Abstract
Background
Intraoperative goal‐directed fluid therapy (GDFT) is recommended in most perioperative guidelines for intraoperative fluid management in patients undergoing elective colorectal surgery. However, the evidence in elective colorectal surgery alone is not well established. The aim of this meta‐analysis was to compare the effects of GDFT with those of conventional fluid therapy on outcomes after elective colorectal surgery.
Methods
A meta‐analysis of RCTs examining the role of transoesophageal Doppler‐guided GDFT with conventional fluid therapy in adult patients undergoing elective colorectal surgery was performed in accordance with PRISMA methodology. The primary outcome measure was overall morbidity, and secondary outcome measures were length of hospital stay, time to return of gastrointestinal function, 30‐day mortality, acute kidney injury, and surgical‐site infection and anastomotic leak rates.
Results
A total of 11 studies were included with a total of 1113 patients (556 GDFT, 557 conventional fluid therapy). There was no significant difference in any clinical outcome measure studied between GDFT and conventional fluid therapy, including overall morbidity (risk ratio (RR) 0·90, 95 per cent c.i. 0·75 to 1·08, P = 0·27; I 2 = 47 per cent; 991 patients), 30‐day mortality (RR 0·67, 0·23 to 1·92, P = 0·45; I 2 = 0 per cent; 1039 patients) and length of hospital stay (mean difference 0·01 (95 per cent c.i. −0·92 to 0·94) days, P = 0·98; I 2 = 34 per cent; 1049 patients).
Conclusion
This meta‐analysis does not support the perceived benefits of GDFT guided by transoesophageal Doppler monitoring in the setting of elective colorectal surgery.
Introduction
Intraoperative goal‐directed fluid therapy (GDFT) using measurements of stroke volume and cardiac output to inform administration of a small volume of fluid (usually 200–250 ml of a colloid, sometimes a crystalloid) to optimize stroke volume has been used for over two decades. A meta‐analysis1 published in 2014 of 22 RCTs that employed GDFT in a variety of elective and emergency operations showed that the risk of developing complications after surgery was reduced by 23 per cent when compared with conventional intraoperative fluid therapy. However, a further meta‐analysis2, which examined 23 RCTs including 2099 patients undergoing elective major abdominal surgery, suggested that GDFT may not be of benefit in all patients, particularly those managed in an enhanced recovery after surgery (ERAS)3, 4 setting. A recent RCT5 including 450 patients at low to moderate risk undergoing major elective surgery demonstrated that oesophageal Doppler‐guided GDFT was associated with a significant reduction in overall complications as well as length of hospital stay.
Most guidelines for perioperative care in colorectal surgery3, 4, 6, 7 recommend that GDFT should be used for patients undergoing colorectal surgery. Furthermore, the UK National Institute for Health and Care Excellence (NICE) has previously issued guidelines stating that intraoperative GDFT with transoesophageal Doppler monitoring should be used in ‘patients undergoing major or high‐risk surgery’8, with evidence supporting both a clinical benefit and cost‐saving based on the literature available when the guideline was published in 2011.
Previous meta‐analyses have been weakened by the inclusion of patients undergoing both emergency and elective surgery1, 9 and a wide range of operations1, 2, 10, and also by the fact that overall standards of perioperative care have changed over the past two decades. Moreover, the device used for monitoring stroke volume and cardiac output varied across the individual RCTs2. Hydroxyethyl starch (HES) was the colloid used frequently in the GDFT arm, but recently there has been a call by the European Medicines Agency for use of this fluid to be suspended11 because of the increased incidence of acute kidney injury (AKI) and the need for renal replacement therapy12, 13, 14.
The aim of this meta‐analysis of RCTs was to examine the effect of GDFT using transoesophageal Doppler monitoring compared with that of conventional intraoperative fluid therapy on postoperative outcome, including AKI, in patients undergoing elective major colorectal surgery.
Methods
The protocol for this meta‐analysis was registered at the outset with the PROSPERO database (www.crd.york.ac.uk/prospero) (registration number CRD42018106818).
Search strategy
A search of the PubMed, MEDLINE, Google™ Scholar and Cochrane Library databases was performed to identify full‐text studies evaluating the impact of intraoperative GDFT on postoperative surgical outcomes in patients undergoing elective colorectal surgery, published between January 1995 and July 2018. The electronic search terms used were [‘goal‐directed fluid therapy’ OR ‘flow‐directed fluid therapy’] AND [‘surgery’ OR ‘intraoperative’] AND [colon OR rectal OR colorectal]. No language restriction was imposed on the search. Only studies including adult patients undergoing elective colorectal surgery were selected. The bibliographies of all studies that met the inclusion criteria were hand‐searched for any additional suitable articles and relevant conference abstracts to ensure study inclusion was as complete as possible. The meta‐analysis was conducted according to the PRISMA statement15.
Selection of articles
Following the exclusion of initial studies on the basis of article title and abstract by two independent researchers, the remaining full‐text articles were screened in detail for inclusion. Studies were included if they examined adult patients undergoing elective colorectal surgery who were randomized to receive either GDFT administered with transoesophageal Doppler monitoring or conventional intraoperative fluid therapy, and if the study reported at least one relevant postoperative outcome. Studies were excluded if any patient had undergone non‐colorectal surgery, or if they included any emergency surgical procedures, employed any device other than the transoesophageal Doppler for the conduct of GDFT, did not include any relevant clinical outcome measures, or if both groups received GDFT. Any studies in which inclusion criteria were not clear were discussed by the authors, with the final decision made by the senior author.
Data extraction
Study data were extracted from the included RCTs by one author and checked by another. The primary outcome measure examined was overall postoperative morbidity; secondary outcome measures included 30‐day mortality, length of hospital stay (LOS), time to return of gastrointestinal function (flatus and stool), incidence of paralytic ileus and AKI, and rates of surgical‐site infection and anastomotic leak. Data were also collated on patient demographics (age, sex, ASA grade), surgical variables (surgical procedure, number of laparoscopic procedures, estimated blood loss) and intraoperative fluid administration (overall, maintenance and bolus fluid volumes, and inotrope administration). Data were extracted on whether the patient was managed using ERAS principles16, 17 or traditional perioperative care, and the method of administration of GDFT was noted. If data necessary for the conduct of the meta‐analysis were not available from the manuscript, the corresponding author was approached to obtain this with the aim of ensuring data collection was as complete as possible. If continuous data were reported only as median (i.q.r.) values and authors did not provide mean(s.d.) values, the technique described by Hozo et al.18 was used to estimate mean(s.d.) from median (i.q.r.) values. This technique uses the median as the best estimate of the mean, with the standard deviation calculated by the following formula: (upper limit of i.q.r. − lower limit of i.q.r.)/1·35.
Risk of bias was assessed using the Cochrane Collaboration tool for assessing bias from Review Manager version 5.3 (RevMan; The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark).
Statistical analysis
Data extracted from the included studies were entered into the RevMan 5.3 software program. Dichotomous variables were analysed using the Mantel–Haenszel random‐effects model and quoted as a risk ratio (RR) with 95 per cent confidence intervals. Continuous variables were analysed using the inverse‐variance random‐effects model and quoted as a weighted mean difference (MD) with 95 per cent confidence intervals. When the Hozo technique18 had been used to estimate mean(s.d.) data from studies included to inform continuous data analysis, two separate meta‐analyses were conducted, one including the estimated data and one excluding these data. Data were used to construct forest plots, with P < 0·050 on two‐tailed testing indicating a statistically significant difference. Study heterogeneity and inconsistency were assessed using the I 2 statistic19, with 25 per cent or less representing low, 25–50 per cent moderate and above 50 per cent high heterogeneity.
Results
Of 831 studies identified initially, 1120, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 were deemed eligible for inclusion (Fig. 1). One published abstract31 was identified that would have been suitable for inclusion, but the data available from the text were insufficient to include in the meta‐analysis. Overall, the risk of bias of the studies included was low and generally the quality of the studies was medium to high (Table 1).
Figure 1.
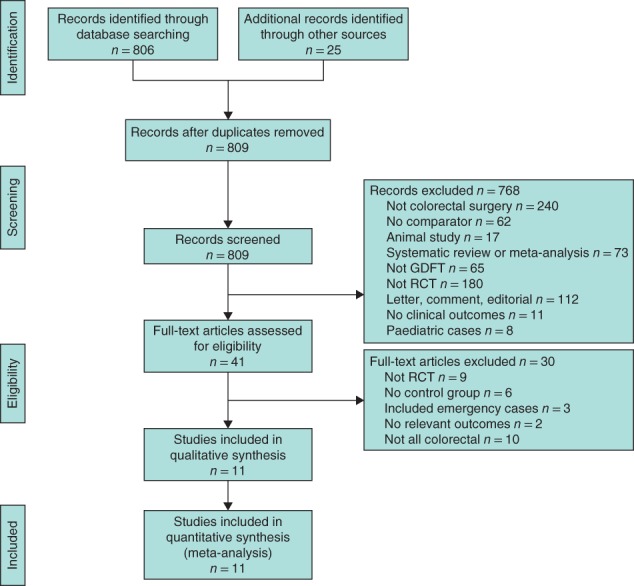
PRISMA diagram for the study GDFT, goal‐directed fluid therapy.
Table 1.
Risk of bias in the 11 included studies
| Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Blinding of outcome data (attrition bias) | Selective reporting (reporting bias) | Other bias | |
|---|---|---|---|---|---|---|---|
| Brandstrup et al.23 | + | + | ? | + | + | + | + |
| Challand et al.25 | ? | ? | ? | + | + | + | + |
| Conway et al.30 | ? | ? | ? | ? | + | + | − |
| Gómez‐Izquierdo et al.26 | + | + | + | + | + | + | + |
| Noblett et al.28 | ? | ? | + | + | + | + | − |
| Phan et al.21 | + | + | − | + | + | + | + |
| Reisinger et al.20 | + | + | − | + | + | + | ? |
| Senagore et al.27 | + | ? | ? | ? | + | + | + |
| Srinivasa et al.22 | + | + | − | + | + | + | + |
| Wakeling et al.29 | + | + | + | ? | + | + | + |
| Zakhaleva et al.24 | + | + | ? | ? | + | + | ? |
+, Low risk of bias; ?, uncertain risk of bias; −, high risk of bias.
Demographics
The 11 RCTs in this meta‐analysis20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 included a total of 1113 adult patients who had undergone elective colorectal surgery, of whom 556 were randomized to intraoperative GDFT using transoesophageal Doppler monitoring and 557 to traditional intraoperative fluid management strategies. In ten studies GDFT was administered as part of an enhanced recovery programme, with just one study30 being conducted within a traditional care programme; thus no analysis was conducted comparing patients managed as part of these differing pathways. Data on laparoscopic surgical approach only were provided by two studies26, 27, with no studies providing data from open‐only approaches. Baseline patient demographics are provided in Table S1 (supporting information), and fluid administered during the perioperative period is detailed in Table S2 (supporting information).
Overall morbidity
Nine studies21, 22, 23, 24, 25, 26, 28, 29, 30 including 487 patients managed with GDFT and 504 who had traditional fluid management reported overall morbidity rates (Fig. 2 a). Overall morbidity was not significantly different between these groups (RR 0·90, 95 per cent 0·75 to 1·08, P = 0·27; I 2 = 47 per cent).
Figure 2.
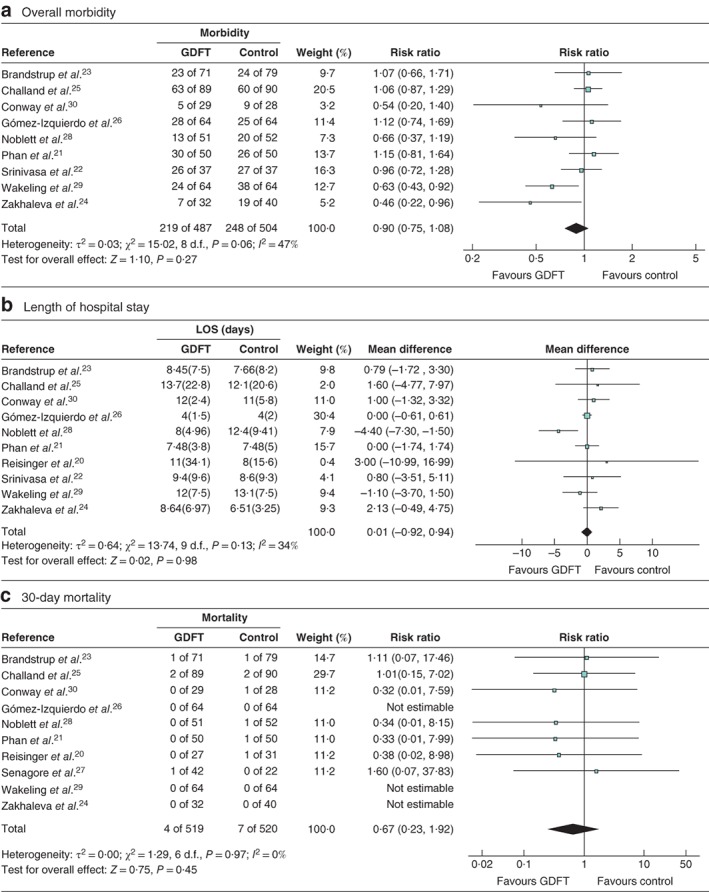
Forest plots of overall morbidity, length of hospital stay and 30‐day mortality a Overall morbidity, b mean(s.d.) length of hospital stay (LOS) and c 30‐day mortality in patients receiving goal‐directed fluid therapy (GDFT) versus controls. a,c Risk ratios are shown with 95 per cent confidence intervals; a Mantel–Haenszel random‐effects model was used for meta‐analysis. b Mean differences are shown with 95 per cent confidence intervals; an inverse‐variance random‐effects model was used to perform the meta‐analysis.
Length of hospital stay
Overall LOS was considered by ten studies20, 21, 22, 23, 24, 25, 26, 28, 29, 30 included in the meta‐analysis (1049 patients, 514 GDFT and 535 traditional) (Fig. 2 b). However, two studies20, 26 included only median (i.q.r.) data and did not provide the authors with mean(s.d.) data for the meta‐analysis. These data were estimated using the technique described by Hozo et al.18 and all data were included in the primary analysis of LOS. A separate meta‐analysis was performed for this outcome excluding the estimated data.
In the first analysis, which included estimated data for LOS, GDFT was not associated with a significant difference in the overall group (MD 0·01 (95 per cent c.i. −0·92 to 0·94) days, P = 0·98; I 2 = 34 per cent) (Fig. 2 b). In the second analysis, which excluded the estimated data, GDFT resulted in no significant change in hospital length of stay (MD −0·01 (−1·38 to 1·35) days, P = 0·99; I 2 = 48 per cent).
Thirty‐day mortality
Mortality rates were detailed in ten studies20, 21, 23, 24, 25, 26, 27, 28, 29, 30, including 519 patients in the GDFT group and 520 in the traditional group (Fig. 2 c). Overall there was no significant difference in 30‐day mortality between GDFT and control patients (RR 0·67, 95 per cent c.i. 0·23 to 1·92, P = 0·45; I 2 = 0 per cent).
Surgical‐site infection
Five studies20, 21, 22, 23, 24 examined the incidence of surgical‐site infection: 217 patients managed with intraoperative GDFT versus 237 controls (Fig. 3 a). Use of GDFT did not affect the rate of surgical‐site infection significantly (RR 0·61, 95 per cent c.i. 0·34 to 1·09, P = 0·10; I 2 = 0 per cent).
Figure 3.
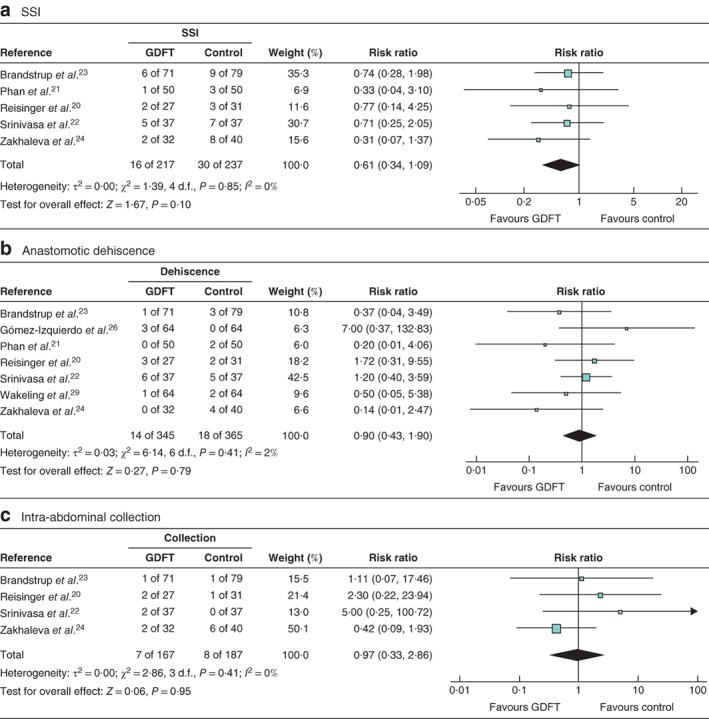
Forest plots of surgical‐site infection, anastomotic dehiscence and intra‐abdominal collection a Surgical‐site infection (SSI), b anastomotic dehiscence and c intra‐abdominal collection in patients receiving goal‐directed fluid therapy (GDFT) versus controls. Risk ratios are shown with 95 per cent confidence intervals. Mantel–Haenszel random‐effects models were used for meta‐analysis.
Anastomotic dehiscence
Seven studies20, 21, 22, 23, 24, 26, 29 included data on the rate of anastomotic dehiscence: 345 patients managed with intraoperative GDFT versus 365 control patients (Fig. 3 b). Intraoperative administration of GDFT did not affect the incidence of anastomotic dehiscence (RR 0·90, 95 per cent c.i. 0·43 to 1·90, P = 0·79; I 2 = 2 per cent).
Intra‐abdominal collection
A total of four studies20, 22, 23, 24 examined the relationship between GDFT and conventional fluid therapy and the rate of intra‐abdominal collection (Fig. 3 c). There was no significant difference between the two groups (RR 0·97, 95 per cent c.i. 0·33 to 2·86, P = 0·95; I 2 = 0 per cent).
Postoperative ileus
Seven studies20, 21, 22, 23, 24, 26, 28 included data on the rate of postoperative ileus: 332 patients managed with intraoperative GDFT versus 353 control patients (Fig. 4 a). The use of GDFT did not affect the incidence of postoperative paralytic ileus significantly (RR 0·89, 95 per cent c.i. 0·49 to 1·60, P = 0·70; I 2 = 36 per cent).
Figure 4.
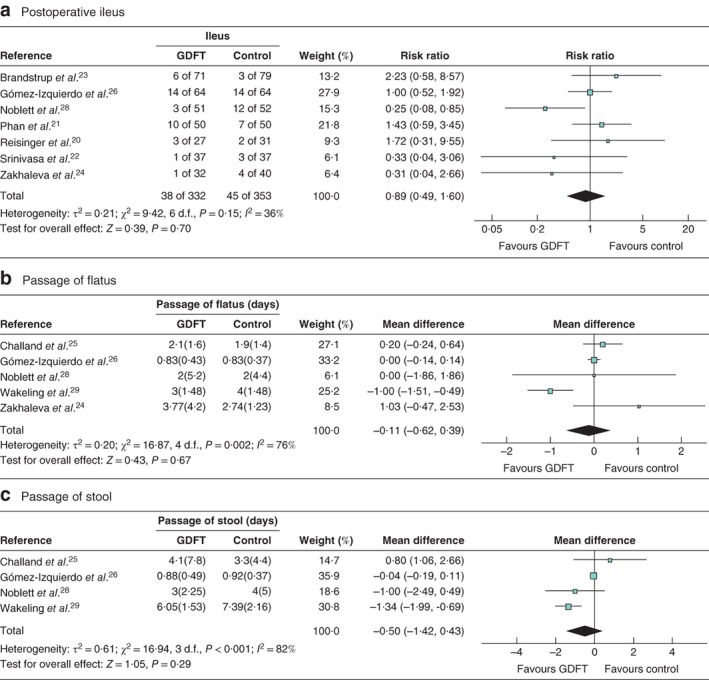
Forest plots of postoperative ileus, time to passage of flatus and time to passage of stool a Postoperative ileus, b mean(s.d.) time to passage of flatus and c mean(s.d.) time to passage of stool in patients receiving goal‐directed fluid therapy (GDFT) versus controls. a Risk ratios are shown with 95 per cent confidence intervals; a Mantel–Haenszel random‐effects model was used for meta‐analysis. b,c Mean differences are shown with 95 per cent confidence intervals; inverse‐variance random‐effects models were used to perform the meta‐analyses.
Return of gastrointestinal function
Five studies examined time to return of gastrointestinal function after surgery, in either the form of flatus24 or both flatus and stool25, 26, 28, 29. Two separate analyses were performed for each outcome as in previous analyses.
For time to flatus in all studies, including those with calculated data (Fig. 4 b), there were 300 patients managed with GDFT and 310 control patients in five studies24, 25, 26, 28, 29. There was no significant difference in the time to return of flatus between the two fluid strategies (MD −0·11 (95 per cent c.i. −0·62 to 0·39) days, P = 0·67; I 2 = 76 per cent). Just two studies25, 29 were left when data calculated using the Hozo technique18 were excluded, so a further meta‐analysis was not attempted.
When time to stool was considered, 268 patients were managed with GDFT and 270 with control intraoperative fluid (Fig. 4 c). There was no significant difference in the overall group (MD −0·50 (95 per cent c.i. −1·42 to 0·43) days, P = 0·29; I 2 = 82 per cent). When data for the single study26 with estimated data were excluded, no difference in time to passage of stool was observed (MD −0·76 (−1·88 to 0·35) days, P = 0·18; I 2 = 56 per cent).
Acute kidney injury
Four studies21, 24, 25, 29 examined the relationship between GDFT and conventional fluid therapy and the incidence of AKI in 479 patients; there was no significant difference between the two groups (RR 1·51, 95 per cent c.i. 0·85 to 2·66, P = 0·16; I 2 = 0 per cent).
Discussion
This meta‐analysis has demonstrated that in patients undergoing elective colorectal surgery GDFT guided by transoesophageal Doppler monitoring was not associated with a significant difference in any postoperative clinical outcome measure compared with conventional intraoperative fluid therapy. There was an indication of difference in terms of a reduced rate of surgical‐site infection when patients received GDFT, and increased rate of AKI, but neither of these trends was statistically significant. Data on the role of GDFT in the open versus the laparoscopic approach for elective colorectal surgery were insufficient, so the decision was taken not to perform a meta‐analysis for this variable. Further high‐quality evidence is necessary to assess the potential role of GDFT between surgical approaches. Only one study30 examined the role of GDFT as part of a traditional care pathway versus ten studies within an ERAS pathway; hence no comparison could be made between these settings.
These findings are more pronounced in the lack of benefit for GDFT than found in a previous meta‐analysis2 examining the role of GDFT in elective abdominal surgery, although that study2 did not limit the papers included by the method of administration of GDFT. The previous meta‐analysis2 found that, overall, GDFT was associated with a significant reduction in morbidity, LOS, duration of stay in the ICU and time to passage of stool, with no significant difference observed in the incidence of postoperative ileus, mortality or time to return of flatus.
An issue raised by other meta‐analyses2, 9, 10 regarding the comparison between GDFT and traditional fluid management strategies is differences in fluid management strategies over the past three decades. In the more historical studies, large volumes tended to be infused in the traditionally managed group, whereas more contemporary fluid management strategies observed in more recently published studies tend to aim for zero balance and for the patient to reach the anaesthetic room in a well hydrated state. The U‐shaped relationship between fluid volume infused and perioperative morbidity that has been postulated previously32 may suggest that in more modern fluid management strategies33, 34 the differences in clinical benefit between GDFT and traditional intraoperative fluid management may not be as pronounced if fluid overload and deficits are avoided.
This study is the largest to examine the impact of GDFT versus traditional fluid management strategies in elective colorectal surgery. The only other previous meta‐analysis35 published on the topic has several methodological issues, as it inadvertently included studies that were not RCTs, despite this being the stated aim, and missed several key studies on the topic. The present study included just one method for the conduct of GDFT: transoesophageal Doppler monitoring. This is the commonest device used to perform GDFT in the UK, as well as in widespread use internationally36, and is the method suggested by NICE in the UK8. Evidence has suggested that transoesophageal Doppler and other methods for GDFT such as lithium dilution techniques37, 38, calibrated pulse contour analysis39 and the pleth variability index40 are not interchangeable; hence the decision was taken to make the study population as homogeneous as possible.
Generally, the degree of heterogeneity within the analyses conducted was low, with five analyses having low levels of heterogeneity, four having moderate levels, and just three having high levels of heterogeneity (those conducted on time to passage of flatus and stool). This large degree of concordance in study results, in addition to the relatively high study quality, adds weight to the conclusions drawn.
This meta‐analysis has a few weaknesses inherent in its design and conduct. The regimens for postoperative fluid management were poorly documented in the included studies. This may have impacted on postoperative outcome, but cannot be accounted for. This study chose to focus on just one technique for the conduct of GDFT, transoesophageal Doppler monitoring, as this was felt to improve the homogeneity of the meta‐analysis. However, this restricts the generalizability of the results to GDFT conducted using other techniques. In addition, efforts to obtain raw data for the continuous variables were not successful. Therefore, the Hozo technique18 was used to estimate mean(s.d.) data for two studies20, 26 in the LOS analysis, three studies26, 28, 29 in the time to return of flatus, and one study26 in the time to return of stool. To negate this potential weakness, an additional analysis, excluding the estimated data, was planned at the outset of the conduct of the meta‐analysis. For the LOS analysis and time to return of stool, excluding the estimated data, no difference was observed in the outcome. However, as three of the five studies required estimated data in the time to return of flatus analysis, no further analysis was conducted due to the poor strength of any conclusion drawn.
A similar meta‐analysis35, including 11 RCTs, was published in 2018 examining the role of GDFT in colorectal surgery. However, two of these 11 studies were not RCTs: one41 was a comparison between a series of patients recruited to undergo GDFT and a historical series of patients managed with traditional fluid management, and the other42 was a matched cohort of patients who were not randomized. In addition, three papers20, 21, 22, which should have been included as they met the inclusion criteria, were missed. They included a study30 of patients undergoing ‘major bowel surgery’, which was excluded from the present meta‐analysis. All but one study used transoesophageal Doppler‐guided GDFT, with the single remaining study43 including central venous oxygen saturation‐guided GDFT; however, this was excluded from the present meta‐analysis. The above‐mentioned methodological flaws in this paper35 weaken the strength of the conclusions.
The nature of the fluid used for the bolus associated GDFT was variable, with HES being the documented fluid administered in seven studies20, 21, 23, 25, 26, 27, 30. However, there is a moratorium on the use of HES owing to concerns of an increased risk of AKI requiring renal replacement therapy12, 13, 14 as well as mortality12, 14, based on recent RCTs. The indication towards increasing rates of AKI in patients receiving GDFT compared with those having traditional fluid management could potentially be related to the larger volume of HES infused in this group and the inherent increased risks. Just four studies21, 24, 25, 29 included data on AKI rates, with the nature of the bolus fluid being variably reported in these. In one study25 HES was administered for boluses, one21 gave a colloid although the choice was ‘at the discretion of the anaesthetist’ and included HES, gelatine or human albumin solution, one29 gave non‐HES colloid, and one24 did not specify the type of colloid given. If just those two studies21, 25 clearly receiving HES were analysed for AKI, the strength of the indication increased; however, based on just two studies, this is far from conclusive evidence. Future studies focusing on the role of GDFT will not include HES as the bolus agent11, and this may result in improved clinical outcomes.
This study has demonstrated no benefit for the routine use of transoesophageal Doppler‐guided GDFT in patients undergoing elective colorectal surgery, contrary to NICE guidance8, which recommends that GDFT technology should be used ‘in patients undergoing major or high‐risk surgery’.
Supporting information
Table S1 Baseline patient demographics for all included studies
Table S2 Intraoperative fluid infused in goal‐directed and control groups
Acknowledgements
This work was supported by the Medical Research Council (grant number MR/K00414X/1) and Arthritis Research UK (grant number 19891).
D.N.L. has received unrestricted research funding from B. Braun and speaker's honoraria from Fresenius Kabi, B. Braun, Shire and Baxter Healthcare for unrelated work.
Disclosure: The authors declare no conflict of interest.
Funding information
Medical Research Council, MR/K00414X/1.
Arthritis Research UK, 19891.
Presented to the Annual Meeting of the Society for Academic and Research Surgery, London, UK, January 2019; published in abstract form as Br J Surg 2019; 106(Suppl 3): 24–25
References
- 1. Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G et al; OPTIMISE Study Group . Effect of a perioperative, cardiac output‐guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014; 311: 2181–2190. [DOI] [PubMed] [Google Scholar]
- 2. Rollins KE, Lobo DN. Intraoperative goal‐directed fluid therapy in elective major abdominal surgery: a meta‐analysis of randomized controlled trials. Ann Surg 2016; 263: 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN et al; Enhanced Recovery After Surgery Society . Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012; 31: 801–816. [DOI] [PubMed] [Google Scholar]
- 4. Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N et al; Enhanced Recovery After Surgery Society . Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012; 31: 783–800. [DOI] [PubMed] [Google Scholar]
- 5. Calvo‐Vecino JM, Ripollés‐Melchor J, Mythen MG, Casans‐Francés R, Balik A, Artacho JP et al; FEDORA Trial Investigators Group . Effect of goal‐directed haemodynamic therapy on postoperative complications in low‐moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial). Br J Anaesth 2018; 120: 734–744. [DOI] [PubMed] [Google Scholar]
- 6. Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L et al Clinical practice guideline for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons (ASCRS) and Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Surg Endosc 2017; 31: 3412–3436. [DOI] [PubMed] [Google Scholar]
- 7. Thiele RH, Raghunathan K, Brudney CS, Lobo DN, Martin D, Senagore A et al; Perioperative Quality Initiative (POQI) I Workgroup . American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on perioperative fluid management within an enhanced recovery pathway for colorectal surgery. Perioper Med (Lond) 2016; 5: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence (NICE) . CardioQ‐ODM Oesophageal Doppler Monitor; March 2011. https://www.nice.org.uk/Guidance/MTG3 [accessed 12 July 2018].
- 9. Som A, Maitra S, Bhattacharjee S, Baidya DK. Goal directed fluid therapy decreases postoperative morbidity but not mortality in major non‐cardiac surgery: a meta‐analysis and trial sequential analysis of randomized controlled trials. J Anesth 2017; 31: 66–81. [DOI] [PubMed] [Google Scholar]
- 10. Chong MA, Wang Y, Berbenetz NM, McConachie I. Does goal‐directed haemodynamic and fluid therapy improve peri‐operative outcomes?: a systematic review and meta‐analysis. Eur J Anaesthesiol 2018; 35: 469–483. [DOI] [PubMed] [Google Scholar]
- 11. European Medicines Agency . Hydroxyethyl‐Starch Solutions for Infusion to be Suspended – CMDh Endorses PRAC Recommendation; 2018. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2018/01/news_detail_002892.jsp&mid=WC0b01ac058004d5c1 [accessed 16 August 2018].
- 12. Brunkhorst FM, Engel C, Bloos F, Meier‐Hellmann A, Ragaller M, Weiler N et al; German Competence Network Sepsis(SepNet) . Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358: 125–139. [DOI] [PubMed] [Google Scholar]
- 13. Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D et al; CHEST Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group . Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012; 367: 1901–1911. [DOI] [PubMed] [Google Scholar]
- 14. Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A et al; 6S Trial Group; Scandinavian Critical Care Trials Group . Hydroxyethyl starch 130/0·42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012; 367: 124–134. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 16. Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K et al Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005; 24: 466–477. [DOI] [PubMed] [Google Scholar]
- 17. Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C et al; Enhanced Recovery After Surgery (ERAS) Group . Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009; 144: 961–969. [DOI] [PubMed] [Google Scholar]
- 18. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 20. Reisinger KW, Willigers HM, Jansen J, Buurman WA, Von Meyenfeldt MF, Beets GL et al Doppler‐guided goal‐directed fluid therapy does not affect intestinal cell damage but increases global gastrointestinal perfusion in colorectal surgery: a randomized controlled trial. Colorectal Dis 2017; 19: 1081–1091. [DOI] [PubMed] [Google Scholar]
- 21. Phan TD, D'Souza B, Rattray MJ, Johnston MJ, Cowie BS. A randomised controlled trial of fluid restriction compared to oesophageal Doppler‐guided goal‐directed fluid therapy in elective major colorectal surgery within an enhanced recovery after surgery program. Anaesth Intensive Care 2014; 42: 752–760. [DOI] [PubMed] [Google Scholar]
- 22. Srinivasa S, Taylor MH, Singh PP, Yu TC, Soop M, Hill AG. Randomized clinical trial of goal‐directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br J Surg 2013; 100: 66–74. [DOI] [PubMed] [Google Scholar]
- 23. Brandstrup B, Svendsen PE, Rasmussen M, Belhage B, Rodt SÅ, Hansen B et al Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near‐maximal stroke volume or zero fluid balance? Br J Anaesth 2012; 109: 191–199. [DOI] [PubMed] [Google Scholar]
- 24. Zakhaleva J, Tam J, Denoya PI, Bishawi M, Bergamaschi R. The impact of intravenous fluid administration on complication rates in bowel surgery within an enhanced recovery protocol: a randomized controlled trial. Colorectal Dis 2013; 15: 892–899. [DOI] [PubMed] [Google Scholar]
- 25. Challand C, Struthers R, Sneyd JR, Erasmus PD, Mellor N, Hosie KB et al Randomized controlled trial of intraoperative goal‐directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth 2012; 108: 53–62. [DOI] [PubMed] [Google Scholar]
- 26. Gómez‐Izquierdo JC, Trainito A, Mirzakandov D, Stein BL, Liberman S, Charlebois P et al Goal‐directed fluid therapy does not reduce primary postoperative ileus after elective laparoscopic colorectal surgery: a randomized controlled trial. Anesthesiology 2017; 127: 36–49. [DOI] [PubMed] [Google Scholar]
- 27. Senagore AJ, Emery T, Luchtefeld M, Kim D, Dujovny N, Hoedema R. Fluid management for laparoscopic colectomy: a prospective, randomized assessment of goal‐directed administration of balanced salt solution or hetastarch coupled with an enhanced recovery program. Dis Colon Rectum 2009; 52: 1935–1940. [DOI] [PubMed] [Google Scholar]
- 28. Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler‐optimized fluid management on outcome after elective colorectal resection. Br J Surg 2006; 93: 1069–1076. [DOI] [PubMed] [Google Scholar]
- 29. Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR et al Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005; 95: 634–642. [DOI] [PubMed] [Google Scholar]
- 30. Conway DH, Mayall R, Abdul‐Latif MS, Gilligan S, Tackaberry C. Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Anaesthesia 2002; 57: 845–849. [DOI] [PubMed] [Google Scholar]
- 31. Zhang L, Wang S, Liu B. Goal‐directed intraoperative fluid therapy guided by mini‐fluid challenge in elective colorectal resection: a prospective randomized study. Br J Anaesth 2016; 116: e924 (Abstract). [Google Scholar]
- 32. Bellamy MC. Wet, dry or something else? Br J Anaesth 2006; 97: 755–757. [DOI] [PubMed] [Google Scholar]
- 33. Doherty M, Buggy DJ. Intraoperative fluids: how much is too much? Br J Anaesth 2012; 109: 69–79. [DOI] [PubMed] [Google Scholar]
- 34. Lobo DN. Fluid overload and surgical outcome: another piece in the jigsaw. Ann Surg 2009; 249: 186–188. [DOI] [PubMed] [Google Scholar]
- 35. Xu C, Peng J, Liu S, Huang Y, Guo X, Xiao H et al Goal‐directed fluid therapy versus conventional fluid therapy in colorectal surgery: a meta analysis of randomized controlled trials. Int J Surg 2018; 56: 264–273. [DOI] [PubMed] [Google Scholar]
- 36. Srinivasa S, Kahokehr A, Soop M, Taylor M, Hill AG. Goal‐directed fluid therapy – a survey of anaesthetists in the UK, USA, Australia and New Zealand. BMC Anesthesiol 2013; 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feldheiser A, Pavlova V, Weimann K, Hunsicker O, Stockmann M, Koch M et al Haemodynamic optimization by oesophageal Doppler and pulse power wave analysis in liver surgery: a randomised controlled trial. PLoS One 2015; 10: e0132715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nordström J, Hällsjö‐Sander C, Shore R, Björne H. Stroke volume optimization in elective bowel surgery: a comparison between pulse power wave analysis (LiDCOrapid) and oesophageal Doppler (CardioQ). Br J Anaesth 2013; 110: 374–380. [DOI] [PubMed] [Google Scholar]
- 39. Feldheiser A, Hunsicker O, Krebbel H, Weimann K, Kaufner L, Wernecke KD et al Oesophageal Doppler and calibrated pulse contour analysis are not interchangeable within a goal‐directed haemodynamic algorithm in major gynaecological surgery. Br J Anaesth 2014; 113: 822–831. [DOI] [PubMed] [Google Scholar]
- 40. Bahlmann H, Hahn RG, Nilsson L. Agreement between pleth variability index and oesophageal Doppler to predict fluid responsiveness. Acta Anaesthesiol Scand 2016; 60: 183–192. [DOI] [PubMed] [Google Scholar]
- 41. Lin Q, Zhou H, Li D, Ye J, Hong J, Hu Y. [Effect of perioperative goal‐directed fluid therapy on clinical outcome in elective colorectal resection.] Zhonghua Wei Chang Wai Ke Za Zhi 2015; 18: 671–675. [PubMed] [Google Scholar]
- 42. McKenny M, O'Malley C, Mehigan B, McCormick P, Dowd N. Introduction of oesophageal Doppler‐guided fluid management in a laparoscopic colorectal surgery enhanced recovery programme: an audit of effect on patient outcome. Ir Med J 2014; 107: 135–138. [PubMed] [Google Scholar]
- 43. Jammer I, Ulvik A, Erichsen C, Lødemel O, Ostgaard G. Does central venous oxygen saturation‐directed fluid therapy affect postoperative morbidity after colorectal surgery? A randomized assessor‐blinded controlled trial. Anesthesiology 2010; 113: 1072–1080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline patient demographics for all included studies
Table S2 Intraoperative fluid infused in goal‐directed and control groups


