Abstract
Parents lay the foundation for their children’s socio-emotional experiences by sensitively responding to their needs. The hormonal and neurobiological changes that occur during transition to parenthood importantly contribute to parents’ caregiving behavior toward their children. Much research has emphasized the relationship between the mother-who is most often the primary caregiver—and her infant, with less focus on the role of fathers in child development. However, recent accounts have suggested that fathers also play an important role in promoting the health, development and psychosocial wellbeing of their children. Evidence from behavioral literature has indicated that there are significant differences between typical mother-infant versus father-infant interactions. The current review aims to outline differences between maternal and paternal caregiving by discussing the differences in their biological mechanisms. First, we focus on the different hormones that are correlated with sensitive parenting behaviors in mothers and fathers. Next, we discuss the differences between neural bases of motherhood and fatherhood. Lastly, we discuss ways in which parental hormones, parental brain, and parental exposure to infant cues interact to shape parental caregiving behavior. In summary, this review highlights the distinct but complementary nature of maternal and paternal caregiving.
Keywords: mothers, fathers, brain, hormones, infant cues
1. Introduction
Across species, parental caregiving is considered critical for the survival and healthy development of one’s progeny. Humans represent a subgroup of mammalian species that exhibit distinct maternal and paternal caregiving behaviors.1 Parenting research has traditionally focused on maternal behavior and positive child development outcomes. Only recently, has there been a burgeoning interest in sensitive fathering and its role for the child’s socio-emotional and cognitive development.2,3 Moreover, behavioral studies have documented sex differences between mother-infant and father-infant interactions.4 For example, mother-infant interactions are mostly characterized by affectionate touch, emotional warmth and support, whereas father-infant interactions are primarily characterized by physical interactions, such as “rough and tumble” stimulatory or exploratory play.4,5 These observed differences in mother-child versus father-child interactions suggest that neuroendocrine differences may exist between the two sexes.
Thus, the aim of the current review is to examine neural and hormonal mechanisms that correlate with maternal and paternal behavior during interactions with their infants. First, we will review the hormonal basis of maternal and paternal caregiving. Second, we will review distinct neural networks that may contribute to maternal and paternal behavior. Lastly, we will consider ways in which unique parenting behaviors may emerge through the interplay of parental hormonal changes, parental brain plasticity, and parental exposure to infant cues during the early postpartum period. We will discuss all of the above with the aim of offering insights into distinct yet complementary roles that mothers and fathers may play in the upbringing of their children.
Our review includes all published papers searched through Pubmed on the neuro-endocrine changes in fathers and their correlation with paternal behaviors (all published between 2000 and 2018). Given the vast extent of literature on the neurobiology of maternal caregiving, we have narrowed our search to only include maternal studies that address overlapping brain areas and hormones that were reviewed in the paternal behavior studies. Finally, we have limited our review to findings from human studies.
2. Hormones and Parenting Behaviors
Humans, especially females, undergo significant hormonal changes during pregnancy, parturition, and postpartum that are often correlated with caregiving behaviors.6 Male partners are also subject to hormonal modifications during these times because of close proximity to their pregnant or lactating partner and contact with the newborn infants post birth. Several hormones are involved in the expression of parenting behaviors. Since there is a significant correlation between oxytocin, testosterone, prolactin, vasopressin and parenting behaviors in animal models, the following section will only focus on these four hormones7–9
2.1. Oxytocin
The neuropeptide, oxytocin is a neurohypophyseal hormone that binds to the G-protein coupled membrane receptors in peripheral tissues, such as mammary glands and the uterus, and in various brain regions10 It has been widely correlated with social behaviors, particularly bond formation.11–13 Specifically, oxytocin has received special attention because of its involvement in the initiation of motherhood.14 Oxytocin is released both peripherally, which leads to uterine contractions during labor and milk let-down during nursing,15 and in the brain, which enables the new mother to approach, nurture, and protect her offspring.16 Furthermore, repeated contact and interactions with her infant increases plasma and salivary oxytocin levels in mothers.17 These increases are associated with mother-infant attachment and synchrony.18 Although new fathers do not experience inherent surges in oxytocin like new mothers, interactions with their pregnant or lactating partner, as well as their newborn infant, have been associated with increases in the levels of oxytocin in fathers.19 Overall, elevated plasma oxytocin levels have been observed in both mothers and fathers during first 6 months of parenting.20
As demonstrated by Feldman and colleagues,17 elevated levels of oxytocin may be associated with different patterns of parental behavior in men and women. For example, increases in maternal oxytocin were associated with displays of affection and warmth toward their infant, whereas increases in paternal oxytocin were specifically correlated with stimulatory—but not affectionate—touch between fathers and their infants. While these findings may reflect an underlying hormonal mechanism which contributes to sex differences in parenting behavior, the reported associations may be independent or merely correlational.
One way to experimentally examine a causal relationship between oxytocin and parenting behavior is to utilize a randomized controlled trial of intranasal oxytocin. While no studies to date have examined the effects of intranasal oxytocin on parenting behavior in mothers, a few have addressed this in fathers. Naber et al. (2010)21 were the first to observe that fathers facilitated their child’s exploratory play more after receiving intranasal oxytocin than in the placebo condition. More recently, Li et al. (2017)22 have shown that intranasal oxytocin increases activation in the brain areas involved in motivating paternal behaviors. However, this was in a small sample of fathers which needs to be replicated and validated in a larger cohort.
2.2. Testosterone
Testosterone is an anabolic steroid hormone which promotes protein synthesis and growth of tissues via androgen receptor binding23. It has been associated with two behaviors in males: both mating and caregiving behavior. Testosterone levels are higher during mating behaviors and significantly lower during parenting behaviors. Moreover, during the transition to parenthood there appears to be a significant decrease in testosterone levels in human fathers.24 Proximity to the pregnant partner and increased contact with the infant post birth both contribute to the decline in testosterone levels.25 Fleming and others26 found that men who had lower testosterone levels were more empathic toward their infant’s cries. A more recent report found a decline in father’s testosterone levels during a strange situation procedure with their infants.27 As infants became distressed during the strange situation procedure, father’s testosterone levels decreased, which was correlated with more empathic or sensitive responses upon reuniting with the infant. While the research described here consistently observed inverse associations between levels of testosterone and paternal behaviors it is unclear as to whether low testosterone influences paternal behavior, or vice versa.
Effects of testosterone are less well defined and more indirect in mothers. Variations in testosterone levels in mothers do not directly translate into differences in maternal caregiving behavior, although testosterone and oxytocin show some interactive effects.19 For example, Gordon and colleagues19 found that when testosterone levels were high, there were positive associations between oxytocin and affectionate touch in mothers, but a negative association between oxytocin and affectionate touch in fathers. This may help explain observed differences in parenting behaviors between males and females with oxytocin and testosterone interacting in a sex-specific manner. More research is needed to better understand the underlying mechanisms of testosterone and its specific role in parenting behaviors, especially in mothers. While it is interesting to see the differential effects of testosterone in men and women, it should be noted that men have higher baseline testosterone levels compared to women and absolute levels of testosterone cannot be directly compared between mothers and fathers.
Although no previous studies have examined the effects of exogenous administration of testosterone in mothers and fathers, one study showed that intranasal testosterone administered to young females activated the thalamocingulate circuit in response to infant cries. This brain circuit has been implicated in the regulation of parental care.28 The findings suggest that increased testosterone may enhance brain activation in regions associated with parental behavior, at least in non-parental females, although the implications for parenting remain unclear.
2.3. Prolactin
Prolactin is another neuropeptide that binds to transmembrane cytokine receptor in a variety of peripheral tissues, including the mammary glands, and the central nervous system.29 It is commonly associated with the onset and conservation of maternal behavior in humans.30 Prolactin facilitates neurogenesis in mothers, which has been correlated with the onset of maternal behavior.31 Additionally, prolactin plays an important role in lactation.32 Prolactin levels increase during pregnancy, soon after the mother establishes contact with her newborn, and at the time of nursing.31
In contrast, males—especially first-time fathers—show an initial dip in prolactin levels approximately 30 minutes after their first exposure to infant cues. This peculiar effect is restricted only to first time fathers, with prolactin levels increasing on exposure to infant cues in second-time fathers.33,34 Thus, males who are experienced in fatherhood show greater increases in prolactin on hearing infant cries than first-time fathers.26 Interestingly, this initial drop in prolactin level is complemented by an initial increase in testosterone when first time fathers hear their infant’s cries. Researchers have speculated that this initial increase in testosterone on hearing infant cries might occur as the father prepares to defend the child against any threat cues that might have elicited the infant cry. Contrary to these earlier accounts, a more recent study found that the levels of prolactin were stable across the first six months of fatherhood and were correlated with father-infant coordinated exploratory play.35 No study to date has examined the effects of administration of exogenous prolactin in mothers and fathers.
2.4. Arginine Vasopressin
Despite ample research describing the role of oxytocin in parenting behaviors, very little is known about its structurally similar neuropeptide, vasopressin, which is also a neurohypophyseal hormone that binds to G-protein coupled membrane receptors.36 Much like oxytocin, vasopressin has also been correlated with socially affiliative behavior.37 While vasopressin appears to regulate male bonding as well as defensive and territorial behavior,38 it has also gained some attention with regard to human paternal caregiving behaviors. For example, intranasal vasopressin increases expectant fathers’ attention toward infant cues, compared with a group of control men.39 In addition, Levi et al40 showed that fathers with high levels of plasma vasopressin exhibited more stimulatory contact with their infants, while fathers with higher levels of plasma oxytocin showed more affectionate touch. This is in contrast to the findings of Feldman and colleagues17 where increased paternal oxytocin was correlated to stimulatory rather than affectionate behavior. With limited research in this area, additional research is warranted to examine the effects of vasopressin on parenting behaviors in both mothers and fathers and to make sense of seemingly contradictory findings.
2.5. Hormones Interplay and Parental Behavior
In summary, the current section has examined key hormones that are critically involved in parenting behaviors. Currently available evidence is generally in support of the view that most of these hormones have sex-specific effects which, in turn, are correlated with different caregiving behaviors. In mothers, these hormones appear to predict behaviors that are characterized as affectionate towards their children, while in fathers, they are correlated with behaviors that may be classified as stimulatory and playful toward their children. While we have examined each of these hormones separately (as did the majority of studies we have reviewed in this section), it is important to note that these hormones do not act in isolation but interact with each other as well as with other hormones that are not focus of the present review and may collectively influence parenting behaviors. For example, in the female brain, testosterone is metabolized to estradiol which in turn increases the number of oxytocin receptors in the brain, thereby facilitating maternal behaviors.28,41 This is consistent with Gordon and colleague’s (2017)19 finding suggesting that high testosterone correlates with high oxytocin levels and affectionate behaviors in mothers. Furthermore, administration of intranasal oxytocin is known to increase the levels of arginine vasopressin in both males and females42. In fact, when present at high levels, oxytocin and vasopressin may activate each other’s receptors. Additional research on the interplay of all these critical hormones is necessary to further clarify the neuroendocrine basis of parenting behaviors.
3. Brain and Parenting Behaviors
Along with hormonal changes, new parents also undergo reorganization in several brain regions in order to prepare for their new caregiving roles. While there is some evidence highlighting similarities between the maternal and paternal brain circuits,43 differences in mother-infant and father-infant interaction might arise from differences in structural and functional changes in the maternal and paternal brains during transition to parenthood. The following section will discuss differences in the structure and function of the maternal and paternal brains, and their relationships with the differences observed in maternal and paternal behaviors.
4.1. Structural Plasticity in New Parents
The parental brain shows increased structural plasticity during the early postpartum period. Although new mothers and new fathers show increased gray matter volume in several overlapping brain areas, such as the midbrain, lateral prefrontal cortex (PFC) and superior temporal regions,44,45 some brain regions show decreases in gray matter volume in fathers but not in mothers. These regions include the medial PFC, posterior cingulate cortex (PCC), inferior parietal cortex, precuneus, right orbitofrontal cortex (OFC) and left insula.45 In particular, Kim and colleagues45 correlated structural decreases in the OFC with paternal stimulatory behavior or high paternal involvement towards infants. While these results are correlational, longitudinal research may help to determine whether the changes in brain structure result in differences in parental behaviors, or vice versa.
4.2. Functional Plasticity in New Parents
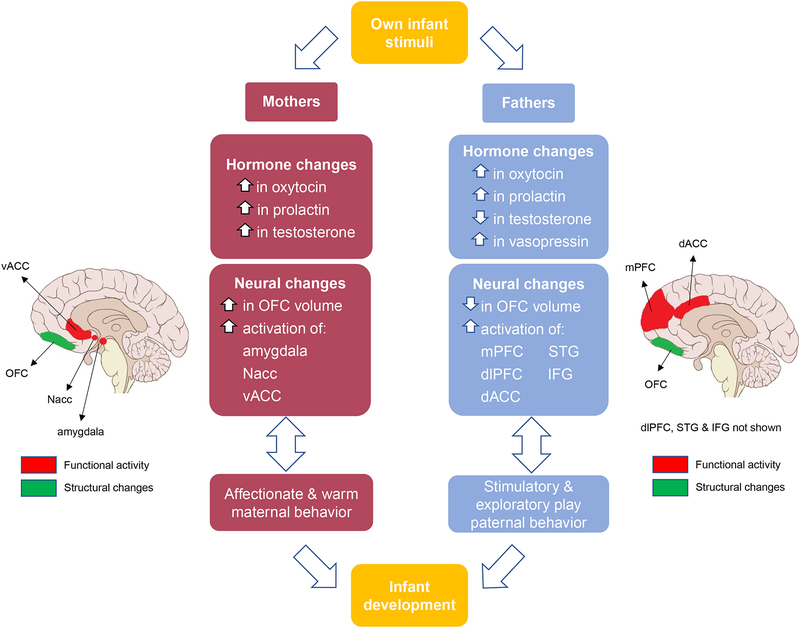
While some preliminary evidence suggests that parents show activation in similar neural circuits when responding to infant cues, more recent work has demonstrated differences in the activation of brain regions between mothers and fathers.20,27 When viewing video clips of own infants versus unknown infants, mothers showed activation in limbic areas such as the amygdala, nucleus accumbens (NAcc), insula, and ventral anterior cingulate cortex (vACC), while fathers showed activations in socio-cognitive areas such as the medial PFC, dorsolateral PFC (dlPFC), dorsal anterior cingulate cortex (dACC), superior temporal gyrus (STG) and inferior frontal gyrus (IFG)20 (Figure 1). Abraham and colleagues46 corroborated this further by using infant video clips and reporting that mothers (in primary caregiving roles) showed activations in the limbic regions while fathers (in secondary caregiving roles) showed activations in the socio-cognitive network. These findings together suggest that primary caregiving mothers have a tendency to demonstrate activation in the emotional processing neural circuitry whereas secondary caregiving fathers demonstrate activation in the socio-cognitive neural circuitry, which may translate to the differences in their parenting behaviors. Abraham and colleagues46 examined a sample of same-sex primary and secondary caregiving fathers and found some interesting results. While the primary caregiving mothers showed activation in the limbic regions and the secondary caregiving fathers showed activation in the socio-cognitive regions, the primary caregiving fathers showed activation in both the limbic as well as the socio-cognitive regions. Given that primary caregiving fathers do not experience the same biological changes that primary caregiving mothers do during pregnancy, birth or lactation, it is remarkable to observe how the paternal brain may, to some degree, adapt to the demands of a primary caregiving role.46 Although some aspects of the parental brain are considered to be hard-wired and sex-specific, the parental brain also appears to be capable of adapting to the social environment in order to better assume specific caregiving responsibilities.
Figure 1:
Model of differences in parenting behaviors between mothers and fathers explained by neuro-hormonal responses to their own infant’s stimuli. OFC = orbitofrontal cortex; Nacc = nucleus accumbens; vACC = ventral anterior cingulate cortex; dACC = dorsal anterior cingulate cortex; mPFC = medial prefrontal cortex; dlPFC = dorsolateral prefrontal cortex; STG = superior temporal gyrus; IFG = inferior frontal gyrus. Brain schematic by P.J. Lynch.
5. Infant Cues, Neuro-hormonal Changes and Parenting Behaviors
As discussed in the sections above, human parents undergo a host of neural and endocrine changes after the birth of their infant which appear to be closely associated with their caregiving responses. Most of these changes occur when parents are in close contact with their infant. In particular, infant faces are socially salient stimuli that capture attention from parents and non-parents, alike.47–49 Studies reviewed in the preceding sections suggest that parental exposure to infant cues, particularly those of one’s own infant, are associated with changes in the parent’s hormonal profiles as well as activation in brain regions associated with reward and attachment.20,46 In other words, parental contact with their own infant may be an important contributor to the neuro-endocrine changes discussed above, which may eventually facilitate parenting behaviors in mothers and fathers.
This raises the question of whether mothers and fathers may process their own infant’s cues differently. A body of literature has looked at parental sex differences in response to infant faces. In their review, Luo et al50 reported that females have a stronger attentional bias to and higher preference for infant faces when compared with males. This is consistent with neuro-imaging findings showing that primary caregiving mothers, when compared to secondary caregiving fathers, show enhanced responses in limbic brain areas associated with reward and motivation while viewing infant video-clips.20,46 However, primary caregiving fathers in same-sex relationships showed increased functional connectivity between both the limbic as well as socio-cognitive networks while viewing infant stimuli. This leads us to understand that parental sex differences in responses to infant cues that are often discussed in the literature might not only be shaped by underlying sex-specific biology but also critically modified by the type and amount of contact the parent has with his/her child during the early postpartum period. This has important implications for adoptive, foster and step-parents who may not undergo biological changes during pregnancy, postpartum, and breastfeeding but spend a great deal of time forming bonds with their infant during the early years of life.
Another important question that arises from the current discussion is whether mothers and fathers differentially respond to different emotions expressed by their infant and if this might translate into distinct behaviors observed in the two sexes. For example, Strathearn and colleagues,51 found activations in the dopaminergic reward-related brain areas in first-time mothers in response to happy but not sad faces of their own infant. The authors argued that happy signals from their infant evoked positive affective arousal in mothers, which may lead to the positive contingent behavioral responses observed in mothers. More recently, Parsons et al52 reported that mothers and fathers may perceive infant emotions differently. In their study, mothers rated the infant’s happy emotion more positively than fathers. The authors suggested that such differences observed in mothers’ and fathers’ perception and processing of the infant’s emotional cues may at least partly explain differences observed in parental behavior. It would be of interest for future studies to examine whether fathers similarly show dopaminergic reward brain response to happy but not sad faces of their own infant, or to more broadly compare maternal and paternal responses to own and unknown infants’ different-valence emotions. This may influence parents’ caregiving responses when infants are faced with emotionally salient experiences and may thus shape the child’s socio-emotional development.
Given that previous work has addressed the similarities between the maternal and paternal brains and their associated hormonal profiles27,53 the current review has focused primarily on the differences that may be associated with sex-specific parenting behaviors. Decades of behavioral research has shown that mothers are more affectionate and warm with their infants, while fathers tend to engage in more stimulatory and exploratory play which contributes uniquely to their child’s development during specific developmental periods.17 We have proposed that the behavioral differences may at least in part be explained by differences in parental brain responses and endocrine functions, although it may equally be true that maternal and paternal behavior may also, in turn, contribute to and/or heighten differences in parental brain and endocrine functions. We have further argued that the ways in which parental brain responses influence specific endocrine functions vary as a function of parental sex and that this may further be associated with different parenting behaviors. Figure 1 summarizes our proposal that each sex brings unique yet complementary neuroendocrine responses and behaviors to parenting, which we believe may have important long-term implications for child development. Bi-directional arrows in the figure reflect mutual influences between parenting behavior and the neural and endocrine changes discussed in the preceding sections.
6. Limitations and Future Directions
While the present review provides important insights into the interplay of hormones, neurobiology and parenting behaviors, currently published studies cannot determine whether changes in hormones and neural circuits contribute to the differences in parenting behaviors or whether the differences in parenting behaviors contribute to changes in the neurobiology. Although exogenous administration of hormones helps us better understand-the causal nature of these effects, one should proceed with caution in interpreting the effects of exogenous hormones as they are dependent upon the individual’s endogenous hormone levels (Weisman et al., 2013). Furthermore, despite the novelty and originality of the studies reported in the current review, one must consider limiting factors such as sample size and individual differences within samples.22 Most of these empirical studies have been published in the last 10 years and require replication in larger and more generalizable cohorts.
From behavioral literature we have learnt that differences in the manner in which mothers and fathers process infants’ emotional cues may have a potential role in shaping unique parenting behaviors.52 Future research should use neuroimaging paradigms to compare whether mothers and fathers differentially process their infant’s emotional cues. A few studies to date have looked at these brain responses separately in mothers and fathers.22,51 However, to our knowledge, no study has addressed the gaps in the literature by specifically comparing brain responses to one’s own infant’s emotional cues between mothers and fathers. Future studies should also compare hormonal responses, including those that have been reviewed in the present paper, in mothers and fathers and their associations with parental brain responses to infant’s emotional stimuli.
Another avenue for future research would be to observe paternal behaviors with infants when the mother’s ability to adequately respond to her infant may be compromised. One such condition would be when mothers experience postpartum depression. To that effect, a recent study has shown the benefits of sensitive fathering and the manner in which it can reduce the effects of maternal depression on the development of child psychopathology.54 Thus, instead of exclusively focusing on the mother-infant dyad for prevention and intervention, as has often been the case in the presence of maternal psychopathology, research should emphasize the mother-father-infant triad as a whole and examine how the father might mitigate some of the long-term negative effects of maternal psychopathology and contribute to their child’s resilience and well-being.
7. Conclusion
Becoming a parent is a critical developmental juncture not only for the parents themselves but also for their infant’s survival and development. Thus, examining sex-differences in the neuro-endocrinology of parenting adds to the larger field’s knowledge base of human development. It helps us understand how two different sexes come together at a critical juncture of life and contribute to a distinct yet complementary manner of parenting. Together, with these distinct yet complementary patterns of neurobiology and behaviors, mothers and fathers may offer a balanced nurturing experience for their young in order to optimize, social, emotional and developmental outcomes.
Acknowledgements
This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development [R01 HD065819 and R03 HD080998]. The content is solely the responsibility of the authors and does not necessarily represent the official views of these institutes or the National Institutes of Health. The authors report no conflicts of interest with respect to the content of this manuscript.
References
- 1.Hrdy SB. Mother nature: A history of mothers, infants, and natural selection. New York: Pantheon Books; 1999. [Google Scholar]
- 2.Allgood SM, Beckert TE, & Peterson C. The Role of Father Involvement in the Perceived Psychological Well-Being of Young Adult Daughters: A Retrospective Study. North American Journal of Psychology. 2012;14(1). [Google Scholar]
- 3.Panter‐Brick C, Burgess A, Eggerman M, McAllister F, Pruett K, & Leckman JF Practitioner review: engaging fathers–recommendations for a game change in parenting interventions based on a systematic review of the global evidence. Journal of Child Psychology and Psychiatry. 2014;55(11):187–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman R Infant–mother and infant–father synchrony: The coregulation of positive arousal. Infant Mental Health Journal: Official Publication of The World Association for Infant Mental Health. 2003;24:1–23. [Google Scholar]
- 5.Fuertes M, Faria A, Beeghly M, & Lopes-dos-Santos P The effects of parental sensitivity and involvement in caregiving on mother–infant and father–infant attachment in a Portuguese sample. Journal of Family Psychology. 2016;30(147). [DOI] [PubMed] [Google Scholar]
- 6.Rilling JK. The neural and hormonal bases of human parentalcare. Neuropsychologia. 2013;51(4):731–747. [DOI] [PubMed] [Google Scholar]
- 7.Storey AE, & Ziegler TE Primate paternal care: interactions between biology and social experience. Hormones and Behavior. 2016;77:260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynne-Edwards KE, & Timonin ME Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Hormones and Behavior. 2007;52(1):114–121. [DOI] [PubMed] [Google Scholar]
- 9.Wynne-Edwards KE. Hormonal changes in mammalian fathers. Hormones and Behavior. 2001;40(2):139–145. [DOI] [PubMed] [Google Scholar]
- 10.Muehlenbein MP. Human evolutionary biology. Cambridge University Press; 2010. [Google Scholar]
- 11.Feldman R Oxytocin and social affiliation in humans. Hormones and behavior. 2012;61(3):380–391. [DOI] [PubMed] [Google Scholar]
- 12.Young LJ, & Wang Z The neurobiology of pair bonding. Nature neuroscience. 2004;7(10):1048. [DOI] [PubMed] [Google Scholar]
- 13.Carter CS, Williams JR, Witt DM, & Insel TR Oxytocin and Social Bonding. Annals of the New York Academy of Sciences. 1992;652(1):204–211. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen CA. Oxytocin control of maternal behavior regulation by sex steroids and offspring stimulia. Annals of the New York Academy of Sciences. 1997;807(1):126–145. [DOI] [PubMed] [Google Scholar]
- 15.Rilling JK, & Young LJ The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345(6198):771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Numan M Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology. 2007;49(1):12–21. [DOI] [PubMed] [Google Scholar]
- 17.Feldman R, Gordon I, Schneiderman I, Weisman O, & Zagoory-Sharon O Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010;35(8):133–1141. [DOI] [PubMed] [Google Scholar]
- 18.Levine A, Zagoory-Sharon O, Feldman R, & Weller A Oxytocin during pregnancy and early postpartum: individual patterns and maternal–fetal attachment. peptides. 2007;28(6):1162–1169. [DOI] [PubMed] [Google Scholar]
- 19.Gordon I, Pratt M, Bergunde K, Zagoory-Sharon O, & Feldman R Testosterone, oxytocin, and the development of human parental care. Hormones and behavior. 2017;93:184–192. [DOI] [PubMed] [Google Scholar]
- 20.Atzil S, Hendler T, Zagoory-Sharon O, Winetraub Y, & Feldman R Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(8):798–811. [DOI] [PubMed] [Google Scholar]
- 21.Naber F, van IJzendoorn MH, Deschamps P, van Engeland H, & Bakermans-Kranenburg MJ Intranasal oxytocin increases fathers’ observed responsiveness during play with their children: a double-blind within-subject experiment. Psychoneuroendocrinology. 2010;35(10):1583–1586. [DOI] [PubMed] [Google Scholar]
- 22.Li T, Chen X, Mascaro J, Haroon E, & Rilling JK Intranasal oxytocin, but not vasopressin, augments neural responses to toddlers in human fathers. Hormones and behavior. 2017;93:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheffield-Moore M Androgens and the control of skeletal muscle protein synthesis. Annals of medicine. 2000;32(3):181–186. [DOI] [PubMed] [Google Scholar]
- 24.Berg SJ, & Wynne-Edwards KE Changes in testosterone, cortisol, and estradiol levels in men becoming fathers. Mayo Clinic Proceedings. 2001;76(6):582–592). [DOI] [PubMed] [Google Scholar]
- 25.Saxbe DE, Edelstein RS, Lyden HM, Wardecker BM, Chopik WJ, & Moors AC Fathers’ decline in testosterone and synchrony with partner testosterone during pregnancy predicts greater postpartum relationship investment. Hormones and behavior. 2017;90:39–47. [DOI] [PubMed] [Google Scholar]
- 26.Fleming AS, Corter C, Stallings J, & Steiner M Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Hormones and behavior. 2002;42(2):399–413. [DOI] [PubMed] [Google Scholar]
- 27.Kuo PX, Carp J, Light KC, & Grewen KM Neural responses to infants linked with behavioral interactions and testosterone in fathers. Biological psychology. 2012;91(2):302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bos PA, Hermans EJ, Montoya ER, Ramsey NF, & van Honk J Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocrinology. 2010;35(1):114–121. [DOI] [PubMed] [Google Scholar]
- 29.Mancini T, Casanueva FF, & Giustina A Hyperprolactinemia and prolactinomas. Endocrinology and metabolism clinics of North America. 2008;37(1):67–99. [DOI] [PubMed] [Google Scholar]
- 30.Russell JA, Douglas AJ, & Ingram CD Brain preparations for maternity—adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Progress in brain research. 2001;133:1–38. [DOI] [PubMed] [Google Scholar]
- 31.Grattan D A mother’s brain knows. Journal of neuroendocrinology. 2011;23(11):1188–1189. [DOI] [PubMed] [Google Scholar]
- 32.Svennersten-Sjaunja K, & Olsson K Endocrinology of milk production. Domestic animal endocrinology. 2005;29(2):241–258. [DOI] [PubMed] [Google Scholar]
- 33.Delahunty KM, McKay DW, Noseworthy DE, & Storey AE Prolactin responses to infant cues in men and women: effects of parental experience and recent infant contact. Hormones and Behavior. 2007;51(2):213–220. [DOI] [PubMed] [Google Scholar]
- 34.Storey AE, Walsh CJ, Quinton RL, & Wynne-Edwards KE Hormonal correlates of paternal responsiveness in new and expectant fathers. Evolution and Human Behavior. 2000;21(2):79–95. [DOI] [PubMed] [Google Scholar]
- 35.Gordon I, Zagoory-Sharon O, Leckman JF, & Feldman R Prolactin, oxytocin, and the development of paternal behavior across the first six months of fatherhood. Hormones and Behavior. 2010;58(3):513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawyer WH. Evolution of neurohypophyseal hormones and their receptors. Federation proceedings. 1977;36(6):1842–1847. [PubMed] [Google Scholar]
- 37.Cho MM, DeVries AC, Williams JR, & Carter CS The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behavioral neuroscience. 1999;113(5):1071. [DOI] [PubMed] [Google Scholar]
- 38.Meyer-Lindenberg A, Domes G, Kirsch P, & Heinrichs M Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12(9):524. [DOI] [PubMed] [Google Scholar]
- 39.Cohen-Bendahan CC, Beijers R, van Doornen LJ, & de Weerth C Explicit and implicit caregiving interests in expectant fathers: Do endogenous and exogenous oxytocin and vasopressin matter? Infant Behavior and Development. 2015;41:26–37. [DOI] [PubMed] [Google Scholar]
- 40.Apter-Levi Y, Zagoory-Sharon O, & Feldman R Oxytocin and vasopressin support distinct configurations of social synchrony. Brain research. 2014;1580:124–132. [DOI] [PubMed] [Google Scholar]
- 41.Cornil CA, Ball GF, & Balthazart J Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain research. 2006;1126(1):2–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisman O, Schneiderman I, Zagoory-Sharon O, & Feldman R Salivary vasopressin increases following intranasal oxytocin administration. Peptides. 2013;40:99–103. [DOI] [PubMed] [Google Scholar]
- 43.Swain JE, Lorberbaum JP, Kose S, & Strathearn L Brain basis of early parent–infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of child psychology and psychiatry. 2007;48(3‐4):262–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, & Swain JE The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behavioral neuroscience. 2010;124(5):695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim P, Rigo P, Mayes LC, Feldman R, Leckman JF, & Swain JE Neural plasticity in fathers of human infants. Social neuroscience. 2014;9(5):522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abraham E, Hendler T, Shapira-Lichter I, Kanat-Maymon Y, Zagoory-Sharon O, & Feldman R Father’s brain is sensitive to childcare experiences. Proceedings of the National Academy of Sciences. 2014;111:9792–9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brosch T, Sander D, & Scherer KR That baby caught my eye. Attention capture by infant faces. Emotion. 2007;7(3):685–689. [DOI] [PubMed] [Google Scholar]
- 48.Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, … & Gur RC Baby schema modulates the brain reward system in nulliparous women. Proceedings of the National Academy of Sciences. 2009;106(22):9115–9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, & Davidson RJ Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21(2):583–592. [DOI] [PubMed] [Google Scholar]
- 50.Luo L, Ma X, Zheng X, Zhao W, Xu L, Becker B, & Kendrick KMF Neural systems and hormones mediating attraction to infant and child faces. Frontiers in psychology. 2015;6:970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strathearn L, Li J, Fonagy P, & Montague PR Maternal brain responses to infant facial cues. Pediatrics. 2008;122(1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parsons CE, Young KS, Elmholdt EMJ, Stein A, & Kringelbach ML Interpreting infant emotional expressions: Parenthood has differential effects on men and women. The Quarterly Journal of Experimental Psychology:Human Experimental Psychology. 2016;70:554–564. [DOI] [PubMed] [Google Scholar]
- 53.Storey A, & Walsh C How fathers evolve: a functional analysis of fathering behavior In: Biosocial foundations of family processes. New York, NY: Springer; 2011. [Google Scholar]
- 54.Vakrat A, Apter-Levy Y, & Feldman R Sensitive Fathering Buffers the Effects of Chronic Maternal Depression on Child Psychopathology. Child Psychiatry & Human Development. 2018:1–7. [DOI] [PubMed] [Google Scholar]