Abstract
Background
Surveillance of individuals at high risk of pancreatic ductal adenocarcinoma (PDAC) and its precursors might lead to better outcomes. The aim of this study was to determine the prevalence and outcomes of PDAC and high‐risk neoplastic precursor lesions among such patients participating in surveillance programmes.
Methods
A multicentre study was conducted through the International CAncer of the Pancreas Screening (CAPS) Consortium Registry to identify high‐risk individuals who had undergone pancreatic resection or progressed to advanced PDAC while under surveillance. High‐risk neoplastic precursor lesions were defined as: pancreatic intraepithelial neoplasia (PanIN) 3, intraductal papillary mucinous neoplasia (IPMN) with high‐grade dysplasia, and pancreatic neuroendocrine tumours at least 2 cm in diameter.
Results
Of 76 high‐risk individuals identified in 11 surveillance programmes, 71 had undergone surgery and five had been diagnosed with inoperable PDAC. Of the 71 patients who underwent resection, 32 (45 per cent) had PDAC or a high‐risk precursor (19 PDAC, 4 main‐duct IPMN, 4 branch‐duct IPMN, 5 PanIN‐3); the other 39 patients had lesions thought to be associated with a lower risk of neoplastic progression. Age at least 65 years, female sex, carriage of a gene mutation and location of a lesion in the head/uncinate region were associated with high‐risk precursor lesions or PDAC. The survival of high‐risk individuals with low‐risk neoplastic lesions did not differ from that in those with high‐risk precursor lesions. Survival was worse among patients with PDAC. There was no surgery‐related mortality.
Conclusion
A high proportion of high‐risk individuals who had surgical resection for screening‐ or surveillance‐detected pancreatic lesions had a high‐risk neoplastic precursor lesion or PDAC at the time of surgery. Survival was better in high‐risk individuals who had either low‐ or high‐risk neoplastic precursor lesions compared with that in patients who developed PDAC.
Abstract
Antecedentes
Se podrían obtener mejores resultados con el seguimiento de individuos de alto riesgo para adenocarcinoma ductal pancreático (pancreatic ductal adenocarcinoma, PDAC) y lesiones precursoras. El objetivo de este estudio fue determinar la prevalencia y los resultados del PDAC y de las lesiones precursoras de alto riesgo neoplásico en pacientes que participaron en programas de seguimiento.
Métodos
Se llevó a cabo un estudio multicéntrico a través del registro internacional del consorcio CAPS (Common Automotive Platform Standard) para identificar a las personas de alto riesgo que se habían sometido a una resección pancreática o habían progresado a PDAC avanzado mientras estaban en seguimiento. Se definieron como lesiones neoplásicas precursoras de alto riesgo la neoplasia intraepitelial pancreática de tipo 3 (PanIN‐3), la neoplasia papilar mucinosa intraductal (intraductal papillary mucinous neoplasia, IPMN) con displasia de alto grado y los tumores neuroendocrinos pancreáticos (pancreatic neuroendocrine tumours, PanNET) de ≥ 2 cm de diámetro.
Resultados
De 76 individuos con lesiones de alto riesgo identificados en 11 programas de seguimiento, 71 fueron tratados quirúrgicamente y 5 fueron diagnosticados de un PDAC inoperable. De las 71 resecciones, 32 (45%) tenían PDAC o una lesión precursora de alto riesgo (19 PDAC, 4 IPMN de conducto principal, 4 IPMN de rama secundaria y 5 PanIN‐3). Los otros 39 pacientes tenían lesiones que se consideraron asociadas con un menor riesgo de progresión neoplásica. La edad ≥ 65 años, el sexo femenino, el ser portador de una mutación genética y la localización de la lesión en la cabeza/proceso uncinado fueron factores asociados a las lesiones precursoras de alto riesgo o al PDAC. No hubo diferencias en la supervivencia de individuos de alto riesgo con lesiones neoplásicas de bajo riesgo frente a aquellos que presentaron lesiones precursoras de alto riesgo. La supervivencia fue peor en los pacientes con PDAC. No hubo mortalidad relacionada con la cirugía.
Conclusión
Un elevado porcentaje de individuos de alto riesgo que se sometieron a resección quirúrgica tras la detección de lesiones pancreáticas en el seguimiento tenían una lesión precursora neoplásica de alto riesgo o un PDAC. La supervivencia fue mejor en individuos de alto riesgo que tenían lesiones precursoras neoplásicas de bajo o alto riesgo en comparación con aquellos pacientes que habían desarrollado un PDAC.
Introduction
Despite improvements in treatments for pancreatic ductal adenocarcinoma (PDAC), it remains the third leading cause of cancer death in the USA, with a 5‐year survival rate of only 8 per cent1. By 2030, PDAC is projected to become the second leading cause of cancer‐related death in the USA2. Advances in screening, prevention and treatment have the potential to change pancreatic cancer incidence and death rates2. Inherited susceptibility is thought to be a major factor in the development of PDAC, accounting for 5–10 per cent of cases3. Surveillance for PDAC and its precursor lesions in asymptomatic high‐risk individuals is increasingly being performed worldwide4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15. These high‐risk individuals can be categorized into two groups: carriers of known PDAC‐associated gene mutations (especially carriers of deleterious mutations in CKDN2A, BRCA2, BRCA1, ATM, TP53, PRSS1 or STK11), and first‐degree relatives in familial PDAC (clustering of at least 2 first‐degree blood relatives with PDAC)16. The goals of surveillance have been described previously17 by the International CAncer of the Pancreas Screening (CAPS) Consortium. They include: detection and treatment of early invasive pancreatic cancer (T1 N0 M0) at baseline or follow‐up; detection and treatment of any invasive resectable cancer at baseline screening; detection and treatment of multifocal pancreatic intraepithelial neoplasia (PanIN) 3; and the detection and treatment of intraductal papillary mucinous neoplasia (IPMN) with high‐grade dysplasia.
Few studies have described the surgical pathology findings in high‐risk patients who have undergone surgery15, 18. The CAPS Consortium Registry was created to gather information rapidly about the experience of surveillance. In this study, the diagnostic yield and outcomes of high‐risk individuals who underwent surgical resection or progressed to invasive cancer were evaluated, and the characteristics of patients who developed high‐risk neoplastic precursor lesions or PDAC were examined.
Methods
All participating centres in the CAPS Consortium (36 centres in 9 countries) were requested to enter patient information data for high‐risk individuals participating in their PDAC surveillance programmes who had either undergone pancreatic surgery because of the detection of a suspicious pancreatic lesion, or progressed to advanced unresectable malignant disease. Data were collected through the use of web‐based electronic data capture software (OmniComm Europe, Bonn, Germany). Anonymized clinical and demographic information was collected relating to age, sex, tobacco and alcohol use, diabetes mellitus, history of pancreatitis, BMI, known gene mutations, and family history of PDAC. In addition, pancreatic imaging modalities that detected the lesions, characteristics of the lesions detected by imaging, timing of detection, therapy, pathology, and outcomes after surgery or diagnosis of advanced PDAC were also recorded.
Research protocols of all participating centres were based largely on the consensus statements of the CAPS Consortium, produced in 201317, acknowledging that the nature of this study and its timespan made it inevitable that differences between protocols of screening centres would exist. Index and follow‐up examinations were carried out using MRI and/or endoscopic ultrasonography. However, when suspect lesions were detected, other modalities, such as CT, were often used for further characterization and staging.
All individuals in this study provided written informed consent for their participation in the respective PDAC surveillance programmes as approved by the ethics committees of the participating centres. The study was conducted in accordance with the Declaration of Helsinki.
Participants with pathologically proven high‐risk neoplastic precursor lesions or PDAC were compared with those who had surgery but in whom the resection specimen harboured no high‐risk precursor lesion or PDAC. High‐risk neoplastic precursor lesions were defined as unifocal or multifocal PanIN‐3 lesions, main‐ and branch‐duct IPMNs with high‐grade dysplasia, and pancreatic neuroendocrine tumours (PanNETs) at least 2 cm in size19, 20.
Statistical analysis
Descriptive statistics were used to characterize patient and lesion characteristics. Univariable analyses (χ2 or Fisher's exact test where indicated for categorical variables, independent‐samples t test for continuous variables) were performed on possible risk factors associated with PDAC or high‐risk neoplastic precursor lesions. All variables with P < 0·200 in univariable analysis were included in the multivariable analysis. Survival comparisons for different subgroups were plotted as Kaplan–Meier curves, and hazard ratios calculated using the log rank test. All analyses were conducted using SPSS® version 21 (IBM, Armonk, New York, USA).
Results
A total of 76 high‐risk individuals were included from 11 PDAC surveillance programmes in four countries (USA, the Netherlands, Israel and Italy). Between the 11 centres, some 1700 patients considered to be at high risk underwent surveillance, of whom approximately 70 per cent were women. Of the 76 included, five were diagnosed with advanced disease during surveillance and 71 underwent surgery for a suspected lesion, of whom two were discovered to have inoperable disease. Baseline characteristics for all 76 high‐risk individuals are summarized in Table 1.
Table 1.
Baseline characteristics of high‐risk individuals who had surgery after detection of a suspicious pancreatic lesion or were diagnosed with advanced pancreatic cancer
| High‐risk individuals who had surgery (n = 71) | High‐risk individuals diagnosed with advanced PDAC (n = 5) | |
|---|---|---|
| Age at surgery or diagnosis (years) * | 60·3(11·6) (59·8; 36–80) | 70·5(6·6) (65–80) |
| Sex ratio (M : F) | 37 : 34 | 1 : 4 |
| Ethnicity | ||
| White | 67 (94) | 5 (100) |
| Black | 3 (4) | ‐ |
| Other | 1 (1) | – |
| Genetic background | ||
| Familial pancreatic cancer | 52 (73) | 4 (80) |
| CDKN2A (FAMMM syndrome) | 7 (10) | – |
| BRCA2 (HBOC) | 3 (4) | – |
| Peutz–Jeghers syndrome | 3 (4) | 1 (20) |
| BRCA1 (HBOC) | 1 (1) | – |
| TP53 (Li–Fraumeni syndrome) | 1 (1) | – |
| MMR (Lynch syndrome) | 1 (1) | – |
| APC | 1 (1) | – |
| ATM | 1 (1) | – |
| PRRS1 (hereditary pancreatitis) | 1 (1) | – |
| No. of first‐degree relatives with PDAC * | 1·5(0·8) (1·0; 0–3) | 1·4(0·9) (0–2) |
| No. of second‐degree relatives with PDAC * | 1·1(1·0) (1·0; 0–4) | 0·3(0·6) (0–1) |
| Youngest family member affected by PDAC * | 55·5(10·8) (33–77) | 63·3(7·5) (52–68) |
| BMI (kg/m 2 ) * | 27·3(5·1) (26·6; 18–48) | 26·1(3·7) (23–31) |
| Personal history of diabetes | 11 (15) | 2 (40) |
| Duration of diabetes before surgery or diagnosis (months) * | 36·6(23·7) (45·0; 0–63) | 66·0(76·4) (12–120) |
| Personal history of pancreatitis | 9 (13) | 1 (20) |
| Smoking behaviour | ||
| Never smoker | 46 (65) | 3 (60) |
| Former smoker | 20 (28) | 2 (40) |
| Current smoker | 3 (4) | – |
| No data | 2 (3) | |
| ≥ 10 pack‐years in total | 11 (15) | 1 (20) |
| ≥ 20 pack‐years in total | 4 (6) | – |
| Alcohol consumption | ||
| Never consumer | 38 (54) | 2 (40) |
| Former consumer | 12 (17) | 1 (20) |
| Current consumer | 19 (27) | 2 (40) |
| No data | 2 (3) | – |
| ≥ 10 units per week (current or past) | 5 (7) | – |
| ≥ 20 units per week (current or past) | 2 (3) | – |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.) (median; range). PDAC, pancreatic ductal adenocarcinoma; FAMMM, familial atypical multiple mole melanoma syndrome; HBOC, hereditary breast and ovarian cancer; MMR, mismatch repair genes; APC, adenomatous polyposis coli; ATM, ataxia telangiectasia mutated.
High‐risk neoplastic precursor lesions and (advanced) pancreatic ductal adenocarcinoma
High‐risk neoplastic precursor lesions or PDAC were present in the surgical specimens of 32 (45 per cent) of the 71 patients who had surgery. Among these, five patients (7 per cent) had PanIN‐3 lesions as the highest‐grade neoplastic lesion, four (6 per cent) had a branch‐duct IPMN with high‐grade dysplasia, four (6 per cent) had a main‐duct IPMN, and 19 (27 per cent) had PDAC. Pathology findings in all 71 high‐risk individuals who underwent surgery are summarized in Table 2, as well as lesion characteristics and types of surgery.
Table 2.
Overview of lesion characteristics, type of surgery and pathology in high‐risk individuals who had surgery after detection of a suspicious pancreatic lesion or were diagnosed with advanced pancreatic cancer
| High‐risk individuals who had surgery (n = 71) | High‐risk individuals diagnosed with advanced PDAC (n = 5) | |
|---|---|---|
| Lesion characteristics | ||
| Time point of lesion detection | ||
| Baseline | 39 (55) | 2 (40) |
| Follow‐up | 32 (45) | 3 (60) |
| Present at previous investigations | 9 (13) | 1 (20) |
| Duration of lesion visualization before resection/diagnosis (months)* | 8·7(9·5) (5·0; 1–32) | 41(41) |
| Overdue for recommended screening | 10 (14) | 1 (20) |
| Months overdue for recommended screening* | 6·7(3·4) (6·0; 1–12) | 3(3) |
| Modality that detected the lesion† | ||
| EUS | 62 (87) | 2 (40) |
| MRI/MRCP | 29 (41) | 3 (60) |
| CT/PET–CT | 28 (39) | 2 (40) |
| ERCP | 8 (11) | 0 (0) |
| Type of lesion as reason for surgery (n = 93) | ||
| Cystic | 44 (47) | |
| Solid | 33 (35) | |
| Hypoechoic | 3 (3) | |
| Dilated pancreatic duct | 2 (2) | |
| Features of chronic pancreatitis | 1 (1) | |
| Other | 10 (11) | |
| Location of lesion (n = 93) | ||
| Head/uncinate region | 35 (38) | |
| Body | 20 (22) | |
| Tail | 29 (31) | |
| No data | 9 (10) | |
| Size of lesion size (mm)* | ||
| All lesions (n = 93) | 14·0(8·8) (11·9; 3–51) | |
| Cystic lesions (n = 44) | 13·6(8·0) (11·6; 3–40) | |
| Solid lesions (n = 33) | 15·5(10·0) (13·0; 4–51) | |
| Neoadjuvant therapy | 4 (6) | n.a. |
| Type of surgery | n.a. | |
| Distal pancreatectomy | 36 (51) | |
| Pancreatoduodenectomy | 18 (25) | |
| Total pancreatectomy | 9 (13) | |
| Pancreatoduodenectomy then completion pancreatectomy | 4 (6) | |
| Central pancreatectomy | 2 (3) | |
| Diagnosis of unresectable disease during surgery | 2 (3) | |
| Complications of surgery † | n.a. | |
| None | 37 (52) | |
| Infectious complications | 10 (14) | |
| Delayed gastric emptying | 6 (8) | |
| Pancreatic fistula | 4 (6) | |
| Bile leak | 2 (3) | |
| Peripancreatic fluid collection | 1 (1) | |
| Other | 6 (8) | |
| No data | 7 (10) | |
| Pathology † | ||
| PDAC | 19 (27) | 5 (100) |
| Main‐duct IPMN with high‐grade dysplasia | 1 (1) | – |
| Main‐duct IPMN with moderate‐grade dysplasia | 4 (6) | – |
| Main‐duct IPMN with low‐grade dysplasia | 1 (1) | – |
| Mixed‐duct IPMN with high‐grade dysplasia | 1 (1) | – |
| Mixed‐duct IPMN with moderate‐grade dysplasia | 0 (0) | – |
| Mixed‐duct IPMN with low‐grade dysplasia | 0 (0) | – |
| Branch‐duct IPMN with high‐grade dysplasia | 5 (7) | – |
| Branch‐duct IPMN with moderate‐grade dysplasia | 9 (13) | – |
| Branch‐duct IPMN with low‐grade dysplasia | 16 (23) | – |
| PanIN‐3, multifocal | 3 (4) | – |
| PanIN‐3, unifocal | 3 (4) | – |
| PanIN‐2, multifocal | 35 (49) | – |
| PanIN‐2, unifocal | 10 (14) | – |
| PanIN‐1, multifocal | 32 (45) | – |
| PanIN‐1, unifocal | 4 (6) | – |
| Pancreatic neuroendocrine tumour ≥ 2 cm | 0 (0) | – |
| Pancreatic neuroendocrine tumour < 2 cm | 8 (11) | – |
| Incipient IPMN | 5 (7) | – |
| Serous cystadenoma | 2 (3) | – |
| Vascular malformation | 1 (1) | – |
| Highest grade of neoplastic lesion per high‐risk individual | ||
| PDAC | 19 (27) | 5 (100) |
| Stage I/II | 16 (23) | 0 (0) |
| Stage III/IV | 3 (4) | 5 (100) |
| Main‐duct IPMN with high‐grade dysplasia | 1 (1) | – |
| Main‐duct IPMN with moderate‐grade dysplasia | 2 (3) | – |
| Main‐duct IPMN with low‐grade dysplasia | 1 (1) | – |
| Branch‐duct IPMN with high‐grade dysplasia | 4 (6) | – |
| Branch‐duct IPMN with moderate‐grade dysplasia | 7 (10) | – |
| Branch‐duct IPMN with low‐grade dysplasia | 9 (13) | – |
| PanIN‐3, multifocal | 3 (4) | – |
| PanIN‐3, unifocal | 2 (3) | – |
| PanIN‐2, multifocal | 9 (13) | – |
| PanIN‐2, unifocal | 7 (10) | – |
| PanIN‐1, multifocal | 1 (1) | – |
| PanIN‐1, unifocal | 1 (1) | – |
| Pancreatic neuroendocrine tumour <2 cm | 3 (4) | – |
| Serous cystadenoma | 2 (3) | – |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.) (median; range).
More than one option possible. PDAC, pancreatic ductal adenocarcinoma; EUS, endoscopic ultrasonography; MRCP, magnetic resonance cholangiopancreatography; ERCP, endoscopic retrograde cholangiopancreatography; n.a., not applicable; IPMN, intraductal papillary mucinous neoplasm; PanIN, pancreatic intraepithelial neoplasia.
In 39 high‐risk individuals (55 per cent), the indication for surgery was detected at the baseline screening evaluation. In the remaining 32 patients (45 per cent), lesions were detected at follow‐up investigations; in nine of these, a lesion had already been present at previous investigations, for a mean of 9 months before resection. These lesions initially did not meet resection criteria, but a changing appearance over time led to resection. For ten of these 32 patients there was a mean delay of 7 months from their recommended screening interval (this ranged from 3 to 24 months, depending on visualization and type of lesion). Endoscopic ultrasonography detected the vast majority of lesions (87 per cent). A total of 93 suspicious lesions were detected in the 71 patients who underwent surgery, of which 44 (47 per cent) were cystic and 33 (35 per cent) were solid in appearance. The mean size of these 93 lesions was 14·0 (range 3–51) mm.
Distal pancreatectomy was performed in 36 patients (51 per cent) and pancreatoduodenectomy in 18 (25 per cent). Complications of surgery were seen in 34 patients (48 per cent). The most common complications were infection (14 per cent), delayed gastric emptying (8 per cent) and pancreatic fistula (6 per cent). There were no surveillance or surgery‐related deaths.
Of the five patients diagnosed with advanced disease during surveillance, three (60 per cent) were identified at follow‐up; the other two were detected at baseline evaluation.
Outcomes
The outcomes for both risk groups are summarized in Table 3. Of all 76 included patients, 61 (80 per cent) are still alive a mean 52 months after surgery or diagnosis of PDAC. Of 71 high‐risk individuals who underwent surgery, 59 (83 per cent) were still alive after a mean follow‐up of 54·3 months. Of the 12 patients who died, eight deaths were PDAC‐related. The survival rate was significantly poorer for individuals with advanced PDAC compared with those who had surgery (40 versus 83 per cent respectively, P = 0·050; mean survival 9·5 versus 54·3 months, P < 0·001). Only two (3 per cent) of the 71 high‐risk patients who underwent surgery died within 1 year (all‐cause 1‐year mortality), compared with two of five patients with advanced PDAC; 52 per cent survived for 3 years or more after surgery.
Table 3.
Outcomes in high‐risk individuals who had surgery or were diagnosed with advanced disease
| High‐risk individuals who had surgery (n = 71) | High‐risk individuals diagnosed with advanced PDAC (n = 5) | P † | |
|---|---|---|---|
| Duration of follow‐up (months) * | 51·6(45·1) (42·0; 0–168) | 8·2(11·1) (3·0; 3–28) | < 0·001‡ |
| Survival | |||
| Alive | 59 (83) | 2 (40) | 0·050 |
| Time after surgery/diagnosis (months)* | 54·3(45·9) (44·0; 0–168) | 9·5(12·3) (3·5; 3–28) | < 0·001‡ |
| Long‐term survival (≥ 3 years) | 37 (52) | 0 (0) | |
| Mortality | |||
| Died | 12 (17) | 3 (60) | 0·050 |
| Time after surgery/diagnosis (months)* | 54·3(56·0) (28·5; 5–164) | 11·3(14·4) (3·0; 3–28) | 0·221‡ |
| Short‐term mortality (≤ 1 year) | 2 (3) | 2 (40) | 0·154 |
| PDAC‐related | 8 (11) | 3 (60) | 0·506 |
| Not PDAC‐related | 2 (3) | 0 (0) | |
| Unknown cause of death | 2 (3) | 0 (0) |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.) (median; range). PDAC, pancreatic ductal adenocarcinoma.
χ2 or Fisher's exact test, except
independent‐samples t test.
Risk factors
Univariable analysis of factors associated with high‐risk neoplastic precursor lesions or PDAC in the resection specimen included age 65 years or more at the time of surgery (odds ratio (OR) 4·11; P = 0·007) and female sex (OR 3·82; P = 0·007) (Table 4). In multivariable analysis, four factors were significantly associated with the presence of a high‐risk precursor lesion or PDAC in the pancreatic resection specimen: age 65 years or above at the time of surgery (OR 7·53; P = 0·010), female sex (OR 5·78; P = 0·017), carriage of a deleterious mutation in a known pancreatic cancer susceptibility gene (OR 4·92; P = 0·040) and location of a lesion in the head/uncinate region of the pancreas (OR 4·23; P = 0·041).
Table 4.
Univariable and multivariable analysis of factors possibly associated with high‐risk neoplastic precursor lesions or pancreatic ductal adenocarcinoma in the resection specimen
| Univariable analyses | Multivariable analyses | |||
|---|---|---|---|---|
| Unadjusted OR | P | Adjusted OR | P | |
| Age ≥ 65 years at surgery | 4·11 (1·44, 11·70) | 0·007 | 7·53 (1·6, 35·0) | 0·010 |
| Female sex | 3·82 (1·42, 10·25) | 0·007 | 5·78 (1·4, 24·3) | 0·017 |
| White ethnicity | 0·25 (0·03, 2·57) | 0·321 | ||
| Carrier of a gene mutation | 2·40 (0·80, 7·16) | 0·113 | 4·92 (1·1, 22·6) | 0·040 |
| ≥ 2 first‐degree relatives affected by PDAC | 2·40 (0·91, 6·36) | 0·076 | 1·71 (0·4, 7·2) | 0·462 |
| Family member aged < 50 years affected by PDAC | 1·15 (0·34, 3·85) | 0·820 | ||
| BMI ≥ 25 kg/m2 | 0·57 (0·20, 1·67) | 0·303 | ||
| Personal history of diabetes | 1·83 (0·49, 6·77) | 0·505 | ||
| Personal history of pancreatitis | 0·69 (0·16, 3·03) | 0·727 | ||
| Current or former smoker | 1·00 (0·36, 2·75) | 1·000 | ||
| > 10 pack‐years of smoking | 0·95 (0·14, 6·28) | 1·000 | ||
| Current or former consumption of alcohol | 0·70 (0·27, 1·84) | 0·470 | ||
| Detection of lesion at follow‐up visit | 1·14 (0·45, 2·92) | 0·782 | ||
| Solid lesion type (versus cystic lesion) | 1·11 (0·40, 3·07) | 0·839 | ||
| Location of lesion in head/uncinate region (versus location in body/tail) | 2·33 (0·83, 6·56) | 0·105 | 4·23 (1·1, 16·9) | 0·041 |
| Lesion size ≥ 1 cm | 1·26 (0·39, 4·12) | 0·702 | ||
| Surgery after 2011 | 1·47 (0·54, 4·01) | 0·448 | ||
Values in parentheses are 95 per cent confidence intervals. OR, odds ratio; PDAC, pancreatic ductal adenocarcinoma.
Survival analysis
The pancreatic neoplasia grade was significantly associated with overall survival in high‐risk individuals. Fig. 1 shows the Kaplan–Meier curves for different pathological subgroups. High‐risk individuals with no or low‐risk neoplastic lesions (group 1; 39 patients) and high‐risk individuals with high‐risk neoplastic precursor lesions (group 2; 13 patients) had the best survival, followed by those with stage I or II PDAC (group 3; 16 patients), and those with stage III or IV PDAC (group 4; 8 patients). The hazard ratio for group 2 was 4·52 (95 per cent c.i. 0·10 to 197·60; P = 0·163) versus group 1, 13·12 (3·03 to 56·75; P < 0·001) versus group 3, and 25·33 (1·17 to 547·20; P < 0·001) versus group 4.
Figure 1.
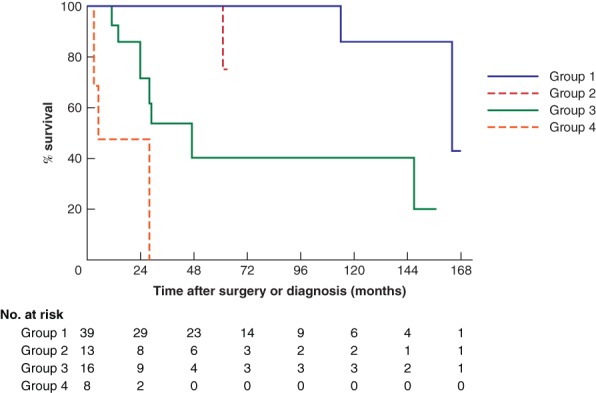
Kaplan–Meier curves for survival of patients in the four pathological subgroups
Group 1, low‐risk neoplastic lesions including pancreatic neuroendocrine tumours smaller than 2 cm; group 2, high‐risk neoplastic lesions including all main‐duct intraductal papillary mucinous neoplasms (IPMNs), branch‐duct IPMNs with high‐grade dysplasia and pancreatic intraepithelial neoplasia type 3 lesions; group 3, stage I–II pancreatic ductal adenocarcinoma (PDAC); group 4, stage III–IV PDAC.
Discussion
In this multicentre international study, high‐risk neoplastic lesions or PDAC were present in 45 per cent of the high‐risk population that underwent surgery in a PDAC surveillance programme. Survival in high‐risk patients with no or low‐risk lesions did not differ significantly from that in patients with high‐risk neoplastic precursor lesions. The patients who developed PDAC had a significantly higher overall mortality rate and poorer survival than those with no or low‐risk neoplastic lesions.
Surveillance of high‐risk individuals has the potential to improve the poor survival of patients with PDAC, and is increasingly being undertaken. In 2010, the CAPS Consortium was formed to help organize global pancreatic surveillance. By pooling data from participating centres, important research questions pertaining to pancreatic surveillance can be assessed readily. The present analysis reports the pooled data of high‐risk individuals for whom surveillance led to the detection of advanced disease or a lesion for which pancreatic surgery was performed.
Goals of surveillance described previously by the CAPS Consortium17 were early invasive cancers (T1 N0 M0), PanIN‐3, main‐duct IPMNs and branch‐duct IPMNs with high‐grade dysplasia. Although PanNETs of 2 cm or more in size were also included in the definition, no such large PanNETs were detected. Timing of intervention is an important issue. In this series, 55 per cent of the resection specimens harboured no high‐risk neoplastic precursor lesion or PDAC, but did contain, for example, low‐risk PanIN lesions (PanIN‐1 or ‐2) or small PanNETs. Only long‐term follow‐up will disclose whether patients with resected low‐risk lesions might have a reduced risk of subsequently developing PDAC. For some patients, surgical resection was performed too late, as only three of the 19 PDACs had T1 status. The main challenge in any surveillance programme is how to distinguish between individuals who can be monitored safely and those who require surgery to resect a neoplastic lesion at a curable stage.
In this study, 55 per cent of lesions that prompted surgery were detected at a baseline visit. This could raise the question whether one‐time screening of high‐risk individuals at a given age is also effective. Alternatively, when an advanced lesion is found at the index investigation, it could be argued that this lesion might have been detected at an earlier stage with potentially a better outcome if surveillance had started at an earlier age. As new lesions were detected in several patients who missed their follow‐up visit by only a few months, it seems appropriate to adhere to an annual surveillance protocol, until more data are available from large prospective cohorts.
Although not all patients with main‐duct IPMN progress to cancer, the overall 10‐year risk is estimated at approximately 25 per cent21. Only two patients in the present study were identified with these lesions before surgery. After pathological evaluation of the resection specimen four cystic lesions were reclassified as main‐duct IPMN. Discrepancy between imaging and pathology is not an uncommon finding in this situation22.
The present study also sought to identify risk factors that can easily be assessed before surgery for association with high‐risk neoplastic precursor lesions or PDAC in the resection specimens. Multivariable analyses showed age at least 65 years, female sex, carriage of a gene mutation and location of a lesion in the head/uncinate region of the pancreas to be associated with the detection of a high‐risk precursor lesion or PDAC in the resection specimen. Among female carriers of a gene mutation aged above 65 years with a lesion suspicious for malignancy in the head/uncinate region of the pancreas, the option of pancreatic surgery versus continuing surveillance should be weighed carefully.
Survival analysis indicated that this was strongly influenced by the stage of disease at diagnosis23. Importantly, the survival of patients with high‐risk neoplastic precursors in the resection specimen was similar to that of those with no or low‐risk neoplastic lesions, emphasizing the need reliably to identify high‐risk precursor lesions more than early cancers.
The strength of this study is the international pooling of data on PDAC surveillance programmes. This yielded a unique and sizeable cohort of high‐risk patients participating in these programmes, in whom either a suspicious lesion was detected for which they underwent surgery, or in whom an inoperable pancreatic cancer developed. The main limitations of this study are its design and potential lead‐time and length bias24. Another limitation is that differences between protocols of the centres existed, particularly before publication of consensus statements of the CAPS Consortium in 201317. Although this is the largest cohort described, its sample size is still too limited to assess differences in survival between R0 and R1 resections. Another limitation is the lack of detailed information of all 1700 high‐risk individuals who underwent surveillance. Attention was focused specifically on the highly selected group of patients who either developed advanced neoplasia or underwent pancreatic surgery, and this study has added new, interesting and valuable data to the literature that provides some rationale to screening individuals at high risk for pancreatic cancer. More research is needed to understand better the risk factors for individuals at high risk of developing PDAC, and improve the selection of high‐risk individuals for surgery. International collaboration in large worldwide prospective studies seems the logical way forward.
Acknowledgements
I.C.A.W.K. and M.I.C. contributed equally to this publication.
The CAPS Consortium involves the following participating centres (in alphabetical order): Amsterdam Medical Centre (the Netherlands), Beaujon Hospital (France), Columbia University Medical Center (USA), Creighton University Hereditary Cancer Center (USA), Dana Farber Cancer Institute (USA), Erasmus MC University Medical Centre Rotterdam (the Netherlands), Icahn School of Medicine at Mount Sinai (USA), Indiana University (USA), Institut Paoli‐Calmettes (France), Jefferson Medical Center (USA), Johns Hopkins Medical Institutions (USA), Karolinska Institutet (Sweden), Kyoto University Hospital (Japan), Mayo Clinic (USA), MD Anderson Cancer Center (USA), Medical College of Wisconsin (USA), Ohio State University (USA), Pancreatic Cancer Action Network (USA), Rambam Healthcare Campus (Israel), St Vincent's Hospital (Australia), Teikyo University Medical Centre (Japan), University Hospital of Santiago de Compostela (Spain), University Hospital of Verona (Italy), University Hospitals Case Medical Center (USA), University of Alabama (USA), University of Arizona (USA), University of California San Diego (USA), University of Colorado Denver (USA), University of Michigan (USA), University of Nebraska (USA), University of Pennsylvania (USA), University of Pittsburgh (USA), University of Southern California (USA), Vita‐Salute San Raffaele University (Italy), Washington University (USA), Yale University (USA).
Disclosure: The authors declare no conflict of interest.
Funding information
No funding
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66 : 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 3. Lynch HT, Smyrk T, Kern SE, Hruban RH, Lightdale CJ, Lemon SJ et al Familial pancreatic cancer: a review. Semin Oncol 1996; 23: 251–275. [PubMed] [Google Scholar]
- 4. Schneider R, Slater EP, Sina M, Habbe N, Fendrich V, Matthäi E et al German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer 2011; 10: 323–330. [DOI] [PubMed] [Google Scholar]
- 5. Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z et al; American Cancer of the Pancreas Screening (CAPS) Consortium . Frequent detection of pancreatic lesions in asymptomatic high‐risk individuals. Gastroenterology 2012; 142: 796–804; quiz e14–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kimmey MB, Bronner MP, Byrd DR, Brentnall TA. Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc 2002; 56(4 Suppl): S82–S86. [DOI] [PubMed] [Google Scholar]
- 7. Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C et al Screening for early pancreatic neoplasia in high‐risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006; 4: 766–781. [DOI] [PubMed] [Google Scholar]
- 8. Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C et al The yield of first‐time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol 2009; 104: 2175–2181. [DOI] [PubMed] [Google Scholar]
- 9. Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD. Pancreatic cancer screening in a prospective cohort of high‐risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 2010; 16: 5028–5037. [DOI] [PubMed] [Google Scholar]
- 10. Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol 2011; 106: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vasen HF, Wasser M, van Mil A, Tollenaar RA, Konstantinovski M, Gruis NA et al Magnetic resonance imaging surveillance detects early‐stage pancreatic cancer in carriers of a p16‐Leiden mutation. Gastroenterology 2011; 140: 850–856. [DOI] [PubMed] [Google Scholar]
- 12. Al‐Sukhni W, Borgida A, Rothenmund H, Holter S, Semotiuk K, Grant R et al Screening for pancreatic cancer in a high‐risk cohort: an eight‐year experience. J Gastrointest Surg 2012; 16: 771–783. [DOI] [PubMed] [Google Scholar]
- 13. Potjer TP, Schot I, Langer P, Heverhagen JT, Wasser MN, Slater EP et al; Leiden Familial Pancreatic Cancer Group; FaPaCa registry . Variation in precursor lesions of pancreatic cancer among high‐risk groups. Clin Cancer Res 2013; 19: 442–449. [DOI] [PubMed] [Google Scholar]
- 14. Harinck F, Konings IC, Kluijt I, Poley JW, van Hooft JE, van Dullemen HM et al; Dutch research group on pancreatic cancer surveillance in high‐risk individuals . A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high‐risk individuals. Gut 2016; 65: 1505–1513. [DOI] [PubMed] [Google Scholar]
- 15. Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthäi E, Carrato A et al Benefit of surveillance for pancreatic cancer in high‐risk individuals: outcome of long‐term prospective follow‐up studies from three European expert centers. J Clin Oncol 2016; 34: 2010–2019. [DOI] [PubMed] [Google Scholar]
- 16. Roberts NJ, Norris AL, Petersen GM, Bondy ML, Brand R, Gallinger S et al Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov 2016; 6: 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I et al; International Cancer of Pancreas Screening (CAPS) Consortium . International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013; 62: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brune K, Abe T, Canto M, O'Malley L, Klein AP, Maitra A et al Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol 2006; 30: 1067–1076. [PMC free article] [PubMed] [Google Scholar]
- 19. Toste PA, Kadera BE, Tatishchev SF, Dawson DW, Clerkin BM, Muthusamy R et al Nonfunctional pancreatic neuroendocrine tumors < 2 cm on preoperative imaging are associated with a low incidence of nodal metastasis and an excellent overall survival. J Gastrointest Surg 2013; 17: 2105–2113. [DOI] [PubMed] [Google Scholar]
- 20. Fitzgerald TL, Mosquera C, Vora HS, Vohra NA, Zervos EE. Indications for surgical resection in low‐grade pancreatic neuroendocrine tumors. Am Surg 2016; 82: 737–742. [PubMed] [Google Scholar]
- 21. Choi SH, Park SH, Kim KW, Lee JY, Lee SS. Progression of unresected intraductal papillary mucinous neoplasms of the pancreas to cancer: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2017; 15: 1509–1520.e4. [DOI] [PubMed] [Google Scholar]
- 22. de Pretis N, Mukewar S, Aryal‐Khanal A, Bi Y, Takahashi N, Chari S. Pancreatic cysts: diagnostic accuracy and risk of inappropriate resections. Pancreatology 2017; 17: 267–272. [DOI] [PubMed] [Google Scholar]
- 23. Witkowski ER, Smith JK, Tseng JF. Outcomes following resection of pancreatic cancer. J Surg Oncol 2013; 107: 97–103. [DOI] [PubMed] [Google Scholar]
- 24. Morrison AS. The effects of early treatment, lead time and length bias on the mortality experienced by cases detected by screening. Int J Epidemiol 1982; 11: 261–267. [DOI] [PubMed] [Google Scholar]


