Abstract
Mantle cell lymphoma (MCL) is an aggressive B-cell malignancy for which novel therapeutics with improved efficacy are greatly needed. To provide support for clinical immune checkpoint blockade, we comprehensively evaluated the expression of therapeutically targetable immune checkpoint molecules on primary MCL cells. MCL cells showed constitutive expression of Programmed Death 1 (PD-1) and Programmed Death Ligand 1 (PD-L1), variable CD200, absent PD-L2, Lymphocyte Activation Gene 3 (LAG-3), and Cytotoxic T-cell Associated Protein 4 (CTLA-4). Effector cells from MCL patients expressed PD-1. Co-culture of MCL cells with T-cells induced PD-L1 surface expression, a phenomenon regulated by IFNγ and CD40:CD40L interaction. Induction of PD-L1 was attenuated by concurrent treatment with ibrutinib or duvelisib, suggesting BTK and PI3K are important mediators of PD-L1 expression. Overall, our data provide further insight into the expression of checkpoint molecules in MCL and support the use of PD-L1 blocking antibodies in MCL patients.
Keywords: Mantle cell lymphoma, immune checkpoint molecules, PD-L1, PD-L2, CTLA-4, LAG3, PD-1, CD200
Introduction
Mantle cell lymphoma (MCL) is an aggressive B-cell non-Hodgkin lymphoma (NHL), and despite decades of research, it remains incurable for most patients [1]. Younger patients are treated with chemo-immunotherapy followed by autologous stem cell transplant consolidation [1], but even with intensive treatment, median overall survival is only 5–7 years [2]. Among older patients who are not candidates for autologous stem cell transplant, outcome is even shorter [3]. Although recent efforts have unveiled a number of targeted therapies, such as the Bruton’s tyrosine kinase (BTK) inhibitors ibrutinib and acalab-rutinib and the BCL2 inhibitor venetoclax, these novel therapies offer only marginal improvements in patient outcome, and survival statistics remain grim [4,5]. Clearly, improved therapies for this aggressive cancer are needed.
For solid tumors and some hematopoietic malignancies, immune escape was recently identified as a mechanism of cancer cell survival (reviewed in [6]). In some instances, researchers have pinpointed specific molecular mechanisms of immune evasion, leading to the development of therapeutics which enhance antitumor immunity and successful treatment of these patients (reviewed in [7]). Of particular interest are immune checkpoint molecules, surface proteins expressed on tumor cells, and immune regulatory cells which serve to modulate the immune system, often dampening antitumor immunity. A variety of immune checkpoint molecules have been shown to play a role in tumor immunology, and these include Programmed Death 1 (PD-1) and its ligands PD-L1 and PD-L2; Cytotoxic T-Lymphocyte Activator 4 (CTLA-4); Lymphocyte Activation Gene 3 (LAG-3), and CD200, among others (reviewed in [7]). PD-1, PD-L1, CTLA-4, and LAG-3 are expressed on T-cells (and rarely other immune effector cells), and in this context, ligation reduces immune cell activation, leading to diminished cytotoxicity, proliferation, and cytokine production [8–11]. Investigation of immune checkpoint molecule expression by cancer cells has heavily focused on PD-L1, with clinical trials aimed at therapeutically blocking its interaction with PD-1. More recently, it has been established that tumor cells can also express CTLA-4 and healthy B-cells LAG-3, both of which are traditionally thought of as being T-cell receptors [12–14], prompting speculation that these molecules may play a direct role in cancer cell survival when expressed within the tumor.
Many immune checkpoint molecules are therapeutically targetable by monoclonal antibody blockade including PD-1, PD-L1, PD-L2, CTLA-4, LAG-3, and CD200. Targeted blockade of two of these molecules, PD-1 and CTLA-4, has resulted in clinical benefit for patients with a variety of solid tumors and Hodgkin lymphoma [15–19]. Surprisingly, there are few data related to expression or function of these molecules in MCL, and published data are often conflicting. For example, four separate studies have shown widespread expression, infrequent expression, or absent expression of PD-L1 on MCL cells [20–23]. Herein, we evaluate expression of several immune checkpoint molecules on MCL cells, showing variable but constitutive expression of PD-L1 on tumor cells. We also show that upon exposure to immune effector cells, as may occur in the tumor microenvironment, PD-L1 is highly upregulated on MCL cells. Finally, we dissect the mechanism of PD-L1 regulation in this disease, also showing that targeted therapeutics, such as ibrutinib and duvelisib, may reduce PD-L1 expression with obvious clinical implications for combination studies.
Methods
Samples
Blood was obtained from healthy donors or from MCL patients in leukemic phase via venipuncture at The Ohio State University following written informed consent under an Institutional Review Board-approved protocol in accordance with the Declaration of Helsinki. Total peripheral blood mononuclear cells (PBMCs) were isolated via Ficoll® Paque PLUS (GE Healthcare Biosciences) density gradient centrifugation according to the manufacturer’s protocol. MCL cells and normal cells were preserved in freezing media composed of 45% FBS, 45% RPMI media, 10% DMSO at −135 °C until further use. After thawing, cells were washed once with phosphate-buffered saline, prior to re-suspending in complete media composed of RPMI media, 10% FBS, 10,000 units penicillin per mL, and 10 mg streptomycin per mL. Information on patient demographics, prior therapy or treatment naïve status, and MCL variant is included in Supplementary Table 1.
Cell selection
Cryopreserved MCL cells were thawed in complete RPMI. Selection of MCL cells was performed using EasySep human B-cell enrichment kit without CD43 depletion (StemCell Technologies, Vancouver BC) according to the manufacturer instructions. Allogeneic and autologous T-cells were selected immediately after collection using human T-cell enrichment cocktail (StemCell Technologies) in combination with Ficoll-Paque PLUS in accordance with the manufacturer instructions.
Co-culture
T-cells were plated with MCL cells at a ratio of 1:1 for allogeneic co-culture (typically 1 × 106 MCL cells and 1 × 106 T-cells) and a ratio of 1:4 for autologous co-culture (typically 1 × 106 MCL cells and 2.5 × 105 T-cells) in a 6-well tissue culture plate. To distinguish T-cells from MCL cells by flow cytometry, MCL cells were stained with CellVue® Claret Far Red Fluorescent Cell Linker Kit (Sigma-Aldrich, St. Louis MO) according to the manufacturer protocol. In preparation for plating, anti-CD3 purified functional grade antibody (Thermo Fisher Scientific, Waltham, MA) was applied at a concentration of 10 μg antibody per 1 mL of PBS to the bottom of a 6-well plate for a minimum of 4h. Anti-CD3 antibody was removed, and the plate washed once with PBS prior to plating cells. Anti-CD28 (Thermo Fisher) purified functional grade antibody was added to media with cells at a concentration of 1 μg/mL. For membrane separated co-culture, Transwell® polycarbonate membrane cell culture inserts (Sigma-Aldrich) were applied to wells with T-cells beneath the insert and MCL cells on top of the insert. For experiments in Figures 2(A–D), 3(B–G) and 5(A), PD-L1 expression was measured after 48 h as this is the time when the most robust induction was apparent. For experiments in Figures 4(A,B), PD-L1 expression was measured after 24 h to preserve the effects of the kinase inhibitors. For experiment 5B, PD-L1 expression was measured after 12 h prior to excessive reduction in cell viability due to α-amanitin treatment. Additional reagents for experiments evaluating IFNγ blockade, CD40 blockade, and α-amanitin treatment are described in the supplement.
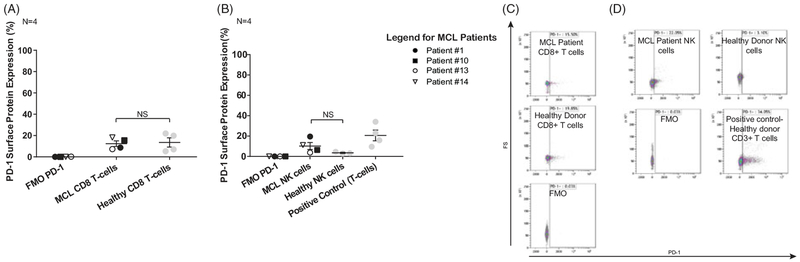
Figure 2.
PD-1 expression of immune effector cells of MCL patients. (A) Flow cytometry staining of CD8+T-cells from peripheral blood of both leukemic and nonleukemic MCL patients shows PD-1 expression similar to healthy donor CD8+ T-cells. Paired T-test, α = 0.05, N = 4. (B) Flow cytometry staining of NK cells from peripheral blood of both leukemic and nonleukemic MCL patients shows PD-1 expression in comparison with healthy donor NK cells, which do not express PD-1. Paired T-test, α = 0.05, N = 4. (C) and (D) Representative flow plots are shown from CD8+ T-cells of patient #10 (C) and NK cells of patient #1 (D).
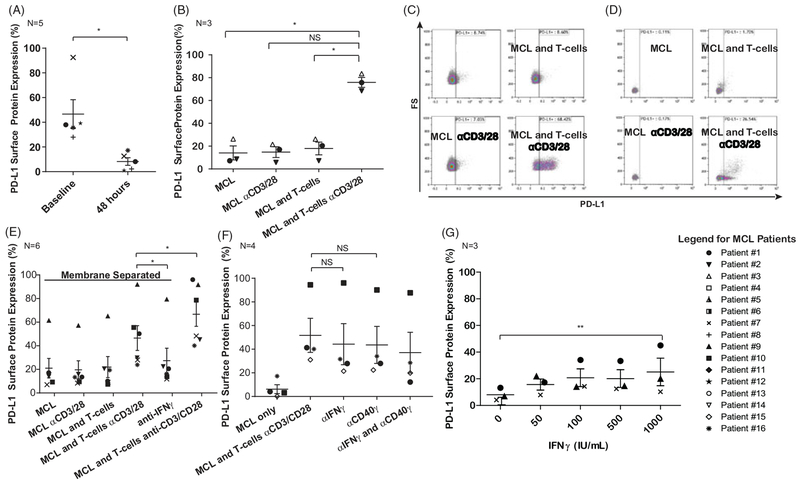
Figure 3.
Activated allogeneic and autologous T-cells modulate PD-L1 surface expression on MCL cells through IFNg secretion and CD40:CD40L interaction. (A) Flow cytometry data of MCL cells immediately after thawing and after 48 h. PD-L1 expression is lost in culture. *p ≤ .05, Paired T-test, α = 0.05, N = 5. (B) Co-culturing the MCL cells with anti-CD3 and anti-CD28 stimulated allogeneic T-cells for 48 h restores PD-L1 surface protein on MCL cells. *p<.0125, Paired T-test, α = 0.0125, N = 3 C. Representative flow cytometry plots from the graph in Figure 3(B) showing PD-L1 induction after co-culture with activated allogeneic T-cells. (D) Induction of PD-L1 surface protein on MCL cells is also observed after autologous co-culture with CD3 and CD28-activated T-cells. N = 1. (E) Co-culture of MCL cells and allogeneic T-cells with (Transwell) membrane separation (0.4 μm pores allow proteins to pass but not cells). There is partial induction of PD-L1 when cells are separated by a transwell insert in comparison with cells co-cultured in contact with each other at the 48-h time point. This proves that both a soluble component and contact-dependent component are responsible for PD-L1 induction. PD-L1 expression is reduced to baseline after antagonizing IFNγ in the transwell separated MCL and T-cells. *p≤.05, Paired T-test with Holm’s procedure, α = 0.05, N = 6. (F) Co-culture of MCL cells and allogenic T-cells with CD40 and IFNγ antagonism. Blockade of IFNγ activity, CD40 activity, or both in the co-culture condition led to a trend toward reduced PD-L1, though small sample size precluded achieving statistically significant results. Linear and mixed-effects model, α = 0.05, N = 4. (G) Recombinant IFNγ can also induce PD-L1 expression of MCL cells after 48 h in a dose-dependent manner. **p≤.01, Paired T-test, α = 0.05, N = 3.
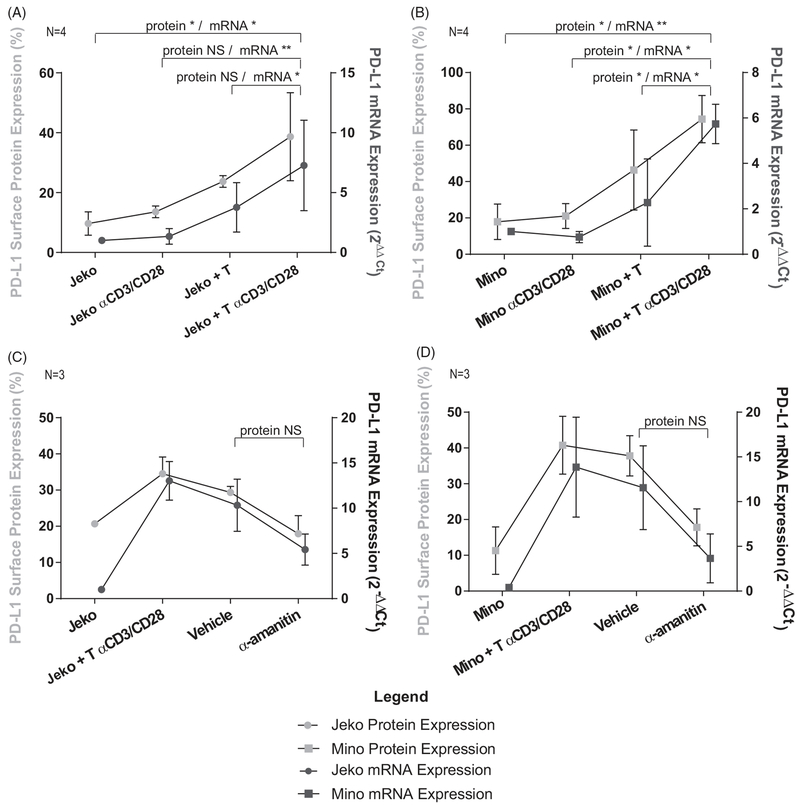
Figure 5.
PD-L1 surface protein expression is regulated by transcriptional activity of RNA polymerase II. (A) Jeko cell line shows inducible PD-L1 surface protein in co-culture with activated allogeneic T-cells similar to primary MCL cells. RT-PCR performed in parallel to the flow cytometry shows that the mRNA levels rise in unison with the surface protein level. *p≤.05, **p≤.01, Paired T-test with Holm’s procedure, α = 0.05, N = 4. (B) Mino cell line shows inducible PD-L1 surface protein in co-culture with activated allogeneic T-cells similar to primary MCL cells. RT-PCR performed in parallel to the flow cytometry shows that the mRNA levels rise in unison with the surface protein level. *p ≤ .05, **p ≤ .01, Paired T-test with Holm’s procedure, α = 0.05, N = 4. (C) Application of α-amanitin, an RNA polymerase II inhibitor, to Jeko cells only in conjunction with activated T-cells showed that inhibition of transcription leads to a simultaneous decrease in surface protein expression though statistical significance was no achieved (p = .228), suggesting transcriptional regulation of PD-L1. mRNA levels were normalized to the housekeeping gene CD52, whose transcript has a long half life and to baseline levels of mRNA transcripts in Jeko cells. Paired T-test, α = 0.05, N = 3. (D) Application of α-amanitin, an RNA polymerase II inhibitor, to Mino cells only in conjunction with activated T-cells showed that inhibition of transcription leads to a simultaneous decrease in surface protein expression though statistical significance was not achieved (p = .195), suggesting transcriptional regulation of PD-L1. mRNA levels are normalized to the housekeeping gene CD52, whose transcript has a long half life and to baseline levels of mRNA transcripts in Mino cells. Paired T-test, α = 0.05, N = 3.
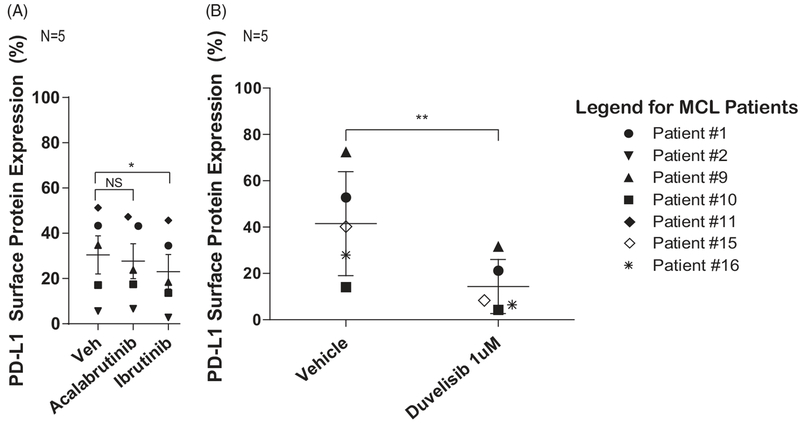
Figure 4.
Inhibitors of the BCR pathway abrogate inducible PD-L1 expression. (A) Reduction of PD-L1 expression on MCL cells in co-culture after treatment with BTK inhibitors. MCL cells co-cultured with activated allogeneic T-cells show reduced PD-L1 expression following treatment of both MCL cells and T-cells with the irreversible BTK inhibitor ibrutinib (*p ≤ .05). There is also a trend toward PD-L1 reduction after treatment of co-cultured MCL and T-cells with acalabrutinib (p>.05). Paired T-test with Holm’s procedure, α = 0.05, N = 5. (B) There is reduction of PD-L1 expression after treatment of co-cultured MCL cells and activated T-cells with the PI3K inhibitor duvelisib. **p≤.01, Paired T-test, α = 0.05, N = 5.
Flow cytometry
For all experiments, 1 × 106 cells were stained for viability using a fixable reactive amine dye and surface markers in a two-step staining process. Detailed staining methods are described in the supplement. Gating strategies are provided in Supplementary Figure 1.
Real-time polymerase chain reaction (RT-PCR)
RNA was extracted, cDNA was synthesized, and RT-PCR was performed as previously described [24]. Primers are as follows (all from Thermo Fisher): PD-L1 (Hs01125301_m1), PD-L2 (Hs01057777_m1), CD200 (Hs01033303_m1), LAG-3 (Hs00158563_m1), CTLA-4 (Hs03044418_m1), TBP (4325803), CD52 (Hs00174349_m1), MCL1 (Hs00172036_m1). For experiments in Figure 5, relative expression was normalized to the endogenous control gene CD52 [25] and calculated by the 2−(ΔΔCt) method [26].
Statistical analysis
Paired T-tests with α = 0.05 were used in Figures 2, 3(A–G), 4(B), and 5(C,D). Paired T-tests with α = 0.0125 (adjusted with a Bonferroni procedure) were used to evaluate the induction of PD-L1 in Figure 3(B). Assays in Figures 3(E–G), 4(A), and 5(A,B) were evaluated using a paired T-test, and the p-value was corrected using Holm’s procedure [27,28]. A linear mixed-effects model including the interaction between CD40 and IFN blockade was used in Figure 3(F).
Results
MCL cells constitutively express immune checkpoint molecules
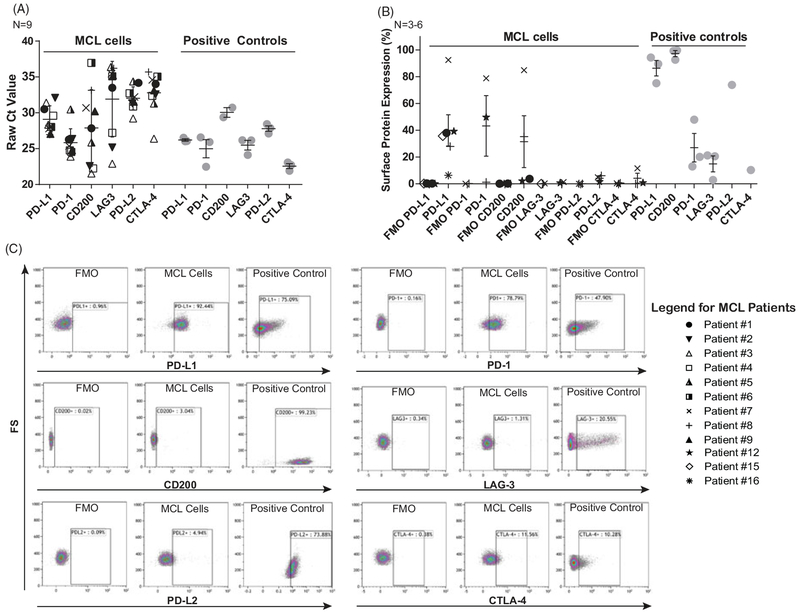
Given the success of immune checkpoint blockade in multiple cancers, we examined surface expression of six therapeutically targetable immune checkpoint molecules on MCL tumor cells. PD-L1, PD-L2, and CD200 were selected due to their well-known expression on healthy B-cells and lymphoid malignancies [29,30]. We also investigated PD-1, CTLA-4, and LAG-3, given the suggestion in several publications that they may play an immune-suppressive role in tumor microenvironment [12–14]. Primary MCL tumor cells isolated from the peripheral blood of MCL patients in leukemic phase were evaluated for these molecules at the mRNA level by RT-PCR and membrane surface protein level by flow cytometry. Figure 1(A) shows raw Ct values from real-time PCR for nine patients. Anti-CD3 and anti-CD28-activated T-cells served as positive controls for PD-L1, PD-1, CTLA-4, and LAG-3; chronic lymphocytic leukemia (CLL) cells for CD200; and the L428 Hodgkin lymphoma cell line for PD-L2. Only PD-L1, PD-1, and CD200 were robustly expressed at the mRNA level with mean Ct values below 30 in primary MCL samples, and Supplementary Figure 2 shows normalized Ct values for these three targets. Furthermore, PD-L1 and PD-1 showed the most consistent mRNA expression, whereas CD200 mRNA expression was variable. CTLA-4, LAG-3, and PD-L2 had very low mRNA expression in these samples. Surface protein expression of PD-L1 in MCL patients complimented the mRNA data (Figure 1(B,C)), showing consistent expression of PD-L1 in six out of six patients evaluated, slightly lower but also consistent expression of PD-1 and inconsistent expression of CD200. In contrast, CTLA-4, LAG-3, and PD-L2 were absent from the cell surface which is consistent with low mRNA expression. Gating strategy for flow experiments is shown in Supplementary Figure 1.
Figure 1.
mRNA and surface protein expression of immune checkpoint molecules in MCL. (A) Raw Ct values from real-time PCR evaluating PD-L1, PD-1, CD200, LAG-3, PD-L2, and CTLA-4 on primary MCL cells from peripheral blood. Only PD-L1, PD-1, and CD200 show robust expression with mean Ct values below 30, N = 9. (B) Surface protein expression of immune checkpoint molecules on MCL cells. Consistent with PCR data, PD-L1 (N = 6), PD-1 (N = 3), and CD200 (N = 4) are expressed, whereas LAG-3 (N = 3), PD-L2 (N = 3), and CTLA-4 (N = 3) are not. (C) Representative plots from MCL patient showing expression of PD-L1 and PD-1 but not CD200, LAG-3, PD-L2, or CTLA-4. Positive controls are CD3/CD28-activated T-cells for PD-L1, PD-1, LAG-3, and CTLA-4; CLL cells for CD200; and L428 cells for PD-L2.
T-cells and NK cells from MCL patients express PD-1
Given the consistent expression of PD-L1 on MCL tumor cells, we next sought to evaluate whether PD-1 was present on immune effector cells in these patients. Figure 2(A,C) showed PD-1 expression levels on CD8+ T-cell from MCL patients to be comparable to that on healthy donor CD8+ T-cells. In Figure 2(B,D), there was increased mean PD-1 expression on NK cells in MCL patients versus healthy donor NK cells, though not statistically significant (p=.279).
PD-L1 surface expression is attenuated following ex vivo culture but can be restored through co-culture with activated T-cells
Though MCL cells express PD-L1, we subsequently discovered that expression is highly labile, and culturing the cells for up to 48 h led to dramatic reduction in surface PD-L1 expression (p=.047) (Figure 3(A)). This change occurred irrespective of cell viability, which was evaluated using amine reactive fluorescent markers (described in Supplementary Figure 1). PD-L1 expression was restored in the MCL cells after exposure to allogeneic healthy donor T-cells activated through T-cell receptor stimulation by anti-CD3 and anti-CD28 antibodies for 48 h (p=.0117, .0149, and .0005) (Figure 3(B,C)). Co-culture of MCL cells with autologous activated T-cells derived from the patient’s peripheral blood showed a similar trend of PD-L1 surface protein induction after 48 h (N = 1) (Figure 3(D)). In a brief pilot experiment, PD-L1 surface protein expression was evaluated both 24- and 48-h time points. Supplementary Figure 3 shows that PD-L1 induction was more robust at 48 h for primary patient cells and the MCL cell line Mino, while the MCL cell line Jeko had slightly higher PD-L1 expression at 24 h. Given these data, the majority of subsequent experiment are conducted using a 48-h time point, except in cases where this prolonged incubation would result in excessive cell death, such as following treatment with small molecule inhibitors or transcriptional inhibitors.
Few data are published regarding the regulation of PD-L1 expression in lymphoma. Thus, we next sought to investigate the mechanism of PD-L1 induction in the co-culture setting. To determine whether induction is dependent on cell to cell contact or soluble mediators, we performed a co-culture using a porous membrane insert within the plate wells (Figure 3(E)). The membranes are perforated with 0.4 μM diameter pores, which allow soluble molecules, such as cytokines, to pass but not permitting T-cells and MCL cells contact with one another [31,32]. Co-culture of activated allogeneic T-cells and MCL cells in this setting resulted in induction of PD-L1. Removal of the membrane led to further increase of PD-L1 expression, suggesting both a soluble mediator and contact dependent mechanism (p=.038). Additionally, anti-interferon gamma (IFNγ) blocking antibodies attenuated PD-L1 induction to baseline in the membrane separated condition, indicating IFNγ is the primary soluble mediator of PD-L1 expression in our MCL cells (p=.038) consistent with previous studies [20].
In healthy B-cells, contact-dependent activation may occur through the interaction between CD40 on B-cells and CD40 ligand on the surface of the T-cell. We therefore hypothesized that this interaction may be inducing PD-L1. In Figure 3(F), blockade of CD40 (p=.051) or IFNγ (p = 0.102) led to a trend toward reduced PD-L1 expression in co-culture, though these results were not statistically significant. Finally, PD-L1 can be independently induced in a dose-dependent manner in the presence of soluble human recombinant IFNγ at 48 h (p=.01) (Figure 3(G)). Taken together, these data suggest that both IFNγ and CD40 contribute to PD-L1 induction in the co-culture system, though additional contact-dependent mechanisms are also at play.
PD-L1 expression is attenuated by inhibition of kinases involved in B-cell receptor signaling
In order to investigate intracellular signaling mechanisms involved in modulation of PD-L1 expression, MCL cells and allogeneic T-cells were treated with the irreversible BTK inhibitors ibrutinib or acalabrutinib (Figure 4(A)). Cells were incubated with ibrutinib or acalabrutinib (1 μM) for 1 h followed by removal of the inhibitor from solution by PBS wash and plating for co-culture. Treatment of both MCL cells and T-cells (N = 5) with ibrutinib resulted in significant reduction of PD-L1 expression (p=.0128), while a significant reduction in PD-L1 was not observed with acalabrutinib (p=.66). Importantly, for all flow cytometry experiments, live cells were identified using an amine reactive dye, and thus, reduction in PD-L1 expression was unrelated to viability. Statistically significant reduction in PD-L1 occurred with only ibrutinib treatment, highlighting the importance of BTK in the expression of PD-L1; PD-L1 reduction was more robust with ibrutinib compared with acalabrutinib, suggesting that alternative T-cell kinases inhibited by ibrutinib but not acalabrutinib also play a role.
Similar reduction in PD-L1 expression was observed following continuous treatment of both MCL cells and T-cells with the dual PI3Kδ and γ inhibitor duvelisib at a concentration of 1 μM (p=.01) (Figure 4(B)). Taken together, these findings indicate that activating prosurvival signals regulated by BCR and T-cell receptor kinases and isoform-specific PI3K contribute to PD-L1 up-regulation in MCL.
PD-L1 surface protein expression is regulated by the transcriptional activity of RNA polymerase II
To further dissect the mechanism of PD-L1 regulation on MCL tumor cells, we evaluated whether its expression was regulated at the transcriptional level. Two commonly used MCL cell lines, Mino and Jeko, were used for these experiments. In a manner similar to primary MCL cells, Jeko (Figure 5(A)) and Mino (Figure 5(B)) cells co-cultured with activated T-cells showed increased PD-L1 surface protein expression in comparison with their respective controls (Jeko: p=.039, .077, .11 and Mino: p=.027, .027, .027). mRNA transcripts of CD274 that encodes PD-L1 increased after co-culture with activated T-cells in a manner similar to the surface protein levels, suggesting regulation at the transcriptional level (Jeko: p=.017, .0014, .021 and Mino: p=.00018, .028, .05). To support this hypothesis, we performed experiments using the irreversible RNA polymerase II inhibitor α-amanitin, which, by virtue of its covalent binding properties, remains bound to its target after washing it out of solution. Mino and Jeko cells treated with α-amanitin prior to co-culture with T-cells showed a trend toward inhibition of mRNA transcripts of PD-L1 alongside a parallel reduction in surface protein (p=. 195 and .228 for protein expression in Mino and Jeko, respectively) (Figure 5(C,D)). Importantly, only the Mino and Jeko cells were treated with α-amanitin, so the transcriptional blockade was specific to the MCL cells and not the T-cells. MCL1 was used as a positive control for α-amanitin due to the short half-life of its mRNA transcript (Supplementary Figure 4) [28]. In summary, these findings support the hypothesis that PD-L1 is regulated at the transcriptional level in MCL.
Discussion
Immune checkpoint inhibitor therapy represents a new wave of immunotherapy, the success of which is evidenced in many solid cancers and Hodgkin lymphoma [15–19]. Though therapeutic strategies with these inhibitors are still being optimized, the presence or absence of specific immune checkpoints on the surface of cancer cells and effector cells appears to play a critical role as a biomarker of response to therapy [19,33,34]. Few prior studies have evaluated the landscape of immune checkpoints in MCL [20–23]. While some of our experiments were statistically underpowered, our findings are meaningful because cell line results were validated in primary MCL patient samples, despite the challenges of obtaining such specimens. Here, we demonstrate that MCL cells express multiple immune checkpoints including PD-L1, PD-1, and more variably CD200. CTLA-4, PD-L2, and LAG-3 were not detected, although this may be the result of small sample size. Expression of PD-L1 on MCL cells and its cognate receptor PD-1 on T-cells and NK cells support therapeutic inhibition of this pathway in MCL.
The relevance of PD-1 expression on MCL cells is uncertain, and this is the first time this molecule has been demonstrated on MCL. A few studies have identified the expression of immune checkpoint molecules traditionally associated with T-cells on the surface of tumor cells. In two investigations, ligation of CTLA-4 on solid tumor cells and leukemia cells induced apoptosis [13,14]. Given these studies, the possibility remains that PD-1 expression on MCL cells may be an important mechanism by which growth and survival signals are regulated in the tumor microenvironment, and this finding carries implications for therapeutic blockade of the PD-1 and PD-L interaction. In addition, PD-L1 is physiologically expressed on the surface of B cells, T-cells, and macrophages, indicating the existence of a reciprocal immune escape mechanism mediated by the interaction between PD-1 on MCL cells and PD-L1 on immune effector cells.
It is interesting that we found some MCL patient samples to express CD200 as a recent publication indicated that a CD200 negative immunophenotype can be used to identify MCL patients and distinguish them from CLL [35]. Importantly, CLL cells were used as a positive control for CD200 in these investigations, and although MCL patients expressed CD200, the CLL cells were invariably much brighter by flow cytometry analysis. Thus, our data also support differential expression of CD200 by these two cancers and findings are not in direct opposition with this previous publication.
Rapid attenuation of PD-L1 in culture speaks to the labile nature of its expression, and the mechanism of PD-L1 regulation in MCL, has not been previously described. Our data show that both IFNγ and CD40:CD40L interaction between MCL cells and T-cells regulates PD-L1 expression. IFNγ has previously been reported to induce PD-L1 expression in normal and cancerous tissues [20,36–38]. Since blockade of both CD40 and IFNγ resulted in incomplete PD-L1 attenuation, other unidentified contact-dependent mechanisms are at play. Our studies confirm the transcriptional regulation of PD-L1 in MCL cells, similar to other cancers [38,39].
Modulation of PD-L1 expression with kinase inhibitors is a novel finding. BTK inhibitors showed a more modest inhibition of PD-L1 expression in comparison with PI3K inhibitors. This may be related to the fact that PI3K is downstream of many growth factor receptors, in comparison with BTK, which is more specific for BCR signaling [40,41]. In addition, PI3K is in closer proximity to NFκB, a known driver of PD-L1 in other cancers [42], in comparison with BTK.
Ibrutinib and acalabrutinib are primarily inhibitors of BTK, but also have numerous alternative kinase targets. Acalabrutinib is more selective for BTK than ibrutinib, but also inhibits the T-cell kinase TXK, the ubiquitously expressed kinase BMX, and ErbB4 [43]. Ibrutinib inhibits the above kinases, in addition to the T-cell kinases ITK and TEC, and additional EGFR family kinases (ErbB1, ErbB2, ErbB3, ErbB4) [44]. Ibrutinib showed a more robust PD-L1 inhibition compared to acalabrutinib, suggesting that T-cell kinases (TEC, ITK) selectively inhibited by ibrutinib play a role in PD-L1 regulation, in addition to kinases that are targeted by both (BTK, TXK, Erb4, and BMX). While it is important to explore novel combination therapeutic strategies to enhance the limited preliminary single agent activity of checkpoint inhibitors in MCL, our data indicate that the effect of targeted therapies on the expression of checkpoint molecules should be taken into consideration in the rational design of novel clinical trials [45].
Together, these data provide novel insight into the landscape of immune checkpoint molecules expressed in MCL and the pathways that control PD-L1 expression. Although consistently present on MCL surface, the expression of PD-L1 can be modulated: Physical interaction mediated by CD40 and CD40L as well as cytokines such as IFNγ can induce up-regulation of PD-L1, while targeted therapy such as BTK inhibitors and PI3K inhibitors can reduce its expression. This has important therapeutic implications for the rational development of combinations strategies that should aim at directly affecting the cancer cells while enhancing the immune-mediated antitumor response [46]. Ongoing clinical trials with checkpoint inhibitors in which serial lymph node biopsies are collected will help to answer the question how the expression of PD-L1 changes over time.
In summary, we have described the expression of the targetable immune checkpoint molecule PD-L1 in MCL. Our data urge the thorough evaluation of PD-1 or PD-L1 blockade in clinical trials with sizable number of MCL patients to affirm whether this pathway represents a viable target in this disease.
Supplementary Material
Acknowledgments
We would like to acknowledge Yuh-Ying Yeh for her contributions toward the validation of methodologies used in the manuscript.
Funding
This was supported the NIH NCI under the Oncology T32 Training Grant T32CA009338 (B.K.H), the CLL Research Consortium (CRC) P01 CA081534, and the OSU Comprehensive Cancer Center Support Grant P30 CA016058. This work was also supported by the Pelotonia Graduate Fellowship (B.K.H.), the CCTS Davis-Bremer Scholar Award (L.A.), and the D. Warren Brown Foundation.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2019.1569231.
Supplemental data for this article can be accessed http://dx.doi.org/10.1080/10428194.2019.1569231.
References
- [1].Dreyling M, Geisler C, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol 2014;25:iii83–iii92. [DOI] [PubMed] [Google Scholar]
- [2].Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973-2010). National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; 2013. [Google Scholar]
- [3].Doorduijn JK, Kluin-Nelemans HC. Management of mantle cell lymphoma in the elderly patient. Clin Interv Aging. 2013;8:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185–S198. [DOI] [PubMed] [Google Scholar]
- [7].Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the Pd-1 immunoinhibitory receptor by a Novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. [DOI] [PubMed] [Google Scholar]
- [10].Golden-Mason L, Klarquist J, Wahed AS, et al. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differences. J Immunol. 2008;180: 3637–3641. [DOI] [PubMed] [Google Scholar]
- [11].Benson DM, Bakan CE, Mishra A, et al. The PD-1/PDL1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010; 116:2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kisielow M, Kisielow J, Capoferri-Sollami G, et al. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol. 2005; 35:2081–2088. [DOI] [PubMed] [Google Scholar]
- [13].Contardi E, Palmisano GL, Tazzari PL, et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer. 2005;117:538–550. [DOI] [PubMed] [Google Scholar]
- [14].Pistillo MP, Tazzari PL, Palmisano GL, et al. CTLA-4 is not restricted to the lymphoid cell lineage and can function as a target molecule for apoptosis induction of leukemic cells. Blood. 2003;101:202–209. [DOI] [PubMed] [Google Scholar]
- [15].Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33: 1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with Ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moskowitz CH, Ribrag V, Michot J-M, et al. PD-1 Blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after Brentuximab vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013). Blood. 2014;124:290–290. [Google Scholar]
- [19].Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer . N Engl J Med. 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- [20].Wang L, Qian J, Lu Y, et al. Immune evasion of mantle cell lymphoma: expression of B7-H1 leads to inhibited T-cell response to and killing of tumor cells. Haematologica. 2013;98:1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vranic S, Ghosh N, Kimbrough J, et al. PD-L1 status in refractory lymphomas. PLoS ONE. 2016;11:e0166266 [cited 2018 Apr 15]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5115714/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gatalica Z, Bilalovic N, Vranic S, et al. PD-L1 and PD1 expression in lymphomas. Blood. 2015;126:3899–3899. [Google Scholar]
- [23].Menter T, Bodmer-Haecki A, Dirnhofer S, et al. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol. 2016;54:17–24. [DOI] [PubMed] [Google Scholar]
- [24].Lapalombella R, Andritsos L, Liu Q, et al. Lenalidomide treatment promotes CD154 expression on CLL cells and enhances production of antibodies by normal B cells through a PI3-kinase-dependent pathway. Blood. 2010;115:2619–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yeh Y-Y, Chen R, Hessler J, et al. Up-regulation of CDK9 kinase activity and Mcl-1 stability contributes to the acquired resistance to cyclin-dependent kinase inhibitors in leukemia. Oncotarget. 2014;6:2667–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25: 402–408. [DOI] [PubMed] [Google Scholar]
- [27].Warton DI, Hui FKC. The arcsine is asinine: the analysis of proportions in ecology. Ecology. 2011;92:3–10. [DOI] [PubMed] [Google Scholar]
- [28].Winter D,. C f J Using the Student’s “t”-test with extremely small sample sizes. Pract. Assess Res Eval. 2013;18:1–12. [Google Scholar]
- [29].Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. [DOI] [PubMed] [Google Scholar]
- [30].Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hurley BP, Pirzai W, Eaton AD, et al. An experimental platform using human intestinal epithelial cell lines to differentiate between hazardous and non-hazardous proteins. Food Chem Toxicol. 2016;92:75–87. [DOI] [PubMed] [Google Scholar]
- [32].Harriff MJ, Cansler ME, Toren KG, et al. Human lung epithelial cells contain mycobacterium tuberculosis in a late endosomal vacuole and are efficiently recognized by CD8þ T Cells. PLOS One. 2014;9:e97515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving Nivolumab. JCO. 2014;32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Carbognin L, Pilotto S, Milella M, et al. Differential activity of Nivolumab, Pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLOS One. 2015;10:e0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alapat D, Coviello-Malle J, Owens R, et al. Diagnostic usefulness and prognostic impact of CD200 expression in lymphoid malignancies and plasma cell myeloma. Am J Clin Pathol. 2012;137:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim J, Myers AC, Chen L, et al. Constitutive and inducible expression of B7 family of ligands by human airway epithelial cells. Am J Respir Cell Mol Biol. 2005; 33:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Abiko K, Matsumura N, Hamanishi J, et al. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112: 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garcia-Diaz A, Shin DS, Moreno BH, et al. Interferon receptor signaling pathways regulating pd-l1 and pdl2 expression. Cell Rep. 2017;19:1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee S-J, Jang B-C, Lee S-W, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett. 2006;580:755–762. [DOI] [PubMed] [Google Scholar]
- [40].Cantley LC. The Phosphoinositide 3-Kinase Pathway. Science. 2002;296:1655–1657. [DOI] [PubMed] [Google Scholar]
- [41].Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120:1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gowrishankar K, Gunatilake D, Gallagher SJ, et al. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-κB. PLOS One. 2015;10:e0123410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib Study. JCO. 2016;34:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ruella M, June CH. Chimeric antigen receptor T cells for B cell neoplasms: choose the right CAR for you. Curr Hematol Malig Rep. 2016;11:368–384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.