Abstract
Objective:
Epidemiologic and clinical research papers often describe the study sample in the first table. If well-executed, this “Table 1” can illuminate potential threats to internal and external validity. However, little guidance exists on best practices for designing a Table 1, especially for complex study designs and analyses. We aimed to summarize and extend the literature related to reporting descriptive statistics.
Study design and setting:
In consultation with existing guidelines, we synthesized and developed reporting recommendations driven by study design and focused on transparency related to potential threats to internal and external validity.
Results:
We describe a basic structure for Table 1, and discuss simple modifications in terms of columns, rows, and cells to enhance a reader’s ability to judge both internal and external validity. We further highlight several analytic complexities common in epidemiologic research (missing data, sample weights, clustered data, and interaction), and describe possible variations to Table 1 to maintain and add clarity about study validity in light of these issues. We discuss considerations and tradeoffs in Table 1 related to breadth and comprehensiveness versus parsimony and reader-friendliness.
Conclusion:
We anticipate that our work will guide authors considering layouts for Table 1, with attention to the reader’s perspective.
Keywords: Descriptive statistics, Tables, Epidemiologic Methods, External validity, Internal validity, Generalizability, Clinical Research
1. INTRODUCTION
“Who is in this study?” is the first question many readers of clinical and epidemiologic studies ask. Readers care about who is in a study because it helps them understand and evaluate the study’s findings: to assess applicability to other patients or populations (i.e. external validity), and risk of bias (i.e. internal validity).1,2 As a result, papers often include a table that describes the study sample; this is commonly the first table in a paper. This “Table 1,” as it is colloquially called, can be designed to shed light on potential threats to both internal and external validity of study findings.
Table 1 can provide insights on threats to internal validity in the traditional epidemiologic framework: a) confounding (i.e. differences in other causes of the outcome between exposed and unexposed), b) selection bias (e.g. differential cohort attrition or control selection), and c) measurement error.3 The primary threats to external validity about which Table 1 can be informative are differences in effect modifiers or other causes of the outcome between the source population and target population; information on these is often insufficient.4
As the study designs and analytic approaches used in modern research have grown in variability and complexity, it has become more challenging to create a Table 1 that serves to inform readers about issues related to study validity. Although brief guidance exists on best practices for reporting descriptive data on study samples,1,2,5,6 these guidelines generally do not address many analytic issues.
To address this gap, we outline considerations in creating a Table 1 that aids readers in judging internal and external validity. In consultation with the limited existing guidelines, we lay out a minimally sufficient Table 1 structure driven by study design, and discuss examples of specific analytic issues for which Table 1 could be modified. Throughout, we focus on validity of studies estimating causal effects, although some considerations may also be relevant for studies with descriptive or predictive goals. To distill and concretize some of the issues discussed, we include an overview figure (Figure 1), and two annotated example tables adapted from published studies,7,8 including a case-control study (Figure 2), and a cohort study with a specific analytic complexity, missing data (Figure 3).
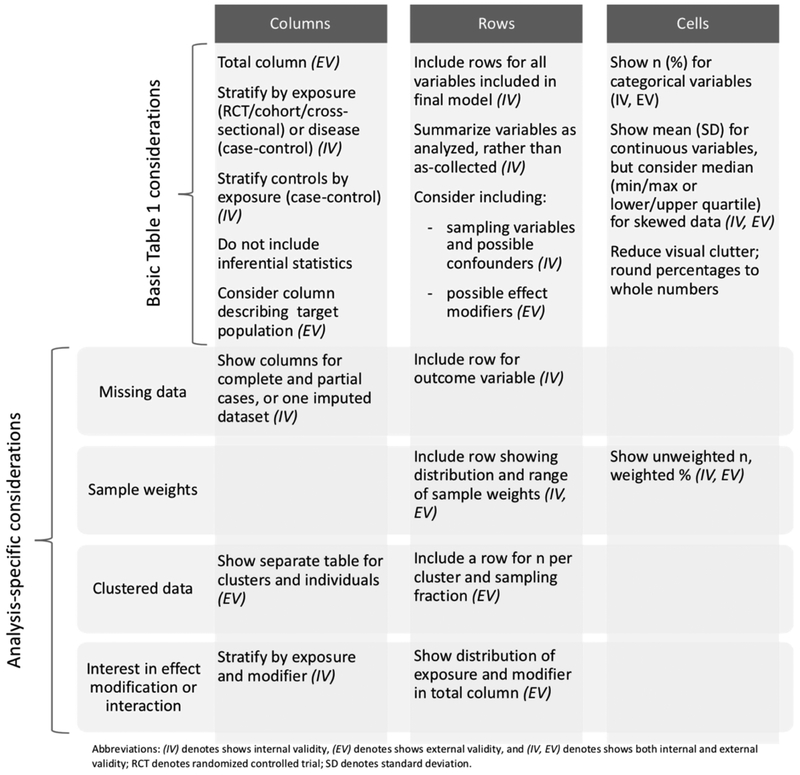
Figure 1.
Basic Table 1 structure and analysis-specific considerations affecting columns, rows, and cells.
Figure 2.
Example construction of Table 1 for a hypothetical case-control study.
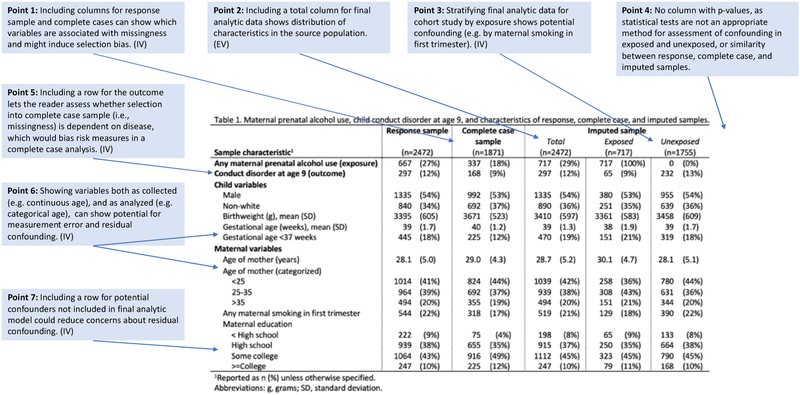
Figure 3.
Example construction of Table 1 for a hypothetical cohort study with missing data.
2. BASIC STRUCTURE OF TABLE 1
The simplest Table 1 is a single column of descriptive statistics for the total study sample, with rows containing key study variables (minimally, all variables included in the final main analysis). Inside each cell, descriptive statistics are typically given as n (%) for categorical variables and mean (standard deviation) or median (25th-75th percentile or minimum-maximum) for continuous variables.1,2 The total column can be useful for assessing external validity, as the reader can examine the characteristics of the whole sample. However, expansions to this basic structure, driven by study design, can provide substantially more insight regarding threats to both internal and external validity (Figure 1) and are more common.
2.1. Considering columns
Table 1 frequently includes columns showing distributions of key variables within sample strata to maximize transparency for assessing internal validity. For example, the primary exposure is a common stratification variable for cohort studies, randomized controlled trials (RCTs), and cross-sectional studies, complementing subsequent tables focused on associations with the outcome. Stratifying by exposure allows assessment of potential confounding,1,2 which may be apparent as an uneven distribution of other causes of the outcome between exposed and unexposed. (When the exposure of interest is continuous, stratification is less straightforward; median or other splits may be used.) In contrast, in case-control studies, stratifying by disease (case vs. control) status is most common; a total column is no longer appropriate as the controls represent the source population (Figure 2, Point 1).2 While stratification by disease status may allow for some assessment of selection bias (e.g. showing whether cases would have reasonably arisen from that source population), it does not allow assessment of confounding because cases and controls are expected to have different distributions of causes of the outcome (some of which are confounders).2 To assess the potential for confounding in a case-control study, the control column can be further stratified by exposure; this allows readers to compare distributions of potential confounders between exposed and unexposed controls, revealing possible confounding resulting from correlations in the source population (Figure 2, Point 2).2
Authors may also add columns to Table 1 to inform readers about external validity. When a study has one or more target populations in mind and data are available for these target populations, including a column showing the distribution of the study variables in each target population allows the reader to make a direct comparison between the study and target populations.6
The appropriateness of including a column containing inferential statistics (e.g. p-values) is a topic of some controversy. Statistical testing of distributions of variables (e.g. between exposed and unexposed) is common and even occasionally required by journals;1,6,9,10 although this is a tempting way to assess confounding, it is not best practice. Statistical significance is often misunderstood: non-significance of a p-value does not indicate that no difference in the distribution of a variable exists, and significance does not mean that the difference is meaningful or that the difference indicates presence of confounding.10–13 As a result, confounder assessment should not be based on p-values (Figure 2, Point 3; Figure 3, Point 4).1,2 Rather, authors should consider whether the relationship between the exposure and hypothesized confounders is as expected according to the causal theory, and consider whether the magnitude of an observed difference for a potential confounder represents a meaningful difference.1,9,10 Similarly, when considering external validity, statistical tests are not a helpful way to assess meaningful differences between source and target populations.
2.2. Considering rows
Reader ability to assess internal validity can also be improved with careful consideration of the rows in Table 1. For all types of study designs, in addition to the key study variables included in the final analyses, it may be helpful to include other potential confounders evaluated (Figure 3, Point 7) and selection variables (e.g., variables used in sampling, variables that may influence study participation or loss to follow up). Inclusion of these variables may inform or pre-empt concerns about residual confounding or selection bias by variables not included in the final analysis. For example, in case-control studies, including rows for variables where differences might indicate the presence of selection biases in enrollment (e.g. distance from home to hospital in a hospital-based case-control study) may be useful (Figure 2, Point 6).
Adding rows can also help readers assess external validity. Even if a target population column is not included as described above, adding rows that show distributions of major baseline demographics,14 including important potential effect modifiers will be useful to a reader in assessing the likelihood of meaningful differences in the effects of interest between the source population and a given target population.15,16 Even if effect modification is not explicitly modeled, showing distributions of possible effect modifiers is still useful for assessing threats to external validity (Figure 2, Point 8).
For studies involving time-to-event analyses, authors should include a summary of person-time, including total and mean or median per person,2,5 as well as a summary of reasons for censoring, stratified by exposure status. The reasons for censoring can inform assessment about internal validity; if reasons for censoring differ between exposed and unexposed, the censoring may be informative, a threat to internal validity. The average person-time can be informative about external validity, as effects of exposures/interventions may differ over different lengths of observation.
When variables are analyzed differently from how they were collected (e.g. a continuous variable is categorized or a categorical variable is collapsed), there are tradeoffs in terms of which version of the variable to show. In general, we suggest including the variable as analyzed in the main analysis to ease navigation between tables. However, other choices may also be appropriate. For example, choices about categorizing continuous variables may introduce measurement error or residual confounding compared to the continuous variable; showing both the analyzed categorical variable and the original variable as measured allows categorization decisions, and any bias introduced by them, to be more transparent to the reader (Figure 2, Point 4; Figure 3, Point 6). In addition, showing only a coarsely categorized variable might also limit the reader’s ability to align categories with an outside data source using different cut points to assess external validity.
2.3. Considering cells
For table readability and easy comparison across columns, the contents of the cells should focus on reducing visual clutter. Some options include showing only percentages for categorical variables, with the N in the column header, or rounding percentages to whole numbers (e.g. 73.1% to 73%, Figure 2, Point 5); more precision should be shown only if it improves a reader’s understanding and is based on a large number of observations.
Cell content decisions can also affect clarity regarding internal and external validity, especially for continuous variables, where authors must decide whether to present mean and standard deviation or percentile-based descriptive data. Although the mean and standard deviation approach is common,1,2 showing a summary including minimum, lower quartile, median, upper quartile, and maximum can be more informative when variables are not normally distributed, or are differently distributed within strata (Figure 2, Point 7). More information about distributions can help readers assess a) whether measurement error could have occurred in data collection, compromising internal validity (e.g. if the distribution of blood pressure measurements is lower than expected), b) whether influential data points could have undue leverage on the reported effect estimate (also potentially compromising internal validity) and c) how the distribution in the sample may compare to a target population, informing the external validity of the results.
A final consideration within cells relates to indicating the absence of a value, which may be due to non-applicability, a measurement that was not completed for all observations (see next section on missing data), or the suppression of values within small strata to preserve confidentiality. The use of shading or a character (e.g., period or dash) rather than a blank cell communicates that the omission is intentional, and may be accompanied with clarifying text in the table footnote.
3. ANALYSIS-SPECIFIC CONSIDERATIONS
3.1. I have missing data
Missing data are a common problem in studies, and may arise from non-response, loss to follow-up/censoring, or other reasons. Patterns such as “missing completely at random,” “missing at random,” or “not missing at random” are commonly used to describe missing data in the literature.17 Missing data is primarily a concern because observations without data on all analytic variables cannot be included in the analysis, and only observations with complete data will be “selected” into the final analytic sample. This selection may or may not cause bias depending on the relationships between the missing data (i.e. selection), exposure, outcome, and other variables in the analysis;18–20 threats to validity due to missing data selection processes are best examined through the lens and language of selection bias.18,19,21 (One exception is that missing outcome data in those who were censored in time-to-event analyses does not preclude inclusion in the sample; nonetheless, selection can occur because only non-censored person-time with complete data on other analytic variables can be included.)
Table 1 can help show the potential (or lack thereof) for missing data to cause selection bias, complementing participant flow diagrams suggested by guidelines.1,2 Flow diagrams typically focus on the proportion and reasons (e.g. loss to follow up) for missing data, rather than their potential to bias results. In contrast, columns in Table 1 showing observations with and without missing data (often called partial and complete cases, respectively) allow the reader to assess whether measured variables are associated with missingness/selection (Figure 3, Point 1). Among these measured variables, including a row to show whether the outcome is associated with selection is particularly important; selection based on disease biases risk measures (Figure 3, Point 5).19 These modifications to Table 1, in combination with the hypothesized causal structure, can allow a reader to evaluate whether selection is associated with variables that could bias results. However, selection associated with unmeasured variables remains a possibility, for which the reasons for missing data, if known, are informative.
Because several analytic options (e.g. complete case or multiple imputation) exist for handling missing data,1,2,17,22 showing complete and partial cases in Table 1 can also help an author clarify the motivation for the analytic approach used. For example, if Table 1 shows no differences between complete and partial cases except for the distribution of the exposure, a complete case analysis will likely be unbiased.19 However, when selection is associated with the outcome, a complete case analysis will be biased and is therefore inappropriate.18,19 Complete case analyses may also be biased when selection is associated with other variables, depending on the causal structure hypothesized.18,19 In these cases, authors must consider multiple imputation, inverse probability weighting, or other approaches that are more robust. Importantly, the rationale for the missing data approach taken in a research paper, along with sensitivity analyses, are recommended or required by some journals.23–25
Once a final analytic sample has been chosen to minimize concerns about selection bias due to missing data, Table 1 can be further expanded to show other threats to internal and external validity as previously described. The best way to do this is to include data on the final analytic sample (e.g. complete case or one imputed dataset17), in accordance with the basic structure outlined above (Figure 3, Point 3).
3.2. My study used sample weights
Many studies, especially surveys designed to produce estimates for a specific population, use sample weights. Sampling weights correct for over- or under-sampling of specific population strata, and after sampling weights have been applied the study sample represents the source population. Therefore, it is not possible to judge the internal validity of a weighted analysis if the weighted distributions are not presented in Table 1; only after weighting is it possible to tell if potential confounder is associated with the exposure in the data as it will be analyzed. To this end, showing unweighted proportions hinders a reader’s ability to judge the presence of confounding in the source population. It may also be helpful to report the number of subjects, minimum and maximum weights, and distribution of weights in Table 1 to allow for assessment of the potential for any observations to have undue influence on the results, potentially affecting internal validity. One approach is to show unweighted N’s and weighted proportions. Fortunately, the external validity of studies utilizing sample weights is also shown most clearly using weighted percentages because the weighted sample reflects the source population to which the results apply; when shown in Table 1 this is more easily compared to a target population.
3.3. I have clustered data
Studies involving clustered data, such as multilevel studies or studies with repeated measures, are increasingly common and present a unique set of challenges, especially related to external validity. In these studies, there are two source populations: one at the cluster level, and one at the individual observation level. Descriptive statistics should be provided for each population (i.e. for the clusters themselves in addition to the observations). This aids in assessing external validity because the reader can determine if the clusters themselves are similar to a target population of clusters on cluster-level variables that affect the outcome. In the event that the sampling fraction of observations is uneven within clusters, a row that describes the number of observations per cluster and sampling fraction per cluster may help readers better understand the sample. Of note, clustering plays different roles in studies involving social or other network analyses versus studies with repeated measures or hierarchical sampling, and best practices therefore differ. In network analyses, a figure displaying network topology, potentially including network summary statistics, may complement an individual-level Table 1.
3.4. I am interested in interaction or effect modification
When interaction, effect modification, or stratified results more generally are of primary interest, Table 1 should show more detail on the strata. For example, for studies of gender differences or racial disparities, it is more informative to present Table 1 within additional strata of gender or race, respectively, rather than across the entire sample. Further, because confounding can occur for the effect of either the exposure or the stratification variable, it is preferred to show distributions of all variables according to strata of both the exposure and the modifier (e.g. 4 columns for dichotomous exposure and stratification variable). Finally, because the overall causal effect is a weighted average of the stratum-specific effects, to allow assessment of external validity, there is value in using Table 1 or a corresponding results paragraph to show the distributions of the exposure and modifier in the total sample.
4. DISCUSSION
Despite the ubiquity of a “Table 1” describing the study sample, there is limited guidance on how to maximize its utility for readers. Drawing on our experience and on existing guidelines,1,2,5,6 we have described a basic structure and potential variations to help Table 1 to inform assessments of both internal and external validity. We included two example tables to concretize several of the points we raised.
A common thread across many of our recommendations is consistency between Table 1 and how the data were analyzed in the main analysis. For example, showing complete cases or imputed data reveals how missing data were handled. Likewise, showing weighted estimates in Table 1 makes the use of weighting throughout more salient. In addition to improving transparency about internal and external validity as discussed above, a Table 1 that is consistent with the analytic approach in the main analysis makes it easy on the reader to understand the main analysis, and can therefore improve a reader’s understanding of the study more broadly.
In the real-world context in which epidemiologists work (e.g. an analysis of a complex survey sample in which some data are missing), our guidance for Table 1 may result in conflicting priorities. In such complex situations, the best approach to provide insight into one threat to validity may not as prominently address others; we especially anticipate that selection of columns will be challenging. For example, inclusion of columns for the final analytic sample and the original study sample to show potential selection bias may compete for space with columns stratified by exposure, which better show confounding. Including all the variations that we discussed to maximize assessment of both internal and external validity would lead to a very large and bulky Table 1 and is likely infeasible. Thus, authors will need to prioritize the data they show in any given manuscript.
We recommend attention to a reader’s perspective. Generally speaking, it is easier for a reader to compare columns when there are fewer of them and cell contents are simpler. We suggest authors focus on the key issues for their study, driven by their study question and main results. For example, if internal validity is of the highest importance, modifications to Table 1 that show issues of confounding may take precedence over those that show external validity. However, if the goal of the study is to recommend policy for a specific target population, showing the external validity of the study to the target population may be crucial. As authors weigh these questions, they may also find it useful to consult more general resources on making tables user-friendly (e.g.26–28). Although journals often dictate style, key principles for readability and ease of comparison across columns and rows include minimizing unnecessary ink (e.g. lines between all rows/columns, or extensive decimals), using judicious shading to accentuate columns or rows in large tables, and aligning data according to decimal location.
Fortunately, many journals now offer the opportunity to include online supplementary materials. Inclusion of additional descriptive data is one excellent use of this space. Authors may include more detailed and exhaustive data, or even alternative Table 1’s that show different stratifications of sample data or describe additional target populations. This offers a way to preserve parsimony of an in-text Table 1 while still providing the reader with thorough descriptive data to aid in their assessment of internal and external validity.
5. CONCLUSION
Despite its role in inferential transparency, there is limited guidance about constructing a “Table 1.” In this article, we identified a basic structure to allow assessment of study validity, and highlighted options for modifications based on specific analytic issues. As study designs and analyses become more complex, thoughtful consideration is needed to develop a Table 1 that optimizes clarity about study internal and external validity. We believe the recommendations in this paper represent a step in this direction, and we anticipate they will lead to better clarity about study validity and therefore more convincing reporting of study findings. However, we welcome and encourage further discussion in the literature about how to achieve this goal.
WHAT IS NEW?
A well-executed “Table 1” (i.e. sample descriptives table) can illuminate potential threats to internal and external validity, but limited guidance exists on best practices for creating Table 1, especially for complex study designs and analyses.
We draw on the existing sparse literature and extend it to make suggestions on best practices for presenting descriptive data.
We find that descriptive data, even for complex analyses, can be of greater use to readers assessing study validity than it typically is, currently. Our paper suggests ways to accomplish this.
We anticipate our results will particularly improve transparency in communicating threats to internal and external validity.
FUNDING SOURCES
KLK is supported by the National Institute of Environmental Health Sciences under the grant T32-ES023772. EHL is supported by the National Institute of Mental Health under the grant T32-MH013043 and previously by the National Institute of Allergy and Infectious Disease under the grant T32-AI114398. SJM is supported by the National Library of Medicine grant K99LM012868 and previously by the Eunice Kennedy Shiver National Institute for Child Health and Human Development grant T32-HD057822. GL is supported by the National Institute on Aging [R01AG049970] and a generous gift from Dana and David Dornsife to the Drexel University Dornsife School of Public Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
REFERENCES
- 1.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. [DOI] [PubMed] [Google Scholar]
- 2.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. December 2014;12(12):1500–1524. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz S, Campbell UB, Gatto NM, Gordon K. Toward a clarification of the taxonomy of “bias” in epidemiology textbooks. Epidemiology. March 2015;26(2):216–222. [DOI] [PubMed] [Google Scholar]
- 4.Malmivaara A Generalizability of findings from randomized controlled trials is limited in the leading general medical journals. J Clin Epi. 2019;107:36–41. [DOI] [PubMed] [Google Scholar]
- 5.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. December 2014;12(12):1495–1499.25046131 [Google Scholar]
- 6.Des Jarlais DC, Lyles C, Crepaz N, and the Trend Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. March 2004;94(3):361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soltoft Larsen K, Pottegard A, Lindegaard HM, Hallas J. Impact of Urate Level on Cardiovascular Risk in Allopurinol Treated Patients. A Nested Case-Control Study. PLoS One. 2016;11(1):e0146172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong SY, Chittleborough CR, Gregory T, Mittinty MN, Lynch JW, Smithers LG. Parenting Practices at 24 to 47 Months and IQ at Age 8: Effect-Measure Modification by Infant Temperament. PLoS One. 2016;11(3):e0152452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knol MJ, Groenwold RHH, Grobbee DE. P-values in baseline tables of randomised controlled trials are inappropriate but still common in high impact journals. Eur J Prev Cardiol. April 2012;19(2):231–232. [DOI] [PubMed] [Google Scholar]
- 10.Palesch YY. Some common misperceptions about P values. Stroke. Dec 2014;45(12):e244–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman S A dirty dozen: twelve p-value misconceptions. Semin Hematol. July 2008;45(3):135–140. [DOI] [PubMed] [Google Scholar]
- 12.Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. April 2016;31(4):337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer MR, Waterlander WE, Kuijper LDJ, Steenhuis IHM, Twisk JWR. Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate. Int J Behav Nutr Phys Act. January 24 2015;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furler J, Magin P, Pirotta M, van Driel M. Participant demographics reported in “Table 1” of randomised controlled trials: a case of “inverse evidence”? Int J Equity Health. March 19 2012;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuart EA, Bradshaw CP, Leaf PJ. Assessing the generalizability of randomized trial results to target populations. Prev Sci. April 2015;16(3):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: The ACTG 320 trial. Am J Epidemiol. July 1 2010;172(1):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison PD. Missing data. Vol 136 Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 18.Daniel RM, Kenward MG, Cousens SN, De Stavola BL. Using causal diagrams to guide analysis in missing data problems. Stat Methods Med Res. June 2012;21(3):243–256. [DOI] [PubMed] [Google Scholar]
- 19.Westreich D Berkson’s bias, selection bias, and missing data. Epidemiology. January 2012;23(1):159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkins NJ, Cole SR, Harel O, Tchetgen Tchetgen EJ, Sun B, Mitchell EM, et al. Principled Approaches to Missing Data in Epidemiologic Studies. Am J Epidemiol. March 1 2018;187(3):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy SE, Allore H, Studenski SA. Missing data: a special challenge in aging research. J Am Geriatr Soc. April 2009;57(4):722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. June 29 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council (US) Panel on Handling Missing Data in Clinical Trials. The Prevention and Treatment of Missing Data in Clinical Trials. Washington (DC)2010. [PubMed] [Google Scholar]
- 24.Ware JH, Harrington D, Hunter DJ, D’Agostino RB. Missing Data. N Engl J Med. 2012;367:1353–1354. [Google Scholar]
- 25.Little RJ, D’Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. October 4 2012;367(14):1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duquia RP, Bastos JL, Bonamigo RR, Gonzalez-Chica DA, Martinez-Mesa J. Presenting data in tables and charts. An Bras Dermatol. Mar-Apr 2014;89(2):280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franzblau LE, Chung KC. Graphs, tables, and figures in scientific publications: the good, the bad, and how not to be the latter. J Hand Surg Am. March 2012;37(3):591–596. [DOI] [PubMed] [Google Scholar]
- 28.Graphics Boers M. and statistics for cardiology: designing effective tables for presentation and publication. Heart. Feb 2018;104(3):192–200. [DOI] [PubMed] [Google Scholar]