Scope
Venous thromboembolism (VTE) is a major complication in cancer patients. The Scientific and Standardization Committee (SSC) through its subcommittee Hemostasis & Malignancy of the International Society for Thrombosis and Hemostasis (ISTH) aims to review emerging data on primary VTE prophylaxis with direct oral anticoagulants (DOACs) for ambulatory cancer patients and provide guidance to clinicians. A guidance document was previously published focusing on using low-molecular-weight heparin (LMWH) for VTE prophylaxis in ambulatory cancer patients [1]. Individualized patient care after mutual discussion and decision making with patients remains important. Three guidance statements are included in this document. Recommendations reflect strong guidance statements supported by evidence from clinical trials. Suggestions reflect weaker guidance statements based on expert opinions and best available evidence.
Definitions
Ambulatory cancer patients. Cancer patients starting a new outpatient systemic therapy regimen are the target population in this guidance statement. Patients with acute leukemia, myeloproliferative neoplasm, planned stem cell transplantation, history of cancer in remission, on hormonal therapy alone, hospitalized or post-surgery are not addressed in this document.
DOACs. There is one thrombin inhibitor, dabigatran, and four factor Xa inhibitors, rivaroxaban, apixaban, edoxaban, and betrixaban, currently approved for the prevention of VTE in multiple countries. Only apixaban and rivaroxaban have been studied in phase III trials of primary thromboprophylaxis in ambulatory cancer patients and are the focus of this guidance statement.
Methods
We performed the pooled analysis using the Review Manager v5.3 (Nordic Cochrane Center) according to the recommendations from the Cochrane Collaboration. The risk ratio (RR) and associated 95% confidence interval (CI) were calculated by the Mantel-Haenszel methods using the random-effect model. The number needed to treat (NNT) and the number needed to harm (NNH) were calculated by dividing 1 by the absolute risk reduction.
Background
Cancer patients have a 4–7 fold increased risk of VTE, and 5–10% of cancer patients develop a VTE within the first year of cancer diagnosis [2]. VTE is associated with significant morbidity and mortality in cancer patients and commonly interferes with cancer treatment [3]. Although various parental anticoagulants have been shown to be effective in preventing VTE in ambulatory cancer patients [4, 5], this has not been adopted nor recommended in routine practice. Enthusiasm for primary thromboprophylaxis with parental anticoagulants is tempered by a high NNT (>40–50) in unselected populations of cancer patients, increased risk of hemorrhage, as well as the burden associated with daily injections.
While the relative benefit of thromboprophylaxis of unselected populations is less certain, recent studies focused on the effectiveness of thromboprophylaxis in cancer populations at higher risk of VTE by limiting enrollment to those with Khorana scores of 2 or higher. The Khorana score includes five readily available clinical and laboratory parameters: site of tumor, body mass index, pre-chemotherapy hemoglobin, leukocyte, and platelet count (Appendix Table 1) [6]. Patients with solid tumor and a Khorana score of 2 or above have a risk of symptomatic VTE of 9.6% in the first 6 months of chemotherapy based on a validation study by the Vienna Cancer And Thrombosis Study group [7]. Prior subgroup and pooled analyses suggest benefit of thromboprophylaxis in patients with Khorana score of 3 or higher but until recently, direct evidence from randomized clinical trials (RCTs) was lacking.
DOACs are effective for the treatment of cancer-associated thrombosis (CAT), but are associated with an increased risk of bleeding, especially in patients with gastrointestinal malignancies [8, 9]. DOACs have the advantage of oral administration without requiring intense laboratory monitoring. AVERT (Apixaban for the Prevention of Venous Thromboembolism in High-Risk Ambulatory Cancer Patients) and CASSINI studies evaluated their benefit for primary VTE prophylaxis in intermediate to high-risk ambulatory cancer patients [10, 11].
The subcommittee for Hemostasis & Malignancy of the SSC has published a guidance document addressing the issue of VTE prevention in cancer outpatients in 2014, mainly with LMWH [1]. This guidance document concentrates on the emerging data of using DOACs in this setting.
Evidence from randomized controlled trials
Low-molecular-weight heparin
Systemic review and meta-analyses have shown that prophylactic LMWH significantly reduced the rate of symptomatic VTE (RR 0.54, 95% CI 0.38–0.75). In the absence of selection based on the Khorana score, the event rate in the control groups was low and the absolute risk reduction was 2–3% translating into a NNT of 32. In addition, a 40% non-significant increased risk of major bleeding (RR 1.44, 95% CI 0.98–2.11) and a NNH of 191 were observed [4]. Routine thromboprophylaxis for unselected ambulatory cancer patients with LMWH is not recommended by major guidelines [1, 12, 13]. However, in selected high-risk patients such as patients with advanced pancreatic cancer starting systemic therapy, ISTH suggested higher doses of LMWH as outpatient thromboprophylaxis in the 2014 guidance document [1].
Direct Oral Anticoagulants
A phase II randomized study previously demonstrated the feasibility and safety of various doses of apixaban in primary VTE prevention in patients with metastatic cancer [14]. Recently, two large randomized controlled studies using DOACs as primary VTE prevention in ambulatory cancer patients were completed and results reported (Table 1). Both studies incorporated the Khorana score to target intermediate to high-risk patients. AVERT utilized apixaban, while CASSINI used rivaroxaban for thromboprophylaxis [10, 11].
Table 1.
Comparison of CASSINI and AVERT studies
| CASSINI | AVERT | |
|---|---|---|
| Anticoagulant | Rivaroxaban 10 mg daily | Apixaban 2.5 mg twice daily |
| Number of patients included in analysis | 841 | 563 |
| Khorana score | ≥2 | ≥2 |
| Male | 428/841 (50.9%) | 236/563 (41.9%) |
| Planned duration | 6 months | 6 months |
| Median treatment duration | 4.3 months | 5.2 months |
| Early discontinuation of study drugs (including early mortality) | R: 177/405 (43.7%) | A: 105/288 (36.5%) |
| P: 203/404 (50.2%) | P: 111/275 (40.4%) | |
| Screening lower extremity ultrasound | Yes (baseline, 8, 16, 24 weeks) | No |
| Myeloma | Excluded | Yes (n=15) |
| Brain tumor | Excluded | Yes (n=24) |
| Primary endpoints | Proximal DVT/PE (symptomatic or asymptomatic), symptomatic distal lower extremity DVT or upper extremity DVT, VTE related death | Proximal upper or lower extremity DVT/PE (symptomatic or asymptomatic), VTE related death |
| VTE | R: 25/420 (6.0%) | A: 12/288 (4.2%) |
| P: 37/421 (8.8%) | P: 28/275 (10.2%) | |
| On treatment VTE | R: 11/420 (2.6%) | A: 3/288 (1.0%) |
| P: 27/421 (6.4%) | P: 20/275 (7.3%) | |
| MB | N/A | A: 10/288 (3.5%) |
| P: 5/275 (1.8%) | ||
| On treatment MB | R: 8/405 (2.0%) | A: 6/288 (2.1%) |
| P: 4/404 (1.0%) | P: 3/275 (1.1%) | |
| CRNMB | R: 11/405(2.7%) | A: 21/288 (7.3%) |
| P: 8/404 (2.0%) | P: 15/275 (5.5%) | |
| Mortality | R: 84/420 (20.0%) | A: 35/288 (12.2%) |
| P: 100/421 (23.8%) | P: 27/275 (9.8%) | |
| NNT VTE | 35 | 17 |
| NNT VTE on treatment | 26 | 16 |
| NNH | N/A | 59 |
| NNH on treatment | 101 | 100 |
Abbreviations: VTE: venous thromboembolism; DVT: deep vein thrombosis; PE: pulmonary embolism; MB: major bleeding; CRNMB: clinically relevant non-major bleeding; AE: adverse event; R: rivaroxaban; A: apixaban; P: placebo; NNT: number needed to treat; NNH: number needed to harm; N/A: not available.
On treatment is defined as only including events occurring while on study drugs and up to 2 days after last dose of study drugs.
Apixaban
AVERT study was a randomized, placebo-controlled, double-blinded clinical trial in 574 ambulatory cancer patients with a slightly modified Khorana score ≥2 (to include patients with brain tumor and myeloma, appendix table 1) starting chemotherapy, to assess the efficacy and safety of apixaban as primary prevention of VTE [10]. Patients were randomized to apixaban 2.5 mg twice daily versus placebo for 6 months. The primary efficacy outcomes were symptomatic proximal deep vein thrombosis (DVT) of upper or lower extremities, symptomatic or incidental pulmonary embolism (PE), or VTE related death, with primary safety outcome as ISTH-defined major bleeding [15]. Over the study period of 180 days, apixaban significantly reduced VTE rate (4.2% on apixaban and 10.2% on placebo, hazard ratio [HR] 0.41; 95% CI, 0.26–0.65; p<0.001). Patients in the apixaban group had a significantly increased risk of major bleeding (3.5% on apixaban and 1.8% on placebo, HR 2.00; 95% CI, 1.01–3.95; p=0.046). The on-treatment major bleeding rates (events occurred while on study drugs or up to 2 days after last dose) were not significantly different (2.1% on apixaban and 1.1% on placebo, HR 1.89; 95% CI, 0.39–9.24). The NNT to prevent one VTE was 17 and the NNH was 59 (on-treatment NNH= 100) (Table 1).
Rivaroxaban
CASSINI study was a randomized, placebo-controlled, double-blinded clinical trial in 841 ambulatory cancer patients with locally advanced or metastatic disease with Khorana score ≥2 starting a new systemic therapy regimen, to assess the efficacy and safety of rivaroxaban in the primary prevention of VTE [11]. Patients were randomized to rivaroxaban 10 mg once daily versus placebo. In contrast to the AVERT study, screening bilateral lower extremity ultrasounds were done at baseline, 8, 16, and 24 weeks. The primary efficacy outcomes included symptomatic or screen-detected proximal lower extremity DVT or PE, symptomatic upper or lower extremity distal DVT, or VTE related death. Primary safety outcome was ISTH-defined major bleeding [15]. Patients with DVT found on baseline screening ultrasound (49/1080, 4.5%) were excluded. Over the study period of 180 days, the primary efficacy outcome occurred in 6.0% of patients on rivaroxaban and 8.8% of patients on placebo, HR 0.66; 95% CI, 0.40–1.09; p=0.10). Patients on rivaroxaban experienced a non-significant increased risk of major bleeding (2.0% on rivaroxaban and 1.0% on placebo, HR 1.96; 95% CI, 0.59–6.49; p=0.26). During the intervention period in the intention-to-treat population of all randomized patients, the primary efficacy outcome was significantly lower in the rivaroxaban group (2.6% on rivaroxaban and 6.4% on placebo, HR 0.40; 95% CI, 0.20–0.80). A pre-specified analysis including composite of primary efficacy endpoints, arterial, and visceral thrombotic events showed a significant reduction in the rivaroxaban group (6.9% on rivaroxaban and 10.7% on placebo, HR 0.62; 95% CI, 0.39–0.99). The NNT with rivaroxaban was 35 (26 if only considered on-treatment) and NNH was 101.
Pooled analysis
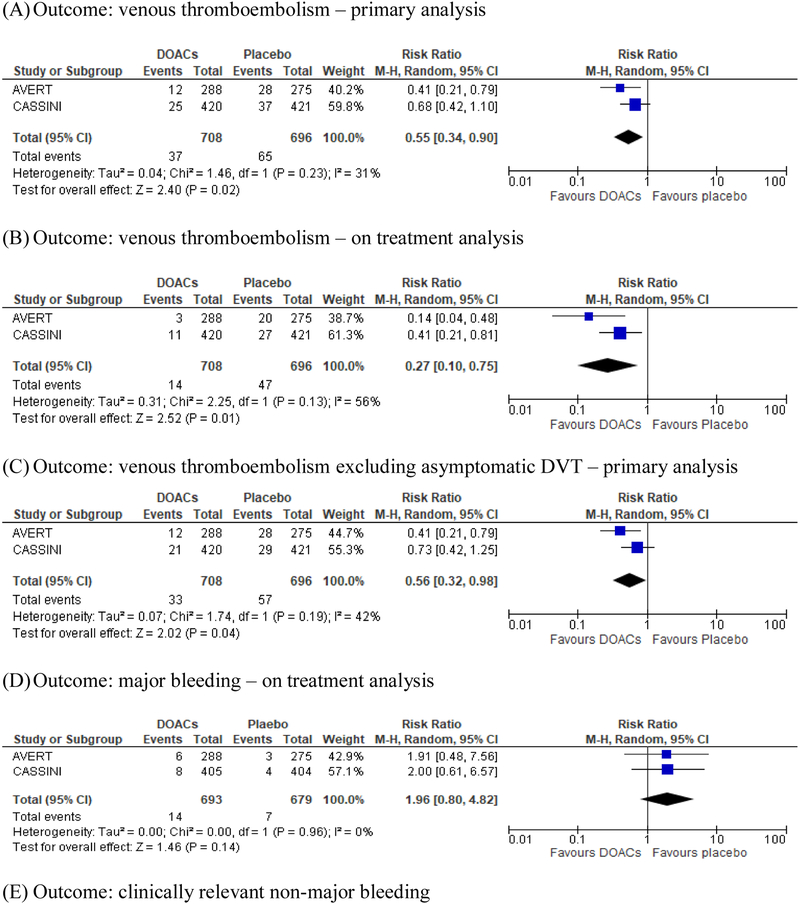
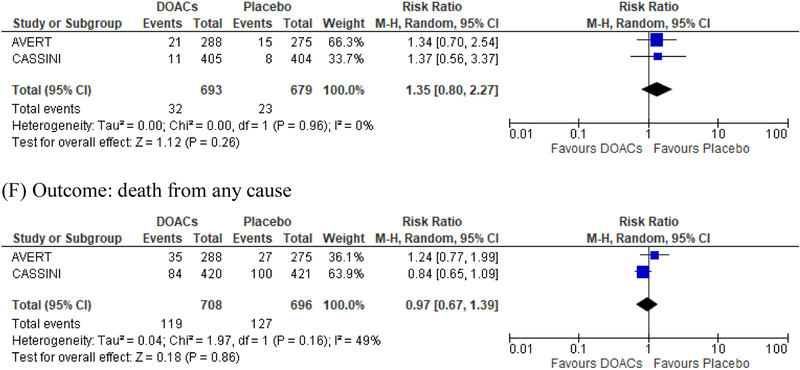
We acknowledge that the two studies have differences such as the use of screening ultrasound and patient population, but the pooled analysis of the two studies showed consistent results (Figure 1). DOACs significantly reduced the risk of VTE in the primary analysis (5.2 % on DOACs and 9.3% on placebo, risk difference [RD] −4.1%; RR 0.55; 95% CI, 0.34–0.90; p=0.02, Figure 1A) and the effects were further accentuated by on-treatment analysis (2.0% on DOACs and 6.8% on placebo, RD −4.8%; RR 0.27; 95% CI 0.10–0.75; p=0.01, Figure 1B). DOACs resulted in a numerically increased risk of major bleeding (2.0 % on DOACs and 1.0% on placebo, RD 1.0%; RR 1.96; 95% CI, 0.80–4.82; p=0.14, Figure 1D) and clinically relevant non-major bleeding (CRNMB) (4.6% on DOACs and 3.4% on placebo, RD 1.2%; RR 1.35; 95% CI 0.80–2.27; p=0.26, Figure 1E). Results excluding asymptomatic DVT were consistent with those from the primary analysis (Figure 1C), and there was no difference in mortality (Figure 1F) [16]. The net benefit of DOACs as primary thromboprophylaxis (VTE prevention vs major bleeding) resulted in an overall risk reduction of 2.8% with DOACs, translating into a NNT of 35. Although DOACs and LMWH achieved comparable reductions in the risk of VTE, the selection of high-risk patients in the DOACs trials resulted in a larger absolute VTE risk reduction of 4–5% by DOACs as compared to 2% by LMWH, while the risk difference in major bleeding was comparable (~1%) [4].
Figure 1.
Pooled analysis of the CASSINI and AVERT studies
Selection of patient population
Both studies enrolled patients with Khorana score ≥ 2 but with some important differences in the inclusion criteria. AVERT study included patients with tumors in the brain (N=24) and myeloma (N=15) while CASSINI study did not (Table 1). CASSINI study focused recruitment of patients with locally advanced or metastatic cancer, while AVERT study included all patients with newly diagnosed or progressive cancer starting chemotherapy. Patients with hematological malignancies accounted for 27.9% (160/574) of the population in the AVERT study, compared with 7.0% (59/841) in the CASSINI study. In the CASSINI study, 32.6% (274/841) of patients had pancreatic cancer, compared with 13.6% (78/574) in the AVERT study. The difference in patient population may provide implications for clinicians when they consider practice adoption. Both studies excluded patients with significant thrombocytopenia (platelet count < 50,000/mm3) or renal dysfunction (creatinine clearance < 30 ml/min). While the prevention of catheter-related thrombosis was not a focus in both studies, both included upper extremity DVT in the primary efficacy outcome.
Selection of type, dose, and duration of anticoagulation
The currently available evidence is only for apixaban and rivaroxaban, so these two agents would be the preferred choice if DOACs were to be considered in this setting. Both studies used the standard prophylactic dosing and planned for 6 months of prophylactic anticoagulants after the initiation of systemic cancer therapy. Although previous studies have investigated higher dose LMWH in the prevention of VTE in pancreatic cancer patients, there are no such data in DOACs. Recently, the results of a subgroup analysis of pancreatic cancer in the CASSINI study were reported at the American Society of Clinical Oncology Annual Meeting [17]. It showed a significant reduction of primary VTE and combined primary and secondary endpoints with standard prophylactic dose of rivaroxaban, with no increased risk of major bleeding. Therefore, approved prophylactic dose for either DOAC remains to be the standard of care. Whether longer duration is needed, the optimal dosing of thromboprophylaxis, or if other DOACs could provide similar results remain to be investigated.
Cautions
In both studies, DOACs were associated with an increased risk of major and CRNMB (Figure 1D and 1E). The hazard ratio of major bleeding was consistently around 2. However, the absolute risk difference remained low (<2%) with a NNH of >100 during the on-treatment period. The location of bleeding appeared to be mainly in the gastrointestinal tract, consistent with results from randomized studies of using DOACs as treatment of CAT [8, 9]. This information could aid in clinicians’ decisions when they are considering primary VTE prophylaxis. In addition, patients with malignancy involving the brain are at high risk of both bleeding and thrombosis, but this population was under-represented (24 patients in AVERT and excluded in CASSINI), and whether this high-risk population could have similar outcomes remains unclear. In patients with brain tumors and CAT, a previous study has shown that therapeutic doses of DOACs were not associated with increased risk of intracranial bleeding relative to LMWH [18]. Although both studies used Khorana score to identify high-risk patients, the included types of malignancies remained heterogeneous and could carry different thrombotic and bleeding risks. However, data from AVERT and CASSINI studies are the highest level of evidence to-date regarding using DOACs as primary thromboprophylaxis for ambulatory cancer patients, and it is likely not feasible to conduct dedicated studies in each tumor type.
Furthermore, DOACs are known substrates of P-glycoprotein (P-gp) and/or cytochrome P-450 (CPY) 3A4, and many targeted cancer therapies such as tyrosine kinase inhibitors are also metabolized through a similar mechanism. Therefore, drug interactions could be a concern. Clinicians should consider potential drug-drug interactions (one example summarized by Kraaijpoel and Carrier et al) when they plan to use DOACs for VTE prevention or treatment [19].
The role of screening ultrasound in intermediate to high-risk ambulatory cancer patients remains controversial. CASSINI study utilized screening ultrasound prior to enrollment and excluded patients with DVT on screening ultrasound (49/1080, 4.5%). Their primary efficacy outcome also included screen-detected asymptomatic proximal lower extremity DVT, while AVERT study had no screening ultrasound either prior to enrollment or throughout the study. It is worth noting that the utilization of screening ultrasound could contribute to the differences of the results in the two studies and is not considered standard of care prior to implementing thromboprophylaxis.
Guidance statement
We suggest the use of DOACs as primary thromboprophylaxis in ambulatory cancer patients starting chemotherapy with Khorana score ≥ 2 in patients with no drug-drug interactions and not at high risk for bleeding (such as patients with gastro-esophageal cancers). Currently, apixaban and rivaroxaban are the only DOACs with evidence from RCTs. A final treatment decision should be made after considering the risk of both VTE and bleeding, as well as patients’ preference and values.
We suggest that if DOACs were to be used for primary thromboprophylaxis in ambulatory cancer patients, it is administered for up to 6 months after the initiation of chemotherapy. We recommend monitoring of platelet counts and risk of bleeding complications while on anticoagulation.
In high-risk ambulatory cancer patients where primary thromboprophylaxis is planned but with concerns for safety of DOAC (such as in patients with concern of drug interaction or high risk of gastrointestinal bleeding), we suggest to use LWMH.
Addendum
T-F. Wang contributed to the concept and design of the study, data interpretation, writing of the manuscript and final approval of the submitted version. J. Zwicker contributed to the concept and design of the study, data interpretation, writing of the manuscript and final approval of the submitted version. S. Noble contributed to the concept and design of the study, data interpretation, writing of the manuscript and final approval of the submitted version. G. Meyer, C. Ay contributed to the concept and design of the study, data interpretation, writing of the manuscript and final approval of the submitted version. A. Falanga, contributed to the concept and design of the study, data interpretation, writing of the manuscript and final approval of the submitted version. I. Pabinger contributed to the concept and design of the study, data interpretation, writing of the manuscript and final approval of the submitted version. D. Antic contributed to the concept and design of the study, data interpretation, writing of the manuscript and final approval of the submitted version. A. A. Khorana contributed to the concept and design of the study, data interpretation, writing of the manuscript and final approval of the submitted version. M. Carrier contributed to the concept and design of the study, data interpretation, writing of the manuscript and final approval of the submitted version.
Acknowledgements
J. Zwicker is the recipient of CLOT UO1 from NHLBI (HL143365). A. Falanga acknowledges research support from the Italian Association of Cancer Research (AIRC). S. Noble holds a Marie Curie Chair in Supportive and Palliative Care from Cardiff University. A. A. Khorana gratefully acknowledges research support from the Sondra and Stephen Hardis Chair in Oncology and the National Heart, Lung and Blood Institute (Consortium Linking Oncology with Thrombosis, CLOT-U01HL143402). M. Carrier is the recipient of Tier 2 Research Chair from the University of Ottawa in Cancer and Thrombosis.
Disclosure of Conflict of Interests
T-F Wang reports consulting honoraria and travel support from Daiichi Sankyo and Pfizer.
J. I. Zwicker reports research support from Incyte, consulting from Parexel, advisory boards from Seattle Genetics, Daiichi, Bayer, Pfizer.
C. Ay reports advisory boards from Bayer, Daiichi-Sankyo, BMS/Pfizer, and Boehringer-Ingelheim and speaker honoraria from Bayer, Daiichi-Sankyo, BMS/Pfizer, and Sanofi.
I. Pabinger reports occasional honoraria for lectures and advisory boards from Bayer, Boehringer-Ingelheim, Pfizer, Daiichi-Sanchyo, Sanofi.
S. Nobel reports speakers bureau from Leo Pharma, Pfizer, Daichi Sankyo, Bayer.
A. A. Khorana reports personal fees and non-financial support from Janssen, Bayer, Parexel, Halozyme, Pfizer, Seattle Genetics, AngioDynamics, Leo Pharma; personal fees from Pharmacyclics, Pharmacyte, Trisalus; personal fees and non-financial support from Medscape/WebMD; research grants to his institution from Merck, Array , Bristol-Myers Squibb, Leap Pharma.
Dr. Carrier reports grants from BMS, Leo Pharma and Pfizer, personal fees from BMS, Leo Pharma, Bayer, Pfizer, Servier and Sanofi.
G. Meyer reports research funding from Leo Pharma and Bayer, and travel support from Leo Pharma, BMS, and Daiichi Sankyo.
Appendix
Table.
Khorana risk scores
| Patient characteristics | Risk score |
|---|---|
| High risk (lung, lymphoma, gynecologic, bladder, testicular) | 1 |
| Pre-chemotherapy platelet count ≥ 350×109/L | 1 |
| Hemoglobin level < 10 mg/mL or use of red cell growth factors | 1 |
| Pre-chemotherapy leukocyte count > 11 ×109/L | 1 |
| BMI ≥ 35 kg/m2 | 1 |
The AVERT study used a modified Khorana to include patients with brain tumor as very high risk cancer (risk score of 2) and myeloma and kidney cancer as high risk cancer (risk score of 1).
Footnotes
The other authors state that they have no conflict of interest.
References
- 1.Khorana AA, Otten HM, Zwicker JI, Connolly GC, Bancel DF, Pabinger I, Subcommittee on H, Malignancy of the S, Standardization Committee of the International Society on T, Hemostasis. Prevention of venous thromboembolism in cancer outpatients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014; 12: 1928–31. 10.1111/jth.12725. [DOI] [PubMed] [Google Scholar]
- 2.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013; 122: 1712–23. 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007; 5: 632–4. DOI 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 4.Di Nisio M, Porreca E, Candeloro M, De Tursi M, Russi I, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2016; 12: CD008500 10.1002/14651858.CD008500.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schunemann H, Ventresca M, Crowther M, Di Nisio M, Briel M, Zhou Q, Noble S, Macbeth F, Griffiths G, Garcia DA, Lyman GH, Iorio A, Mbuagbaw L, Neumann I, Van Es N, Brozek J, Guyatt G, Streiff MB, Brouwers M, Baldeh T, Marcucci M, Florez I, Solh Z, Ageno W, Bleker S, Bozas G, Buller H, Klerk C, Lebeau B, Lecumberri R, McBane RD, Sideras K, Maraveyas A, Pelzer U, Loprinzi C, Bossuyt P, Kahale L, Akl EA, Zulian G. An Individual Participant Data Meta-Analysis of 13 Randomized Trials to Evaluate the Impact of Prophylactic Use of Heparin in Oncological Patients. Blood. 2017; 130. [Google Scholar]
- 6.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008; 111: 4902–7. 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, Quehenberger P, Zielinski C, Pabinger I. Prediction of venous thromboembolism in cancer patients. Blood. 2010; 116: 5377–82. 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 8.Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang TF, Yeo E, Zhang G, Zwicker JI, Weitz JI, Buller HR, Hokusai VTECI. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018; 378: 615–24. 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 9.Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C, Hale D, Dunn JA, Lyman GH, Hutchinson C, MacCallum P, Kakkar A, Hobbs FDR, Petrou S, Dale J, Poole CJ, Maraveyas A, Levine M. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol. 2018; 36: 2017–23. 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 10.Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, Kuruvilla P, Hill D, Spadafora S, Marquis K, Trinkaus M, Tomiak A, Lee AYY, Gross PL, Lazo-Langner A, El-Maraghi R, Goss G, Le Gal G, Stewart D, Ramsay T, Rodger M, Witham D, Wells PS, Investigators A. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med. 2018. 10.1056/NEJMoa1814468. [DOI] [PubMed] [Google Scholar]
- 11.Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, Streiff MB, Garcia DA, Liebman HA, Belani CP, O’Reilly EM, Patel JN, Yimer HA, Wildgoose P, Burton P, Vijapurkar U, Kaul S, Eikelboom J, McBane R, Bauer KA, Kuderer NM, Lyman GH, Investigators C. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med. 2019; 380: 720–8. 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- 12.Easaw JC, Shea-Budgell MA, Wu CMJ, Czaykowski PM, Kassis J, Kuehl B, Lim HJ, MacNeil M, Martinusen D, McFarlane PA, Meek E, Moodley O, Shivakumar S, Tagalakis V, Welch S, Kavan P. Canadian consensus recommendations on the management of venous thromboembolism in patients with cancer. Part 1: prophylaxis. Curr Oncol. 2015; 22: 133–43. 10.3747/co.22.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE, Kakkar AK, Key NS, Levine MN, Liebman HA, Tempero MA, Wong SL, Somerfield MR, Falanga A. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update 2014. Journal of Clinical Oncology. 2015; 33: 654–U174. 10.1200/Jco.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine MN, Gu C, Liebman HA, Escalante CP, Solymoss S, Deitchman D, Ramirez L, Julian J. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost. 2012; 10: 807–14. 10.1111/j.1538-7836.2012.04693.x. [DOI] [PubMed] [Google Scholar]
- 15.Schulman S, Kearon C, Haemostasis IST. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005; 3: 692–4. DOI 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 16.Agnelli G Direct Oral Anticoagulants for Thromboprophylaxis in Ambulatory Patients with Cancer. N Engl J Med. 2019; 380: 781–3. 10.1056/NEJMe1816060. [DOI] [PubMed] [Google Scholar]
- 17.Vadhan-Raj S, McNamara MG, Venerito M, Riess H, O’Reilly EM, Overman MJ, Zhou X, Vijapurkar U, Kaul S, Wildgoose P, Khorana AA. Rivaroxaban thromboprohylaxis in ambulatory patients with pancreatic cancer: Results from a prespecified subgroup analysis of the CASSINI study. J Clin Oncol. 2019; 37: 4016 10.1200/JCO.2019.37.15_suppl.4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carney BJ, Uhlmann EJ, Puligandla M, Mantia C, Weber GM, Neuberg DS, Zwicker JI. Intracranial hemorrhage with direct oral anticoagulants in patients with brain tumors. J Thromb Haemost. 2019; 17: 72–6. 10.1111/jth.14336. [DOI] [PubMed] [Google Scholar]
- 19.Kraaijpoel N, Carrier M. How I treat cancer-associated venous thromboembolism. Blood. 2019; 133: 291–8. 10.1182/blood-2018-08-835595. [DOI] [PubMed] [Google Scholar]