Abstract
Cardiovascular diseases (CVD) remain the leading cause of morbidity and mortality in both men and women in developed societies. Age is the greatest risk factor for CVD due largely to adverse changes to arteries that include stiffening of the large elastic arteries (aortic and carotid arteries) and endothelial dysfunction. Vascular aging is driven by oxidative stress, which reduces nitric oxide (NO) bioavailability and stimulates changes in the extracellular matrix. In women, reductions in circulating estrogens with menopause interact with aging processes to induce vascular dysfunction. Regular aerobic exercise is the most evidence-based strategy for reducing CVD risk with aging in both men and women. Much of this CV-protective effect of aerobic exercise is likely due to its vascular health-enhancing influence. Large elastic artery stiffening with advancing age is attenuated in healthy adults engaged in aerobic exercise training, and aerobic exercise interventions improve arterial stiffness in previously sedentary middle-aged and older men and postmenopausal women. Regular aerobic exercise also enhances endothelial function with aging in men (by reducing oxidative stress and preserving NO bioavailability), but not consistently in estrogen-deficient postmenopausal women. In postmenopausal women, treatment with estradiol appears to restore the ability of aerobic exercise to improve NO-mediated endothelial function by reducing oxidative stress. Several research gaps exist in our understanding of potential sex differences in the vascular adaptations to regular aerobic exercise. More information is needed on the factors that are responsible for sex differences, including the role of circulating estrogens in transducing the aerobic exercise training “stimulus”.
Keywords: arterial stiffness, endothelial function, estrogen, oxidative stress
Despite recent declines in prevalence, cardiovascular diseases (CVD) remain the leading cause of morbidity and mortality in men and women in both developed societies and most developing nations (Wang et al., 2016; Benjamin et al., 2018). By far, the greatest risk factor for CVD is advancing age with most of the burden of CVD falling on middle-aged and older adults (Benjamin et al., 2018). Given projected increases in the absolute numbers of middle-aged and older men and women in the future, a sharp upward trend in CVD is forecasted in the absence of effective population-wide prevention efforts (Heidenreich et al., 2011).
A major target for the prevention of CVD in middle-aged and older adults is vascular aging (Najjar et al., 2005; Lakatta, 2015; Niiranen et al., 2017; Nowak et al., 2018; R. Seals et al., 2018; Rossman et al., 2018; Seals et al., 2018). With advancing age, several adverse changes occur in the arterial system that drive the development of CVD. One clinically important event is stiffening of the large elastic arteries, i.e., the aorta and carotid arteries (Lakatta & Levy, 2003) (Figure 1). This age-associated arterial stiffening is mediated in part by changes to the extracellular matrix within the walls of arteries including degradation of elastin fibers, which are replaced by collagen deposition (fibrosis), and increased formation of crosslinking molecules such as advanced glycation end-products (AGEs) (Lakatta, 2003; Lakatta & Levy, 2003; Fleenor, 2013; Nowak et al., 2018). Greater arterial smooth muscle tone, likely mediated by an imbalance between bioavailability and/or action of vasodilatory and vasoconstrictor signaling, is also believed to contribute to age-associated arterial stiffening (Lakatta & Levy, 2003; McEniery et al., 2003; Wilkinson et al., 2004; Lyle & Raaz, 2017; Nowak et al., 2018). This imbalance of the vasoactive molecular milieu, most prominently a decrease in nitric oxide (NO) bioavailability, induces a second negative change with vascular aging—endothelial dysfunction, as typically indicated by impaired endothelium-dependent dilation in response to chemical or mechanical (increased flow) stimuli (Celermajer et al., 1994; Taddei et al., 1996; Taddei et al., 2001; Seals et al., 2011) (Figure 1).
Figure 1. Mechanisms of age-associated vascular dysfunction and subsequent cardiovascular diseases.
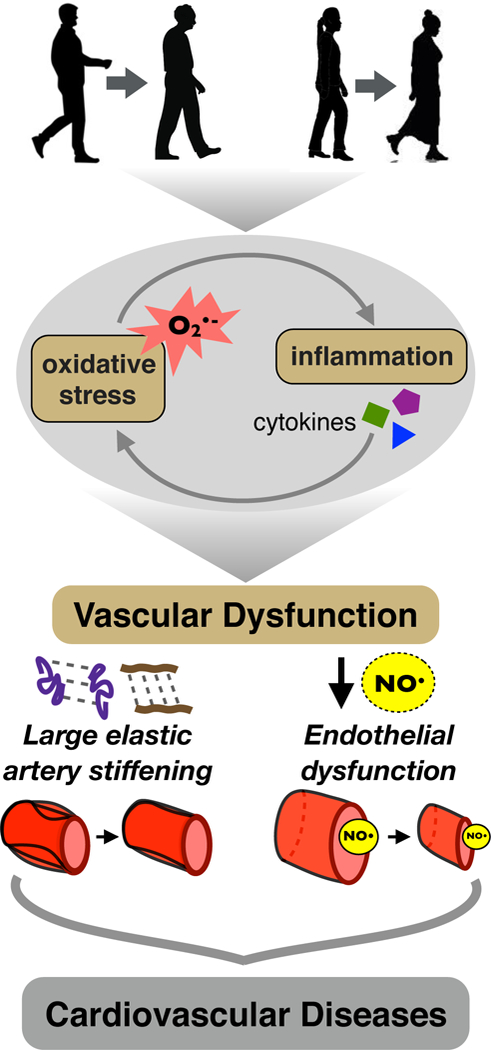
Aging in both men and women is associated with increased oxidative stress and inflammation, marked by increased superoxide (O-2) bioactivity and inflammatory mediators. Together, these processes induce vascular dysfunction, featuring (lower left) large elastic artery stiffening mediated by degradation of elastin fibers (purple), increased deposition of collagen fibers (brown), and greater crosslinking of elastin and collagen molecules by advanced glycation endproducts (dashed connecting lines); and (right) vascular endothelial dysfunction characterized by reduced nitric oxide (NO) bioavailability and endothelium-dependent dilation. These and other changes to arteries, in turn, increase the risk of developing clinical cardiovascular diseases.
Although the cellular mechanisms that mediate arterial stiffening and endothelial dysfunction with aging remain under investigation, there is extensive evidence supporting important roles for vascular oxidative stress and chronic low-grade inflammation (Taddei et al., 2001; Eskurza et al., 2004b; Moreau et al., 2005; Donato et al., 2007; Donato et al., 2009; Pierce et al., 2009; Hildreth et al., 2013; Moreau et al., 2013a; Walker et al., 2014) (Figure 1). In women, reductions in circulating estrogens associated with menopause interact with other cellular aging processes to influence vascular function via mechanisms that whilst incompletely understood, also appear to involve oxidative stress and inflammation (Moreau et al., 2012; Hildreth et al., 2013; Moreau et al., 2013a; Moreau & Hildreth, 2014).
Collectively, these observations suggest that strategies that suppress the development of oxidative stress and inflammation, maintain vasodilatory/vasoconstrictor balance, preserve endothelial function and inhibit arterial stiffening would be highly effective for preventing age-associated CVD (Figure 1). In this context, physical activity, in general, and regular aerobic exercise, in particular, are strongly associated with reduced CVD risk, especially in middle-aged and older men and women (Eckel et al., 2014). The cardiovascular-protective effect of aerobic exercise likely is mediated by multiple mechanisms, including a more favorable cardiovascular risk factor profile (Mora et al., 2007; Eckel et al., 2014). However, the latter explains no more than 50% of the effects of physical activity on CVD risk (Mora et al., 2007). The unexplained variance in the influence of aerobic exercise on CVD appears to be mediated, at least in part, via favorable modulation of vascular aging (Joyner & Green, 2009; Seals, 2014).
The purpose of this symposium review article, which summarizes a presentation at the 2018 Europhysiology meeting in London, is to discuss similarities as well as potential differences in how regular aerobic exercise influences vascular function with aging in healthy men and women. Particular attention will be paid to the potential influence of reductions in circulating estrogens in observed sex differences. Broader reviews of the topic of exercise and vascular aging are available elsewhere (Seals et al., 2008; Seals et al., 2009; Moreau & Hildreth, 2014; Santos-Parker et al., 2014; Seals, 2014; Moreau & Ozemek, 2017).
Regular Aerobic Exercise and Arterial Stiffness with Aging
The effects of regular aerobic exercise on large elastic artery stiffness with aging have been investigated using both carotid-to-femoral artery pulse wave velocity (cfPWV), a measure of aortic stiffness, and carotid artery compliance or its blood pressure-modified reciprocal measure, the carotid beta-stiffness index (Vaitkevicius et al., 1993; Tanaka et al., 1998; Tanaka et al., 2000; Moreau et al., 2003). Increases in cfPWV and reductions in carotid artery compliance (increases in beta-stiffness index) indicate arterial stiffening; changes in the opposite direction indicate greater compliance (reduced stiffness)
Among healthy sedentary men, cfPWV increases with aging, but middle-aged and older men who perform regular aerobic exercise have a cfPWV closer to young men than their sedentary peers (Vaitkevicius et al., 1993). Similarly, among healthy females, sedentary postmenopausal women demonstrate greater cfPWV compared with sedentary premenopausal women, whereas no significant differences are observed in cfPWV between endurance exercise-trained postmenopausal and premenopausal women (Tanaka et al., 1998). Collectively, these findings indicate that aortic stiffening with aging is attenuated in healthy men and women who perform regular aerobic exercise compared with sedentary adults.
Carotid artery compliance decreases (carotid beta-stiffness index increases) with aging in both sedentary and aerobic exercise-trained healthy men and women; however, the magnitude of the changes with age in exercising men and women is only ~50% of that observed with age in sedentary adults (Tanaka et al., 2000; Moreau et al., 2003; Matsubara et al., 2013; Tanahashi et al., 2014) (Figure 2A). To assess the potential modulatory influence of circulating estrogen status on carotid artery elasticity with aging in women, we studied eumenorrheic premenopausal controls and groups of postmenopausal women differing in hormone therapy and aerobic exercise status. The impairment in carotid artery compliance observed in postmenopausal vs. premenopausal women was smaller in postmenopausal women who were taking estrogen-based hormone therapy compared with their estrogen-deficient peers (Moreau et al., 2003). Carotid artery stiffness was similar in estrogen-treated sedentary women and endurance exercise-trained estrogen-deficient women; that is, both estrogen treatment without regular aerobic exercise and regular aerobic exercise without estrogen treatment were associated with more favorable carotid artery compliance compared with their sedentary estrogen-deficient peers.
Figure 2. Aging, aerobic exercise and carotid artery compliance.
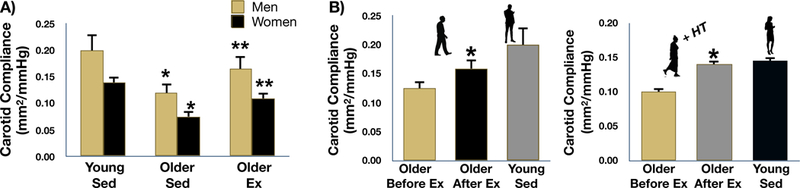
A: carotid artery compliance is reduced in both sedentary (Sed) and aerobic exercise trained (Ex) healthy older men and women compared to young controls; however, the age-related reduction in carotid compliance is attenuated by ~50% in Ex older adults. *P<0.05 vs. Young Sed, **P<0.05 vs Older Sed. B: carotid artery compliance increased by ~25–30% after 12 weeks of moderate-intensity aerobic exercise in previously sedentary healthy older men and estrogen-treated postmenopausal women. P<0.05 vs Before Ex. Data are from Tanaka et al. (Tanaka et al., 2000) and Moreau et al. (Moreau et al., 2003).
In addition to such cross-sectional observations, aerobic exercise interventions increase carotid artery compliance (reduce carotid artery beta-stiffness index) to a similar extent (25–30%) in previously sedentary healthy middle-aged and older men (Tanaka et al., 2000) and estrogen-treated postmenopausal women (Moreau et al., 2003) (Figure 2B). Improvements in arterial stiffness with aerobic exercise training also have been reported in estrogen-deficient postmenopausal women (Matsubara et al., 2013; Tanahashi et al., 2014).
Mechanisms of Action
The mechanisms by which regular aerobic exercise counteracts arterial stiffening with aging in humans are largely unknown, in part because of the difficulty in accessing and/or manipulating the aorta and carotid arteries without the confounding influence of altering blood pressure and triggering vascular tone-modulating cardiovascular reflexes (Seals, 2014). There is some evidence, however, that aerobic exercise training may modulate oxidative stress and/or inflammation.
Oxidative Stress
Lower concentrations of circulating markers of oxidative stress, such as plasma oxidized LDL, and lower expression of genes related to oxidant production in peripheral blood mononuclear cells have been reported in the aerobic exercise-trained vs. sedentary state in middle-aged and older men and women (Moreau et al., 2006; Gano et al., 2011; Pierce et al., 2011a; Santos-Parker et al., 2017). Such findings support the possibility that the lower arterial stiffness of the exercising middle-aged and older adults may be related to lower systemic oxidative stress. Moreover, observations in healthy estrogen-deficient postmenopausal women using supra-physiological infusions of the potent antioxidant vitamin C (ascorbic acid) to acutely suppress excess superoxide bioavailability support the view that lower oxidative stress contributes to the greater carotid artery compliance observed in endurance exercise-trained versus sedentary women (Moreau et al., 2006). This is consistent with findings in old male mice in which reductions in carotid artery stiffness in response to voluntary wheel running were associated with reduced oxidative stress-related collagen expression (Fleenor et al., 2010). However, the role of excessive superoxide-associated oxidative stress in carotid artery stiffening with aging in healthy sedentary men is unclear. In contrast to postmenopausal women (Moreau et al., 2006), systemic infusion of vitamin C did not affect carotid artery compliance in healthy sedentary middle-aged and older men (Eskurza et al., 2004a), and no data presently are available in exercising vs. sedentary middle-aged and older men.
Inflammation
The lower arterial stiffness in middle-aged and older men and women who exercise regularly may also be related to less age-associated inflammation. Several investigations have reported lower concentrations of circulating markers of inflammation such as C-reactive protein and inflammatory cytokines and/or higher concentrations of anti-inflammatory cytokines in middle-aged and older men and women who regularly perform vigorous aerobic exercise compared with non-exercising controls (Kasapis & Thompson, 2005; Nicklas et al., 2008; Pierce et al., 2011b; Santos-Parker et al., 2017). Moreover, lower expression of genes coding for nuclear factor κ B (NFκB), a master pro-inflammatory transcription factor, and pro-inflammatory cytokines have been observed in peripheral blood mononuclear cells of middle-aged and older men and women after vs. before aerobic exercise training (Gano et al., 2011). These findings are consistent with the concept of lower systemic inflammation with aging in the middle-aged and older exercising adults. Similar observations have been made for expression of pro-inflammatory cytokines in breast tissue of exercising vs. sedentary postmenopausal women (Hanna et al., 2018). The results of preclinical investigations in mice extend these observations to vascular tissue. Voluntary wheel running in older male mice reduced expression of NFκ B and pro-inflammatory cytokines in whole aortic lysate to levels observed in young mice (Lesniewski et al., 2011). Running wheel exercise also normalized age-related increases in macrophage and T lymphocyte infiltration, another marker of vascular inflammation, in the adventitial and perivascular regions of the arterial wall (Lesniewski et al., 2011).
Summary
Collectively these observations suggest that the lower levels of large elastic artery stiffness observed in middle-aged and older adults who perform regular aerobic exercise may, at least in some cases, be associated with lower oxidative stress and/or inflammation compared with their sedentary peers. However, additional research is needed to support such conclusions, and sex differences in the respective roles of these mechanisms in modulating age-and exercise-related differences in arterial stiffness remain a possibility.
Regular Aerobic Exercise and Vascular Endothelial Function with Aging
Healthy Middle-Aged and Older Men
Regular aerobic exercise is generally associated with preserved vascular endothelial function with aging in healthy men, as assessed by endothelium-dependent dilation (Seals, 2014). We and others have shown that the forearm blood flow responses to intra-brachial artery infusion of the endothelium-dependent dilator acetylcholine, a clinically-relevant measure of endothelial function of the forearm resistance arteries (microvasculature) (Halcox Julian et al., 2002; Deanfield et al., 2007; Seals et al., 2014), is markedly lower in healthy sedentary middle-aged and older versus young adult men but is well-maintained with aging in endurance exercise-trained middle-aged and older men (DeSouza et al., 2000; Taddei et al., 2000) (left panel Figure 3A). Similarly, we have also shown that brachial artery flow-mediated dilation (FMD), a well-established measure of conduit artery (macrovascular) NO-mediated endothelium-dependent dilation and predictor of future clinical CV outcomes (Yeboah et al., 2007; Rossi et al., 2008), in healthy sedentary middle-aged and older men is only ~50% of that observed in young adult controls but is well-preserved in middle-aged and older men engaged in endurance exercise-training (Eskurza et al., 2004b). Importantly, in previously sedentary healthy middle-aged and older men, 8–12 weeks of conventional moderate-intensity aerobic exercise (brisk walking) improves both forearm resistance artery and brachial artery endothelial function to levels not significantly different from young healthy men (DeSouza et al., 2000; Pierce et al., 2011b) (left panels Figure 3A and 3B).
Figure 3. Aging, aerobic exercise and endothelial function.
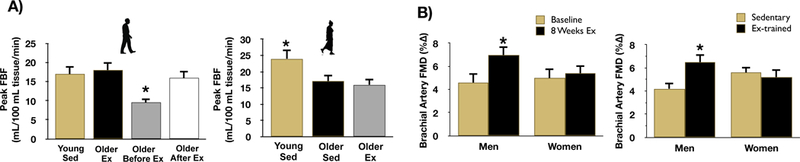
A: peak forearm blood flow (FBF) to acetylcholine (a measure of microvascular endothelial function) is reduced in healthy sedentary (Sed) older men (Older Before Ex) vs. Young Sed, older aerobic exercise trained (Older Ex) and older Sed men after aerobic exercise training (Older After Ex) (left). *P<0.05 vs. other groups. In women, peak FBF to acetylcholine is reduced in both Older Sed and Older Ex estrogen-deficient (postmenopausal) vs. Young Sed (premenopausal) (right). *P<0.05 vs. Young Sed. B: brachial artery flow-mediated dilation (FMD, a measure of macrovascular endothelial function) increased after 8 weeks of moderate-intensity aerobic exercise training in previously sedentary middle-aged/older men but not in estrogen-deficient postmenopausal women (left). Brachial artery FMD is greater in aerobic exercise-trained (Ex) compared to sedentary middle-aged/older men, but there is no difference in FMD between sedentary and Ex estrogen-deficient postmenopausal women (right). *P<0.05 vs Before Ex. Data are from DeSouza et al. (DeSouza et al., 2000), Santos-Parker et al. (Santos-Parker et al., 2017) and Pierce et al. (Pierce et al., 2011b).
The robust endothelium-dependent dilation observed in middle-aged and older exercising men is achieved by maintaining NO bioavailability at young adult levels (Taddei et al., 2000; Seals et al., 2008). This healthy endothelial function phenotype observed in endurance exercise-trained middle-aged and older men compared with their sedentary peers is associated with lower oxidative stress, as indicated by a significant increase in endothelium-dependent dilation in response to acute vitamin C infusion in sedentary, but not in exercising men (Taddei et al., 2001; Eskurza et al., 2004b). These observations are unaffected when endothelium-dependent dilation is corrected for arterial blood pressure and expressed as increased vascular conductance (DeSouza et al., 2000; Taddei et al., 2001). Moreover, endothelium-independent dilation in response to a NO “donor” (infusion of nitroprusside or sublingual administration of nitroglycerine), a measure of vascular smooth muscle sensitivity to NO, is unaffected by aerobic exercise status in healthy middle-aged and older men (DeSouza et al., 2000; Taddei et al., 2001; Eskurza et al., 2004b; Pierce et al., 2011b), suggesting that the enhanced endothelium-dependent dilation found in the exercising men is attributable to adaptations of the vascular endothelium per se.
Healthy Estrogen-Deficient Postmenopausal Women
In healthy women, vascular endothelial function, as assessed by brachial artery FMD, declines progressively from the premenopausal state of young adult females across the menopause transition, or “perimenopause” period, to the postmenopausal state in which menstrual periods have stopped for at least one year (Moreau et al., 2012). The menopause transition starts in the mid-to late 40s and features the initial change in menstrual cycle regularity and alterations in the sex hormone environment (Santoro, 2016; Moreau, 2018). As such, strategies that are effective in preserving or enhancing endothelial function as women transition through and into the postmenopausal period of life may lessen the marked increase in CVD prevalence observed after menopause (Santoro & Sutton-Tyrrell, 2011; Moreau & Hildreth, 2014; Moreau, 2018).
Unfortunately, the effects of regular aerobic exercise on vascular endothelial function in postmenopausal women are not nearly as clear as in middle-aged and older men. We found that an 8-week program of brisk walking that improved brachial artery FMD by ~50% in previously sedentary but healthy middle-aged and older men had no effect on brachial FMD in healthy estrogen-deficient women who had been postmenopausal for ~10 years (Pierce et al., 2011b) (left panel Figure 3B). Upon post hoc analysis we could find no biological or exercise training-related factor that explained the lack of improvement in the women, including pre-training (baseline) brachial FMD. No changes were observed in endothelium-independent dilation with exercise training.
Consistent with our findings, some other studies also have reported no improvement in brachial artery FMD with aerobic exercise training in estrogen-deficient postmenopausal women (Casey et al., 2007; Swift et al., 2013). However, improvements in brachial FMD with aerobic exercise have been reported, particularly in women with more greatly impaired endothelial function at baseline (Black et al., 2009; Swift et al., 2011; Akazawa et al., 2012; Swift et al., 2013). Of note, a more recent study in healthy estrogen-deficient women who had only been postmenopausal for an average of 3 years, found that a 12-week program of vigorous leg cycle ergometry exercise training increased femoral artery endothelium-dependent dilation (increase in femoral vascular conductance) to intra-femoral artery infusion of both acetylcholine and the prostacyclin analog epoprostenol, indicating improved leg conduit artery endothelial function in response to leg cycling-based aerobic exercise training (Nyberg et al., 2016).
Potential Role of Exercise Training Stimulus
We considered the possibility that the exercise training “stimulus” used in our aerobic exercise intervention study (Pierce et al., 2011b), although the same exercise duration (~50 min/d), intensity (70–75% of maximal exercise heart rate), frequency (6 d/wk) and treatment duration (8–9 weeks) used in the middle-aged and older men, was somehow insufficient to evoke an adaptation in the postmenopausal women. To determine this, we assessed brachial artery FMD in a large cohort of healthy middle-aged and older men and estrogen-deficient postmenopausal women who were either sedentary or had been performing strenuous endurance exercise training for at least 5 consecutive years (Pierce et al., 2011b). Consistent with the results of our aerobic exercise intervention study (Pierce et al., 2011b), middle-aged and older endurance exercise-trained men demonstrated a 50% greater brachial artery FMD compared with their sedentary peers (right panel Figure 3B). In contrast, yet consistent with our exercise intervention results, no differences were observed in brachial FMD between endurance exercise-trained and sedentary estrogen-deficient postmenopausal women (right panel Figure 3B). These findings suggest that an insufficient exercise training stimulus (intensity, duration and/or volume of aerobic exercise performed) does not obviously explain the absence of an effect on vascular endothelial function in estrogen-deficient postmenopausal women compared with middle-aged and older men.
Potential Role of Resistance Versus Conduit Artery Adaptations
As described above, aerobic exercise training induces significant increases in endothelium-dependent dilation (endothelial function) in both forearm resistance arteries and the brachial artery in healthy middle-aged and older men. Because the studies described above in estrogen-deficient postmenopausal women all assessed endothelium-dependent dilation in conduit arteries, primarily brachial artery FMD, it is possible that regular aerobic exercise improves endothelial function in resistance arteries, but not in conduit arteries.
To address this possibility, we recently conducted an investigation that compared the forearm blood flow responses to intra-brachial artery infusions of acetylcholine in 3 groups: (1) premenopausal, (2) estrogen-deficient postmenopausal sedentary, and (3) estrogen-deficient endurance exercise-trained postmenopausal women (Santos-Parker et al., 2017). In agreement with our previous observations when assessing brachial artery FMD, we found that the postmenopausal sedentary women had significantly lower forearm blood flow responses to acetylcholine compared with the premenopausal controls, indicating impaired endothelium-dependent dilation of resistance arteries with the combination of aging and menopause (right panel Figure 3A). Most importantly, and also in keeping with our prior findings, the forearm blood flow responses to acetylcholine were similar in the sedentary and endurance exercise-trained postmenopausal women; expression of the responses as (blood pressure-corrected) forearm vascular conductance provided the same results. As in our studies using brachial FMD, there were no group differences in endothelium-independent dilation (intra-brachial artery infusion of nitroprusside). These findings support the idea that, in contrast to middle-aged and older men, regular aerobic exercise does not consistently enhance vascular endothelial function in either resistance or conduit arteries of healthy estrogen-deficient postmenopausal women.
Healthy Estrogen-Treated Postmenopausal Women
The postmenopausal women studied in previous investigations on the effects of regular aerobic exercise and vascular endothelial function were estrogen-deficient, i.e., they had significantly lower circulating concentrations of estrogens compared with the premenopausal women serving as controls. It is possible that in sedentary postmenopausal women, improvements in endothelial function with aerobic exercise training require higher circulating concentrations of estrogen to properly transduce the stimulus created by regular exercise (Parker et al., 2010; Pierce et al., 2011b).
Accordingly, we conducted a randomized, placebo-controlled, double-blind clinical trial (Moreau et al., 2013b) in which groups of sedentary estrogen-deficient postmenopausal women (~8 years post-menopause on average) were given either: a daily oral estradiol tablet and a weekly transdermal placebo patch; a weekly transdermal estradiol patch and a daily oral placebo tablet; or a daily placebo tablet and a weekly placebo patch, for 12 weeks. These initial 12-week periods were followed in each case by 12 weeks of aerobic exercise training (brisk walking for 40–45 min/d, 5–7 d/wk) during which time the women maintained their initial estradiol or placebo treatment condition. Compared with baseline values, circulating estrogens (i.e., estradiol and estrone) concentrations were increased significantly at 12 and 24 weeks in the two estradiol-treated groups and unchanged in the placebo group.
Brachial artery FMD was increased after the initial 12 weeks of treatment (no exercise) in both estradiol groups, but not in the placebo controls. Most importantly, brachial FMD increased further in response to the subsequent 12-week period of aerobic exercise training only in the estradiol-treated groups; no improvement was observed in the placebo treated (estrogen-deficient) women, consistent with our previous findings (Pierce et al., 2011b) (Figure 4). The combination of estradiol treatment and aerobic exercise training produced a similar overall increase in brachial artery FMD as that observed in our previous study in middle-aged and older men (Pierce et al., 2011b), whereas the response in the placebo-treated group was identical to that reported in our prior exercise intervention trial in estrogen-deficient postmenopausal women (Pierce et al., 2011b) (Figure 4).
Figure 4. Sex differences in improvements in endothelial function to aerobic exercise training in middle-aged/older adults.
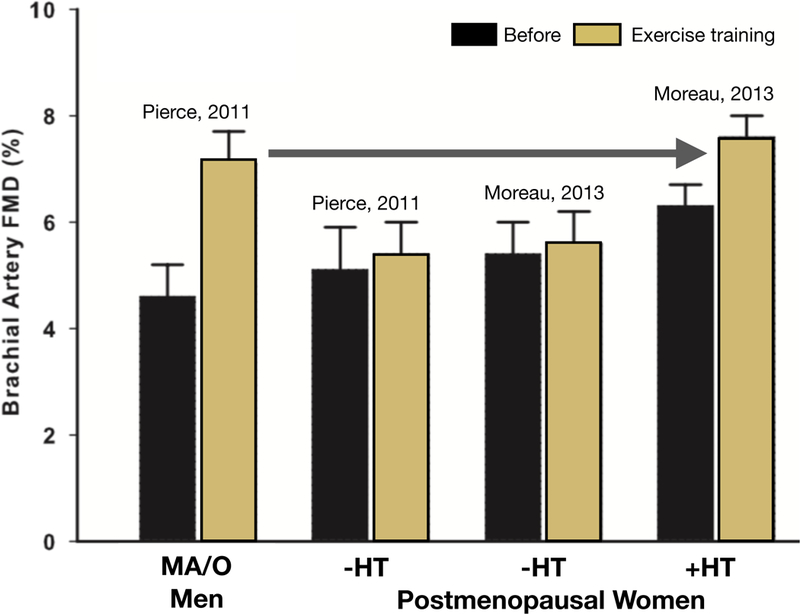
Brachial artery flow-mediated dilation (FMD) increased after 8–12 weeks of exercise training in healthy middle-aged/older (MA/O) men (far left) and postmenopausal women treated with estrogen-based hormone therapy (+HT, far right), but not in estrogen-deficient postmenopausal women (-HT) (middle left and middle right). Data are from Pierce et al. (Pierce et al., 2011b) and Moreau et al. (Moreau et al., 2013b). Reproduced from Moreau et al. (Moreau et al., 2013b).
Oxidative Stress
To determine the influence of oxidative stress in explaining the different effects of aerobic exercise training in the presence and absence of high circulating estrogen concentrations, we used acute vitamin C infusions to temporarily reduce superoxide bioavailability (Moreau et al., 2013b). At baseline (i.e., prior to any treatment), vitamin C infusion increased brachial artery FMD in all 3 groups of sedentary estrogen-deficient postmenopausal women, suggesting excessive superoxide-associated tonic suppression of endothelial function (Figure 5). As an internal control, an increase in brachial FMD with vitamin C infusion was also documented in a separate group of estrogen-deficient postmenopausal women who were endurance exercise-trained. This observation confirmed that in the absence of high circulating estrogen, habitual aerobic exercise has no obvious effect on vascular oxidative stress and its tonic inhibition of endothelium-dependent dilation in postmenopausal women. After 12 weeks of regular aerobic exercise, vitamin C infusion still produced an increase in brachial artery FMD in the placebo-treated women, but not in the 2 estradiol-treated groups, suggesting amelioration of oxidative stress-related suppression of endothelial function with exercise training in healthy postmenopausal women with restored circulating estrogen concentrations (Figure 5). The latter findings are consistent with the lack of effect of vitamin C infusion on brachial artery FMD and the reduced expression of nitrotyrosine, a marker of oxidative stress, in brachial artery endothelial cells in middle-aged and older men who regularly perform aerobic exercise compared with their sedentary peers (Eskurza et al., 2004b; Pierce et al., 2011a).
Figure 5. Mechanisms of aerobic exercise training on endothelial function in the absence and presence of estrogen in postmenopausal women.
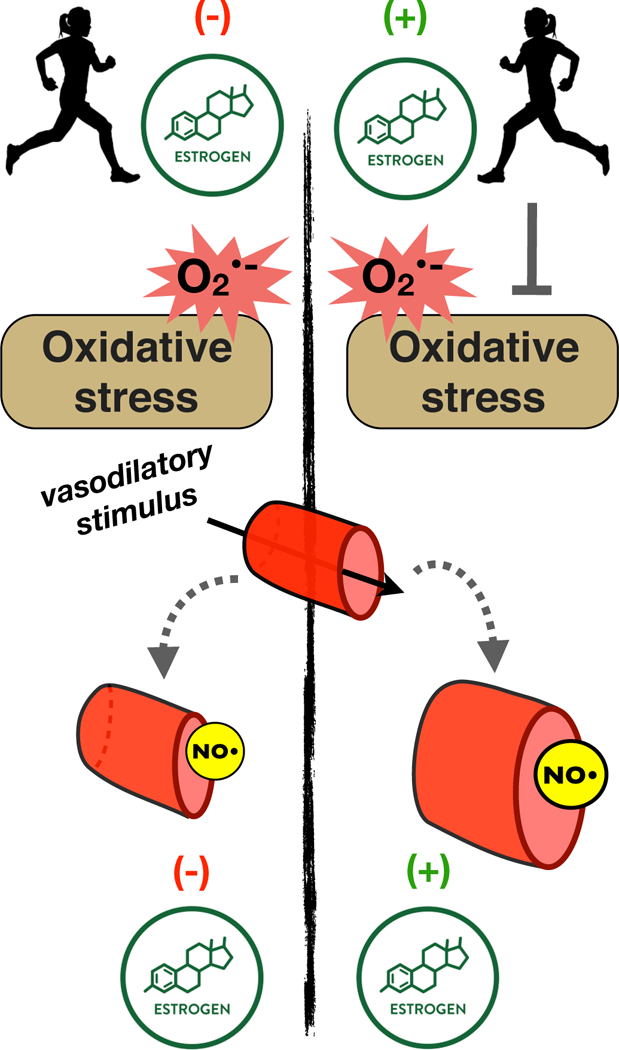
In healthy estrogen-deficient postmenopausal women, aerobic exercise has no obvious effect on oxidative stress-suppression of vascular endothelium-dependent vasodilation. In contrast, estrogen treatment in postmenopausal women ameliorates the oxidative stress-related suppression of endothelial function with exercise training.
Summary
Taken together, these results support the hypothesis that regularly performed aerobic exercise alone, whether short-term moderate-intensity exercise or longer-term vigorous endurance exercise training, does not consistently improve vascular endothelial function in healthy estrogen-deficient postmenopausal women. However, the combination of estradiol treatment and regular aerobic exercise appears to induce improvements in endothelial function similar to those produced by exercise training alone in middle-aged and older men (Pierce et al., 2011b) (Figure 5). These findings suggest that some currently undetermined circulating level of estrogen (greater than that typically occurring in an untreated state) may be required to “transduce” the physiological stimulus created by regular aerobic exercise for improving endothelial function in postmenopausal women.
In this context, 3 additional observations are worth noting. One is that, in general, middle-aged and older men actually have higher circulating concentrations of estradiol compared with untreated postmenopausal women (Bjørnerem et al., 2004). Thus, it is possible that estrogen is also required to transduce exercise signals to the vascular endothelium in middle-aged and older men, but they have sufficient circulating estrogen concentrations to do so, whereas non-estrogen treated postmenopausal women may not. A second point is that our findings in postmenopausal women are consistent with results showing amelioration of vascular endothelial dysfunction in amenorrheic premenopausal endurance athletes in response to estrogen treatment (oral contraceptive pills) or restoration of menses (Rickenlund et al., 2005b, a). Finally, it is possible that sex differences in vascular remodeling might play a role in the inconsistent results reported to date on endothelial function and exercise training in postmenopausal women. Some work indicates that large conduit arteries like the brachial artery may remodel over time during an aerobic exercise training program (Tinken et al., 2008). These structural changes may mask an improvement in conduit artery endothelial function at a particular point in time. Other investigators have not observed changes in, for example, brachial artery diameter in response to aerobic exercise training in middle-aged and older adults (Pierce et al., 2011b; Moreau et al., 2013b). Moreover, such a mechanism would not explain the absence of an improvement in resistance artery endothelial function in estrogen-deficient postmenopausal women (Santos-Parker et al., 2017).
Role of Reduced Inflammation in the Effects of Regular Aerobic Exercise on Vascular Endothelial Function in Middle-Aged and Older Men and Women
As discussed above, findings based on circulating concentrations of inflammatory markers, as well as results from studies of inflammatory gene expression in peripheral blood mononuclear cells, suggest the possibility of reduced systemic inflammation in middle-aged and older men and women in the aerobic exercise-trained vs. sedentary state (Kasapis & Thompson, 2005; Nicklas et al., 2008; Pierce et al., 2011b; Santos-Parker et al., 2017). As such, lower systemic inflammation may contribute to aerobic exercise training-associated improvements in vascular endothelial function in both middle-aged and older men and estrogen-treated postmenopausal women. Moreover, observations showing reduced expression of NFκ B in aorta of older male mice after several weeks of voluntary wheel running indicate a strong anti-inflammatory effect of aerobic exercise training with aging in whole (large) arteries (Lesniewski et al., 2011).
Other findings extend these observations to the vascular endothelium. Brachial artery FMD was decreased and expression of NFκ B in brachial artery endothelial cells was increased in healthy middle-aged and older vs. young adult sedentary men, whereas middle-aged and older aerobic exercise-trained men had levels of brachial FMD and endothelial cell expression of NFκ B similar to young sedentary men (Pierce et al., 2011a). Furthermore, short-term treatment with the NFκ B inhibitor salsalate reduced endothelial cell expression of NFκ B and improved brachial artery FMD in healthy non-exercising middle-aged and older men and estrogen-deficient postmenopausal women but had no effect in aerobic exercise-trained middle-aged and older adults (both middle-aged and older groups were ~30% postmenopausal women, all estrogen-deficient) or in non-exercising young adult controls (Walker et al., 2014). These findings are consistent with the idea of a tonic pro-inflammatory suppression of vascular endothelial function in sedentary, but not in aerobically exercising middle-aged and older adults. However, relatively few postmenopausal women were included in the latter study; as such, a more definitive understanding of the interactive effects of inflammation and aerobic exercise training on vascular endothelial function in this group will require investigation in a larger cohort.
Conclusions
As illustrated in the abstract figure, findings from our laboratory and others support the view that regular aerobic exercise inhibits arterial stiffening with advancing age in healthy men and women. Moreover, aerobic exercise interventions reduce arterial stiffness in previously sedentary middle-aged and older men and, possibly, in both estrogen-treated and estrogen-deficient postmenopausal women, although evidence is limited in women. Observations from well-controlled studies indicate that aerobic exercise training also preserves vascular endothelial function with aging in healthy men by enhancing NO bioavailability and reducing oxidative stress. In contrast, at present we lack consistent evidence for a similarly beneficial effect of aerobic exercise in estrogen-deficient postmenopausal women. However, the results of an initial investigation suggest that estradiol treatment may restore the ability of an aerobic exercise stimulus to evoke improvements in endothelial function in postmenopausal women by reducing oxidative stress.
Future Directions
Presently there are several biomedically significant gaps in our understanding of potential sex differences in the beneficial effects of regular aerobic exercise on vascular aging. The following represent some of the possible future research directions in this area (Figure 6).
Figure 6.
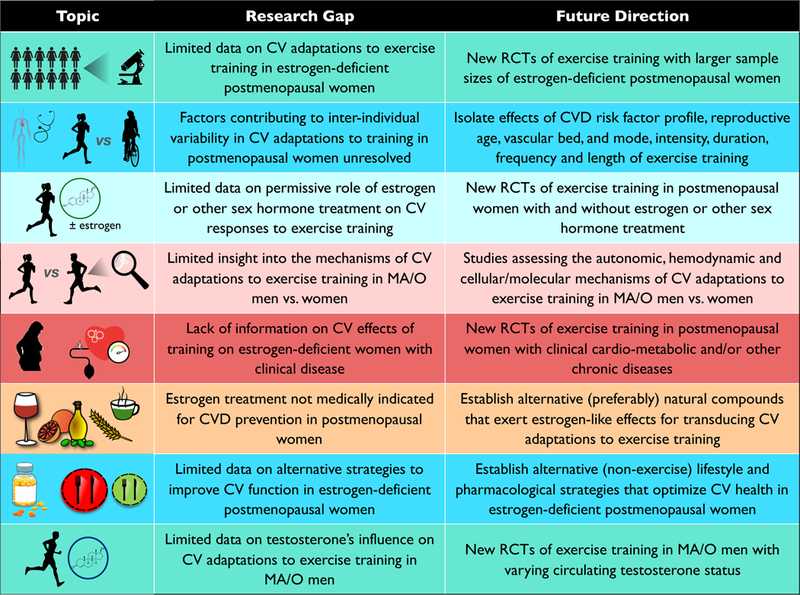
Gaps in knowledge and future research directions.
-
1)
The limited information we have on vascular adaptations to aerobic exercise interventions in postmenopausal women is based on studies with very small samples sizes. A larger randomized placebo-controlled trial will be necessary to more definitively establish the effects of regular aerobic exercise on arterial stiffness and endothelial function in estrogen-deficient postmenopausal women.
-
2)
At least some of the inconsistencies in the results of studies to date in estrogen-deficient postmenopausal women likely are due to differences in the participants studied and/or research design. It will therefore be important to identify the factors that contribute to inter-individual variability in the vascular adaptations to exercise in this population, including, but not limited to the initial CVD risk factor profile of the participants, the stage of reproductive aging (perimenopausal; early vs. late postmenopausal); the specific vascular beds studied (microvascular vs. macrovascular circulations; arm vs. leg arteries); the intensity, duration and frequency of the exercise stimulus; and length of the exercise training intervention.
-
3)
Only one clinical trial performed in small groups of estrogen-deficient postmenopausal women has assessed the potential permissive role of circulating estrogen concentrations in transducing the beneficial effects of aerobic exercise on vascular function. Again, a larger randomized clinical trial is needed to confirm these initial results.
-
4)
Limited results available on the mechanisms that mediate vascular adaptations to aerobic exercise with aging in men and women also suggest the possibility of sex differences. Accordingly, additional studies are needed to establish the mechanisms of action by which regular aerobic exercise enhances vascular function in middle-aged and older men compared with estrogen-deficient and/or estrogen-treated postmenopausal women.
-
5)
Randomized clinical trials are needed to determine if aerobic exercise training improves vascular function in postmenopausal women at high risk of cardiometabolic disorders or with diagnosed clinical disease.
-
6)
Currently, estrogen treatment is clinically contraindicated for purposes other than: a) short-term medical management of menopausal symptoms; b) prevention of bone loss and fractures in women at elevated risk (in whom alternative treatments are not tolerated); or c) hypoestrogenism caused by hypogonadism, surgical menopause, or primary ovarian insufficiency. As such, establishing the mechanisms by which estrogen supplementation facilitates transduction of exercise-induced signals for stimulating vascular health might lead to the identification of alternative natural compounds that could serve the same role. Putative compounds that may enhance exercise signaling in postmenopausal women must be tested for efficacy, as well as risk.
-
7)
If aerobic exercise training is determined to be less effective for enhancing vascular function in postmenopausal women than in middle-aged and older men, it will be essential to establish alternative evidence-based lifestyle and pharmacological prevention and treatment strategies to optimize cardiovascular health in women, as reviewed elsewhere (Moreau & Hildreth, 2014; LaRocca et al., 2016; Martens & Seals, 2016; Moreau & Ozemek, 2017; Moreau, 2018; Nowak et al., 2018; Seals et al., 2018).
-
8)
Presently it is unknown if sex hormone-associated modulation of the cardiovascular benefits of aerobic exercise extend to middle-aged and older men. For example, it will be important to determine if the circulating concentration of testosterone (or estrogens) influences and, thus, contributes to the inter-individual variability in the vascular adaptations to aerobic exercise training in middle-aged and older men. By extension, we need to establish if the vascular adaptations to aerobic exercise training are blunted in hypogonadal men.
Acknowledgments
Grants
Work from the authors’ research was supported by National Institutes of Health awards AG013038, HL134887, AG049451, AG000279, HL107120, HL107105, AG042795, AG006537, AG20683, AG027678, AG019339–03A1S1, AG049762; R56HL114073 and RR000051/TR001082; and University of Colorado Denver Center for Women’s Health Research and Eastern Colorado VA GRECC.
References
- Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R & Maeda S. (2012). Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res 32, 795–799. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, Ferranti SDd Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS & Muntner P. (2018). Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137, e67–e492. [DOI] [PubMed] [Google Scholar]
- As Bjørnerem, Straume B, Midtby M, Fønnebø V, Sundsfjord J, Svartberg J, Acharya G, Øian Pl & Berntsen GKR. (2004). Endogenous Sex Hormones in Relation to Age, Sex, Lifestyle Factors, and Chronic Diseases in a General Population: The Tromsø Study. The Journal of Clinical Endocrinology & Metabolism 89, 6039–6047. [DOI] [PubMed] [Google Scholar]
- Black MA, Cable NT, Thijssen DHJ & Green DJ. (2009). Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. American Journal of Physiology -Heart and Circulatory Physiology 297, H1109–H1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey D, Pierce G, Howe K, Mering M & Braith R. (2007). Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol 100, 403–408. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J & Deanfield JE. (1994). Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24, 471–476. [DOI] [PubMed] [Google Scholar]
- Deanfield JE, Halcox JP & Rabelink TJ. (2007). Endothelial Function and Dysfunction: Testing and Clinical Relevance. Circulation 115, 1285–1295. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H & Seals DR. (2000). Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102, 1351–1357. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE & Seals DR. (2007). Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100, 1659–1666. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Pierce GL, Lesniewski LA & Seals DR. (2009). Role of NFkappaB in age-related vascular endothelial dysfunction in humans. Aging (Albany NY) 1, 678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Svetkey LP, Wadden TA & Yanovski SZ. (2014). 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 63, 2960–2984. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA & Seals DR. (2004a). Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. Am J Physiol Heart Circ Physiol 286, H1528–1534. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA & Seals DR. (2004b). Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556, 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS. (2013). Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis 4, 76–83. [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA & Seals DR. (2010). Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol 588, 3971–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano LB, Donato AJ, Pierce GL, Hamza M, Magerko KA, Roeca C & Seals DR. (2011). Increased proinflammatory and oxidant gene expression in circulating mononuclear cells in older adults: amelioration by habitual exercise. Physiol Genomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halcox Julian PJ, Schenke William H, Zalos G, Mincemoyer R, Prasad A, Waclawiw Myron A, Nour Khaled RA & Quyyumi Arshed A. (2002). Prognostic Value of Coronary Vascular Endothelial Dysfunction. Circulation 106, 653–658. [DOI] [PubMed] [Google Scholar]
- Hanna M, Dumas I, Orain M, Jacob S, Têtu B & Diorio C. (2018). Association between physical activity and the expression of mediators of inflammation in normal breast tissue among premenopausal and postmenopausal women. Cytokine 102, 151–160. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF & Woo YJ. (2011). Forecasting the Future of Cardiovascular Disease in the United States. Circulation 123, 933–944. [DOI] [PubMed] [Google Scholar]
- Hildreth KL, Kohrt WM & Moreau KL. (2013). Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner MJ & Green DJ. (2009). Exercise protects the cardiovascular system: effects beyond traditional risk factors. The Journal of Physiology 587, 5551–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasapis C & Thompson PD. (2005). The Effects of Physical Activity on Serum C-Reactive Protein and Inflammatory Markers: A Systematic Review. Journal of the American College of Cardiology 45, 1563–1569. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. (2003). Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107, 490–497. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. (2015). So! What’s aging? Is cardiovascular aging a disease? Journal of Molecular and Cellular Cardiology 83, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG & Levy D. (2003). Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107, 139–146. [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Martens CR & Seals DR. (2016). Nutrition and other lifestyle influences on arterial aging. Ageing Res Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ & Seals DR. (2011). Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301, H1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle AN & Raaz U. (2017). Killing Me Unsoftly: Causes and Mechanisms of Arterial Stiffness. Arterioscler Thromb Vasc Biol 37, e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens CR & Seals DR. (2016). Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J Physiol 594, 7177–7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T, Miyaki A, Akazawa N, Choi Y, Ra S-G, Tanahashi K, Kumagai H, Oikawa S & Maeda S. (2013). Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. American Journal of Physiology -Heart and Circulatory Physiology. [DOI] [PubMed] [Google Scholar]
- McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR & Wilkinson IB. (2003). Endothelin-1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol 42, 1975–1981. [DOI] [PubMed] [Google Scholar]
- Mora S, Cook N, Buring JE, Ridker PM & Lee I-M. (2007). Physical Activity and Reduced Risk of Cardiovascular Events. Circulation 116, 2110–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL. (2018). The intersection between gonadal function and vascular aging in women. J Appl Physiol (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, Deane KD, Meditz AL & Kohrt WM. (2013a). Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 230, 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, DeSouza CA & Tanaka H. (2003). Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res 57, 861–868. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Gavin KM, Plum AE & Seals DR. (2005). Ascorbic Acid Selectively Improves Large Elastic Artery Compliance in Postmenopausal Women. Hypertension 45, 1107–1112. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Gavin KM, Plum AE & Seals DR. (2006). Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause 13, 951–958. [DOI] [PubMed] [Google Scholar]
- Moreau KL & Hildreth KL. (2014). Vascular Aging across the Menopause Transition in Healthy Women. Advances in Vascular Medicine 2014, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, Hildreth KL, Meditz AL, Deane KD & Kohrt WM. (2012). Endothelial Function Is Impaired across the Stages of the Menopause Transition in Healthy Women. J Clin Endocrinol Metab 97, 4692–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL & Ozemek C. (2017). Vascular Adaptations to Habitual Exercise in Older Adults: Time for the Sex Talk. Exerc Sport Sci Rev 45, 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, Stauffer BL, Kohrt WM & Seals DR. (2013b). Essential Role of Estrogen for Improvements in Vascular Endothelial Function With Endurance Exercise in Postmenopausal Women. Journal of Clinical Endocrinology & Metabolism 98, 4507–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A & Lakatta EG. (2005). Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46, 454–462. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Hsu F-C, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB & Pahor M. (2008). Exercise Training and Plasma C-Reactive Protein and Interleukin-6 in Elderly People. Journal of the American Geriatrics Society 56, 2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiranen TJ, Lyass A, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF & Vasan RS. (2017). Prevalence, Correlates, and Prognosis of Healthy Vascular Aging in a Western Community-Dwelling Cohort. Hypertension 70, 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak KL, Rossman MJ, Chonchol M & Seals DR. (2018). Strategies for Achieving Healthy Vascular Aging. Hypertension 71, 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Egelund J, Mandrup CM, Nielsen MB, Mogensen AS, Stallknecht B, Bangsbo J & Hellsten Y. (2016). Early Postmenopausal Phase Is Associated With Reduced Prostacyclin-Induced Vasodilation That Is Reversed by Exercise Training: The Copenhagen Women Study. Hypertension 68, 1011–1020. [DOI] [PubMed] [Google Scholar]
- Parker BA, Kalasky MJ & Proctor DN. (2010). Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. Eur J Appl Physiol 110, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE & Seals DR. (2011a). Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 10, 1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Eskurza I, Walker AE, Fay TN & Seals DR. (2011b). Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Lesniewski LA, Lawson BR, Beske SD & Seals DR. (2009). Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 119, 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Brunt V & Rossman M. (2018). Strategies for Optimal Cardiovascular Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenlund A, Eriksson MJ, Schenck-Gustafsson K & Hirschberg AL. (2005a). Amenorrhea in Female Athletes Is Associated with Endothelial Dysfunction and Unfavorable Lipid Profile. The Journal of Clinical Endocrinology & Metabolism 90, 1354–1359. [DOI] [PubMed] [Google Scholar]
- Rickenlund A, Eriksson MJ, Schenck-Gustafsson K & Hirschberg AL. (2005b). Oral Contraceptives Improve Endothelial Function in Amenorrheic Athletes. Journal of Clinical Endocrinology & Metabolism 90, 3162–3167. [DOI] [PubMed] [Google Scholar]
- Rossi R, Nuzzo A, Origliani G & Modena MG. (2008). Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol 51, 997–1002. [DOI] [PubMed] [Google Scholar]
- Rossman MJ, LaRocca TJ, Martens CR & Seals DR. (2018). Healthy Lifestyle-Based Approaches for Successful Vascular Aging. J Appl Physiol (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro N (2016). Perimenopause: From Research to Practice. J Womens Health (Larchmt) 25, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro N & Sutton-Tyrrell K. (2011). The SWAN Song: Study of Women’s Health Across the Nation’s Recurring Themes. Obstetrics and Gynecology Clinics of North America 38, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Parker JR, LaRocca TJ & Seals DR. (2014). Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Advances in physiology education 38, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Parker JR, Strahler TR, Vorwald VM, Pierce GL & Seals DR. (2017). Habitual aerobic exercise does not protect against micro-or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. Journal of Applied Physiology 122, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR. (2014). Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (1985) 117, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Brunt VE & Rossman MJ. (2018). Keynote lecture: strategies for optimal cardiovascular aging. American Journal of Physiology-Heart and Circulatory Physiology 315, H183–H188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Desouza CA, Donato AJ & Tanaka H. (2008). Habitual exercise and arterial aging. J Appl Physiol 105, 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL & Donato AJ. (2011). Aging and vascular endothelial function in humans. Clin Sci (Lond) 120, 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Kaplon RE, Gioscia-Ryan RA & LaRocca TJ. (2014). You’re only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda) 29, 250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Walker AE, Pierce GL & Lesniewski LA. (2009). Habitual exercise and vascular ageing. J Physiol 587, 5541–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift DL, Earnest CP, Blair SN & Church TS. (2011). The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. British Journal of Sports Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift DL, Weltman JY, Patrie JT, Saliba SA, Gaesser GA, Barrett EJ & Weltman A. (2013). Predictors of Improvement in Endothelial Function after Exercise Training in a Diverse Sample of Postmenopausal Women. J Womens Health (Larchmt). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C & Salvetti A. (2000). Physical Activity Prevents Age-Related Impairment in Nitric Oxide Availability in Elderly Athletes. Circulation 101, 2896–2901. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S & Salvetti A. (1996). Menopause is associated with endothelial dysfunction in women. Hypertension 28, 576–582. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A & Salvetti A. (2001). Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38, 274–279. [DOI] [PubMed] [Google Scholar]
- Tanahashi K, Akazawa N, Miyaki A, Choi Y, Ra S-G, Matsubara T, Kumagai H, Oikawa S & Maeda S. (2014). Aerobic Exercise Training Decreases Plasma Asymmetric Dimethylarginine Concentrations With Increase in Arterial Compliance in Postmenopausal Women. American Journal of Hypertension 27, 415–421. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA & Seals DR. (1998). Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 18, 127–132. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA & Seals DR. (2000). Aging, habitual exercise, and dynamic arterial compliance. Circulation 102, 1270–1275. [DOI] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DHJ, Black MA, Cable NT & Green DJ. (2008). Time course of change in vasodilator function and capacity in response to exercise training in humans. The Journal of Physiology 586, 5003–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC & Lakatta EG. (1993). Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88, 1456–1462. [DOI] [PubMed] [Google Scholar]
- Walker AE, Kaplon RE, Pierce GL, Nowlan MJ & Seals DR. (2014). Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor kappaB. Clin Sci (Lond) 127, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, Coggeshall M, Dandona L, Dicker DJ, Erskine HE, Ferrari AJ, Fitzmaurice C, Foreman K, Forouzanfar MH, Fraser MS, Fullman N, Gething PW, Goldberg EM, Graetz N, Haagsma JA, Hay SI, Huynh C, Johnson CO, Kassebaum NJ, Kinfu Y, Kulikoff XR, Kutz M, Kyu HH, Larson HJ, Leung J, Liang X, Lim SS, Lind M, Lozano R, Marquez N, Mensah GA, Mikesell J, Mokdad AH, Mooney MD, Nguyen G, Nsoesie E, Pigott DM, Pinho C, Roth GA, Salomon JA, Sandar L, Silpakit N, Sligar A, Sorensen RJD, Stanaway J, Steiner C, Teeple S, Thomas BA, Troeger C, VanderZanden A, Vollset SE, Wanga V, Whiteford HA, Wolock T, Zoeckler L, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Abreu DMX, Abu-Raddad LJ, Abyu GY, Achoki T, Adelekan AL, Ademi Z, Adou AK, Adsuar JC, Afanvi KA, Afshin A, Agardh EE, Agarwal A, Agrawal A, Kiadaliri AA, Ajala ON, Akanda AS, Akinyemi RO, Akinyemiju TF, Akseer N, Lami FHA, Alabed S, Al-Aly Z, Alam K, Alam NKM, Alasfoor D, Aldhahri SF, Aldridge RW, Alegretti MA, Aleman AV, Alemu ZA, Alexander LT, Alhabib S, Ali R, Aa Alkerwi, Alla F, Allebeck P, Al-Raddadi R, Alsharif U, Altirkawi KA, Martin EA, Alvis-Guzman N, Amare AT, Amegah AK, Ameh EA, Amini H, Ammar W, Amrock SM, Andersen HH, Anderson BO, Anderson GM, Antonio CAT, Aregay AF, Ärnlöv J, Arsenijevic VSA, Artaman A, Asayesh H, Asghar RJ, Atique S, Avokpaho EFGA, Awasthi A, Azzopardi P, Bacha U, Badawi A, Bahit MC, Balakrishnan K, Banerjee A, Barac A, Barker-Collo SL, Bärnighausen T, Barregard L, Barrero LH, Basu A, Basu S, Bayou YT, Bazargan-Hejazi S, Beardsley J, Bedi N, Beghi E, Belay HA, Bell B, Bell ML, Bello AK, Bennett DA, Bensenor IM, Berhane A, Bernabé E, Betsu BD, Beyene AS, Bhala N, Bhalla A, Biadgilign S, Bikbov B, Abdulhak AAB, Biroscak BJ, Biryukov S, Bjertness E, Blore JD, Blosser CD, Bohensky MA, Borschmann R, Bose D, Bourne RRA, Brainin M, Brayne CEG, Brazinova A, Breitborde NJK, Brenner H, Brewer JD, Brown A, Brown J, Brugha TS, Buckle GC, Butt ZA, Calabria B, Campos-Nonato IR, Campuzano JC, Carapetis JR, Cárdenas R, Carpenter DO, Carrero JJ, Castañeda-Orjuela CA, Rivas JC, Catalá-López F, Cavalleri F, Cercy K, Cerda J, Chen W, Chew A, Chiang PP-C, Chibalabala M, Chibueze CE, Chimed-Ochir O, Chisumpa VH, Choi J-YJ, Chowdhury R, Christensen H, Christopher DJ, Ciobanu LG, Cirillo M, Cohen AJ, Colistro V, Colomar M, Colquhoun SM, Cooper C, Cooper LT, Cortinovis M, Cowie BC, Crump JA, Damsere-Derry J, Danawi H, Dandona R, Daoud F, Darby SC, Dargan PI, das Neves J, Davey G, Davis AC, Davitoiu DV, de Castro EF, de Jager P, Leo DD, Degenhardt L, Dellavalle RP, Deribe K, Deribew A, Dharmaratne SD, Dhillon PK, Diaz-Torné C, Ding EL, dos Santos KPB, Dossou E, Driscoll TR, Duan L, Dubey M, Duncan BB, Ellenbogen RG, Ellingsen CL, Elyazar I, Endries AY, Ermakov SP, Eshrati B, Esteghamati A, Estep K, Faghmous IDA, Fahimi S, Faraon EJA, Farid TA, Farinha CSeS, Faro A, Farvid MS, Farzadfar F, Feigin VL, Fereshtehnejad S-M, Fernandes JG, Fernandes JC, Fischer F, Fitchett JRA, Flaxman A, Foigt N, Fowkes FGR, Franca EB, Franklin RC, Friedman J, Frostad J, Fürst T, Futran ND, Gall SL, Gambashidze K, Gamkrelidze A, Ganguly P, Gankpé FG, Gebre T, Gebrehiwot TT, Gebremedhin AT, Gebru AA, Geleijnse JM, Gessner BD, Ghoshal AG, Gibney KB, Gillum RF, Gilmour S, Giref AZ, Giroud M, Gishu MD, Giussani G, Glaser E, Godwin WW, Gomez-Dantes H, Gona P, Goodridge A, Gopalani SV, Gosselin RA, Gotay CC, Goto A, Gouda HN, Greaves F, Gugnani HC, Gupta R, Gupta R, Gupta V, Gutiérrez RA, Hafezi-Nejad N, Haile D, Hailu AD, Hailu GB, Halasa YA, Hamadeh RR, Hamidi S, Hancock J, Handal AJ, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Havmoeller R, Heckbert SR, Heredia-Pi IB, Heydarpour P, Hilderink HBM, Hoek HW, Hogg RS, Horino M, Horita N, Hosgood HD, Hotez PJ, Hoy DG, Hsairi M, Htet AS, Htike MMT, Hu G, Huang C, Huang H, Huiart L, Husseini A, Huybrechts I, Huynh G, Iburg KM, Innos K, Inoue M, Iyer VJ, Jacobs TA, Jacobsen KH, Jahanmehr N, Jakovljevic MB, James P, Javanbakht M, Jayaraman SP, Jayatilleke AU, Jeemon P, Jensen PN, Jha V, Jiang G, Jiang Y, Jibat T, Jimenez-Corona A, Jonas JB, Joshi TK, Kabir Z, Kamal R, Kan H, Kant S, Karch A, Karema CK, Karimkhani C, Karletsos D, Karthikeyan G, Kasaeian A, Katibeh M, Kaul A, Kawakami N, Kayibanda JF, Keiyoro PN, Kemmer L, Kemp AH, Kengne AP, Keren A, Kereselidze M, Kesavachandran CN, Khader YS, Khalil IA, Khan AR, Khan EA, Khang Y-H, Khera S, Khoja TAM, Kieling C, Kim D, Kim YJ, Kissela BM, Kissoon N, Knibbs LD, Knudsen AK, Kokubo Y, Kolte D, Kopec JA, Kosen S, Koul PA, Koyanagi A, Krog NH, Defo BK, Bicer BK, Kudom AA, Kuipers EJ, Kulkarni VS, Kumar GA, Kwan GF, Lal A, Lal DK, Lalloo R, Lallukka T, Lam H, Lam JO, Langan SM, Lansingh VC, Larsson A, Laryea DO, Latif AA, Lawrynowicz AEB, Leigh J, Levi M, Li Y, Lindsay MP, Lipshultz SE, Liu PY, Liu S, Liu Y, Lo L-T, Logroscino G, Lotufo PA, Lucas RM, Lunevicius R, Lyons RA, Ma S, Machado VMP, Mackay MT, MacLachlan JH, Razek HMAE, Magdy M, Razek AE, Majdan M, Majeed A, Malekzadeh R, Manamo WAA, Mandisarisa J, Mangalam S, Mapoma CC, Marcenes W, Margolis DJ, Martin GR, Martinez-Raga J, Marzan MB, Masiye F, Mason-Jones AJ, Massano J, Matzopoulos R, Mayosi BM, McGarvey ST, McGrath JJ, McKee M, McMahon BJ, Meaney PA, Mehari A, Mehndiratta MM, Mejia-Rodriguez F, Mekonnen AB, Melaku YA, Memiah P, Memish ZA, Mendoza W, Meretoja A, Meretoja TJ, Mhimbira FA, Micha R, Millear A, Miller TR, Mirarefin M, Misganaw A, Mock CN, Mohammad KA, Mohammadi A, Mohammed S, Mohan V, Mola GLD, Monasta L, Hernandez JCM, Montero P, Montico M, Montine TJ, Moradi-Lakeh M, Morawska L, Morgan K, Mori R, Mozaffarian D, Mueller UO, Murthy GVS, Murthy S, Musa KI, Nachega JB, Nagel G, Naidoo KS, Naik N, Naldi L, Nangia V, Nash D, Nejjari C, Neupane S, Newton CR, Newton JN, Ng M, Ngalesoni FN, de Dieu Ngirabega J, Nguyen QL, Nisar MI, Pete PMN, Nomura M, Norheim OF, Norman PE, Norrving B, Nyakarahuka L, Ogbo FA, Ohkubo T, Ojelabi FA, Olivares PR, Olusanya BO, Olusanya JO, Opio JN, Oren E, Ortiz A, Osman M, Ota E, Ozdemir R, Pa M, Pain A, Pandian JD, Pant PR, Papachristou C, Park E-K, Park J-H, Parry CD, Parsaeian M, Caicedo AJP, Patten SB, Patton GC, Paul VK, Pearce N, Pedro JM, Stokic LP, Pereira DM, Perico N, Pesudovs K, Petzold M, Phillips MR, Piel FB, Pillay JD, Plass D, Platts-Mills JA, Polinder S, Pope CA, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Qorbani M, Quame-Amaglo J, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman M, Rahman MHU, Rahman SU, Rai RK, Rajavi Z, Rajsic S, Raju M, Rakovac I, Rana SM, Ranabhat CL, Rangaswamy T, Rao P, Rao SR, Refaat AH, Rehm J, Reitsma MB, Remuzzi G, Resnikoff S, Ribeiro AL, Ricci S, Blancas MJR, Roberts B, Roca A, Rojas-Rueda D, Ronfani L, Roshandel G, Rothenbacher D, Roy A, Roy NK, Ruhago GM, Sagar R, Saha S, Sahathevan R, Saleh MM, Sanabria JR, Sanchez-Niño MD, Sanchez-Riera L, Santos IS, Sarmiento-Suarez R, Sartorius B, Satpathy M, Savic M, Sawhney M, Schaub MP, Schmidt MI, Schneider IJC, Schöttker B, Schutte AE, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Shackelford KA, Shaddick G, Shaheen A, Shahraz S, Shaikh MA, Shakh-Nazarova M, Sharma R, She J, Sheikhbahaei S, Shen J, Shen Z, Shepard DS, Sheth KN, Shetty BP, Shi P, Shibuya K, Shin M-J, Shiri R, Shiue I, Shrime MG, Sigfusdottir ID, Silberberg DH, Silva DAS, Silveira DGA, Silverberg JI, Simard EP, Singh A, Singh GM, Singh JA, Singh OP, Singh PK, Singh V, Soneji S, Søreide K, Soriano JB, Sposato LA, Sreeramareddy CT, Stathopoulou V, Stein DJ, Stein MB, Stranges S, Stroumpoulis K, Sunguya BF, Sur P, Swaminathan S, Sykes BL, Szoeke CEI, Tabarés-Seisdedos R, Tabb KM, Takahashi K, Takala JS, Talongwa RT, Tandon N, Tavakkoli M, Taye B, Taylor HR, Ao BJT, Tedla BA, Tefera WM, Have MT, Terkawi AS, Tesfay FH, Tessema GA, Thomson AJ, Thorne-Lyman AL, Thrift AG, Thurston GD, Tillmann T, Tirschwell DL, Tonelli M, Topor-Madry R, Topouzis F, Towbin JA, Traebert J, Tran BX, Truelsen T, Trujillo U, Tura AK, Tuzcu EM, Uchendu US, Ukwaja KN, Undurraga EA, Uthman OA, Dingenen RV, van Donkelaar A, Vasankari T, Vasconcelos AMN, Venketasubramanian N, Vidavalur R, Vijayakumar L, Villalpando S, Violante FS, Vlassov VV, Wagner JA, Wagner GR, Wallin MT, Wang L, Watkins DA, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Westerman R, White RA, Wijeratne T, Wilkinson JD, Williams HC, Wiysonge CS, Woldeyohannes SM, Wolfe CDA, Won S, Wong JQ, Woolf AD, Xavier D, Xiao Q, Xu G, Yakob B, Yalew AZ, Yan LL, Yano Y, Yaseri M, Ye P, Yebyo HG, Yip P, Yirsaw BD, Yonemoto N, Yonga G, Younis MZ, Yu S, Zaidi Z, Zaki MES, Zannad F, Zavala DE, Zeeb H, Zeleke BM, Zhang H, Zodpey S, Zonies D, Zuhlke LJ, Vos T, Lopez AD & Murray CJL. (2016). Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 388, 1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson IB, Franklin SS & Cockcroft JR. (2004). Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension 44, 112–116. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu F-C, Burke GL & Herrington DM. (2007). Brachial Flow-Mediated Dilation Predicts Incident Cardiovascular Events in Older Adults: The Cardiovascular Health Study. Circulation 115, 2390–2397. [DOI] [PubMed] [Google Scholar]


