Abstract
ACSL4 is a member of the ACSL family that catalyzes the conversion of long-chain fatty acids to acyl-coenzyme As, which are essential for fatty-acid incorporation and utilization in diverse metabolic pathways, including cholesteryl ester synthesis. Steroidogenic tissues such as the adrenal gland are particularly enriched in cholesteryl esters of long-chain polyunsaturated fatty acids, which constitute an important pool supplying cholesterol for steroid synthesis. The current studies addressed whether ACSL4 is required for normal steroidogenesis. CYP11A1 promoter‒mediated Cre was used to generate steroid tissue‒specific ACSL4 knockout (KO) mice. Results demonstrated that ACSL4 plays an important role in adrenal cholesteryl ester formation, as well as in determining the fatty acyl composition of adrenal cholesteryl esters, with ACSL4 deficiency leading to reductions in cholesteryl ester storage and alterations in cholesteryl ester composition. Statistically significant reductions in corticosterone and testosterone production, but not progesterone production, were observed in vivo, and these deficits were accentuated in ex vivo and in vitro studies of isolated steroid tissues and cells from ACSL4-deficient mice. However, these effects on steroid production appear to be due to reductions in cholesteryl ester stores rather than disturbances in signaling pathways. We conclude that ACSL4 is dispensable for normal steroidogenesis.
Steroid hormones, including glucocorticoids, mineralocorticoids, and gonadal steroids, are essential for carbohydrate metabolism, inflammation and stress responses, blood pressure maintenance, and salt and water balance, as well as fertility and reproduction (1–5). The adrenal, ovary, and testis are the main sites for synthesis of steroid hormones (steroidogenesis). Steroidogenesis, which involves coordinated functioning of multiple pathways as well as actions of many steroidogenic enzymes (6–15), is controlled primarily by cell/tissue-specific trophic hormones (ACTH, LH, and FSH) (7, 9, 13, 14) and is subject to both acute (7–10, 12–15) and chronic regulation (6–8, 14, 16–18). Unlike peptide hormones, which are typically stored within secretory vesicles in cells and acutely secreted upon stimulation, steroid hormones are not appreciably stored within cells; in addition, their release requires rapid synthesis from their precursor cholesterol in response to extracellular stimuli. The mobilization of the cholesteryl esters (CEs) stored in lipid droplets (LDs), or CE-rich LDs, appears to be the initially preferred source of cholesterol for steroidogenesis upon acute hormone stimulation. In steroidogenic tissues, CE-rich LDs can accumulate either through the uptake of intact CEs from circulating lipoproteins mediated by SR-B1 or through the esterification of free cholesterol. Sources of free cholesterol include de novo synthesis in the endoplasmic reticulum; the hydrolysis of lipoprotein CEs that are taken up via low-density lipoprotein receptor-mediated endocytosis into lysosomes, where they are acted on by lysosomal lipase; or the hydrolysis of lipoprotein CEs taken up via SR-B1 and then acted on by hormone-sensitive lipase in the cytosol (19). The esterification of cholesterol is mediated by acyl-coenzyme A (CoA):cholesterol acyltransferase and requires the activation of fatty acids to fatty acyl-CoA, mediated through the actions of the family of long-chain fatty acid‒CoA synthetases (ACSLs). CEs stored within LDs are hydrolyzed by hormone-sensitive lipase to free cholesterol for transport into mitochondria for steroid synthesis.
The ACSL family consists of 11 enzymes that differ in their fatty-acid substrate specificities and in their subcellular compartmentation (20). As opposed to other tissues and circulating lipoproteins in which cholesteryl oleate is the predominant form of CE, the CEs in steroidogenic tissues such as the adrenal are particularly enriched in CEs of long-chain polyunsaturated fatty acids (21). Among ACSL family members, ACSL4 appears to display the highest preference for generating several long-chain polyunsaturated fatty acyl-CoAs and thus appears to be important in allowing the formation of CEs in adrenal LDs (22–26). Indeed, it has been proposed that ACSL4 is required for normal steroidogenesis because the knockdown of ACSL4 in vitro in adrenal and Leydig cell lines reportedly markedly reduced steroid production (27, 28), suggesting either that CE formation is necessary as a substantial source of substrate or the intermediate pathway of cholesterol for steroidogenesis or that long-chain polyunsaturated fatty acyl-CoAs or their products are required for signaling. However, it is noteworthy that although a number of in vitro knockdown studies initially suggested the important involvement of a particular gene product, for instance StarD3 (29) or translocator protein (30), in steroidogenesis, this finding has not been reproduced in vivo (29, 30). In this article, we report the generation of tissue-specific ACSL4 KO mice and the examination of its role in steroidogenesis in vivo.
Materials and Methods
Chemicals and reagents
Reagents were obtained from the following sources: Cholesterol LiquiColor Test (Enzymatic) from Stanbio (Boerne, TX); bicinchoninic acid assay protein kit from Pierce Biotechnology, Inc. (Rockford, IL); organic solvents from J. T. Baker (Phillipsburg, NJ); TRIzol reagent and SuperScript II from Invitrogen (Carlsbad, CA); RNeasy kit from QIAGEN (Valencia, CA); SyBr Green Taqman PCR kit from Applied Biosystems (Foster City, CA); Odyssey blocking buffer, goat anti-mouse IgG-IRDye 800 (catalog no. 925-32210) (31), donkey anti-goat IgG-IRDye 680 (catalog no. 926-68074) (32), and goat anti-rabbit IgG-IRDye 680 (catalog no. 925-68021) (33) from Li-Cor Biosciences (Lincoln, NE); corticosterone ELISA kit from Cayman Chemical (Ann Arbor, MI); progesterone and testosterone ELISA kits from ALPCO (Salem, NH); pregnant mare’s serum gonadotropin (PMSG)/equine chorionic gonadotropin (eCG) and Bt2cAMP from Sigma (St. Louis, MO). Cosyntropin was from Amphastar Pharmaceuticals Inc. (Rancho Cucamonga, CA).
Animals
Animal studies were performed in accordance with National Institutes of Health guidelines, and all procedures were approved by the institutional animal care and use committee of Veterans Affairs Palo Alto Health Care System. Mice in which exon 3 and exon 4 of the ACSL4 gene were floxed were generated as previously described (26). The animals carrying floxed ACSL4 were crossed with transgenic mice expressing Cre recombinase under control of the CYP11A1 promoter on a C57BL6 background obtained from Jackson Laboratories (Bar Harbar, ME; stock number: 010988). Cre activity is expressed in the adrenals of both sexes and in the testes of these animals, leading to the knockdown of ACSL4 in the adrenals and testes. All animals were maintained on a chow diet (PMI 5015) with a 12-hour/12-hour dark/light cycle. Genotypes were identified by PCR analysis using genomic DNA from tail biopsies as described. Both male and female mice were studied and analyzed separately for sex-specific differences in responses. ACSL4 floxed mice and CYP11A1-Cre mice were analyzed separately as control animals. ACSL4 KO and control mice aged 16 to 24 weeks were used for the experiments. To assess serum corticosterone production, control and ACSL4 KO mice were injected with ACTH (cosyntropin, 2.5 IU per mouse) subcutaneously once. Blood was drawn from mice before and 1 hour after ACTH injection. To assess serum testosterone production, male animals were treated with human chorionic gonadotropin (hCG) (2.5 IU per mouse), and serum was collected 4 hours later. To assess progesterone production, female mice were treated with 5 IU of PMSG/eCG, and serum was collected 5 hours later.
For detection of ACSL4 mRNA expression in different tissues, mice were injected with saline or ACTH (cosyntropin, 2.5 IU) subcutaneously once, and various tissues were collected 1 hour after injection, frozen in liquid nitrogen, and stored at −80°C for later use.
Ex vivo adrenal steroid production
ACSL4 KO and control mice were euthanized at 24 weeks of age, and the adrenals were removed. The adrenals were cut in half, and each half adrenal was preincubated in one tube of 500 µL medium (RPMI 1640 supplemented with 10% fetal bovine serum) at 37°C 5% CO2 for 1 hour, then transferred to another tube and treated in 500 µL medium with or without 2.5 mM of Bt2cAMP at 37°C 5% CO2 for another 1 hour. Some experiments included the presence or absence of human apoE-free high-density lipoprotein (HDL; 500 µg CE/mL) during the incubation period. Media were collected after incubation and subjected to ELISA analysis of corticosterone.
Preparation and treatment of granulosa and Leydig cells
Granulosa cells from control and ACSL4 KO mice were prepared as described previously (34, 35). Briefly, immature female mice (22 to 25 days old) were injected once with 5 IU of PMSG/eCG for 48 hours. After hormone treatment, the ovaries were excised and placed in basal medium [DMEM:F12 with 20 mm of HEPES (pH, 7.4)] supplemented with 100 U/mL of penicillin and 100 mg/mL of streptomycin. Clumps of mural granulosa cells and oocyte-cumulus complexes were released into the medium by puncturing follicles with a 25-gauge needle. The mural granulosa cells were collected in DMEM/F12/HEPES/BSA medium and dispersed by being gently drawn in and out of a Pasteur pipette. The granulosa cells were washed and resuspended in fresh medium and cultured as described previously (36–38). The cells were then incubated with or without Bt2cAMP and/or human apoE-free HDL (500 μg CE/mL) for 5 hours. After incubation, media were collected and progesterone production was measured using RIA.
Testicular interstitial cells containing Leydig cells were isolated by mechanical dispersion of decapsulated testes obtained from control and ACSL4 KO mice. Highly purified (>85%) Leydig cell preparations were obtained by subjecting interstitial cell suspensions to Percoll density gradient centrifugation as previously described (35, 39). To assay steroidogenesis, freshly purified Leydig cells were incubated without (basal) or with Bt2cAMP (2.5 mm) for 5 hours in triplicate, and secreted testosterone was assayed by RIA (40).
RNA isolation and quantitative real-time PCR analysis
For RNA isolation, tissues were homogenized in 1 mL of TRIzol reagent using a power homogenizer (Ultra-Turrax T25; Labortechnik, Gottingen, Germany). RNA was isolated following the protocol for TRIzol reagent, and after the isopropanol precipitation step, total RNA was dissolved in 20 µL of RNase-free water, RNA concentration and quality were analyzed, and then 1 µg of RNA was converted to cDNA using SuperScript II reverse transcription (Invitrogen) for real-time PCR analysis. Real-time PCR was performed with the cDNA prepared as described previously using an ABI Prism 8500 System and SYBR Green Master Mix reagent. The relative mass of specific RNA was calculated by the comparative cycle of threshold detection method according to the manufacturer’s instructions. Genes examined included ACSL4, ACSL1, ACSL3, ACSL5, StAR, SNAP23, SNAP25, NSF, αSNAP, CYP11A1, CYP11B1, 3βHSD, CYP21A1, and CYP21A2. The primer sets used for each gene are shown in an online repository (41).
Lipid extraction
Lipid extraction from adrenals was performed as described previously (42). Briefly, adrenal tissue extract was mixed with 20 times the volume of chloroform/methanol (2:1) and vortexed. After that, chloroform was added to the mixture and subjected to centrifugation. The combined lower layers were washed and transferred to a new tube and dried under N2. The lipids were dissolved with 500 µL of isopropanol. Cholesterol was measured using a Cholesterol LiquiColor Test (Enzymatic) kit (EKF Diagnostic USA, Boerne, TX). In addition, adrenal samples were sent to the National Institutes of Health West Coast Metabolomics Center at University of California, Davis, for lipid extraction and lipidomics analysis by charged surface hybrid column–electrospray quadrupole time of flight tandem mass spectrometry.
Immunoblotting
Immunoblotting was performed as described previously (35). Briefly, tissues were homogenized in 50 mm Tris-HCl (pH, 7.4), 8% sucrose, 1 mm EDTA, 0.1 mm Na3VO4, and 50 mm NaF with 10 μg/mL leupeptin. After determination of protein concentrations by bicinchoninic acid protein assays, 20 μg of proteins was loaded onto and resolved by 4% to 15% gradient SDS-PAGE gel. After electrophoresis, proteins were transferred onto nitrocellulose membranes, and the membranes were blocked with Odyssey blocking buffer for 1 hour at room temperature. The membranes were incubated overnight with the following primary antibodies: anti-ACSL4 (1:1000; catalog no. PA5-27137; Thermo Fisher Scientific, Waltham, MA) (43), antiβ-actin (1:1000; catalog no. 4970; Cell Signaling Technology, Danvers, MA) (44), and anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1000; catalog no. 2118; Cell Signaling Technology) (45). Membranes were then incubated with the appropriate secondary antibody conjugated to infrared (IR) dye [goat anti-mouse IgG-IR dye 800 cw (31), donkey anti-goat IgG-IR dye 680 cw (32), and goat anti-rabbit IgG-IR dye 680 cw (33)] at room temperature for 1 hour, washed three times with PBS (0.1% Tween 20), rinsed with PBS, and then detected by an Odyssey IR fluorescent imaging system (Li-Cor Biosciences).
Statistics
Data are expressed as means ± SEM. Statistical analyses were performed by one-way ANOVA and by independent t test using SPSS 20.0 and GraphPad Prism 7.0 (GraphPad, La Jolla, CA). Differences between groups were considered statistically significant when P < 0.05.
Results
ACSL4 is highly expressed in steroidogenic tissue and is responsive to hormone stimulation
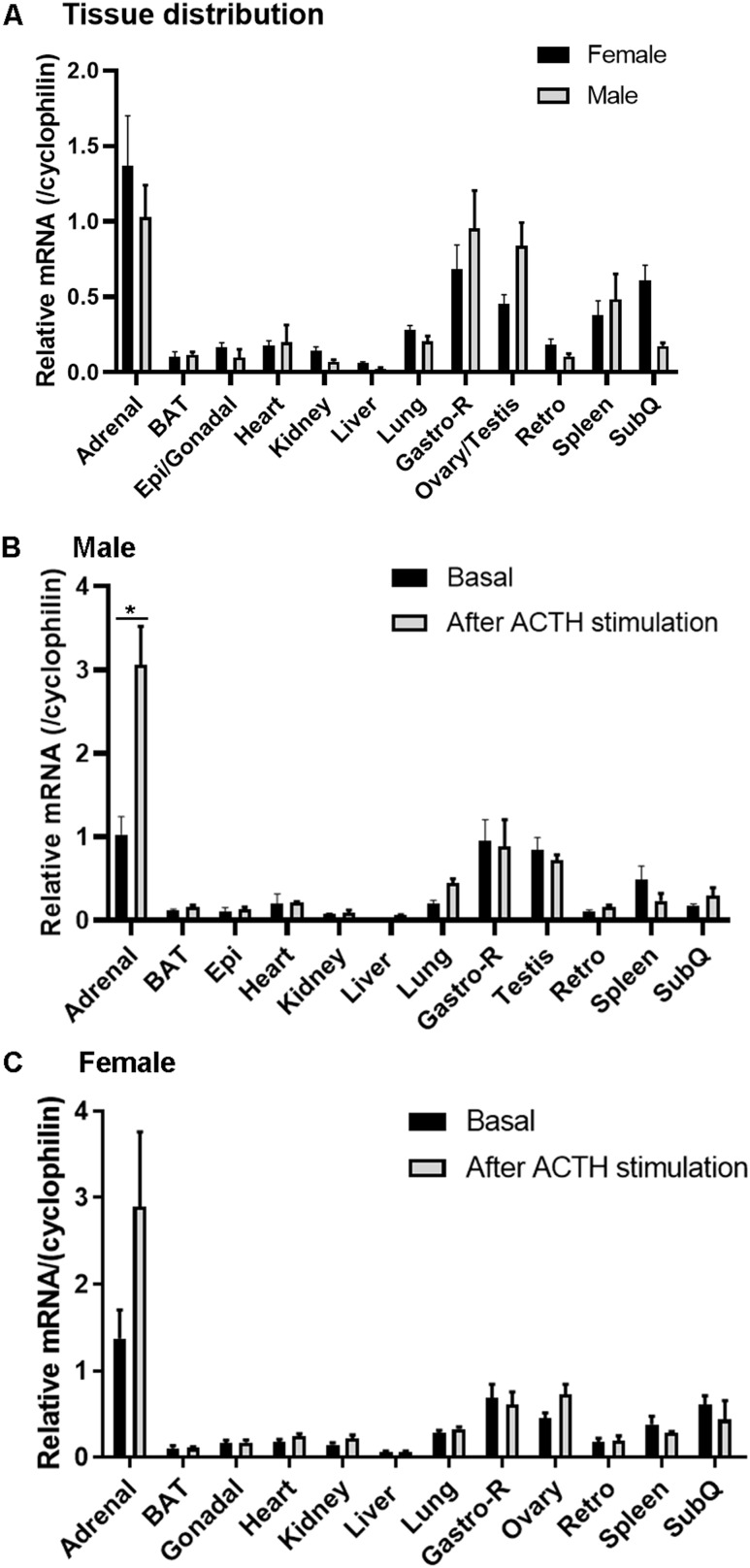
Before the importance of ACSL4 for steroidogenesis was examined, we analyzed the expression levels of ACSL4 in different tissues of wild-type mice using quantitative TaqMan RT-PCR. In general, expression of ACSL4 was detected in almost all of the tissues examined, with steroidogenic tissues (adrenal and gonadal tissues) having higher expression than other tissues. As shown in Fig. 1A, the adrenals had the highest expression level of ACSL4 in both male and female mice, followed by skeletal muscle from the hind limb. In gonadal tissues, the testes and ovaries of male and female mice, respectively, expression of ACSL4 was also very high. In agreement with a potential role in steroid production, upon ACTH stimulation, ACSL4 expression in the adrenal increased significantly in male mice (Fig. 1B; P < 0.05) and showed a trend toward increase in female mice (Fig. 1C). The expression of ACSL4 in the other tissues examined was not changed with ACTH stimulation.
Figure 1.
ACSL4 mRNA expression in various tissues under different physiological conditions. (A) mRNA was isolated from various tissues of 16- to 24-wk-old male and female mice (n = 3 to 6), and levels of ACSL4 mRNA were analyzed using RT-PCR. (B) Male mice that were 16 to 24 wk old were injected with ACTH or carrier, and tissues were collected 1 h later for analysis of ACSL4 mRNA levels (n = 3 to 6). (C) Female mice that were 16 to 24 wk old were injected with ACTH or carrier, and various tissues were collected 1 h later for analysis of ACSL4 mRNA levels (n = 3 to 6). Data are expressed as means ± SEM. *P < 0.05. BAT, brown adipose tissue; Epi, epididymal adipose tissue; Gastro-R; gastrocnemius muscle-red; Retro, retroperitoneal adipose tissue; SubQ, subcutaneous adipose tissue.
Generation of steroidogenic tissue-specific ACSL4 KO mice
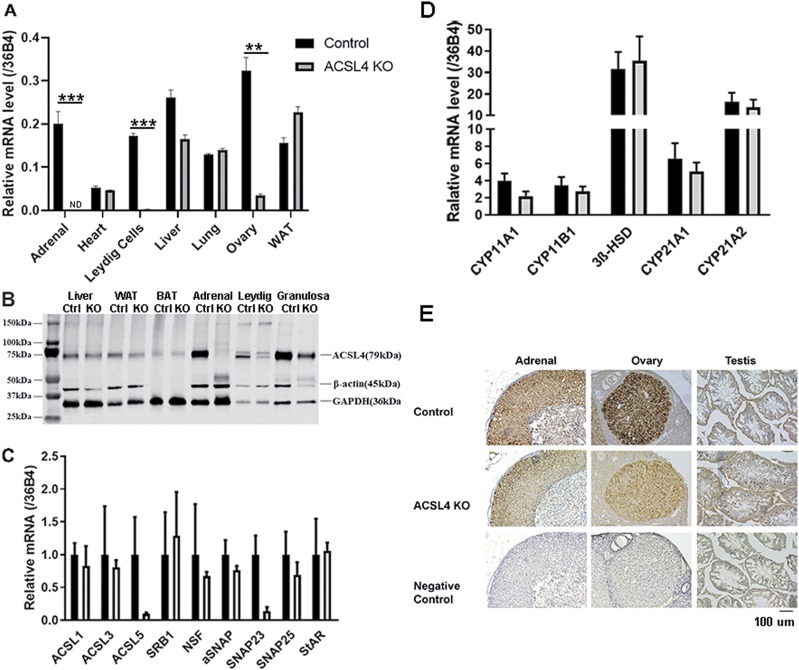
To generate steroidogenic tissue-specific knockout of ACSL4 mice, animals carrying floxed ACSL4 (26) were crossed with CYP11A1-Cre mice, in which Cre expression is restricted primarily to steroid-producing cells. After confirmation of genotype by PCR from tail genomic DNA, the expression of ACSL4 mRNA in the adrenal and other tissues was analyzed using TaqMan quantitative PCR. As shown in Fig. 2A, the expression of ACSL4 mRNA was reduced >90% in the adrenal (P < 0.001), Leydig cells (P < 0.001), and ovary (P < 0.01) of KO mice compared with controls, whereas ACSL4 mRNA expression was unchanged in heart, liver, lung, or white adipose tissue. Further analysis of protein levels using anti-ACSL4 antibody (43) confirmed the ablation of ACSL4 in adrenal tissue extracts (>95%; P < 0.005) and the absence of any change in extracts of liver and adipose tissues (Fig. 2B). Relative to GAPDH (45) in isolated Leydig cells, the protein levels of ACSL4 were significantly decreased (∼65%; P < 0.01) in ACSL KO mice, whereas protein levels of ACSL4 relative to GAPDH in isolated granulosa cells trended to decrease (∼60%), though this did not reach statistical significance. Quantification of ACSL4 protein expression relative to GAPDH and β-actin (44) is shown in Supplemental Fig. 1 (41).
Figure 2:
Generation of tissue-specific ACSL4 KO mice. (A) Levels of ACSL4 mRNA in control and ACSL4 KO mice. mRNA was isolated from adrenals and other tissues of 16- to 24-week-old mice (n = 3), and levels of ACSL4 mRNA were analyzed using RT-PCR. Experiments were repeated three times. (B). Western blot analysis of ACSL4 protein in various tissues and isolated cells from control and ACSL4 KO mice. Total cell extracts were prepared from tissues of 16- to 24-week-old control and ACSL4 KO mice (n = 3 to 5). Leydig and granulosa cells were isolated from male and female mice, respectively, following the protocol described in the Materials and Methods. Protein levels of ACSL4 were analyzed by immunoblotting with ACSL4 antibody. (C) Analysis of expression of various ACSL genes and selected genes that are involved in steroidogenesis in the adrenal using TaqMan quantitative PCR. (D) Analysis of expression of various genes involved in steroidogenesis in the adrenal using TaqMan quantitative PCR. (E) Histochemical analysis of ACSL4 in the adrenal, ovary, and testis of control and ACSL4 KO mice. Data are expressed as means ± SEM. **P < 0.01; ***P < 0.005. BAT, brown adipose tissue; Ctrl, control; ND, not detected; WAT, white adipose tissue.
To further analyze the effect of ACSL4 ablation, the expression of various other ACSL genes and some select genes that are involved in steroidogenesis were analyzed using TaqMan quantitative PCR. As shown in Fig. 2C, the mRNA levels of most of the genes analyzed (i.e., ACSL1, ACSL3, SR-B1, NSF, α-SNAP, SNAP25, and StAR) showed no differences between control and ACSL4 KO mice. The mRNA levels of ACSL5 and SNAP23, however, appeared to be decreased in the adrenals of ACSL4 KO mice. As shown in Fig. 2D, mRNA levels of several genes encoding enzymes in the steroidogenic pathway (i.e., CYP11A1, 3βHSD, CYP21A1, CYP21A2) showed no differences between control and ACSL4 KO mice. Histochemical staining of sections from steroidogenic tissues (Fig. 2E) showed distinct staining of ACSL4 in the adrenal of control mice, but these signals were not present in adrenal sections from ACSL4 KO mice or when nonimmune antibody was used. ACSL4 staining was detected in sections of ovary samples from control animals, whereas the signal for ACSL4 was reduced, but detectable, in the ovary of ACSL4 KO mice, reflecting a lack of complete knockdown at the protein level. This finding is consistent with the observation that the Cre recombinase driven by the CYP11A1 promoter failed to drive substantial GFP expression in the ovary in heterozygous CYP11A1-Cre-GFP mice (46, 47) even though CYP11A1 is expressed in the ovary. The ACSL4 signals in the sections of testes were not very clear, which probably reflects the fact that it is difficult to identify Leydig cells in the sections of whole testis at low magnification.
Lipidomics analysis of adrenal lipids from control and ACSL4 KO mice
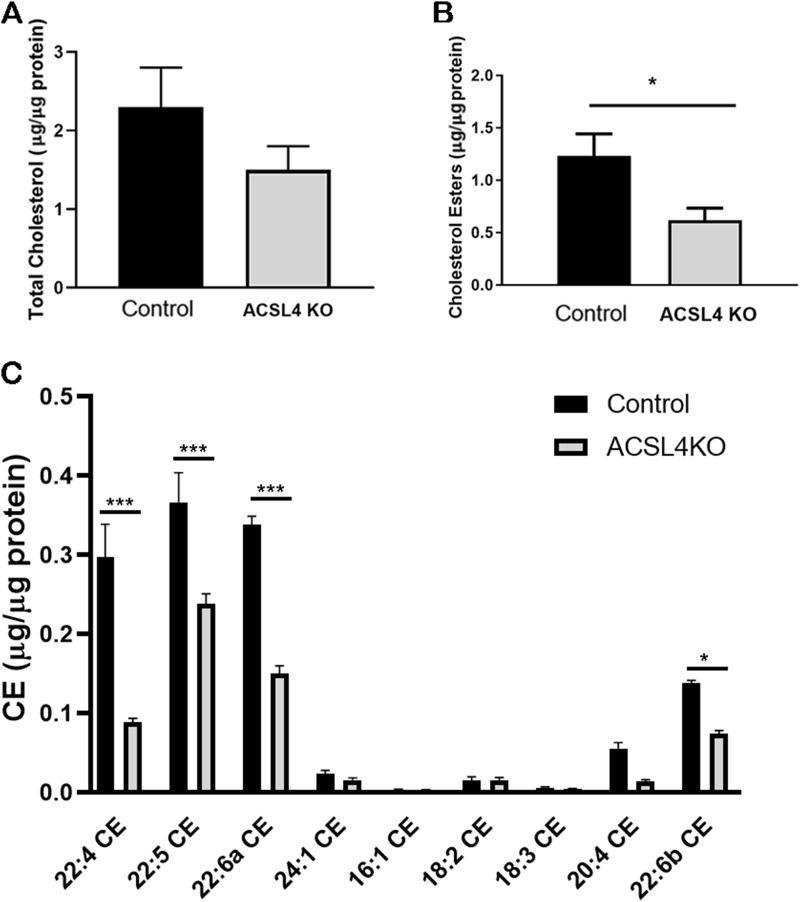
Because ACSL4 was successfully knocked down, but not completely eliminated, in the adrenal, the effects of ACSL4 deficiency on adrenal lipid content were examined, focusing on CEs in view of their importance in steroid production. CE content was reduced ∼50% (P < 0.05) in ACSL4 KO adrenals (Fig. 3A), whereas total cholesterol content trended to be reduced but did not reach statistical significance (Fig. 3B), consistent with the importance of products of ACSL4 in the formation of CEs in the adrenal. CEs accounted for ∼50% to 60% of total adrenal cholesterol.
Figure 3.
Total cholesterol and cholesteryl esters in control and ACSL4 KO mice adrenals. Total lipids were extracted from the adrenals of 12- to 24-week-old female control and ACSL4 KO mice (n = 7 or 8) using the Folch method. (A) Total cholesterol and (B) cholesteryl esters were assayed. (C) Cholesteryl ester compositions were analyzed using mass spectrometry (n = 5 or 6). Data are expressed as means ± SEM. *P < 0.05; ***P < 0.005.
To explore the effects of ACSL4 deficiency on adrenal lipid composition in more detail, complete lipidomics analysis of adrenal lipids was performed by charged surface hybrid column-electrospray quadrupole time of flight tandem mass spectrometry (41). Figure 3C highlights the composition of CEs found in control and ACSL4 KO adrenals. The dominant CEs identified in control mouse adrenals were cholesteryl adrenate (C22:4; ∼24%), cholesteryl docosapentaenoate (C22:5; ∼30%), and cholesteryl docosahexaenoate (C22:6; ∼27%), with minor components of cholesteryl arachidonate (C20:4; ∼4.5%), cholesteryl nervonate (C24:1; ∼2%), and cholesteryl linoleate (C18:2; ∼1%) along with smaller amounts of other CEs. Consistent with fatty acid preferences of ACSL4 (48), the total CEs as cholesteryl adrenate, cholesteryl docosapentaenoate, and cholesteryl docosahexaenoate were significantly reduced (P < 0.005) in ACSL4 KO adrenals, with cholesteryl arachidonate trending to be reduced (P < 0.07); however, the reduction in cholesteryl docosapentaenoate was relatively less than that observed for the other long-chain polyunsaturated fatty acyl CEs. Thus, ACSL4 deficiency resulted in significant alterations in adrenal CE content and composition. Effects of ACSL4 deficiency on lipid composition in ovaries and testes were not examined because steroid-producing cells targeted by CYP11A1-Cre constitute a smaller percentage of total cells in the gonads than in the adrenals; thus, lipid measurements would have been substantially skewed by nontargeted cells.
Effects of tissue-specific ablation of ACSL4 on stimulated adrenal steroids
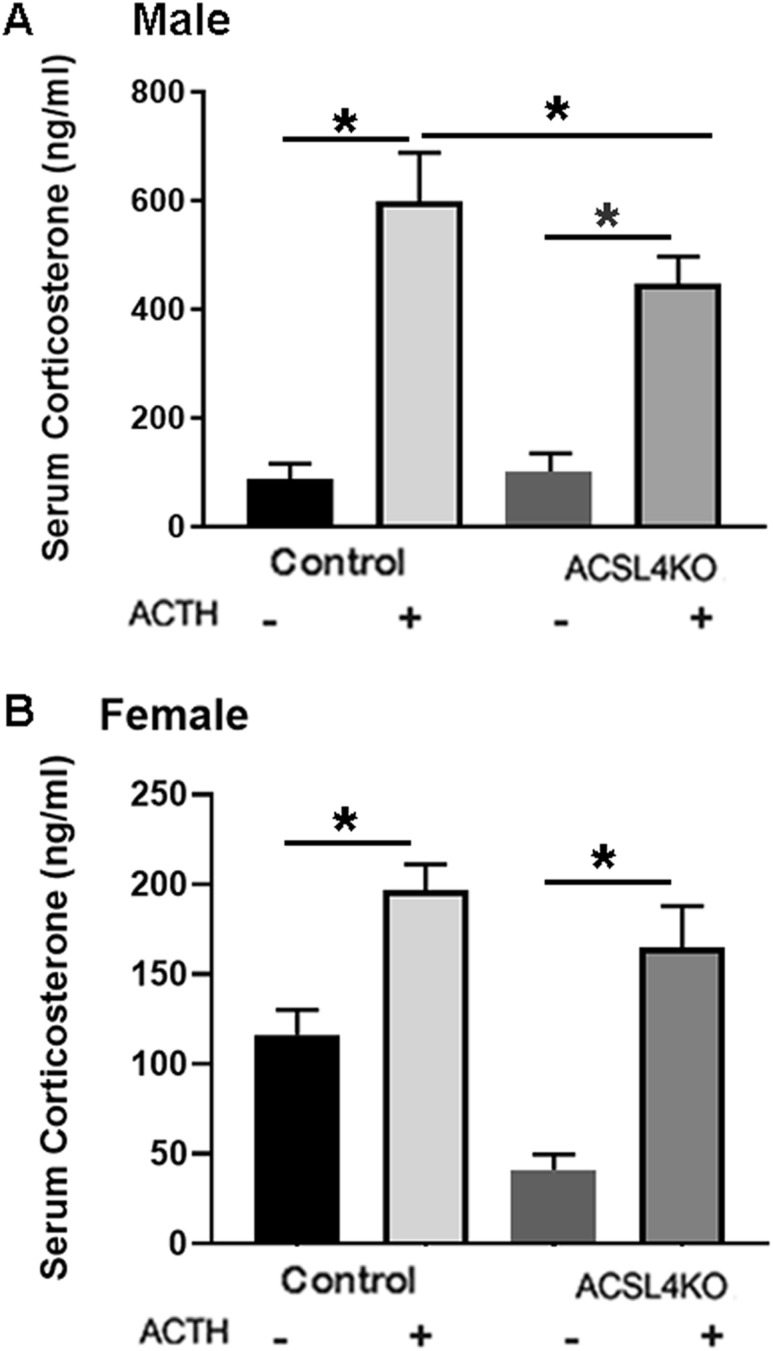
To analyze the role of ACSL4 in adrenal steroidogenesis, both male and female control and ACSL4 KO mice were treated with cosyntropin, and serum was collected 1 hour later for analysis of corticosterone levels. As shown in the male mice (Fig. 4A), maximal corticosterone levels were reduced 25% in male ACSL4 KO (P < 0.05) compared with control mice. In the female mice, there were no statistically significant differences in maximum levels of corticosterone after ACTH stimulation between control and ACSL4 KO mice, though there was a trend toward a decrease in ACSL4 KO mice (Fig. 4B).
Figure 4.
Serum corticosterone in control and ACSL4 KO mice. (A) Serum corticosterone levels in control and ACSL4 KO male mice. Male control and ACSL4 KO mice that were 16 to 24 wk old (n = 10) were treated with cosyntropin (2.5 IU per mouse, 1 h), and serum was collected before and 1 h after the treatment. (B) Serum corticosterone levels in control and ACSL4 KO female animals: 12- to 24-wk-old female control and ACSL4 KO mice (n = 9) were treated with cosyntropin (2.5 IU per mouse, 1 h), and serum was collected before and 1 h after the treatment. Serum corticosterone level was assayed using an ELISA kit. Data are presented as means ± SEM. *P < 0.05. -, without; +, with.
Decreased steroidogenesis ex vivo in adrenals from ACSL4 KO mice
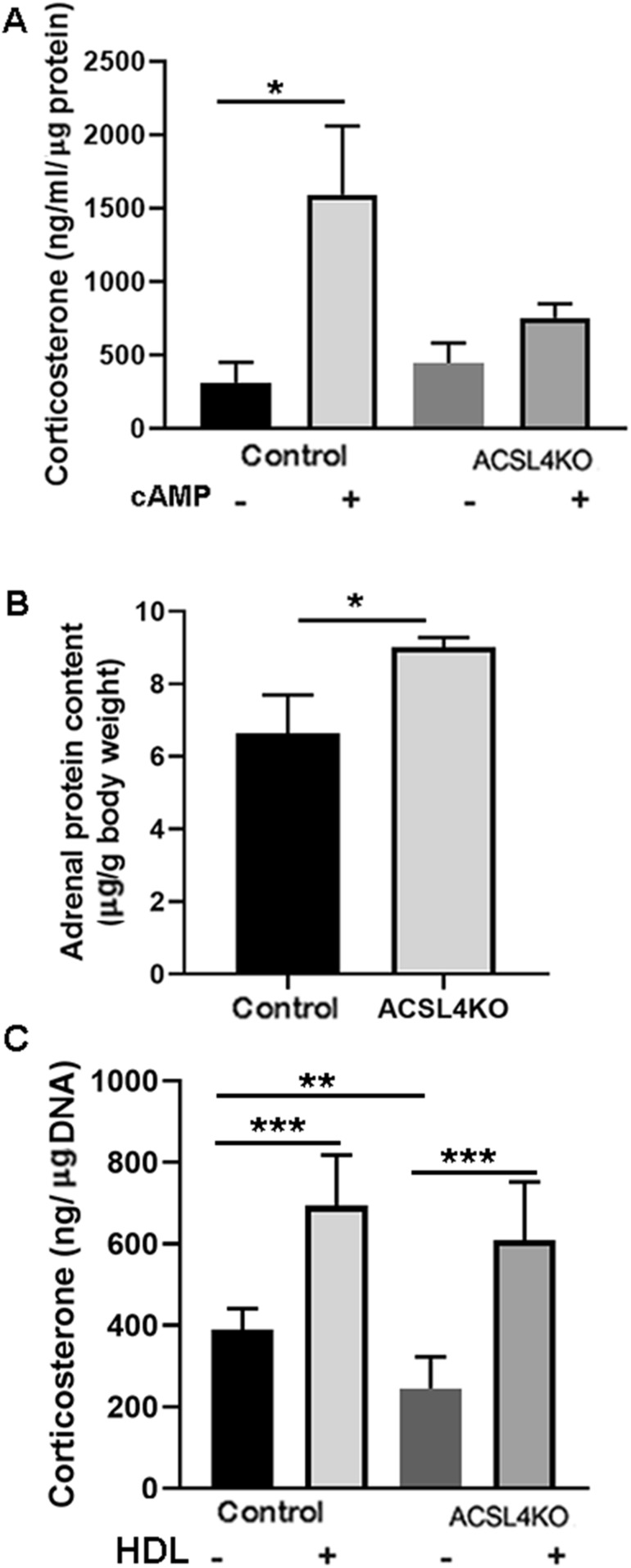
To further explore potential changes in circulating serum corticosterone levels, adrenals from female control and ACSL4 KO mice were dissected and placed in culture medium after being dissected in half. Corticosterone levels in the media were measured before and after treatment with 2.5 mM of Bt2cAMP for 1 hour. As shown in Fig. 5A, cAMP treatment significantly increased the production of corticosterone from control adrenals; however, in contrast to the in vivo observation, the response of the adrenals from ACSL4 KO mice to cAMP treatment was significantly blunted. This difference between the observations in vivo and ex vivo is likely due to the incubation conditions for examining ex vivo steroid production, whereby steroid synthesis is dependent entirely on cellular cholesterol and CEs as substrate, as opposed to in vivo, whereby circulating lipoproteins are abundantly available as a major source of cholesterol substrate. Analysis of the protein content of the adrenals showed significantly more protein in ACSL4 KO adrenals than in control adrenals (Fig. 5B), indicating possible compensatory hypertrophy in the adrenals of ACSL4 KO mice.
Figure 5.
Decreased ex vivo adrenal steroidogenesis in ACSL4 KO mice. (A) Ex vivo corticosterone production. Adrenals from 12- to 24-wk-old female mice (n = 9) were cut in half and cultured ex vivo. After 0.5-h preincubation, media were changed with fresh media with or without 2.5 mM of Bt2cAMP and incubated for another 1 h. Corticosterone levels in the media were measured. (B) Total adrenal protein. Protein content in tissue extracts of adrenals was assayed using bicinchoninic acid reagent. Total protein content of the adrenals was assayed and expressed as a percentage of the animal’s body weight. (C) Effects of HDL on ex vivo corticosterone production. Adrenals from 12- to 24-wk-old female mice (n = 9) were cut in half and cultured ex vivo. After 0.5-h preincubation, media were changed with fresh media with 2.5 mM of Bt2cAMP and with or without HDL (500 µg CE/mL) and incubated for another 1 h. Corticosterone levels in the media were measured. Data are expressed as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.005. -, without; +, with.
To further address the basis for the difference between the observations in vivo and ex vivo, adrenals from female control and ACSL4 KO mice were dissected, placed in culture medium after being dissected in half, and incubated with cAMP in the absence or presence of HDL (500 µg CE/mL) as a source of cholesterol. As shown in Fig. 5C, the production of corticosterone was again reduced in adrenals from ACSL4 KO mice compared with adrenals from controls; however, the presence of HDL in the incubation period normalized corticosterone production in the adrenals of ACSL4 KO mice, consistent with most or all of the reduction in ex vivo production of corticosterone in ACSL4 KO adrenals as a result of reduced cellular CE stores.
Effects of tissue-specific ablation of ACSL4 on stimulated gonadal steroids
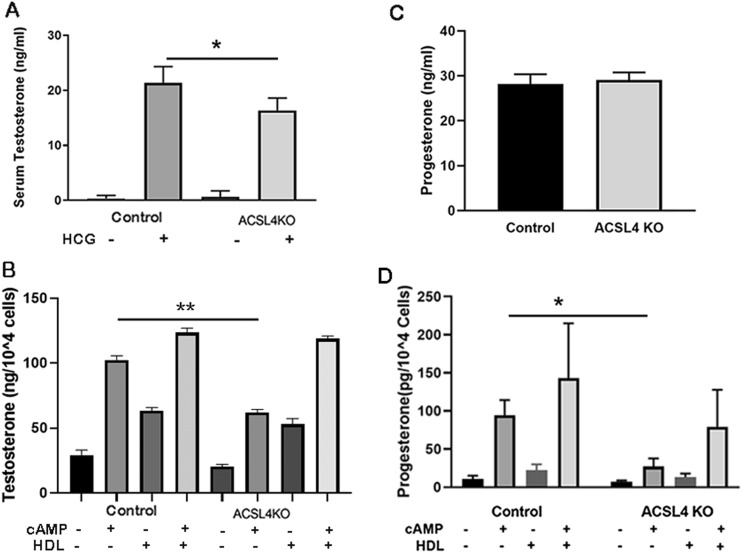
To examine the role of ACSL4 in gonadal steroidogenesis, male control and ACSL4 KO mice were treated with hCG, and serum was collected 4 hours later for analysis of testosterone levels (Fig. 6A). After hCG treatment, testosterone levels were reduced ∼22% in male ACSL4 KO mice (P < 0.05). To further explore potential changes in testosterone production, Leydig cells were prepared from age-matched control and ACSL4 KO mice, and cells were treated with Bt2cAMP. As shown in Fig. 6B, basal testosterone production from isolated Leydig cells was very low, and cAMP treatment resulted in significant increases in testosterone production in cells isolated from control mice (P < 0.05); however, similar to observations with adrenals ex vivo, testosterone production from isolated Leydig cells from ACSL4 KO mice was significantly blunted (P < 0.05). Again, similar to observations with adrenals ex vivo, testosterone production from isolated Leydig cells from ACSL4 KO mice was normalized in the presence of cAMP and HDL, consistent with most or all of the reduction in ex vivo production of testosterone in ACSL4 KO testes, probably because of reduced cellular CE stores (though not measured).
Figure 6.
Gonadal steroid production in ACSL4 KO mice. (A) Serum testosterone in control and ACSL4 KO male mice. Male control and ACSL4 KO mice that were 16 to 24 wk old (n = 10) were treated with hCG (2.5 IU per mouse), and serum was collected before and 4 h after the treatment for measurement of testosterone. (B) Testosterone production in isolated Leydig cells. Leydig cells were isolated from 16- to 24-wk-old male mice (n = 6) using Percoll gradients after the procedures described in “Materials and Methods.” Freshly purified Leydig cells were incubated without (basal) or with Bt2cAMP (2.5 mM) and/or HDL (500 µg CE/mL) for 5 h, and incubation media were collected and frozen until assayed by RIA for secreted testosterone. Three separate experiments were performed, with triplicate samples for each treatment. (C) Serum progesterone in control and ACSL4 KO female mice. Female control and ACSL4 KO mice that were 16 to 24 wk old (n = 10) were treated with PMSG/hCG (2.5 IU per mouse), and serum was collected before and 5 h after the treatment for measurement of progesterone. (D) Progesterone production in isolated granulosa cells. Age-matched female control and ACSL4 KO mice (n = 3 to 5) were injected twice with 5 IU of PMSG for 48 h. Granulosa cells were isolated after the procedures described in the Materials and Methods. Freshly purified granulosa cells were incubated without (basal) or with Bt2cAMP (2.5 mM) and/or human apoE-free HDL (500 µg CE/mL) for 5 h. After incubation, media were collected and frozen until assayed by ELISA for secreted progesterone. Three separate experiments were performed, with triplicate samples for each treatment. Data are expressed as means ± SEM. *P < 0.05; **P < 0.01. -, without; +, with.
To examine the role of ACSL4 in ovarian steroidogenesis, female control and ACSL4 KO mice were treated with PMSG/hCG, and serum was collected 4 hours later for analysis of progesterone levels (Fig. 6C). After PMSG/hCG treatment, progesterone levels were similar in female control and ACSL4 KO mice. To further explore potential changes in progesterone production, granulosa cells were prepared from age-matched control and ACSL4 KO mice, and cells were treated with Bt2cAMP (Fig. 6D). Basal progesterone production from isolated granulosa cells was very low, and cAMP treatment resulted in significant increases in progesterone production in cells isolated from control mice. However, similar to observations with adrenals ex vivo and isolated Leydig cells, cAMP-stimulated progesterone production from isolated granulosa cells from ACSL4 KO mice was significantly different from that of control mice (P < 0.05). Again, similar to observations with adrenals ex vivo and isolated Leydig cells, progesterone production in the presence of cAMP and HDL was increased and was not statistically different between granulosa cells isolated from ACSL4 KO and control mice.
Discussion
In the current studies, we examined the effects of deficiency of ACSL4 in steroidogenic tissues on CE storage and steroidogenesis. ACSL4 is a member of the ACSL family that catalyzes the conversion of long-chain polyunsaturated fatty acids to acyl-CoAs, which are essential for fatty acid incorporation and utilization in metabolic pathways (23, 49, 50). ACSL4 was originally identified as being highly expressed in the adrenal, ovary, and testis and is reported to have a preference for long-chain polyunsaturated fatty acids, in particular arachidonate (C20:4) and eicosapentaenoate (C20:5) among the fatty acids examined (23, 26, 48, 51, 52). However, it is important to note that ACSL4 displays activity against a number of other unsaturated, as well as saturated, fatty acids, albeit with generally lower affinities (50, 53). Moreover, other ACSLs display overlapping activities against similar fatty acid species, but with differing affinities.
As opposed to macrophages, circulating lipoproteins, and tissues such as the liver in which cholesteryl oleate is the predominant form of CEs, steroidogenic tissues such as the adrenal are particularly enriched in CEs of long-chain polyunsaturated fatty acids. Earlier studies of fatty acid composition of adrenal CEs examined rat adrenals using targeted methods such as gas-liquid chromatography (54–58) or HPLC (21). Although the described CE compositions varied somewhat among studies, the predominant fatty acids consistently reported were adrenate (C22:4) and arachidonate (C20:4), with smaller amounts of oleate (C18:1), palmitate (C16:0), and stearate (C18:0), as well as some highly unsaturated longer chain species. In the current studies, we used HPLC-electrospray quadrupole time-of-flight tandem mass spectrometry for a modern, unbiased lipidomics approach to analyze CE species in the adrenals of normal mice that had been maintained on normal chow diets. Our results showed that the dominant fatty acids of adrenal CEs were adrenate (C22:4; ∼24%), docosapentaenoate (C22:5; ∼30%), and docosahexaenoate (C22:6; ∼27%), with minor components of arachidonate (C20:4; ∼4.5%), nervonate (C24:1; ∼2%), and linoleate (C18:2; ∼1%) along with smaller amounts (<1%) of other fatty acids. The reasons for our observed differences in CE fatty acid composition compared with that of previous reports are likely attributable to both species differences (mice vs rats) and, importantly, methodological differences, whereby some fatty acid species were not typically identified using earlier techniques.
By crossing floxed ACSL4 mice with transgenic mice expressing Cre recombinase under control of the CYP11A1 promoter, we successfully generated mice with markedly reduced expression of ACSL4 in the adrenal and lesser reductions in Leydig cells and the ovary. Of note, the deficiency of ACSL4 in the adrenal resulted in alterations in both adrenal CE content and composition, documenting that products of ACSL4 are important in adrenal CE formation; this is consistent both with the preferences of ACSL4 for long-chain polyunsaturated fatty acids and with long-chain polyunsaturated fatty acids being the dominant components of adrenal CEs. Thus, ACSL4 deficiency resulted in an ∼50% reduction in total adrenal CE content, which was reflected by decreases in cholesteryl adrenate, cholesteryl docosapentaenoate, cholesteryl docosahexaenoate, and cholesteryl arachidonate. These reductions suggest that ACSL4 plays an important role in generating/activating these long-chain polyunsaturated fatty acids in the adrenal.
ACSL4 deficiency resulted in diminished in vivo corticosterone response to ACTH in male, but not female, mice and diminished testosterone response to hCG in vivo in male mice; however, no alterations in PMSG-stimulated progesterone production was observed in vivo in female mice. Of note, in ACSL4 KO mice no alterations were observed in mRNA levels of genes encoding enzymes involved in steroidogenesis that could possibly contribute to defective steroidogenesis. ACSL4 was previously reported to be essential for the production of prostaglandins, which function as downstream signaling molecules for the induction of StAR and subsequently for steroidogenesis (22). Indeed, in vitro knockdown of ACSL4 in adrenal cells or in Leydig cells (28) was reported to abrogate steroid production (28, 59, 60). The current observations with ACSL4 KO mice do not seem to be compatible with this conclusion. Rather, it would appear that the deficits observed in steroid production in ACSL4 KO mice are more likely secondary to reductions in CE stores than to effects on signaling pathways. It should be noted that reduced CE stores in ACSL4 KO mice were documented only in adrenals in the current studies, and CE stores were not quantified in Leydig cells or granulosa cells of ACSL4 KO mice. Nonetheless, the conclusion that deficits observed in steroid production in ACSL4 KO mice are likely secondary to reductions in CE stores in each of the steroid-producing tissues is further supported by ex vivo experiments of adrenal slices and in vitro experiments of isolated Leydig cells and granulosa cells, wherein more marked defects in steroid production were displayed in both male and female mice than in the in vivo setting.
The experimental conditions of both the ex vivo and in vitro experiments would be expected to accentuate any defects in steroid production that are due to reductions in CE stores because lipoproteins are not available under this situation, thus creating conditions with greater reliance on stored CEs and/or cellular cholesterol synthesis for supplying cholesterol substrate for steroidogenesis. Indeed, including HDL as another source of cholesterol substrate in the ex vivo incubation normalized the corticosterone response of ACSL4 KO adrenals, normalized the testosterone response of ACSL4 KO Leydig cells, and normalized the progesterone response of isolated ACSL4 KO granulosa cells. Thus, the ex vivo and in vitro observations reinforce the importance of CE stores as an essential source of cholesterol for steroidogenesis and further emphasize the importance of stored CEs as the initial source of cholesterol for steroidogenesis.
In summary, the current studies using CYP11A1 promoter–mediated Cre to generate steroid tissue‒specific ACSL4 KO mice demonstrated that ACSL4 plays an important role in adrenal CE formation, as well as in determining the fatty acyl composition of adrenal CEs. Although statistically significant reductions in corticosterone and testosterone production, but not in progesterone production, were observed in vivo, these effects appear to be due to reductions in CE stores rather than disturbances of signaling pathways. We conclude that ACSL4 is dispensable for steroidogenesis.
Acknowledgments
Financial Support: This work was supported in part by Merit Review Awards I01BX001923 (to S.A.) and I01BX000398 (to F.B.K.) and by Senior Research Career Scientist Award IK6B004200 (to S.A.) from the US Department of Veterans Affairs, Biomedical Laboratory Research Development Program; by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants P30 DK116074 (to F.B.K.), P30 DK046200 (to A.S.G.), and R01 DK108722 (to A.S.G.); and by USDA Grants 1950-5100-071-02S and 1R21HD098056 (both to A.S.G.).
Additional Information
Current Affiliations: W. Wang’s current affiliation is the Department of Endocrinology, Peking University First Hospital, Beijing, China. X. Hao’s current affiliation is the Department of Endocrinology, First Affiliated Hospital of the Medical College of Zhengzhou University, Zhengzhou, China. L. Han’s current affiliation is the Department of Ultrasound, Sichuan University, Chengdu, China. Z. Yan’s current affiliation is the Division of Endocrinology, Sichuan University, Chengdu, China. K Hasbargen’s current affiliation is Medical Department IV–Grosshadern Clinic, Ludwig-Maximilians University, Munich, Germany.
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Glossary
Abbreviations:
- ACSL
long-chain fatty acid‒coenzyme A synthetase
- CE
cholesteryl ester
- eCG
equine chorionic gonadotropin
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- hCG
human chorionic gonadotropin
- HDL
high-density lipoprotein
- IR
infrared
- KO
knockout
- LD
lipid droplet
- PMSG
pregnant mare’s serum gonadotropin
References and Notes
- 1. Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18(4):502–519. [DOI] [PubMed] [Google Scholar]
- 2. Hiort O, Holterhus PM, Nitsche EM. Physiology and pathophysiology of androgen action. Baillieres Clin Endocrinol Metab. 1998;12(1):115–132. [DOI] [PubMed] [Google Scholar]
- 3. Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55(7):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74(5):279–285. [DOI] [PubMed] [Google Scholar]
- 5. Pearce D, Bhargava A, Cole TJ. Aldosterone: its receptor, target genes, and actions. Vitam Horm. 2003;66:29–76. [DOI] [PubMed] [Google Scholar]
- 6. Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25(6):947–970. [DOI] [PubMed] [Google Scholar]
- 7. LaVoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med (Maywood). 2009;234(8):880–907. [DOI] [PubMed] [Google Scholar]
- 8. Miller WL. Steroidogenic enzymes. Endocr Dev. 2008;13:1–18. [DOI] [PubMed] [Google Scholar]
- 9. Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17(3):221–244. [DOI] [PubMed] [Google Scholar]
- 10. Stocco DM. Intramitochondrial cholesterol transfer. Biochim Biophys Acta. 2000;1486(1):184–197. [DOI] [PubMed] [Google Scholar]
- 11. Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43(10):1585–1594. [DOI] [PubMed] [Google Scholar]
- 12. Papadopoulos V, Miller WL. Role of mitochondria in steroidogenesis. Best Pract Res Clin Endocrinol Metab. 2012;26(6):771–790. [DOI] [PubMed] [Google Scholar]
- 13. Hu J, Zhang Z, Shen WJ, Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab (Lond). 2010;7(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52(12):2111–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jefcoate CR, McNamara BC, Artemenko I, Yamazaki T. Regulation of cholesterol movement to mitochondrial cytochrome P450scc in steroid hormone synthesis. J Steroid Biochem Mol Biol. 1992;43(8):751–767. [DOI] [PubMed] [Google Scholar]
- 16. Simpson E, Lauber M, Demeter M, Means G, Mahendroo M, Kilgore M, Mendelson C, Waterman M. Regulation of expression of the genes encoding steroidogenic enzymes in the ovary. J Steroid Biochem Mol Biol. 1992;41(3-8):409–413. [DOI] [PubMed] [Google Scholar]
- 17. Payne AH, Youngblood GL. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol Reprod. 1995;52(2):217–225. [DOI] [PubMed] [Google Scholar]
- 18. Sewer MB, Dammer EB, Jagarlapudi S. Transcriptional regulation of adrenocortical steroidogenic gene expression. Drug Metab Rev. 2007;39(2-3):371–388. [DOI] [PubMed] [Google Scholar]
- 19. Kraemer FB. Adrenal cholesterol utilization. Mol Cell Endocrinol. 2007;265-266:42–45. [DOI] [PubMed] [Google Scholar]
- 20. Cooper DE, Young PA, Klett EL, Coleman RA. Physiological consequences of compartmentalized acyl-CoA metabolism. J Biol Chem. 2015;290(33):20023–20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng B, Kowal J. Analysis of adrenal cholesteryl esters by reversed phase high performance liquid chromatography. J Lipid Res. 1994;35(6):1115–1121. [PubMed] [Google Scholar]
- 22. Kang MJ, Fujino T, Sasano H, Minekura H, Yabuki N, Nagura H, Iijima H, Yamamoto TT. A novel arachidonate-preferring acyl-CoA synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis. Proc Natl Acad Sci USA. 1997;94(7):2880–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soupene E, Kuypers FA. Mammalian long-chain acyl-CoA synthetases. Exp Biol Med (Maywood). 2008;233(5):507–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewin TM, Van Horn CG, Krisans SK, Coleman RA. Rat liver acyl-CoA synthetase 4 is a peripheral-membrane protein located in two distinct subcellular organelles, peroxisomes, and mitochondrial-associated membrane. Arch Biochem Biophys. 2002;404(2):263–270. [DOI] [PubMed] [Google Scholar]
- 25. Küch EM, Vellaramkalayil R, Zhang I, Lehnen D, Brügger B, Sreemmel W, Ehehalt R, Poppelreuther M, Füllekrug J. Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim Biophys Acta. 2014;1841(2):227–239. [DOI] [PubMed] [Google Scholar]
- 26. Killion EA, Reeves AR, El Azzouny MA, Yan QW, Surujon D, Griffin JD, Bowman TA, Wang C, Matthan NR, Klett EL, Kong D, Newman JW, Han X, Lee MJ, Coleman RA, Greenberg AS. A role for long-chain acyl-CoA synthetase-4 (ACSL4) in diet-induced phospholipid remodeling and obesity-associated adipocyte dysfunction. Mol Metab. 2018;9:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cornejo Maciel F, Maloberti P, Neuman I, Cano F, Castilla R, Castillo F, Paz C, Podestá EJ. An arachidonic acid-preferring acyl-CoA synthetase is a hormone-dependent and obligatory protein in the signal transduction pathway of steroidogenic hormones. J Mol Endocrinol. 2005;34(3):655–666. [DOI] [PubMed] [Google Scholar]
- 28. Maloberti P, Castilla R, Castillo F, Cornejo Maciel F, Mendez CF, Paz C, Podestá EJ. Silencing the expression of mitochondrial acyl-CoA thioesterase I and acyl-CoA synthetase 4 inhibits hormone-induced steroidogenesis. FEBS J. 2005;272(7):1804–1814. [DOI] [PubMed] [Google Scholar]
- 29. Kishida T, Kostetskii I, Zhang Z, Martinez F, Liu P, Walkley SU, Dwyer NK, Blanchette-Mackie EJ, Radice GL, Strauss JF III. Targeted mutation of the MLN64 START domain causes only modest alterations in cellular sterol metabolism. J Biol Chem. 2004;279(18):19276–19285. [DOI] [PubMed] [Google Scholar]
- 30. Tu LN, Morohaku K, Manna PR, Pelton SH, Butler WR, Stocco DM, Selvaraj V. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J Biol Chem. 2014;289(40):27444–27454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. RRID:AB_2687825, https://scicrunch.org/resolver/AB_2687825.
- 32. RRID:AB_10956736, https://scicrunch.org/resolver/AB_10956736.
- 33. RRID:AB_2713919, https://scicrunch/resolver/AB_2713919.
- 34. Azhar S, Nomoto A, Leers-Sucheta S, Reaven E. Simultaneous induction of an HDL receptor protein (SR-BI) and the selective uptake of HDL-cholesteryl esters in a physiologically relevant steroidogenic cell model. J Lipid Res. 1998;39(8):1616–1628. [PubMed] [Google Scholar]
- 35. Shen WJ, Zaidi SK, Patel S, Cortez Y, Ueno M, Azhar R, Azhar S, Kraemer FB. Ablation of vimentin results in defective steroidogenesis. Endocrinology. 2012;153(7):3249–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu Z, Shen WJ, Kraemer FB, Azhar S. Regulation of adrenal and ovarian steroidogenesis by miR-132. J Mol Endocrinol. 2017;59(3):269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Azhar S, Tsai L, Reaven E. Uptake and utilization of lipoprotein cholesteryl esters by rat granulosa cells. Biochim Biophys Acta. 1990;1047(2):148–160. [DOI] [PubMed] [Google Scholar]
- 38. Reaven E, Tsai L, Azhar S. Intracellular events in the “selective” transport of lipoprotein-derived cholesteryl esters. J Biol Chem. 1996;271(27):16208–16217. [DOI] [PubMed] [Google Scholar]
- 39. Schumacher M, Schäfer G, Holstein AF, Hilz H. Rapid isolation of mouse Leydig cells by centrifugation in Percoll density gradients with complete retention of morphological and biochemical integrity. FEBS Lett. 1978;91(2):333–338. [DOI] [PubMed] [Google Scholar]
- 40. Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88(1):61–67. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Hao X, Han L, Yan Z, Shen W-J, Dong D, Hasbargen K, Bittner S, Cortez Y, Greenberg A, Azhar S, Kraemer F. doi: 10.6084/m9.figshare.9404102. Data from: Tissue-specific ablation of ACSL4 results in disturbed steroidogenesis. figshare 2019. Deposited 8 September 2019. [DOI] [PMC free article] [PubMed]
- 42. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 43. RRID:AB_2544613, https://scicrunch.org/resolver/AB_2544613.
- 44. RRID:AB_2223172, https://scicrunch.org/resolver/AB_2223172.
- 45. RRID:AB_561053, https://scicrunch.org/resolver/AB_561053.
- 46. O’Hara L, York JP, Zhang P, Smith LB. Targeting of GFP-Cre to the mouse Cyp11a1 locus both drives cre recombinase expression in steroidogenic cells and permits generation of Cyp11a1 knock out mice. PLoS One. 2014;9(1):e84541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dumbell R, Leliavski A, Matveeva O, Blaum C, Tsang AH, Oster H. Dissociation of molecular and endocrine circadian rhythms in male mice lacking Bmal1 in the adrenal cortex. Endocrinology. 2016;157(11):4222–4233. [DOI] [PubMed] [Google Scholar]
- 48. Klett EL, Chen S, Yechoor A, Lih FB, Coleman RA. Long-chain acyl-CoA synthetase isoforms differ in preferences for eicosanoid species and long-chain fatty acids [published correction appears in J Lipid Res. 2017;58:2365]. J Lipid Res. 2017;58(5):884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol. 2010;21(3):212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maloberti P, Cornejo Maciel F, Castillo AF, Castilla R, Duarte A, Toledo MF, Meuli F, Mele P, Paz C, Podestá EJ. Enzymes involved in arachidonic acid release in adrenal and Leydig cells. Mol Cell Endocrinol. 2007;265-266:113–120. [DOI] [PubMed] [Google Scholar]
- 51. Klett EL, Chen S, Edin ML, Li LO, Ilkayeva O, Zeldin DC, Newgard CB, Coleman RA. Diminished acyl-CoA synthetase isoform 4 activity in INS 832/13 cells reduces cellular epoxyeicosatrienoic acid levels and results in impaired glucose-stimulated insulin secretion. J Biol Chem. 2013;288(30):21618–21629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maloberti P, Lozano RC, Mele PG, Cano F, Colonna C, Mendez CF, Paz C, Podestá EJ. Concerted regulation of free arachidonic acid and hormone-induced steroid synthesis by acyl-CoA thioesterases and acyl-CoA synthetases in adrenal cells. Eur J Biochem. 2002;269(22):5599–5607. [DOI] [PubMed] [Google Scholar]
- 53. Midzak A, Papadopoulos V. Adrenal mitochondria and steroidogenesis: from individual proteins to functional protein assemblies. Front Endocrinol (Lausanne). 2016;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boyd GS, Trzeciak WH. Cholesterol metabolism in the adrenal cortex: studies on the mode of action of ACTH. Ann N Y Acad Sci. 1973;212(1 Multienzyme S):361–377. [DOI] [PubMed] [Google Scholar]
- 55. Young AK, Walker BL. Cholesteryl ester hydrolase activity in adrenal homogenates from normal and essential fatty acid-deficient female rats. Lipids. 1982;17(9):634–638. [DOI] [PubMed] [Google Scholar]
- 56. Gidez LI. Occurrence of a docosatrienoic acid in the cholesterol esters of adrenals of rats on essential fatty acid deficient diets. Biochem Biophys Res Commun. 1964;14(5):413–418. [DOI] [PubMed] [Google Scholar]
- 57. Dailey RE, Swell L, Field H Jr, Treadwell CR. Adrenal cholesterol ester fatty acid composition of different species. Proc Soc Exp Biol Med. 1960;105(1):4–6. [DOI] [PubMed] [Google Scholar]
- 58. Vahouny GV, Chanderbhan R, Bisgaier C, Hodges VA, Naghshineh S. Essential fatty acids and adrenal steroidogenesis. Prog Lipid Res. 1981;20:233–240. [DOI] [PubMed] [Google Scholar]
- 59. Wang X, Walsh LP, Reinhart AJ, Stocco DM. The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J Biol Chem. 2000;275(26):20204–20209. [DOI] [PubMed] [Google Scholar]
- 60. Wang XJ, Dyson MT, Jo Y, Eubank DW, Stocco DM. Involvement of 5-lipoxygenase metabolites of arachidonic acid in cyclic AMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J Steroid Biochem Mol Biol. 2003;85(2-5):159–166. [DOI] [PubMed] [Google Scholar]