Abstract
Stress during adolescence is a risk factor for neuropsychiatric diseases, including schizophrenia. We recently observed that peripubertal male rats exposed to a combination of daily footshock plus restraint stress exhibited schizophrenia-like changes. However, numerous studies have shown sex differences in neuropsychiatric diseases as well as on the impact of coping with stress. Thus, we decided to evaluate, in adolescent female rats, the impact of different stressors (restraint stress [RS], footshock [FS], or the combination of FS and RS [FS+RS]) on social interaction (3-chamber social approach test/SAT), anxiety responses (elevated plus-maze/EPM), cognitive function (novel object recognition test/NOR), and dopamine (DA) system responsivity by evaluating locomotor response to amphetamine and in vivo extracellular single unit recordings of DA neurons in the ventral tegmental area (VTA) in adulthood. The impact of FS+RS during early adulthood was also investigated. Adolescent stress had no impact on social behavior, anxiety, cognition and locomotor response to amphetamine. In addition, adolescent stress did not induce long-lasting changes in VTA DA system activity. However, a decrease in the firing rate of VTA DA neurons was found 1–2 weeks post-adolescent stress. Similar to adolescent stress, adult stress did not induce long-lasting behavioral deficits and changes in VTA DA system activity, but FS+RS decreased VTA DA neuron population activity 1–2 weeks post-adult stress. Our results are consistent with previous studies showing that female rodents appear to be more resilient to developmental stress-induced persistent changes than males and may contribute to the delayed onset and lesser severity of schizophrenia in females.
Keywords: female, sex differences, stress, schizophrenia, depression, VTA recording
1. Introduction
Stress in early life is a well-known risk factor for the development of neuropsychiatric disorders in adulthood, like schizophrenia or depression (Kessler et al., 2010a; Sanchez et al., 2001). Given that adolescence is a phase during which genetic and environmental dynamics lead to the maturation of the developing brain, the brain is particularly sensitive to disruptive factors which can lead to psychiatric disorders (Sisk and Foster, 2004; Spear, 2000; Thompson et al., 2004). Childhood trauma is a well-documented predictor of psychosis onset, which typically manifests during late adolescence and early adulthood (Yung et al., 2015). Moreover, chronic stress in general is a risk factor in the establishment of neuropsychiatric diseases (Chrousos, 2009) such as depression and schizophrenia (Beards et al., 2013; Kendler et al., 1999) and part of the pathology of schizophrenia and of depression is the alteration of the mesolimbic dopamine (DA) system, which originates in the ventral tegmental area (VTA) (Grace, 2016; Heckers and Konradi, 2010; Lodge and Grace, 2007; Marinelli and McCutcheon, 2014; Nestler and Carlezon, 2006).
Besides these known interactions, it is well-known that prevalence and manifestation of psychiatric diseases (Bangasser and Valentino, 2014; Goldstein et al., 2002; Leung and Chue, 2000) as well as impact of stress and age (Bale and Epperson, 2015) can be sex-dependent (reviewed in (Novais et al., 2017; Rincon-Cortes et al., 2019). In contrast, the baseline activity of VTA DA neurons is similar in male and female rats (Rincon-Cortes and Grace, 2017). Sex differences are present in nearly all aspects of psychiatric disorders, such as prevalence, age of onset, symptom expression and severity (Castle et al., 1995). In the case of stress, the difference is also multifarious, from physiological changes, such as changes in levels of corticosterone (CORT) and glucocorticoids (GC) (Critchlow et al., 1963; Galea et al., 1997) or behavior (Luine et al., 2017) to cellular responses and structural changes (Bangasser et al., 2010; Goel and Bale, 2009). It has been suggested that these sex differences may involve the hypothalamic-pituitary-adrenal axis response to stress (Kudielka and Kirschbaum, 2005) and a possible interaction with the DA system (Dalla et al., 2008; Gillies et al., 2014).
Although females are more susceptible to affective dysfunction and exhibit greater sensitivity to stress than males, as suggested by the higher incidence of depression and anxiety disorders (Kessler et al., 2010b; Kudielka and Kirschbaum, 2005; Parker and Brotchie, 2010), the preclinical research is dominated by results obtained in male animals (Beery and Zucker, 2011) and the effects of adolescent stress on VTA DA neuron activity in females have not been examined. To get a more complete picture of the role of stress in developing neuropsychiatric diseases, this study evaluated the impact of the exposure of female rats to different stressors during adolescence or early adulthood on social interaction, anxiety, cognitive function, as well as the activity of the DA system. The experimental paradigm is similar to our previous study (Gomes and Grace, 2017b), which found that adolescent stressors resulted in schizophrenia-like pathology (in terms of behavior and VTA DA neuron activity) in adult male rats.
2. Experimental Procedures
Animals
For the adolescent stress protocol, pregnant Sprague–Dawley rats at gestational day 14 were purchased from Envigo (Indianapolis, IN) and gave birth in our animal facility. On postnatal day (PD) 21–23, litters were weaned and housed 2–3 per cage on a 12-h dark/light cycle (lights on from 7AM to 7PM). Only female offspring (a total of 80 rats) were used in this study. For the adult stress procedure, adult females (PD58, total 32 rats) were ordered from Envigo (Indianapolis, IN) and allowed to acclimate for a period of 7 days before the start of the stress regimen. Seven days before the behavioral tests, animals were moved to a reversed cycle (for acclimatisation) and were housed there throughout behavioral testing. Afterwards, the animals were moved back to the normal cycle and electrophysiological recordings of VTA DA neurons started 1-week later to ensure consistency with our previous study (Gomes and Grace, 2017b). All animals received food and water ad libitum. All experimental procedures were conducted according to National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Experimental Design
Adolescent female rats were exposed to: restraint stress (RS; at PD31, PD32, and PD40); footshock (FS; daily PD31–40); or a combination of FS and RS (FS+RS). This period (PD31–40) was chosen based on our previous work investigating both the increased responsivity to stress in the MAM model of schizophrenia (Du and Grace, 2013; Zimmerman et al., 2013) and the impact of different stressors applied to normal male animals on the VTA DA system (Gomes and Grace, 2017b). Thus, animals were exposed to psychological (restraint) and physical stressors (shock), as well as the combination of both stressors. Naïve animals remained in their home cages. These stressor types and time-points were selected to match the stress regimen used in our previous study in male rats (Gomes and Grace, 2017b). Electrophysiological recordings of VTA DA neurons were performed in a subset of animals 1–2 weeks after stress exposure (PD47–52). In adulthood, the rest of the animals were tested for social behavior in the 3-chamber social approach test (SAT, PD63–64), anxiety-like behavior (elevated plus-maze – EPM, PD65), cognitive function (novel-object recognition test, NOR; PD66–67), DA system activity (locomotor response to amphetamine, PD68–69; and recordings of VTA DA neurons, PD75–102). We also evaluated, in a separate cohort of animals, the impact of FS (from PD65–74) + RS (PD65, PD66, and PD74) administered in adulthood. This experimental design is similar to that reported previously (Gomes and Grace, 2017b).
Stress Procedures
Footshock (FS)
Adolescent females were exposed to daily session of FS (PD31–40) as previously described (Gomes and Grace, 2017b). In each session, 25 scrambled FS (1.0 mA, 2 s) were delivered every 60 ± 20 seconds.
Restraint Stress (RS)
Animals were exposed to 3 RS sessions as previously described (Gomes and Grace, 2017b). Each rat was placed in a Plexiglas cylindrical size-adjusted restraint tube. Cylinders measured 14 cm × 3.9 cm (length × diameter) for rats at PD31–32, 20.3 cm × 5.1 cm and for rats at PD40 or adults 23 cm × 6.3 cm. Each RS session was 1-hour in duration. For the combination of stressors, RS was done immediately after the FS session.
Behavioral tests
3-chamber Social approach test (SAT)
A three-chambered apparatus (total: L92 cm × W45 cm × H44.5 cm; side chambers: L36 cm × W45 cm × H44.5cm; center chamber: L20 cm × W45 cm × H44.5 cm), was used to assess social approach behavior, also known as social motivation (Fairless et al., 2011; Sandi and Haller, 2015). Rats were acclimated to the testing room for at least 1-h prior to testing. The test started with a 5-minute habituation to the apparatus in which the rat started in the center chamber and could freely explore all chambers. Afterwards, an unfamiliar younger same-sex rat (i.e., social stimulus), which had been habituated to the wire cage inside the testing apparatus for 15 min 1-day before, was placed in one of the wire cages, and a wire cage with an inanimate object (stuffed rat toy) was placed in the opposite chamber. The experimental rat was allowed to explore the entire apparatus for 10-min (Rincon-Cortes et al., 2018). The primary measurement was the social sniff time, which was the time the test rat was in close proximity to the social stimulus with its snout directed toward the cage (i.e., cage time), as described in prior studies assessing sociability in rodents (Fairless et al., 2011; Hanks et al., 2013). Total crossings between the chambers was also recorded and used as an index for locomotor activity.
Elevated Plus-Maze (EPM)
The EPM consisted of 2 opposite open arms (50 cm × 10 cm) which are crossed in a right angle by 2 arms that are enclosed by 40 cm high opaque walls; and 50 cm above the floor. For testing each rat was placed in the center of the EPM facing an enclosed arm, and the movement was recorded for 5-minutes. The percentage of time the animal spent in the open arms was calculated and used as an index of anxiety. The total number of entries into all arms was also measured as an index of locomotor activity.
Novel-Object Recognition (NOR) Test
The NOR test was conducted in a rectangular test box (L70 cm × W40 cm × H30 cm). The animals were habituated to the arena for 10-min one day before. On the test day, animals were subjected to 2 trials (each 5-min) separated by 1-hour. In the first trial (acquisition trial, T1), rats were placed in the arena and explored 2 identical objects. For the second trial (retention trial, T2), one of the objects presented in T1 was replaced by an unknown (i.e. novel) object. Object exploration was defined as conditions where the animal is directing its face to the object at a distance of approximately 2 cm while watching, licking, or touching it with the forepaws during sniffing. Recognition memory was assessed using the discrimination index (DI/discrimination index = [novel − familiar / novel + familiar]), corresponding to the difference between the time exploring the novel and the familiar object, corrected for total time exploring both objects.
Locomotor Response to Amphetamine
Rats were tested in an open-field chamber in which locomotor activity was determined by beam breaks and recorded with TruScan software (Coulbourn Instruments). Spontaneous activity was recorded for 30-minutes. Afterwards, rats were injected with D-amphetamine sulfate (0.75 mg/kg, i.p.; Sigma) and their locomotor activity was recorded for another 60-minutes. Total distance travelled was computed at 5-minute intervals.
VTA DA neuron extracellular recordings
Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.; Sigma) and mounted on a stereotaxic frame. The coordinates for the VTA were 5.3mm posterior from the bregma (AP), 0.6mm lateral to the midline (ML), and 6.5–9.0mm ventral from the brain surface (DV) for adult animals and 5.0mm AP, 0.5mm ML, and 6.0–8.5mm DV for the adolescent animals. Electrodes were lowered through 6–9 vertical tracks in a predetermined pattern within the VTA. DA neurons were identified according to well-established electrophysiological features (Grace and Bunney, 1983; Ungless and Grace, 2012). Three parameters were measured: population activity, i.e., the number of spontaneously active DA neurons recorded per electrode track; average firing rate, and the percentage of action potentials occurring in bursts (Grace and Bunney, 1984). Single-unit recording data was analysed using LabChart (Version 8.1; AD Instruments) and exported to Neuroexplorer (NEX Technologies, NexTech Systems) software to analyse firing rate and spikes in burst.
At the end, the recording site was marked via electrophoretic ejection of Chicago Sky Blue (Sigma) from the tip of the electrode (20μA constant negative current, 25–30-min) for subsequent histological confirmation. Afterwards, the brains were removed, fixed in 8% paraformaldehyde for at least 48-h and transferred to a 25% sucrose solution for cryoprotection before being sectioned using a cryostat (Leica Frigocut 2800). The brains were sliced coronally (thickness: 60μm) and mounted onto gelatin-chromalum-coated slides. After a preselection of slides containing brain slices with the Chicago Sky Blue marking, these slides were stained with a combination of neutral red and cresyl violet. Only rats with verified placement within the targeted region were included in the data analysis.
Statistical Analysis
All data were represented as mean ± SEM. Data analysis was conducted using ANOVA (1- or 2-way ANOVA) for 3 or more groups and t-tests for pairwise comparisons. Post hoc analysis were performed using the Tukey’s test when appropriate (i.e. one-way ANOVAs indicating a main effect or two-way ANOVAs indicating an interaction). Results of statistical tests with P < 0.05 were considered significant.
3. Results
In this experimental design, one female cohort was exposed to adolescent stress (PD31–40) and tested in a behavioral battery which was performed 25 days later (PD63–69) followed by recordings (PD75–90). A second female cohort was recorded 1–2 weeks (PD47–54) after adolescence stress (PD31–40). Additionally, this design was used to stress female rats in early adulthood (PD65–74), but here just the combination of the stressors was used.
Effects of adolescent stress on adult behavior
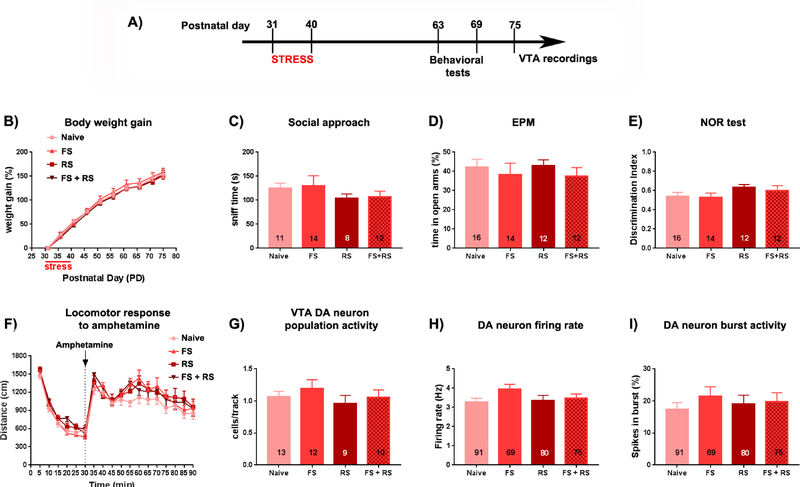
Adult females that were exposed to adolescent stress (FS, RS or FS+RS from PD31–40) were tested in a behavioral battery during early adulthood (PD63–69) (Figure 1A). No effect of stress (stress effect: F(3,48)=0.215, p=0.885, two-way ANOVA) or interaction between stress vs. time (age) on weight gain was observed among groups (interaction: F(27,432)=0.735, p=0.832, two-way ANOVA; Figure 1B). Adult females that experienced adolescent stress (FS, RS or FS+RS) exhibited social sniff time in the SAT comparable to naïve rats (F(3,40)=1.062, p=0.376, one-way ANOVA; Figure 1C). On the following day (PD65) the animals were tested for anxiety responses in the EPM. Again, no effects of stress exposure were found for the percentage of time in the open arms (F(3,50)=0.357, p=0.784; one-way ANOVA; Figure 1D). These findings indicate that, different from males (Gomes and Grace, 2017b), the exposure of females to these stress paradigms during adolescence did not induce anxiety responses at adulthood. Furthermore, rats were also tested for cognitive function in NOR test (PD66–67), but no difference in the discrimination index was observed among the groups (F(3,49)=1.305, p=0.283, one-way ANOVA; Figure 1E). Finally, all rats were tested for locomotor response to amphetamine (PD68–69). No difference was observed in the basal locomotor activity or in the response to amphetamine administration (stress effect: F(3,49)=0.924, p=0.435, two-way ANOVA; interaction stress vs. time: F(51,833)=1.07, p=0.355, two-way ANOVA; Figure 1F).
Figure 1: After exposure to adolescent stress, female rats did not show any differences in terms of behavioral measures and VTA DA system activity tested at adulthood.
(A) Adolescent female rats were submitted to restraint stress (RS; at postnatal day [PD]31, PD32, and PD40), footshock (FS; daily through PD31–40); or a combination of FS + RS. At adulthood, animals were tested in the social approach test (PD62–65), elevated plus-maze (EPM) (PD65), novel-object recognition (NOR) test (PD66–67), and locomotor response to amphetamine (PD68–69). Extracellular recordings of ventral tegmental area (VTA) dopamine (DA) neurons started 1 week after the behavioral experiments (>PD77). No difference induced by the adolescent stress was observed for (B) body weight gain, (C) sociability (indicated by no change in the sniff time in the social approach test), (D) anxiety responses in the EPM (indicated by no change in the percentage (%) time animals spent in the open arms), (E) cognitive function (indicated by no change in the discrimination index in the NOR test), (F) Locomotor response to amphetamine (baseline locomotion was recorded for 30 min (0–30 min), than amphetamine was injected (i.p) and another 60 min (35–90 min) the locomotion was recorded), (G) population activity of the VTA DA neurons, and (H) mean frequency and (I) % of spike in burst of the identified VTA DA cells.
Short and long-term effects of adolescent stress on VTA DA neuron activity
To examine if there were long-term changes in DA activity in rats that underwent adolescent stress, electrophysiological recordings were conducted seven days (i.e., PD75) after the behavior to assess DA neuron activity. While the exposure of male rats during adolescence to FS+RS lead to an increased VTA DA neuron population activity at adulthood (Gomes and Grace, 2017b), the exposure of adolescent females to the same stressors did not change the number of spontaneously active DA neurons per electrode track (i.e. population activity) (Naive: 13 rats, 1.1 ± 0.3 cells per track; FS: 12 rats, 1.2 ± 0.4 cells per track; RS: 9 rats, 1.0 ± 0.4 cells per track; FS+RS: 10 rats, 1.1 ± 0.4 cells per track; F(3,40)=0.608, p=0.614, one-way ANOVA; Figure 1G). Furthermore, no differences in firing rate (Naive: 91 cells, 3.3 ± 1.9 Hz; FS: 69 cells, 3.9±2.0 Hz; RS: 80 cells, 3.4 ± 2.3 Hz; FS+RS: 75 cells, 3.5 ± 1.8 Hz; F(3,311)=1.646, p=0.179, one-way ANOVA; Figure 1H) or burst firing (Naive: 91 cells, 17.3 ± 20.4 % of spike in burst; FS: 69 cells, 21.5 ± 24.6 % of spike in burst; RS: 80 cells, 19.0 ± 25.5 % of spike in burst; FS+RS: 75 cells, 19.1±24.1 % of spike in burst; F(3,319)=0.283, p=0.838, one-way ANOVA; Figure 1I) were found between groups. These data suggest that there is no long-term effect on DA neuron activity due to exposing females to these kinds of stressors.
Given that an increase in VTA DA system activity was present 1–2 weeks after the exposure of adolescent male rats to FS+RS (Gomes and Grace, 2017b; Gomes et al., 2019), we also tested if similar changes would be observed in females (Figure 2A).
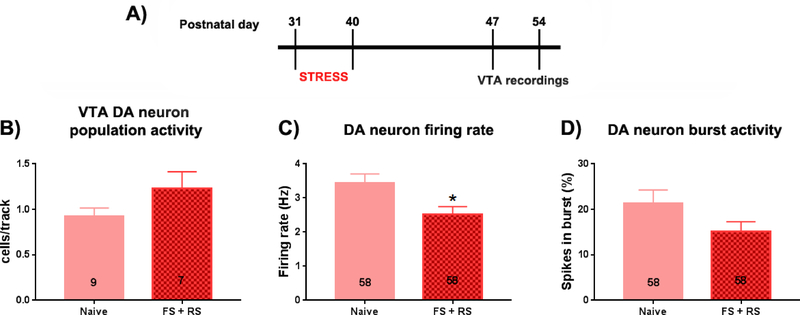
Figure 2: VTA DA neurons showed lower firing frequencies in female rats 1–2 weeks after adolescent stress exposure.
(A) Timeline of the experiment. Adolescent female rats were submitted to a combination of FS + RS (PD31–40). Extracellular recordings of ventral tegmental area (VTA) dopamine (DA) neurons were performed between 1 and 2 week post-stress (PD47–54). (B) Population activity of DA neurons measured in cells/track, (C) Mean frequency and (D) % of spike in burst of the identified DA cells were analyzed. No differences in (B) cells/track or (D) burst firing were noted; however there was a significant decrease (C) in the mean frequency of the VTA DA neurons recorded in stressed female rats. * p < 0.05, t-test.
There was no significant difference in DA neuron activity in terms of population activity or burst firing in the FS+RS group compared to controls (Population activity: Naive: 9 rats, 0.93 ± 0.28 cells per track; FS+RS: 7 rats, 1.3 ± 0.49 cells per track; t(14)=1.585; p=0.135, t-test; Figure 2B; Burst firing: Naive: 58 cells, 21.4 ± 22.0 % of spike in burst; FS+RS: 58 cells, 15.2 ± 16.3 % of spike in burst; t(114)=1.723, p=0.088, t-test; Figure 2D). However, a significant decrease in the firing rate of VTA DA neurons in females exposed to FS+RS was observed (Naive: 58 cells, 3.4 ± 2.1 Hz; FS+RS: 58 cells, 2.5 ± 1.7 Hz; t(114)=2.580, p=0.011, t-test; Figure 2C). These results suggest that there is a transient decrease in the firing rate of VTA DA neurons of females exposed to FS+RS during adolescence, but this change did not persist into adulthood (>PD75).
Effects of early adult stress on adult behavior
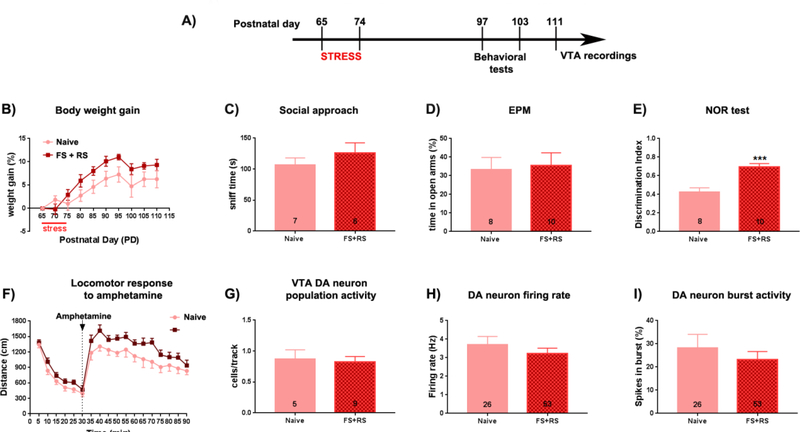
To evaluate whether the effect of stress was dependent on developmental stage, a separate cohort of female rats were stressed during early adulthood (PD65–74) and tested in a behavioral battery, similar to the females exposed to the adolescent stress, 25 days later in late adulthood (PD97–103); Figure 3A). Similar to females exposed to the adolescence stress, the females exposed to adult stress showed no differences in weight gain (F(18,225)=0.956, p=0.512, two-way ANOVA; Figure 3B) and in the social sniff time in the SAT (t(11)=0.99, p=0.3435, t-test; Figure 3C) or total number of chamber crossings in the SAT (t(11)=1.339, p=0.2077, t-test; data not shown) at adulthood (PD97–98). These data suggest comparable SAT behavior and locomotor activity in females exposed to early adult stress compared with controls. On the following day (PD99) the animals were tested in the EPM. Again, no effects of early adult stress exposure were found for time in open arms (t(15)=0.22, p=0.826, t-test, Figure 3D) or total number of entries (t(15)=0.14, p=0.315, t-test; data not shown). This suggests that females exposed to early adult stress did not exhibit a higher anxiety level or locomotion differences compared to the naïve females. Furthermore, the rats exposed to the early adult stress showed a significant higher discrimination index which indicates a significant increase in cognitive performance, compared with naïve females (t(16)=4.59, p < 0.001, t-test; Figure 3G) at PD100–101. Finally, there was an effect of stress on locomotor response to amphetamine (F(1,16)=4.722, p=0.045, two-way ANOVA), but with no interaction between stress and time (F(17,272)=0.834, p=0.653, two-way ANOVA; Figure 3F). Post hoc analysis did not indicate any significant difference at a specific time point (Bonferroni’s test, p > 0.05).
Figure 3: Adult female rats exposed to early adulthood stress showed a significant increased performance in the NOR test.
A: (A) Adult female rats were submitted to restraint stress a combination of FS + RS (PD65–74). Twenty-three days later, animals were tested in the social approach test, elevated plus-maze (EPM), novel-object recognition (NOR) test, and locomotor response to amphetamine. Extracellular recordings of ventral tegmental area (VTA) dopamine (DA) neurons started 1 week after the behavioral experiments (>PD111). No difference induced by the adult stress was observed for (B) body weight gain, (C) sociability (indicated by no change in the sniff time in the social approach test), and (D) anxiety responses in the EPM (indicated by no change in the percentage (%) time animals spent in the open arms). However, stressed animals showed an increased discrimination index in the NOR test). In terms of DA system activity, (F) there was an effect of stress to increase the locomotor response to amphetamine, but with no change in (G) VTA DA neuron population activity, and (H) mean frequency and (I) % of spike in burst of the identified VTA DA cells. * p < 0.05, t-test.
Short- and long-term effects of early adult stress on VTA DA activity
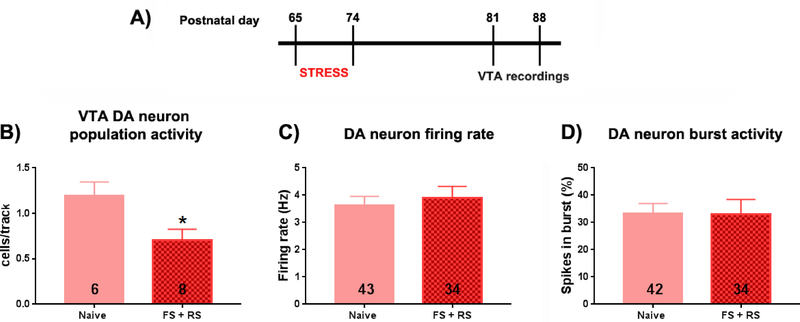
Electrophysiological recordings were started 7 days after the behavior (PD111–125). No differences between females exposed to early adult stress and controls in VTA DA neuron population activity (Naive: 5 rats, 0.9 ± 0.3 cells per track; FS+RS: 9 rats, 0.8 ± 0.3 cells per track; t(12)=0.25, p=0.807, t-test; Figure 3G) in firing rate (Naive: 26 cells, 3.7 ± 2.3 Hz; FS+RS: 53 cells, 3.2 ± 2.1 Hz; t(77)=0.91, p=0.368, t-test; Figure 3H) or spikes in burst (Naive: 26 cells, 28.3 ± 29.5 SPB; FS+RS: 53 cells, 23.2 ± 25.3 SPB; t(77)=0.79, p=0.4310, t-test; Figure 3I) were found. VTA population activity in females exposed to early adult stress, as with behavior, shifted back to levels observed in controls. A separate cohort of female rats was recorded 1–2 weeks after the early adult stress (PD81–88) to examine the short-term effects on VTA DA system activity (Figure 4A). The females exposed to early adult stress showed a significant decrease in DA neuron population activity when compared to the naïve females (Naive: 6 rats, 1.2 ± 0.4 cells per track; FS+RS: 8 rats, 0.7 ± 0.3 cells per track; t(12)=2.586, p=0.024, t-test; Figure 4B). No differences between the groups were found in firing rate (Naive: 43 cells, 3.6 ± 2.1 Hz; FS+RS: 34 cells, 3.9 ± 2.4 Hz; t(75)=0.535, p=0.594, t-test; Figure 4C) or burst firing (Naive: 43 cells, 33.1 ± 24.6 % of spike in burst; FS+RS: 34 cells, 33.0 ± 32.1 % of spike in burst; t(75)=0.028, p=0.977, t-test Figure 4D). Therefore, the impact of early adult stress in female rats characterized by a decreased VTA DA neuron population activity appears to be transient in nature.
Figure 4: VTA DA neuron population activity was significantly lower in female rats 1–2 weeks after early adulthood stress exposure.
(A) Timeline of the experiment. Adolescent female rats were submitted to a combination of FS + RS (PD65–74). Extracellular recordings of ventral tegmental area (VTA) dopamine (DA) neurons were performed between 1 and 2 week post-stress (PD81–88). (B) Population activity of DA neurons measured in cells/track, (C) Mean frequency and (D) % of spike in burst of the identified DA cells were analyzed. (B) A significant decrease in VTA DA neuron population activity was found in stressed female rats. In contrast no differences in (C) mean firing frequency or (D) burst firing were noted. No differences in (B) cells/track or (D) burst firing were noted. * p < 0.05, t-test.
4. Discussion
Stress during adolescence increases susceptibility for psychiatric disorders, such as schizophrenia and depression (Kessler et al., 2010a; Sanchez et al., 2001). In this context, we recently showed that the exposure of male Sprague Dawley rats to either 3 RS sessions or daily FS given at PD31–40, a period corresponding to mid-adolescence in humans (Keshavan et al., 2014), leads to a heightened anxiety state in adult rats, but only RS+FS leads to changes resembling a schizophrenia-like phenotype, which includes higher anxiety in the EPM, lower scores in the NOR and a hyperdopaminergic state in terms of increased VTA DA neuron population activity and increased locomotor response to amphetamine (Gomes and Grace, 2017a, b). These changes are similar to those observed in the MAM model of schizophrenia as well as the correlates in schizophrenia patients (Du and Grace, 2016a; Grace, 2017; Modinos et al., 2015; Moore et al., 2006). However, the exposure of adult rats (PD65–74) to the same combination of stressors only caused a transient decrease in VTA DA system activity (Gomes and Grace, 2017b). Given that sex differences are shown in several psychiatric disorders (Goldstein et al., 2002; Leung and Chue, 2000) and in the way stress influences physiological and behavioral changes (Luine and Gomez, 2016), in the present study we extended our previous findings by investigating the impact of stress on adolescent and adult female rats.
We found that adult females previously exposed to adolescent stress did not show any behavioral changes associated with social motivation, anxiety, and cognitive function. These findings are in contrast to that previously reported in adult male rats exposed to adolescent stress. However, our results are in accordance with some prior reports. Other studies have found no effect in females (different strains, stressors and ages of exposure to stress) in the EPM (Barna et al., 2003; Prusator and Greenwood-Van Meerveld, 2015; Slotten et al., 2006). Although examined at an earlier time points (i.e., PD3, PD9 or PD11) and different stressors, others have also found no impact of early life stress on female rats with respect to the EPM, NOR, and object recognition location test (Loi et al., 2017). A recent study conducted in female MAM rats also found no differences in anxiety-like behavior in the EPM (Perez et al., 2019). Additionally, maternal deprivation (MD; PD9), chronic unpredictable stress (CUS; PD28–43) and the combination of MD and CUS causes a decrease of the DI, which is an indicator of the cognitive performance, in the NOR (Llorente et al., 2011). Decreased social motivation has also been observed in female MAM rats, although this effect is dependent on estrous stage (Perez et al., 2019). This may suggest that early life stressors prior to weaning, but not adolescent stressors, have long-lasting effects on social behavior and that social behavior may be influenced by estrogen levels. Collectively, these data indicate that different stressors and timepoints can causes different outcomes in behavior later in life.
In terms of DA system activity, no difference in the locomotor response to amphetamine was observed in females exposed either to daily FS alone, or 3 sessions of RS alone, or to FS+RS during adolescence. This is different from that previously reported in female rats exposed to earlier periods of social isolation shortly after weaning (Lampert et al., 2017). Furthermore, this is different from the increased locomotor response to amphetamine that was observed in male rats exposed to FS +RS during adolescence (Gomes and Grace, 2017b). Unfortunately, not a lot is known about the electrophysiological properties of VTA DA neurons of females following stress exposure at different developmental stages. Our previous study showed a decrease of DA population activity in the VTA of females after chronic mild stress in adulthood, which is an animal model of depression (Rincon-Cortes and Grace, 2017). A few other studies measured the metabolites of DA after stress exposure in females and did not find differences (Bowman et al., 2009; Lampert et al., 2017). The absence of an increased response to amphetamine is consistent with the absence of impact on VTA DA neuron activity, which contrasts with that observed in male rats exposed to FS+RS during adolescence (Gomes and Grace, 2017b). Given that a hyperdopaminergic state induced by the FS+RS in adolescent male rats was also present between 1–2 weeks after the stress (Gomes et al., 2018), when animals were at late adolescence (PD47–54), we decided to evaluate the impact of the exposure of adolescent female rats to the FS+RS on the VTA DA system activity by performing recordings at the same timeframe. Interestingly, we found that adolescent stress exposure decreased the average firing rate of identified VTA DA neurons with a trend to decrease the burst activity, but with no change in population activity. It is noteworthy that these changes in the firing rate were transient since no change was observed when animals were tested at adulthood (>5 weeks post-stress). Such a decrease may indeed be protective to the deleterious effects of stress observed in males.
Another cohort of females was exposed to the FS+RS during early adulthood (PD65–74) and were tested in the same behavioral and electrophysiological experiments starting at PD97. When male rats were exposed to a similar protocol neither behavioral nor electrophysiological changes in the VTA DA system were observed 5–6 weeks post-stress (Gomes and Grace, 2017b). In females, similar to adolescent stress, early adult stress exposure did not affect social and anxiety-like behaviors. The results for anxiety were in accordance with Bowman and colleagues, who showed that adult stress did not affect anxiety responses of females in the EPM (Bowman et al., 2009). In contrast to females that underwent adolescent stress, females exposed to early adult stress had increased cognitive performance. Bowmann and colleagues also showed that stress in adult females either enhanced or did not alter cognitive function in females, although this depended on task, stressor, and length of stress exposure. They showed that performance in the NOR test was not altered after 7 and 21 days of daily RS (Bowman et al., 2003; Bowman et al., 2009; Luine et al., 2017). The underlying mechanisms are unclear, but others have hypothesized that a shift of cognition strategies especially for the anxiety part of the test seems to be important (Bowman et al., 2009; Luine et al., 2017), and this could be the reason for sex differences in different tasks.
Regarding changes in the activity of VTA DA neurons after the adult stress, we did not find persistent changes induced by the adult stress on any parameters measured (population activity, firing rate, and burst activity). These findings are similar to those observed in adult females exposed to stress during adolescence, indicating that both adolescence and adult stress did not induce long-lasting changes in the VTA DA system activity. However, the adult stress decreased VTA DA neuron population activity when the recordings were performed between 1 and 2 weeks post-adult stress. In males, the adult stress also did not induce long-lasting changes in the VTA DA system activity, but did induce a similar decrease in VTA DA neuron population activity when the recordings were conducted 1–2 weeks post-stress (Gomes et al., 2018).This hypodopaminergic state is similar to that found in male and female rats exposed to animal models of depression, such as the chronic mild stress and learned helplessness paradigms (Belujon and Grace, 2014; Chang and Grace, 2014; Rincon-Cortes and Grace, 2017) and is thought to underlie the pathology of depression (Grace, 2016). Similar to what was found in males, this hypodopaminergic state was followed by a recovery since no change in the VTA DA system activity was observed more than 5 weeks post-adult stress.Taken together, these data suggest that female animals recover from adolescent and adult stress exposure over time. This is consistent with the idea that time point of stress is important for the type of pathophysiology observed, predisposing adolescent males towards schizophrenia-like traits and DA dysfunction, but not females.
Collectively, our findings suggest that females are more resilient to adolescent stress than males and even stress during early adulthood showed sex-dependent differences in behavioral tasks. Several factors can mediate these sex differences. For example, females mature earlier than males (Spear, 2000). Thus, one possibility is that the sensitive period of greater susceptibility to stress occurred prior to the time point when females were exposed to the stressors. Another factor is the stressor itself; it has been shown that behavioral responses to stress can be sex-dependent and also influenced by the type of stressor (Hodes, 2018; Rincon-Cortes et al., 2019). Alternately, it may be that females require more extended stress exposure. For example, the behavior in the EPM and object placement task of females is altered by prolonged period of stress (Bowman and Kelly, 2012). Additionally, the differential role of sexual hormones should also be taken into account (Bellanti et al., 2013). Estrogen is known as a powerful mediator of branching and density of neurons, such as GABAergic interneurons expressing the calcium binding protein parvalbumin (PV) (Ross and Porter, 2002; Wu et al., 2014). The reduction of PV positive interneurons is present in the pathology of schizophrenia as well as in animal models of this disorder (Du and Grace, 2016b; Konradi et al., 2011; Lodge et al., 2009). In fact, the hyperdopaminergic state in males induced by the adolescent stress seems to be driven by PV interneuron loss in the ventral hippocampus (Zhu et al., 2018). In males, these interneurons will be in a mature state only at adulthood and it has been suggested that they are more susceptible to stress-induced damage at earlier time points (Cabungcal et al., 2013; Do et al., 2015; Steullet et al., 2010). Notably, it was observed that female mice exhibit a gradual increase in hippocampal PV expression during adolescence that is dependent on the levels of sex steroid hormones, which is not observed in males (Wu et al., 2014). Thus, the protection of PV interneurons at earlier periods when compared to males could be a reason for the unaltered behavioral and electrophysiological results after adolescent stress in females and could be a factor in the delayed onset of schizophrenia in women (Leung and Chue, 2000). Moreover, it is known that stress changes the female brain in a different way than males. For example, chronic stress induces retraction of dendrites in the CA3 region of the hippocampus and dendritic atrophy in the prefrontal cortex in males but not in females (Galea et al., 1997; Garrett and Wellman, 2009). Another study showed that stress in males causes a decrease in dendritic length in parts of the hippocampus but not in females (Suenaga et al., 2012). These sex differences to stress can be found in differential modulation of the HPA, differences in glucocorticoid levels, neuronal morphology (Bangasser and Valentino, 2014), and monoaminergic activity (Bowman et al., 2003). Furthermore, stress-induced changes in the estrous cycle have yet to be monitored, as some studies suggest cycle-dependent changes (Mora et al., 1996) and some not (Loi et al., 2017).
In sum, this study showed that, in contrast to males, adolescent stress failed to lead to adult behavioral and neurophysiological alterations consistent with those observed in animal models of schizophrenia. Intriguingly, after exposure to adolescent stress there was no induction of a hyperdopaminergic state (i.e. increased number of active DA cells or population activity) in the VTA of female rats, which is in contrast with what is observed in males (Gomes and Grace, 2017b). However, in contrast to the activation observed in males shortly after adolescent stress, female rats exhibited an attenuation of DA neuron activity. The fact that adolescent stress transiently attenuates DA activity in female rats may contribute to the protection against the impact of adolescent stress, and could point to a greater resilience to schizophrenia-like traits resulting from developmental stress.
Highlights.
Female rats are resistant to long-term behavior changes after adolescent stress
Female rats are resistant to long-term DA changes induced by adolescent stress
Female rats are resistant to long-term behavior changes induced by adult stress
Female rats are resistant to long-term DA system changes induced by adult stress
Adolescent and adult stress have short-term effects on VTA DA system activity
Acknowledgments
Funding for this study was provided by the National Institutes of Health (NIH MH57440) to AAG. We thank Niki MacMurdo and Christy Smolak for technical assistance.
Author Disclosure
Role of Funding Source. Funding for this study was provided by the National Institutes of Health (NIH MH57440) to AAG. The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest. AAG has received funds from Lundbeck, Pfizer, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, Janssen, Alkermes, Newron, and Takeda. KK, FVG, and MRC declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katharina Klinger, Departments of Neuroscience, Psychiatry and Psychology, University of Pittsburgh.
Felipe V. Gomes, Departments of Neuroscience, Psychiatry and Psychology, University of Pittsburgh.
Millie Rincón-Cortés, Departments of Neuroscience, Psychiatry and Psychology, University of Pittsburgh.
Anthony A. Grace, Departments of Neuroscience, Psychiatry and Psychology, University of Pittsburgh.
References
- Bale TL, Epperson CN, 2015. Sex differences and stress across the lifespan. Nat Neurosci 18, 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ, 2010. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15, 877, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ, 2014. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna I, Balint E, Baranyi J, Bakos N, Makara GB, Haller J, 2003. Gender-specific effect of maternal deprivation on anxiety and corticotropin-releasing hormone mRNA expression in rats. Brain Res Bull 62, 85–91. [DOI] [PubMed] [Google Scholar]
- Beards S, Gayer-Anderson C, Borges S, Dewey ME, Fisher HL, Morgan C, 2013. Life events and psychosis: a review and meta-analysis. Schizophr Bull 39, 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I, 2011. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanti F, Matteo M, Rollo T, De Rosario F, Greco P, Vendemiale G, Serviddio G, 2013. Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biology, 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA, 2014. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76, 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN, 2003. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav 43, 48–59. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Kelly R, 2012. Chronically stressed female rats show increased anxiety but no behavioral alterations in object recognition or placement memory: a preliminary examination. Stress 15, 524–532. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN, 2009. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiol Behav 97, 21–29. [DOI] [PubMed] [Google Scholar]
- Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ, 2013. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry 73, 574–582. [DOI] [PubMed] [Google Scholar]
- Castle DJ, Abel K, Takei N, Murray RM, 1995. Gender differences in schizophrenia: hormonal effect or subtypes? Schizophr Bull 21, 1–12. [DOI] [PubMed] [Google Scholar]
- Chang CH, Grace AA, 2014. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry 76, 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, 2009. Stress and disorders of the stress system. Nat Rev Endocrinol 5, 374–381. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS, 1963. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol 205, 807–815. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z, 2008. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol Behav 93, 595–605. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cuenod M, Hensch TK, 2015. Targeting Oxidative Stress and Aberrant Critical Period Plasticity in the Developmental Trajectory to Schizophrenia. Schizophr Bull 41, 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA, 2013. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology 38, 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA, 2016a. Amygdala Hyperactivity in MAM Model of Schizophrenia is Normalized by Peripubertal Diazepam Administration. Neuropsychopharmacology 41, 2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA, 2016b. Loss of Parvalbumin in the Hippocampus of MAM Schizophrenia Model Rats Is Attenuated by Peripubertal Diazepam. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless AH, Shah RY, Guthrie AJ, Li H, Brodkin ES, 2011. Deconstructing sociability, an autism-relevant phenotype, in mouse models. Anat Rec (Hoboken) 294, 1713–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS, 1997. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81, 689–697. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL, 2009. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience 162, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, Virdee K, McArthur S, Dalley JW, 2014. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: A molecular, cellular and behavioral analysis. Neuroscience 282, 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Bale TL, 2009. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol 21, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O’Brien LM, Horton NJ, Kennedy DN, Makris N, Caviness VS Jr., Faraone SV, Tsuang MT, 2002. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry 59, 154–164. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Grace AA, 2017a. Adolescent Stress as a Driving Factor for Schizophrenia Development-A Basic Science Perspective. Schizophr Bull 43, 486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Grace AA, 2017b. Prefrontal Cortex Dysfunction Increases Susceptibility to Schizophrenia-Like Changes Induced by Adolescent Stress Exposure. Schizophr Bull 43, 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Zhu X, Grace AA, 2018. The impact of stress on the dopamine system is dependent on the state of the critical period plasticity. Society for Neuroscience, Washington D.C. [Google Scholar]
- Gomes FV, Zhu X, Grace AA, 2019. Stress during critical periods of development and risk for schizophrenia. Schizophr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, 2016. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 17, 524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, 2017. Dopamine System Dysregulation and the Pathophysiology of Schizophrenia: Insights From the Methylazoxymethanol Acetate Model. Biol Psychiatry 81, 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, 1983. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons−-1. Identification and characterization. Neuroscience 10, 301–315. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, 1984. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4, 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks AN, Dlugolenski K, Hughes ZA, Seymour PA, Majchrzak MJ, 2013. Pharmacological disruption of mouse social approach behavior: relevance to negative symptoms of schizophrenia. Behav Brain Res 252, 405–414. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C, 2010. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci 4, 529–553. [DOI] [PubMed] [Google Scholar]
- Hodes G, 2018. A primer on sex differences in the behavioral response to stress. Current Opinion in Behavioral Sciences 23, 75–83. [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA, 1999. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156, 837–841. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Giedd J, Lau JY, Lewis DA, Paus T, 2014. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry 1, 549–558. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB, 2007. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 20, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Aguilar-Gaxiola S, Alhamzawi AO, Alonso J, Angermeyer M, Benjet C, Bromet E, Chatterji S, de Girolamo G, Demyttenaere K, Fayyad J, Florescu S, Gal G, Gureje O, Haro JM, Hu CY, Karam EG, Kawakami N, Lee S, Lepine JP, Ormel J, Posada-Villa J, Sagar R, Tsang A, Ustun TB, Vassilev S, Viana MC, Williams DR, 2010a. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry 197, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ruscio AM, Shear K, Wittchen HU, 2010b. Epidemiology of anxiety disorders. Curr Top Behav Neurosci 2, 21–35. [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S, 2011. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res 131, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C, 2005. Sex differences in HPA axis responses to stress: a review. Biol Psychol 69, 113–132. [DOI] [PubMed] [Google Scholar]
- Lampert C, Arcego DM, de Sa Couto-Pereira N, Dos Santos Vieira A, Toniazzo AP, Krolow R, Garcia E, Vendite DA, Calcagnotto ME, Dalmaz C, 2017. Short post-weaning social isolation induces long-term changes in the dopaminergic system and increases susceptibility to psychostimulants in female rats. Int J Dev Neurosci 61, 21–30. [DOI] [PubMed] [Google Scholar]
- Leung A, Chue P, 2000. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl 401, 3–38. [DOI] [PubMed] [Google Scholar]
- Llorente R, Miguel-Blanco C, Aisa B, Lachize S, Borcel E, Meijer OC, Ramirez MJ, De Kloet ER, Viveros MP, 2011. Long term sex-dependent psychoneuroendocrine effects of maternal deprivation and juvenile unpredictable stress in rats. J Neuroendocrinol 23, 329–344. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA, 2009. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci 29, 2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA, 2007. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci 27, 11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M, Mossink JC, Meerhoff GF, Den Blaauwen JL, Lucassen PJ, Joels M, 2017. Effects of early-life stress on cognitive function and hippocampal structure in female rodents. Neuroscience 342, 101–119. [DOI] [PubMed] [Google Scholar]
- Luine V, Gomez J, Beck K, Bowman R, 2017. Sex differences in chronic stress effects on cognition in rodents. Pharmacol Biochem Behav 152, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Gomez JL, 2016. Sex differences in rodent cognitive processing and responses to chronic stress, in: Shansky RM (Ed.), Sex Differences in the Central Nervous System. Academic Press. [Google Scholar]
- Marinelli M, McCutcheon JE, 2014. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 282, 176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Allen P, Grace AA, McGuire P, 2015. Translating the MAM model of psychosis to humans. Trends Neurosci 38, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA, 2006. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry 60, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G, 1996. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology 21, 609–620. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA Jr., 2006. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Novais A, Monteiro S, Roque S, Correia-Neves M, Sousa N, 2017. How age, sex and genotype shape the stress response. Neurobiol Stress 6, 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, Brotchie H, 2010. Gender differences in depression. Int Rev Psychiatry 22, 429–436. [DOI] [PubMed] [Google Scholar]
- Perez SM, Donegan JJ, Lodge DJ, 2019. Effect of estrous cycle on schizophrenia-like behaviors in MAM exposed rats. Behav Brain Res 362, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusator DK, Greenwood-Van Meerveld B, 2015. Gender specific effects of neonatal limited nesting on viscerosomatic sensitivity and anxiety-like behavior in adult rats. Neurogastroenterol Motil 27, 72–81. [DOI] [PubMed] [Google Scholar]
- Rincon-Cortes M, Gagnon KG, Dollish HK, Grace AA, 2018. Diazepam reverses increased anxiety-like behavior, social behavior deficit, and dopamine dysregulation following withdrawal from acute amphetamine. Neuropsychopharmacology 43, 2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M, Grace AA, 2017. Sex-Dependent Effects of Stress on Immobility Behavior and VTA Dopamine Neuron Activity: Modulation by Ketamine. Int J Neuropsychopharmacol 20, 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M, Herman JP, S. L, Maguire J, Shansky RM, 2019. Stress: Influence of sex, reproductive status and gender. Neurobiology of Stress 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross NR, Porter LL, 2002. Effects of dopamine and estrogen upon cortical neurons that express parvalbumin in vitro. Brain Res Dev Brain Res 137, 23–34. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM, 2001. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol 13, 419–449. [DOI] [PubMed] [Google Scholar]
- Sandi C, Haller J, 2015. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 16, 290–304. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL, 2004. The neural basis of puberty and adolescence. Nat Neurosci 7, 1040–1047. [DOI] [PubMed] [Google Scholar]
- Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KC, 2006. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: gender-dependent effects. Brain Res 1097, 123–132. [DOI] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, Cuenod M, Do KQ, 2010. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci 30, 2547–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga T, Yukie M, Gao S, Nakahara D, 2012. Sex-specific effects of prenatal stress on neuronal development in the medial prefrontal cortex and the hippocampus. Neuroreport 23, 430–435. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Pogue-Geile MF, Grace AA, 2004. Developmental pathology, dopamine, and stress: a model for the age of onset of schizophrenia symptoms. Schizophr Bull 30, 875–900. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Grace AA, 2012. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35, 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Du X, van den Buuse M, Hill RA, 2014. Sex differences in the adolescent developmental trajectory of parvalbumin interneurons in the hippocampus: a role for estradiol. Psychoneuroendocrinology 45, 167–178. [DOI] [PubMed] [Google Scholar]
- Yung AR, Cotter J, Wood SJ, McGorry P, Thompson AD, Nelson B, Lin A, 2015. Childhood maltreatment and transition to psychotic disorder independently predict long-term functioning in young people at ultra-high risk for psychosis. Psychol Med 45, 3453–3465. [DOI] [PubMed] [Google Scholar]
- Zhu X, Gomes FV, Grace AA, 2018. Adolescent stress during critical periods alters the developmental trajectory of hippocampal parvalbumin interneurons and recapitulates circuit dysfunction implicated in schizophrenia, Society for Neuroscience, Washington D.C. [Google Scholar]
- Zimmerman EC, Bellaire M, Ewing SG, Grace AA, 2013. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology 38, 2131–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]