Abstract
Fatty liver is common in men and post-menopausal women, suggesting that estrogen may be involved in liver lipid metabolism. The aim of this study is to be clear the role of estrogen and estrogen receptor alpha (ERα) in fat accumulation during liver regeneration using the 70% partial hepatectomy (PHX) model in male, female, ovariectomized (OVX) and E2-treated OVX (OVX-E2) rats. Liver tissues were sampled at 0–48 hr after PHX and fat accumulation, fatty acid translocase (FAT/CD36), sterol regulatory element-binding protein (SREBP1c), peroxisome proliferator-activated receptor α (PPARα), proliferative cell nuclear antigen (PCNA) and ERα were examined by Oil Red O, qRT-PCR and immunohistochemistry, respectively. Hepatic fat accumulation was abundant in female and OVX-E2 compared to male and OVX rats. FAT/CD36 expression was observed in female, OVX and OVX-E2 at 0–12 hr after PHX, but not in male rats. At 0 hr, SREBP1c and PPARα were elevated in female and male rats, respectively, but were decreased after PHX in all rats. The PCNA labeling index reached a maximum at 36 hr and 48 hr in OVX-E2 and OVX rats, respectively. ERα expression in OVX-E2 was higher than OVX at 0–36 hr after PHX. In conclusion, these results indicated that estrogen and ERα might play an important role in fat accumulation related to FAT/CD36 during early phase of rat liver regeneration.
Keywords: lipid metabolism, hepatic fat accumulation, estrogen, fatty acid translocase/CD36, liver regeneration
I. Introduction
The liver plays an important role in the homeostasis of lipid metabolism because it regulates cholesterol synthesis and the production of triglycerides by lipogenesis, as well as the formation of lipoproteins that act as fatty acid transports [1]. Clinically, fatty liver, mainly non-alcoholic fatty liver disease (NAFLD), is a common cause of chronic liver disease and is closely associated with obesity, insulin resistance and dyslipidemia [11, 14]. The prevalence and severity of NAFLD are higher in men than in women during the reproductive years, but are increased in post-menopausal women, which suggests that estrogen is a protective factor [19, 35]. This sex difference affects liver lipid metabolism and the development of hepatic steatosis [25]. Furthermore, the effects of estrogen, mediated through estrogen receptor α (ERα), may be closely related with lipid metabolism [6, 26, 28]. In fact, it has been reported that the loss of estrogen promotes liver fat accumulation and leads to NAFLD and dyslipidemia [26].
We previously reported that estrogen accelerates hepatocyte proliferation through ERα in female rats, and enhances liver regeneration compared to male rats after 70% partial hepatectomy (PHX) [3]. During liver regeneration, fatty acids and triglycerides are required for hepatocyte proliferation [16, 17, 22], while cholesterol acts as a structural lipid for new cell membrane formation [7, 18, 27]. Recent reports also suggest that the transient fat accumulation in hepatocyte after PHX is thought to be a critical energy source for liver regeneration [12, 15, 29, 37]. Furthermore, the disruption of hepatic fat accumulation is associated with suppressed liver regeneration [8, 15, 30].
Generally, free fatty acids are taken up by hepatocytes via transport proteins including fatty acid translocase (FAT/CD36), fatty acid transport protein and liver fatty acid binding protein [23, 34]. FAT/CD36 is a transmembrane glycoprotein that plays an important role in lipid homeostasis by transporting long-chain fatty acids into hepatocytes [4], and its expression is higher in female compared to male rat liver [33]. In addition, estrogen induces FAT/CD36 expression in ovariectomized (OVX) female and male rat livers [5, 32]. However, the biological role of lipid accumulation and estrogen effects during liver regeneration is not well defined.
To characterize the effect of estrogen on lipid metabolism in liver regeneration, male, female, OVX and estrogen-treated OVX (OVX-E2) rats were subjected to PHX and hepatic fat accumulation, hepatic triglyceride and cholesterol levels were analyzed. The expression of lipid metabolism related genes, such as FAT/CD36, sterol regulatory element-binding protein (SREBP1c) and peroxisome proliferator-activated receptor α (PPARα) were evaluated using quantitative reverse transcription (qRT)-PCR. Additionally, FAT/CD36, proliferative cell nuclear antigen (PCNA) and ERα were examined by immunohistochemistry. In this study, we investigated the expression of FAT/CD36 in fat accumulation and the involvement of estrogen and ERα during early phase rat liver regeneration.
II. Materials and Methods
Animals and tissue preparation
Eight-week-old male (240–260 g) and female (190–210 g) Wistar rats were used in this study (Clea Japan, Shizuoka, Japan). Rats were housed in a specific pathogen-free facility under constant 12-hour dark/light conditions and allowed to acclimatize for one week prior to PHX and fed normal chow and drinking water ad libitum, except during an overnight fast before surgery. 70% PHX was performed according to the method described by Higgins and Anderson [9]. Briefly, left lateral and median lobes were excised and the liver tissues were retained for use as control samples (0 hr). After PHX, the rats were allowed free access to food and water until being sacrificed at 6, 12, 24, 36 and 48 hr post PHX. Rats were assigned to each group in a randomized manner and consisted of three rats. In one group of female rats, bilateral OVX was performed 18–20 days before PHX (OVX group). To confirm successful OVX, we performed vaginal smear. The other group received a single intraperitoneal injection of 17β-estradiol (9 μg/g bodyweight, Sigma-Aldrich, MO, USA) 12 hr before PHX (OVX-E2 group). The experimental protocol was approved by the Animal Ethics Review Committee of the University of Miyazaki (2012-502-5).
After sacrificing the animals, the liver tissues were isolated and fixed overnight in 4% paraformaldehyde (PFA, Merck, Darmstadt, Germany) in phosphate-buffered saline (PBS, pH 7.4) at room temperature (RT) and subsequently embedded in paraffin using standard methods. Some pieces of liver tissues were embedded in O.C.T compound (Sakura Finetek, Tokyo, Japan) to prepare fresh frozen liver sections. The remaining tissues were snap frozen on dry-ice and stored at −80°C until use in lipid extraction and qRT-PCR.
Liver lipid analysis
Hepatic fat accumulation was determined by Oil Red O staining. Briefly, liver cryosections (5 μm thick) were fixed with 4% PFA in PBS for 20 min and incubated in freshly prepared Oil Red O working solution (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) for 10 min and counterstained with hematoxylin for 15 sec. The random images of each Oil Red O stained liver frozen tissue sections (n = 3 per group) were captured at ×200 magnification. Relative areas of steatosis, expressed as % of fat accumulation, were quantified by histomorphometry using WinROOF image analyzer (Version 7, Mitani Corp, Tokyo, Japan). Moreover, total hepatic lipid was extracted from fresh frozen liver tissues using a lipid extraction kit (STA-612; Cell biolabs, San Diego, CA, USA). Hepatic triglycerides and cholesterol were then quantitated using colorimetric assay kits, according to the manufacturer’s instructions (Fujifilm Wako).
Immunohistochemistry
Paraffin-embedded tissues were cut into 5-μm-thick sections and placed onto silane-coated glass slides. For detection of FAT/CD36 and ERα, the sections were heated to 120°C for 15 min in 10 mM citrate buffer (pH 6.0). After inhibition of endogenous peroxidase activity with 3% H2O2 (Fujifilm, Wako) in methanol for 15 min, the sections were pre-incubated with normal goat IgG in 1% Bovine serum albumin (Sigma-Aldrich) in PBS to block non-specific binding then reacted with the primary antibodies for FAT/CD36 (NB400-144; 5 μg/ml, Novus biologicals, Colorado, USA), PCNA (PC-10; 6.5 μg/ml, Dako, Glostrup, Denmark) and ERα (6F11; 1:50, Thermo Fisher Scientific, MA, USA) overnight at RT. After washing with 0.075% Brij L23 (Sigma-Aldrich) in PBS, each slide was reacted with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Dako, 1:100) or HRP-conjugated goat anti-rabbit IgG (Dako, 1:200) antibody for 1 hr, then visualized with 3,3′-diaminobenzidine (DAB, Dojindo Chemicals, Kumamoto, Japan) and H2O2 or DAB, Ni, Co and H2O2. Hematoxylin or methylgreen was used for counterstaining. The negative control consisted of normal mouse or rabbit IgG (Dako) used at the same concentration instead of the primary antibodies. The number of PCNA or ERα positive cells was counted at ×400 magnification. PCNA positive cell per total number of counted cell was represented by a labeling index (PCNA-LI) while the number of ERα positive cell was expressed as percentage of positive cell per total number of hepatocyte. At least 2000 cells were counted in random fields.
RNA extraction and qRT-PCR analysis
Total RNA was extracted from snap frozen liver tissues using Isogen II (Nippon Gene, Tokyo, Japan) as reported previously [2]. RNA was reverse transcribed to cDNA using a Moloney murine leukemia virus reverse transcriptase enzyme (Invitrogen, Carlsbad, CA, USA). Transcript expression levels were analyzed by an ABI StepOne plus Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) using Fast SYBR Green (Applied Biosystems). GAPDH was used for normalization and the relative gene expression was calculated using the 2−ΔΔct method. The primer pairs were listed below. FAT/CD36 F-5'-CACACGCCGACCCTCACAACT-3' and R-5'-TCCGCCTTCTCTTTGACAGCC-3'; SREBP1c F-5'-AGAAGGCCAGTGGGTACCTG-3' and R-5'-TGCGGGCCACAAGAATAG-3'; PPARα F-5'-ATGCCCTCGAACTGGATGAC-3' and R-5'-CCCTCCTGCAACTTCTCAATG-3'; GAPDH F-5'-CCGCATCTTCTTGTGCGTG-3' and R-5'-GAGAAGGCAGCCCTGGTAAC-3'.
Statistical analysis
All data were expressed as the mean ± standard error (SE). Differences between experimental groups were assessed by Student’s t-test. P < 0.05 was considered statistically significant. All analyses were performed with the Statistical Package for Social Sciences (version 20.0 software, SPSS Inc., Chicago, IL, USA).
III. Results
Analysis of hepatic fat accumulation in rat liver after PHX
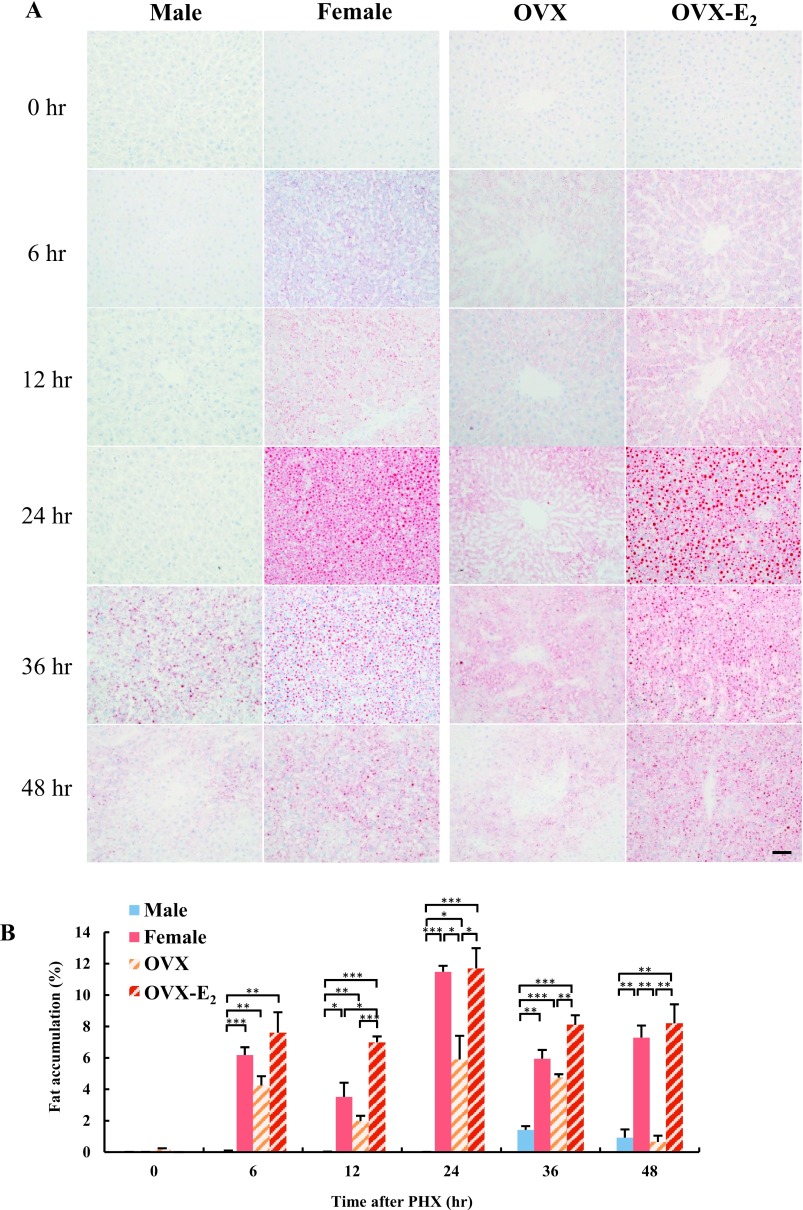
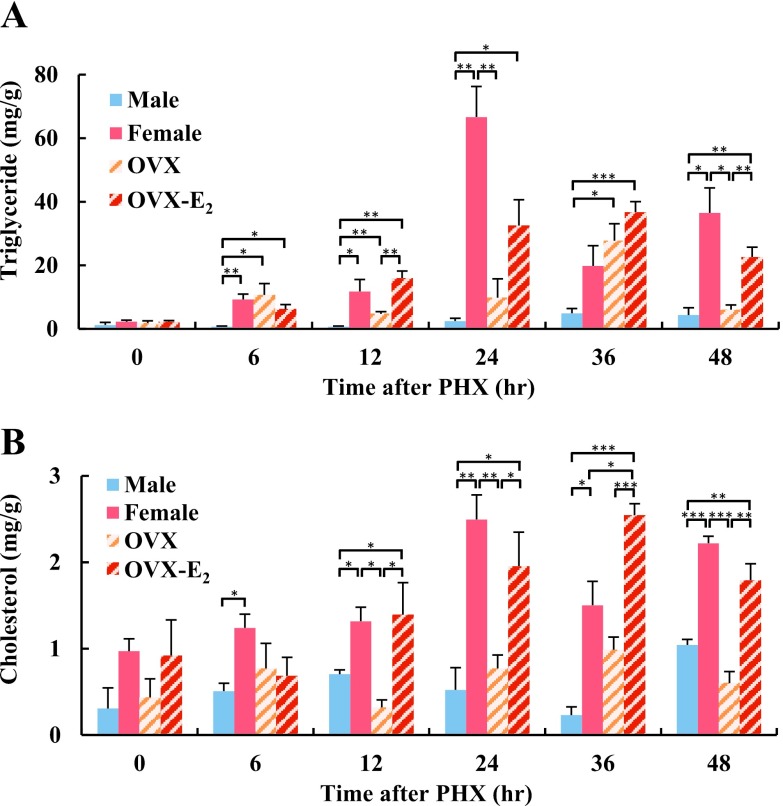
To investigate lipid metabolism during liver regeneration, we examined hepatic fat accumulation in male and female rat livers after PHX using Oil Red O staining. No fat accumulation was observed in male and female rat livers at 0 hr (Fig. 1A). In male rat livers, fat accumulation was observed at 36 hr after PHX, and was decreased at 48 hr. Interestingly, fat accumulation in female rat livers was observed from 6 hr and peaked at 24 hr, then gradually decreased until 48 hr after PHX. We hypothesized that differential levels of endogenous estrogen were likely to have caused the different temporal patterns of fat accumulation in male and female rat livers. To address this question, OVX and OVX-E2 rat livers were also stained with Oil Red O. Although fat accumulation was observed in OVX rat livers after PHX, more intense staining was found in OVX-E2 livers; indicating that estrogen may induce hepatic fat accumulation during liver regeneration. Semi-quantitative analysis confirmed that hepatic fat accumulation was significantly higher in female and OVX-E2 rats compared to male and OVX rats (Fig. 1B). Oil Red O staining detects lipids and neutral triglycerides in tissue sections, and subsequent analyses were performed to quantitate lipid components. In female rat livers, triglyceride and cholesterol levels were significantly higher, particularly at 24 hr after PHX, compared to male rat livers (Fig. 2A, B). In OVX-E2 rat livers, triglyceride levels were significantly higher at 12 and 48 hr, and cholesterol levels were higher at 12, 24, 36 and 48 hr compared to OVX rat livers. Altogether, these findings indicate that estrogen induced hepatic fat accumulation in female and OVX-E2 rat livers following PHX.
Fig. 1.
Fat accumulation in rat liver at different time points after PHX. (A) Fat accumulation in hepatocytes following PHX in male, female, OVX and OVX-E2 was examined by Oil Red O staining. Magnification 400×. Bar = 50 μm. (B) Semi-quantitative analysis of Oil Red O stained liver tissue sections to quantify fat deposition as a percentage of red stained area compared with the total section area. Blue, red, diagonal bar on white background and diagonal bar on red background graphs represent male, female, OVX and OVX-E2, respectively. Data represent the mean ± SE from 3 rats per group and time point. Asterisks indicate statistically significant differences (*p < 0.05, **p < 0.01 and ***p < 0.001).
Fig. 2.
Hepatic lipid content in regenerating liver after PHX at different time points. (A) Triglycerides and (B) cholesterol in regenerating liver after PHX were assessed using enzymatic assays. Blue, red, diagonal bar on white background and diagonal bar on red background graphs represent male, female, OVX and OVX-E2, respectively. Data represent the mean ± SE from 3 rats per group and time point. Asterisks indicate statistically significant differences (*p < 0.05, **p < 0.01 and ***p < 0.001).
Analysis of the expression of genes involved in lipid metabolism in rat livers after PHX
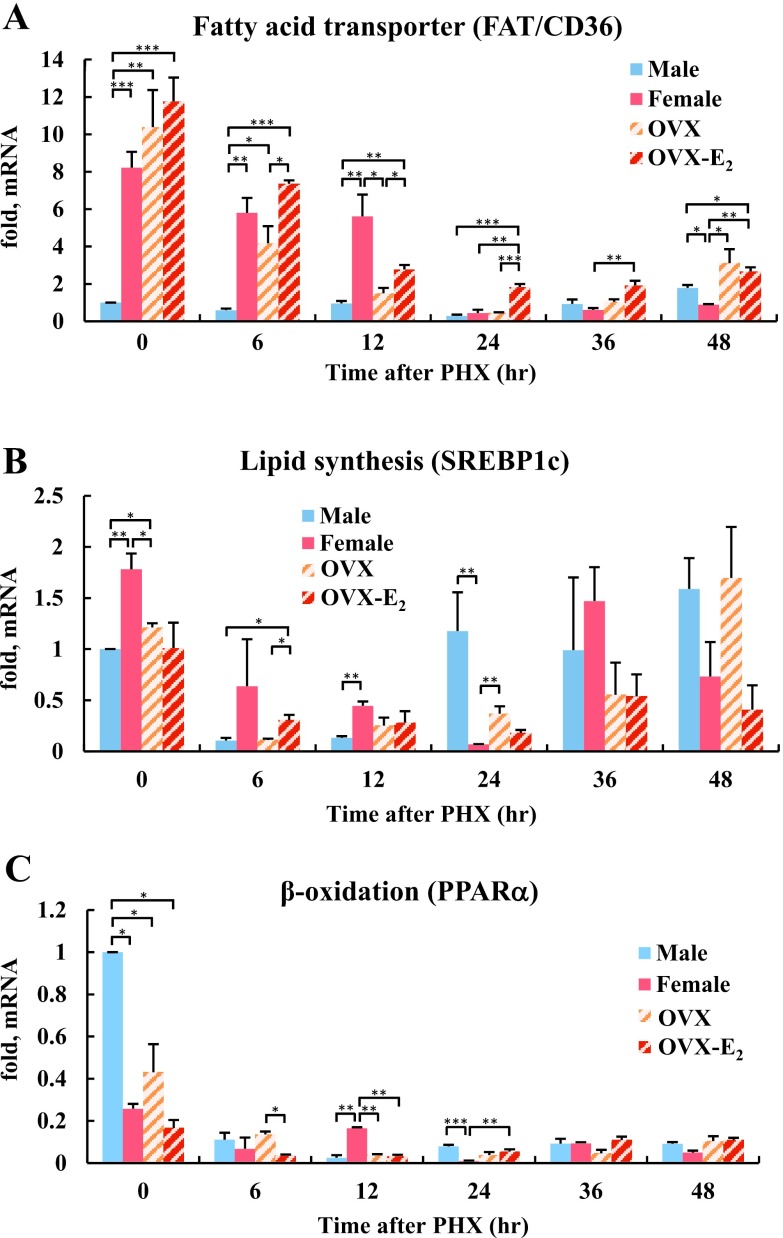
Estrogen dependent genes involved in lipid metabolism, such as FAT/CD36, SREBP1c and PPARα, were examined by qRT-PCR. In female rat livers at 0 hr, FAT/CD36 mRNA expression was 8-fold higher than in male rat livers (Fig. 3A). Even after PHX, marked expression of FAT/CD36 was observed in female rats and continued until 12 hr after PHX. Dramatic decreases in FAT/CD36 expression in female rat liver was correlated with peak hepatic fat accumulation at 24 hr after PHX (Fig. 1A). At 48 hr after PHX, hepatic FAT/CD36 mRNA was 2-fold higher in male rats than in female rats. In addition, hepatic FAT/CD36 mRNA in OVX-E2 rats was also significantly higher than in OVX rats at 6–24 hr after PHX. Lipid synthesis gene, SREBP1c mRNA expression was also higher at 0 and 12 hr after PHX in female compared to male rat livers (Fig. 3B). Interestingly, SREBP1c mRNA was increased in male rat livers, whereas it was decreased in female rat livers 24 hr after PHX. At 6 hr after PHX, SREBP1c mRNA expression was significantly higher in OVX-E2 rats compared to OVX rats. β-oxidation gene, PPARα mRNA expression was higher in male compared to female rat livers at 0 hr (Fig. 3C). However, PPARα mRNA expression was markedly decreased after PHX in all rats. PPARα was elevated in female rat livers at 12 hr, while its expression become predominant again at 24 hr in male rats. Taken together, these data revealed that FAT/CD36 and SREBP1c mRNA increased in female and OVX-E2 rats, while PPARα mRNA decreased in all rats during the early phase of liver regeneration.
Fig. 3.
The expression of genes involved in lipid metabolism during rat liver regeneration. (A) Quantitative RT-PCR analysis of mRNA levels for the fatty acid transporter gene FAT/CD36, (B) lipid synthesis gene SREBP1c and (C) the β-oxidation gene PPARα. The mRNA expression levels are represented as fold change compared with male rat liver at 0 hr after PHX. Blue, red, diagonal bar on white background and diagonal bar on red background graphs represent male, female, OVX and OVX-E2, respectively. Data represent the mean ± SE from 3 rats per group and time point. Asterisks indicate statistically significant differences (*p < 0.05, **p < 0.01 and ***p < 0.001).
Immunohistochemical analysis of FAT/CD36 levels in rat livers after PHX
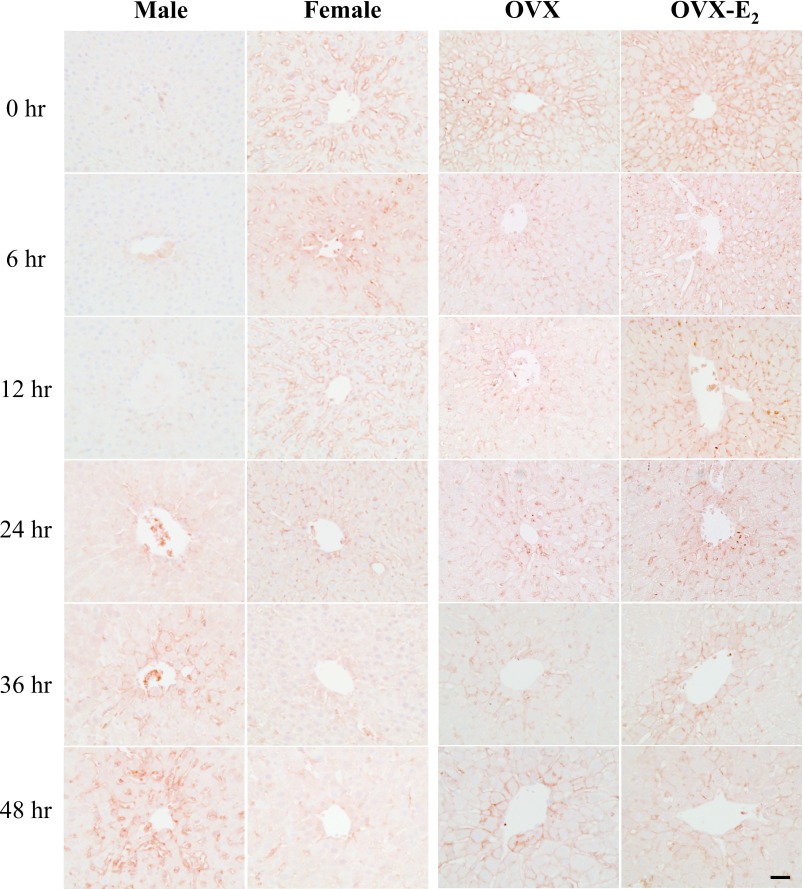
The hepatic FAT/CD36 expression was examined by immunohistochemistry. In male rat livers, FAT/CD36 was not observed at 0–12 hr, weak expression was detected at 24 hr and gradually increased at 36 and 48 hr after PHX (Fig. 4). However, the opposite expression pattern was observed in female rats; FAT/CD36 was strongly expressed at 0–12 hr and decreased at 24 hr, and was not observed at 36–48 hr after PHX. FAT/CD36 expression was found in both OVX and OVX-E2 rat livers, however staining intensity was higher in OVX-E2 rat livers.
Fig. 4.
Immunohistochemical detection of FAT/CD36 in rat livers after PHX. Immunohistochemical localization of FAT/CD36 in paraffin-embedded sections of male, female, OVX and OVX-E2 rat livers at different time points after PHX. Magnification 400×. Bar = 50 μm.
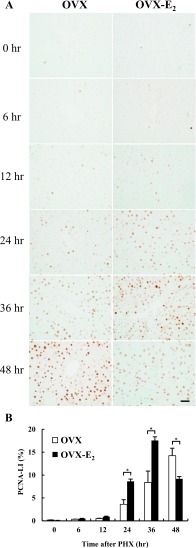
Immunohistochemical detection of PCNA in rat livers after PHX
We previously reported that accelerated liver regeneration was found in female rats compared to male rats [3]. The effect of estrogen in liver regeneration was confirmed in OVX and OVX-E2 rat livers using immunohistochemistry for PCNA (Fig. 5A). In OVX rat livers, PCNA positive cells were significantly increased at 24 hr and peaked at 48 hr. Interestingly, PCNA positive cells were significantly increased at 24 hr and reached a peak at 36 hr after PHX in OVX-E2 rat livers. The PCNA-LI indicated that the peak number of proliferating cells in OVX and OVX-E2 rats was observed at 48 and 36 hr after PHX, respectively (Fig. 5B). These results indicated that exogenous estrogen accelerated liver regeneration for 12 hr in OVX-E2 rats compared to OVX rats.
Fig. 5.
Hepatocyte proliferation in rat livers after PHX. (A) Immunohistochemical analysis of PCNA staining in paraffin-embedded sections from OVX and OVX-E2 rat livers at different time points after PHX. Magnification 400×. Bar = 50 μm. (B) PCNA-LI in OVX and OVX-E2 rat livers after PHX. White and black bar-graphs represent OVX and OVX-E2, respectively. Data represent the mean ± SE from 3 rats per group and time point. Asterisks indicate statistically significant differences (*p < 0.05).
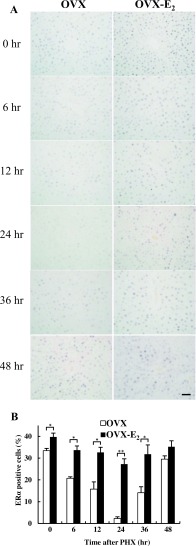
Immunohistochemical detection of ERα in rat livers after PHX
It has been previously reported that estrogen accelerates liver regeneration via ERα [3]. Therefore, the expression of ERα was examined by immunohistochemistry. In normal state at 0 hr, the number of ERα positive cells was significantly lower and exhibited weaker intensity in OVX rat livers compared to OVX-E2 rat livers. After PHX, the number of ERα positive cells was decreased until 24 hr, increased at 36 hr and 48 hr in OVX rat livers (Fig. 6A). In contrast, ERα was expressed at all-time points in OVX-E2 rat livers, exhibiting stronger staining intensity compared to OVX rat livers after PHX. Quantitative analysis revealed that the number of ERα positive cells was significantly higher at 0–36 hr in OVX-E2 compared to OVX rats after PHX. At 48 hr, the number of ERα positive cells was not significantly different in OVX and OVX-E2 rat livers (Fig. 6B). These results indicated that the ERα expression was downregulated during liver regeneration in the absence of estrogen. However, exogenous estrogen could maintain the expression of ERα and its effects during rat liver regeneration.
Fig. 6.
ERα expression in rat livers after PHX. (A) Immunohistochemical analysis of ERα staining in paraffin-embedded sections from OVX and OVX-E2 rat livers at different time points after PHX. Magnification 400×. Bar = 50 μm. (B) The number of ERα positive cells in OVX and OVX-E2 rat livers after PHX. White and black bar-graphs represent OVX and OVX-E2, respectively. Data represent the mean ± SE from 3 rats per group and time point. Asterisks indicate statistically significant differences (*p < 0.05 and **p < 0.01).
IV. Discussion
In this study, the upregulation of FAT/CD36 and hepatic fat accumulation were found in female rat livers after PHX. Multiple studies have reported that hepatic fat accumulation occurs from 24 to 48 hr after 70% PHX in rats [17, 21, 36], which is concomitant with hepatocellular proliferation [10, 39]. Our results revealed that female rat livers exhibit marked fat accumulation in regenerating liver that started from 6 hr and peaked at 24 hr after PHX, whereas fat accumulation in male rat livers started from 36 hr after PHX. Although estrogen treatment of OVX mice has been reported to suppress lipogenesis and liver lipid infiltration in several fatty liver models [13, 38], our data suggests that female and OVX-E2 regenerating livers have much greater fat accumulation after PHX than male and OVX rats. Our data further suggests that estrogen may be involved in the regulation of hepatic fat accumulation that serves in energy storage during liver regeneration after PHX. These results correlated with the peak number of proliferating hepatocytes, which occurred earlier in OVX-E2 compared to OVX rats. In general, glucose is the main energy source for cellular metabolism when it is sufficiently available. PHX is known to induce systemic metabolic changes, including hypoglycemia and glycogen depletion, and leads to a change to the use of fat as energy substrate [17, 22]. Therefore, hepatic fat accumulation during early liver regeneration is considered to be an important process that is closely linked to the liver regenerative process.
The results presented in this study revealed that maximal hepatocyte proliferation in OVX-E2 occurred earlier than that in OVX rats after PHX, which recapitulated an essential role for estrogen during liver regeneration. In OVX-E2 rat livers, the number of ERα positive cells was higher and staining intensity was stronger compared to OVX rat livers. These results indicate that liver ERα expression is inducible by estrogen administration in OVX rats. Therefore, estrogen and ERα may play an important role for liver regeneration [3]. On the other hand, androgen and its receptor, but not estrogen, are related to liver diseases. It is reported that the serum testosterone is frequently reduced in men with cirrhosis and falls progressively with increasing severity of liver disease [31]. Furthermore, testosterone replacement reduced NAFLD in castrated male rats [24]. In addition, androgen receptor suppresses hepatocellular carcinoma (HCC) progression in a mouse model [20]. However, in this study, we did not evaluate the biological effect of androgen during liver regeneration. Therefore, androgen and its receptor should be evaluated in future studies to further clarify the detailed mechanism of liver regeneration.
The mechanism underlying the hepatic fat accumulation observed after PHX can be attributed to three different major pathways: 1) increased hepatic uptake of fatty acids 2) decreased β-oxidation and 3) increased de novo lipogenesis. It has been reported that during the early stage of liver regeneration, de novo lipogenesis represents an initial supply of lipid in the liver followed by uptake of lipolytic products from peripheral adipose tissue, leading to increased hepatic lipid levels at 24 hr after PHX [15]. Our data revealed that rather than de novo lipogenesis, increased fatty acid uptaken by hepatocyte occurred through FAT/CD36 in female and OVX-E2 rats at the early 12 hr time point. This coincided with rapidly decreased β-oxidation through PPARα after PHX, leading to hepatic steatosis in female and OVX-E2 rat livers. On the other hand, FAT/CD36 expression was negligible in male rats during early liver regeneration after PHX. All these findings are emphasizing the effects of estrogen on FAT/CD36 expression. Although, it has been reported that increased hepatic FAT/CD36 levels are observed in estrogen treated OVX rats [32], our results indicate that there is no significant difference in hepatic FAT/CD36 levels between OVX and OVX-E2 at 0 hr. However, hepatic FAT/CD36 mRNA was significantly higher at 6–24 hr after PHX in OVX-E2 rats, compared to OVX rats. This discrepancy may be due to differences in dosing and duration of estrogen administration.
In conclusion, these results suggest that the effect of estrogen on hepatic fat accumulation, maybe related to FAT/CD36 expression, during the early stage of liver regeneration after PHX in female rats.
V. Conflicts of Interest
The authors declare that there are no conflicts of interest.
VI. Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 16K08471 to Y. Hishikawa).
VII. References
- 1.Abu Rmilah A., Zhou W., Nelson E., Lin L., Amiot B. and Nyberg S. L. (2019) Understanding the marvels behind liver regeneration. Wiley Interdiscip. Rev. Dev. Biol. 8; e340. doi: 10.1002/wdev.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali M. N., Choijookhuu N., Takagi H., Srisowanna N., Nguyen Nhat Huynh M., Yamaguchi Y., Synn Oo P., Tin H. K. M., Sato K., Yamaguchi R. and Hishikawa Y. (2018) The HDAC inhibitor, SAHA, prevents colonic inflammation by suppressing pro-inflammatory cytokines and chemokines in DSS-induced colitis. Acta Histochem. Cytochem. 51; 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batmunkh B., Choijookhuu N., Srisowanna N., Byambatsogt U., Synn Oo, P., Noor Ali M., Yamaguchi Y. and Hishikawa Y. (2017) Estrogen accelerates cell proliferation through estrogen receptor alpha during rat liver regeneration after partial hepatectomy. Acta Histochem. Cytochem. 50; 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonen A., Han X. X., Narendra N. N., Glatz J. F., Lally J., Snook L. A. and Luiken J. J. (2009) FAT/CD36 expression is not ablated in spontaneously hypertensive rats. J. Lipid Res. 50; 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung L., Andersen M., Gustavsson C., Odeberg J., Fernández-Pérez L., Norstedt G. and Tollet-Egnell P. (2007) Hormonal and nutritional regulation of alternative CD36 transcripts in rat liver—a role for growth hormone in alternative exon usage. BMC Mol. Biol. 8; 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow J. D., Jones M. E., Prelle K., Simpson E. R. and Boon W. C. (2011) A selective estrogen receptor α agonist ameliorates hepatic steatosis in the male aromatase knockout mouse. J. Endocrinol. 210; 323–334. doi: 10.1530/JOE-10-0462. [DOI] [PubMed] [Google Scholar]
- 7.Delgado-Coello B., Briones-Orta M. A., Macías-Silva M. and Mas-Oliva J. (2011) Cholesterol: recapitulation of its active role during liver regeneration. Liver Int. 31; 1271–1284. [DOI] [PubMed] [Google Scholar]
- 8.Hamano M., Ezaki H., Kiso S., Furata K., Egawa M., Kizu T., Chatani N., Kamada Y., Yoshids Y. and Takehara T. (2014) Lipid overloading during liver regeneration causes delayed hepatocyte DNA replication by increasing ER stress in mice with simple hepatic steatosis. J. Gastroenterol. 49; 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins G. M. and Anderson R. M. (1931) Experimental pathology of the liver. 1. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. 12; 186–202. [Google Scholar]
- 10.Huang J. and Rudnick D. A. (2014) Elucidating the metabolic regulation of liver regeneration. Am. J. Pathol. 184; 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung U. J. and Choi M. S. (2014) Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15; 6184–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kachaylo E., Tschuor C., Calo N., Borgeaud N., Ungethüm U., Limani P., Piguet A. C., Dufour J. F., Foti M., Graf R., Clavien P. A. and Humar B. (2017) PTEN down-regulation promotes β-oxidation to fuel hypertrophic liver growth after hepatectomy in mice. Hepatology 66; 908–921. [DOI] [PubMed] [Google Scholar]
- 13.Kamada Y., Kiso S., Yoshida Y., Chatani N., Kizu T., Hamano M., Tsubakio M., Takemura T., Ezaki H., Hayashi N. and Takehara T. (2011) Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am. J. Physiol. Gastrointest. Liver Physiol. 301; G1031–1043. doi: 10.1152/ajpgi.00211.2011. [DOI] [PubMed] [Google Scholar]
- 14.Kitade H., Chen G., Ni Y. and Ota T. (2017) Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients 9; E387. doi: 10.3390/nu9040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohjima M., Tsai T. H., Tackett B. C., Thevananther S., Li L., Chang B. H. and Chan L. (2013) Delayed liver regeneration after partial hepatectomy in adipose differentiation related protein-null mice. J. Hepatol. 59; 1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai H. S., Chen W. J. and Chen K. M. (1992) Energy substrate for liver regeneration after partial hepatectomy in rats: effects of glucose vs fat. JPEN. J. Parenter. Enteral. Nutr. 16; 152–156. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Yang F., Li J., Wang J., Wang X., Zhang Y., Yuan X., Zhu W. and Shi X. (2018) Mesenchymal stem cells enhance liver regeneration via improving lipid accumulation and hippo signaling. Stem Cells Int. 13; 7652359. doi: 10.1155/2018/7652359. e Collection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo S. G., Celli N., Caboni M., Murzilli S., Salvatore L., Morgano A., Vacca M., Pagliani T., Parini P. and Moschetta A. (2010) Down-regulationof the LXR transcriptome provides the requisite cholesterol levels to proliferating hepatocytes. Hepatology 51; 1334–1344. [DOI] [PubMed] [Google Scholar]
- 19.Lonardo A., Nascimbeni F., Ballestri S., Fairweather D., Win S., Than T. A., Abdelmalek M. F. and Suzuki A. (2019) Sex differences in NAFLD: State of the art and identification of research gaps. Hepatology. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma W. L., Lai H. C., Yeh S., Cai X. and Chang C. (2014) Androgen receptor roles in hepatocellular carcinoma, fatty liver, cirrhosis and hepatitis. Endocr. Relat. Cancer 21; R165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray A. B., Strecker W. and Silz S. (1981) Ultrastructural changes in rat hepatocytes after partial hepatectomy, and comparison with biochemical results. J. Cell Sci. 50; 433–448. [DOI] [PubMed] [Google Scholar]
- 22.Nakatani T., Ozawa K., Asano M., Ukikusa M., Kamiyama Y. and Tobe T. (1981) Differences in predominant energy substrate in relation to the resected hepatic mass in the phase immediately after hepatectomy. J. Lab. Clin. Med. 97; 887–898. [PubMed] [Google Scholar]
- 23.Nguyen P., Leray V., Diez M., Serisier S., Le Bloc’h J., Siliart B. and Dumon H. (2008) Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. (Berl). 92; 272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaenko L., Jia Y., Wang C., Diaz-Arjonilla M., Yee J. K., French S. W., Liu P. Y., Laurel S., Chong C., Lee K., Lue Y., Lee W. N. and Swerdloff R. S. (2014) Testosterone replacement ameliorates nonalcoholic fatty liver disease in castrated male rats. Endocrinology 155; 417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmisano B. T., Zhu L., Eckel R. H. and Stafford J. M. (2018) Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 15; 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmisano B. T., Zhu L. and Stafford J. M. (2017) Role of estrogens in the regulation of liver lipid metabolism. Adv. Exp. Med. Biol. 1043; 227–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng J., Yu J., Xu H., Kang C., Shaul P. W., Guan Y., Zhang X. and Su W. (2018) Enhanced liver regeneration after partial hepatectomy in sterol regulatory element-binding protein (SREBP)-1c-null mice is associated with increased hepatocellular cholesterol availability. Cell. Physiol. Biochem. 47; 784–799. [DOI] [PubMed] [Google Scholar]
- 28.Qiu S., Vazquez J. T., Boulger E., Liu H., Xue P., Hussain M. A. and Wolfe A. (2017) Hepatic estrogen receptor α is critical for regulation of gluconeogenesis and lipid metabolism in males. Sci. Rep. 7; 1661. doi: 10.1038/s41598-017-01937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudnick D. A. and Davidson N. O. (2012) Functional relationships between lipid metabolism and liver regeneration. Int. J. Hepatol. 2012; 549241. doi: 10.1155/2012/549241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shteyer E., Liao Y., Muglia L. J., Hruz P. W. and Rudnick D. A. (2004) Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology 40; 1322–1332. [DOI] [PubMed] [Google Scholar]
- 31.Sinclair M., Gow P. J., Grossmann M., Shannon A., Hoermann R. and Angus P. W. (2016) Low serum testosterone is associated with adverse outcome in men with cirrhosis independent of the model for end-stage liver disease score. Liver Transpl. 22; 1482–1490. [DOI] [PubMed] [Google Scholar]
- 32.Singhal R., Shankar K., Badger T. M. and Ronis M. J. (2009) Hepatic gene expression following consumption of soy protein isolate in female Sprague-Dawley rats differs from that produced by 17{beta}-estradiol treatment. J. Endocrinol. 202; 141–152. [DOI] [PubMed] [Google Scholar]
- 33.Ståhlberg N., Rico-Bautista E., Fisher R. M., Wu X., Cheung L., Flores-Morales A., Tybring. G., Norstedt G. and Tollet-Egnell P. (2004) Female-predominant expression of fatty acid translocase/CD36 in rat and human liver. Endocrinology 145; 1972–1979. [DOI] [PubMed] [Google Scholar]
- 34.Storch J. and Thumser A. E. (2010) Tissue-specific functions in the fatty acid-binding protein family. J. Biol. Chem. 285; 32679–32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki A. and Abdelmalek M. F. (2009) Nonalcoholic fatty liver disease in women. Womens Health (Lond). 5; 191–203. [DOI] [PubMed] [Google Scholar]
- 36.VanNoorden C. J., Vogels I. M. and James J. (1994) Adaptive sex-dependent changes in the zonation of carbohydrate and lipid metabolism in rat liver lobules after partial hepatectomy. Hepatology 20; 714–724. [DOI] [PubMed] [Google Scholar]
- 37.Wu H., Ploeger J. M., Kamarajugadda S., Mashek D. G., Mashek M. T., Manivel J. C., Shekels L. L., Lapiro J. L. and Albrecht J. (2019) Evidence for a novel regulatory interaction involving cyclin D1, lipid droplets, lipolysis, and cell cycle progression in hepatocytes. Hepatol. Commun. 3; 406–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu L., Brown W. C., Cai Q., Krust A., Chambon P., McGuinness O. P. and Stafford J. M. (2013) Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 62; 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou Y., Bao Q., Kumar S., Hu M., Wang G. Y. and Dai G. (2012) Four waves of hepatocyte proliferation linked with three waves of hepatic fat accumulation during partial hepatectomy-induced liver regeneration. PLoS One 7; e30675. doi: 10.1371/journal.pone.0030675 [DOI] [PMC free article] [PubMed] [Google Scholar]