Abstract
Background.
Numerous studies have suggested a possible role for acute kidney injury (AKI) biomarkers in predicting renal recovery both before and after renal replacement therapy (RRT). However, definitions for recovery and whether to include patients dying but free of RRT may influence results.
Objectives.
To validate plasma neutrophil gelatinase-associated lipocalin (pNGAL) as a useful biomarker for predicting or improving the ability of clinical predictors alone to predict recovery following AKI, including in our model plasma B-type natriuretic peptide (pBNP) to account for cardiovascular events.
Methods.
We analyzed 69 patients enrolled in the Acute Renal Failure Trial Network study. pNGAL and pBNP were measured on days 2, 7 and 14. We analyzed their predictive ability for subsequent recovery, defined as alive and independent from dialysis at 60 days. In sensitivity analyses we explored changes in results with alternative definitions of recovery.
Results.
Twenty-nine patients (42%) recovered from AKI. Neither pNGAL nor pBNP, alone or in combination, were accurate predictors of renal recovery—the best area under the receiver-operating characteristics (ROC) curve (AUC) were for pNGAL using the largest relative change (AUC 0.59, 95%CI 0.45–0.74). The best clinical model achieved superior performance to biomarkers (AUC 0.69, 95%CI 0.56 to 0.81). The AUC was greatest (0.75, 95%CI 0.60 to 0.91) when pNGAL + pBNP at day 14 were added to the clinical model but this increase did not achieve statistical significance. However, integrated discrimination improvement (IDI) analysis showed that the addition of pNGAL and pBNP at day 14 to the clinical model significantly improved the prediction of renal recovery (p=0.008).
Conclusions.
pNGAL and pBNP can improve the accuracy of clinical parameters in predicting AKI recovery and a full model using biomarkers together with age achieved adequate discrimination.
Keywords: acute kidney injury, biomarkers, renal recovery, renal replacement therapy, NGAL
Introduction
While recovery after severe acute kidney injury (AKI) receiving renal replacement therapy (RRT) may still occur in some patients, a significant proportion of patients can show partial recovery or do not recover at all. Non-recovery of renal function carries significant negative effects on quality of life and health care costs [1, 2], prolonged hospitalization, increased risk for chronic comorbidities and mortality [3]. Recovery from AKI varied in several clinical trials due to several factors, such as differences in the study population and in the definition of recovery [4]. Hence, the ability to accurately predict the possibility of recovery after AKI would have significant impact in the clinical management of critically ill patients [5]. Clinical predictors could help physicians to predict renal recovery and to assess clinical decisions, such as defining the timing of initiation and/or discontinuation of RRT [1] [6]. Several urinary and plasma biomarkers have been investigated in predicting AKI development and severity, but only few studies explored their ability in predicting renal recovery [7, 8]. We previously tested plasma neutrophil gelatinase-associated lipocalin (pNGAL) in patients with community-acquired pneumonia and found that elevated NGAL levels were associated with renal non-recovery [5]. We also reported results from the Biological Markers of Recovery for the Kidney (BioMaRK) study, where urinary biomarkers were evaluated and compared between patients who did or did not recover from AKI requiring RRT [9]: significant differences were found between the two groups and urinary hepatocyte growth factor (uHGF) and urinary NGAL improved clinical risk prediction for renal recovery [9]. In the present study, we sought to validate pNGAL as a useful biomarker for improving the ability of clinical predictors alone to predict recovery following AKI. Finally, decreased survival after AKI is strongly influenced by cardiovascular events [10]. We included plasma B-type natriuretic peptide (pBNP) to account for the cardiovascular component in our models.
Materials and Methods
Study design
The present study was a prospective cohort study conducted as an ancillary analysis to the Acute Renal Failure Trial Network (ATN) study. ATN was a multicenter, prospective study that aimed to compare two different strategies of RRT in critically ill patients with AKI [11]. Briefly, patients with AKI and failure of at least one non-renal organ or sepsis were randomized between November 2003 and July 2007 to receive either intensive treatment (intermittent hemodialysis [IHD] and sustained low-efficiency dialysis [SLED] provided six times per week, and continuous venovenous hemodiafiltration [CVVHDF] providing a total effluent flow rate of 35ml/kg of body weight per hour) or less intensive RRT (IHD and SLED provided three times per week, and CVVHDF providing a total effluent flow rate of 20ml/kg of body weight per hour) from 27 VA and university-affiliated medical centers. The present cohort includes patients with availability of plasma biomarkers (pNGAL and pBNP) at specific time points (day 2, 7 and 14). The analysis was based only on day 2 and 7 measurements for patients without samples on day 14. Patients with pre-existing chronic kidney disease (CKD) were excluded from the ATN study. The institutional review boards at all participating sites approved the parent study, and written informed consent was obtained from all participants or their proxies.
Data collection and laboratory measurements
Clinical records of participants were prospectively collected in order to analyze baseline clinical characteristics, renal function, causes of AKI and severity of illness scores. Sepsis was defined according to International Sepsis Definitions recommendations [12]. The primary outcome was recovery from AKI, defined as survival and dialysis independence at 60 days.
Plasma NGAL measurement was performed using the Triage NGAL Assay (Alere Inc, San Diego, CA, USA); it is a fluorescence-based sandwich immunoassay, which measures pNGAL with a measurable range from 15ng/ml to 1,300ng/ml. After addition of the sample to the device, the filter separated blood cells from plasma and the analyzer displayed the results in about 15 minutes. Plasma BNP testing was also performed on the Triage platform using a standard commercially available assay (Alere Triage BNP Test, Alere Inc).
Statistical analyses
We compared clinical characteristics between patients who recovered and patients who failed to recover from AKI at 60 days. Continuous data were presented as mean and standard deviation (SD) and compared with Student t-test or Mann-Whitney test, as appropriate. Categorical data were reported as proportions and compared using the chi-squared or Fisher exact test. Plasma biomarkers (pNGAL, alone or in combination with pBNP, as markers of patient recovery) were measured at specific time-points (days 2, 7 and 14 after enrollment); furthermore, we analyzed the largest relative change between day 2 and day 7 or day 14. We examined the association between each marker and renal recovery with logistic regression models and generated area under the receiver-operating characteristics (ROC) curves (AUC) to analyze the accuracy in predicting recovery. We used clinical risk prediction models identified in this population in our previous publication [9], based on age, Acute Physiology and Chronic Health Evaluation (APACHE II) score and Charlson comorbidity index; then, we added each biomarker model individually to the best clinical model and compared the composite models using AUC analysis. We performed sensitivity analyses using alternative definitions of recovery. Finally, we calculated the integrated discrimination improvement (IDI) to examine the improvement in reclassification with the biomarkers compared to the clinical model alone. Analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC), with statistical significance set at P<0.05.
Results
Baseline patient characteristics and outcomes
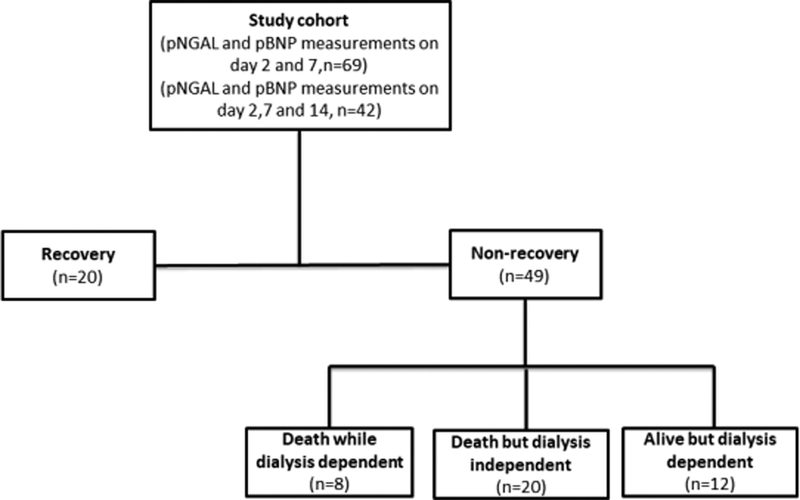
A total of 69 patients had complete clinical data available and 29 patients (42%) achieved the primary endpoint, while the remaining 40 patients did not recover (12 patients were alive but dialysis dependent, 8 patients died while dialysis dependent, and the remaining 20 subjects were dialysis independent but died) (Figure 1). Baseline clinical and demographic characteristics were shown in Table 1. Patients who recovered from AKI were younger than those who failed to recover (mean age 54.1 ± 15.5 vs 64.6 ± 14.2, p=0.008). The primary etiology of AKI was ischemia in both groups (overall 84.1%), followed by sepsis (59.4%) and nephrotoxic exposures (23.2%). No significant differences between recovery and non-recovery groups were observed with regards to gender, race, baseline renal function, renal function at the initiation of RRT, causes of AKI, length of ICU and hospital stay, severity of illness scores and intensity of RRT.
Figure 1.
Flow chart of study population
Table 1.
Baseline clinical characteristics of the study population
| All patients (n=69) |
Non-recovery (n=40) |
Recovery (n=29) |
P | |
|---|---|---|---|---|
| Age, years, mean (SD) | 60.2 (15.5) | 64.6 (14.2) | 54.1 (15.5) | 0.008 |
| Female gender (%) | 26 (37.7%) | 15 (37.5%) | 11 (37.9%) | >0.99 |
| White race (%) | 54 (78.3%) | 30 (75.0%) | 24 (82.8%) | 0.56 |
| Serum creatinine prior to randomization (mg/dl) | 4.1 (1.6) | 3.9 (1.5) | 4.3 (1.8) | 0.43 |
| Baseline serum creatinine (mg/dl) | 1.1 (0.4) | 1.1 (0.4) | 1.1 (0.4) | 0.83 |
| CKD-EPI Estimated GFR (ml/min/1.73m2) | 17 (8.9) | 17.2 (9.3) | 16.6 (8.5) | 0.78 |
| BUN at admission (mg/dl) | 36.2 (27.1) | 40.7 (31.4) | 30.1 (18.7) | 0.28 |
| Causes of acute kidney injury | ||||
| ischemia (%) | 58 (84.1%) | 36 (90.0%) | 22 (75.9%) | 0.19 |
| nephrotoxins (%) | 16 (23.3%) | 7 (17.5%) | 9 (31%) | 0.25 |
| sepsis (%) | 41 (59.4%) | 22 (55%) | 19 (65.5%) | 0.46 |
| multifactorial causes (%) | 47 (69.1%) | 27 (69.2%) | 20 (69%) | >0.99 |
| APACHE II score a | 24.9 (7.1) | 25.6 (6.9) | 23.8 (7.5) | 0.39 |
| Non-renal SOFA organ-system score b | ||||
| respiratory | 2.3 (1) | 2.2 (1.0) | 2.4 (0.9) | 0.49 |
| coagulation | 1.4 (1.2) | 1.4 (1.3) | 1.4 (1.2) | 0.84 |
| liver | 1.4 (1.3) | 1.5 (1.5) | 1.2 (1.1) | 0.42 |
| cardiovascular | 2.4 (1.7) | 2.6 (1.7) | 2.2 (1.7) | 0.36 |
| central nervous system | 2.4 (1.4) | 2.2 (1.4) | 2.6 (1.4) | 0.33 |
| total | 14.4 (3.5) | 14.7 (3.3) | 13.9 (3.8) | 0.42 |
| Cleveland Clinic ICU ARF score c | 11.5 (3.2) | 11.5 (3.2) | 11.5 (3.1) | 0.95 |
| Intensive strategy (%)d | 33 (47.8%) | 18 (45%) | 15 (51.7%) | 0.62 |
| Length of ICU stay before randomization (days) | 6.5 (9.2) | 7.9 (11.6) | 4.5 (3.2) | 0.08 |
| Hospital length of stay before randomization (days) | 10.3 (11.6) | 12.4 (13.8) | 7.5 (6.6) | 0.13 |
| Charlson comorbidity index e | 2.5 (2.2) | 2.6 (2) | 2.4 (2.4) | 0.53 |
| Mechanical ventilation (%) | 60 (87%) | 35 (85.7%) | 25 (86.2%) | >0.99 |
| Severe sepsis (%) | 45 (65.2%) | 25 (62.5%) | 20 (69%) | 0.62 |
Data presented as mean (SD). BUN, blood urea nitrogen; ICU intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; ARF acute renal failure.
According to the method of Knaus et al.[13]
Non-renal SOFA score was assessed on the first day[14]
According to the method of Thakar et al.[15]
Intensive strategy: intermittent hemodialysis and sustained low-efficiency dialysis six times per week, and continuous venovenous hemodiafiltration at 35 ml per kilogram of body weight per hour[11]
According to the method of Charlson et al.[16]
Biomarker levels and recovery status
No significant differences in pNGAL or pBNP were found at any time-point between recovery and non-recovery patients. No differences in pNGAL were observed in the non-recovery group between patients who were alive but dialysis dependent, those who died while dialysis dependent and those who died while dialysis independent (Supplementary Figure 1 and 2).
Prediction of renal recovery by biomarker models alone
The AUCs for each plasma biomarker for prediction of renal recovery are shown in Table 2. Neither pNGAL nor pBNP alone were good predictors of recovery from AKI and, interestingly, the best predictive performance was given by pNGAL and pBNP using the largest relative change (AUC 0.59, 95% confidence interval (CI) 0.45–0.74; and 0.59, 95%CI 0.46–0.73, respectively). When adding pNGAL to pBNP, the greatest AUC was 0.63 (95%CI 0.44–0.81) on day 14 and 0.60 (95%CI 0.47–0.74) for the largest relative change of pNGAL+pBNP. However, we did not find significant differences in predictive accuracy between models with pBNP alone and those including both pBNP and pNGAL. We found similar results in sensitivity analyses, excluding patients who died, but who were dialysis independent or including this group of patients in the recovery group (Supplementary Table 1 and 2).
Table 2.
Area under the receiver-operating characteristics curve for biomarker models on day 2, 7, 14 and for the largest relative changes for predicting recovery from AKI.
| Plasma biomarker | Time point | AUC | 95%CI | P* |
|---|---|---|---|---|
| pNGAL | Day 2 | 0.54 | 0.41 to 0.69 | |
| Day 7 | 0.50 | 0.36 to 0.64 | ||
| Day 14 | 0.55 | 0.37 to 0.73 | ||
| Largest relative change# | 0.59 | 0.45 to 0.74 | ||
| pBNP | Day 2 | 0.50 | 0.36 to 0.65 | |
| Day 7 | 0.58 | 0.45 to 0.72 | ||
| Day 14 | 0.59 | 0.41 to 0.78 | ||
| Largest relative change# | 0.59 | 0.46 to 0.73 | ||
| pNGAL and pBNP | Day 2 | 0.55 | 0.41 to 0.68 | 0.89 |
| Day 14 | 0.63 | 0.44 to 0.81 | 0.53 | |
| Largest relative change# | 0.60 | 0.47 to 0.74 | 0.86 |
AUC, area under the receiver-operating characteristic curve; CI, confidence interval, pNGAL, plasma neutrophil gelatinase-associated lipocalin; pBNP, plasma B-type natriuretic peptide.
Largest relative change in the first 7 or 14 days as compared to day 2
Compared with pNGAL model on day 2, day 14 and for the largest relative change Biomarkers analysis on day 14 was performed on only 42 patients
Clinical risk prediction models and combination of clinical and biomarker models
We analyzed several clinical risk prediction models previously identified in the BioMaRK study and based on age, Charlson comorbidity index and Acute Physiology and Chronic Health Evaluation II (APACHE II) [9]. We described the accuracy in predicting renal recovery for each variable individually and then together (Table 3a). The clinical model including only age (model A) had the best AUC in predicting the primary outcome (AUC 0.69, 95%CI 0.56–0.81) compared to those for Charlson comorbidity index (model B) and APACHE II (model C). Furthermore, the composite clinical model including all variables (model M2) had a similar accuracy (AUC 0.70, 95%CI 0.57–0.84, p=0.84) compared to model A. Similarly, the model including age and Charlson index (model M1) did not significantly differ from the others (AUC 0.70, 95%CI 0.58–0.83, p=0.61).
Table 3.
Area under the receiver-operating characteristics curve for clinical risk prediction models alone (3a) and for the combination of the best clinical model and biomarker models (3b) for each time point for predicting renal recovery.
| Clinical variables | OR (95%CI) | Model | AUC | 95%CI | P* |
|---|---|---|---|---|---|
| AGE | 0.95 (0.92 to 0.99) | A | 0.69 | 0.56 to 0.81 | - |
| CHARLSON COMORBIDITY INDEX | 0.95 (0.75 to 1.2) | B | 0.55 | 0.39 to 0.70 | - |
| APACHE II | 0.96 (0.90 to 1.0) | C | 0.56 | 0.42 to 0.71 | - |
| M1= A + C | 0.70 | 0.58 to 0.83 | 0.61 | ||
| M2+ A + B + C | 0.70 | 0.57 to 0.84 | 0.84 | ||
| OR, odds ratio; AUC area under the curve; CI confidence interval; APACHE II Acute Physiology and Chronic Health Evaluation II. | |||||
| *Compared with model A | |||||
| Time point | Model | AUC | 95%CI | P* |
|---|---|---|---|---|
| Day 2 | Age + pNGAL | 0.71 | 0.58 to 0.83 | 0.54 |
| Age + pBNP | 0.69 | 0.56 to 0.82 | 0.86 | |
| Age + pNGAL + pBNP | 0.70 | 0.58 to 0.83 | 0.57 | |
| Day 14 | Age + pNGAL | 0.74 | 0.58 to 0.90 | 0.56 |
| Age + pBNP | 0.75 | 0.59 to 0.91 | 0.74 | |
| Age + pNGAL + pBNP | 0.75 | 0.60 to 0.91 | 0.62 | |
| Largest relative change# | Age + pNGAL | 0.70 | 0.57 to 0.82 | 0.75 |
| Age + pBNP | 0.71 | 0.59 to 0.84 | 0.48 | |
| Age + pNGAL + pBNP | 0.73 | 0.61 to 0.85 | 0.35 | |
| AUC, area under the curve; CI confidence interval; pNGAL plasma neutrophil gelatinase-associated lipocalin; pBNP, plasma B-type natriuretic peptide. | ||||
| # Largest relative change in the first 14 days as compared to day 2 | ||||
| *Compared with Age alone | ||||
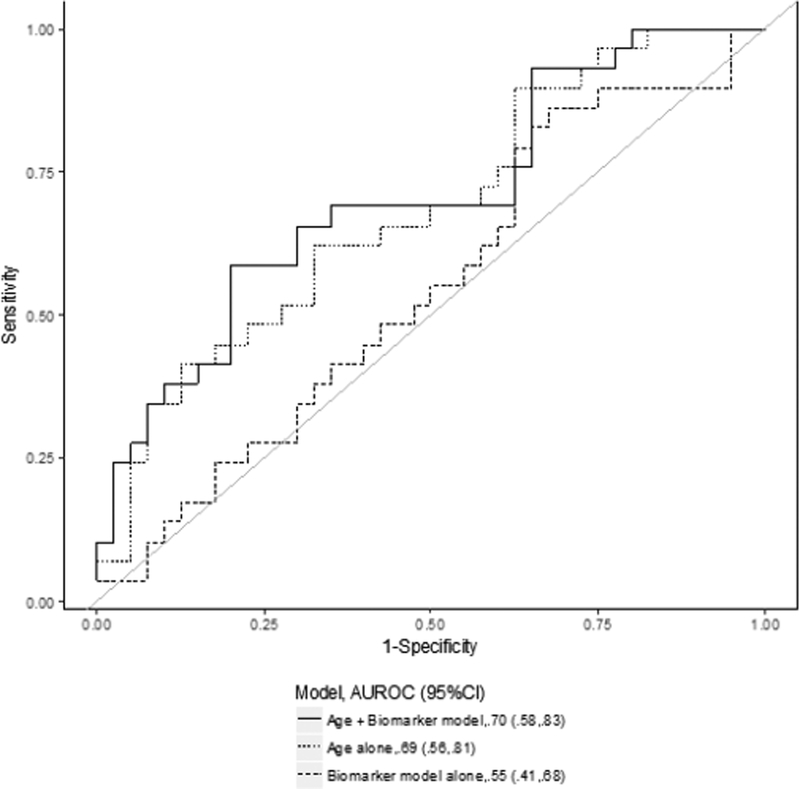
Next, we analyzed the predictive value of plasma biomarkers when added to the clinical model and compared with the best clinical model (model A). Overall, the combination of the clinical model with biomarkers improved the ability to predict renal recovery, although this difference did not achieve statistical significance. As shown in Table 3b, we found that on day 2 the combined model of pNGAL and age had similar accuracy to predict renal recovery (AUC 0.71, 95%CI 0.58–0.83) compared to age alone (ROC contrast p-value=0.57) (Figure 2a).
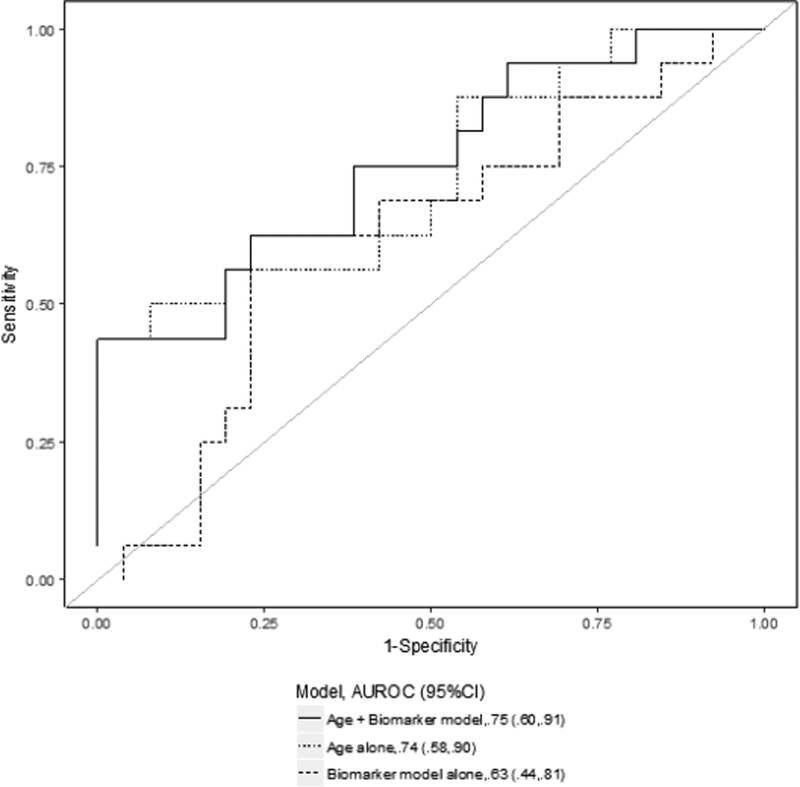
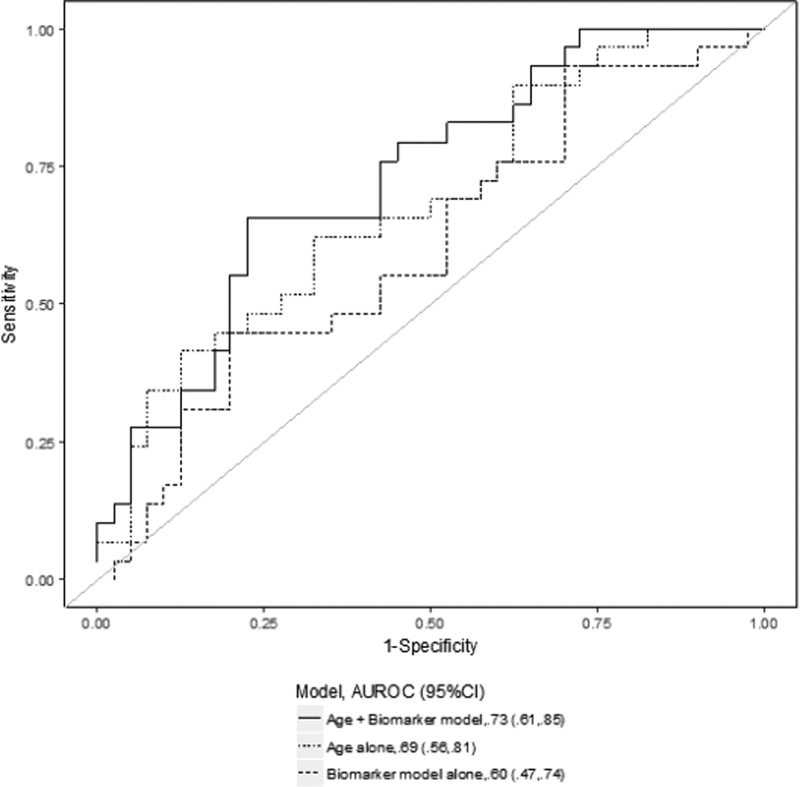
Figure 2. Receiver-operating characteristic (ROC) curves for several prediction models for renal recovery.
The data shown represent the comparison between the best biomarker model alone (thick dashed line), the clinical model alone (thin dashed line) and the combined clinical and biomarker model (solid line) on day 2 (2a), day 14 (2b) and for the largest relative change (2c). The clinical prediction model included age alone. The area under the ROC curve (AUC) values and 95% CI are also reported.
Similarly, the AUC for the composite model including pNGAL, pBNP and age on day 14 (AUC 0.75, 95%CI 0.60–0.91) was not significantly higher than that for age alone (ROC contrast p-value=0.62) (Figure 2b). Moreover, the model combining the largest relative change of pNGAL and pBNP with age presented a predictive ability (AUC 0.73, 95%CI 0.61–0.85) similar to the clinical model alone (ROC contrast p-value=0.35) (Figure 2c). In sensitivity analysis, when excluding patients who died while dialysis independent from the non-recovery group, the models including biomarkers had a discrete ability in predicting renal recovery compared to those in the primary analysis and the greatest AUC was for age+ largest relative change of pNGAL and pBNP (AUC 0.78, 95%CI 0.66–0.91) (Supplementary Table 1). Finally, we also examined the incremental value of biomarkers in assessing renal recovery compared to the clinical model alone by IDI analysis: there was significant improvement in prediction of renal recovery for the model age+pNGAL at day 14 (p=0.008) and age+pBNP at day 14 (p=0.008).
Discussion
The present study examined one of the most important clinical challenges in critical care medicine, the prediction of recovery after AKI. We found that the integration of plasma biomarkers with clinical parameters can improve the accuracy in identifying subgroups of patients with different likelihood of renal recovery.
Much evidence suggested that patients who fail to recover from AKI have a reduced quality of life and an increased risk for chronic disease [3, 17–19]. The multinational Beginning and Ending Supportive Therapy for the kidney (BEST kidney) study showed that hospital mortality in patients with AKI was very high (60.3%) and dialysis dependence at hospital discharge was significant as well (13.8%) [20]. The ATN study described a high percentage of patients who did not recover renal function by day 28 (75.8% in the intensive RRT group, 72.6% in the less-intensive RRT group), and only 16% were discharged to home without dialysis by day 60 [11]. However, our recent data in patients with septic shock showed that those with complete or partial recovery from AKI may have a similar one-year survival to patients without AKI [21]. These data emphasize the urgent need for new tools to improve recovery and reduce the incidence of negative outcomes in patients with AKI. Clinical parameters (increasing age, comorbidities, severity and duration of AKI and timing and dose of RRT) have been correlated with renal recovery [2]; however, the ATN study did not describe differences in recovery rate between the intensive and less-intensive RRT strategy groups [11]. In this scenario, a useful approach could be to evaluate certain biomarkers in predicting renal recovery [6]. In this study, we focused on a well-recognized plasma biomarker for renal disease, pNGAL. NGAL has been tested as an early diagnostic tool for AKI [22–25], and it could be an optimal marker of renal recovery. Kusaka et al. evaluated serum NGAL in predicting functional recovery after kidney transplantation and showed that the decrease of NGAL values after transplantation well correlated with the renal recovery and discriminated patients with immediate, slow or delayed graft function [26]. Recently, Liu et al. showed that in 49 adult patients with living donor kidney transplants, serum NGAL on the day of transplantation was an independent predictor of graft function recovery [27]. Previously, we analyzed pNGAL and renal recovery in a post hoc analysis performed as part of the Genetic and Inflammatory Markers of Sepsis (GenIMS) study, a multicenter prospective study with patients with community-acquired pneumonia [5, 28]. In this cohort, pNGAL alone predicted failure to recover with an AUC of 0.74 and a clinical model including age, serum creatinine, pneumonia severity and nonrenal organ failure score presented a similar AUC (0.78); the AUC did not significantly increase when combining pNGAL and clinical model, but the reclassification of risk of renal recovery significantly improved by 17% [5].
In this study, we also focused on pBNP to control for cardiovascular effects on recovery. pBNP is a neuropeptide hormone released from myocytes in response to ventricular stretching; it is a well-known biomarker of cardiac volume and hemodynamics, but it has been recently evaluated as an AKI biomarker, particularly in patients with heart failure [29]. Recently, Howell et al hypothesized that point-of-care pBNP and pNGAL could be useful for earlier assessment of intravascular volume and renal function in severe burn injury during resuscitation, in order to predict inadequate resuscitation strategies and to define cardio-renal syndrome early [30]. Plasma BNP levels were significantly higher in patients with AKI compared to control patients, as well as in over-resuscitated patients (23.1 ± 21.9 vs 13.9 ± 13.4 pg/ml, p<0.001) [30].
In sensitivity analyses, when we focused on a specific renal definition for recovery (excluding patients who died while dialysis independent from the non-recovery group), the accuracy of biomarkers increased and a full model that included biomarkers and age achieved an adequate accuracy in predicting renal recovery at each time point (AUC 0.77 at day 2, 0.76 at day 14, 0.78 using the largest relative change between day 2 and 14). While death and non-recovery are frequently combined as a composite endpoint (e.g. major adverse kidney events) [31], patients may be interested in understanding the risks of requiring dialysis even if mortality risk is uncertain. There is no agreement about the best definition for renal recovery and the conflicting results about the application of biomarkers among different studies may be related to this issue. Since our analysis focused on a limited cohort, larger studies are needed to confirm the utility of these biomarkers in informing clinical decision-making in critically ill patients [32]. For example, timing cessation of RRT is a very important clinical decision, since unnecessary treatment should be avoided to reduce dialysis complications [7]. In the ATN study, the mean duration of RRT was about 13.4±9.6 for the intensive RRT and 12.8±9.3 for the less-intensive strategy group[1]. For this analysis, we decided not to focus on RRT duration in predicting renal recovery since patients who do not recover obviously undergo a longer RRT duration. Furthermore, since failure to recover is associated with worse outcomes (CKD), these patients can be targeted for closer follow-up and for specific interventions (placement of permanent vascular access and kidney transplantation for those who develop end-stage renal disease).
Our study has some important limitations. First, sample size was limited by availability of biomarker measurements, so we did not explore comparison of biomarker levels between complete and partial recovery. Second, this is an ancillary analysis from the ATN study and the data are relatively old (between 2003 and 2007), although samples have been maintained at −80°. Also, we did not have long-term follow up data. Third, we only measured two biomarkers. Other serum and urinary biomarkers, including cell-cycle arrest biomarkers [33, 34], might also be useful for predicting AKI and renal recovery. Moreover, we did not analyze the potential differences in NGAL removal between different dialysis techniques. Finally, the ATN study did not collect data on fluid balance and this could limit the interpretation of pBNP data since prior literature has described its relevance in the setting of fluid overload.
Conclusions
In conclusion, our results demonstrate that models including plasma NGAL and BNP and clinical information can provide reasonably robust prediction of patients more or less likely to recover after AKI requiring RRT, especially when focusing on a specific renal definition for recovery. Additional studies will be required to determine whether these results can be incorporated into clinical protocols to improve patient care.
Supplementary Material
Funding
This work was funded by institutional grants. The content is solely the responsibility of the authors. BioMaRK was supported by a grant (R01DK070910) from the National Institute of Diabetes, and Digestive, and Kidney Diseases (NIDDK). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of NIDDK or NIH. The VA/NIH ATN study was supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development (CSP #530) and by NIDDK by interagency agreement Y1-DK-3508.
Footnotes
Trial Registration. The present study was a prospective cohort study conducted as an ancillary analysis to the Acute Renal Failure Trial Network (ATN) study. The institutional review boards at all participating sites approved the parent study, and written informed consent was obtained from all participants or their proxies. NCT number: , first posted January 19, 2004 (retrospectively registered).
Ethics approval and consent to participate
The present study was a prospective cohort study conducted as an ancillary analysis to the Acute Renal Failure Trial Network (ATN) study. The institutional review boards at all participating sites approved the parent study, and written informed consent was obtained from all participants or their proxies.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure Statement
JAK and RM report grant support from Bioporto. MF reports consulting fees from Bioporto.
References
- 1.Koraishy FM, Coca SG: Can we predict recovery from severe acute kidney injury with biomarkers? Seminars in dialysis 2014, 27(3):236–239. [DOI] [PubMed] [Google Scholar]
- 2.Godin M, Macedo E, Mehta RL: Clinical determinants of renal recovery. Nephron Clinical practice 2014, 127(1–4):25–29. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein SL, Chawla L, Ronco C, Kellum JA: Renal recovery. Critical care (London, England) 2014, 18(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellum JA: How can we define recovery after acute kidney injury? Considerations from epidemiology and clinical trial design. Nephron Clinical practice 2014, 127(1–4):81–88. [DOI] [PubMed] [Google Scholar]
- 5.Srisawat N, Murugan R, Lee M, Kong L, Carter M, Angus DC, Kellum JA: Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney international 2011, 80(5):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srisawat N, Murugan R, Kellum JA: Repair or progression after AKI: a role for biomarkers? Nephron Clinical practice 2014, 127(1−4): 185–189. [DOI] [PubMed] [Google Scholar]
- 7.Endre ZH: Recovery from acute kidney injury: the role of biomarkers. Nephron Clinical practice 2014, 127(1−4):101–105. [DOI] [PubMed] [Google Scholar]
- 8.Coca SG, Yalavarthy R, Concato J, Parikh CR: Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney international 2008, 73(9):1008–1016. [DOI] [PubMed] [Google Scholar]
- 9.Srisawat N, Wen X, Lee M, Kong L, Elder M, Carter M, Unruh M, Finkel K, Vijayan A, Ramkumar M et al. : Urinary biomarkers and renal recovery in critically ill patients with renal support. Clinical journal of the American Society of Nephrology : CJASN 2011, 6(8):1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL: Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clinical journal of the American Society of Nephrology : CJASN 2014, 9(3):448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM et al. : Intensity of renal support in critically ill patients with acute kidney injury. The New England journal of medicine 2008, 359(1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM et al. : The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 2016, 315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG,Sirio CA, Murphy DJ, Lotring T, Damiano A et al. : The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991, 100(6):1619–1636. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996, 22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 15.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. Journal of the American Society of Nephrology : JASN 2005, 16(1):162–168. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 1987, 40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 17.Doyle JF, Forni LG: Long-Term Follow-up of Acute Kidney Injury. Critical care clinics 2015, 31(4):763–772. [DOI] [PubMed] [Google Scholar]
- 18.Kellum JA: Diagnostic Criteria for Acute Kidney Injury: Present and Future.Critical care clinics 2015, 31(4):621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes: KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Inter 2012, Suppl. 2(2):1–138. [Google Scholar]
- 20.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E et al. : Acute renal failure in critically ill patients: a multinational, multicenter study. Jama 2005, 294(7):813–818. [DOI] [PubMed] [Google Scholar]
- 21.Kellum JA, Chawla LS, Keener C, Singbartl K, Palevsky PM, Pike FL, Yealy DM, Huang DT, Angus DC: The Effects of Alternative Resuscitation Strategies on Acute Kidney Injury in Patients with Septic Shock. American journal of respiratory and critical care medicine 2016, 193(3):281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q: Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clinical practice 2008, 108(3):c176–181. [DOI] [PubMed] [Google Scholar]
- 23.Lipcsey M, Hayward P, Haase M, Haase-Fielitz A, Eastwood G, Peck L,Matalanis G, Bellomo R: Neutrophil gelatinase-associated lipocalin after off pump versus on pump coronary artery surgery. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals 2014, 19(1):22–28. [DOI] [PubMed] [Google Scholar]
- 24.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J et al. : Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet (London, England) 2005, 365(9466): 1231–1238. [DOI] [PubMed] [Google Scholar]
- 25.Huang CY, Shih CC, Chung K, Kao KC, Wu HP: Predictive value of plasma neutrophil gelatinase-associated lipocalin for acute renal failure in patients with severe sepsis. Journal of the Chinese Medical Association : JCMA 2016, 79(8):428–434. [DOI] [PubMed] [Google Scholar]
- 26.Kusaka M, Iwamatsu F, Kuroyanagi Y, Nakaya M, Ichino M, Marubashi S,Nagano H, Shiroki R, Kurahashi H, Hoshinaga K: Serum neutrophil gelatinase associated lipocalin during the early postoperative period predicts the recovery of graft function after kidney transplantation from donors after cardiac death. The Journal of urology 2012, 187(6):2261–2267. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Li HX, Ying ZW, Guo JJ, Cao CY, Jia W, Yang HR: Serum Neutrophil Gelatinase-Associated Lipocalin and Cystatin C for Assessing Recovery of Graft Function in Patients Undergoing Living-Donor Kidney Transplantation. Clinical laboratory 2016, 62(1–2):155–163. [DOI] [PubMed] [Google Scholar]
- 28.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC: Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Archives of internal medicine 2007, 167(15):1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palazzuoli A, Ruocco G, Pellegrini M, De Gori C, Del Castillo G, Franci B, Nuti R, Ronco C: Comparison of Neutrophil Gelatinase-Associated Lipocalin Versus B- Type Natriuretic Peptide and Cystatin C to Predict Early Acute Kidney Injury and Outcome in Patients With Acute Heart Failure. The American journal of cardiology 2015, 116(1):104–111. [DOI] [PubMed] [Google Scholar]
- 30.Howell E, Sen S, Palmieri T, Godwin Z, Bockhold J, Greenhalgh D, Tran NK: Point-of-care B-type natriuretic peptide and neutrophil gelatinase-associated lipocalin measurements for acute resuscitation: a pilot study. Journal of burn care & research : official publication of the American Burn Association 2015, 36(2):e26–33. [DOI] [PubMed] [Google Scholar]
- 31.Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, Chertow GM, Murray PT, Parikh CR, Shaw AD et al. : Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clinical journal of the American Society of Nephrology : CJASN 2012, 7(5):844–850. [DOI] [PubMed] [Google Scholar]
- 32.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL et al. : Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nature reviews Nephrology 2017, 13(4):241–257. [DOI] [PubMed] [Google Scholar]
- 33.Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K et al. : Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. American journal of respiratory and critical care medicine 2014, 189(8):932–939. [DOI] [PubMed] [Google Scholar]
- 34.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS et al. : Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Critical care (London, England) 2013, 17(1):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.