishing an immunization platform for 16-year-olds in the United States. J Adolesc Health. 2017;60(4):475–76. doi: 10.1016/j.jadohealth.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 86.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination coverage among children aged 19-35 months - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(43):1171–77. doi: 10.15585/mmwr.mm6643a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18(2s):S72–S78. doi: 10.1016/j.acap.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Immunization of adolescents Recommendations of the Advisory Committee on Immunization practices, the American academy of pediatrics, the American academy of family physicians, and the American Medical Association. MMWR Recomm Rep. 1996;45(RR–13):1–16. [PubMed] [Google Scholar]
- 89.Robinson CL, Romero JR, Kempe A, Pellegrini C, Advisory Committee on Immunization Practices Child/Adolescent Immunization Work Group . Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger - United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(5):134–35. doi: 10.15585/mmwr.mm6605e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bilukha OO, Rosenstein N, National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease . Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005;54(RR–7):1–21. [PubMed] [Google Scholar]
- 91.Ostergaard L, Vesikari T, Absalon J, Beeslaar J, Ward BJ, Senders S, Eiden JJ, Jansen KU, Anderson AS, York LJ, et al. A bivalent meningococcal B vaccine in adolescents and young adults. N Engl J Med. 2017;377:2349–62. doi: 10.1056/NEJMoa1614474. [DOI] [PubMed] [Google Scholar]
- 92.Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388(10061):2775–82. doi: 10.1016/S0140-6736(16)31921-3. [DOI] [PubMed] [Google Scholar]
- 93.Block SL, Szenborn L, Daly W, Jackowska T, D’Agostino D, Han L, Dull PM, Smolenov I. A comparative evaluation of two investigational meningococcal ABCWY vaccine formulations: results of a phase 2 randomized, controlled trial. Vaccine. 2015;33(21):2500–10. doi: 10.1016/j.vaccine.2015.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Centers for Disease Control and Prevention Enhanced meningococcal disease surveillance report, 2016. [accessed 2018 April2] https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf.


