The Toll-like receptor 3 (TLR3) pathway is a key component of the innate immunity that prevents replication of viruses in the CNS. Inborn errors of this pathway (TLR3-pathway deficiency), which includes defects in the genes TLR3, UNC93B1, TRIF, TRAF3, TBK1 and IRF3, occur in 10% of patients with herpes simplex encephalitis (HSE),1,2 and about 66% of these patients develop relapses of HSE.1 A recent study showed that 27% of patients with HSE develop autoimmune encephalitis (AE) in the weeks or months ensuing the infection.3 It is unknown whether TLR3-pathway deficient patients can also develop AE post-HSE. Here we report a patient with TLR3-pathway deficiency who developed HSE and a relapse of the viral infection followed by AE post-HSE, highlighting the fact that TLR3-pathway deficient patients should be carefully followed for both HSE relapses and AE.
Case
A 6-year-old healthy Caucasian girl, with a family history of maternal grandfather with recurrent herpetic keratitis, developed acute-onset headache, fever, decreased level of consciousness, aphasia, and right hemiparesis. Brain MRI showed bilateral hemorrhagic temporal lesions leading to suspect HSE. Signs of cranial hypertension precluded CSF studies; however, a positive herpes simplex virus 1 (HSV-1) PCR was obtained in the blood samples. IV acyclovir resulted in symptom improvement, and 3 weeks later the patient was discharged home with residual aphasia.
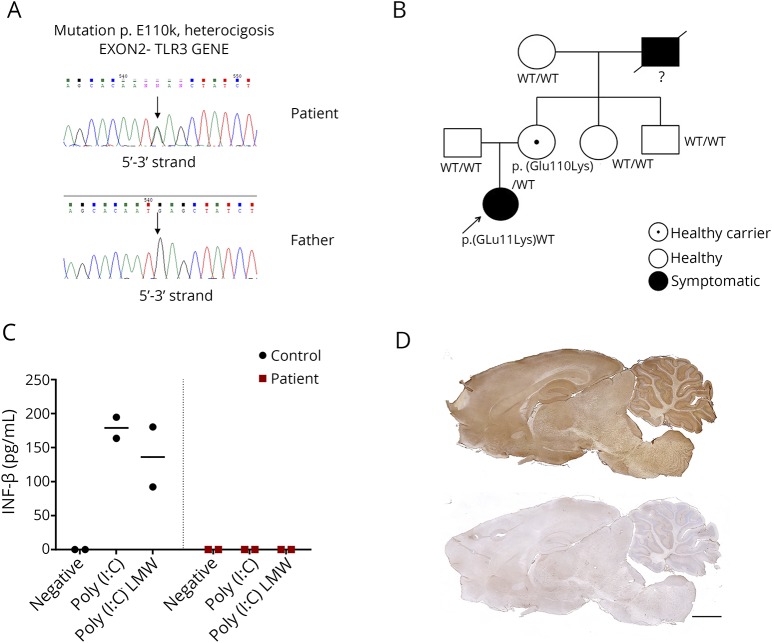
Five months later, she was readmitted with severe headache and decreased level of consciousness. Brain CT showed new hemorrhagic temporal lesions. She required urgent decompressive craniectomy, and a brain tissue sample was HSV-1 PCR positive. Genetic studies showed that the patient and the mother carried a heterozygous missense mutation p.Glu110Lys (c.328G>A) in exon 2 of the gene TLR3 (NM_003265.2) (figure, A and B). Such mutation shows a very low allelic frequency (0.00082%, 1/121,316). Functional studies on patient's fibroblasts (figure, C) and monocyte-derived dendritic cells showed a decrease in TLR3-mediated activation (methods in appendix e-1, links.lww.com/NXI/A141). The patient received a new 21-day course of IV acyclovir followed by oral valganciclovir.
Figure. Genetic, functional, and immunologic studies in a 6-year-old child with TLR3-pathway deficiency who developed HSE, and a relapse of the viral infection followed by AE post-HSE.
(A and B) Genetic studies identified a missense heterozygous mutation p.Glu110Lys (c.328G>A) in exon 2 of the TLR3 gene (NM_003265.2) in the patient and her mother (who was asymptomatic). The patient's grandfather had history of severe recurrent herpetic keratitis but at the time our patient was studied, he was deceased, and no genetic studies were available. (C) IFN-β production by fibroblasts of the patient (red squares) and a healthy control (black dots) in 2 independent experiments after 24 hours stimulation with 2 different TLR3 agonists: Poly(I:C) or Poly(I:C) LMW; stimulation with its own culture media was used as negative control (negative). IL-6 production by TLR3-agonists stimulated MDDCs was also decreased in the patient compared with a healthy control in 2 independent experiments (data not shown). (D) Rat brain immunostaining with CSF of the patient (upper panel) indicating the presence of antibodies against neuronal surface antigens, compared with that of a healthy control individual (lower panel). Scale bar = 2,000 μm. AE = autoimmune encephalitis; HSE = herpes simplex encephalitis; IFN-β = interferon-beta; LMW = low molecular weight; MDDC = monocyte-derived dendritic cell; Poly(I:C) = polyinosinic-polycytidylic acid; TLR3 = Toll-like receptor 3.
One year later, 17 months after the initial episode of HSE, she developed behavioral changes characterized by aggressivity and paranoid thoughts. CSF studies showed pleocytosis (15 cells/μL) and elevated protein concentration (100 mg/dL) but were HSV-1 PCR negative. CSF immunochemistry studies on rat brain tissue (figure, D, upper panel) and cultured live neurons showed strong reactivity revealing the presence of neuronal antibodies. Autoantibodies against N-methyl-D-aspartate, γ-aminobutyric acid A, and other known receptors and cell surface proteins were all negative (methods in appendix e-1, links.lww.com/NXI/A141). The indicated patient's antibodies were absent in samples of CSF obtained during HSE (data not shown). With the diagnosis of AE post-HSE, she was started on high-dose IV steroids and immunoglobulins without a clear improvement. Subsequently, rituximab (2 doses 500 mg/m2 2 weeks apart) resulted in neurologic improvement. A CSF sample obtained 4 months later was no longer reactive with brain.
Discussion
We report a young girl with recurrent HSE and a new TLR3 mutation associated with absent interferon-β responses to TLR3 agonist who several months after the first HSE episode developed recurrent HSE followed by AE. TLR3-pathway deficient patients, especially TLR3-deficient, are prone to develop HSE relapses, putting them at risk of developing AE. Indeed, a recent series of patients with HSE showed that 27% subsequently developed AE; none of them were investigated for TLR3-pathway deficiency but the current case indicates that patients with this deficiency are also at risk of AE. The symptoms of our patient (predominant behavioral abnormalities) are typical of AE post-HSE in patients older than 4 years3,4; yet the long interval between HSE and AE (17 months since the onset of HSE and 12 months since the relapse of HSE) is somewhat atypical (median 26 days in children aged 4 years or younger and 43 days in patients older than 4 years),3,5 but similar prolonged intervals occurred in 7% of patients in a recently reported series.3 The exact mechanisms underlying this severe immune-mediated complication are unknown. It has been postulated that the neuronal destruction caused by the virus leads to a release of antigens, which in the context of severe inflammation results in neuronal autoimmunity, such as that shown in our patient.3 In patients with TLR3-pathway deficiency, the increased number of episodes of HSE theoretically increases the risk of this autoimmune complication. Our case suggests that patients with recurrent HSE or family history of HSE or HSV keratitis should be assessed for TLR3-pathway deficiency.1,6,7 If the function of this pathway is impaired, patients should be carefully followed for potential relapses of HSE or AE post-HSE.
Acknowledgment
The authors thank Maria Rodés, Marta Muñoz, Mercè Alba, Eva Caballero, and Esther Aguilar for excellent administrative and technical support.
Appendix. Authors
Study funding
This study was supported in part by Mutua Madrileña Foundation Award (AP162572016, T.A.); Plan Nacional de I+D+i and cofinanced by the ISCIII—Subdirección General de Evaluación y Formento de la Investigación Sanitaria—and the Fondo Europeo de Desarrollo Regional (ISCIII-FEDER; PI18-00486 to T.A.; 17/00234 to J.D., PI15/01094, PFIS0200 (AC16/00025), and PI18/00223 to L.A.; PI14/00616 and PI17/00543 to R.P.d.D.); Pla estratègic de recerca i innovació en salut (PERIS), Departament de Salut, Generalitat de Catalunya (SLT006/17/00362 to T.A.; SLT006/17/00199 to L.A.); a 2017 Leonardo Grant for Researchers and Cultural Creators, BBVA Foundation to L.A.; Ramon Areces Foundation, through a grant from the XVII Concurso Nacional de Ayudas a la Investigación to L.A.; and Fundació CELLEX to J.D.
Disclosure
J. Dalmau holds patents for the use of Ma2, NMDAR, GABAbR, GABAaR, DPPX, and IgLON5 as autoantibody tests and receives royalties related to autoantibody tests from Athena Diagnostics and Euroimmun, Inc., and is editor of Neurology®: Neuroimmunology & Neuroinflammation. The other authors declare no conflict of interest related to this manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Lim HK, Seppänen M, Hautala T, et al. . TLR3 deficiency in herpes simplex encephalitis: high allelic heterogeneity and recurrence risk. Neurology 2014;83:1888–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafaille FG, Pessach IM, Zhang SY, et al. . Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 2012;491:769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armangue T, Spatola M, Vlagea A, et al. . Frequency, syndromes, risk factors, and outcome of autoimmune encephalitis following herpes simplex encephalitis: a prospective observational study and a retrospective analysis of cases. Lancet Neurol 2018;17:760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armangue T, Moris G, Cantarín-Extremera V, et al. . Autoimmune post–herpes simplex encephalitis of adults and teenagers. Neurology 2015;85:1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nosadini M, Mohammad S, Corazza F, et al. . Herpes simplex virus-induced anti-N-methyl-d-aspartate receptor encephalitis: a systematic literature review with analysis of 43 cases. Dev Med Child Neurol 2017;59:796–805. [DOI] [PubMed] [Google Scholar]
- 6.Routes J, Abinun M, Al-Herz W, et al. . ICON: the early diagnosis of congenital immunodeficiencies. J Clin Immunol 2014;34:398–424. [DOI] [PubMed] [Google Scholar]
- 7.Abel L, Plancoulaine S, Jouanguy E, et al. . Age-dependent Mendelian predisposition to herpes simplex virus type 1 encephalitis in childhood. J Pediatr 2010;157:623–629, 629.e1. [DOI] [PubMed] [Google Scholar]