Abstract
Background
Superior mesenteric artery syndrome (SMAS) is often associated with gastric dilatation but, very rarely, it can be associated with gastric emphysema. In addition, there are few reported cases accompanied by septic shock.
Case Presentation
A 64‐year‐old man was transferred to our hospital with vomiting and abdominal distention. He went into shock and showed impaired consciousness. Blood biochemistry tests showed elevated levels of C‐reactive protein. An abdominal computed tomography scan revealed a dilated stomach and proximal duodenum, and constriction of the third portion of the duodenum with gastric and portal emphysema. We thus diagnosed him with gastric and portal emphysema associated with SMAS that progressed to septic shock. We treated him conservatively by giving antibiotics and undertaking gastric drainage and feeding by way of the jejunum through a double elementary diet tube.
Conclusion
This is the first report to describe a case of SMAS with gastric and portal emphysema that progressed to septic shock.
Keywords: Gastric emphysema, septic shock, superior mesenteric artery syndrome (SMAS)
Introduction
Superior mesenteric artery syndrome (SMAS) is rare form of upper gastrointestinal obstruction with an incidence ranging between 0.013% and 0.3%.1, 2 The predominant symptom of SMAS is generally repetitive vomiting. Superior mesenteric artery syndrome is often associated with gastric dilatation but, very rarely, it can be associated with gastric emphysema.3, 4 In addition, there are few reported cases accompanied by septic shock. We report a very rare case of SMAS with gastric emphysema and portal emphysema that progressed to septic shock.
Case report
A 64‐year‐old man presented to his local doctor with vomiting and abdominal distention. He had a medical history of depression and Parkinson's disease. His arterial blood gas analysis results on room air showed severe hypoxemia and acidemia (pH 6.96, PaO2 60 mmHg, PaCO2 63 mmHg, HCO3− 14 mmol/L, base excess −18 mmol/L), and abdominal computed tomography (CT) showed gastric wall pneumatosis and portal emphysema. He was intubated and transferred to the Department of Emergency and Critical Care Medicine, Kansai Medical University (Hirakata, Japan).
On physical examination, he had a Glasgow Coma Scale score of 5 (E1VTM4). His blood pressure was 80/59 mmHg, and his heart rate was 133 b.p.m. under administration of 0.2 μg/kg/min norepinephrine. He had abdominal distention, and 3000 mL of reddish‐brown gastric juice was aspirated through his nasogastric (NG) tube. Blood biochemistry tests showed decreased levels of white blood cells (2100/μL) and elevated levels of C‐reactive protein (15.8 mg/dL) and procalcitonin (6.6 ng/mL).
An abdominal CT revealed a dilated stomach and proximal duodenum, with extensive gastric wall pneumatosis and portal emphysema. This was unlikely to be bowel necrosis because the gastric wall was highlighted (Fig. 1). Upper gastrointestinal endoscopy showed acute gastric mucosal lesions (AGMLs) over a wide range of focus on the gastric fornix (Fig. 2). Although the culture of gastric acid and gastric mucosa, and blood culture were negative, levels of C‐reactive protein and procalcitonin were high. Our provisional diagnosis at that time was gastric wall pneumatosis, portal emphysema, and septic shock derived from emphysematous gastritis.
Figure 1.
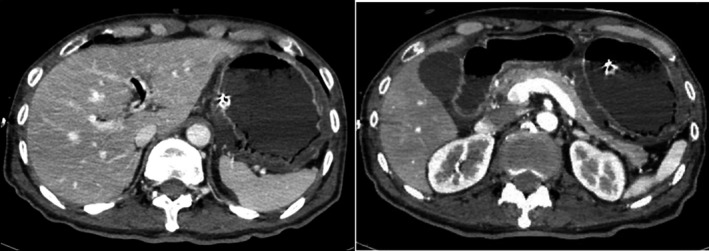
Abdominal computed tomography scan of a 64‐year‐old man with superior mesenteric artery syndrome with gastric and portal emphysema that progressed to septic shock. The images reveal a dilated stomach and proximal duodenum, with extensive gastric wall pneumatosis and portal emphysema. The gastric wall is highlighted.
Figure 2.
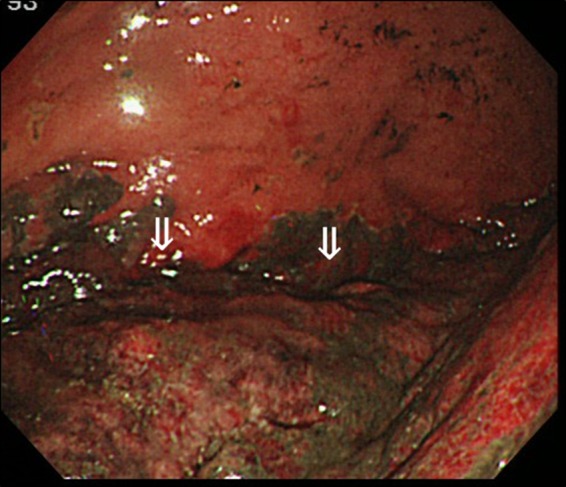
Upper gastrointestinal endoscopy of a 64‐year‐old man with superior mesenteric artery syndrome with gastric and portal emphysema that progressed to septic shock. The image shows acute gastric mucosal lesions (arrows) over a wide range of focus on the gastric fornix.
We treated him conservatively by giving antibiotics and antacids, and undertaking gastric drainage with a NG tube. His respiratory and circulatory conditions improved, and he was taken off of the pressor agent on hospital day 3. On hospital day 4, the abdominal CT showed a decrease in the gastric wall pneumatosis and portal emphysema. In addition, upper gastrointestinal endoscopy showed improvement of AGMLs, and we could culture no organisms from his gastric juice or gastric mucosa. We thus started him on NG tube feeding. Shortly thereafter, however, he went into shock and showed impaired consciousness. An abdominal CT and gastrointestinal endoscopy revealed exacerbation of the gastric wall pneumatosis, portal emphysema, and AGMLs. Therefore, we started gastric drainage and feeding by way of the jejunum through a double elementary diet (W‐ED) tube. On hospital day 13, feeding was changed back to an NG tube because there was no appearance of digestive symptoms and improvement of AGMLs. But, again, shortly thereafter, the same findings and septic shock recurred. Because of the repeat of his symptoms following NG tube feeding, we re‐examined his medical history and CT findings.
Due to the patient's lack of appetite, his body weight had decreased by 30 kg over 2 years and his body mass index on admission was 17 kg/m2. An abdominal CT showed an SMA of 7.7 mm in diameter arising from the aorta at a narrow aortomesenteric angle (AMA) of 9.8°, and constriction of the third portion of the duodenum (Fig. 3). We thus diagnosed him as having SMAS and obstructive gastric emphysema and started gastric drainage and feeding by way of the jejunum through a W‐ED tube. He began to gain weight and a CT showed an increase in the AMA. On hospital day 49, we changed from the W‐ED tube to an NG tube, and the patient's general condition improved further. He was transferred to a rehabilitation hospital on hospital day 60.
Figure 3.
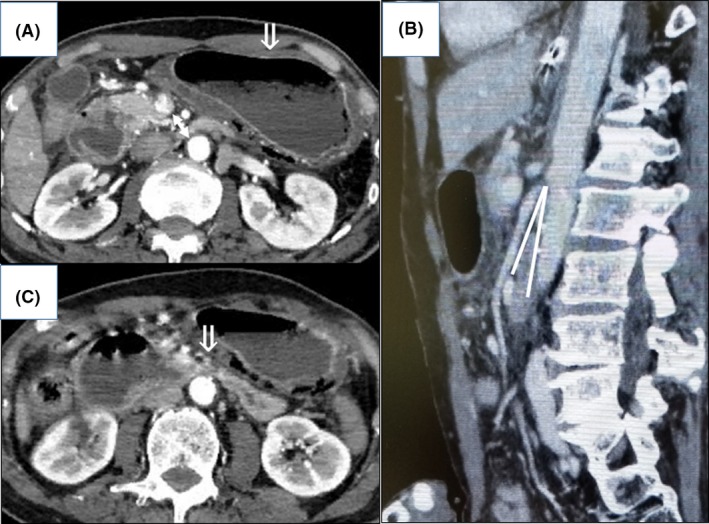
Abdominal computed tomography scans on admission of a 64‐year‐old man with superior mesenteric artery syndrome with gastric and portal emphysema that progressed to septic shock. A, The superior mesenteric artery arising from the aorta is short (arrow). B, Narrow aortomesenteric angle, indicated by white lines. C, Constricting of the third portion of duodenum (arrow).
Discussion
Superior mesenteric artery syndrome is a defined disorder whereby gastrointestinal obstruction occurs due to compression of the third portion of the duodenum at the root of the SMA.5 In ultrasonography and CT findings, we observe that the SMA arising from the aorta is less than 8 mm in diameter and the AMA is less than 22°.6 Superior mesenteric artery syndrome has a predilection for patients aged 10–30 years old and is precipitated by severe wasting diseases or dietary disorders, severe injuries that lead to prolonged bed rest, spinal conditions, postoperative obstruction, and congenital anomalies.7 Superior mesenteric artery syndrome is often treated conservatively, such as with gastric drainage and weight gain through the administration of added nutrition. However, when conservative treatment is ineffective or SMAS recurs repeatedly, surgical treatment is necessary.
The SMAS in this patient was precipitated by low body weight and prolonged bed rest because of a loss of motivation combined with depression and was treated conservatively.
Gastric wall pneumatosis is typically divided into two types: emphysematous gastritis and gastric emphysema. Emphysematous gastritis is an infectious gastritis caused by gas‐forming organisms and has an extremely poor prognosis. Gastric emphysema is typically divided into three categories: traumatic, obstructive, and pulmonary.8 Emphysema caused by an increase in internal gastric pressure, such as in SMAS, is categorized as obstructive type. The emphysema is not associated with infection and can often be treated conservatively.
We surmise that this was a case of obstructive gastric emphysema because of the facts that NG tube feedings led to an exacerbation of the emphysema and the cultures of gastric juice and gastric mucosa were negative. The possible pathogenesis in this case was that the combination of gastric mucosal disturbance and an increase in gastric pressure due to SMAS led to the spread of air bubbles in the submucosal tissue, that is, obstructive gastric emphysema, and invasion of bacteria into the blood vessels that resulted in septic shock.
There are few reported cases of SMAS with gastric emphysema or SMAS with septic shock. A MEDLINE search found only one case reported of SMAS complicated by septic shock. Furthermore, this is the first report, to our knowledge, to describe a case of SMAS with gastric emphysema and portal emphysema that progressed to septic shock. Clinicians should bear in mind that septic shock can be complicated by SMAS and gastric emphysema without the presence of gas‐forming organisms.
Disclosure
Approval of the research protocol: N/A.
Informed consent: The patient provided consent for publication.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Funding information
No funding information provided.
References
- 1. Anderson JR, Earnshaw PM, Fraser GM. Extrinsic compression of the third part of the duodenum. Clin. Radiol. 1982; 33: 75–81. [DOI] [PubMed] [Google Scholar]
- 2. Biank V, Werlin S. Superior mesenteric artery syndrome in children: a 20‐year experience. J. Pediatr. Gastroenterol. Nutr. 2006; 42: 522–5. [DOI] [PubMed] [Google Scholar]
- 3. Lim JE, Duke GL, Eachempati SR. Superior mesenteric artery syndrome presenting with acute massive gastric dilatation, gastric wall pneumatosis, and portal venous gas. Surgery 2003; 134: 840–3. [DOI] [PubMed] [Google Scholar]
- 4. Sakamoto Y, Mashiko K, Matsumoto H et al Gastric pneumatosis and portal venous gas in superior mesenteric artery syndrome. Indian J. Gastroenterol. 2006; 25: 265–6. [PubMed] [Google Scholar]
- 5. Gustafsson L, Falk A, Lukes PJ et al Diagnosis and treatment of superior mesenteric artery syndrome. Br. J. Surg. 1985; 71: 499–501. [DOI] [PubMed] [Google Scholar]
- 6. Unal B, Aktaş A, Kemal G et al Superior mesenteric artery syndrome: CT and ultrasonography findings. Diagn Interv Radiol. 2005; 11: 90–5. [PubMed] [Google Scholar]
- 7. Barnes JB, Lee M. Superior mesenteric artery syndrome in an intravenous drug abuser after rapid weight loss. South. Med. J. 1996; 89: 331–4. [DOI] [PubMed] [Google Scholar]
- 8. Agha FP. Gastric emphysema: an etiologic classification. Australas. Radiol. 1984; 28: 346–52. [DOI] [PubMed] [Google Scholar]


