Abstract
Introduction
Neointimal hyperplasia (NIH) and restenosis after percutaneous transluminal coronary angioplasty (PTCA) and intravascular stenting remains a problem on a long term basis by causing endothelial denudation and damage to the intima and media. Vascular sterile inflammation has been attributed to the formation of NIH. Cathepsin L (CTSL), a lysosome protease, is associated with diet-induced atherogenesis. Vitamin D regulates the actions and regulatory effects of proteases and protease inhibitors in different cell types. Objectives of this study are: to evaluate the modulatory effect of vitamin D on CTSL activity in post PTCA coronary arteries of atherosclerotic swine.
Methods
Yucatan microswine were fed with a high cholesterol atherosclerotic diets. The swine were stratified to receive 3 diets 1) vitamin D-deficient diet, 2) vitamin D-sufficient diet, and 3) vitamin D-supplement diet. After 6 months, PTCA was performed in the left circumflex coronary artery (LCX). After one year, angiography and optical coherence tomography (OCT) imaging were performed and swine euthanized. Coronary arteries were embedded in paraffin. Tissue sections-stained with H&E. Expression of Ki67, CTSL were evaluated by Immunofluorescence.
Results
Increased number of Ki67+ cells were observed in the post angioplasty LCx in vitamin D-deficient compared to -sufficient or -supplemented swine. Notably, the expression of CTSL was significantly increased in post angioplasty LCx of vitamin D-deficient swine compared to the vitamin D-sufficient or -supplemented animal groups.
Conclusion
Increased expression of CTSL correlates with the formation of NIH in the PTCA-injured coronary arteries. However, in the presence of sufficient or supplemented levels of vitamin D in the blood, CTSL expression was significantly reduced.
Keywords: Cathepsin L, Vitamin D, Intimal hyperplasia, Restenosis
I. Introduction
Percutaneous transluminal coronary angioplasty (PTCA) for occlusive artery disease is a widely used safe and effective therapeutic intervention to treat coronary artery disease (CAD). One of the major limitations to this procedure is the development of restenosis in the arterial lumen. This occurs as a result of damage to the endothelium, intima, and media of the coronary artery secondary to the PTCA. This therapeutic intervention induces inflammation causing a significant increase in the influx of inflammatory cytokines and growth factors which stimulate proliferation of smooth muscle cells. These events result in the development of intimal hyperplasia leading to narrowing of the lumen1. Inflammatory cytokines and proangiogenic growth factors have been reported to stimulate cathepsin L (CTSL) expression in vascular smooth muscle cells (VSMCs) which can contribute to the progression of intimal hyperplasia and restenosis by degrading the extracellular matrix2. CTSL is a cysteine protease which has a potent elastase and collagenase activity promoting atherogenesis and arterial wall remodeling2–3.
Vitamin D is a fat-soluble secosteroid that exerts its function through vitamin D receptors (VDR’s). Evidence of VDR’s is present widely in a variety of cells and tissues like VSMCs4, cardiomyocytes5, endothelium6 and cells of immune system7. Vitamin D has been shown to regulate proteases and protease inhibitors in different cell types by contributing to its regulatory effects on cell physiology8. In recent studies, vitamin D was found to inhibit the expression of CTSL in breast cancer cells9 and also in fibroblasts by inhibition of CTSL activity10.
We hypothesized that vitamin D deficiency enhances the production of CTSL in the VSMCs and contributes to the development of neointimal hyperplasia (NIH). However, by supplementing vitamin D in the diet, the expression of CTSL decreases. The objectives of this study are to evaluate the role of vitamin D supplementation and expression of CTSL in the post angioplasty coronary arteries of atherosclerotic swine, as well as the effect of calcitriol on CTSL production in the presence of inflammatory cytokine and angiogenic growth factor in porcine coronary artery smooth muscle cells.
II. Material and methods
Swine model
Yucatan female microswine of 30–40 lbs were obtained from Sinclair Laboratories, Columbia, MO, USA. Female micropigs are less aggressive, safer and easier to handle than males. Commercially available males are castrated for safety of the personnel, as hormonal changes which may be a confounding factor in cardiovascular studies. Swine were housed in the Animal Resource Facility of Creighton University and treated according to NIH and USDA guidelines. Our animal study protocol (0830.2) was approved by Creighton University’s Institutional Animal Care and Use Committee.
The different diets were selected, and animals stratified to these diets. Three microswine were fed a mixture of Teklad swine diets 8753 and 10125 (Envigo, USA), a vitamin D-deficient high cholesterol diet composed of 19% “Vitamin-Free” Test Casein (to precisely control vitamin D content), 23.5% sucrose, 23.9% corn starch, 13% maltodextrin, 4% soybean oil, 4% cholesterol, and 10% cellulose, with the addition of 0.55% sodium cholate and 9% chocolate. These microswine received 500 IU of vitamin D3/per day (Vitamin D-deficient diet). The six microswine were fed a mixture of Teklad swine diets 8753 and 9487 (Envigo, USA), a high cholesterol diet (HC) that consisted of 37.2% corn (8.5% protein), 23.5% soybean meal (44% protein), 5% alfalfa, 4% cholesterol, 4% peanut oil, 1.5% sodium cholate, and 1% lard; with the addition of 8% chocolate. These six microswine were equally divided into two groups: Vitamin D-sufficient diet group where 1,500 IU of vitamin D3/per day was administered (Vitamin D-sufficient diet), and vitamin D-supplemented diet group received 4,500–5, 000 IU of vitamin D3/per day (vitamin D-supplemented diet). Blood was routinely collected at baseline, before the coronary intervention and before the time of euthanasia from the auricular vein to monitor the serum 25(OH)D levels.
Percutaneous Transluminal Coronary Angioplasty (PTCA) procedure
In this established animal model, all study subjects were monitored for the development of atherosclerosis. After 4–6 months of the high cholesterol diet, PTCA was performed. All the animals were given aspirin (325 mg/day) and ticlopidine (250 mg/day) or clopidogrel (75 mg) orally 3 days before the planned PTCA procedure and were kept on overnight fast before the procedure.
Animals were initially anesthetized with intramuscular anesthetic first, followed with inhaled isoflurane (3–4% isoflurane mixed with 2 L/min oxygen using a face mask). After 15 mins, endotracheal intubation was performed, and inhalational isoflurane (1–3%) was continued throughout the procedure. A prophylactic pre-operative intramuscular dose of cefazolin (3–5 mg/kg) was administered. Intravenous catheters were placed in the auricular vein on both sides for normal saline (NS) and the administration of prophylactic anti-arrhythmic (Amiodarone 4–5 mg/kg followed by 0.05 mg/kg/hr).
Groin was shaved and prepped in a sterile fashion with betadine and alcohol scrubs and prepped sterile. A 5–6 cm incision was made, femoral vessels were identified, and the femoral artery was punctured. A 4F sheath was introduced followed by 6F sheath to maintain the vascular access patent for insertion of cardiac catheters. Prophylactic anti-arrhythmic (Amiodarone 4–5 mg/kg followed by 0.05 mg/kg/hr IV) was started prior to PTCA. An angiography guiding catheter (6F AR2/100 cm) was advanced to the left coronary ostium, coronary angiography was performed by injecting a non-ionic contrast media and imaged using C arm (OEC 9900 Elite Vas 8, GE Healthcare).
After introducing a guide wide into the left circumflex coronary artery (LCx), a balloon angioplasty catheter-balloon size of 2.5 mm × 15 mm was advanced to reach the LCx and inflated to 10–15 atmospheres (ATMs) utilizing the inflation device with a pressure gauge (Basix Touch™). The angioplasty balloon was subsequently deflated, and angiography performed to evaluate the patency of the artery. All catheters were removed, and the femoral artery puncture site was repaired using a 4–0 polypropylene (Prolene) sutures. The groin and skin incisions were closed with polyglycolic-acid (Vicryl) sutures. After completion of the procedure, the animal was extubated after return of reflexes.
During post-operative recovery, the animal was covered with a warm blanket and IV fluids administered until the swine was able to stand unassisted. A single dose of analgesic (buprenorphine) was given to relieve the pain which was continued every 12 hours for the first three days. During the first ten post-operative days, the animals were monitored daily for signs of pain or any other discomfort.
After 6 months from the PTCA procedure, follow-up angiography and optical coherence tomography (OCT) imaging were performed using a C7-XR OCT intravascular imaging system (St. Jude Medical, St. Paul, MN). The percentage (%) of neointima was calculated using this formula: [(mean reference lumen area−minimum lumen area) ÷ mean reference lumen area] ×100. Animals were then euthanized by injecting a high dose of barbiturates (Beuthanasia-D, 0.1 ml/lb, i.v.). The swine heart was extirpated, and the coronary vessels were harvested for analysis. Tissues were handled in a Sakura Tissue Tek VIP Tissue Processor and embedded in paraffin. Sections were sectioned at a thickness of 5μm using a microtome (Leica, Germany) and subsequently placed on slides for immunofluorescence evaluation.
Hematoxylin and Eosin
Obtained tissue sections were stained with hematoxylin and eosin (H&E) and were viewed with a Nikon™ Eclipse Ci microscope. Images were photographed with a Nikon™ DS-L3 camera.
Immunofluorescence
Tissue sections were deparaffinized in xylene and rehydrated in decreasing concentration of ethanol then washed with double-distilled water. The slides were then treated with citrate buffer (AP-9003–500, Lab Vision™) in a steamer for 20 minutes. After a 20 minute cool down period, they were washed with phosphate buffered saline (PBS) and then blocked in normal horse serum from Vector™ Laboratories for two hours at room temperature in a humid chamber.
Primary antibodies included: rabbit anti-TNF-α (Ab6671; Abcam™), rabbit anti-Ki67 (Ab15580; Abcam™). For double immunofluorescence staining, mouse anti-α smooth muscle cell actin (αSMA) (Ab7817; Abcam™) and rabbit anti-cathepsin L (LS-C293267; LSBio™) were added overnight at 4°C. On the next day, the slides were washed with PBS and the secondary antibody (Invitrogen Alexa-Fluor® 594 Donkey anti-rabbit IgG A21207 and/or Invitrogen Alexa-Fluor® 488 Donkey anti-mouse A21202) were added and incubated on the slides for two hours at room temperature. Sections were washed in 1x PBS and nuclei were counterstained with Vector™ laboratories DAPI. Images were obtained in Olympus VS120 Slide scanner.
The mean pixel intensity of CTSL+ cells, Ki67+ cells in the post angioplasty coronary sections was determined for each swine treatment (n=3 in each swine treatment). Images captured at 20x magnification were converted to a grayscale image in Image J. Mean gray value equaling the sum of the gray values, of all the pixels in the selection, divided by the number of pixels (http://imagej.sourceforge.net/docs/menus/analyze.html). In this case, the sum of the gray values was calculated for all the pixels in each neointimal nuclei. Hence, the mean pixel intensity was obtained from 20x images of the PTCA-LCx from three swine per treatment. The mean pixel intensity values for each swine treatment was averaged and statistically analyzed.
Vascular smooth muscle cell isolation and culture
Coronary arteries harvested from the group of microswine administered the vitamin-sufficient diet and were used for vascular smooth muscle cell isolation. After removing the adventitia (fibroblasts) and intima (endothelial cells), the media were sectioned into pieces and then digested with Gibco™ Trypsin-EDTA (0.25%), Phenol red (Cat #25200–056, Fisher Scientific™) for 30min and Collagenase Type I (Cat #C0130, Sigma-Aldrich™) incubated for 3 hours at 37°C. Digested tissues was plated in T25 flask of SMC media (1101, ScienCell™), with 10% fetal bovine serum (0010, ScienCell™), Antibiotic Antimycotic Solution (100 μg/mL Streptomycin, 100 U/mL Penicillin and 2.5 μg/ml amphotericin B, and smooth muscle cell growth supplement (1152, ScienCell™) at 37°C in 5% CO2. Cell passage between P3 and P7 are used for treatment. VSMCs were treated with IGF-1 100 ng/ml (100–11, PeproTech™) and TNF-α 100 ng/ml (300–01A, PeproTech™) cultured in smooth muscle cell (SMC) media with antibiotic antimycotic solution 24h at 37°C in 5% CO2, and then added 0, 50, 100 nM 1α,25-dihydroxyvitamin D3 (Calcitriol) (D1530, Sigma-Aldrich™) for 1 hour.
Western blot
The vascular smooth muscle cells were lysed with RIPA buffer (R0278, Sigma-Aldrich™). The concentrations of protein in the cell lysates were measured using Bicinchoninic Acid Kit (Cat #B9643, Sigma-Aldrich™) and copper (II) sulfate solution (C2284, Sigma-Aldrich™). For SDS PAGE, 4–15% Mini-PROTEAN® TGXTM Precast Protein Gels (456–1084, BIO-RADTM) were used. Electrophoresis was performed for a period of 90 minutes at 100 volts, or until the dye front reached the bottom end of the gel. Subsequently, the resolved proteins in the gel were transferred onto PVDF Transfer Membrane, 0.45 μm (88518, ThermoFisher™). The transferred blot was then blocked in Tris-buffered saline with Tween-20 (TBST) buffer containing 3% Bovine Serum Albumin (A7906, Sigma-Aldrich™) for 1 hour. The membrane was then probed with Primary antibody specific to CTSL (1:200, LS-C293267, LifeSpan Biosciences™) or GAPDH Antibody (1D4) (1:1000, NB300-221, Novus Biologicals™) for 16h at 4°C. After three TBST washings, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody at a 1:5000 dilution of rabbit anti-mouse IgG Antibody [HRP] (NB7544, Novus Biologicals™) or goat anti-rabbit IgG antibody (NB7160, Novus Biologicals™) for 1 hour. PierceTM ECL Western Blotting Substrate (32106, ThermoFisher™) was used to detect protein expression on the membrane through chemiluminescence.
Vascular smooth muscle cell proliferation assay
The vascular smooth muscle cells were cultured to about 70% confluence in SMC media containing 10% FBS, and then serum starved overnight. Subsequently, the cells were treated with 100 ng/ml of TNF-α or 100 ng/ml of IGF-1 or both together for 24 hours. The effects of chemokine and growth factor on cell proliferation was measured by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Briefly, the control and treated cells were incubated with MTT reagent for 4 hours at 37°C. After washing with PBS, acidified isopropanol was added to the cells to dissolve the formazan crystals. The extent of cell proliferation was assessed through colorimetric assay using a spectrophotometer.
Statistical analysis
Immunofluorescence data were analyzed by GraphPad Prism 6.0 (GraphPad Software, Inc). The values are presented as mean ± SEM. One-way ANOVA with Tukey’s multiple comparison test was used to analyze significant differences in mean grey intensity value in CTSL, TNF-α, Ki67 within the experimental groups. A p value of <0.05 was selected as statistically significant.
III. Results
The baseline serum 25 hydroxy vitamin D levels were measured before starting the experimental diets were 29 ± 1 ng/ml in vitamin D-deficient group, 34 ± 3 ng/ml in vitamin D-sufficient group, and 28 ± 2 ng/ml in vitamin D-supplemental group. After feeding the animals with vitamin D modified diets, the serum 25(OH)D levels at the time of euthanasia were measured. The levels of serum 25(OH)D among the vitamin D-deficient group was 7 ± 0.2 ng/ml, for the vitamin D-sufficient group was 39 ± 0.9 ng/ml and for the vitamin D-supplemental group was 82 ± 16 ng/ml. These vitamin D levels were maintained in the corresponding animals of each group throughout the study which was controlled primarily through diet.
Optical coherence tomography and Percent Restenosis
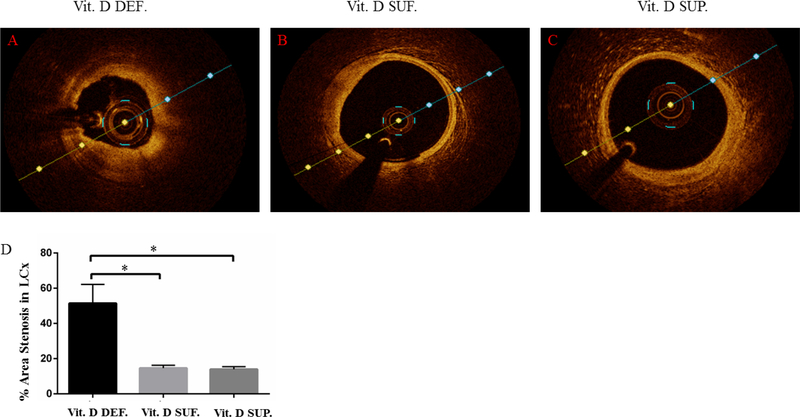
Optical coherence tomography (OCT) was performed just before euthanasia. The optical quantification showed the greatest restenosis in the PTCA-LCx of vitamin D-deficient group (51 ± 11%) (see Fig. 1A) compared to PTCA-LCx of vitamin D-sufficient (15 ± 2%) (p=0.01) (see Fig. 1B) and PTCA-LCx of vitamin D-supplemental group (14 ± 2%) (p=0.01) (see Fig. 1C).
Figure 1. Vitamin D deficiency enhances coronary restenosis: Optical coherence tomography (OCT) imaging.
Panel A shows restenosis occurred in the coronary arteries after angioplasty in animals that were fed with vitamin D deficient diet (Vit. D DEF.).
Panel B shows the extent of restenosis after angioplasty seen in the coronary arteries of animals that were fed with sufficient levels of vitamin D sufficient (Vit. D SUF.).
Panel C shows restenosis in the coronary arteries of vitamin D supplemented animal group.
The degree of post-interventional percentage area restenosis in the coronary arteries of all animal groups was calculated using OCT integrated software and the data was represented as a bar diagram (D). The number of animals in each group were n=3. Data were compared with t- test analysis where a p-value of less than 0.05 was considered statistically significant. Actual p-values noted here were less than 0.01; *p<0.01.
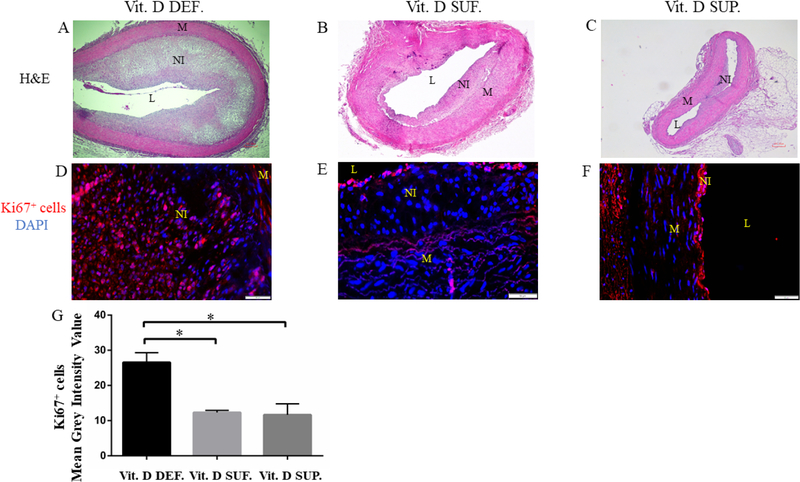
Immunofluorescence Ki67 and Hematoxylin Eosin Stain
From the histological characterization by H&E (see Fig. 2A–C) of PTCA-LCx, greater neointimal region and immunopositivity to Ki67 (see Fig. 2D) were seen in the neointimal regions of vitamin D-deficient group animals. Few cells in the neointima that were closer to the lumen also showed immunopositivity to Ki67 in vitamin D-sufficient (see Fig. 2E) and - supplemented group (see Fig. 2F). The mean grey scale intensity value of Ki67 expressing cells in PTCA-LCx was found to be greater in vitamin D-deficient swine (27 ± 2.8) compared to vitamin D-sufficient swine (12 ± 0.59) (p=0.014) and vitamin D-supplemented swine (12 ± 3.1) (p=0.011).
Figure 2. H&E and Ki67 staining of the PTCA LCx in microswine fed a High cholesterol diet.
Figure shows H&E staining that reveals the morphology of the coronary arteries.
Panel A shows the histomorphometric features in the LCx of Vitamin D-deficient animals (Vit. D DEF.).
Panel B shows the extent of neointimal hyperplasia in Vitamin D sufficient animal groups (Vit. D SUF.).
Panel C shows restenosis in the Vitamin D supplemented animal group (Vit. D SUP.). Expression of Ki67 with DAPI counterstain was shown in panels D-F. The mean grey scale intensity of Ki67 expressing cells was measured for each treatment group (panel G). The number of animals in each group were n=3. The observed p-value was *p <0.01. L=lumen, M=media, NI=neointima, A adventitia.
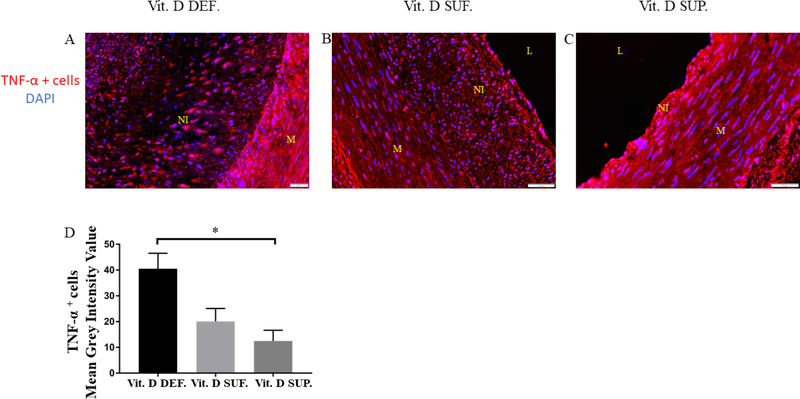
Immunofluorescence of TNF-α in PTCA-LCx
Expression of TNF-α was seen in the neointima of all the post-angioplasty coronary arteries in all groups (see Fig.3A–C). The mean grey scale intensity value of TNF-α (Fig.3D) expressing cells was significantly greater in vitamin D-deficient (see Fig.3A) compared to the vitamin D-supplemented swine (see Fig. 3C) (12 ± 4) (p=0.02). Although not statistically significant it was greatly lower in the vitamin D-sufficient swine (see Fig. 3B) (20 ± 5) (p=0.07).
Figure 3. Expression of TNF-α in the PTCA-injured LCX of microswine fed a High cholesterol diet.
Panel A–C shows TNF-α expressing cell in the neointima of the PTCA LCx. Expression of TNF-α is in vitamin D deficient (Vit. D DEF.) swine greatest compared to vitamin D sufficient (Vit. D SUF.) and vitamin D supplemented swine (Vit. D SUP.). The mean grey scale intensity of TNF-α expressing cells was measured for each treatment group (panel D). (n=3 in each treatment group) *p=0.02. L=lumen, M=media, NI=neointima
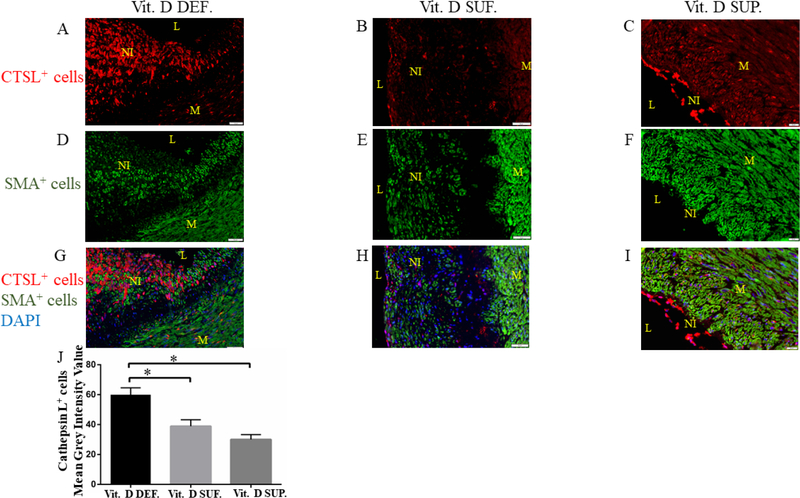
Immunofluorescence dual staining with α-SMA and Cathepsin L Cells in the neointima showed the expression of CTSL (See Fig. 3A–C). Immunopositivity to alpha smooth muscle cell actin (α-SMA) was seen distributed throughout the neointima and media (See Fig. 3D–F). Co-localization of α-SMA and CTSL was found in cells within the central region of the neointima. It was observed that as the neointima grew, more co-localized expression of α-SMA and CTSL was seen in the vitamin D-deficient group (See Fig. 3G). Co-localization of α-SMA and CTSL was mainly found restricted to the luminal side of the neointima in the vitamin D-sufficient group (See Fig. 3H) and in vitamin D-supplemental group (Fig 3I). The mean grey scale intensity values of CTSL positive cells in PTCA-LCx were found to be greater in vitamin D-deficient swine (60 ± 4.6) compared to vitamin D-sufficient swine (39 ± 4.3) (p=0.03) and to vitamin D-supplemented swine (27 ± 3.8) (p=0.003).
TNF-α and IGF-1 promotes proliferation of VSMCs
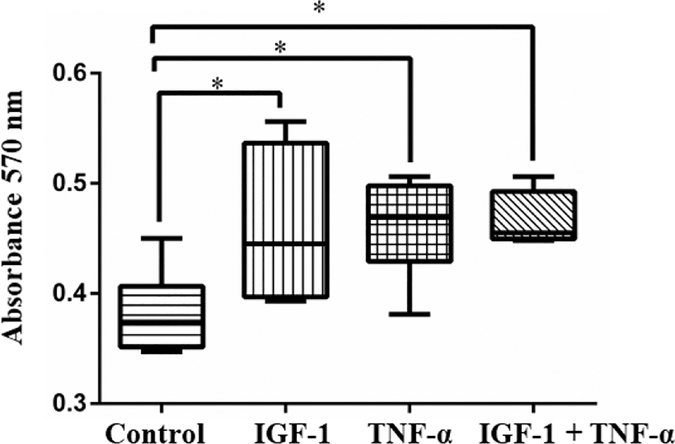
The effects of the angiogenic growth factor, IGF-1, and the cytokine, TNF-α, were tested for their ability to promote cell proliferation. Cell culture experiments were carried out in 37°C incubator containing 5% CO2. After serum starving overnight, the VSMCs were treated with both TNF-α and IGF-1 both independently and in combination at 100 ng/ml concentration for 24 hours. Subsequently, their effects on cell proliferation were measured by MTT assay and the cell proliferation was assessed by measuring the absorbance at 570 nm. Absorbance values obtained from the cells treated with either TNF-α or IGF-1 or both together along with untreated control. These absorbance values directly correlate with the extent of cell proliferation observed in different treatment conditions (See Fig. 5).
Figure 5: IGF-1 and TNF-α promotes smooth muscle cell proliferation.
The effect of TNF-α and IGF-1 on proliferation of vascular smooth muscle cells (VSMCs) were evaluated through MTT assay. Increased cell proliferation was observed when VSMCs were treated with both TNF-α and IGF-1 as compared with untreated control. Significant increase in cell proliferation was also seen in cells that were treated with either TNF-α or IGF-1. *p<0.02
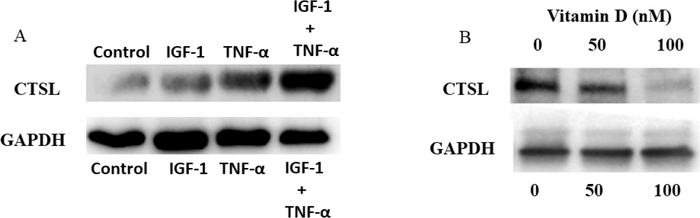
Calcitriol treatment prevents TNF-α and IGF-1-induced expression of cathepsin L in VSMCs.
The effects of TNF-α and IGF-1 in inducing the expression of CTSL was examined. VSMCs were treated with TNF-α and IGF-1 either independently or together and the expression of CTSL was evaluated through western blotting. Increased expression of CTSL was observed in cells that were treated with both TNF-α and IGF-1 compared to individual treatments. Also, the effect of Calcitriol at 0 nM, 50 nM or 100 nM on TNF-α- and IGF-1-induced CTSL expression was examined. In the absence of calcitriol, increased expression of CTSL was observed in cells that were treated with TNF-α and IGF-1. In cells that were treated with 50 nM concentration of calcitriol, the expression of CTSL induced by TNF-α and IGF-1 was greatly reduced. Furthermore, complete inhibition of TNF-α and IGF-1-induced CTSL was observed in cells that were treated with 100 nM concentration of calcitriol (See Fig. 6B).
Figure 6: Calcitriol prevents TNF-a and IGF-1 induced expression of cathepsin L in swine VSMCs.
Panel A shows western blot analysis of Cathepsin L expression in VSMCs that were treated with or without TNF-α and/or IGF1.
Panel B shows Cathepsin L expression in VSMCs that were treated with TNF-α and IGF1, and with various concentrations of Calcitriol (0 nM, 50 nM and 100 nM). The expression of Cathepsin L was found completely inhibited in presence of Calcitriol at concentrations above the sufficient levels.
IV. Discussion
Therapeutic coronary artery intervention with percutaneous transluminal coronary angioplasty (PTCA) induces a balloon related injury promoting proliferation of VSMCs that can result in NIH and compromise the patency of the vessel. In our study, PTCA was performed in three groups of Yucatan microswine that were treated with atherogenic inducing diets followed by angioplasty to induce arterial injury. All the animals developed NIH after PTCA in the LCx artery, which was evident from the OCT imaging and histological characterization by H&E (See Figs. 1A, 2A). Furthermore, due to this intervention, and the resulting denudation of the endothelium stimulates active division of smooth muscle cells at the intimal region. As a consequence, the internal elastic lamina was reported to develop fenestrations which is a notable feature observed. This eventually leads to the development of NIH11. Due to the injury resulting from this interventional procedure, increased expression of different chemokines and growth factors have been reported.
Vitamin D has been reported to exert its biological functions through it specific intracellular VDR receptor. Some of our earlier studies reported the expression of VDR’s to be significantly decreased in the actively proliferating VSMCs of the neointimal lesions that were induced due to post PTCA intervention in the coronary arteries of atherosclerotic swine12. Results from our previous studies have also demonstrated that the development of NIH due to PTCA negatively correlates with serum levels of vitamin D. We also demonstrated that PTCA injury in the coronary arteries causes increases expression of TNF-α expression in neointimal lesions12.
Several reports have showed that TNF-α confers a stimulatory effect on VSMCs and assists in cell migration. Therefore, it is reasonable to presume that TNF-α is directly associated with intimal growth in newly divided VSMCs thus compromising the vascular lumen. This phenomenon was observed during plaque formation13–14. Our group has previously demonstrated TNF-α as one of the important effector molecules promoting different intracellular signaling mechanisms which are associated with increased proliferation of VSMCs both in-vitro and in-vivo in swine.
After PTCA in our atherogenic animals, we observed increased expression of TNF-α in-vivo (See Fig. 3A–C). Although TNF-α alone can significantly induce cell proliferation (See Fig. 3A–C), there is substantial clinical and experimental evidence suggesting IGF-1 to be intimately linked to the processes that result in the accumulation of VSMCs15–16. The pathogenic role of IGF-1 has been further substantiated by experimental in vivo models showing that the VSMCs at the site of injury exhibit an increased expression of IGF-117. Therefore, in the present study, direct cell proliferating effects of the combined treatment with TNF-α and IGF-1 was tested through in-vitro cell culture of VSMCs. We observed that in the presence of both TNF-α and IGF-1 the cell proliferation was significantly enhanced when compared with the control group (See Fig. 5).
CTSL is an endosomal and lysosomal protease with potent elastase and collagenase activity that has been associated in vascular remodeling in aortic aneurysms2, atherosclerosis18, and cardiac valvular disease which are regulated by inflammation after an injury2,19. TNF-α and interferon gamma (IFN-γ) showed selective effect on the secretion of CTSL in synovial fibroblast like cells20. In addition, the proinflammatory cytokine, IL-6, was also shown to induce the expression of CTSL in human lung epithelial cells21. Since increased expression of CTSL was found to be associated with different cytokines and growth factors, injury-induced production of these cell growth promoting agents may play an active role in enhancing NIH. Previous studies have demonstrated that arterial injury in mice also induced expression of CTSL19. Thus, induced expression of the CTSL by VSMCs in coronary arteries may contribute to the internal elastic lamina fragmentation and hasten SMC migration and accumulation into the neointima resulting in vascular wall expansion.
In our in vivo results in (See Fig. 4A–C), it is evident that CTSL is significantly increased in the neointimal regions of atherosclerotic coronary arteries of microswine. These results confirm previous reports and suggest that the expression of CTSL is tightly associated with matrix remodeling with elastinolytic and collagenolytic properties, contributing to NIH in the coronary vessels. Our in-vitro study which demonstrates that TNF-α and IGF-1 also stimulate VSMCs and induce the expression of CTSL, supporting the in-vivo observations.
Figure 4: Expression of cathepsin L and α-smooth muscle actin in the PTCA LCx of microswine fed a High cholesterol diet.
Panel A–C shows cathepsin L (CTSL) (red) positive cells in the neointima of the PTCA LCx. The expression of CTSL in the vitamin D deficient (Vit. D DEF.) swine is significantly more than the vitamin D sufficient (Vit. D SUF.) and vitamin D supplemented swine (Vit. D SUP.).
Panel D–F shows the α-smooth muscle cell actin (αSMA) (green) in the neointima of the PTCA LCx.
Panel G–I shows co-localization of CTSL (red) and αSMA (green) in the neointima (DAPI background, blue). The mean grey scale intensity of CTSL expressing cells was measured for each treatment group (panel J). (n=3 in each treatment group) *p <0.03. L=lumen, M=media, NI=neointima
Vitamin D by interacting with the vitamin D receptors (VDR’s) exerts its effects on the expression of several important cellular proteins including proteases and protease inhibitors. Cystatins are endogenous inhibitors of endosomal and lysosomal proteases. Earlier studies have shown that 1,25(OH)2D3 regulates cystatin A22, cystatin B23, and cystatin D24. In the present study, we have identified that the presence of TNF-α and IGF-1 stimulates expression of CTSL in VMSCs and calcitriol treatment inhibits the induced CTSL (See Fig. 6B). The presence of vitamin D at or above the sufficient levels in-vivo was found to inhibit the expression of CTSL. In our in-vivo study, we observed that in the PTCA injured coronary arteries of atherosclerotic microswine, CTSL expression was drastically reduced when the animals were administered levels of dietary vitamin D at sufficient and above level (See Fig. 4B,C). Previously, dosing of vitamin D and CTSL activity was unknown but the further decrease in CTSL activity evidenced with supplemented vitamin D may indicate a dose-dependent relationship. Furthermore, the expression of CTSL in the vitamin D-deficient animals correlated with the expression of smooth muscle actin which suggests that CTSL promotes proliferation of VSMCs during NIH.
Our in-vitro findings strongly correlate with the in-vivo observations and demonstrates a possible cellular mechanism associated with CTSL expression and cell proliferating agents, including TNF-α and IGF-1. Furthermore, vitamin D appears to act directly on the inhibition of CTSL production in VSMCs. These observations clearly indicate a direct correlation between the expression of CTSL in the absence of vitamin D and progression of NIH. Our results support previous evidence in small animal models that showed that the inhibition of CTSL would lead to reduced internal elastic lamina degradation and impaired SMC accumulation in the neointimal lesions. These reports were demonstrated in LDL receptor-deficient and CTSL-deficient mice25.
A positive correlation was previously reported between MMP-9 activity and the activity of CTSL which leads to an increased proteolytic environment26. Further study into understanding the interplay of inflammatory cytokines, CTSL and vitamin D are warranted to explore the global matrisomal changes occurring in the absence of vitamin D. We anticipate that these studies would provide additional insights into the extracellular milieu that are playing a vital role in the development of NIH in addition to other matrix metalloproteinases and cysteine cathepsins. Importantly, with recent evidence that CTSL deficiency impairs the turnover of autophagolysosomes, Cai et al. hypothesized that alterations in autophagy may explain their findings of reduced NIH in CTSL knockout mice27. With direct findings of the induction of autophagy by vitamin D28, including its upregulation in anti-inflammatory M2 macrophages,29 it is plausible to consider autophagy mechanistically and therapeutically given our findings related to the immunomodulatory effects of vitamin D towards CTSL. Such investigation may find vitamin D or other means of CTSL inhibition to be a safer alternative to the successful activation of autophagy to prevent NIH witnessed with biolimus recently30.
V. Conclusion
The findings from this study support the concept that proliferation of vascular smooth muscle cells is induced in the presence of inflammatory cytokine (TNF-α) and angiogenic growth factor(IGF-1). This leads to increased expression of CTSL by the vascular smooth muscle cells which induces formation of NIH in the PTCA injured coronary arteries. However, in the presence of vitamin D-sufficient or vitamin D-supplemented levels, cathepsin L expression can be inhibited. Specific mechanisms of suppression of CTSL activity require further investigation and may benefit approaches to NIH.
Study Limitations
The limitations in this study consist of not extending our investigation further into underlying mechanisms linking vitamin D with the inhibition of cathepsin L. Nevertheless, our findings do demonstrate that a cellular mechanism exists. Additionally, the sole use of female micropigs in our study does raise the possibility that our results could be influenced by the differential expression of sex hormones or of sex chromosome genes unknown to current knowledge. However, the ubiquitous cardioprotective effects of estrogen may have diminished the magnitude of the anti-inflammatory effect of vitamin D. Therefore, these investigations are worthwhile and are certainly warranted in further studies.
Funding
This work was supported by research grants R01 HL112597, R01 HL116042, and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this original research article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the State of Nebraska.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Presented at 14th Academic Surgical Congress (Association of Academic Surgery and Society of University surgeons) on February 07, 2019 at Houston Texas.
References
- 1.Schwartz RS, Holmes DR, Topol EJ. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J Am Coll Cardiol. 1992;20(5):1284–1293. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Sukhova GK, Yang J-T, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006;184(2):302–311. doi: 10.1016/j.atherosclerosis.2005.05.012 [DOI] [PubMed] [Google Scholar]
- 3.Li W, Kornmark L, Jonasson L, Forssell C, Yuan X-M. Cathepsin L is significantly associated with apoptosis and plaque destabilization in human atherosclerosis. Atherosclerosis. 2009;202(1):92–102. doi: 10.1016/j.atherosclerosis.2008.03.027 [DOI] [PubMed] [Google Scholar]
- 4.Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186(1):20–28. doi: 10.1016/j.atherosclerosis.2005.06.046 [DOI] [PubMed] [Google Scholar]
- 5.O’Connell TD, Berry JE, Jarvis AK, Somerman MJ, Simpson RU. 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272(4 Pt 2):H1751–1758. doi: 10.1152/ajpheart.1997.272.4.H1751 [DOI] [PubMed] [Google Scholar]
- 6.Jamali N, Sorenson CM, Sheibani N. Vitamin D and regulation of vascular cell function. Am J Physiol Heart Circ Physiol. 2018;314(4):H753–H765. doi: 10.1152/ajpheart.00319.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewison M Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39(2):365–379, table of contents. doi: 10.1016/j.ecl.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Álvarez-Díaz S, Larriba MJ, López-Otín C, Muñoz A. Vitamin D: Proteases, protease inhibitors and cancer. Cell Cycle. 2010;9(1):32–37. doi: 10.4161/cc.9.1.10266 [DOI] [PubMed] [Google Scholar]
- 9.Swami S, Raghavachari N, Muller UR, Bao YP, Feldman D. Vitamin D Growth Inhibition of Breast Cancer Cells: Gene Expression Patterns Assessed by cDNA Microarray. Breast Cancer Res Treat. 2003;80(1):49–62. doi: 10.1023/A:1024487118457 [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Suarez I, Redwood AB, Grotsky DA, et al. A new pathway that regulates 53BP1 stability implicates cathepsin L and vitamin D in DNA repair. EMBO J. 2011;30(16):3383–3396. doi: 10.1038/emboj.2011.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morimoto S, Yamada K, Hiramitsu S, et al. Fragmentation of internal elastic lamina and spread of smooth muscle cell proliferation induced by percutaneous transluminal coronary angioplasty. Jpn Circ J. 1993;57(5):388–394. [DOI] [PubMed] [Google Scholar]
- 12.Gupta GK, Agrawal T, Del Core MG, Hunter WJ, Agrawal DK. Decreased Expression of Vitamin D Receptors in Neointimal Lesions following Coronary Artery Angioplasty in Atherosclerotic Swine. Zirlik A, ed. PLoS ONE. 2012;7(8):e42789. doi: 10.1371/journal.pone.0042789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovinge S, Hultgårdh-Nilsson A, Regnström J, Nilsson J. Tumor necrosis factor-alpha activates smooth muscle cell migration in culture and is expressed in the balloon-injured rat aorta. Arterioscler Thromb Vasc Biol. 1997;17(3):490–497. [DOI] [PubMed] [Google Scholar]
- 14.Mitra AK, Del Core MG, Agrawal DK. Cells, cytokines and cellular immunity in the pathogenesis of fibroproliferative vasculopathies. Can J Physiol Pharmacol. 2005;83(8–9):701–715. doi: 10.1139/y05-080 [DOI] [PubMed] [Google Scholar]
- 15.Grant MB, Wargovich TJ, Ellis EA, Caballero S, Mansour M, Pepine CJ. Localization of insulin-like growth factor I and inhibition of coronary smooth muscle cell growth by somatostatin analogues in human coronary smooth muscle cells. A potential treatment for restenosis? Circulation. 1994;89(4):1511–1517. [DOI] [PubMed] [Google Scholar]
- 16.Kitamoto S, Sukhova GK, Sun J, et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115(15):2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523 [DOI] [PubMed] [Google Scholar]
- 17.Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: A review of atherosclerosis and restenosis. Circ Res. 2000;86(2):125–130. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Li X, Peng D, et al. Usefulness of Serum Cathepsin L as an Independent Biomarker in Patients With Coronary Heart Disease. Am J Cardiol. 2009;103(4):476–481. doi: 10.1016/j.amjcard.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai J, Zhong H, Wu J, et al. Cathepsin L promotes Vascular Intimal Hyperplasia after Arterial Injury. Mol Med Camb Mass. 2017;23:92–100. doi: 10.2119/molmed.2016.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemaire R, Huet G, Zerimech F, et al. Selective induction of the secretion of cathepsins B and L by cytokines in synovial fibroblast-like cells. Br J Rheumatol. 1997;36(7):735–743. [DOI] [PubMed] [Google Scholar]
- 21.Gerber A, Wille A, Welte T, Ansorge S, Bühling F. Interleukin-6 and Transforming Growth Factor-β1 Control Expression of Cathepsins B and L in Human Lung Epithelial Cells. J Interferon Cytokine Res. 2001;21(1):11–19. doi: 10.1089/107999001459114 [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Ibe M, Honma M, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. 1,25-dihydroxyvitamin D(3) increases human cystatin A expression by inhibiting the Raf-1/MEK1/ERK signaling pathway of keratinocytes. Arch Dermatol Res. 2003;295(2):80–87. doi: 10.1007/s00403-003-0396-5 [DOI] [PubMed] [Google Scholar]
- 23.Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58(4):367–376. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez-Díaz S, Valle N, García JM, et al. Cystatin D is a candidate tumor suppressor gene induced by vitamin D in human colon cancer cells. J Clin Invest. 2009;119(8):2343–2358. doi: 10.1172/JCI37205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiro Kitamoto, Sukhova Galina K., Sun Jiusong, et al. Cathepsin L Deficiency Reduces Diet-Induced Atherosclerosis in Low-Density Lipoprotein Receptor–Knockout Mice. Circulation. 2007;115(15):2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523 [DOI] [PubMed] [Google Scholar]
- 26.Abisi S, Burnand KG, Waltham M, Humphries J, Taylor PR, Smith A. Cysteine protease activity in the wall of abdominal aortic aneurysms. J Vasc Surg. 2007;46(6):1260–1266. doi: 10.1016/j.jvs.2007.08.015 [DOI] [PubMed] [Google Scholar]
- 27.Cai J, Zhong H, Wu J, et al. Cathepsin L promotes Vascular Intimal Hyperplasia after Arterial Injury. Mol Med. 2017;23:92–100. doi: 10.2119/molmed.2016.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao S, Zhang H, Xue L, et al. Vitamin D protects against particles-caused lung injury through induction of autophagy in an Nrf2-dependent manner. Environmental Toxicology. 2019;34(5):594–609. doi: 10.1002/tox.22726. [DOI] [PubMed] [Google Scholar]
- 29.Das LM, Binko AM, Traylor ZP, Peng H, Lu KQ. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15(5):813–826. doi: 10.1080/15548627.2019.1569298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y, Park JK, Seo J-H, et al. A rapamycin derivative, biolimus, preferentially activates autophagy in vascular smooth muscle cells. Scientific Reports. 2018;8(1). doi: 10.1038/s41598-018-34877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]