Abstract
Background
The evidence regarding the prognostic impact of a positive circumferential resection margin (CRM) in oesophageal cancer is conflicting, and there is global variability in the definition of a positive CRM. The aim of this study was to determine the impact of a positive CRM on survival in patients undergoing oesophagectomy for oesophageal cancer.
Methods
A systematic review and meta‐analysis was performed. PubMed and Embase databases were searched for articles to May 2018 examining the effect of a positive CRM on survival. Cohort studies written in English were included. Meta‐analyses of univariable and multivariable hazard ratios (HRs) were performed using both Royal College of Pathologists (RCP) and College of American Pathologists (CAP) criteria. Risk of bias was assessed using the Newcastle–Ottawa Scale. Egger regression, and Duval and Tweedie trim‐and‐fill statistics were used to assess publication bias.
Results
Of 133 studies screened, 29 incorporating 6142 patients were finally included for analysis. Pooled univariable HRs for overall survival in patients with a positive CRM were 1·68 (95 per cent c.i. 1·48 to 1·91; P < 0·001) and 2·18 (1·84 to 2·60; P < 0·001) using RCP and CAP criteria respectively. Subgroup analyses demonstrated similar results for patients by T category, neoadjuvant therapy and tumour type. Pooled HRs from multivariable analyses suggested that a positive CRM was independently predictive of a worse overall survival (RCP: 1·41, 1·21 to 1·64, P < 0·001; CAP: 2·37, 1·60 to 3·51, P < 0·001).
Conclusion
A positive CRM is associated with a worse prognosis regardless of classification system, T category, tumour type or neoadjuvant therapy.
Introduction
The incidence of oesophageal adenocarcinoma has increased steadily over the past 30 years and now accounts for up to one‐sixth of cancer‐related mortality1. Owing to its aggressive nature, late symptomatology, early haematological and lymphatic spread, curative surgery remains a treatment for selected patients only, with significant risks of morbidity and mortality2, 3. It is therefore important to select good candidates for surgery. Lymph node involvement, depth of tumour invasion, and distal and proximal resection margin involvement have been shown consistently to be associated with a poor prognosis in patients who undergo resection4, 5, 6, 7, 8, 9, 10.
Most authors11, 12 have reported that circumferential resection margin (CRM) involvement is associated with an increased risk of local recurrence and poorer long‐term survival in oesophageal cancer. This association, however, was not observed in all studies13, 14, 15. This discrepancy may be explained partly by the ongoing dispute regarding the exact pathological classification of CRM involvement. The Royal College of Pathologists (RCP) in the UK defines positive CRM as cases where tumour is found within 1 mm of the surgical margin, whereas the College of American Pathologists (CAP) defines CRM as positive if tumour is found at the cut margin of resection (a 0‐mm margin). The optimal definition of a positive CRM remains unknown. In addition, many studies have had a relatively small sample size and short follow‐up12, 16. These factors may have biased the true effect of a positive CRM on outcomes.
A former meta‐analysis17 on this topic from 2014 comprised 19 studies published between 1993 and 2013, and concluded that a positive CRM according to either the RCP or the CAP definition was associated with a poor prognosis. Since then, there have been advancements in neoadjuvant and adjuvant treatment regimens, and further studies8, 13, 14, 15, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 examining CRM status in oesophageal cancer have been published. The aim of the present systematic review and meta‐analysis was to examine further the role of CRM status in the current era of multimodality treatment of patients with oesophageal cancer.
Methods
Patients undergoing oesophagectomy for oesophageal cancer formed the population for this meta‐analysis. CRM‐positive and ‐negative pathology specimens, defined by both CAP and RCP criteria, formed the intervention and comparator, with overall survival (OS) defined as the outcome. PRISMA guidelines45 were used to design the study and report the findings. The protocol of this meta‐analysis was registered in the Prospective Register of Systematic Reviews (PROSPERO identification code CRD42017078901).
Search strategy and selection
A literature search was conducted using MEDLINE (PubMed) and Embase online databases, using the search terms ‘oesophageal/esophageal cancer’ or ‘oesophageal/esophageal carcinoma’ or ‘oesophageal/esophageal neoplasm’ or ‘oesophagectomy/esophagectomy’, and ‘circumferential resection margin’ or ‘radial resection margin’ or ‘lateral resection margin’. The search was limited to articles in English and a publication date up to May 2018. The bibliographies of relevant studies were examined for further publications not found during initial database searching.
To be included, studies had to: report on a population of patients undergoing curative oesophagectomy for oesophageal cancer exclusively; investigate the relationship between CRM status and survival; and either report hazard ratios (HRs) for OS or present Kaplan–Meier plots from which HRs could be estimated46, 47. Reviews and case reports were excluded. Conference proceedings were also examined.
Data extraction and outcome definitions
All eligible studies were identified by two reviewers, who independently extracted data. Data extracted included author name, year of publication, country of study, total number of patients, mean/median age, proportion of male participants, tumour T category, tumour histology, neoadjuvant treatment, definition of CRM (RCP, CAP or both), length of follow‐up (mean/median and range), as well as HRs for OS, with 95 per cent c.i. and P values. Any disputes were resolved by a third reviewer.
The RCP defined a positive CRM as the presence of tumour within 1 mm of the margin12. The CAP defined tumour at the CRM as positive16.
All studies were graded for methodological and reporting quality using the Newcastle–Ottawa Scale (NOS). This involves scoring the selection of patients into the study, the comparability of the two included cohorts and the assessment of outcomes. Two reviewers scored each paper and disputes were resolved by a third reviewer.
Statistical analysis
If studies reported survival data using Kaplan–Meier curves, a HR with 95 per cent c.i. was estimated according to the method described by Parmar and colleagues46, with survival rates extracted from plots at yearly intervals and constant censoring assumed. Where studies stratified patients into three categories (no tumour within 1 mm of the CRM, tumour within 1 mm of the CRM but not within 0 mm of the CRM, and tumour at the CRM), these were combined by taking weighted means of the survival curves in order to estimate the HRs for RCP and CAP definitions.
The HRs of studies were then pooled using a random‐effects model with inverse variance weighting. Studies were summarized using forest plots, and I 2 statistics were calculated as measures of heterogeneity. To investigate causes of heterogeneity, the following subgroup analyses were performed: category pT3 only; adenocarcinoma or squamous cell carcinoma (SCC) only; neoadjuvant therapy; studies reporting HRs; and studies reporting data by Kaplan–Meier analysis. In addition, for studies that performed multivariable analyses, the resulting HRs for CRM were pooled to assess the independent association between CRM and survival.
Publication biases were examined using funnel plots, Begg and Mazumdar rank correlation analysis, and Egger linear regression tests. The Duval and Tweedie trim‐and‐fill method48 was used to impute studies to correct for publication biases.
Statistical analysis was performed using Comprehensive Meta‐Analysis® version 3 (Biostat, Englewood, New Jersey, USA) and RevMan® version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
A total of 127 studies were initially found. Cross‐references led to a further six studies that were possibly eligible. After removing duplicates, initial screening and full‐text review 31 studies remained (Fig. 1). Some 29 studies8, 13, 14, 15, 19, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 from ten different countries, published between 2001 and 2018, were included in the meta‐analysis (Table S1, supporting information).
Figure 1.
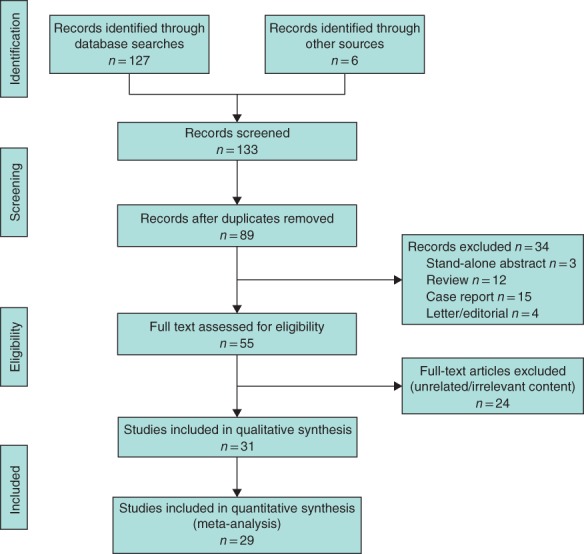
PRISMA diagram for the review
The median number of patients per study was 172 (range 59–479), with a total population of 6142 patients. Mean/median age ranged from 58 to 68 years across the studies (median 64 years), and a male preponderance was observed, with cohorts being 65–96 per cent men (median 79 per cent). There was a degree of variation across the studies of the defined histopathological inclusion criteria (Table S2, supporting information). Twelve studies included all histological types of oesophageal malignancy, ten included both adenocarcinoma and SCC, four included adenocarcinoma alone and three included SCC alone. Twelve studies included only patients with category T3 disease. Seventeen studies defined margins using both the RCP and CAP criteria, whereas 11 used RCP criteria alone and a single study used solely CAP criteria.
The use of neoadjuvant chemotherapy and chemoradiotherapy (CRT) varied across studies and within the studies themselves. Eight studies did not use neoadjuvant therapy, 13 administered chemotherapy only, four used CRT only, and four used both chemotherapy and CRT. Across the studies, the median rate of a positive CRM was 45·3 (range 18·2–78·3) per cent using the RCP definition and 17·6 (4·9–30·0) per cent according to the CAP definition. Reporting on the duration of patient follow‐up was sporadic and varied markedly across studies. NOS scores varied from 5 to 9 (median 7) (Table 1).
Table 1.
Newcastle–Ottawa Scale grading for eligible studies
| Newcastle–Ottawa Scale grading | ||||
|---|---|---|---|---|
| Reference | Selection | Comparability | Outcome | Score |
| Dexter et al.19 | 4 | 0 | 2 | 6 |
| Khan et al.21 | 3 | 1 | 3 | 7 |
| Roh et al.22 | 4 | 1 | 2 | 7 |
| Griffiths et al.23 | 3 | 2 | 2 | 7 |
| Thompson et al.8 | 3 | 2 | 3 | 8 |
| Sujendran et al.24 | 4 | 0 | 1 | 5 |
| Deeter et al.25 | 3 | 1 | 2 | 6 |
| Scheepers et al.26 | 4 | 0 | 2 | 6 |
| Saha et al.27 | 4 | 1 | 2 | 7 |
| Sillah et al.28 | 4 | 0 | 2 | 6 |
| Mirnezami et al.29 | 4 | 1 | 1 | 6 |
| Pultrum et al.30 | 3 | 1 | 2 | 6 |
| Chao et al.31 | 3 | 2 | 2 | 7 |
| Verhage et al.32 | 2 | 1 | 3 | 6 |
| Harvin et al.33 | 3 | 2 | 2 | 7 |
| Rao et al.34 | 2 | 0 | 3 | 5 |
| Reid et al.35 | 4 | 1 | 3 | 8 |
| Salih et al.36 | 3 | 1 | 2 | 6 |
| O'Farrell et al.13 | 3 | 1 | 2 | 6 |
| O'Neill et al.37 | 3 | 1 | 1 | 5 |
| Ahmad et al.38 | 3 | 1 | 3 | 7 |
| Theologou et al.14 | 3 | 2 | 3 | 8 |
| Gilbert et al.39 | 4 | 1 | 2 | 7 |
| Lee et al.40 | 3 | 1 | 1 | 5 |
| Okada et al.41 | 2 | 1 | 3 | 6 |
| Ghadban et al.42 | 3 | 1 | 3 | 7 |
| Depypere et al.43 | 3 | 1 | 3 | 7 |
| Quinn et al.15 | 4 | 2 | 3 | 9 |
| Knight et al.44 | 3 | 1 | 3 | 7 |
Meta‐analysis
Analysis of the 26 studies reporting univariable survival analyses, using the RCP definition, found OS to be shorter in patients with a positive CRM (pooled HR 1·68, 95 per cent c.i. 1·48 to 1·91; P < 0·001) (Fig. 2). Analysis of the 14 studies that used the CAP definition returned a similar result: pooled HR 2·18 (1·84 to 2·60; P < 0·001) (Fig. 3). Significant heterogeneity between studies was observed, with I 2 values of 70 and 63 per cent for the RCP and CAP definitions respectively.
Figure 2.
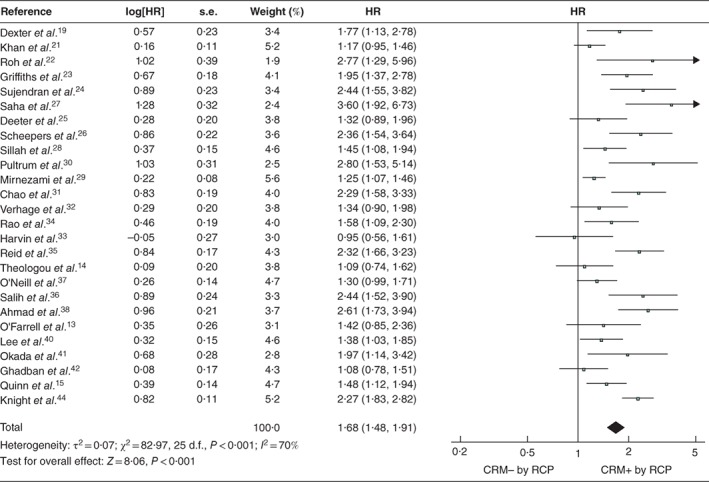
Forest plot of all studies assessing the influence of a positive circumferential resection margin in accordance with the Royal College of Pathologists' definition An inverse‐variance random‐effects model was used for meta‐analysis. Hazard ratios (HRs) are shown with 95 per cent confidence intervals. CRM−/+, negative/positive circumferential resection margin; RCP, Royal College of Pathologists.
Figure 3.
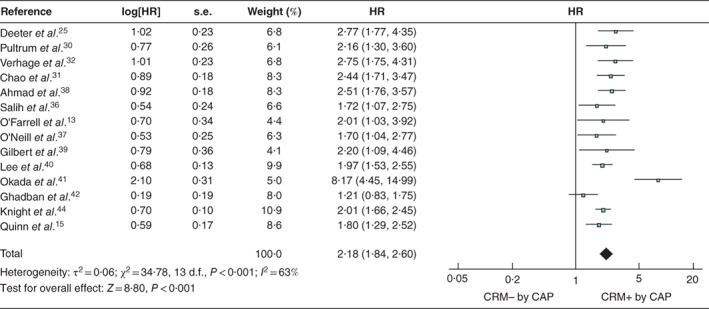
Forest plot of all studies assessing the influence of a positive circumferential resection margin in accordance with the College of American Pathologists' definition An inverse‐variance random‐effects model was used for meta‐analysis. Hazard ratios (HRs) are shown with 95 per cent confidence intervals. CRM−/+, negative/positive circumferential resection margin; CAP, College of American Pathologists.
Publication bias
Potential publication bias was detected in the studies using the RCP definition of a positive CRM (Fig. 4), with significance on the Begg and Mazudmar rank test (P = 0·013) and Egger's test of the intercept (P < 0·001). In an attempt to correct for this, the Duval and Tweedie trim‐and‐fill approach was used, which identified a potential eight missing studies with effect sizes below that of the pooled HR. After accounting for these, the difference in survival between the CRM‐positive and ‐negative groups remained significant, with an imputed point estimate HR of 1·37 (95 per cent c.i. 1·19 to 1·58). Assessment of the studies reporting outcomes using the CAP definition of a positive CRM found minimal publication bias (Fig. 5), with a non‐significant Begg and Mazudmar rank test (P = 0·213) and Egger's test of the intercept (P = 0·132).
Figure 4.
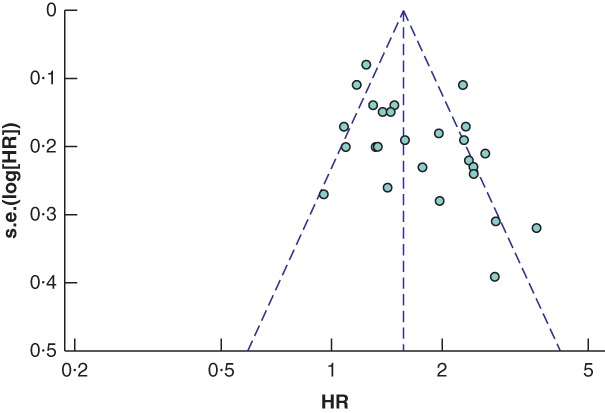
Funnel plot of all studies assessing the influence of a positive circumferential resection margin in accordance with the Royal College of Pathologists' definition HR, hazard ratio.
Figure 5.
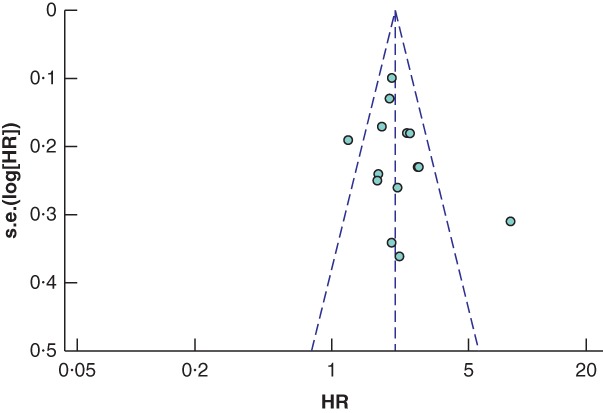
Funnel plot of all studies assessing the influence of a positive circumferential resection margin in accordance with the College of American Pathologists' definition HR, hazard ratio.
Subgroup analyses
Subgroup analysis of studies reporting OS for category T3 tumours led to a pooled HR of 1·43 (95 per cent c.i. 1·20 to 1·71; P < 0·001) according to RCP and 2·25 (1·62 to 3·10; P < 0·001) according to CAP. Other subgroup analyses by tumour histology, use of neoadjuvant therapy and type of summary statistics reported by the study (HR or Kaplan–Meier curve) returned consistent results, all finding survival to be significantly shorter in those with a positive CRM (Table 2).
Table 2.
Meta‐analysis of specific subgroups
| RCP | CAP | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of studies | I 2 (%) | Pooled HR* | P | No. of studies | I 2 (%) | Pooled HR* | P | |
| Univariable analysis | ||||||||
| All studies | 26 | 70 | 1·68 (1·48, 1·91) | < 0·001 | 14 | 63 | 2·18 (1·84, 2·60) | < 0·001 |
| pT category | ||||||||
| pT3 only | 10 | 49 | 1·43 (1·20, 1·71) | < 0·001 | 8 | 81 | 2·25 (1·62, 3·10) | < 0·001 |
| Histological type | ||||||||
| AC | 4 | 81 | 1·78 (1·11, 2·86) | 0·001 | 2 | 35 | 2·19 (1·67, 2·88) | < 0·001 |
| SCC | 3 | 13 | 1·79 (1·27, 2·54) | 0·001 | 3 | 89 | 3·20 (1·67, 6·15) | < 0·001 |
| Neoadjuvant therapy | ||||||||
| Yes | 7 | 50 | 2·03 (1·56, 2·64) | < 0·001 | 4 | 71 | 2·99 (1·68, 5·33) | < 0·001 |
| No | 9 | 45 | 1·48 (1·25, 1·75) | < 0·001 | 3 | 51 | 2·85 (1·91, 4·24) | < 0·001 |
| HR estimation | ||||||||
| Derived from Kaplan–Meier curves | 14 | 66 | 1·74 (1·58, 1·91) | < 0·001 | 6 | 0 | 2·12 (1·84, 2·45) | < 0·001 |
| Reported | 12 | 69 | 1·52 (1·27, 1·82) | < 0·001 | 8 | 78 | 2·25 (1·66, 3·06) | < 0·001 |
| Multivariable analysis † | ||||||||
| All studies | 13 | 41 | 1·41 (1·21, 1·64) | < 0·001 | 8 | 80 | 2·37 (1·60, 3·51) | < 0·001 |
Values in parentheses are 95 per cent confidence intervals.
Pooled hazard ratios (HRs) are derived from random‐effects meta‐analysis models and are for patients with a positive circumferential resection margin (CRM) relative to those with a negative CRM.
Meta‐analysis of HRs reported from multivariable analyses; the factors accounted for in the multivariable models for the included studies are summarized in Table 3. RCP, Royal College of Pathologists; CAP, College of American Pathologists; AC, adenocarcinoma; SCC, squamous cell carcinoma.
Multivariable analyses
The variables most commonly adjusted for in the multivariable models of included studies were node status, presence of lymphovascular invasion, tumour grade, and patient age and sex (Table 3). After accounting for these factors, a positive CRM remained significantly associated with shorter survival across the 13 studies using the RCP definition (pooled HR 1·41, 95 per cent c.i. 1·21 to 1·64; P < 0·001) (Fig. 6) and the eight studies that used the CAP definition (pooled HR 2·37, 1·60 to 3·51; P < 0·001) (Fig. 7).
Table 3.
All factors included in multivariable analyses alongside circumferential resection margin status
| No. of studies including factor in multivariable analysis | ||
|---|---|---|
| RCP (n = 13) | CAP (n = 8) | |
| Demographic factors | ||
| Increasing age | 7 | 3 |
| Male sex | 5 | 2 |
| Worsening preoperative lung function | 1 | 0 |
| Staging | ||
| Increasing T category | 5 | 1 |
| Increasing N category | 12 | 6 |
| LVI+ | 7 | 6 |
| Operative factors | ||
| Operation type (transhiatal versus transthoracic) | 1 | 1 |
| Pathology | ||
| Positive CRM | 13 | 8 |
| Tumour subtype (adenocarcinoma) | 3 | 0 |
| Increasing tumour grade | 4 | 3 |
| Increasing Mandard score | 1 | 1 |
| Increasing tumour diameter | 0 | 1 |
| Increasing tumour length | 0 | 1 |
| Presence of Barrett's mucosa | 0 | 1 |
| Concurrent treatment | ||
| No neoadjuvant therapy | 4 | 3 |
| No adjuvant therapy | 2 | 1 |
| Preoperative stenting | 1 | 1 |
RCP, Royal College of Pathologists; CAP, College of American Pathologists; LVI+, presence of lymphovascular invasion; CRM, circumferential resection margin.
Figure 6.
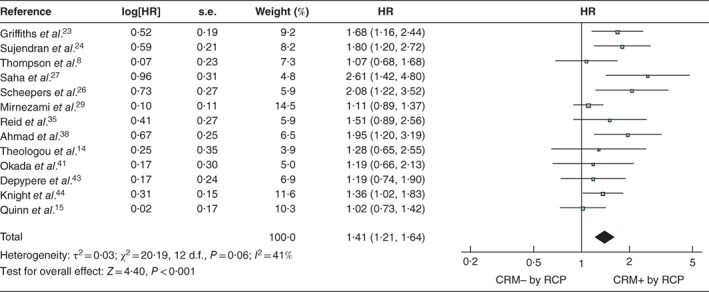
Forest plot of all studies assessing, via multivariable analysis, the influence of a positive circumferential resection margin in accordance with the Royal College of Pathologists' definition An inverse‐variance random‐effects model was used for meta‐analysis. Hazard ratios (HRs) are shown with 95 per cent confidence intervals. CRM−/+, negative/positive circumferential resection margin; RCP, Royal College of Pathologists.
Figure 7.
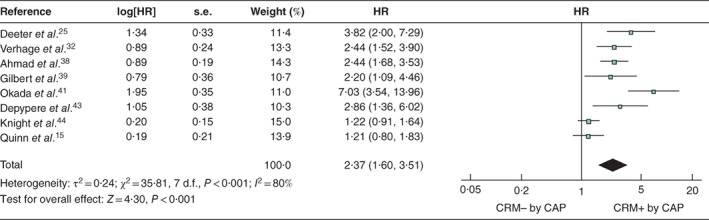
Forest plot of all studies assessing, via multivariable analysis, the influence of a positive circumferential resection margin in accordance with the College of American Pathologists' definition An inverse‐variance random‐effects model was used for meta‐analysis. Hazard ratios (HRs) are shown with 95 per cent confidence intervals. CRM−/+, negative/positive circumferential resection margin; CAP, College of American Pathologists.
Discussion
This study showed that a positive CRM, as defined by both RCP and CAP definitions, was associated with a worse overall prognosis. This association was also observed in the subgroups of patients with category T3 disease, adenocarcinoma and SCC, and in those receiving neoadjuvant therapy. Both RCP and CAP criteria for a positive CRM were associated with decreased survival, but patients positive according to CAP criteria appeared to have worse OS. Previous studies have suggested that this may be due to the effect of a positive CRM on distant recurrence. Chao and colleagues31 found that, although both RCP and CAP criteria defined a subgroup at increased risk of local recurrence, CAP criteria also defined a subgroup that was likely also to have distant recurrence, which markedly shortens OS in this group. Another theory is that close and involved CRM margins are a proxy for more aggressive tumour phenotypes. This effect was seen in a recent study44 that stratified patients by distance of tumour from the CRM, finding that as the distance to the CRM increased so did patient survival.
In the meta‐analysis of HRs of multivariable analyses, both RCP and CAP criteria still defined subgroups with worse prognosis, suggesting that these definitions are predictive of poorer survival independently of other factors, such as lymph node metastasis. This result seems logical, but must be considered with caution as the methodology and inclusion criteria of the individual studies were variable.
This review contained thorough synthesis of up‐to‐date literature, building on work by Wu and co‐workers17. Nine papers on the influence of a positive CRM on survival were added, including one that was not included by Wu et al.17, despite prior publication, and eight more recent publications14, 15, 22, 39, 40, 41, 42, 43, 44. The present study had several limitations. It is increasingly recognized that adenocarcinoma and SCC of the oesophagus are completely different disease entities with differing risk factors, treatment options, genetic basis and prognosis, and should therefore be analysed separately49. Although reporting in the additional papers improved over time, mixed analyses of different histological types of malignancy, without reporting of the effect of either subgroup on prognosis, is still commonplace14, 38, 39, 42. A subgroup analysis was conducted in the present study, but this was limited by the lack of separate reporting by histological type in each paper. Achieving a clear CRM in proximal and middle third tumours, which are usually SCC, is difficult, as the margins consist of the airways and major mediastinal vessels. In the lower third of the oesophagus, the CRM consists of the diaphragmatic crura and pericardial fat, which can be resected more easily. In subgroup analysis, a positive CRM was prognostic in both SCC and adenocarcinoma. In future studies in this area, SCC and adenocarcinoma should be analysed as separate groups to avoid introducing confounding variables.
A further limitation is that oncological and surgical treatment modalities for oesophageal cancer have changed over time. The increase in patients receiving chemotherapy and CRT over the review period has the potential to reduce the positive CRM rate, in particular with the recent introduction of fluorouracil/leucovorin, oxaliplatin and docetaxel (FLOT)‐style neoadjvuant chemotherapy, which has a high complete pathological response rate50, 51. Likewise, operative techniques have developed, with an increasing number of minimally invasive oesophagectomies performed. Studies15, 44 assessing CRM status and minimally invasive surgical techniques have not, however, shown any differences in oncological adequacy, so far.
Patients with T1 or T2 disease do not have a positive CRM, unless the resectional field has been violated. Therefore, papers with high numbers of patients with T1 or T2 tumours may have falsely inflated the prognostic role of CRM status. For this reason, a predefined subgroup analysis only of patients with category T3 tumours was undertaken. A positive CRM was still found to have a significant negative prognostic impact on OS. Several recent papers13, 14, 37, 38, 40, 41, 42 have examined exclusively the effect of CRM positivity on prognosis in patients with T3 disease.
Positive margins after surgery in patients undergoing neoadjuvant therapy and in those with multiple lymph node‐positive disease are known to carry a poor prognosis. The impact of neoadjuvant therapy and lymph node status as confounders of the predictive value of a positive CRM is, however, uncertain. Lymph node status is critical to survival in oesophageal cancer, and some studies have tried to account for this in reporting the influence of CRM status. One relatively small study42 found that CRM status was predictive of OS and locoregional recurrence, but not independently of lymph node status. In addition, another study23 found that a positive CRM was associated with decreased survival only in patients with a lower lymph node burden (less than 25 per cent), suggesting that a positive CRM has greater prognostic influence in those with lower stage disease. Unfortunately, owing to limited reporting in other studies13, 14, 15, 29, 33, 42, it was not possible to perform subgroup analyses of patients stratified by lymph node status, overall stage or other possible confounders, and so future research into the relationship between these variables and the CRM is warranted.
Supporting information
Table S1 Key characteristics and demographics of eligible studies
Table S2 Pathology and outcome data for eligible studies
Acknowledgements
R.E. and J.R.B. contributed equally to this work.
This study was completed with funding from the Queen Elizabeth Hospital Birmingham Charity (Upper Gastrointestinal Fund) and the Upper GI Blues Charity, Sandwell, Birmingham.
Disclosure: The authors declare no conflict of interest.
Funding information
Queen Elizabeth Hospital Birmingham Charity (Upper Gastrointestinal Fund)
Upper GI Blues Charity
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt HM, Gisbertz SS, Moons J, Rouvelas I, Kauppi J, Brown A et al Defining benchmarks for transthoracic esophagectomy: a multicenter analysis of total minimally invasive esophagectomy in low risk patients. Ann Surg 2017; 266: 814–21. [DOI] [PubMed] [Google Scholar]
- 3. Busweiler LA, Wijnhoven BP, van Berge Henegouwen MI, Henneman D, van Grieken NC, Wouters MW et al; Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group . Early outcomes from the Dutch Upper Gastrointestinal Cancer Audit. Br J Surg 2016; 103: 1855–1863. [DOI] [PubMed] [Google Scholar]
- 4. Davarzani N, Hutchins GGA, West NP, Hewitt LC, Nankivell M, Cunningham D et al Prognostic value of pathological lymph node status and primary tumour regression grading following neoadjuvant chemotherapy – results from the MRC OE02 oesophageal cancer trial. Histopathology 2018; 72: 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qureshi YA, Sarker SJ, Walker RC, Hughes SF. Proximal resection margin in Ivor‐Lewis oesophagectomy for cancer. Ann Surg Oncol 2017; 24: 569–577. [DOI] [PubMed] [Google Scholar]
- 6. Shapiro J, Biermann K, van Klaveren D, Offerhaus GJ, Ten Kate FJ, Meijer SL et al Prognostic value of pretreatment pathological tumor extent in patients treated with neoadjuvant chemoradiotherapy plus surgery for esophageal or junctional cancer. Ann Surg 2017; 265: 356–362. [DOI] [PubMed] [Google Scholar]
- 7. Akutsu Y, Matsubara H. The significance of lymph node status as a prognostic factor for esophageal cancer. Surg Today 2011; 41: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 8. Thompson SK, Ruszkiewicz AR, Jamieson GG, Esterman A, Watson DI, Wijnhoven BP et al Improving the accuracy of TNM staging in esophageal cancer: a pathological review of resected specimens. Ann Surg Oncol 2008; 15: 3447–3458. [DOI] [PubMed] [Google Scholar]
- 9. Wijnhoven BP, Tran KT, Esterman A, Watson DI, Tilanus HW. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg 2007; 245: 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siewert JR, Stein HJ, Feith M, Bruecher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1000 consecutive resections at a single center in the Western world. Ann Surg 2001: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Birbeck KF, Macklin CP, Tiffin NJ, Parsons W, Dixon MF, Mapstone NP et al Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg 2002; 235: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Royal College of Pathologists . Standards and Datasets for Reporting Cancers: Dataset for the Histopathological Reporting of Oesophageal Carcinoma (2nd edn); 2007. https//www.rcpath.org/resourceLibrary/g006oesophagealdatasetfinalfeb07‐pdf.html [accessed 21 May 2019].
- 13. O'Farrell NJ, Donohoe CL, Muldoon C, Costelloe JM, King S, Ravi N et al Lack of independent significance of a close (< 1 mm) circumferential resection margin involvement in esophageal and junctional cancer. Ann Surg Oncol 2013; 20: 2727–2733. [DOI] [PubMed] [Google Scholar]
- 14. Theologou T, Diab M, Kyaw PA, Gosney JR, McShane J, Howes N et al The impact of positive circumferential margin on survival following oesophagectomy using the new 7th TNM classification. Eur J Cardiothorac Surg 2013; 44: 855–859. [DOI] [PubMed] [Google Scholar]
- 15. Quinn LM, Hollis AC, Hodson J, Elshafie MA, Hallissey MT, Whiting JL et al Prognostic significance of circumferential resection margin involvement in patients receiving potentially curative treatment for oesophageal cancer. Eur J Surg Oncol 2018; 44: 1268–1277. [DOI] [PubMed] [Google Scholar]
- 16. College of American Pathologists . Protocol for the Examination of the Esophagus; 2016. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/cp‐esophagus‐16protocol‐3200.pdf [accessed 12 November 2018].
- 17. Wu J, Chen QX, Teng LS, Krasna MJ. Prognostic significance of positive circumferential resection margin in esophageal cancer: a systematic review and meta‐analysis. Ann Thorac Surg 2014; 97: 446–453. [DOI] [PubMed] [Google Scholar]
- 18. Sagar PM, Johnston D, McMahon MJ, Dixon MF, Quirke P. Significance of circumferential resection margin involvement after oesophagectomy for cancer. Br J Surg 1993; 80: 1386–1388. [DOI] [PubMed] [Google Scholar]
- 19. Dexter SP, Sue‐Ling H, McMahon MJ, Quirke P, Mapstone N, Martin IG. Circumferential resection margin involvement: an independent predictor of survival following surgery for oesophageal cancer. Gut 2001; 48: 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zafirellis K, Dolan K, Fountoulakis A, Dexter SP, Martin IG, Sue‐Ling HM. Multivariate analysis of clinical, operative and pathologic features of esophageal cancer: who needs adjuvant therapy? Dis Esophagus 2002; 15: 155–159. [DOI] [PubMed] [Google Scholar]
- 21. Khan OA, Fitzgerald JJ, Soomro I, Beggs FD, Morgan WE, Duffy JP. Prognostic significance of circumferential resection margin involvement following oesophagectomy for cancer. Br J Cancer 2003; 88: 1549–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roh MS, Lee JI, Choi PJ. Significance of circumferential resection margin involvement following esophagectomy for esophageal cancer. Korean J Pathol 2004; 38: 23–28. [Google Scholar]
- 23. Griffiths EA, Brummell Z, Gorthi G, Pritchard SA, Welch IM. The prognostic value of circumferential resection margin involvement in oesophageal malignancy. Eur J Surg Oncol 2006; 32: 413–419. [DOI] [PubMed] [Google Scholar]
- 24. Sujendran V, Wheeler J, Baron R, Warren BF, Maynard N. Effect of neoadjuvant chemotherapy on circumferential margin positivity and its impact on prognosis in patients with resectable oesophageal cancer. Br J Surg 2008; 95: 191–194. [DOI] [PubMed] [Google Scholar]
- 25. Deeter M, Dorer R, Kuppusamy MK, Koehler RP, Low DE. Assessment of criteria and clinical significance of circumferential resection margins in esophageal cancer. Arch Surg 2009; 144: 618–624. [DOI] [PubMed] [Google Scholar]
- 26. Scheepers JJ, van Der Peet DL, Veenhof AA, Cuesta MA. Influence of circumferential resection margin on prognosis in distal esophageal and gastroesophageal cancer approached through the transhiatal route. Dis Esophagus 2009; 22: 42–48. [DOI] [PubMed] [Google Scholar]
- 27. Saha AK, Sutton C, Rotimi O, Dexter S, Sue‐Ling H, Sarela AI. Neoadjuvant chemotherapy and surgery for esophageal adenocarcinoma: prognostic value of circumferential resection margin and stratification of N1 category. Ann Surg Oncol 2009; 16: 1364–1370. [DOI] [PubMed] [Google Scholar]
- 28. Sillah K, Pritchard SA, Watkins GR, McShane J, West CM, Page R et al The degree of circumferential tumour involvement as a prognostic factor in oesophageal cancer. Eur J Cardiothorac Surg 2009; 36: 368–373. [DOI] [PubMed] [Google Scholar]
- 29. Mirnezami R, Rohatgi A, Sutcliffe RP, Hamouda A, Chandrakumaran K, Botha A et al Multivariate analysis of clinicopathological factors influencing survival following esophagectomy for cancer. Int J Surg 2010; 8: 58–63. [DOI] [PubMed] [Google Scholar]
- 30. Pultrum BB, Honing J, Smit JK, van Dullemen HM, van Dam GM, Groen H et al A critical appraisal of circumferential resection margins in esophageal carcinoma. Ann Surg Oncol 2010; 17: 812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chao YK, Yeh CJ, Chang HK, Tseng CK, Chu YY, Hsieh MJ et al Impact of circumferential resection margin distance on locoregional recurrence and survival after chemoradiotherapy in esophageal squamous cell carcinoma. Ann Surg Oncol 2011; 18: 529–534. [DOI] [PubMed] [Google Scholar]
- 32. Verhage RJ, Zandvoort HJ, ten Kate FJ, van Hillegersberg R. How to define a positive circumferential resection margin in T3 adenocarcinoma of the esophagus. Am J Surg Pathol 2011; 35: 919–926. [DOI] [PubMed] [Google Scholar]
- 33. Harvin JA, Lahat G, Correa AM, Lee J, Maru D, Ajani J et al Neoadjuvant chemoradiotherapy followed by surgery for esophageal adenocarcinoma: significance of microscopically positive circumferential radial margins. J Thorac Cardiovasc Surg 2012; 143: 412–420. [DOI] [PubMed] [Google Scholar]
- 34. Rao VS, Yeung MM, Cooke J, Salim E, Jain PK. Comparison of circumferential resection margin clearance criteria with survival after surgery for cancer of esophagus. J Surg Oncol 2012; 105: 745–749. [DOI] [PubMed] [Google Scholar]
- 35. Reid TD, Chan DS, Roberts SA, Crosby TD, Williams GT, Lewis WG. Prognostic significance of circumferential resection margin involvement following oesophagectomy for cancer and the predictive role of endoluminal ultrasonography. Br J Cancer 2012; 107: 1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salih T, Jose P, Mehta SP, Mirza A, Udall G, Pritchard SA et al Prognostic significance of cancer within 1 mm of the circumferential resection margin in oesophageal cancer patients following neo‐adjuvant chemotherapy. Eur J Cardiothorac Surg 2013; 43: 562–567. [DOI] [PubMed] [Google Scholar]
- 37. O'Neill JR, Stephens NA, Save V, Kamel HM, Phillips HA, Driscoll PJ et al Defining a positive circumferential resection margin in oesophageal cancer and its implications for adjuvant treatment. Br J Surg 2013; 100: 1055–1063. [DOI] [PubMed] [Google Scholar]
- 38. Ahmad J, Loughrey MB, Donnelly D, Ranaghan L, Shah R, Napolitano G et al Prognostic value of added stratification of circumferential resection margin status in oesophageal carcinoma. Histopathology 2013; 62: 752–763. [DOI] [PubMed] [Google Scholar]
- 39. Gilbert S, Martel AB, Seely AJ, Maziak DE, Shamji FM, Sundaresan SR et al Prognostic significance of a positive radial margin after esophageal cancer resection. J Thorac Cardiovasc Surg 2015; 149: 548–555. [DOI] [PubMed] [Google Scholar]
- 40. Lee GD, Lee SE, Kim KM, Kim YH, Ahn JH, Jung S et al New 3‐tiered circumferential resection margin criteria in esophageal squamous cell carcinoma. Ann Surg 2015; 262: 965–971. [DOI] [PubMed] [Google Scholar]
- 41. Okada N, Fujii S, Fujita T, Kanamori J, Kojima T, Hayashi R et al The prognostic significance of the positive circumferential resection margin in pathologic T3 squamous cell carcinoma of the esophagus with or without neoadjuvant chemotherapy. Surgery 2016; 159: 441–450. [DOI] [PubMed] [Google Scholar]
- 42. Ghadban T, Reeh M, Koenig AM, Nentwich MF, Bellon E, Izbicki JR et al Prognostic significant or not? The positive circumferential resection margin in esophageal cancer: impact on local recurrence and overall survival in patients without neoadjuvant treatment. Ann Surg 2017; 266: 988–994. [DOI] [PubMed] [Google Scholar]
- 43. Depypere L, Moons J, Lerut T, De Hertogh G, Peters C, Sagaert X et al Prognostic value of the circumferential resection margin and its definitions in esophageal cancer patients after neoadjuvant chemoradiotherapy. Dis Esophagus 2018; 31: dox117. [DOI] [PubMed] [Google Scholar]
- 44. Knight WRC, Zylstra J, Wulaningsih W, Van Hemelrijck M, Landau D, Maisey N et al; Guy's and St Thomas' Oesophago‐Gastric Research Group . Impact of incremental circumferential resection margin distance on overall survival and recurrence in oesophageal adenocarcinoma. BJS Open 2018; 2: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Stat Med 1998; 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 47. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000; 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 49. Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol 2007; 17: 38–44. [DOI] [PubMed] [Google Scholar]
- 50. Pauligk C, Tannapfel A, Meiler J, Luley KB, Kopp HG, Homann N et al Pathological response to neoadjuvant 5‐FU, oxaliplatin, and docetaxel (FLOT) versus epirubicin, cisplatin, and 5‐FU (ECF) in patients with locally advanced, resectable gastric/esophagogastric junction (EGJ) cancer: Data from the phase II part of the FLOT4. J Clin Oncol 2015; 33 (Suppl 15): 4016. [Google Scholar]
- 51. Burdall OC, Boddy AP, Fullick J, Blazeby J, Krysztopik R, Streets C et al A comparative study of survival after minimally invasive and open oesophagectomy. Surg Endosc 2015; 29: 431–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Key characteristics and demographics of eligible studies
Table S2 Pathology and outcome data for eligible studies


