Abstract
Advanced age is a primary risk factor for cardiovascular disease (CVD), the leading cause of death in the industrialized world. Two major components of arterial ageing are stiffening of the large arteries and impaired endothelium dependent dilation in multiple vascular beds. These two alterations are major contributors to the development of overt CVD. Increasing inflammation with advanced age likely plays a role in this arterial dysfunction. The purpose of this review is to synthesize what is known about inflammation and its relationship to age-related arterial dysfunction. This review discusses both the initial observational evidence for the relationship of age-related inflammation and arterial dysfunction as well as evidence that inflammatory autoimmune diseases are associated with a premature arterial ageing phenotype. We next discuss interventional and mechanistic evidence linking inflammation and age-related arterial dysfunction in older adults. We also attempt to summarize the relevant evidence from preclinical models. Lastly, we discuss interventions in both humans and animals that have been shown to ameliorate age-related arterial inflammation and dysfunction. The available evidence provides a strong basis for the role of inflammation in both large artery stiffening and impairment of endothelium dependent dilation; however, the specific inflammatory mediators, the initiating factors, and the relative importance of the endothelium, smooth muscle cells, perivascular adipose and immune cells in arterial inflammation are not well understood. With the expansion of the ageing population, ameliorating age-related arterial inflammation represents an important potential strategy for preserving vascular health in the elderly.
Keywords: Ageing, Endothelium, Vascular, Smooth Muscle, Perivascular Adipose
Introduction
Recently several lines of evidence have demonstrated that advanced age is associated with chronic low-grade inflammation and that this inflammation is associated with numerous age-related pathologies that lead to morbidity and mortality. As ageing is the major risk factor for cardiovascular disease (CVD) and most of these diseases are either entirely vascular or have a significant vascular component, there has been substantial attention to the aetiology of dysfunction in the aged artery. Arterial ageing manifests itself primarily through: 1) increases in large artery stiffness (Lakatta and Levy, 2003); and 2) impairments in endothelium dependent dilation (EDD) due to decreased nitric oxide (NO)-bioavailability and greater influence of the endothelial derived vasoconstrictor, endothelin-1 (Diehl, et al, 2012, Donato, et al, 2007, Gerhard, et al, 1996). Both large elastic artery stiffness (Lakatta and Levy, 2003, Mitchell, et al, 2010, Vlachopoulos, et al, 2010) and endothelial dysfunction (Ras, et al, 2012, Widlansky, et al, 2003, Yeboah, et al, 2007) are predictive of future cardiovascular events and are present in patients with CVD. Numerous investigations implicate age-associated inflammation in the initiation or propagation of diseases of the arteries (discussed in detail below). Despite these findings, it is less clear how the cells of the cells of the artery wall, circulating inflammatory factors and immune cells interact with age. The purpose of this review is to synthesize data in humans, animals and in vitro models to summarize the current state of knowledge regarding arterial inflammation with advanced age and how it relates to arterial dysfunction and risk of CVD. The topics covered include: observational evidence for the relationship of age-related inflammation and arterial dysfunction, insight from autoimmune inflammatory diseases and their effects on arterial function, interventional evidence linking inflammation and age-related arterial dysfunction, insight into age-related arterial inflammation from preclinical models and interventions to ameliorate age-related inflammation and arterial dysfunction. Lastly, a summary and future directions for research on aging, inflammation and arterial dysfunction is provided.
Overview of inflammation
Inflammation, first identified by the Roman physician Cornelius Celsus in the 1st century AD, is known by the classic signs of heat, pain, redness and swelling. In 1858 Rudolph Virchow added an important fifth sign, loss of function (Medzhitov, 2010). Shortly after the addition of loss of function to the signs of inflammation, Augustus Walter and Julius Cohnheim discovered that leukocytes emigrate out of the vasculature into resident tissues and that the leaky vasculature results in plasma loss and swelling (Medzhitov, 2010).
For a comprehensive description of the normal inflammatory response, readers are referred to the excellent review of Medzhitov, 2010. Briefly summarized, the typical inflammatory response is initiated by the appearance of molecules from either invasive pathogens (pathogen associated molecular patterns, PAMPS) or tissue damage (damage associated molecular patterns, DAMPS). These initiating molecules are detected by Toll like receptors (TLR) on many different cell types. The TLRs activate the transcription factor, Nuclear Factor κ B (NFκB) which translocates to the nucleus and induces transcription of multiple pro-inflammatory cytokine genes. Of particular importance is interleukin (IL)-1β, which can subsequently induce production of other notable pro-inflammatory cytokines including Tumour Necrosis Factor (TNF)-α and IL-6 (Willebrords, et al, 2016). This combination of cytokines reduces endothelial barrier function and upregulates endothelial cell surface molecules (Intracellular adhesion molecule, ICAM; vascular cell adhesion molecule, VCAM; platelet endothelial cell adhesion molecule, PECAM; e-Selectin and p-selectin) that allow for adhesion, rolling and extravasation of immune cells to enable recruitment to the inflammatory site. These cells include phagocytic cells from the innate immune system including neutrophils, monocytes (which can differentiate to macrophages), and dendritic cells. In addition, T cells and B cells from the adaptive immune system can also extravasate to the site of inflammation. Notably, Interferon (IFN)-γ and IL-17 production from T cells potentiate inflammation by recruiting and activating other leukocytes (Onishi and Gaffen, 2010, Schroder, et al, 2004). In a typical healthy inflammatory response, neutrophils undergo apoptosis following completion of their phagocytic actions at the site of inflammation and macrophages ingest apoptotic neutrophils (Ortega‐Gómez, et al, 2013). This prompts a switch in macrophages from pro- to anti-inflammatory characterized by an increase in anti-inflammatory IL-10 production (Ortega‐Gómez, et al, 2013). T regulatory cells, which also produce IL-10, are recruited by these anti-inflammatory macrophages and help to orchestrate the resolution of inflammation. In addition to these primary inflammatory factors, this review will also address the roles of: C reactive protein (CRP), a marker of inflammation which is synthesized by the liver in response to IL-6, IL-1 and TNF-α (Kolb-Bachofen, 1991); chemokines, which broadly defined, serve to recruit leukocytes to the site of inflammation including the CC and CXC motif chemokine ligand (CCL and CXCL) families as well as Monocyte Colony Stimulating Factor (MCSF) and Granulocyte Monocyte Colony Stimulating Factor (GM-CSF) which recruit and activate monocytes and granulocytes; Matrix Metalloproteinases which are peptidases that are induced by inflammation that can break down elastin; Transforming Growth Factor (TGF)-β a cytokine that contributes to collagen deposition; and, Nrf2, a transcription factor that regulates antioxidant and anti-inflammatory genes. The inflammatory mediators discussed in this review are summarized in Table 1. Figure 1 depicts the cellular locations, age-related alterations and interactions of these mediators in the arterial wall that are most strongly supported by the literature. These alterations are discussed in detail in the subsequent paragraphs.
Table.
Inflammatory Mediators and their role(s) in arterial ageing
| Mediator | Abbreviation | Localization | Species | Role in arterial ageing | Key References |
|---|---|---|---|---|---|
| Adhesion molecules | |||||
| e-selectin | N/A | Circulation | Human | Associated with microvascular EDD impairment | (Lind, et al, 2008) |
| Intracellular adhesion molecule | ICAM | Endothelial, circulation | Human, rat, mouse | Associated with impaired EDD (primarily microvascular), promotes leukocyte adhesion to endothelium | (Csiszar, et al, 2009, Vita, et al, 2004a, Yepuri, et al, 2012) |
| Vascular cell adhesion molecule | VCAM | Endothelial, circulation | Human, mouse | Associated with impaired cerebral blood flow, promotes leukocyte adhesion to endothelium | (Tchalla, et al, 2015, Yepuri, et al, 2012) |
| Cytokines & Chemokines | |||||
| Interferon-γ | IFN-γ | Whole artery | Human, mouse | Gene polymorphisms associated with CVD, EDD impairment | (Annoni, et al, 2009, Lesniewski, et al, 2011, Stokes, et al, 2007) |
| Interluekin-1β | IL-1β | Endothelial, whole artery, circulation | Human, non-human primate, rat, mouse | Major role in CVD | (Csiszar, et al, 2003, Csiszar, et al, 2012, Lesniewski, Durrant, Connell, Henson, et al., 2011, Ridker, Paul M., et al, 2017) |
| Interluekin-6 | IL-6 | Endothelial, circulation | Human,rat | Associated with CVD & large artery stiffness, role in impaired EDD | (Csiszar, et al, 2003, Donato, et al, 2008, Schnabel, et al, 2008, Vita, et al, 2004b, Wassmann, et al, 2004) |
| Interleukin-17 | IL-17 | Smooth muscle | Rat | EDD impairment | (Csiszar, et al, 2003, Nguyen, et al, 2013) |
| Interleukin-10 | IL-10 | Circulation, whole artery | Human, mouse | Low levels associated with CVD, large artery stiffness, EDD impairment | (Freitas, et al, 2011, Kinzenbaw, et al, 2013) |
| Transforming Growth Factor-β | TGF-β | Whole artery | Mouse | Collagen deposition, arterial stiffening | (Fleenor, et al, 2010) |
| Tumour Necrosis Factor-α | TNF-α | Endothelial, smooth muscle, circulation | Human, rat, mouse | Associated with CVD, impaired EDD, role in large artery stiffening | (Belmin, et al, 1995, Csiszar, et al, 2003, Donato, et al, 2008, Machado-Silva, et al, 2016, Moreau, et al, 2013) |
| CC motif chemokine ligand 2 | CCL2, aka Monocyte Chemoattractant protein 1 | Endothelial, Smooth muscle, perivascular adipose | Human, mouse | Leukocyte recruitment to artery, promotes large artery stiffening | (Donato, et al, 2008, Wang, et al, 2007, Wang, et al, 2011) |
| CC motif chemokine ligand 5 | CCL5, aka RANTES | Circulating | Human, mouse | Associated with CVD, may promote arterial stiffening | (Grufman, et al, 2014, Lozhkin, et al, 2017) |
| CXC motif chemokine ligand 2 | CXCL2 | Smooth muscle, perivascular adipose | Mouse | Recruitment of monocytes to the artery | (Fleenor, et al, 2014, Song, et al, 2012) |
| CXC motif chemokine ligand 10 | CXCL10 | Whole artery, smooth muscle | Mouse | Recruitment of T cells to the artery | (Song, et al, 2012, Trott, et al, 2017) |
| Granuolocyte Macrophage Colony Stimulating Factor | GM-CSF | Whole artery, perivascular adipose | Mouse | Macrophage recruitment to the artery | (Fleenor, et al, 2014, Trott, et al, 2017) |
| Immune cells | |||||
| B cells | N/A | Circulation, Perivascular adipose | Mouse | Infiltration of perivascular adipose | (Trott, et al, 2018) |
| T cells | N/A | Circulation, Perivascular adipose | Human, mouse | Proinflammatory cells associated with large artery stiffness, infiltration of perivascular adipose | (Lesniewski, et al, 2011, Trott, et al, 2018, Yu, et al, 2017) |
| Monocytes/ Macrophages | N/A | Circulation, Perivascular adipose | Human, mouse | Associated with EDD impairment, infiltration of perivascular adipose promote atherosclerosis | (Du, et al, 2016, Lesniewski, et al, 2011, Trott, et al, 2018, Walker, et al, 2010) |
| Neutrophils | N/A | Circulation, other? | Human | Associated with microvascular EDD impairment | (Walker, et al, 2010) |
| Transcription Factors | |||||
| Nuclear Factor κ B | NFκB | Endothelial, Smooth muscle | Human, non-human primate, rodent | Induces transcription of pro-inflammatory cytokines and chemokines and ROS producing enzymes. Associated with impaired flow mediated dilation and microvascular EDD impairment | (Donato, et al, 2007, Donato, et al, 2008, Donato, et al, 2013, Rodríguez-Mañas, et al, 2009, Ungvari, et al, 2011) |
| Nuclear factor (erythroid-derived 2)-like 2 | Nrf2 | Endothelial, smooth muscle | Non-human primate, mouse | Decrease in antoxidant and antiinflammatory gene expression, EDD impairment | (Csiszar, et al, 2012, Fulop, et al, 2018, Ungvari, et al, 2011) |
| Other | |||||
| Matrix Metalloproteinase 2 | MMP2 | Endothelial | Human | Elastin degradation, arterial stiffening | (Wang, et al, 2007) |
| Matrix Metalloproteinase 9 | MMP9 | Endothelial, whole artery | Human, mouse | Elastin degradation, arterial stiffening | (Donato, et al, 2013, Wang, et al, 2007) |
| Toll Like Receptor 4 | TLR | Smooth muscle | Rat | Upregulation of IL-6 production | (Song, et al, 2012) |
| C reactive protein | CRP | Circulation | Human | Associated with CVD & large artery stiffness, role in impaired EDD (primarily microvascular) | (Fichtlscherer, et al, 2000, Hein, et al, 2009, Lind, et al, 2008, Mattace-Raso, et al, 2004) |
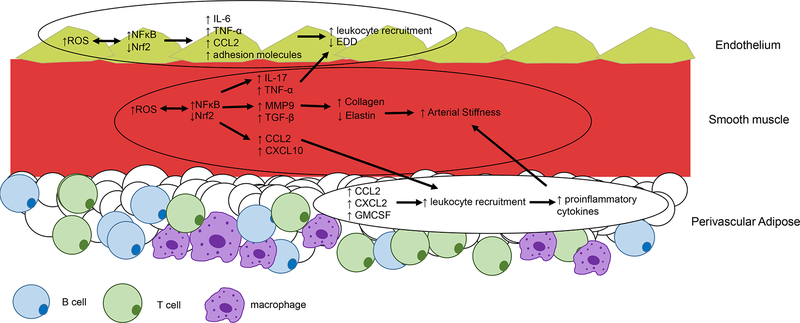
Figure 1. Age-related alterations in inflammatory mediators in the arterial wall.
In the endothelial layer increased reactive oxygen species (ROS) mediate increased Nuclear Factor κ B (NFκB) and decreased Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activity which results in increased inflammatory cytokine (interleukin (IL)-6, Tumour Necrosis Factor (TNF)-α), chemokine (CC motif chemokine ligand 2 (CCL2) and adhesion molecule production, leukocyte recruitment and subsequent impaired endothelium dependent dilation (EDD). In the smooth muscle layer ROS similarly affects NFκB and Nrf2 which mediate increased cytokine (IL-17, TNF-α, Transforming Growth Factor (TGF)-β), chemokine (CCL2 and CXC motif chemokine ligand 10 (CXCL10)) as well as Matrix Metalloproteinase (MMP) 9. These cytokines can interact with the endothelium to blunt EDD, contribute to collagen deposition and elastin degradation leading to increased arterial stiffness and recruitment of proinflammatory leukocytes. In the perivascular adipose, aging results in an increase in chemokines CCL2, CXCL2, and Granuolocyte Macrophage Colony Stimulating Factor (GM-CSF), these chemokines contribute to infiltration of inflammatory leukocytes that appear to play a role in increased arterial stiffness.
Age-related alterations in immune function and inflammation
As mentioned above, the immune system is composed of both innate and adaptive arms. The innate immune system is broadly composed of cells that differentiate along the myeloid lineage and include neutrophils, monocytes, macrophages, natural killer and dendritic cells. In contrast, adaptive immune cells differentiate along the lymphoid linage. Lymphoid cells have the capacity to generate T cell receptors (T cells) and antibodies (B cells) against specific antigens. This allows specificity, in contrast to the general capabilities of the innate immune system. Aging induces profound alterations in both the innate and adaptive immune system. First, there is a shift in the differentiation in hematoepoietic stem cells away from lymphoid (adaptive) and toward myeloid (innate) cells (Pang, et al, 2011). The innate immune system exhibits alterations including: impaired neutrophil chemotaxis, bacterial killing and phagocytosis; impairments in activation of monocytes and macrophages; increased basal monocyte and macrophage cytokine production; and, impairments in dendritic cell antigen and co-stimulatory molecule presentation. These alterations are expertly reviewed elsewhere by Shaw, et al, 2013. The major change that occurs in the adaptive immune system with advanced age is a decline in thymic naïve T cell output (Palmer, 2013). Other T cell changes include an inverted CD4:CD8 ratio, impaired T cell proliferation in response to mitogens, and accumulation of end differentiated memory T cells, likely driven by latent cytomegalovirus infection and increased basal proinflammatory cytokine production (Chou and Effros, 2013, Ferguson, et al, 1995, Pawelec, et al, 2009). There is also evidence for increased proportions of T regulatory cells but lower frequency of suppressive B cells (Duggal, et al, 2013, Jagger, et al, 2014). Collectively these alterations lead to both a reduction in immunity and the ability to fight infection and; paradoxically, increased inflammation and risk for autoimmune diseases.
These changes in the immune system and other changes associated with organismal stress resistance are synthesized in the “Inflammaging” hypothesis (Franceschi, et al, 2000). This hypothesis states that the reduction in ability to cope with stressors and concomitant increases in inflammatory status is a hallmark of the aging process (Franceschi, et al, 2000). Further, the hypothesis posits that inflammation in response to antigenic stressors early in life confers stress resistance, but later in life the accumulation of exposure to these stressors and resultant inflammation contributes to progression of age-related diseases. In addition to the changes described above, senescent cells accumulate in many tissues with advanced age. These cells produce many factors collectively referred to as the Senescence Associated Secretory Phenotype (SASP), reviewed in by Coppé, et al, 2010. Although far from exhaustive, major SASP components include IL-6, IL-1β, TNF-α and CCL2 (Monocyte chemoattractant protein-1). Importantly, NFκB is an important regulator of the SASP mediator production (Chien, et al, 2011, Salminen, et al, 2012). In addition to the SASP, senescent cells also produce ROS (particularly superoxide anion (O2−) and hydrogen peroxide (H2O2)). This is important because elevated ROS have been implicated as a major contributor to arterial ageing primarily by decreasing the bioavailability of NO (Donato, et al, 2018, Seals, 2014). ROS also induces activation of NFκB and subsequent inflammation (Schreck, et al, 1992), linking these two key processes in arterial ageing (Figure 1).
Observational evidence supporting a relationship between age-related inflammation and arterial dysfunction
Correlational Evidence of inflammation and CVD
Atherosclerosis is characterized by endothelial injury, which allows the initiation of inflammation with significant macrophage and T cell accumulation in atherosclerotic plaques (Ross, 1999). Because ageing is the single most predictive risk factor for the development of CVD, a natural hypothesis is that ageing is associated with increased inflammation that subsequently drives the development of CVD. Higher plasma levels of multiple proinflammatory mediators are present in older adults even in the absence of traditional CVD risk factors (Bonfante, et al, 2017, Cohen, et al, 1997, Gerli, et al, 2001, Shurin, et al, 2007). Further, inflammatory genes represent the largest cluster of increased whole blood gene expression with advanced age (Peters, et al, 2015).
Over the past 20 years a relatively robust literature on the association of inflammatory markers and arterial dysfunction or risk of CVD has emerged. Perhaps the most common clinical measurement of inflammation is CRP, which is synthesized in the liver in response to increases in IL-1, IL-6 and TNF-α during an inflammatory response (Pepys and Hirschfield, 2003). Plasma CRP levels are positively correlated with pulse wave velocity (PWV, a measure of large artery stiffness) and inversely correlated with endothelial dependent dilation (EDD) in forearm resistance arteries (Fichtlscherer, et al, 2000, Lind, et al, 2008, Mattace-Raso, et al, 2004). In a study of over 2700 older subjects (mean age = 61) in the Framingham offspring study, circulating concentrations of CRP, IL-6 and soluble ICAM-1 were negatively correlated to forearm reactive hyperaemia; an index of microvascular EDD (Vita, et al, 2004). Other data from the Framingham study indicates that IL-6 is positively associated with PWV (Schnabel, et al, 2008). In older adults, circulating VCAM-1 and e-selectin are associated with impaired endothelial function in the cerebral and forearm circulations, respectively (Lind, et al, 2008, Tchalla, et al, 2015). Brachial artery flow mediated dilation (FMD) is negatively related to circulating white blood cell counts (specifically neutrophils, eosinophils and monocytes) in older adults and decreased NO bioavailability appears to involved (Walker, et al, 2010). Together, these data provide substantial correlational evidence for the influence of inflammation on arterial function with ageing.
Polymorphisms that promote production of IL-6 and IFN-γ and decreased production of IL-10 are associated with development of carotid plaques in older adults (Annoni, et al, 2009, Giacconi, et al, 2004). In octogenarians, high levels of TNF-α, CRP, IL-1β, IL-6 and low levels of IL-10 are associated with increased coronary artery calcification and carotid artery intima media thickness (Freitas, et al, 2011, Machado-Silva, et al, 2016, Quaglia, et al, 2014). It should be noted that IFN-γ and IL-17a, pro-inflammatory cytokines for which there is evidence of participation in age-related arterial dysfunction, were not associated with carotid artery disease in older adults (Machado-Silva, et al, 2016). Serum levels of CCL5 (RANTES), a potent T cell recruiting chemokine, is associated with arterial plaque development as well as serum CRP, IL-1β, and IL-6 in middle aged men (Koh, et al, 2009). Interestingly, carotid plaques in subjects > 70 years of age analysed ex vivo had lower plaque specific concentrations of IFN-γ and TNF-α (Grufman, et al, 2014). An important consideration when interpreting these studies is that these associations differ between arteries (i.e. carotid vs. coronary) and signs of CVD (i.e. plaque development, calcification, medial thickening) and localization of inflammatory mediator(s). It appears that various inflammatory mediators may have heterogeneous effects on the arterial tree and that the specific systemic vs. local effects (i.e. serum TNF-α associated with carotid disease, yet carotid plaques from older adults generating less TNF-α (Freitas, et al, 2011, Grufman, et al, 2014)) and interactions of these mediators are complex not completely understood.
Other population-based evidence indicates that seropositivity for cytomegalovirus (CMV) or CagA-positive Helicobactor pylori increase risk for CVD and cardiovascular mortality and CMV positivity is also associated with elevated PWV. (Mayr, et al, 2003, Roberts, et al, 2010, Spyridopoulos, et al, 2016, Yu, et al, 2017). These data, in accordance with the “Inflammageing” theory, support the concept that lifelong accumulated antigen exposure increases inflammation, contributing to CVD. Although this body of evidence strongly implicates inflammation as a factor in CVD, there may be a critical interaction between western lifestyle and inflammation that mediates arterial ageing. In this regard, the Tismaine people of the Bolivian Amazon are horticulturalist/foragers and exhibit greater CRP and white blood cell counts but markedly lower systolic blood pressure and risk for CVD compared to Americans (Gurven, et al, 2009). There is some evidence that western diet mediated changes in the microbiome may be responsible for these societal differences (see further discussion in the ‘Targeted Antioxidant and Pharmacological interventions’ section below). Collectively, these findings underscore the complexity by which inflammation and its multiple mediators interact with the vasculature as one ages.
Direct Evidence of Age-related Arterial Inflammation
Building on the base of correlational evidence, two seminal studies directly demonstrate the upregulation of NFκB in both venous and arterial endothelial cells of older humans (Donato, et al, 2007, Donato, et al, 2008). Further, venous endothelial cells from older adults exhibit increased IL-6, TNF-α and the pro-inflammatory chemokine CCL2 (Morgan, et al, 2013, Rossman, et al, 2017). The upregulation of these endothelial inflammatory factors that are components of the SASP may be due to increased age-associated arterial cell senescence (Morgan, et al, 2013, Rossman, et al, 2017). In human mesenteric arterioles, ageing is associated with increased NFκB and this increase is negatively correlated to EDD in these same vessels (Rodríguez-Mañas, et al, 2009). These observations demonstrate that endothelial specific inflammation occurs with advanced age. Complementing these data, aortas from healthy older adults exhibit greater intimal and medial CCL2 and macrophage staining as well as increased Matrix Metalloproteinase (MMP)2, MMP9 (proteinase enzymes that degrade elastin) and collagen deposition (Wang, et al, 2007). This process appears to be inflammation dependent (discussed further in the preclinical models section below) and promotes large artery stiffening. In older adults, aortic 18FDG PET, a non-invasive measure of infiltrating inflammatory cells, is related to large artery stiffness and increases in the 18FDG signals around large arteries are observed in older adults over time (Joly, et al, 2016, Orellana, et al, 2013). Together these data provide direct evidence of endothelial inflammation, inflammation related arterial remodelling and arterial immune cell infiltration in older adults.
Insight from autoimmune inflammatory diseases and arterial function
There are a number of parallels between autoimmune disease and immune ageing. These similarities are reviewed extensively elsewhere (Weyand, et al, 2014). Briefly, a major change that occurs in both ageing and many autoimmune diseases, such as rheumatoid arthritis is the accumulation of CD28- T cells. These T cells are associated with increased arterial stiffness, impaired EDD and are predictive of stroke and cardiovascular mortality (Gerli, et al, 2004, Nadareishvili, et al, 2004, Youn, et al, 2013, Yu, et al, 2017). Further, patients with rheumatoid arthritis are at greater risk for cardiovascular disease and this is not explained by traditional CVD risk factors (del Rincón, et al, 2001). Patients with inflammatory bowel disease and Systemic lupus erythematosus exhibit greater large artery stiffness (Barnes, et al, 2011, Zanoli, et al, 2012). Perhaps the most relevant autoimmune disease is Giant Cell Arteritis, which is an autoinflammatory syndrome that is characterized by T cell and macrophage mediated intimal hyperplasia and stenosis of medium and large arteries that feed the eye, optic nerve and brain. Detailed pathophysiology of Giant Cell Arteritis is described elsewhere (Weyand, et al, 2012). Importantly, for this review, the strongest risk factor for the development of Giant Cell Arteritis is advanced age.
There are a number of relevant clinical trials using anti-inflammatory interventions and assessing cardiovascular outcomes in patients with autoimmune diseases (Askling and Dixon, 2011). In patients with rheumatoid arthritis, ankylosing spondylitis, or psoriatic arthritis, TNF-α antagonism improves large artery stiffness (Angel, et al, 2010). Further, patients with rheumatoid arthritis treated with TNF-α antagonists exhibit decreased arterial inflammation as assessed by 18FDG imaging that is accompanied by improved large artery stiffness. (Mäki-Petäjä, et al, 2012). Brachial artery FMD was improved in rheumatoid arthritis patients twelve weeks following a single course of Ritiuximab to deplete B cells (Hsue, et al, 2014). In contrast, IL-6 receptor blockade and inhibition of T cell co-stimulation do not improve PWV in rheumatoid arthritis patients (Mathieu, et al, 2013, McInnes, et al, 2015). Together these data indicate that some anti-inflammatory interventions designed to improve autoimmune symptoms have the secondary effect of improving arterial function.
Interventional evidence linking inflammation and age-related arterial dysfunction
Paul Ridker has led several highly impactful clinical trials to determine whether inflammation can directly influence cardiovascular outcomes. In a cohort from the Physicians Health Study his team found that aspirin lowered risk of cardiovascular events in subjects with high, but not low, plasma CRP (Ridker, et al, 1997). Further, in The JUPITER trial, statin treatment reduced CVD risk in subjects with high CRP but normal blood lipids, suggesting an anti-inflammatory effect of statins independent of cholesterol lowering (Ridker, et al, 1997, Ridker, et al, 2008). Most recently, the IL-1β antagonist canakinumab (The CANTOS trial) has been shown to be effective in preventing cardiovascular events (Ridker, et al, 2017). There are several other clinical trials that are ongoing, for a full review see Ferrucci and Fabbri, 2018.
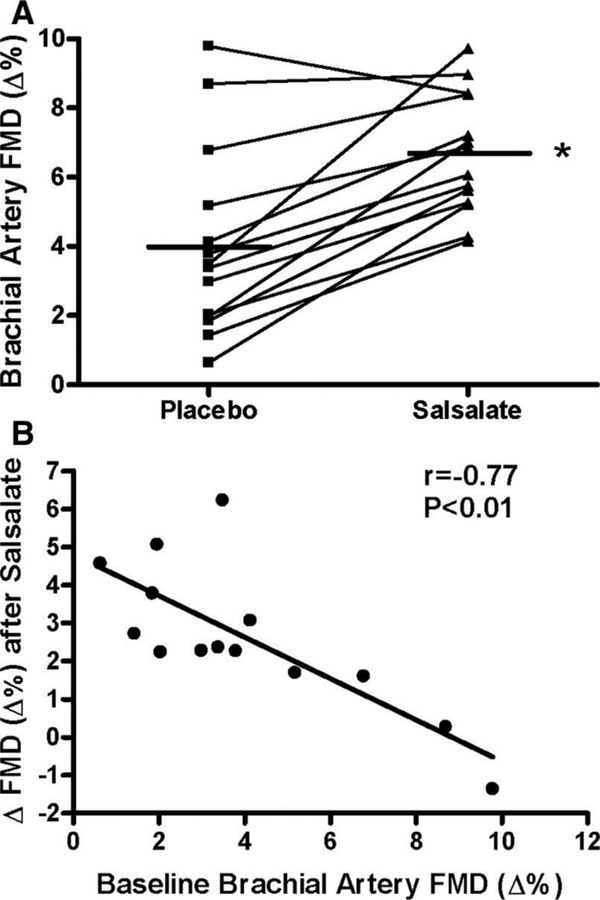
In smaller mechanistic studies, short-term inhibition of NFκB with salsalate improved EDD in older adults (Figure 2) (Pierce, et al, 2009). Interestingly, in this cohort, salsalate reduced endothelial cell NFκB, oxidative stress and ROS producing enzymes, but not endothelial cell or systemic cytokines/chemokines (Pierce, et al, 2009). In a subsequent investigation, the same short-term inhibition of NFκB improved PWV in older adults (Jablonski, et al, 2015). Supporting this line of evidence, induced inflammation via vaccination, acutely impairs EDD in young healthy subjects (Hingorani, et al, 2000). The combination of data from these large clinical trials and smaller mechanistic studies provide support for the concept that inflammation is causal in arterial dysfunction with age.
Figure 2. Inhibition of Nuclear Factor κB improved endothelium dependent dilation in middle aged to older adults.
(A) Individual values for endothelium-dependent dilation (n=14; brachial artery FMD; percentage change, Δ%) in subjects after placebo and salsalate conditions; (B) Relation between baseline (placebo condition) brachial artery FMD and the change (Δ) in FMD from placebo to salsalate conditions. *P<0.05 vs placebo. Reproduced with permission from Pierce et al., (2009).
In older post-menopausal women, short-term treatment with the TNF-α antagonist etanercept improves both large artery stiffness and EDD independent of exogenous oestrogen treatment (Moreau, et al, 2013). However, in uterine arteries, in vitro treatment with 17β-estradiol reduces production of endothelial adhesion molecules, IL-6 and CCL2 in arteries from women who are less than 5 years, but not greater than 10 years, from the onset of menopause (Novella, et al, 2012). Interestingly, physical activity reduces systemic inflammation in oestrogen-deficient postmenopausal women but does not improve endothelial function (Santos-Parker, et al, 2017). Collectively, these data suggest that the interaction of sex hormones, inflammation and arterial function in postmenopausal women is complex and is an important topic of further investigation.
Insight into age-related arterial inflammation from preclinical models
The first evidence of age-related arterial inflammation in animals was introduced 25 years ago when it was shown that aortas from old rats produce more TNF-α and IL-6 than young controls (Belmin, et al, 1995). Since then, it has been found that aortas from old rodents also produce more IL-1β, IFN-γ, endothelial adhesion molecules (ICAM1 and VCAM1) as well as T cell, granulocyte and monocyte recruiting chemokines (CCL2, CXCL10, GM-CSF, MCSF) (Lesniewski, et al, 2011, Sindler, et al, 2011, Trott, et al, 2017, Yepuri, et al, 2012). Most of these mediators are downstream of NFκB. Whole aortas of old mice and carotid arteries from non-human primates also demonstrate greater NFκB activity (Lesniewski, et al, 2011, Sindler, et al, 2011, Ungvari, et al, 2011). In endothelial cells from old rats, NFκB activity and inflammatory cytokine production is dependent upon mitochondrial ROS production (Ungvari, et al, 2007). Further, inhibition of NFκB with salicylate ameliorates age-related increases in aortic proinflammatory cytokine concentrations and endothelial dysfunction (Lesniewski, et al, 2011). These data indicate that NFκB is a major mediator of age-related inflammation and endothelial dysfunction.
Older IL-10 (an anti-inflammatory cytokine) knockout mice exhibit greater large artery stiffness and greater impairments in carotid artery EDD and compared to old wild type mice (Kinzenbaw, et al, 2013, Sikka, et al, 2013). Arteries from old IL-10 knockout mice exhibit greater NADPH oxidase (a ROS producing enzyme) and IL-6 gene expression (Kinzenbaw, et al, 2013). In wild type mice, we have found that aortic IL-10 production is greater in old mice compared to young (Trott, et al, 2017). Together, these data suggest that upregulated IL-10 may be an attempt to constrain age related arterial inflammation and dysfunction.
In addition to endothelial effects, there are also direct pro-inflammatory effects on smooth muscle cells with ageing. For example, in primary smooth muscle cells from old non-human primates, ROS upregulates NFκB and IL-6 to a greater extent than in young animals, due to impairments in Nrf2 activity (Ungvari, et al, 2011). Nrf2 is a transcription factor that regulates expression of numerous antioxidant and anti-inflammatory genes and can oppose the proinflammatory effects of NFκB (Ahmed, et al, 2017). Inflammatory processes orchestrate multiple changes in both the intimal and medial layer that lead to elastin breakdown and collagen deposition. NFκB promotes MMP9 production in large arteries from older mice; MMP9 degrades elastin and promotes arterial stiffness (Donato, et al, 2013). Carotid arteries from older mice exhibit greater levels of the cytokine Transforming Growth Factor (TGF)-β1 which leads to increased collagen deposition and arterial stiffness (Fleenor, et al, 2010). This increase in TGF-β may be due to a feed forward cascade that involves age-related impairments in NO bioavailability, increases in arterial ROS, upregulation of leukocyte attracting chemokines (CCL2, CCL5 and CXCL2), upregulation of adhesion molecules, monocyte infiltration and MMP2 production (Du, et al, 2016, Koyanagi, et al, 2000, Lozhkin, et al, 2017, Song, et al, 2012, Wang, et al, 2011). Both mineralacortocoid receptor/aldosterone signalling and TLRs also appear to be involved in this process (Krug, et al, 2010, Song, et al, 2012). Collectively, these data suggest that inflammation dependent alterations can occur in the medial layer of large arteries. Perivascular adipose also appears to play a role in arterial ageing as transplantation of perivascular adipose from old mice induces large artery stiffness in young recipient mice (Fleenor, et al, 2014). In this same study, cultured perivascular adipose tissue from old mice generated greater amounts of IL-6 and chemokines GM-CSF, CCL2, and CXCL2 (Fleenor, et al, 2014). Others have found relatively few age-related inflammatory changes in perivascular adipose tissue but when older mice were fed a high fat diet to induce obesity perivascular adipose inflammation was markedly enhanced (Bailey-Downs, et al, 2013). These age-related alterations in arterial wall inflammation are summarized in Figure 1.
Most of the above findings are in the aorta or other large-elastic arteries; however, since the arterial tree exhibits marked heterogeneity in both anatomy and function, it is important to consider the role of inflammation other vascular beds. In the cerebro-microvasculature old rats exhibit greater in vivo endothelial leukocyte adhesion and rolling during ischemia reperfusion along with decreased shear rate (Ritter, et al, 2008). Ageing results in a leakier hippocampal blood brain barrier and greater hippocampal inflammation (both cytokines and inflammatory microglia) (Tucsek, et al, 2014). Genetic deletion of the anti-inflammatory transcription factor Nrf2 results in impaired cerebral artery EDD and increased brain inflammatory gene expression (Fulop, et al, 2018). In coronary arteries from old rats, IL-1β and IL-6 are predominantly localized in the endothelium whereas TNF-α and IL-17 are localized in the smooth muscle (Csiszar, et al, 2003). Importantly, CRP, IL-6, IL-17, TNF-α, IL-1β and IFN-γ have all been shown to directly induce resistance artery impairments in EDD and/or increase endothelial leukocyte adhesion in experimental models (Bevilacqua, et al, 1985, Hein, et al, 2009, Nguyen, et al, 2013, Stokes, et al, 2007, Wassmann, et al, 2004, Watson, et al, 1996, Zhang, et al, 2006). These data suggest that with age, locally produced cytokines may contribute to impairments in resistance artery function. The potential differential effects of age-related inflammation on large elastic and resistance arteries are depicted in Figure 3. The relative lack of information about age-related inflammation in vascular beds other than the large elastic arteries represent an important area of future study.
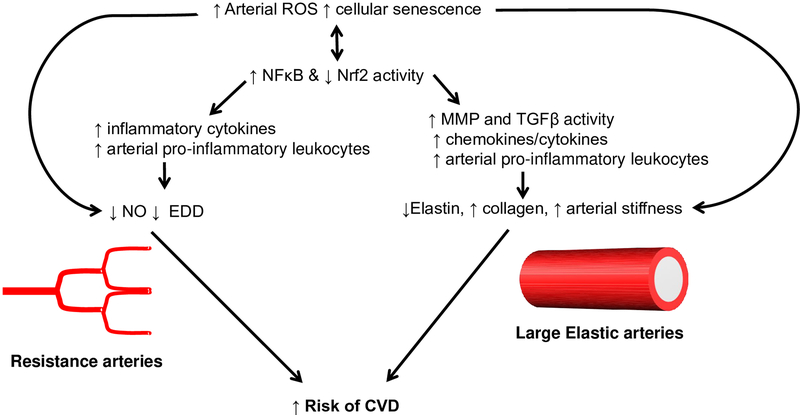
Figure 3. Schematic for proposed model of inflammation mediated arterial dysfunction with advanced age.
We propose that increased arterial reactive oxygen species (ROS) and increased cellular senescence result in increased NFκB and decreased Nrf2 activity resulting in upregulation of inflammatory and ROS producing gene expression. In the resistance vasculature, the upregulation of inflammatory cytokines act directly to suppress Nitric Oxide (NO) and endothelium dependent dilation (EDD). In the large elastic arteries, inflammation leads to increased Matrix Metalloproteinase (MMP) and Transforming Growth Factor (TGF)-β activity as well as increased infiltration of proinflammatory leukocytes. These changes lead to breakdown of elastin, increased collagen deposition and resultant arterial stiffness. Together the impairments in EDD and increase in large artery stiffness contribute to increased risk for cardiovascular disease (CVD).
Immune system contribution to arterial inflammation
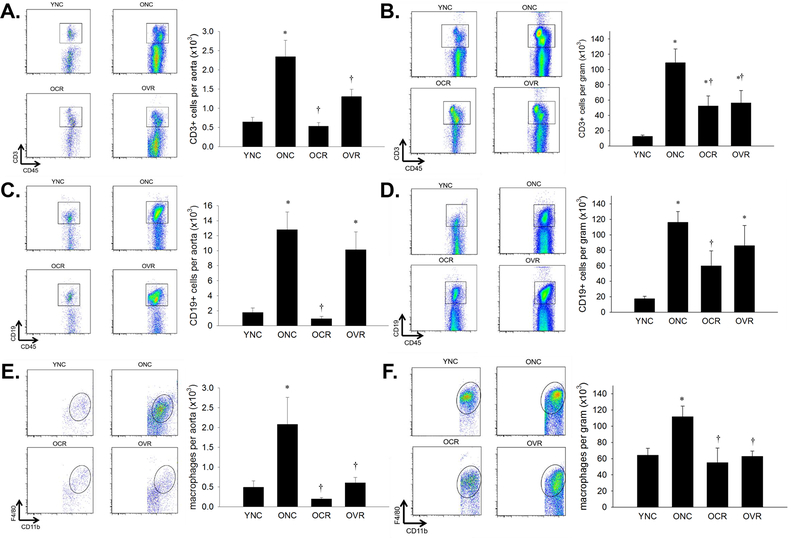
Although there is a sizeable literature on inflammation in the artery per se, there is a relative lack of information on whether the immune system contributes directly to age-related arterial inflammation. We have found that T cells, B cells and Macrophages accumulate in the aorta and in the mesenteric vascular arcade of old mice (Figure 4) (Lesniewski, et al, 2011, Trott, et al, 2018). In the atherosclerosis Ldlr−/− mouse model, ageing promotes macrophage infiltration of the aorta beyond that observed in younger Ldlr−/− mice (Du, et al, 2016). Immune cell recruitment to arteries of mice that overexpress smooth muscle p22phox (a component of the ROS producing NADPH oxidase) is age-dependent up to early middle age, suggesting that the recruitment of inflammatory immune cells to the artery is dependent on arterial ROS (Wu, et al, 2016). Further, increased large artery stiffness in these mice is dependent on the presence of lymphocytes (Wu, et al, 2016). In experimental stroke models, old mice exhibit greater memory T cell mediated inflammation in the brain as well as increased infiltrating neutrophils, which produce MMP-9 and ROS, leading to impairments in functional recovery (Ritzel, et al, 2016, Ritzel, et al, 2018). When these mice were irradiated and received a bone marrow transplant from a younger donor much of the inflammation and functional deficits were ameliorated (Ritzel, et al, 2018). In a similar experimental stroke model, in old mice, splenectomy resulted in smaller infarct sizes when compared to old mice with spleens intact (Chauhan, et al, 2018). Collectively, these data suggest that ageing results in recruitment of immune cells to both the large elastic arteries and smaller arteries of the gut and brain circulation where these cells can affect arterial structure and function (Figures 1 & 3).
Figure 4. Leukocyte infiltration of aorta with age, caloric restriction and voluntary running.
Aortas and mesenteric vascular arcades from young normal chow (YNC), old- (O) normal chow (NC), voluntary running (VR) and calorie restricted (CR) mice were digested to a single cell suspension and incubated with antibodies against CD45 and CD3 to assess total T cells in aorta (A) and mesentery (B); CD45 and CD19 to assess total B cells in aorta (C) and mesentery (D) and; CD45, CD11b and F4/80 to assess total macrophages in aorta (E) and mesentery (F). Representative flow cytometry plots are shown on the left of each panel, summary data is shown on the right. n = 5–11/group. Differences were assessed with one-way ANOVA with LSD post hoc tests. * different from YNC, † different from ONC, p ≤ 0.05. Data are means ± SEM. Adapted with permission from Trott et al., (2018).
Interventions to ameliorate age-related inflammation and arterial dysfunction
Dietary interventions
Caloric restriction, a life extending and vasculoprotective intervention improves endothelial function and decreases NFκB activity as well as ICAM gene expression in aortas from old rats (Csiszar, et al, 2009). Further, in vitro monocyte adhesion is blunted in the presence of serum from old caloric restricted rats when compared to serum from old ad libitum fed rats (Csiszar, et al, 2009). Caloric restriction also improves PWV and reverses the age-related increase of MMP-9 in mouse large elastic arteries (Donato, et al, 2013) as well as normalizing age-related infiltration of aortic T cells, B cells and macrophages (Figure 4) and aortic production of the T cell recruiting chemokine CXCL10 (Trott, et al, 2017, Trott, et al, 2018). Similar effects of caloric restriction on immune cell infiltration was observed in the mesenteric vasculature (Figure 4) (Trott, et al, 2018).
In addition, a number of dietary interventions have been shown to be effective in reducing age-related inflammation and arterial dysfunction. In smooth muscle cells isolated from non-human primates resveratrol reverses age-related increases in IL-1β, CCL2, TNF-α, mitochondrial ROS production and NFκB activity, as well as increasing Nrf2 activity (Csiszar, et al, 2012). Sodium nitrate supplementation improves EDD and normalizes PWV in old mice (Sindler, et al, 2011). These improvements were associated with lower aortic ROS and IL-1β, IL-6, IFN-γ and TNF-α (Sindler, et al, 2011). In older adults nitrate-rich beetroot juice decreased blood pressure and monocyte/platelet aggregation (Raubenheimer, et al, 2017). Trehalose, a disaccharide which has autophagy stimulating effects, improves EDD in both older humans and mice and the aortas from old mice treated with trehalose exhibit lower IL-1β, IL-6 and TNF-α production (LaRocca, et al, 2012). Curcumin, a polyphenol found in turmeric, improves both resistance and conduit artery EDD in older adults independent of changes in systemic CRP, IL-6 or TNF-α (Santos-Parker, et al, 2017). Flaxseed and nut consumption appear to improve arterial function and reduce inflammation in middle-aged cohorts with cardiovascular and metabolic diseases (Khandouzi, et al, 2019, Neale, et al, 2017).
Physical Activity
Habitually exercising older adults exhibit lower PWV compared to their sedentary counterparts and improvements in PWV induced by inhibition of NFκB occur only in sedentary older adults (Jablonski, et al, 2015), suggesting that NFκB activity is restrained by chronic exercise. Habitually exercising older adults also exhibit blunted endothelial senescence and production of SASP components compared to their sedentary counterparts (Rossman, et al, 2017). In a cross sectional study, exercise trained postmenopausal women exhibited lower CRP when compared to sedentary postmenopausal women; however, the exercise trained women did not exhibit improved EDD in either the macro- or microvasculature of the forearm (Santos-Parker, et al, 2017). In old mice, voluntary wheel running ameliorates aortic NFκB activity, IL-1β, IL-6, TNF-α and IFN-γ production as well as aortic and mesenteric T cell and macrophage infiltration (Figure 4) (Lesniewski, et al, 2011, Trott, et al, 2018). Physically active Systemic Lupus Erythematosus patients exhibit improved large artery stiffness and lower inflammation compared to sedentary patients (Barnes, et al, 2011). Together, these data suggest that physical activity can act to restrain arterial inflammation; however, there appear to be differential effects in men and women. Elucidating mechanistically how physical activity might influence arterial inflammation with age is understudied and an important topic for further investigation.
Role of ROS and Antioxidant Interventions
As discussed previously, with age, increases in arterial ROS likely activate NFκB and supress Nrf2 signalling resulting in increased production of both ROS producing enzymes and inflammatory cytokines. ROS are also involved in MMP-9 production and arterial remodelling (Wang, et al, 2015). Scavenging of O2− with TEMPOL both improves arterial function and normalizes production of IL-1β, IL-6 and TNF-α production in older mice (Fleenor, et al, 2012). Further, TEMPOL abolished the increases in large artery stiffness induced by old to young perivascular adipose tissue transplants or using an ex vivo perivascular adipose tissue/large artery incubation assay (Fleenor, et al, 2014). Mitochondrial targeted antioxidants have been shown to improve both large artery stiffness and EDD in old mice and older adults: however, in contrast to the results with TEMPOL described above, these improvements are largely independent of alterations in circulating and aortic cytokine production (Gioscia-Ryan, et al, 2018, Gioscia-Ryan, et al, 2014, Rossman, et al, 2018, Zinovkin, et al, 2014). Mitochondrial targeted antioxidants decrease aortic ICAM in old mice (Zinovkin, et al, 2014) and in rat endothelial cells scavenging of mitochondrial ROS attenuates NFkB activity and inflammatory cytokine production (Ungvari, et al, 2007). The age-related increase in aortic VCAM is dependent on the NAPDH oxidase subunit Nox2 (Fan, et al, 2017). Leukocyte recruitment to arteries is greater in 9 month old mice that overexpress p22phox in smooth muscle compared to 9 month old controls (Wu, et al, 2016). Further, the increase in large artery stiffness in these mice is dependent on the presence of lymphocytes as Rag-1 knockout mice, which lack T and B cells, were protected against increases in stiffness, even in the presence of smooth muscle p22phox overexpression (Wu, et al, 2016). Chemerin, an adipose derived cytokine that is increased with advanced age, mediates increases in endothelial/monocyte adhesion in vitro in a ROS dependent manner (Neves, et al, 2015). Paradoxically, the NADPH oxidase subunit Nox4 is decreased in vascular smooth muscle cell senescence and down regulation of Nox4 led to increases in IL-6 and IL-8 secretion (Przybylska, et al, 2016). These data suggest an important link between ROS and inflammation. Collectively the data suggest that cytosolic and extracellular scavenging of ROS may be more effective than scavenging mitochondrial specific ROS; however, the localization of ROS both at the tissue and subcellular level that contribute to increased inflammation as well as what characterizes optimal vs. pathogenic ROS production has yet to be fully elucidated. It should also be noted that most studies have only assessed the influence of ROS on a small number of inflammatory mediators, it is likely that different ROS and their localization effect inflammation differently.
Pharmacological interventions
In a model of Alzheimer’s disease (amyloid precursor protein transgenic mice), treatment with low dose simvastatin decreases brain microglia activation and also improved cerebral artery reactivity and blood flow (Tong, et al, 1994). Treatment with exogenous parathyroid hormone improves EDD in bone principal nutrient arteries of old rats, this effect was associated with greater bone marrow production of IL-10 (Lee, et al, 2018). Clearance of senescent cells with the senolytic drug cocktail Dasatinib + Quercetin has recently been shown to improve several age-related pathologies including impaired endothelium dependent relaxation in both old and hypercholesterolemic mice (Roos, et al, 2016). In this investigation, the authors found that the improvements in arterial function were independent of changes in arterial macrophage specific gene expression in the hypercholesterolemic mice but did not assess other inflammatory outcomes in either the hypercholesterolemia or old mice (Roos, et al, 2016). Pharmacological activation of SIRT1, a member of the sirtuin family of enzymes that contribute to lifespan extension, improves EDD and ameliorates age-related increases in aortic ROS, NFκB activity and TNF-α in old mice (Gano, et al, 2014). Supplementation with nictotinamide mononucleotide, which also contributes to SIRT1 activity improves EDD and large-artery stiffness in old mice with a concomitant decrease in NFκB activity (de Picciotto, et al, 2016)
A possible explanation for the role of the western lifestyle discussed in the ‘Observational evidence’ section above is the interaction of diet and the gut microbiome. In mice, a western diet induces gut dysbiosis that is accompanied by systemic and arterial inflammation and arterial dysfunction (Battson, et al, 2018). These deleterious effects on the arteries were abolished with broad spectrum antibiotics, indicating that the microbiome plays a critical role in inflammation and arterial dysfunction. The microbiome also appears to play a role in age-related arterial dysfunction. In old mice broad spectrum antibiotics improve EDD, large artery stiffness and ameliorate age-related increases in aortic IL-6, TNF-α and IFN-γ production (Brunt, et al, 2019). In this investigation, antibiotic treatment also reduced systemic levels of the microbiota derived molecule trimethylamine N-oxide (TMAO) which has been shown to be proatherogenic (Brunt, et al, 2019, Tang, et al, 2013). There is a clear need for more interventional studies targeting age-related inflammation to improve arterial dysfunction.
Summary and Future Directions
Collectively the data described in this review lends a strong basis for the concept that inflammation contributes to age-related arterial dysfunction. However, the origin and onset of age-related arterial inflammation, the mechanisms by which inflammation is a direct driver of arterial dysfunction, and the effects of inflammation at different points in the arterial tree remain unclear. This review proposes the model depicted in Figure 3. With advancing age both the increases in ROS and the accumulation of senescent cells in the artery mediate NFκB activation. Notably, whether the increase in ROS or accumulating cellular senescence is the initiating factor in arterial inflammation is unknown. The ability of Nrf2 to promote antioxidant and antiinflammatory mediators is impaired with age resulting in the production of proinflammatory cytokines and chemokines. Several of the mediators discussed throughout the review (CRP, IL-6, IL-17, TNF-α, IL-1β, IFN-γ) can directly induce resistance artery impairments in EDD and/or increase endothelial leukocyte adhesion in preclinical models (Bevilacqua, et al, 1985, Hein, et al, 2009, Nguyen, et al, 2013, Stokes, et al, 2007, Wassmann, et al, 2004, Watson, et al, 1996, Zhang, et al, 2006). Which of these mediators is most critical in induction of impairments in resistance artery EDD and the mechanism(s) are important topics for further study. In addition to these direct effects on the endothelium, it appears that inflammation in the perivascular adipose and smooth muscle media layers of the artery mediate leukocyte infiltration, collagen deposition and arterial stiffening in large elastic arteries (Donato, et al, 2013, Fleenor, et al, 2010, Fleenor, et al, 2014, Lesniewski, et al, 2011, Wang, et al, 2011). These immune cells may generate more inflammatory cytokines further promoting inflammation and impairing vascular function. Which immune cell subtypes and the inflammatory cytokines and chemokines involved in this process have yet to be fully identified. Lastly, the contributions of other factors, such as increased sympathetic nerve activity, which is known to play a role in both vascular aging (Kaplon, et al, 2011) and arterial inflammation (Marvar, et al, 2010), are unknown. These gaps in knowledge represent several important lines of future investigation.
In conclusion, it appears that age-related arterial inflammation contributes to both endothelial dysfunction and impaired NO-bioavailability as well as elastin breakdown and collagen deposition leading to arterial stiffening. These factors together contribute to increased risk of CVD. Importantly, the initiating factor(s) of arterial inflammation; the relative importance of inflammation in the large arteries, resistance arteries and microcirculation; the relative importance of the endothelium smooth muscle, perivascular adipose, and immune cells; and the differential effects of inflammation along the arterial tree are unknown. Lastly, as major suppression of inflammation and the immune system is not practical in older adults, identifying interventions to ameliorate arterial specific inflammation represents a potentially fruitful area for intervention to prevent and treat CVD in the elderly.
New Findings.
What is the topic of this review?
This review summarizes and synthesizes what is known about the contribution of inflammation to age-related arterial dysfunction.
What advances does it highlight?
This review details observational evidence for the relationship of age-related inflammation and arterial dysfunction, insight from autoimmune inflammatory diseases and their effects on arterial function, interventional evidence linking inflammation and age-related arterial dysfunction, insight into age-related arterial inflammation from preclinical models and interventions to ameliorate age-related inflammation and arterial dysfunction.
Acknowledgements
The authors are grateful to Dr. Jody Greaney for helpful feedback.
Funding
NIH K01AG061271-01 (DWT, PI), NIH R01 HL127071-01A1 (PJF, PI) and R15HL130906-01 (PJF, Co-PI).
Footnotes
Competing Interests
None.
References:
- Ahmed SMU, Luo L, Namani A, Wang XJ & Tang X, (2017). Nrf2 signaling pathway: Pivotal roles in inflammation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1863, 585–597. [DOI] [PubMed] [Google Scholar]
- Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK & Atar D, (2010). Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension 55, 333–338. [DOI] [PubMed] [Google Scholar]
- Annoni G, Annoni F, Arosio B, et al. , (2009). Asymptomatic carotid plaque and pro-inflammatory genetic profile in the elderly. Aging Clin Exp Res 21, 431–436. [DOI] [PubMed] [Google Scholar]
- Askling J & Dixon W, (2011). Influence of biological agents on cardiovascular disease in rheumatoid arthritis. Annals of the Rheumatic Diseases 70, 561–562. [DOI] [PubMed] [Google Scholar]
- Bailey-Downs LC, Tucsek Z, Toth P, et al. , (2013). Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J.Gerontol.A Biol.Sci.Med.Sci. 68, 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JN, Nualnim N, Sugawara J, Sommerlad SM, Renzi CP & Tanaka H, (2011). Arterial stiffening, wave reflection, and inflammation in habitually exercising systemic lupus erythematosus patients. Am.J.Hypertens. 24, 1194–1200. [DOI] [PubMed] [Google Scholar]
- Battson ML, Lee DM, Jarrell DK, et al. , (2018). Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction. Am. J. Physiol. Endocrinol. Metab 314, E477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmin J, Bernard C, Corman B, Merval R, Esposito B & Tedgui A, (1995). Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. American Journal of Physiology-Heart and Circulatory Physiology 268, H2293. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS & Gimbrone MA, (1985). Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am. J. Pathol 121, 394–403. [PMC free article] [PubMed] [Google Scholar]
- Bonfante HdL, Almeida CdS, Abramo C, Grunewald STF, Levy RA & Teixeira HC, (2017). CCL2, CXCL8, CXCL9 and CXCL10 serum levels increase with age but are not altered by treatment with hydroxychloroquine in patients with osteoarthritis of the knees. Int J Rheum Dis 20, 1958–1964. [DOI] [PubMed] [Google Scholar]
- Brunt VE, Gioscia‐Ryan RA, Richey JJ, et al. , (2019). Suppression of the gut microbiome ameliorates age‐related arterial dysfunction and oxidative stress in mice. The Journal of Physiology 597, 2361–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Al Mamun A, Spiegel G, Harris N, Zhu L & McCullough LD, (2018). Splenectomy protects aged mice from injury after experimental stroke. Neurobiol.Aging 61, 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y, Scuoppo C, Wang X, et al. , (2011). Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 25, 2125–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JP & Effros RB, (2013). T cell replicative senescence in human aging. Curr. Pharm. Des 19, 1680–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, Harris T, Rao KM & Currie MS, (1997). The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J. Gerontol. A Biol. Sci. Med. Sci 52, 201. [DOI] [PubMed] [Google Scholar]
- Coppé J, Desprez P, Krtolica A & Campisi J, (2010). The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, et al. , (2009). Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mechanisms of ageing and development 130, 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE & Ungvari Z, (2012). Age-Associated Proinflammatory Secretory Phenotype in Vascular Smooth Muscle Cells From the Non-human Primate Macaca mulatta: Reversal by Resveratrol Treatment. J Gerontol A Biol Sci Med Sci 67, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG & Kaley G, (2003). Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 17, 1183–1185. [DOI] [PubMed] [Google Scholar]
- de Picciotto NE, Gano LB, Johnson LC, et al. , (2016). Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rincón ID, Williams K, Stern MP, Freeman GL & Escalante A, (2001). High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 44, 2737–2745. [DOI] [PubMed] [Google Scholar]
- Diehl KJ, Weil BR, Greiner JJ, Stauffer BL & Desouza CA, (2012). White blood cell count and endothelin-1 vasoconstrictor tone in middle-aged and older adults. Artery Res. 6, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB & Seals DR, (2008). Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, et al. , (2007). Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ. Res 100, 1659–1666. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Machin DR & Lesniewski LA, (2018). Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res 123, 825–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, Magerko KA, et al. , (2013). Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12, 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Wong C, Song Y, et al. , (2016). Age‐associated vascular inflammation promotes monocytosis during atherogenesis. Aging Cell 15, 766–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal NA, Upton J, Phillips AC, Sapey E & Lord JM, (2013). An age-related numerical and functional deficit in CD19(+) CD24(hi) CD38(hi) B cells is associated with an increase in systemic autoimmunity. Aging Cell 12, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LM, Cahill-Smith S, Geng L, Du J, Brooks G & Li J, (2017). Aging-associated metabolic disorder induces Nox2 activation and oxidative damage of endothelial function. Free Radic. Biol. Med 108, 940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson FG, Wikby A, Maxson P, Olsson J & Johansson B, (1995). Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J. Gerontol. A Biol. Sci. Med. Sci 50, 378. [DOI] [PubMed] [Google Scholar]
- Ferrucci L & Fabbri E, (2018). Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15, 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S & Zeiher AM, (2000). Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation 102, 1000–1006. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, Seals DR, Zigler ML & Sindler AL, (2012). Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 11, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Eng JS, Sindler AL, Pham BT, Kloor JD & Seals DR, (2014). Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell 13, 576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA & Seals DR, (2010). Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J. Physiol. (Lond.) 588, 3971–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, et al. , (2000). Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci 908, 244–254. [DOI] [PubMed] [Google Scholar]
- Freitas WM, Quaglia LA, Santos SN, et al. , (2011). Association of systemic inflammatory activity with coronary and carotid atherosclerosis in the very elderly. Atherosclerosis 216, 212–216. [DOI] [PubMed] [Google Scholar]
- Fulop GA, Kiss T, Tarantini S, et al. , (2018). Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience 40, 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano LB, Donato AJ, Pasha HM, Hearon CM, Sindler AL & Seals DR, (2014). The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol 307, H1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard M, Roddy MA, Creager SJ & Creager MA, (1996). Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27, 849–853. [DOI] [PubMed] [Google Scholar]
- Gerli R, Monti D, Bistoni O, et al. , (2001). Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mechanisms of Ageing and Development 121, 37–46. [DOI] [PubMed] [Google Scholar]
- Gerli R, Schillaci G, Giordano A, et al. , (2004). CD4+CD28- T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation 109, 2744–2748. [DOI] [PubMed] [Google Scholar]
- Giacconi R, Cipriano C, Albanese F, et al. , (2004). The −174G/C polymorphism of IL-6 is useful to screen old subjects at risk for atherosclerosis or to reach successful ageing. Experimental Gerontology 39, 621–628. [DOI] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP & Seals DR, (2018). Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J.Appl.Physiol.(1985) 124, 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP & Seals DR, (2014). Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol. (Lond.) 592, 2549–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grufman H, Schiopu A, Edsfeldt A, et al. , (2014). Evidence for altered inflammatory and repair responses in symptomatic carotid plaques from elderly patients. Atherosclerosis 237, 177–182. [DOI] [PubMed] [Google Scholar]
- Hein TW, Singh U, Vasquez-Vivar J, Devaraj S, Kuo L & Jialal I, (2009). Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis 206, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani AD, Cross J, Kharbanda RK, et al. , (2000). Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation 102, 994–999. [DOI] [PubMed] [Google Scholar]
- Hsue PY, Scherzer R, Grunfeld C, et al. , (2014). Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J Am Heart Assoc 3, e001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski KL, Donato AJ, Fleenor BS, et al. , (2015). Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor κ B signalling. Journal of Hypertension 33, 2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger A, Shimojima Y, Goronzy JJ & Weyand CM, (2014). 4878402; Regulatory T cells and the immune aging process: a mini-review. Gerontology 60, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly L, Mandry D, Verger A, et al. , (2016). Influence of Thoracic Aortic Inflammation and Calcifications on Arterial Stiffness and Cardiac Function in Older Subjects. J.Nutr.Health Aging 20, 347–354. [DOI] [PubMed] [Google Scholar]
- Kaplon RE, Walker AE & Seals DR, (2011). Plasma norepinephrine is an independent predictor of vascular endothelial function with aging in healthy women. Journal of Applied Physiology 111, 1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandouzi N, Zahedmehr A, Mohammadzadeh A, Sanati HR & Nasrollahzadeh J, (2019). Effect of flaxseed consumption on flow-mediated dilation and inflammatory biomarkers in patients with coronary artery disease: a randomized controlled trial. Eur J Clin Nutr 73, 258–265. [DOI] [PubMed] [Google Scholar]
- Kinzenbaw DA, Chu Y, Pena Silva RA, Didion SP & Faraci FM, (2013). Interleukin-10 protects against aging-induced endothelial dysfunction. Physiol.Rep 1, e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SJ, Kim JY, Hyun YJ, et al. , (2009). Association of serum RANTES concentrations with established cardiovascular risk markers in middle-aged subjects. Int. J. Cardiol 132, 102–108. [DOI] [PubMed] [Google Scholar]
- Kolb-Bachofen V, (1991). A Review on the Biological Properties of C-Reactive Protein. Immunobiology 183, 133–145. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Egashira K, Kubo-Inoue M, et al. , (2000). Role of transforming growth factor-beta1 in cardiovascular inflammatory changes induced by chronic inhibition of nitric oxide synthesis. Hypertension 35, 86–90. [DOI] [PubMed] [Google Scholar]
- Krug AW, Allenhöfer L, Monticone R, et al. , (2010). Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension 55, 1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG & Levy D, (2003). Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107, 139–146. [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL & Seals DR, (2012). Translational evidence that impaired autophagy contributes to arterial ageing. J.Physiol. 590, 3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Bice A, Hood B, Ruiz J, Kim J & Prisby RD, (2018). Intermittent PTH 1–34 administration improves the marrow microenvironment and endothelium-dependent vasodilation in bone arteries of aged rats. J.Appl.Physiol.(1985) 124, 1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ & Seals DR, (2011). Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J. Gerontol. A Biol. Sci. Med. Sci 66, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, et al. , (2011). Aerobic exercise reverses arterial inflammation with aging in mice. Am. J. Physiol. Heart Circ. Physiol 301, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L, Siegbahn A, Hulthe J & Elmgren A, (2008). C-reactive protein and e-selectin levels are related to vasodilation in resistance, but not conductance arteries in the elderly: the prospective investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis 199, 129–137. [DOI] [PubMed] [Google Scholar]
- Lozhkin A, Vendrov AE, Pan H, Wickline SA, Madamanchi NR & Runge MS, (2017). NADPH oxidase 4 regulates vascular inflammation in aging and atherosclerosis. J.Mol.Cell.Cardiol. 102, 10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Hillard Kaplan, Jeffrey Winking, et al. , (2009). Inflammation and Infection Do Not Promote Arterial Aging and Cardiovascular Disease Risk Factors among Lean Horticulturalists. PLoS One 4, e6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Silva W, Henriques AD, Souza GD, et al. , (2016). Serum Immune Mediators Independently Associate with Atherosclerosis in the Left (But Not Right) Carotid Territory of Older Individuals. J.Stroke Cerebrovasc Dis. 25, 2851–2858. [DOI] [PubMed] [Google Scholar]
- Mäki-Petäjä KM, Elkhawad M, Cheriyan J, et al. , (2012). Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 126, 2473–2480. [DOI] [PubMed] [Google Scholar]
- Marvar PJ, Thabet SR, Guzik TJ, et al. , (2010). 2921936; Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ.Res. 107, 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu S, Couderc M, Glace B, et al. , (2013). Effects of 6 months of abatacept treatment on aortic stiffness in patients with rheumatoid arthritis. Biologics : targets & therapy 7, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace-Raso FUS, van der Cammen Tischa J. M., van der Meer Irene M., et al. , (2004). C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis 176, 111–116. [DOI] [PubMed] [Google Scholar]
- Mayr M, Kiechl S, Mendall MA, Willeit J, Wick G & Xu Q, (2003). Increased risk of atherosclerosis is confined to CagA-positive Helicobacter pylori strains: prospective results from the Bruneck study. Stroke 34, 610–615. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Thompson L, Giles JT, et al. , (2015). Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann. Rheum. Dis 74, 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, (2010). Inflammation 2010: New Adventures of an Old Flame. Cell 140, 771–776. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Hwang S, Vasan RS, et al. , (2010). Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, Deane KD, Meditz AL & Kohrt WM, (2013). Tumor necrosis factor-alpha inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 230, 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RG, Ives SJ, Lesniewski LA, et al. , (2013). Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am. J. Physiol. Heart Circ. Physiol 305, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadareishvili ZG, Li H, Wright V, et al. , (2004). Elevated pro-inflammatory CD4+CD28- lymphocytes and stroke recurrence and death. Neurology 63, 1446–1451. [DOI] [PubMed] [Google Scholar]
- Neale EP, Tapsell LC, Guan V & Batterham MJ, (2017). The effect of nut consumption on markers of inflammation and endothelial function: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 7, e016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves KB, Nguyen Dinh Cat A, Lopes RAM, et al. , (2015). Chemerin Regulates Crosstalk Between Adipocytes and Vascular Cells Through Nox. Hypertension 66, 657–666. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ & Mitchell BM, (2013). Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc. Res 97, 696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novella S, Heras M, Hermenegildo C & Dantas AP, (2012). Effects of estrogen on vascular inflammation: a matter of timing. Arterioscler. Thromb. Vasc. Biol 32, 2035–2042. [DOI] [PubMed] [Google Scholar]
- Onishi RM & Gaffen SL, (2010). Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana MR, Bentourkia M, Sarrhini O, et al. , (2013). Assessment of inflammation in large arteries with 18F-FDG-PET in elderly. Comput.Med.Imaging Graph. 37, 459–465. [DOI] [PubMed] [Google Scholar]
- Ortega‐Gómez A, Perretti M & Soehnlein O, (2013). Resolution of inflammation: an integrated view. EMBO Molecular Medicine 5, 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DB, (2013). The effect of age on thymic function. Front Immunol 4, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang WW, Price EA, Sahoo D, et al. , (2011). Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. U.S.A. 108, 20012–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J & Wikby A, (2009). Cytomegalovirus and human immunosenescence. Rev. Med. Virol 19, 47–56. [DOI] [PubMed] [Google Scholar]
- Pepys MB & Hirschfield GM, (2003). C-reactive protein: a critical update. The Journal of clinical investigation 111, 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MJ, Joehanes R, Pilling LC, et al. , (2015). The transcriptional landscape of age in human peripheral blood. Nat Commun 6, 8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Lesniewski LA, Lawson BR, Beske SD & Seals DR, (2009). Nuclear factor-{kappa}B activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 119, 1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybylska D, Janiszewska D, Goździk A, et al. , (2016). NOX4 downregulation leads to senescence of human vascular smooth muscle cells. Oncotarget 7, 66429–66443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglia LA, Freitas W, Soares AA, et al. , (2014). C-reactive protein is independently associated with coronary atherosclerosis burden among octogenarians. Aging Clin.Exp.Res. 26, 19–23. [DOI] [PubMed] [Google Scholar]
- Ras R, Streppel M & Draijer R, (2012). Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. International Journal of Cardiology 168, 344–351. [DOI] [PubMed] [Google Scholar]
- Raubenheimer K, Hickey D, Leveritt M, et al. , (2017). Acute Effects of Nitrate-Rich Beetroot Juice on Blood Pressure, Hemostasis and Vascular Inflammation Markers in Healthy Older Adults: A Randomized, Placebo-Controlled Crossover Study. Nutrients 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP & Hennekens CH, (1997). Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med 336, 973–979. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FAH, et al. , (2008). Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med 359, 2195–2207. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, et al. , (2017). Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med 377, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Ritter L, Funk J, Schenkel L, et al. , (2008). Inflammatory and hemodynamic changes in the cerebral microcirculation of aged rats after global cerebral ischemia and reperfusion. Microcirculation 15, 297–310. [DOI] [PubMed] [Google Scholar]
- Ritzel RM, Crapser J, Patel AR, et al. , (2016). Age-Associated Resident Memory CD8 T Cells in the Central Nervous System Are Primed To Potentiate Inflammation after Ischemic Brain Injury. J.Immunol. 196, 3318–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Lai YJ, Crapser JD, et al. , (2018). Aging alters the immunological response to ischemic stroke. Acta Neuropathol. 136, 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB & Aiello AE, (2010). Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am. J. Epidemiol 172, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Mañas L, El-Assar M, Vallejo S, et al. , (2009). Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 8, 226–238. [DOI] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, et al. , (2016). Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, (1999). Atherosclerosis--an inflammatory disease. N. Engl. J. Med 340, 115–126. [DOI] [PubMed] [Google Scholar]
- Rossman MJ, Kaplon RE, Hill SD, et al. , (2017). Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am. J. Physiol. Heart Circ. Physiol 313, H895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman MJ, Santos-Parker JR, Steward CAC, et al. , (2018). Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension 71, 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A & Kaarniranta K, (2012). Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell. Signal 24, 835–845. [DOI] [PubMed] [Google Scholar]
- Santos-Parker JR, Strahler TR, Vorwald VM, Pierce GL & Seals DR, (2017). Habitual aerobic exercise does not protect against micro- or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J.Appl.Physiol.(1985) 122, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB & Seals DR, (2017). Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 9, 187–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel R, Larson MG, Dupuis J, et al. , (2008). Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension 51, 1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R, Albermann K & Baeuerle PA, (1992). Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic. Res. Commun 17, 221–237. [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T & Hume DA, (2004). Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol 75, 163–189. [DOI] [PubMed] [Google Scholar]
- Seals DR, (2014). Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J. Appl. Physiol 117, 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AC, Goldstein DR & Montgomery RR, (2013). Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol 13, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR & Lokshin AE, (2007). Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine 39, 123–129. [DOI] [PubMed] [Google Scholar]
- Sikka G, Miller KL, Steppan J, et al. , (2013). Interleukin 10 knockout frail mice develop cardiac and vascular dysfunction with increased age. Exp.Gerontol. 48, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, et al. , (2011). Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10, 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Shen H, Schenten D, Shan P, Lee PJ & Goldstein DR, (2012). Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol 32, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyridopoulos I, Martin-Ruiz C, Hilkens C, et al. , (2016). 4783336; CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell 15, 389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes KY, Gurwara S & Granger DN, (2007). T-cell derived interferon-gamma contributes to arteriolar dysfunction during acute hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol 27, 1998–2004. [DOI] [PubMed] [Google Scholar]
- Tang WHW, Wang Z, Levison BS, et al. , (2013). Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. The New England Journal of Medicine 368, 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchalla AE, Wellenius GA, Travison TG, et al. , (2015). Circulating vascular cell adhesion molecule-1 is associated with cerebral blood flow dysregulation, mobility impairment, and falls in older adults. Hypertension 66, 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Nicolakakis N, Fernandes P, et al. , (1994). Simvastatin improves cerebrovascular function and counters soluble amyloid-beta, inflammation and oxidative stress in aged APP mice. Neurobiology of disease 35, 406–414. [DOI] [PubMed] [Google Scholar]
- Trott DW, Henson GD, Ho MHT, Allison SA, Lesniewski LA & Donato AJ, (2018). Age-related arterial immune cell infiltration in mice is attenuated by caloric restriction or voluntary exercise. Exp.Gerontol. 109, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Lesniewski LA & Donato AJ, (2017). Selected life-extending interventions reduce arterial CXCL10 and macrophage colony-stimulating factor in aged mouse arteries. Cytokine 96, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowska D, et al. , (2014). Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J.Gerontol.A Biol.Sci.Med.Sci. 69, 1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Gautam T, et al. , (2011). Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J.Gerontol.A Biol.Sci.Med.Sci. 66, 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Labinskyy N, et al. , (2007). Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am. J. Physiol. Heart Circ. Physiol 293, 37. [DOI] [PubMed] [Google Scholar]
- Vita JA, Keaney JF, Larson MG, et al. , (2004). Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation 110, 3604–3609. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K & Stefanadis C, (2010). Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol 55, 1318–1327. [DOI] [PubMed] [Google Scholar]
- Walker AE, Seibert SM, Donato AJ, Pierce GL & Seals DR, (2010). Vascular endothelial function is related to white blood cell count and myeloperoxidase among healthy middle-aged and older adults. Hypertension 55, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Kim SH, Monticone RE & Lakatta EG, (2015). Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 65, 698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Spinetti G, Monticone RE, et al. , (2011). A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PLoS ONE 6, e16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang J, Jiang L, et al. , (2007). Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50, 219–227. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Stumpf M, Strehlow K, et al. , (2004). Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res 94, 534–541. [DOI] [PubMed] [Google Scholar]
- Watson C, Whittaker S, Smith N, Vora AJ, Dumonde DC & Brown KA, (1996). IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin. Exp. Immunol 105, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand CM, Liao YJ & Goronzy JJ, (2012). The immunopathology of giant cell arteritis: diagnostic and therapeutic implications. J.Neuroophthalmol 32, 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand CM, Yang Z & Goronzy JJ, (2014). T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol 26, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF,J & Vita JA, (2003). The clinical implications of endothelial dysfunction. J.Am.Coll.Cardiol. 42, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Willebrords J, Crespo Yanguas S, Maes M, et al. , (2016). Connexins and their channels in inflammation. Crit. Rev. Biochem. Mol. Biol 51, 413–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Saleh MA, Kirabo A, et al. , (2016). Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J. Clin. Invest 126, 50–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu F, Burke GL & Herrington DM, (2007). Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115, 2390–2397. [DOI] [PubMed] [Google Scholar]
- Yepuri G, Velagapudi S, Xiong Y, et al. , (2012). Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell. 11, 1005–1016. [DOI] [PubMed] [Google Scholar]
- Youn J, Yu HT, Lim BJ, et al. , (2013). Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62, 126–133. [DOI] [PubMed] [Google Scholar]
- Yu HT, Youn J, Kim JH, et al. , (2017). Arterial Stiffness Is Associated With Cytomegalovirus-Specific Senescent CD8+ T Cells. J Am Heart Assoc 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoli L, Cannavo M, Rastelli S, et al. , (2012). Arterial stiffness is increased in patients with inflammatory bowel disease. J.Hypertens. 30, 1775–1781. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W, Ren Y, Shipley RD & Kuo L, (2006). Activation of JNK and xanthine oxidase by TNF-alpha impairs nitric oxide-mediated dilation of coronary arterioles. J. Mol. Cell. Cardiol 40, 247–257. [DOI] [PubMed] [Google Scholar]
- Zinovkin RA, Romaschenko VP, Galkin II, et al. , (2014). Role of mitochondrial reactive oxygen species in age-related inflammatory activation of endothelium. Aging (Albany NY) 6, 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]