Myelin oligodendrocyte glycoprotein antibody (MOG-Ab)-associated demyelination has been associated with a range of clinical phenotypes, including acute disseminated encephalomyelitis (ADEM), ADEM followed by recurrent optic neuritis, aquaporin 4–negative neuromyelitis optica spectrum disorder, and less commonly, brainstem encephalitis.1 In contrast, chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) is a clinical, radiologic, and pathologic diagnosis without an associated antibody.2,3 There have been few reported cases of pediatric CLIPPERS,4 and most have not clearly met the strict case definition of CLIPPERS as proposed in 2017.3 Here, we present a pediatric case, ultimately diagnosed as MOG-Ab–associated demyelination, that was initially concerning for CLIPPERS due to subacute brainstem encephalitis with MRI showing curvilinear and punctate contrast enhancement in the pons.
Case presentation
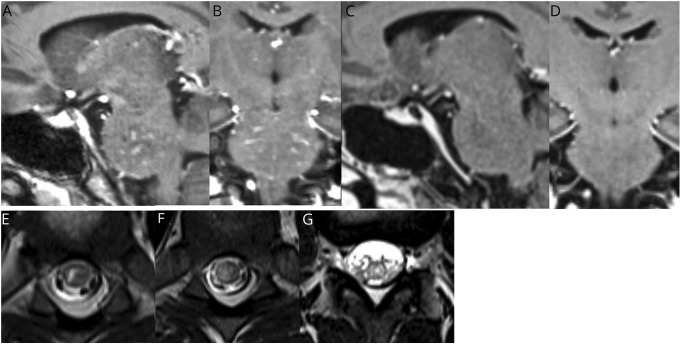
A 17-year-old previously healthy boy developed headache and transient horizontal diplopia. Over the next 3 days, he developed paresthesias in his left hand, arm, and face. The symptoms rapidly progressed to include dysarthria, gait imbalance, and urinary retention. MRI of the brain demonstrated T2 prolongation in the pons with extension into the cerebral peduncles, with multiple areas of curvilinear and punctate contrast enhancement (figure). MRI of the spine revealed multifocal, patchy T2 signal abnormality throughout the thoracic cord and conus (figure). Although a diagnosis of diffuse midline glioma was initially considered, this was ultimately thought to be less likely due to the subacute onset of symptoms, the noted punctate and curvilinear enhancement, and the presence of spinal cold lesions. CSF analysis was notable for 64/µL white blood cells and a protein of 65 mg/dL. Flow cytometric analysis of the CSF showed that 58% of cells were mature lymphocytes. Of the lymphocytes, 90% were T-lymphocytes, with an elevated CD4:CD8 ratio of 8:1. Serum MOG-IgG1 testing, analyzed via a live cell–based flow cytometry assay at Mayo Clinic laboratories, was then sent, following a single dose of dexamethasone.
Figure. Sagittal (A) and coronal (B) 3D T1-weighted postcontrast images showing punctate and curvilinear enhancement in the pons.
Follow-up sagittal (C) and coronal (D) 3D T1-weighted postcontrast images approximately 3 weeks later showing resolution of enhancement after treatment with IV methylprednisolone and rituximab. Axial T2-weighted images of the thoracic spinal cord (E and F) and conus (G) at the time of presentation showing patchy T2 hyperintense lesions involving gray and white matter.
Because of clinical concern for CLIPPERS, IV methylprednisolone was started on hospital day 2. The patient's symptoms rapidly improved, and his neurologic examination at discharge was normal. Rituximab was started on hospital day 6 as a steroid-sparing agent, and a steroid taper was initiated. Following discharge, the MOG-IgG1 testing sent during admission returned positive with a titer of 1:1,000. A repeat brain and spine MRI performed 3 weeks later demonstrated near-complete resolution of previously seen abnormalities. Repeat MOG-IgG1 antibody testing sent 3 months after presentation remained positive, though with a decreased titer of 1:100. He remains symptom-free 6 months after presentation with no further lesion accrual.
Discussion
Given the rarity of CLIPPERS in the pediatric population, recognition and evaluation of possible alternative diagnoses is key. The differential for CLIPPERS is broad and includes infectious, inflammatory, and neoplastic processes. Although the case presented above was characterized by a subacute presentation of brainstem symptoms, dramatic response to steroids, and curvilinear enhancement predominating in the pons and cerebellum, it did not meet the strict criteria for CLIPPERS proposed by Tobin et al., as the T2 signal abnormality exceeded the area of contrast enhancement and the MOG-Ab positivity provided an alternative diagnosis. Clinically, as with CLIPPERS, MOG-Ab–associated demyelination is often steroid responsive, and relapses can occur when steroids are weaned.1 Radiologically, specific features can be suggestive of MOG-Ab–associated disease in the setting of a brainstem encephalitis such as lesions in the posterior fossa greater than 2 cm or lesions with ill-defined margins. As many patients with MOG-Ab–associated disease will also have supratentorial lesions involving the gray and white matter, optic pathway lesions, or spinal cord lesions, comprehensive imaging of the neuroaxis should be performed.5
Two previous case reports have reported positive MOG antibodies at the time of relapse in patients previously thought to have CLIPPERS.6,7 Although the pathologic role of MOG antibodies produced by B cells requires further study, the underlying biology of relapses may be different in MOG-Ab–associated demyelination than in CLIPPERS, which is characterized by a predominantly T-cell infiltrate. These differences may have implications for the safety, use, and duration of long-term steroid-sparing therapies.
Early identification of brainstem predominant MOG-Ab–associated disease will lead to better understanding of the clinical phenotype, prognosis, and treatment response. We recommend consideration of MOG-Ab testing in pediatric patients where there is clinical concern for CLIPPERS due to subacute brainstem encephalitis with punctate and curvilinear contrast enhancement, as the prognosis and treatment of MOG-Ab–associated disease and CLIPPERS may ultimately differ.
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
The authors report no relevant disclosures. Disclosures available: Neurology.org/NN.
References
- 1.Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation 2018;15:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittock SJ, Debruyne J, Krecke KN, et al. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). Brain 2010;133:2626–2634. [DOI] [PubMed] [Google Scholar]
- 3.Tobin WO, Guo Y, Krecke KN, et al. Diagnostic criteria for chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). Brain 2017;140:2415–2425. [DOI] [PubMed] [Google Scholar]
- 4.Sa M, Green L, Abdel‐Mannan O, et al. Is chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) in children the same condition as in adults? Dev Med Child Neurol 2019;61:490–496. [DOI] [PubMed] [Google Scholar]
- 5.Jurynczyk M, Geraldes R, Probert F, et al. Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain 2017;140:617–627. [DOI] [PubMed] [Google Scholar]
- 6.Berzero G, Taieb G, Marignier R, et al. CLIPPERS mimickers: relapsing brainstem encephalitis associated with anti-MOG antibodies. Eur J Neurol 2018;25:e16–e17. [DOI] [PubMed] [Google Scholar]
- 7.Symmonds M, Waters PJ, Küker W, Leite MI, Schulz UG. Anti-MOG antibodies with longitudinally extensive transverse myelitis preceded by CLIPPERS. Neurology 2015;84:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]