Abstract
Objective:
Failure of antipsychotic medications to resolve symptoms in schizophrenia patients creates a clinical challenge that is known as “treatment resistance”. Its causes are unknown, but it is associated with an earlier age of onset and more severe cognitive deficits. This study tested the hypothesis that white matter deficits, that are involved in both neurodevelopment and severity of cognitive deficits in schizophrenia, are associated with a higher risk for treatment resistance.
Method:
The sample included schizophrenia patients (N=122, age=38.2±13.3, N=45/40/37 of treatment initiation, treatment-responsive, and treatment-resistant patients, respectively) and healthy controls (N=78, age=39.2±14.0). We tested white matter “regional vulnerability index” (RVI) as a predictor of treatment resistance and cognitive deficits. Higher RVI is indicative of a better agreement between diffusion tensor imaging (DTI) fractional anisotropy (FA) across the brain in an individual and the pattern identified by the largest-to-date meta-analysis of white matter deficits in schizophrenia.
Results:
Treatment-resistant patients showed the highest white matter RVI (0.38±0.2), which was significantly higher than the treatment-responsive patients (0.30±0.02, p=0.01). At the onset of treatment, patients showed significantly higher RVI than normal controls (0.18±0.03 and 0.13±0.02, respectively, p=0.01). RVIs were significantly correlated with performance on processing speed and negative symptoms.
Conclusions:
Schizophrenia affects white matter microstructure in specific regional patterns. Susceptibility to white matter regional deficits is associated with an increased likelihood of treatment resistance. Developments to overcome schizophrenia treatment resistance should consider white matter as one of the important targets.
Introduction
Modern antipsychotic pharmaceuticals fail to resolve clinical symptoms in approximately a third of people with schizophrenia, a condition known as “treatment resistance” (1). This clinical challenge has motivated attempts to develop more effective, next-generation antipsychotics for several decades (2-6). However, research has been hindered by insufficient understanding of the neurobiological mechanisms and by a lack of valid brain biomarkers for treatment resistance. Current antipsychotic drugs share their neurotransmitter targets in dopaminergic systems. Their ineffectiveness in treatment-resistant patients suggests other mechanisms and neurotransmitter systems although the evidence is still preliminary (7-10). Some structural neuroimaging studies suggest that treatment resistance is associated with reduced gray matter and cortical thickness (7, 11, 12). However, a recent meta-analysis found no replicated neuroimaging findings when comparing treatment-resistant and treatment-responsive patients (13).
Patients with treatment-resistant schizophrenia share two consistent features: earlier age of disease onset and more severe cognitive deficits (14-17); this suggests both neurodevelopmental and cognitive contributions to treatment resistance. Therefore, brain measures that track with neurodevelopment and cognitive dysfunctions in schizophrenia may help to identify treatment resistance biomarkers. White matter shows promise as a research focus in schizophrenia and the largest meta-analytic study of white matter deficits in schizophrenia conducted by the Enhancing Neuro Imaging Genetics Meta-Analysis (ENIGMA) consortium determined a region-specific pattern of white matter deficits (18). The ENIGMA pattern of regional differences confirmed findings from previous DTI imaging studies that demonstrated significant deficits in the frontal associative white matter regions, including the anterior corona radiate and the genu and body of the corpus callosum (19-23), as well as with histological findings of reduced glial cell density and myelination in the frontal lobe of schizophrenia patients (24-26). This ENIGMA regional white matter deficit pattern contributes to core cognitive dysfunctions, especially in processing speed deficits in schizophrenia (27-30). Interestingly, among all the cognitive abnormalities associated with treatment-resistance, processing speed shows the largest deficit (16). Both white matter and processing speed development follow an inverse-U neurodevelopmental trajectory and the peak of myelination for associative white matter overlaps with the peak of processing speed abilities (31, 32). The origin of the white matter deficit pattern in schizophrenia is not fully understood, but the disorder by development interaction that prevents normal development of the late-myelinating areas likely prevents the establishment of the normal pattern of inter-neuronal communication (33). This may lead to the observed contrast in regional deficits between late and early myelinating white matter regions in this disorder, as observed by ENIGMA and other studies (18, 34, 35). Therefore, we hypothesized that the regional white matter deficit pattern may represent a neurobiological mechanism leading to treatment resistance in schizophrenia patients. We tested this hypothesis by assessing if white matter regional deficits index treatment resistance by comparing treatment-resistant and treatment-responsive patients. In parallel, we compared regional deficits in patients who were imaged within two weeks of the initiation of antipsychotic treatment with controls. We used this comparison to control for potential chronic antipsychotic medication effects on white matter deficits and to determine whether the identified white matter deficits were also associated with schizophrenia, independent of chronic disease and treatment courses. Evidence supporting this hypothesis would imply white matter as a contributing factor of treatment resistance.
Methods
Clinical characteristics
The study was performed in 200 participants that included 122 patients (57M/65F, average age=38.2±13.3) and 78 healthy controls (37M/41F, average age=39.2±14.0) (Table 1). Patients included treatment-resistant schizophrenia (N=17M/20F, average age=47.8±8.9) and the primary comparison group of treatment-responsive schizophrenia (N=18M/22F, average age=46.3±11.5) groups, who were frequency-matched on age and sex (all p>0.2). A group of schizophrenia patients within the first two weeks of treatment initiation (N=22M/23F, average age=28.6±10.1) was recruited to determine whether any identified white matter deficits were also associated with schizophrenia independent of chronic disease and treatment courses. This group was necessarily younger, however, the combined patients and controls were frequency-matched for age and sex distribution (all p>0.2). Data were collected between 2017-2018. The patients were recruited from Beijing Huilongguan Hospital. Controls were recruited through local advertisement. All patients met DSM-IV criteria for schizophrenia. Participants had a homogeneous Chinese background, which is considered advantageous for identifying treatment resistance biomarkers because different ethnicities may have significant effects on treatment resistance(36). All participants provided written informed consent according to the Helsinki Declaration. The research protocol was approved by local Ethics Committees.
Table 1.
Subject demographics for three patient groups and controls. Values are mean ± SD. PANSS: The Positive and Negative Syndrome Scale
| Controls | Treatment Initiation Patients |
Treatment Responsive Patients |
Treatment Resistant Patients |
Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Four Groups | Treatment Resistant vs. Responsive |
|||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | t-score | p | |
| Age (years) | 39.2 | 13.4 | 28.6 | 10.1 | 46.3 | 11.5 | 47.8 | 8.9 | 17.1 | 10−10 | 0.6 | 0.5 |
| PANSS (total) | N/A | N/A | 74.7 | 13.0 | 49.6 | 12.2 | 69.0 | 19.5 | n/a | n/a | 11.8 | 10−19 |
| Illness Duration (years) | N/A | N/A | 2.5 | 1.7 | 23.4 | 5.0 | 22.9 | 6.3 | n/a | n/a | 0.4 | 0.7 |
| Education (years) | 13.0 | 2.9 | 12.7 | 3.5 | 12.3 | 2.9 | 12.0 | 3.0 | 2.6 | 0.11 | 0.5 | 0.6 |
| Medication Dose (CPZ) | N/A | N/A | 176.4 | 117 | 404 | 345 | 781 | 354 | n/a | n/a | 4.1 | 10−5 |
| Current Smokers | 35% | 29% | 27% | 30% | 2.2 | 0.15 | 0.7 | 0.5 | ||||
The treatment-resistant and treatment-responsive groups were defined based on consensus guidelines(1). Treatment-resistant patients met criteria of little response to treatment with at least 2 different antipsychotic medications with a dose equivalence of chlorpromazine (CPZ) ≥600 mg/day for ≥12 weeks; the Brief Psychiatric Rating Scale (BPRS) score ≥45; and the Clinical Global Impression-severity of illness (CGI-SI) scale score ≥4 during the current assessment. The treatment-responsive group was defined by periods of good clinical response to antipsychotics as measured by CGI-SI score <3 over the duration ≥12 weeks. The two groups were frequency-matched on age, sex, years of education, and duration of treatment. Patients who did not meet either criterion were excluded.
The treatment initiation group had no prior antipsychotic medication exposure until enrollment and were included to identify whether treatment resistance biomarkers, if found, were present at the onset of the disorder with minimal antipsychotics exposure. Patients were treated without delay upon admission; and treatment helped to stabilize patients for undergoing the MRI. Imaging data were collected within two-weeks of treatment initiation. All participants had no current or past neurological conditions, unstable major medical conditions, or current or previous substance (except nicotine) dependence. Demographic information is presented in Table 1.
Six patients were medication-free (n=4/2/0 for treatment-initiation, treatment-responsive, and treatment-resistant groups, respectively; same below). Seven (4/3/0) patients were on first generation antipsychotics. The remaining patients were on the following second-generation antipsychotics: risperidone (35: 17/11/7); clozapine (31: 0/9/22); olanzapine (27: 9/11/7); aripiprazole (15: 8/5/2); paliperidone (5: 2/0/3); amisulpride, iloperisone, lurazidone, or quetiapine (9: 1/4/4); and 45 (4, 7, 34) of them were also on more than one antipsychotic medications. CPZ was calculated(37) and treatment-resistant patients had nearly twice the CPZ of treatment-responsive patients (p<0.001) (Table 1). Treatment-initiation patients were on a much smaller dose and were medicated for an average of 4.2±2.3 days (range: 0 to 12 days).
Symptom and neurocognitive evaluations
Patients were evaluated using the Positive and Negative Syndrome Scale (PANSS), BPRS and CGI-SI by one of three attending psychiatrists who maintained inter-rater reliability above 0.80. BPRS and CGI-SI were used for group-definition only; PANSS was used for symptoms assessment. Cognitive function was assessed with the MATRICS Consensus Cognitive Battery (MCCB) covering 7 cognitive domains and a composite score(38-40). The eight raw scores were converted to Chinese normative T-scores(40).
Diffusion tensor imaging and data processing
Imaging data was collected using a 3 T Prisma MRI scanner (Erlangen, Germany) at the Imaging Research Center of the Beijing Huilongguan Hospital, equipped with a 64-channel RF head coil. DTI data was collected using a spin-echo, EPI sequence with a spatial resolution of 17×1.7×1.7mm. The sequence parameters were: TE/TR=87/8000 ms, FOV=200 mm, axial slice orientation with 82 slices and no gaps, 98 isotropically distributed diffusion-weighted directions, two diffusion weighting values (b=0 and 1000 s/mm2) and five b=0 images. Subjects’ head movement was minimized with restraining padding. DTI data was processed using the ENIGMA-DTI analysis pipeline (https://www.nitrc.org/projects/enigma_dti (41). All data included in the analysis passed the ENIGMA-DTI QA/QC. Regional white matter fractional anisotropy (FA) were generated for twenty-one major regions based on ENIGMA-DTI atlas, averaged across hemispheres. These regions are listed in Figure 1 legends.
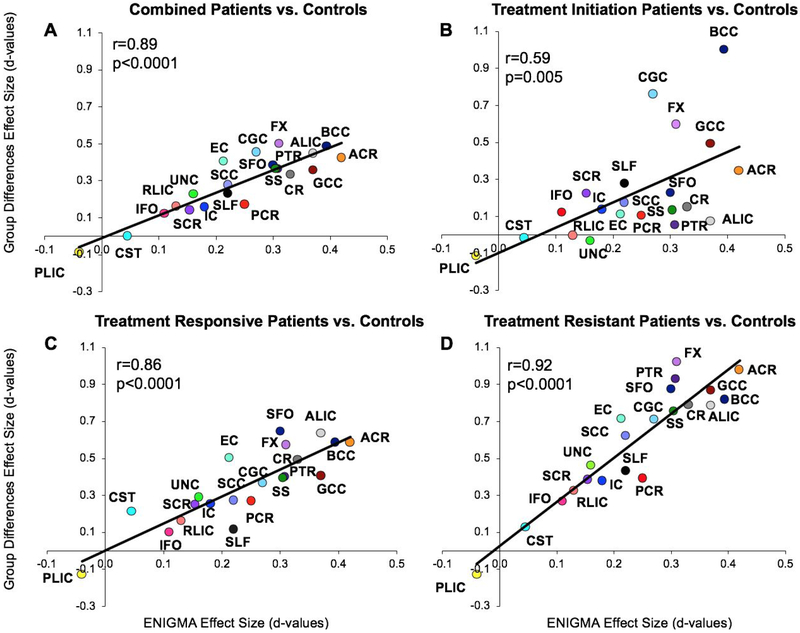
Figure 1.
The mean effect size of group (Cohen’s d) of the twenty-one white matter regions were plotted against the effects sizes of the ENIGMA region for all patients (A) and three subgroups (B-D). The significant correlations indicate that the pattern of FA deficits in the Chinese sample were consistent with the ENIGMA pattern. The data (mean and s.d.) of all the effect sizes are in Supplementary Table 1. Abbreviations: Anterior Corona Radiata (ACR), Anterior Limb of Internal Capsule (ALIC), Body of Corpus Callosum (BCC), Cingulum (CGC), Corona Radiata (CR), Cortico-Spinal Tract (CST), External Capsule (EC), Fornix (FX), Genu of Corpus Callosum (GCC), Internal Capsule (IC), Inferior Frontal Occipital fasciculus (IFO), Posterior Corona Radiata (PCR), Posterior Limb of Internal Capsule (PLIC), Posterior Thalamic Radiation (PTR), Retrolenticular Limb of the Internal Capsule (RLIC), Splenium of Corpus Callosum (SCC), Superior Corona Radiata (SCR), Superior Fronto-Occipital Fasciculus (SFO), Superior Longitudinal Fasciculus (SLF), Sagittal Striatum (SS), Uncinate Fasciculus (UNC).
This cohort is independent from the samples used in ENIGMA and our prior study (18, 27). As such, an ancillary aim was to use the sample to replicate the association between the ENIGMA regional deficit pattern and cognitive deficits in schizophrenia(27).
Statistics
The ENIGMA study provided the meta-analysis based ranks of the severity of impairment associated with schizophrenia in 21 major white matter regions as measured by region-specific effect sizes in Cohen’s d statistics (Supplemental Table 1). We developed an index of regional impairment at the individual level, the regional vulnerability index (RVI) as a simple measure of agreement between an individual’s pattern of FA in these 21 regions and the expected pattern of schizophrenia across these 21 regions as shown in ENIGMA. FA for each of the white matter regions was converted to z values by (1) calculating the residual values following the regression of effects of age and sex and (2) for each individual, we subtracted the average value for a region and divided it by the standard deviation calculated from the healthy controls. This produced a vector of normalized z-values (one for each region) for every individual in the sample. The RVI was then calculated as the correlation coefficient (normalized dot product) between the vector of region-wise z values for the subject and the vector of regional schizophrenia-controls effect sizes in ENIGMA. We used the term “vulnerability” here to imply a narrow definition: whether the regional white matter deficit pattern identified by ENIGMA is associated with an increased likelihood for treatment resistance. Higher RVI values imply that the pattern of white matter regional values followed the regional vulnerability pattern for schizophrenia as determined by the ENIGMA meta-analysis. All group comparisons of imaging and cognitive measures were performed using the general linear model while controlling for age and sex. Bonferroni corrections were applied to correct for the number of regions examined. Associations of RVI and clinical measures were examined by bivariate correlation analyses while controlling for age and sex, and Bonferroni corrections were applied.
Results
Specific white matter region and treatment-resistance
Patients had significantly lower whole-brain average FA than controls (Cohen’s d=0.69, t=5.1, p=10−6), and the regional effect sizes were significant in 4 of 21 regions after correcting for multiple (n=21) comparisons (Supplementary Table 1). The average effect size in this sample was not significantly different from ENIGMA (t=1.2, p=0.2). The effect sizes of the regional patient-control differences were correlated with those in ENIGMA (r=0.85, p=10−5) (Figure 1A). This pattern was also observed when comparing the three patient groups individually to controls (Figure 1B-1D), with the strongest effect in the treatment-resistant group (r=0.92, p=10−8) (Figure 1B). Frontal associative tracts such as the anterior corona radiata and genu of the corpus callosum showed the largest schizophrenia-control effect sizes in ENIGMA, and they were also the regions that showed the largest FA reduction in the treatment-resistant group compared to controls (Figure 1B). However, there were no significant differences in the whole-brain average or regional FA measurements between treatment-resistant and treatment-responsive groups (Table 2) or between the treatment-initiation group and controls (Table 2).
Table 2.
MCCB cognitive domain and total scores, and associations with treatment stages and RVI. All analyses included age and sex as covariates.
| A: MCCB Associations with Treatment Status | B: MCCB Associations with RVI in Patients | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testing schizophrenia-related cognitive deficits |
Testing treatment-resistance related cognitive deficits |
|||||||||||||||||
| Treatment Initiation (N=45) |
Controls (N=78) |
Analysis | Treatment Resistant (N=37) |
Treatment Responsive (N=40) |
Analysis | Treatment Initiation (N=45) |
Treatment Responsive (N=40) |
Treatment Resistant (N=37) |
||||||||||
| Mean | SD | Mean | SD | t | p | Mean | SD | Mean | SD | t | p | r | p | r | p | r | p | |
| Visual Learning | 47.8 | 8.6 | 54.9 | 7.4 | 4.7 | 1·10−5** | 42.4 | 11.8 | 46.0 | 11.5 | 1.4 | 0.2 | 0.0 | 0.9 | −0.44 | 5·10−3* | −0.10 | 0.5 |
| Verbal Learning | 50.5 | 10.2 | 54.5 | 8.7 | 2.3 | 0.02 | 46.9 | 10.0 | 48.8 | 11.3 | 0.8 | 0.4 | 0.0 | 0.9 | −0.39 | 7·10−3* | −0.12 | 0.4 |
| Social Cognition | 48.4 | 9.3 | 53.1 | 10.0 | 2.4 | 0.02 | 42.1 | 12.4 | 46.3 | 10.5 | 1.6 | 0.1 | 0.0 | 0.9 | −0.08 | 0.6 | 0.0 | 0.9 |
| Reasoning and Problem Solving | 43.9 | 7.8 | 57.9 | 7.4 | 9.1, | 2·10−15** | 45.3 | 9.8 | 45.7 | 9.8 | 0.2 | 0.9 | −0.15 | 0.3 | −0.11 | 0.5 | −0.10 | 0.5 |
| Processing Speed | 47.3 | 7.3 | 57.2 | 7.9 | 6.3 | 6·10−9** | 43.8 | 11.9 | 45.3 | 9.9 | 1.0 | 0.3 | −0.10 | 0.4 | −0.53 | 7·10−4** | −0.16 | 0.3 |
| Working Memory | 47.0 | 9.8 | 47.0 | 6.9 | 7.1 | 1·10−10** | 39.3 | 14.1 | 46.8 | 10.7 | 2.9 | 0.004* | −0.05 | 0.6 | −0.24 | 0.12 | −0.12 | 0.4 |
| Total Score | 46.8 | 7.9 | 58.9 | 6.8 | 8.2 | 1·10−12** | 40.0 | 11.1 | 45.2 | 11.5 | 2.5 | 0.1 | 0.0 | 0.9 | −0.42 | 5·10−3* | −0.10 | 0.5 |
White matter regional vulnerability index (RVI) and treatment resistance
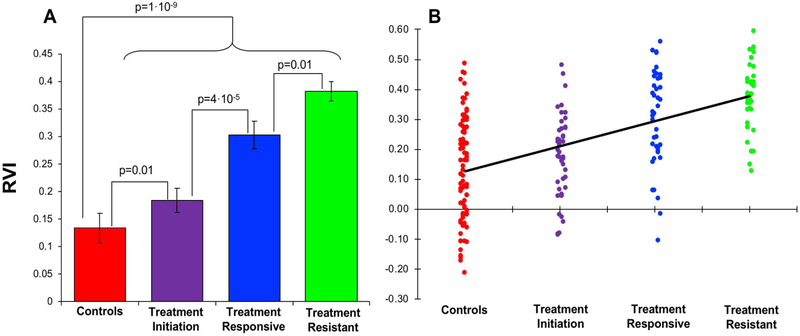
Average RVI was significantly higher in the combined patient group compared to the controls (0.29±0.01 vs 0.13±0.02, t=6.4, df=198, p=10’9). Treatment-resistant group showed the highest RVI (0.38±0.02), followed by treatment-responsive (0.30±0.03) and treatment-initiation patients (0.18±0.02), where all patient subgroups showed significant differences compared to the controls (0.13±0.02; all t>2.7, p ≤0.01) (Figure 2A).
Figure 2.
A: Average coefficients of regional vulnerability index (RVI) plotted by group. B: There was a significance ordinal trend of elevation of RVI with respect to group assignment (p<0.001).
The treatment-resistant group showed significantly higher RVI than the treatment-responsive group (t=2.7, df=75, p=0.01), suggesting that higher RVI was associated with treatment resistance. Treatment-initiation patients showed significantly higher RVI than controls (t=2.8, df=121, p=0.007), suggesting that higher RVI was unlikely to be primarily due to chronic disease or chronic antipsychotic medication exposure. Chronic patients (combined treatment-resistant and responsive groups) also showed significant higher RVI compared with the treatment-initiation group (t=4.2, df=153, p=4. 10−5), suggesting perhaps additional disease progression and/or chronic medication effect (Figure 2).
These relationships can be further illustrated by examining the ordinal trend of group RVI in the order of control, treatment initiation, treatment responsive, and treatment-resistant. We applied mid-ranks scoring to assign scores for the group variable and performed general linear regression analysis of the ordinal trend (42), and found that the trend was significant (F(1,198)=57.2, p<0.001) (Figure 2B).
Cognition and white matter regional vulnerability
The MCCB total score was most severely reduced in treatment-resistant (t=11.6, df=113, p=3.10−17), followed by treatment-responsive (t=8.4, df=116, p=7.10−13) and treatment initiation (t=8.2, df=121, p=2.10−12) patients compared to controls (Table 2A). Treatment-resistant patients showed no significant impairment compared to treatment-responsive patients in MCCB total or domain scores after Bonferroni correction; however, working memory was nominally significantly different between the two groups (Table 2A). There were significant differences in cognitive measures between treatment-initiation patients and controls (Table 2A). RVI was significantly associated with processing speed only for the treatment-responsive group (r=−0.53, p=7.10−4) after correcting for multiple comparisons (Table 1B). We also explored MCCB correlations with the whole brain average FA and 21 regional values. However, there were no significant regional FA – MCCB correlations in combined or individual patient groups after the correction for multiple comparisons, with the exception of significant correlations between whole brain average FA and the processing speed scores (r=0.31, r=5.10−4) in the combined patient sample after correcting for multiple comparisons.
Clinical correlates
There were no significant associations between RVIs and PANSS total, positive or general symptoms scores (all r<0.1, all p>0.2), but there was a significant correlation with negative symptom in the combined patient group (r=0.26, p=0.002). However, none of the regional FA values were significantly correlated with negative symptoms after Bonferroni correction (all r<0.27, all p>0.003). None was significant within each patient subgroup (all p>0.1). RVIs were not significantly correlated with illness duration (all p>0.20), CPZ (all p>0.41) or smoking status (all p>0.15) in any combined or individual patient groups. Over half of the treatment-resistant patients were on clozapine (n=22 vs. n=15 who were not), but RVI values were not different between patients on or not on clozapine (t=0.2, df=35, p=0.8).
Replications for the association between regional deficit pattern and processing speed
ENIGMA regional FA effect sizes were significantly correlated with regional FA-processing speed correlation coefficients (r=0.90, p<0.001) (Figure 3A) and regional FA-working memory (r=0.84, p<0.001) correlation coefficients (Figure 3C), replicating findings from another sample(27). When controlling for working memory the ENIGMA-based regional effect sizes remained significant in explaining regional FA-processing speed correlation coefficients (partial r=0.88, p<0.001) (Figure 3B) but the opposite was not significant (partial r=0.25, p=0.4; Figure 3D). Permutation analysis showed that the difference in the partial correlation coefficients was significant (p=0.01), suggesting the relationships between cognitions and white matter were driven primarily by processing speed rather than working memory. Similar trends were observed in patients (Figure 3E-H) and controls (Figure 3I-L), separately. The findings in this Chinese sample (Figure 3) largely replicate those found in the U.S. sample (Supplementary Figure 1).
Figure 3.
Replication of the relationship between cognition and white matter vulnerability to schizophrenia that were reported in Kochunov et al 2017 (for convenience to the readers, the figure of original findings in the U.S. sample is reproduced in Supplementary Figure 1). X-axis: Effect sizes of the 21 major white matter regions in ENIGMA, higher values indicate more severe impairment in schizophrenia compared with controls in the ENIGMA meta-analysis. Y-axis: The correlation coefficients between FA of each region and cognitive measures in processing speed (PS) or working memory (WM) in the current Chinese sample. Full sample: all participants combined. Patients: all patients combined. A: Relationship between correlation coefficients for regional FA - processing speed (PS) (y-axis) and regional FA effect sizes of schizophrenia from ENIGMA-Schizophrenia (x-axis, Cohen’s d). B: partial correlation coefficients of A after corrected for working memory (WM). C: Relationship between correlation coefficients for regional FA values and working memory (y-axis) and regional FA effect sizes of schizophrenia (x-axis). D: partial correlation coefficients of C after corrected for PS. This was tested in the full sample (A to D) and then patients (E to H) and controls (I to L) separately.
Discussion
The white matter regional deficit pattern in schizophrenia was found to be significantly associated with treatment resistance. The white matter regional vulnerability index (RVI) significantly separated treatment-resistant from treatment-responsive patients, even with matched treatment duration and in the absence of global FA differences. The RVI measure further significantly separated patients at treatment initiation from controls. These results suggest that the extent of regional white matter vulnerability, as defined by RVI, can be observed in schizophrenia at initial diagnosis and treatment, and may mark the liability for treatment resistance to the currently available antipsychotic medications. Follow up longitudinal studies will be required to test if higher RVI at the onset would track with the development of treatment resistance.
Treatment resistance has been linked to cognitive deficits, especially in processing speed (15, 16) and negative symptoms(14), although the underlying mechanism remain unknown(28-30). Data from this study suggests that white matter may represent a shared underlying neurobiology, as the regional vulnerability pattern appears associated with treatment resistance and with more severe negative symptoms. We also replicated a prior study where the ENIGMA schizophrenia pattern predicted the association between FA and processing speed and working memory(27) (Figure 3 vs. Supplementary Figure 1). That study employed only two cognitive tasks, which was considered a limitation(27). This study used the MCCB cognitive battery to replicate these findings, and the similar pattern across two diverse (U.S. and Chinese) samples further validates white matter regional vulnerability in indexing core cognitive deficits in schizophrenia.
A white matter association with treatment resistance was initially suggested by white matter volume reduction findings(43), but opposite results were reported(11). Several DTI studies further suggested a white matter effect in treatment-resistant patients(44, 45). However, without a direct comparison with age-, sex-, and treatment duration-matched treatment-responsive patients using sufficient sample sizes, it is difficult to interpret whether these previous findings were related to schizophrenia in general or specific to treatment-resistant schizophrenia. We showed that multiple white matter regions had robust and significant reduction in FA in patients compared with controls (Supplementary Table 1), consistent with many previous studies (19-23, 27, 46, 47). No individual white matter region could consistently separate the treatment–resistant and treatment–responsive patients after correction for multiple comparisons. Why RVI, but not individual regional measures, capture treatment resistance (by comparing with treatment response) is not immediately clear. RVI is a readily obtainable index that correlates normalized regional FA of each person to the ENIGMA schizophrenia effect sizes and is assumed to reflect the contrast between the high vulnerability of late-developing, associative white matter regions and the lower vulnerability of early-developing regions to schizophrenia(34, 48, 49). We speculate that by taking into account white matter across the whole brain, RVI may have reduced nonspecific effects that impact all white matter regions and further accentuates the regional effects specific to schizophrenia (Figure 1A-1C) and treatment resistance (Figure 1D). Therefore, higher RVI in patients may have identified individuals with more severe patterns of neurodevelopmental white matter impairment, who, in turn, may be more vulnerable to treatment resistance.
Treatment-resistant patients are invariably given higher doses of medications, including multiple medications, and are more likely to be prescribed clozapine, compared to treatment-responsive patients. These factors could confound the discovery of the neurobiology of treatment resistance by hindering the isolation of biomarkers for treatment resistance from the medication effects. We analyzed a group of patients who were assessed within two weeks of initiating antipsychotic treatment to mitigate this concern. We observed a significant heterogeneity in the RVI values within this group. However, the candidate treatment-resistant biomarker was present in patients with minimal antipsychotic medication exposure, ruling out that higher RVI is a chronic disease effect but is associated with schizophrenia even at the onset of treatment. However, the current study is limited by its cross-sectional nature, and follow-up studies are required to test whether RVI observed at this stage would predict treatment-resistant vs. treatment-response in this group of patients.
The white matter pathways most strongly associated with treatment resistance, for example, the fornix (FX, the main white matter bundle of axonal fibers in and out the hippocampus), and the anterior corona radiate (ACR, the main white matter connecting ipsilateral prefrontal cortices) (effect sizes 1.0 range, Figure 1D), are well-known associative pathways for supporting cognitive functions (50, 51). In treatment responsive patients, the effect sizes of the same tracts were weaker (e.g., 0.5-0.6 range for FX and ACR, Figure 1C), suggesting that impairments in associative tracts that support cognition may play a larger role in treatment resistance. The agreement between white matter FA regional effect sizes in treatment-resistant patients and ENIGMA patients (r=0.92) also provides a novel perspective on the ENIGMA findings. Currently available antipsychotic medications have failed to treat symptoms in treatment-resistant schizophrenia, having only limited effects on cognitive deficits (52, 53). The ENIGMA DTI sample was worldwide, and the meta-analytical aggregation likely removed specific local medication and environmental, yielding schizophrenia-related neurobiology that is untreated and shared across the sites. The remarkable alignment between ENIGMA and the treatment-resistant patients in the current study indicates that the ENIGMA white matter regional effect size pattern may underlie the critical unmet treatment targets, i.e., treatment resistance, in addition to cognitive deficits(27) in schizophrenia. Meanwhile, it may also suggest that the ENIGMA white matter regional effect size pattern has its limitation, as it is less strongly associated with schizophrenia during treatment initiation (r=0.58) and more strongly associated with the treatment resistant aspect of schizophrenia.
Another potential limitation is that treatment-resistant patients are prescribed clozapine as the treatment of choice. Post hoc analyses did not show an association between RVI and clozapine, and elevated RVI was already present in the treatment-initiation. This study focused on differences between treatment resistant and treatment responsive groups that were also age and sex matched. The treatment initiation group had a significantly lower average age than treatment resistant and responsive groups, which is a potential limitation. However, the RVI was developed to be independent of age and sex and all analyses were performed while controlling for age and sex. Finally, only FA was used to index white matter abnormalities and other diffusion parameters (axial, radial, and mean diffusivities) were not explored. We chose FA as it showed higher sensitivity to schizophrenia deficits compared to these other parameters(18). Future studies should re-examine the findings using more advanced diffusion weighted imaging parameters(54).
Conclusion
Treatment resistance in schizophrenia may have part of its etiopathological origins in white matter deficits. Patients showing the pattern of regional white matter impairment in the late-developing, frontal associative fibers and no or limited impairment in the early developing sensory and motor fibers, were more likely to show resistance to contemporary antipsychotic medications. Development of new treatments and therapies to overcome schizophrenia treatment resistance should more strongly consider strategies that target white matter related mechanisms.
Supplementary Material
Acknowledgments
Support was received from the National Key R & D Program of China (2016YFC1307000), National Natural Science Foundation of China grants 81071086, 81461130016 and the National Institutes of Health grants R01MH112180, R01MH116948, S10OD023696, R01EB015611, U54 EB020403, T32MH067533, and U01MH108148. These funding sources provided financial supports to enable design and conduct of the study and collection, management, and analysis of the data. None of the funding agencies had a role in the interpretation of the data. None has a role in the preparation, review, or approval of the manuscript. None has a role in the decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
LEH has received or plans to receive research funding or consulting fees on research projects from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho, Heptares, Pfizer, Sound Pharma, Takeda, and Regeneron. None was involved in the design, analysis or outcomes of the study. PT and NJ have received research funding, unrelated to this work, from Biogen. All other authors declare no conflicts of interest.
References
- 1.Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, Bloomfield MA, Bressan RA, Buchanan RW, Carpenter WT, Castle DJ, Citrome L, Daskalakis ZJ, Davidson M, Drake RJ, Dursun S, Ebdrup BH, Elkis H, Falkai P, Fleischacker WW, Gadelha A, Gaughran F, Glenthoj BY, Graff-Guerrero A, Hallak JE, Honer WG, Kennedy J, Kinon BJ, Lawrie SM, Lee J, Leweke FM, MacCabe JH, McNabb CB, Meltzer H, Moller HJ, Nakajima S, Pantelis C, Reis Marques T, Remington G, Rossell SL, Russell BR, Siu CO, Suzuki T, Sommer IE, Taylor D, Thomas N, Ucok A, Umbricht D, Walters JT, Kane J, Correll CU. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry. 2017;174:216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samara MT, Dold M, Gianatsi M, Nikolakopoulou A, Helfer B, Salanti G, Leucht S. Efficacy, Acceptability, and Tolerability of Antipsychotics in Treatment-Resistant Schizophrenia: A Network Meta-analysis. JAMA Psychiatry. 2016;73:199–210. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni J, Gavrilidis E, Wang W, Worsley R, Fitzgerald PB, Gurvich C, Van Rheenen T, Berk M, Burger H. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry. 2015;20:695–702. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN, Rachid F, Carroll K, Krystal JH. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60:49–56. [DOI] [PubMed] [Google Scholar]
- 5.Heresco-Levy U, Ermilov M, Shimoni J, Shapira B, Silipo G, Javitt DC. Placebo-controlled trial of D-cycloserine added to conventional neuroleptics, olanzapine, or risperidone in schizophrenia. Am J Psychiatry. 2002;159:480–482. [DOI] [PubMed] [Google Scholar]
- 6.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. [DOI] [PubMed] [Google Scholar]
- 7.Mouchlianitis E, Bloomfield MA, Law V, Beck K, Selvaraj S, Rasquinha N, Waldman A, Turkheimer FE, Egerton A, Stone J, Howes OD. Treatment-Resistant Schizophrenia Patients Show Elevated Anterior Cingulate Cortex Glutamate Compared to Treatment-Responsive. Schizophr Bull. 2016;42:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein ME, Anderson VM, Pillai A, Kydd RR, Russell BR. Glutamatergic neurometabolites in clozapine-responsive and -resistant schizophrenia. Int J Neuropsychopharmacol. 2015;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, McGuire PK. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75:e11–13. [DOI] [PubMed] [Google Scholar]
- 10.Berretta S, Heckers S, Benes FM. Searching human brain for mechanisms of psychiatric disorders. Implications for studies on schizophrenia. Schizophrenia research. 2015;167:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson VM, Goldstein ME, Kydd RR, Russell BR. Extensive Gray Matter Volume Reduction in Treatment-Resistant Schizophrenia. International Journal of Neuropsychopharmacology. 2015;18:pyv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White TP, Wigton R, Joyce DW, Collier T, Fornito A, Shergill SS. Dysfunctional Striatal Systems in Treatment-Resistant Schizophrenia. Neuropsychopharmacology. 2016;41:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima S, Takeuchi H, Plitman E, Fervaha G, Gerretsen P, Caravaggio F, Chung JK, Iwata Y, Remington G, Graff-Guerrero A. Neuroimaging findings in treatment-resistant schizophrenia: A systematic review: Lack of neuroimaging correlates of treatment-resistant schizophrenia. Schizophr Res. 2015;164:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demjaha A, Weinstein S, Stahl D, Day F, Valmaggia L, Rutigliano G, De Micheli A, Fusar-Poli P, McGuire P. Formal thought disorder in people at ultra-high risk of psychosis. BJPsych Open. 2017;3:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bartolomeis A, Balletta R, Giordano S, Buonaguro EF, Latte G, Iasevoli F. Differential cognitive performances between schizophrenic responders and non-responders to antipsychotics: Correlation with course of the illness, psychopathology, attitude to the treatment and antipsychotics doses. Psychiatry research. 2013;210:387–395. [DOI] [PubMed] [Google Scholar]
- 16.Frydecka D, Beszlej JA, Goscimski P, Kiejna A, Misiak Bae. Profiling cognitive impairment in treatment-resistant schizophrenia patients. Psychiatry research. 2016;235:133–138. [DOI] [PubMed] [Google Scholar]
- 17.Elkis H, Buckley PF. Treatment-Resistant Schizophrenia. Psychiatric Clinics of North America. 2016;39:239–265. [DOI] [PubMed] [Google Scholar]
- 18.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Hibar D, Chen J, Agartz I, Alloza C, Andreassen O, Arango C, Banaj N, Bouix S, Bousman C, Brouwer R, Bruggemann J, Bustillo J, Cahn W, Calhoun V, Cannon D, Carr V, Catts S, Chen J, Chen X, Chiapponi C, Cho K, Ciullo V, Corvin A, Crespo-Facorro B, Cropley V, De Rossi P, Diaz-Caneja C, Dickie E, Doan N, Fan F, Faskowitz J, Fatouros-Bergman H, Flyckt L, Ford J, Fouche J, Fukunaga M, Gill M, Glahn D, Gollub R, Goudzwaard E, Guo H, Gur R, Gur R, Hashimoto R, Hatton S, Henskens F, Hickie I, Hong L, Horacek J, Howells F, Hulshoff Pol H, Hyde C, Isaev D, Whitford T, Jablensky A, Jansen P, Janssen J, Jonsson E, Kahn R, Kikinis R, Liu K, Klauser P, Knöchel M, Kubicki M, Kwon J, Lagopoulos J, Langen C, Lawrie S, Lenroot R, Lim K, López-Jaramillo C, Lyall A, Magnotta V, Mandl R, Mathalon D, McCarley R, McCarthy-Jones S, McDonald C, McEwen S, Mcintosh A, Melicher T, Mesholam-Gately R, Michie P, Mowry B, Mueller B, Newell D, O'Donnell P, Oertel V, Oestreich L, Paciga S, Pantelis C, Pasternak O, Pearlson G, Pereira A, Pineda J, Piras F, Piras F, Potkin S, Preda A, Rasser A, Roalf D, Roiz-Santiañez R, Pellicano G, Roos A, Rotenberg D, Satterthwaite T, Savadjiev P, Schall U, Scott R, Seal M, Seidman L, Weickert C, Shenton M, Spalletta G, Spaniel F, Sprooten E, Stäblein M, Stein D, Sundram S, Tan Y, Tan S, Tang S, Temmingh H, Tønnesen S, Tordesillas-Gutierrez D, Vaidya J, Haren D, Vargas C, Vecchio D, Velakoulis D, Voineskos A, Voyvodic J, Wang Z, Wang P, Wei D, Weickert T, Westlye L, Whalley H, White T, Wojcik J, Xiang H, Xie Z, Yamamori H, Yang F, Yao N, Zhang D, Zhao J, van Erp T, Turner J, Ehrlich S, Jung L, Thompson P, Donohue G. Widespread white matter microstructural differences in schizophrenia across 4,375 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2017;23:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazeri A, Mallar Chakravarty M, Felsky D, Lobaugh NJ, Rajji TK, Mulsant BH, Voineskos AN. Alterations of Superficial White Matter in Schizophrenia and Relationship to Cognitive Performance. Neuropsychopharmacology. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, Golembo S, Kanellopoulou I, Ng J, Hof PR, Harvey PD, Tsopelas ND, Stewart D, Davis KL. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–1032. [DOI] [PubMed] [Google Scholar]
- 21.Alba-Ferrara LM, de Erausquin GA. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front Integr Neurosci. 2013;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. [DOI] [PubMed] [Google Scholar]
- 24.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. [DOI] [PubMed] [Google Scholar]
- 25.Hof PR, Haroutunian V, Friedrich VL Jr., Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. 2003;53:1075–1085. [DOI] [PubMed] [Google Scholar]
- 26.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. [DOI] [PubMed] [Google Scholar]
- 27.Kochunov P, Coyle TR, Rowland LM, Jahanshad N, Thompson PM, Kelly S, Du X, Sampath H, Bruce H, Chiappelli J, Ryan M, Fisseha F, Savransky A, Adhikari B, Chen S, Paciga SA, Whelan CD, Xie Z, Hyde CL, Chen X, Schubert CR, O'Donnell P, Hong LE. Association of White Matter With Core Cognitive Deficits in Patients With Schizophrenia. JAMA Psychiatry. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowles EE, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167:828–835. [DOI] [PubMed] [Google Scholar]
- 29.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. [DOI] [PubMed] [Google Scholar]
- 30.Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57:688–691. [DOI] [PubMed] [Google Scholar]
- 31.Bartzokis G Neuroglialpharmacology: myelination as a shared mechanism of action of psychotropic treatments. Neuropharmacology. 2012;62:2137–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiology of aging. 2010;31:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kochunov P, Ganjgahi H, Winkler A, Kelly S, Shukla DK, Du X, Jahanshad N, Rowland L, Sampath H, Patel B, O'Donnell P, Xie Z, Paciga SA, Schubert CR, Chen J, Zhang G, Thompson PM, Nichols TE, Hong LE. Heterochronicity of white matter development and aging explains regional patient control differences in schizophrenia. Hum Brain Mapp. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tishler TA, Bartzokis G, Lu PH, Raven EP, Khanoyan M, Kirkpatrick CJ, Pyle MH, Villablanca JP, Altshuler LL, Mintz J, Ventura J, Casaus LR, Subotnik KL, Nuechterlein KH, Ellingson BM. Abnormal Trajectory of Intracortical Myelination in Schizophrenia Implicates White Matter in Disease Pathophysiology and the Therapeutic Mechanism of Action of Antipsychotics. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2018;3:454–462. [DOI] [PubMed] [Google Scholar]
- 36.Lally J, Ajnakina O, Di Forti M, Trotta A, Demjaha A, Kolliakou A, Mondelli V, Reis Marques T, Pariante C, Dazzan P, Shergil SS, Howes OD, David AS, MacCabe JH, Gaughran F, Murray RM. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. 2016;46:3231–3240. [DOI] [PubMed] [Google Scholar]
- 37.Gardner KN, Bostwick JR. Antipsychotic treatment response in schizophrenia. Am J Health Syst Pharm. 2012;69:1872–1879. [DOI] [PubMed] [Google Scholar]
- 38.Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220. [DOI] [PubMed] [Google Scholar]
- 39.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 40.Zou YZCJ, Wang J. Clinical reliability and validity of the version of Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery. Chin J Psychiatry. 2009;42:29–33. [Google Scholar]
- 41.Jahanshad N, Kochunov P, Sprooten E, Mandl RC, Nichols TE, Almassy L, Blangero J, Brouwer RM, Curran JE, de Zubicaray GI, Duggirala R, Fox PT, Hong LE, Landman BA, Martin NG, McMahon KL, Medland SE, Mitchell BD, Olvera RL, Peterson CP, Starr JM, Sussmann JE, Toga AW, Wardlaw JM, Wright MJ, Hulshoff Pol HE, Bastin ME, McIntosh AM, Deary IJ, Thompson PM, Glahn DC. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: A pilot project of the ENIGMA-DTI working group. Neuroimage. 2013;doi:pii: S1053-8119(13)00408-4. 10.1016/j.neuroimage.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agresti A: Categorical Data Analysis. 3rd edition ed, Wiley & Sons; 2013. [Google Scholar]
- 43.Molina V, Reig S, Sanz J, Palomo Ts, Benito C, SÃ!nchez J, Sarramea F, Pascau J, Desco M. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophrenia research. 2005;80:61–71. [DOI] [PubMed] [Google Scholar]
- 44.Vanes LD, Mouchlianitis E, Wood TC, Shergill SS. White matter changes in treatment refractory schizophrenia: Does cognitive control and myelination matter? NeuroImage : Clinical. 2018;18:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holleran L, Ahmed M, Anderson-Schmidt H, McFarland J, Emsell L, Leemans A, Scanlon C, Dockery P, McCarthy P, Barker GJ, McDonald C, Cannon DM. Altered Interhemispheric and Temporal Lobe White Matter Microstructural Organization in Severe Chronic Schizophrenia. Neuropsychopharmacology. 2014;39:944–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I, de Lucas EM, Rodriguez-Sanchez JM, Ayesa-Arriola R, Vazquez-Barquero JL, Crespo-Facorro B. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2011;167:451–458. [DOI] [PubMed] [Google Scholar]
- 47.Phillips KA, Rogers J, Barrett EA, Glahn DC, Kochunov P. Genetic contributions to the midsagittal area of the corpus callosum. Twin Res Hum Genet. 2012;15:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinberger DR. On the plausibility of "the neurodevelopmental hypothesis" of schizophrenia. Neuropsychopharmacology. 1996;14:1S–11S. [DOI] [PubMed] [Google Scholar]
- 49.Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res. 1995;16:87–110. [DOI] [PubMed] [Google Scholar]
- 50.Shamy JL, Carpenter DM, Fong SG, Murray EA, Tang CY, Hof PR, Rapp PR. Alterations of white matter tracts following neurotoxic hippocampal lesions in macaque monkeys: a diffusion tensor imaging study. Hippocampus. 2010;20:906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu J, Lee TMC, Frontotemporal Lobar Degeneration Neuroimaging I. The longitudinal decline of white matter microstructural integrity in behavioral variant frontotemporal dementia and its association with executive function. Neurobiol Aging. 2018;76:62–70. [DOI] [PubMed] [Google Scholar]
- 52.Manschreck TC, Boshes RA. The CATIE Schizophrenia Trial: Results, Impact, Controversy. Harvard Review of Psychiatry. 2007;15:245–258. [DOI] [PubMed] [Google Scholar]
- 53.Mishara AL, Goldberg TE. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia: opening a closed book. Biological Psychiatry. 2004;55:1013–1022. [DOI] [PubMed] [Google Scholar]
- 54.Kochunov P, Rowland LM, Fieremans E, Veraart J, Jahanshad N, Eskandar G, Du X, Muellerklein F, Savransky A, Shukla D, Sampath H, Thompson PM, Hong LE. Diffusion-weighted imaging uncovers likely sources of processing-speed deficits in schizophrenia. Proc Natl Acad Sci U S A. 2016;113:13504–13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.